Abstract
This study investigated the association between COVID-19 infection and host metabolic signatures as prognostic markers for disease severity and mortality. We enrolled 82 patients with RT-PCR confirmed COVID-19 infection who were classified as mild, moderate, or severe/critical based upon their WHO clinical severity score and compared their results with 31 healthy volunteers. Data on demographics, comorbidities and clinical/laboratory characteristics were obtained from medical records. Peripheral blood samples were collected at the time of clinical evaluation or admission and tested by quantitative mass spectrometry to characterize metabolic profiles using selected metabolites. The findings in COVID-19 (+) patients reveal changes in the concentrations of glutamate, valeryl-carnitine, and the ratios of Kynurenine/Tryptophan (Kyn/Trp) to Citrulline/Ornithine (Cit/Orn). The observed changes may serve as predictors of disease severity with a (Kyn/Trp)/(Cit/Orn) Receiver Operator Curve (ROC) AUC = 0.95. Additional metabolite measures further characterized those likely to develop severe complications of their disease, suggesting that underlying immune signatures (Kyn/Trp), glutaminolysis (Glutamate), urea cycle abnormalities (Cit/Orn) and alterations in organic acid metabolism (C5) can be applied to identify individuals at the highest risk of morbidity and mortality from COVID-19 infection. We conclude that host metabolic factors, measured by plasma based biochemical signatures, could prove to be important determinants of Covid-19 severity with implications for prognosis, risk stratification and clinical management.
Introduction
On December 31, 2019, a cluster of atypical pneumonia cases was reported in Wuhan, Hubei province, China. By mid-January 2020, the first case of this SARS/MERS variant dubbed COVID-19 was reported in the United States. Over time, this coronavirus variant rapidly spread around the world resulting in one of worst pandemics in modern history [1]. While 80% of infected individuals show mild symptoms, approximately 20% progress to pneumonia, ARDS, multi-organ failure or death [2], with the highest risk of symptoms and complications occurring among persons with pre-existing co-morbidities including obesity, diabetes mellitus, hypertension, and cardiovascular disease [3]. The association between these cardio-metabolic conditions and disease severity suggested the possibility of a metabolic predisposition [4].
We had previously examined the association between retroviral infection with the HIV-Lentivirus and the levels of 186 different metabolites quantified using tandem mass spectrometry (MS/MS) conducted upon plasma. We identified metabolomic signatures that could distinguish HIV rapid-progressors and immunologic-non-responders from controls, suggesting that host metabolic factors strongly influenced the severity of HIV infection [5].
To determine whether similar metabolic signatures are found in patients with COVID-19 infection and to examine the impact of these signatures upon clinical outcome, we conducted a prospective study on the plasma of 82 patients positive for COVID-19 infection by RT-PCR and compared the results with 31 plasma samples from healthy volunteers using quantitative tandem MS/MS.
Materials and methods
Study design and patient accrual
A cross sectional and prospective observational study was conducted at CASSEMS General Hospital in the city of Campo Grande, Mato Grosso do Sul State (southwestern Brazil), in collaboration with investigators from the Federal University of São Paulo (EPM-UNIFESP), São Paulo, Brazil; Nagourney Institute and Metabolomycs, Inc., both in Long Beach, California, USA.
The protocol was approved by the Institutional Review Board from the Federal University of São Paulo (CEP/UNIFESP—approval CAAE: 37348020.3.0000.5505) and was conducted in compliance with the World Medical Association Declaration of Helsinki. Written informed consents were obtained from all participants.
All patients who were accrued to the study tested positive for SARS-CoV2 and were followed for clinical outcome, categorized as mild (n = 20), moderate (n = 32) or severe (n = 30) according to World Health Organization classification of severity [6]. The control group (n = 31) was composed of healthy volunteers who tested negative for SARS-CoV2. All patients and controls submitted an EDTA-purple-top tube collected from peripheral blood samples, obtained at the time of protocol accrual. All patients and control subjects provided written informed consent for participation in the study protocol.
Inclusion and exclusion criteria
Between November 30th, 2020, and January 20th, 2021, all patients over the age 18 who presented to the Cassems Hospital for evaluation of respiratory symptoms, who tested positive for COVID-19 by RT-PCR were eligible for inclusion.in the study. Patients positive for COVID-19 were stratified as mild, moderate, and severe/critical according to WHO criteria [6]. Healthy volunteers (consisting of CASSEMS Hospital healthcare providers) who tested negative for SARS-CoV2 served as controls.
Clinical and laboratory data assessment
Nasal and pharyngeal swab specimens were collected either in the emergency room (ER) or during hospitalization, and a confirmed case of COVID-19 was defined as having detectable SARS-CoV-2 virus on real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay, carried out according to validated protocols [7].
Clinical data was extracted by chart review from physician notes and medical records in the CASSEMS healthcare database. Data on symptoms and vital signs were collected at initial presentation in the ER or as part of the admission history. Data on past medical history and comorbidities were collected from medical records. Fever was defined as forehead temperature >37.4°C (>99.3 F), and hypoxemia was defined as pulse oximetry reading from finger oximeter <90%. Hypotension was defined as mean arterial pressure (MAP) <65 mmHg and tachycardia were defined as heart rate (HR) >100 beats per minute (bpm). All laboratory values on the day of admission or during hospitalization were collected from the Medical Records. Laboratory values included complete blood counts, blood chemistry including renal function, C-reactive protein (C-RP), d-dimer, arterial blood gas. Details of radiologic examinations such as computed tomography (CT) scanning of the chest were also collected [8, 9]. Clinical and laboratory data was collected and analyzed by the healthcare team at CASSEMS Hospital who vouched for accuracy and completeness of data and for adherence of study to protocol.
Study outcomes
The primary composite endpoint is recovery or WHO-classified severity of illness defined as the need for mechanical ventilation, use of inotrope support, intensive care unit (ICU) admission, or death. Secondary endpoints are development of acute respiratory distress syndrome (ARDS), secondary pneumonia; acute renal failure, acute cardiac injury, and length of hospital stay [10].
