Abstract
Background
Patients undergoing bronchoscopic procedures may develop hypoxemia and severe complications. High-flow nasal cannula (HFNC) may prevent hypoxemic events during bronchoscopy. We conducted a systematic review of randomized controlled trials (RCTs) to evaluate the effectiveness of HFNC in these patients.
Methods
We conducted a search in PubMed, Embase, and the Cochrane Library for RCTs published before November 2021. Individual effect sizes were standardized, and a meta-analysis was performed to calculate the pooled effect size using random-effects models. The primary outcome was the incidence of hypoxemic events (oxygen saturation [SpO2] < 90%) during bronchoscopy. Secondary outcomes included the incidence of interrupted bronchoscopy due to desaturation, lowest SpO2 during bronchoscopy, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), end-tidal CO2 (EtCO2) at the end of bronchoscopy, and the incidence of intubation after the procedure.
Results
Five trials involving 257 patients were reviewed. The incidence of hypoxemic events was lower in the HFNC group than in the conventional oxygen therapy group (risk ratio, 0.25; 95% confidence interval [CI], 0.14–0.42). The lowest SpO2 during the procedure was significantly higher in the HFNC group than in the conventional oxygen therapy group (weighted mean difference [WMD], 7.12; 95% CI, 5.39–8.84). PaO2 at the end of the procedure was significantly higher in the HFNC group than in the conventional oxygen therapy group (WMD, 20.36; 95% CI, 0.30–40.42). The incidence of interrupted bronchoscopy due to desaturation, PaCO2 and EtCO2 at the end of the procedure, and the incidence of intubation after the procedure were not significantly different between groups.
Conclusions
HFNC may reduce the incidence of hypoxemic events and improve oxygenation in patients undergoing bronchoscopy.
Introduction
Hypoxemia is one of the complications in patients undergoing bronchoscopy. Sedation and occlusion of the bronchi during the procedure reduce the respiratory drive and lead to hypoventilation [1]. Patients with pulmonary complications after bronchoscopic procedures occasionally have a risk of hypoxemic events that require rescue airway interventions [2]. Complications of bronchoscopy, such as refractory hypoxemia and respiratory depression, can be debilitating without careful monitoring [2–4]. Therefore, oxygen supplementation during bronchoscopy is crucial for these patients.
In the past, conventional oxygen therapy was usually adopted for patients undergoing diagnostic or therapeutic bronchoscopy, but desaturation would occasionally occur due to impaired respiratory drive and hypoventilation [2–4]. For safer bronchoscopic procedures, high-flow nasal cannula (HFNC) may replace conventional oxygen supply due to the more consistent fraction of inspired oxygen [5, 6]. According to a previous study, in patients using conventional oxygen therapy, the complication rate can reach 35% after bronchoscopy [7]. Thus, conventional oxygen therapy may not be as useful as expected for maintaining oxygenation.
Currently, HFNC is used to prevent respiratory failure. HFNC enhances secretion clearance and reduces bronchoconstriction by using heated and humidified gas [8], decreases dead space [9], and restores functional residual capacity to improve ventilation/perfusion mismatch (V/Q mismatch) [10]. In the past, several feasibility studies and reviews have assessed whether HFNC can maintain oxygenation and avoid endotracheal intubation in patients undergoing bronchoscopy [7, 11, 12]. Since then, several randomized controlled trials (RCTs) have been reported [13–17], but no systematic review or meta-analysis has encompassed them all. Thus, the aim of this systematic review was to compare the efficacy of HFNC with conventional oxygen therapy in maintaining oxygenation (SpO2 ≥ 90%) and avoiding endotracheal intubation in patients undergoing bronchoscopy.
Materials and methods
Inclusion criteria
RCTs that compared HFNC with conventional oxygen therapy in patients undergoing bronchoscopy were included in the analysis. We excluded trials in which (1) patients were <18 years old, (2) patients underwent bronchoscopy for intubation, (3) comparisons different from the one of interest were performed, or (4) duplicate patient cohorts were reported.