Collection of blood samples
Peripheral venous blood samples from each patient/volunteer were collected using tubes with anti-clotting factor (EDTA). Immediately after blood collection, samples were centrifuged (5 min at 4000 rpm). After centrifugation, the plasma was aliquoted, frozen, and stored at −80°C for targeted mass spectrometry analysis.
Metabolomic analysis workflow
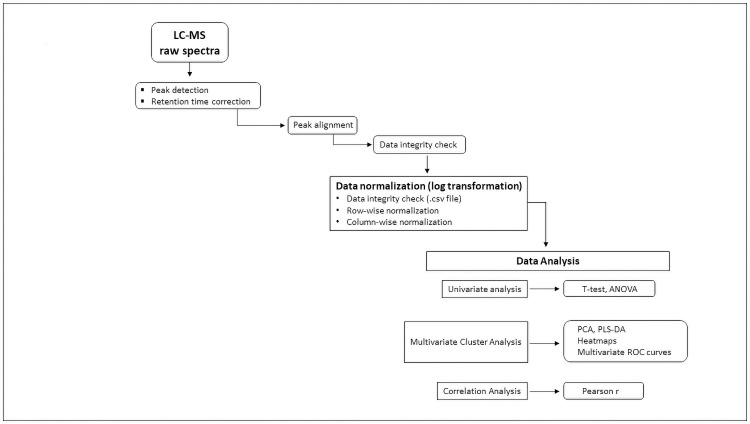
Fig 1 provides sample processing flowsheet from plasma receipt to liquid chromatography and mass spectrometry through data analysis.
Fig 1. Flowchart illustrating workflow and data processing.
Individual metabolite absolute concentrations measured by targeted mass spectrometry (MS/MS) transmitted in.csv data-files were log transformed for normalization and then uploaded into MetaboAnalyst 5.0 bio-informatic data analytic platform. Univariate (t-test, ANOVA), multivariate (PCA, PLS-DA, Heatmaps, Multivariate ROC Curve Analysis) and correlation coefficients (Pearson r) then applied to identify metabolites and ratios associated with COVID-19.
Targeted quantitative MS/MS analysis
In this study, targeted metabolomic analyses of plasma samples were performed using Absolute IDQ® P180 kit from Biocrates (Biocrates, Life Science AG, Innsbruck, Austria). This validated targeted assay allows simultaneous detection and absolute quantification of metabolites in plasma in a high throughput manner. This kit can be used on a variety of LC-MS/MS instruments and has already been applied to many studies of human serum and plasma, including several large-scale prospective cohort studies [11–15]. Absolute quantification (μmol/L) of blood metabolites was achieved by targeted quantitative profiling of 186 annotated metabolites by electrospray ionization (ESI) tandem mass spectrometry (MS/MS) in 113 biological samples, blinded to any phenotype information, on a centralized, independent, fee-for-service basis at the quantitative metabolomics platform from BIOCRATES Life Sciences AG, Innsbruck, Austria. Briefly, a targeted profiling scheme was used to quantitatively screen for fully annotated metabolites using multiple reaction monitoring, neutral loss, and precursor ion scans. Quantification of metabolite concentrations and quality control assessment were performed with the MetIQ software package (BIOCRATES Life Sciences AG, Innsbruck, Austria), which implies proof of reproducibility within a given error range. An MS Excel file (.xls) was then generated, which contained sample identification and 186 metabolite names and concentrations with the unit of μmol/L of plasma [16].
Metabolite panel
In total, 186 annotated metabolites were quantified using the Biocrates AbsoluteIDQ® p180 kit (Biocrates Life Sciences AG, Innsbruck, Austria), being 40 acylcarnitines (ACs), 21 amino acids (AAs), 19 biogenic amines (BA), sum of hexoses (Hex), 76 phosphatidylcholines (PCs), 14 lysophosphatidylcholines (LPCs) and 15 sphingomyelins (SMs), glycerophospholipids were further differentiated with respect to the presence of ester (a) and ether (e) bonds in the glycerol moiety, where two letters denote that two glycerol positions are bound to a fatty acid residue (aa = diacyl, ae = acyl-alkyl), while a single letter indicates the presence of a single fatty acid residue (a = acyl or e = alkyl) [17].
Validation tests
For Metabolomic Data Analysis, log-transformation was applied to all quantified metabolites to normalize the concentration distributions and uploaded into the web based analytical pipelines MetaboAnalyst 5.0 (www.metaboanalyst.ca/faces/upload/RocUploadView.xhtml) and Receiver Operating Characteristic Curve Explorer & Tester (ROCCET) available at (https://www.metaboanalyst.ca/resources/data/metabolomics2012_xia.pdf) for the generation of uni- and multivariate Receiver Operating Characteristic (ROC) curves obtained through Support Vector Machine (SVM), Partial Least Squares-Discriminant Analysis (PLS-DA) and Random Forests as well as Logistic Regression Models were used to calculate Odds Ratios of specific metabolites. ROC curves were generated by Monte-Carlo Cross Validation (MCCV) using balanced sub-sampling where two thirds (2/3) of the samples were used to evaluate the feature importance. Significant features were then used to build classification models, which were validated on the 1/3 of the samples that were left out on the first analysis. The same procedure was repeated 10–100 times to calculate the performance and confidence interval of each model. To further validate the statistical significance of each model, ROC calculations included bootstrap 95% confidence intervals for the desired model specificity as well as accuracy after 1000 permutations and false discovery rates (FDR) calculation [18].
Statistical analysis
Sample characteristics were evaluated with continuous variables expressed as means and standard deviation and categorical variables as frequencies and percentages. Logistic regression models were fit to compare the effects of each metabolite measure as a potential predictor on each clinical outcome, both with and without control for age, sex, and BMI to estimate unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CIs) for the association between each metabolite and each outcome. P-Statistical significance was set at values P <0.05 Statistical analyses for these clinical outcome models were performed using R version 4.0.1.
Results and discussion
The COVID-19 pandemic has had a profound impact upon every aspect of human existence with over 224,588,128 cases and 4,628,882 deaths reported by the WHO as of September 2021. The resulting disruptions have devastated economies, overwhelmed health care delivery and severely restricted international trade and travel.
The medical community’s response to COVID-19 has largely focused upon the infecting agent’s virulence, mode of transmission, infectivity, and molecular features. While we have come to understand the virus’s capacity to gain entry to the cell via the ACE-2 receptor, characterized the structure of the Spike Protein, identified mutational variants, and developed vaccines to prevent infection and transmission [19, 20], less is known about the effectiveness of the host’s response to the infection.
Severe complications of COVID-19 including coagulopathies, ARDS, hepatic and renal failure and multisystem damage are shared by other infectious processes [21].
To better understand the physiologic response of the host to COVID-19 infection we used plasma metabolic signatures to examine the intrinsic features of each patient’s mechanisms of defense. Our question being: Is it the pathogenicity of the infecting organism or the host’s response and defenses that determine the ultimate morbidity and mortality of the disease? With insights from our prior work in HIV [5] and that of Davanzo et al. [22], we explored metabolic signatures in the plasma of COVID-19 patients.
The study sample had mean age 48.6 years (SD = 12.5 years), 51% male, 75% overweight or obese and had high prevalence of comorbid health conditions, notably hypertension (33%) and diabetes mellitus (12%). COVID-related symptoms were very common at the time of presentation to the hospital, with half of the sample presenting with cough, and several other known symptoms commonly reported (fever: 43%, asthenia: 31%, dyspnea: 29% and myalgia: 29%). While the majority of patients recovered or were discharged (68%), several patients required supplemental oxygen (16%) or intubation with mechanical ventilation (16%). Sample characteristics overall and by disease severity are shown in Table 1.
Table 1. Patient demographics and clinical characteristics.
| Variables | All Groups | Severe/Critical | Moderate | Mild | Control |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 113 (100%) | 30 (27%) | 32 (28%) | 20 (18%) | 31 (100%) |
| Demographics | |||||
| Age (years), mean (SD) | 48.58 (12.53) | 56.77 (10.2) | 53.25 (10.4) | 43.25 (10.5) | 39.29 (10.28) |
| Male | 58 (51.3%) | 23 (76.7%) | 18 (56.2%) | 7 (35.0%) | 10 (32.3%) |
| Female | 55 (48.7%) | 7 (23.3%) | 14 (43.8%) | 13 (65.0%) | 21 (67.7%) |
| BMI categories | |||||
| Normal | 28 (24.8%) | 3 (10%) | 3 (9.4%) | 9 (45%) | 13 (41.9%) |
| Overweight | 43 (38.1%) | 12 (40%) | 12 (37.5%) | 6 (30%) | 13 (41.9%) |
| Obese | 42 (37.2%) | 15 (50%) | 17 (53.1%) | 5 (25%) | 5 (16.1%) |
| Comorbidities | |||||
| Cardiovascular disease | 9 (8%) | 7 (23.3%) | 2 (6.2%) | 0 (0%) | - |
| Hypertension | 37 (32.7%) | 19 (63.3%) | 17 (53.1%) | 1 (5%) | - |
| Chronic pulmonary disease (asthma, COPD) | 4 (3.5%) | 2 (6.7%) | 2 (6.2%) | 0 (0%) | - |
| Dyslipidemia | 3 (2.7%) | 1 (3.3%) | 2 (6.2%) | 0 (0%) | - |
| Diabetes mellitus | 14 (12.4%) | 7 (23.3%) | 7 (21.9%) | 0 (0%) | - |
| History of smoking | 7 (6.2%) | 4 (13.3%) | 2 (6.2%) | 1 (5%) | - |
| COVID symptoms at presentation | - | ||||
| Cough | 57 (50.4%) | 21 (70%) | 25 (78.1%) | 11 (55%) | - |
| Shortness of breath | 22 (19.5%) | 8 (26.7%) | 11 (34.4%) | 3 (15%) | - |
| Dyspnea | 33 (29.2%) | 19 (63.3%) | 13 (40.6%) | 1 (5%) | - |
| Fever | 48 (42.5%) | 19 (63.3%) | 19 (59.4%) | 10 (50%) | - |
| Myalgia | 33 (29.2%) | 12 (40%) | 15 (46.9%) | 6 (30%) | - |
| Odinophagy | 23 (20.4%) | 11 (36.7%) | 4 (12.5%) | 8 (40%) | - |
| Rhinorrhea | 14 (12.4%) | 2 (6.7%) | 6 (18.8%) | 6 (30%) | - |
| Diarrhea | 12 (10.6%) | 7 (23.3%) | 3 (9.4%) | 2 (10%) | - |
| Vomit | 3 (2.7%) | 2 (6.7%) | 1 (3.1%) | - | - |
| Ageusia | 10 (8.8%) | 5 (16.7%) | 4 (12.5%) | 1 (5%) | - |
| Anosmia | 11 (9.7%) | 5 (16.7%) | 5 (15.6%) | 1 (5%) | - |
| Asthenia | 35 (31%) | 16 (53.3%) | 15 (46.9%) | 4 (20%) | - |
| Vital signs and laboratory measures | - | ||||
| Oxygen saturation > 95% | 67 (59.3%) | 1 (3.3%) | 15 (46.9%) | - | - |
| D-dimer, mean (SD) | 3.92 (19.89) | 1.98 (5.44) | 0.75 (0.47) | 36.49 (72.34) | - |