Search strategy and study selection
Relevant trials published before November 2021 were identified from the PubMed, Embase, and Cochrane Library databases. The following Medical Subject Headings (MeSH) were used in the search: “HFNC”, “high flow nasal cannula,” “high flow oxygen,” “high flow nasal oxygen,” “bronchoscopy,” and “bronchoscope.” These were combined into the search strategy detailed in S1 Appendix. No language restrictions were imposed. The ClinicalTrials.gov registry was searched for ongoing trials. This systematic review was registered in the online PROSPERO International Prospective Register of Systematic Reviews of the National Institute for Health Research (registration number CRD42021254176). The completed PRISMA checklist was provided in S2 Appendix.
Data extraction
Two authors independently extracted baseline and outcome data, study designs, study population characteristics, inclusion and exclusion criteria, HFNC settings, sedative or anesthetic agents, and post-treatment parameters from the studies retrieved by the database search. The reviewers’ individually recorded decisions were compared, and disagreements concerning data extraction were resolved through discussion with a third reviewer.
Methodological quality appraisal
Two reviewers independently assessed the methodological quality of each study using the risk-of-bias tool, version 2, as recommended by the Cochrane Collaboration [18]. For randomized trials, we assessed allocation, performance, attrition, measurement, reporting, and overall bias. Disagreements regarding the assessment of risk of bias were resolved through a comprehensive discussion.
Outcomes
The primary outcome was the incidence of hypoxemic events during bronchoscopy (oxygen saturation [SpO2] < 90%). Secondary outcomes included the incidence of interrupted bronchoscopy due to desaturation, lowest SpO2 during bronchoscopy, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), end-tidal CO2 (EtCO2) at the end of bronchoscopy, and the incidence of intubation after the procedure.
Grading evidence quality
Two reviewers independently assessed the evidence quality for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [19]. Evidence quality was classified as high, moderate, low, or very low based on the assessed risk of bias, inconsistency, indirectness, imprecision, and publication bias. Discrepancies were resolved by consensus.
Statistical analyses
We analyzed the data using Review Manager version 5.4 (Cochrane Collaboration, Oxford, England) in accordance with the PRISMA guidelines [20]. Risk ratios (RRs) for binary outcomes and weighted mean differences (WMDs) for continuous outcomes with the corresponding 95% confidence intervals (CIs) were computed. Standard deviations were estimated from CI limits or standard errors. If the mean and variance were not reported in a trial, they were estimated from the median, interquartile range (IQR), and sample size if the skewness was acceptable [21]. All outcomes were analyzed using a random-effects model [22]. Cochran’s Q and I2 statistics were calculated to evaluate the statistical heterogeneity and inconsistency of treatment effects, respectively, across trials. Statistical significance was set at p < .10 for Cochran’s Q tests. Statistical heterogeneity across trials was assessed using the I2 test, which quantifies the proportion of total outcome variability across trials [23]. Trial sequential analysis was performed to reduce type I errors, and sensitivity analysis was used to manage heterogeneity [18, 24, 25].
Results
Trial characteristics
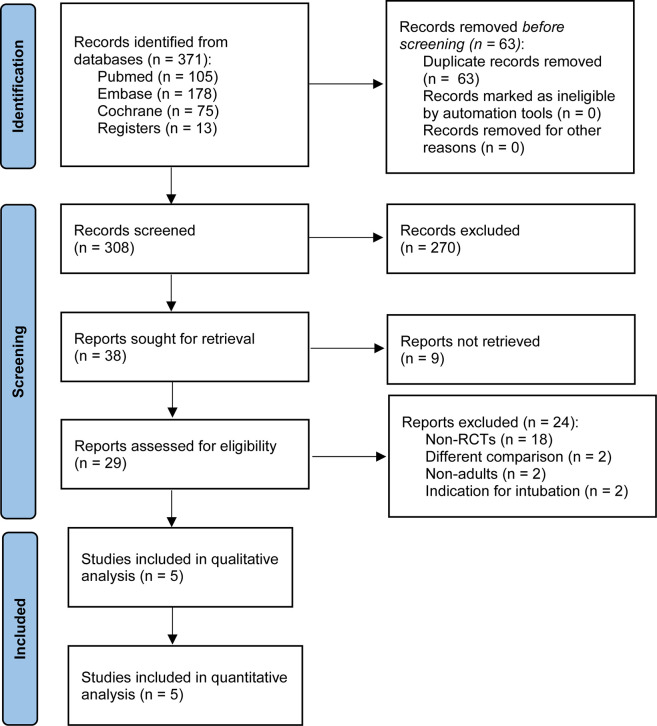
Fig 1 illustrates the study selection process. The initial search yielded 371 citations. After removal of duplicates, 308 studies were left, of which 270 did not compare HFNC with conventional oxygen therapy in patients undergoing bronchoscopy. Among these reports, nine registrations that were not retrieved were excluded. After the remaining 29 studies were reviewed, 24 were excluded. Of these, 18 were not RCTs, two included patients aged <18 years, two investigated bronchoscopy for intubation, and two conducted different comparisons. Hence, five trials were eligible for this study [10–14]. The search strategy and list of 23 major exclusions are reported in the S1 Appendix.