| Creatinine, mean (SD) | 0.94 (0.35) | 1.04 (0.48) | 0.87 (0.2) | 0.87 (0.15) | - |
| Urea, mean (SD) | 36.8 (17.15) | 43.55 (22.55) | 32.43 (10.01) | 30.11 (6.85) | - |
| C-reactive protein, mean (SD) | 63.7 (88.1) | 142.67 (99.14) | 75.72 (77.87) | 6.02 (4.21) | 2.77 (3.87) |
| Chest CT category (Ground Glass Opacities) | - | ||||
| 0 –no GG opacities | 2 (1.8%) | 0 (0%) | 0 (0%) | 2 (15.4%) | - |
| I–up to 25% | 32 (28.3%) | 3 (10%) | 18 (56.2%) | 11 (84.6%) | - |
| II– 25%–50% | 23 (20.4%) | 12 (40%) | 11 (34.4%) | - | - |
| III–> 50% | 18 (15.9%) | 15 (50%) | 3 (9.4%) | - | - |
| Metabolite and ratio measures, mean (SD) | |||||
| IDO (Kyn/Trp) | 0.07 (0.07) | 0.12 (0.1) | 0.06 (0.03) | 0.03 (0.01) | - |
| (Cit/Orn) | 0.24 (0.11) | 0.21 (0.08) | 0.21 (0.09) | 0.35 (0.12) | - |
| [(Kyn/Trp)/(Cit/Orn)] | 0.39 (0.37) | 0.6 (0.47) | 0.36 (0.25) | 0.11 (0.08) | - |
| (IDO/lysoPC a C18:0) | 0.0027 (0.0038) | 0.0046 (0.0056) | 0.0020 (0.0014) | 0.00092 (0.00080) | - |
| (Glu/PC aa C34:3) | 11 (9.28) | 14.93 (10.36) | 11.24 (8.54) | 4.73 (4.27) | - |
| (Asp/PC aa C34:3) | 0.75 (0.56) | 0.92 (0.46) | 0.81 (0.68) | 0.39 (0.31) | - |
| (IDO/PC aa C34:3) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0) | 0 (0) | - |
| C5 | 0.38 (0.31) | 0.5 (0.39) | 0.36 (0.24) | 0.24 (0.18) | - |
| COVID-19-related Outcomes | |||||
| Recovered/discharged | 77 (68.1%) | 25 (83.3%) | 32 (100%) | 20 | - |
| Required oxygen mask | 18 (15.9%) | 15 (50%) | 18 (15.9%) | - | - |
| OTI + Mechanical Ventilation | 18 (15.9%) | 18 (60%) | 18 (15.9%) | - | - |
| Tracheostomy | 7 (6.2%) | 7 (23.3%) | 7 (6.2%) | - | - |
| Death | 5 (4.4%) | 5 (16.7%) | 5 (4.4%) | - | 0 (0%) |
Plasma samples were obtained on all patients accrued to the study but processing errors in sample cryopreservation resulted in the loss of 5 samples leaving 77/82 (94%) of the samples fully evaluable.
Table 2 provides the most discriminating lipid ratios identified from the initial set of 186 metabolites.
Table 2. The most discriminating lipid ratios obtained from the data set of 186 metabolites.
| METABOLITE RATIOS | AUC | T-tests |
|---|---|---|
| [(lysoPC a C26:0/PC ae C42:0)/PC aa C40:3] | 0.99 | 2.10e-109 |
| [(lysoPC a C26:0/PC aa C40:3)/PC ae C42:0] | 0.99 | 2.10e-109 |
| (lysoPC a C26:0/PC ae C42:0) | 0.99 | 8.59e-115 |
| (lysoPC a C28:0/PC ae C42:0) | 0.99 | 1.48e-124 |
| (lysoPC a C26:0/PC aa C40:3) | 0.99 | 3.07e-105 |
| (lysoPC a C28:1/PC ae C42:0) | 0.99 | 9.78e-108 |
| (lysoPC a C26:0/PC aa C34:2) | 0.99 | 5.01e-93 |
| (lysoPC a C26:0/PC aa C42:6) | 0.99 | 8.91e-93 |
| (lysoPC a C26:0/PC aa C36:3) | 0.99 | 2.46e-93 |
| (lysoPC a C26:0/PC aa C36:4) | 0.99 | 3.05e-86 |
| (lysoPC a C26:0/PC ae C42:1) | 0.99 | 6.76e-99 |
| (lysoPC a C26:0/PC aa C40:2) | 0.99 | 1.19e-92 |
| (lysoPC a C26:0/PC aa C42:5) | 0.98 | 7.55e-83 |
| (lysoPC a C26:0/PC aa C36:2) | 0.98 | 1.05e-92 |
| (lysoPC a C28:1/PC aa C32:3) | 0.98 | 1.52e-97 |
Ratios utilized in multivariate ROC curve analysis 100-fold Cross Validation = 0.99, Permutation Test Statistics = p < 3.52e-6.
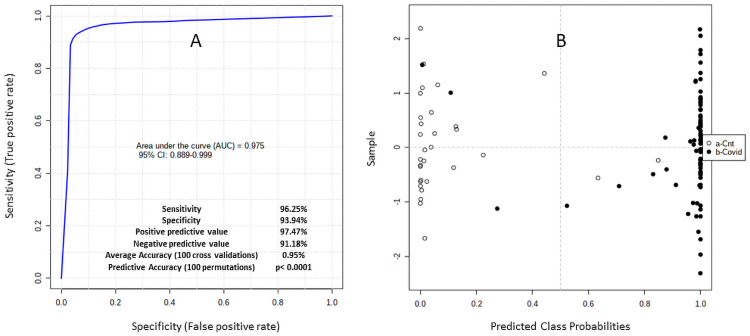
These profiles segregated Covid-19 (+) patients from controls. Fig 2A provides the results of an unsupervised clustering analysis using the ratios from Table 2 to segregate controls (n = 31) from COVID-19 (+) patients (n = 77). Fig 2B shows the results of a multivariate analysis providing a receiver operator curve (ROC) AUC = 0.975 (95% CI 0.889–0.999).
Fig 2.
(A, B) reflect unsupervised clustering analysis using the most discriminating ratios that segregate controls (n = 31) from COVID-19 (+) patients (n = 77). The average accuracy based on 100 cross validations is 0.95 with an ROC AUC = 0.975 (95% CI 0.889–0.999) and permutation test statistic: p<0.0001.
With the ROC curve AUC = 0.975 that clearly identified a metabolic signature for Covid 19 infection, we included additional metabolites that were identified in the 77 Covid-19 (+) patient cohort to compare the signatures of 18 patients with mild infection to 59 patients with moderate or severe infection as defined by WHO criteria [6].
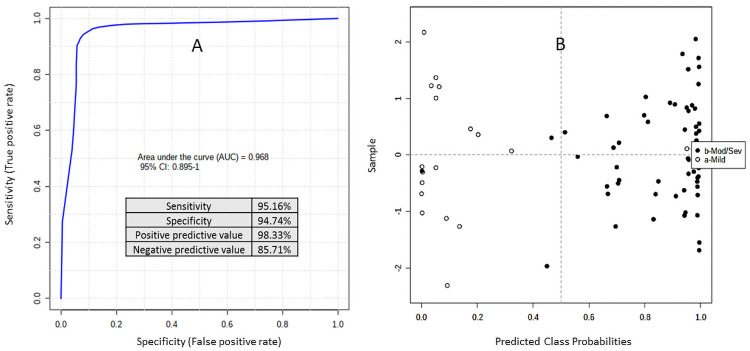
Fig 3A and 3B represents prediction, at base line, of patients with mild (n = 18) [empty circles] and moderate/severe Covid-19 (n = 59) [black circles]. This multivariate ROC Curve analysis used [(Glu/PC ae C42:1)/Taurine] and {[IDO/(Cit/Orn)]/(PC ae C36:4). The average accuracy based on 100 cross validations is 0.90, Permutation Test (x500) statistics = p< 7.10e-05. The ROC curve with an AUC = 0.968 (95% CI 0.895–1.0) sensitivity = 95.16%, Specificity = 94.74%, (+) predictive value = 98.33% and (-) predictive value = 85.71% indicates that plasma samples provide a robust predictor of Covid 19 morbidity and mortality.
Fig 3.
(A, B) provide base line predictions separating mild from moderate/severe using multivariate ROC Curve analysis applying the ratios obtained from the training set (Fig 2A and 2B): [(Glu/PC ae C42:1)/Taurine] and [IDO/(Cit/Orn)]/(PC ae C36:4). The average accuracy based on 100 cross validations is 0.90, Permutation Test (x500) statistics = p< 7.10e-05.
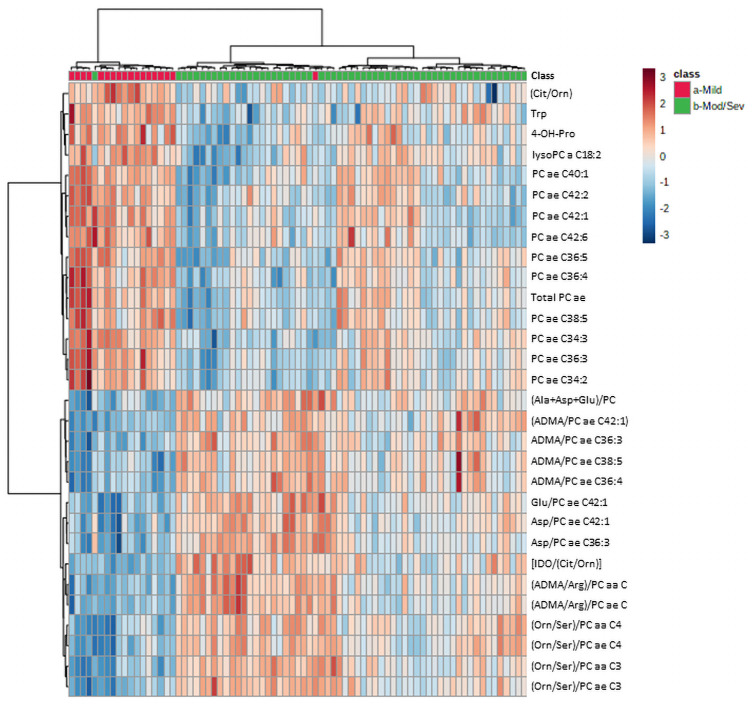
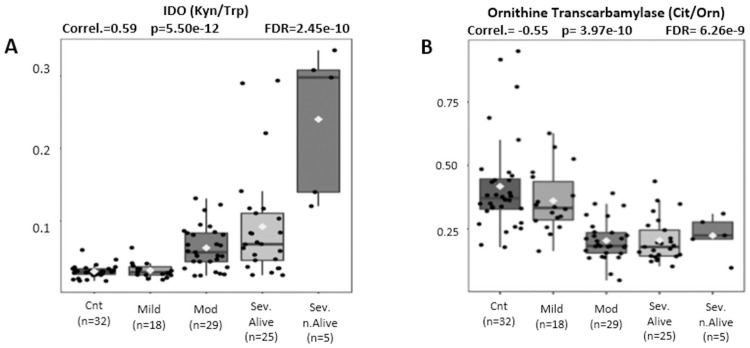
Fig 4 provides an unsupervised clustering analysis as a heat map comparing mild (red) to moderate/severe (green) Covid infection. The results indicate that Covid 19 severity is associated with a decline in tryptophan (Trp) reflecting immune dysregulation. Early evidence that tryptophan metabolism regulated immunity (D) has more recently led to the observation that kynurenine/tryptophan ratios correlate with carbohydrate metabolism and cardio-metabolic risk [23] both associated with COVID-19 severity [24].
Fig 4. Heatmap of unsupervised clustering analysis using 30 most discriminating metabolites and ratios comparing mild (red) vs moderate/severe (green) Covid 19 outcomes.
Alterations in liver function reflected by changes in the urea cycle (Cit/Orn), are consistent with the prior observations that patients with underlying liver disease are at significantly increased risk of morbidly and mortality from COVID-19 infection [25].
Increased inflammation associated with a decline in phosphatidyl cholines and a rise in lysophosphatidyl cholines, the result of phospholipase activation [26], reflects the inflammatory response to COVID-19 characteristic of hyper-immunity and an increased risk of morbidity and mortality as recently reported [27].
Finally, the results reveal increases in ADMA, a marker of epigenetic reprogramming that is associated with inflammation-related release of endothelial Nitric Oxide (NO) and has been shown to predict in-hospital mortality in COVID-19 patients [28].
While the measurement of individual metabolites provided insights into Covid-19 severity, ratios of analytes proved superior for the prediction of disease severity as they combined a multitude of metabolic perturbations into highly discriminating signatures.