Fig 1. Flowchart of study selection.
RCT, randomized controlled trial.
Five trials on patients undergoing bronchoscopy were published between 2012 and 2021 [13–17]. They had sample sizes ranging from 36 to 76, with a total sample size of 257. All the trials enrolled adult patients with indications for diagnostic or therapeutic interventions. Three trials measured baseline oxygenation by examining SpO2 [13–15], one by investigating PaO2 [16], and one by examining PaO2/FiO2 [17]. Ben-Menachem et al. performed local anesthesia with nebulized 2% lidocaine and sedation with midazolam, propofol, and alfentanil [13]. Douglas et al. conducted sedation with midazolam, opioids, or propofol [14]. Irfan et al. conducted moderate sedation with midazolam and alfentanil [15]. Longhini et al. administered an anesthetic spray containing 10% lidocaine [16]. Lucangelo et al. performed local anesthesia nebulized lidocaine 2% through the mouth and nostrils [17]. Notably, Ben-Menachem et al. investigated post-lung transplant patients [13]. All the included trials categorized patients into two groups: those undergoing HFNC and those undergoing conventional oxygen therapy. Regarding intervention timing, HFNC was conducted during bronchoscopy in all trials. The baseline characteristics of the patients in each trial are summarized in Table 1.
Table 1. Characteristics of the selected randomized controlled trials.
| Study | Inclusion criteria | No. of patients (% male) | Age, years, mean ± SD | Baseline oxygenation | Sedative or anesthetic agents; duration of bronchoscopy (min) | Interventions |
|---|---|---|---|---|---|---|
| Ben-Menachem [13] | Age ≥ 18 years; lung transplant recipients; undergoing TBLB; able to provide informed consent; English speaking | H: 37 (40.5) | H: 54.9 ± 11.7 | H: 98 (97–99)†,a | Local Topicalized anesthesia with nebulized 2% lidocaine and midazolam (1 to 3 mg) sedation with midazolam, propofol (321 mg in intervention group and 337 mg in control group) and alfentanil (586 mcg in intervention group and 691 mcg in control group) to keep ASA score II–III; H: 33 ± 10, C: 34 ± 8 | H: FiO2: 100%, flow rate: 30–50 LPM through the nasal cannula |
| C: 39 (25.6) | C: 55.8 ± 11.9 | C: 98 (97–99)†,a | C: Flow rate: 4–10 LPM through standard oxygen tubing | |||
| Douglas [14] | Age ≥ 18 years; able to provide informed consent; sedation planned; English speaking | H: 30 (63) | H: 62.8 ± 14.1 | H: 96 (95–99)†,a | Topical 2% lignocaine to patient’s nasopharynx and oropharynx Sedation with midazolam, opioids or propofol to keep MOAA/S = 4; I: 24 (26–28)†, C: 21 (17–32)† | H: FiO2: 100%, flow rate: 30–50 LPM (up to 70 LPM if necessary) through the nasal cannula |
| C: 30 (63) | C: 63.4 ± 14.3 | C: 96 (94–98)†,a | C: Flow rate: 10 LPM (up to 15 LPM if necessary) through the bite block | |||
| Irfan [15] | Age ≥ 18 years; SpO2 ≥ 90%; able to breathe spontaneously throughout the procedure | H: 20 (60) | H: 61.9 ± 12 | H: 98.4 ± 2.7a | Local anesthesia sedation with midazolam (5.6 mg in intervention group and 5.5 mg in control group) and alfentanil (300 mcg in intervention group and 287 mcg in control group) varied by assessing purposeful response to verbal and/or tactile stimuli while preserving spontaneous respiratory efforts; NI | H: FiO2: 36%, flow rate: 30 LPM through the nasal cannula |
| C: 20 (60) | C: 64.5 ± 14 | C: 96.9 ± 1.9a | C: Nasal prong to maintain SpO2 ≥ 94% | |||