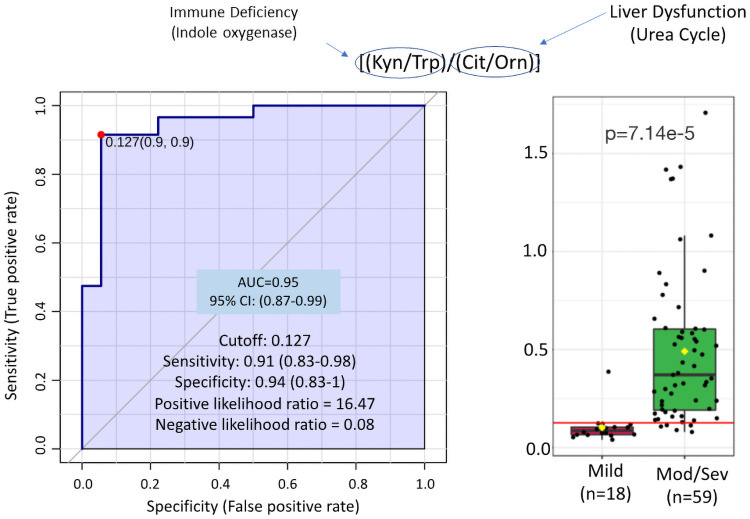
Fig 5 provides the ratio of Kynurenine/Tryptophan (Kyn/Trp) divided by Citrulline/Ornithine (Cit/Orn) comparing mild (n = 18) to moderate/severe (n = 59) Covid-19 infection. By combining the IDO/TDO (indoleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO) immune-ratio of Kynurenine/Tryptophan [29] with the liver-dysfunction-urea-cycle ratio of Citrulline/Ornithine [30] the receiver operator curve (ROC) AUC = 0.95 (95% CI 0.87–0.99) provides a more discriminating measure of disease severity confirming the multi-factorial nature of effective response to Covid-19.
Fig 5. Ratio of immune dysfunction reflected by indole oxygenase activity (Kyn/Trp) over liver dysfunction reflected by ornithine transcarbamylase (Cit/Orn) discriminates patients with mild vs moderate/severe outcomes.
100-fold Cross Validation = 0.82, Predictive Accuracy (100 permutations) p = 1.00E-03.
Multivariate regression models were fit for each of four outcomes (moderate/severe vs. mild COVID-19); need for ventilator; complications besides pneumonia; and death), with independent variables including the metabolite measured, controlled for age, sex, and BMI. Models could not be run for several of the outcomes due to low numbers. However, our findings in Table 3 indicate that the ratio of glutamate and PC ae C34:3 was significantly positively associated with risk of developing moderate/severe COVID (OR = 1.283, 95% CI = 1.07, 1.68). The ratio of (Kynurenine/Tryptophan)/(Citrulline/Ornithine) (Kyn/Trp)/(Cit/Orn) was associated with increased risk of complications other than pneumonia (OR = 73.9, 95% CI 8.2, 1282.7) and need for ventilator (OR = 20.6, 95% CI = 3.1, 206.9). Valeryl-carnitine (C5) levels were strongly associated with risk for each outcome, with ORs ranging between 8.3 to 48.4. No other metabolite measures were good predictors for any of the other outcomes.
Table 3. Logistic regression models for selected COVID-19-related outcomes adjusted for age, sex, and BMI.
| Moderate/Severe COVID | Needed ventilator | Any complications besides pneumonia | Death | |
|---|---|---|---|---|
| Value | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| IDO (Kyn/Trp) | - | - | - | - |
| (Cit/Orn) | - | 0.02 (0.00, 9.53) | 0.01 (0.00, 5.10) | - |
| [(Kyn/Trp)/(Cit/Orn)] | - | 20.62 (3.07, 206.96) * | 73.91 (8.20, 1282.65) * | 10.10 (0.51, 504.43) |
| (IDO/lysoPC a C18:0) | - | - | - | - |
| (Glu/PC ae C34:3) | 1.283 (1.07, 1.68) * | 1.05 (0.99, 1.12) | 1.02 (0.95, 1.09) | 1.04 (0.90, 1.12) |
| (Asp/PC aa C34:3) | 28.61 (2.95, 891.34) * | 2.03 (0.60, 5.93) | 1.46 (0.34, 4.70) | 1.964 (0.08, 41.91) |
| (IDO/PC aa C34:3) | - | - | - | - |
| C5 | 48.44 (1.62, 6094.69) * | 8.35 (1.12, 71.91) * | 21.51 (2.66, 248.73) * | 8.95 (0.12, 1613.24) |
Note
* p <0.05.
As host response to viral infection reflects immune competence, we compared our Coronavirus COVID-19 signatures with those associated with the lentivirus HIV. It has been shown that certain subpopulations of HIV (+) individuals can tolerate the infection without progressing to AIDS. These individuals, known as “elites” [31] have been shown to have distinct metabolic features [5].
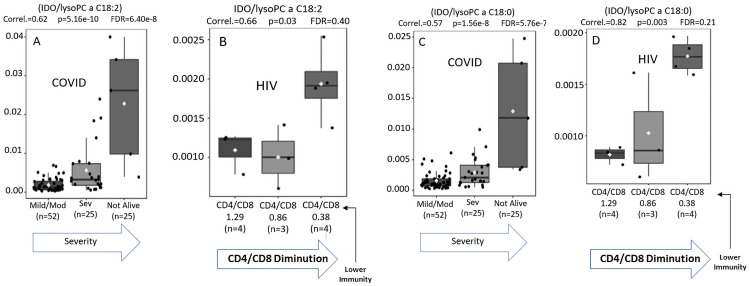
Fig 6A–6D compare the metabolic signatures of patients of mild versus moderate/severe Covid-19 infection with those obtained from individuals with HIV. As the ratio of CD4/CD8 is an established parameter of HIV severity [22] we used cut offs of CD4/CD8 ratios to compare HIV severity with COVID-19 severity (mild/moderate vs. severe) using WHO criteria [6].
Fig 6.
(A-D). Comparison of immune signatures for Covid 19 vs. HIV using immune IDO (Kyn/Trp) ratio divided by the inflammatory markers (lyso PC a 18:2 and 18:0) correlates Covid 19 severity with HIV progression.