| Longhini [16] | Age ≥ 18 years; outpatients undergoing flexible bronchoscopy for bronchoalveolar lavage | H: 18 (83) | H: 61.9 ± NI | H: 10.8 (8.7–12.0)†,b | Topical Aanesthetic spray containing 10% lidocaine over tongue and nasopharynx; gargles with 10 mL of 2% lidocaine hydrochloride solution guaranteed further anesthesia of the oropharynx; H: 11 min 30 s ± NI, C: 12 min 50 s ± NI | H: FiO2 set to reach SpO2 ≥ 95%, flow rate: 60 LPM through the nasal cannula |
| C: 18 (67) | C: 64.5 ± NI | C: 11.1 (10.5–12.1)†,b | C: Nasal cannula to keep SpO2 ≥ 94% | |||
| Lucangelo [17] | Age ≥ 18 years; BMI ranging from 21 to 30 | H60: 15 (47) | H60: 64 (63–70)† | H60: 350.9 (304.3–363.8)†,c | Anesthesia by nNebulized 2% lidocaine 2% through the mouth and nostrils to guarantee fully developed local anesthesia; 4mg midazolam in each group delivered as demanded by each patient, reaching a maximum dose of 0.1 mg/kg BW; H60: 15 (9–21)†, H40: 15 (12–16)†, C: 14 (10–16)† | H60: FiO2: 50%, flow rate: 60 LPM through the nasal cannula |
| H40: 15 (53) | H40: 70 (61–76)† | H40: 342.8 (295.7–371.9) †,c | H40: FiO2: 50%, flow rate: 40 LPM through the nasal cannula | |||
| C: 15 (60) | C: 68 (62–78)† | C: 322.4 (295.6–374.3)†,c | C: FiO2: 50%, flow rate: 40 LPM through the venturi mask |
†Data reported as median (IQR); aData reported as SpO2; bData reported as PaO2 in kPa; cData reported as PaO2/FiO2 ratio.
Abbreviations: BMI, body mass index; C, conventional oxygen therapy; H, high-flow nasal cannula; H60, high-flow nasal cannula with flow of 60 LPM; H40, high-flow nasal cannula with flow of 40 LPM; LPM, liters per minute; MOAA/S, Modified Observer’s Assessment of Alertness/Sedation Scale; NI, no information; TBLB, transbronchial lung biopsy.
The methodological quality of the included studies is summarized in Table 2. Regarding allocation bias, one trial presented an imbalance in the comorbidity of pulmonary carcinoma and SpO2 following preoxygenation at baseline [14], one trial showed an imbalance for the diagnosis of metastatic cancer at baseline [15], and one trial did not provide information on age [16]. All trials had acceptable management of performance, attrition, measurement, and reporting biases. Overall, two trials were rated as having a low risk of bias [13, 17], and three trials had some concerns regarding bias [14–16].
Table 2. Methodological quality assessment of the randomized trials.
| Randomized controlled trials evaluated using the revised Cochrane Risk of Bias (RoB 2.0) tool | ||||||
|---|---|---|---|---|---|---|
| Study | Allocation bias | Performance bias | Attrition bias | Measurement bias | Reporting bias | Overall bias |
| Ben-Menachem [13] | Low risk | Low riskd | Low risk | Low risk | Low risk | Low risk |
| Douglas [14] | Some concernsa | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Irfan [15] | Some concernsb | Low risk | Low risk | Low risk | Low riske | Some concerns |
| Longhini [16] | Some concernsc | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Lucangelo [17] | Low risk | Low risk | Low risk | Low risk | Low riske | Low risk |
aThe baseline of comorbidity of pulmonary carcinoma, procedural data, and the SpO2 following pre-oxygenation were imbalanced.
bThe baseline of diagnosis of metastatic cancer was imbalanced.
cThe baseline of age was not available.
dOne patient crossed over from the conventional oxygen group to the HFNC group in Ben-Menachem’s trial, but they performed adequate analysis on an intend-to-treat (ITT) basis. Hence, we did not raise the risk of bias.
eAlthough these two trials did not provide protocol registration, they investigated the same outcomes as other trials registered in ClinicalTrials.gov. Obvious selective reporting was not detected. Hence, we did not raise the risk of bias.