Using the immune (IDO) ratio divided by the lipid specie LysoPCa18:2, a measure of inflammation, we found a strong correlation between these two related but distinct retroviral infections.
Fig 7A and 7B correlate immune dysfunction measured by IDO (kyn/Trp) and liver dysfunction measured by ornithine transcarbamylase activity (Cit/Orn) with disease severity from controls to mild, moderate, severe, and lethal clinical outcomes.
Fig 7.
(A, B). Pearson Moment correlations of IDO activity (Kyn/Trp) and Ornithine transcarbamylase activity (Cit/Orn) for disease severity comparing controls, mild, moderate, and severe Covid patients.
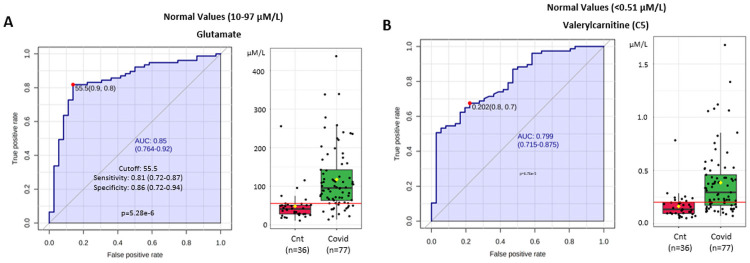
Fig 8A and 8B compare metabolic signatures for controls vs Covid 19 (+) patients using measures of glutaminolysis (Glutamate) and mitochondrial dysfunction reflected by organic acidemia (Valeryl-carnitine C5) to provide ROC curves with an AUC = 0.85 (95% CI 0.764–0.92) and an AUC = 0.799 (95% CI 0.715–0.875) respectively that clearly distinguish the two groups.
Fig 8.
(A, B). Glutamate and Valeryl-carnitine (C5) concentrations comparing controls (red) n = 36 to Covid 19 patients (green) n = 77 provide ROC AUC = 0.85 (95% CI 0.764–0.92) and AUC = 0.799 (95% CI 0.715–0.875) respectively.
Our findings are in agreement with the recent study reported by Herrera-Van Oostdam et al. [27] that identified immune-metabolic signatures as predictors of COVID-19 progression to sepsis. Among the similarities are perturbations in the Kynurenine/Tryptophan ratios, changes in phosphatidylcholine / lyso-phosphatidylcholine ratios and alterations in valeryl-carnitine. Italian investigators using targeted lipidomics have shown that COVID-19 is associated with alterations in sphingolipids, specifically ceramides [28].
The association between COVID-19 severity and obesity, diabetes, and cardiovascular disease [4] suggests that metabolic stress contributes to the morbidity and mortality of this infection [32, 33]. Recognizing that effective immune response draws upon numerous physiologic reserves, we found that COVID-19 severity could be predicted using algorithms that incorporate multiple aspects of altered metabolism. Combining lipid ratios with measures of liver dysfunction (Citrulline/Ornithine); mitochondrial dysfunction (Valerylcarnitine), glutaminolysis and immune response (Kyn/Trp) provided the most discriminating signatures.
To examine whether these findings extended to other infections, we compared the COVID-19 signatures with those associated with HIV infection. Correlations between the severity of HIV measured as CD4/CD8 ratios with the severity of COVID-19 by WHO criteria suggest that defense against these two distinct but related retroviral infections reflect shared features of human immune response.
Our findings suggest that host factors play an important role in COVID-19 pathogenicity. Metabolic changes may predispose certain individuals to higher risk of morbidity and mortality. In keeping with the recent findings of other investigators in the field, metabolomic analyses may provide important tools as we confront new challenges in the ongoing COVID-19 pandemic.
Limitations of the study
The study was undertaken as an exploratory analysis with patients accrued from a single institution in southwestern Brazil during the COVID-19 resurgence (second wave). Newly diagnosed patients were compared with suffering more severe illness. We recognize that pharmacologic interventions in the severe group including dexamethasone, supplemental oxygen, heparin, antibiotics and two patients who received tocilizumab could have had an impact on the observed metabolic signatures. No patients received Remdesivir. Future studies will accrue patients at first presentation to control for these variables.
Our control group consisted of PCR negative, healthy hospital staff who were regularly screened as part of hospital policy. Our controls could also have included patients presenting with respiratory symptoms who were then proven PCR negative, and this will be examined in future studies.
The principal limitation of the study was sample size that precluded a more thorough examination of clinical parameters of severity against biochemical measures. Logistic regression did reveal correlations, but the confidence intervals were large leaving many of the findings as hypothesis-generating.
Conclusions
We conclude that the severity of COVID-19 infection represents the complex interaction between the organisms’ innate pathogenicity and the hosts’ response. Commonalities between COVID-19 and HIV suggest a critical role for the host’s metabolic wellbeing as a determinant of clinical severity in these and perhaps many infectious processes. The metabolic signatures associated with COVID-19 severity may offer new diagnostic and prognostic determinations that could lead to novel interventions for the treatment or prevention of the biochemical frailties that predispose individuals to severe disease.
Acknowledgments
The completion of this study could not have been possible without the participation and assistance of Internal Medicine and Intensive Care Staff, Internal Medicine Residents and Nurses and Laboratory Staff members at Cassems Hospital, in the city of Campo Grande, Mato Grosso do Sul State, southwestern Brazil, involved in the treatment and sample collection of covid-19 patients accrued in the protocol since December 2020. Their contributions are sincerely appreciated and gratefully acknowledged.
Data Availability
All data is in the manuscript.
Funding Statement
Supported by a Research Grant from the Nagourney Institute, Long Beach, California, USA (ID# 95-4731388, Grant 001-2021). AMP was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funder provided support in the form of consulting fees for authors PD and IDCGS, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020. Mar;579(7798):265–269. Epub 2020 Feb 3. Erratum in: Nature. 2020 Apr;580(7803):E7. doi: 10.1038/s41586-020-2008-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020. May;39(5):405–407. Epub 2020 Mar 20. doi: 10.1016/j.healun.2020.03.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020. Dec;13(12):1833–1839. Epub 2020 Aug 4. doi: 10.1016/j.jiph.2020.07.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020. Jun;16(6):297–298. doi: 10.1038/s41574-020-0353-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarpellini B, Zanoni M, Sucupira MC, Truong HM, Janini LM, da Silva ID, et al. Correction: Plasma Metabolomics Biosignature According to HIV Stage of Infection, Pace of Disease Progression, Viremia Level and Immunological Response to Treatment. PLoS One. 2017. Feb 24;12(2):e0173164. Erratum for: PLoS One. 2016 Dec 12;11(12):e0161920. doi: 10.1371/journal.pone.0173164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The WHO COVID-19 Clinical management: living guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 7.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020. Jan;25(3):2000045. Erratum in: Euro Surveill. 2020 Apr;25(14): Erratum in: Euro Surveill. 2020 Jul;25(30): Erratum in: Euro Surveill. 2021 Feb;26(5): doi: 10.2807/1560-7917.ES.2020.25.3.2000045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020. Jul;92(7):797–806. Epub 2020 Apr 1. doi: 10.1002/jmv.25783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meirelles GSP. COVID-19: a brief update for radiologists. Radiol Bras. 2020. Sep-Oct;53(5):320–328. doi: 10.1590/0100-3984.2020.0074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez MM, et al. Cholinergic and lipid mediators crosstalk in Covid-19 and the impact of glucocorticoid therapy. MedRxiv 2021.01.07.20248970. doi: 10.1101/2021.01.07.20248970 [DOI] [Google Scholar]
- 11.Floegel A, Kühn T, Sookthai D, Johnson T, Prehn C, Rolle-Kampczyk U, et al. Serum metabolites and risk of myocardial infarction and ischemic stroke: a targeted metabolomic approach in two German prospective cohorts. Eur J Epidemiol. 2018. Jan;33(1):55–66. Epub 2017 Nov 27. doi: 10.1007/s10654-017-0333-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kühn T, Floegel A, Sookthai D, Johnson T, Rolle-Kampczyk U, Otto W, et al. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016. Jan 28;14:13. doi: 10.1186/s12916-016-0552-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013. Feb;62(2):639–48. Epub 2012 Oct 4. doi: 10.2337/db12-0495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carayol M, Licaj I, Achaintre D, Sacerdote C, Vineis P, Key TJ, et al. Reliability of Serum Metabolites over a Two-Year Period: A Targeted Metabolomic Approach in Fasting and Non-Fasting Samples from EPIC. PLoS One. 2015. Aug 14;10(8):e0135437. doi: 10.1371/journal.pone.0135437 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010. Nov 11;5(11):e13953. doi: 10.1371/journal.pone.0013953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr Protoc Bioinformatics. 2019. Dec;68(1):e86. doi: 10.1002/cpbi.86 . [DOI] [PubMed] [Google Scholar]
- 17.Paglia G, Del Greco FM, Sigurdsson BB, Rainer J, Volani C, Hicks AA, et al. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin Chim Acta. 2018. Nov;486:320–328. Epub 2018 Aug 13. doi: 10.1016/j.cca.2018.08.014. . [DOI] [PubMed] [Google Scholar]
- 18.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021. May 21:gkab382. Epub ahead of print. doi: 10.1093/nar/gkab382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020. Aug 25;324(8):782–793. doi: 10.1001/jama.2020.12839 . [DOI] [PubMed] [Google Scholar]
- 20.Van Egeren D, Novokhodko A, Stoddard M, Tran U, Zetter B, Rogers M, et al. Risk of rapid evolutionary escape from biomedical interventions targeting SARS-CoV-2 spike protein. PLoS One. 2021. Apr 28;16(4):e0250780. doi: 10.1371/journal.pone.0250780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bos LDJ, Brodie D, Calfee CS. Severe COVID-19 Infections-Knowledge Gained and Remaining Questions. JAMA Intern Med. 2021. Jan 1;181(1):9–11. doi: 10.1001/jamainternmed.2020.6047 . [DOI] [PubMed] [Google Scholar]
- 22.Davanzo et al. SARS-CoV-2 Uses CD4 to Infect T Helper Lymphocytes. MedRxiv September, 2020. doi: 10.1101/2020.09.25.20200329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiluk M, Lewkowicz J, Pawlak D, Tankiewicz-Kwedlo A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J Clin Med. 2021. Jun 4;10(11):2484. doi: 10.3390/jcm10112484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan MR, Suleiman M, Pérez-López A. Metabolomics in the Diagnosis and Prognosis of COVID-19. Front Genet. 2021. Jul 23;12:721556. doi: 10.3389/fgene.2021.721556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020. Aug;159(2):768–771.e3. Epub 2020 May 4. doi: 10.1053/j.gastro.2020.04.064 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int J Mol Sci. 2019. Mar 6;20(5):1149. doi: 10.3390/ijms20051149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera-Van Oostdam AS, Castañeda-Delgado JE, Oropeza-Valdez JJ, Borrego JC, Monárrez-Espino J, Zheng J, et al. Immunometabolic signatures predict risk of progression to sepsis in COVID-19. PLoS One. 2021. Aug 30;16(8):e0256784. doi: 10.1371/journal.pone.0256784 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caterino M, Gelzo M, Sol S, Fedele R, Annunziata A, Calabrese C, et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci Rep. 2021. Feb 3;11(1):2941. doi: 10.1038/s41598-021-82426-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020. Jul 23;5(14):e140327. doi: 10.1172/jci.insight.140327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012. May 29;7:32. doi: 10.1186/1750-1172-7-32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarrete-Muñoz MA, Restrepo C, Benito JM, Rallón N. Elite controllers: A heterogeneous group of HIV-infected patients. Virulence. 2020. Dec;11(1):889–897. doi: 10.1080/21505594.2020.1788887 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020. Aug;159(2):768–771.e3. Epub 2020 May 4. doi: 10.1053/j.gastro.2020.04.064 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayres JS. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020. Jul;2(7):572–585. Epub 2020 Jun 30. doi: 10.1038/s42255-020-0237-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is in the manuscript.