Incidence of hypoxemic events during bronchoscopy and of interrupted bronchoscopy due to desaturation
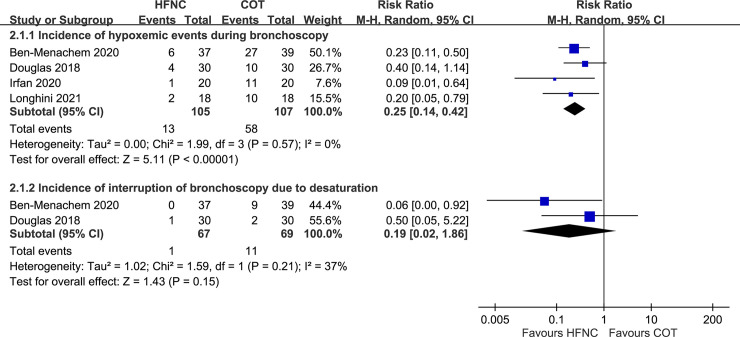
A total of four trials measured the incidence of hypoxemic events after bronchoscopy [13–16]. All trials defined hypoxemic events as SpO2 < 90%. The incidence of hypoxemic events was significantly lower among patients who underwent HFNC than among those who underwent conventional oxygen therapy (RR, 0.25; 95% CI, 0.14–0.42; Fig 2). Furthermore, the results of trial sequential analysis showed that the Z-curve crossed the O’Brien-Fleming boundaries after the fifth cumulative significance testing. These findings indicate that HFNC may reduce hypoxemic events (SpO2 < 90%) in patients undergoing bronchoscopy (S3 Appendix). Two trials measured the incidence of interrupted bronchoscopy due to desaturation [13, 14], which was lower among patients who underwent HFNC than among those who underwent conventional oxygen therapy (RR, 0.19; 95% CI, 0.02–1.86), although the result was not statistically significant (Fig 2).
Fig 2. Forest plot for comparison: High-flow nasal cannula versus conventional oxygen therapy.
Outcomes: incidence of hypoxemic events and incidence of interrupted bronchoscopy due to desaturation. HFNC, high-flow nasal cannula; COT, conventional oxygen therapy; CI, confidence interval; M-H, Mantel-Haenszel.
Lowest SpO2 during bronchoscopy
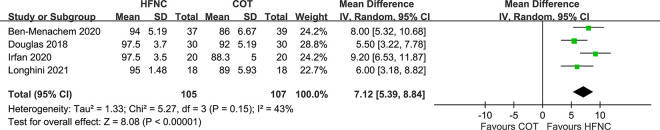
Four trials measured the lowest SpO2 during the procedure in patients undergoing bronchoscopy [13–16]. The lowest SpO2 was significantly higher in patients on HFNC than in those on conventional oxygen therapy (WMD, 7.12; 95% CI, 5.39–8.84; Fig 3).
Fig 3. Forest plot for comparison: High-flow nasal cannula versus conventional oxygen therapy.
Outcome: lowest SpO2 during bronchoscopy. HFNC, high-flow nasal cannula; COT, conventional oxygen therapy; CI, confidence interval; SD, standard deviation.
PaO2 at the end of bronchoscopy
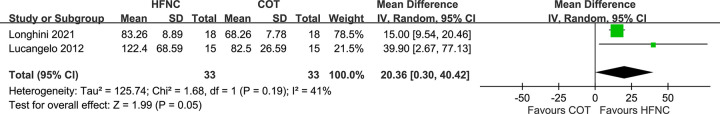
Two trials measured PaO2 at the end of the procedure in patients undergoing bronchoscopy [16, 17]. We compared the HFNC group with a flow rate of 60 liters per minute (LPM) with the conventional oxygen therapy group in Lucangelo’s study [17]. The unit was converted from kPa to mmHg. PaO2 was significantly higher in patients on HFNC than in those on conventional oxygen therapy (WMD, 20.36; 95% CI, 0.30–40.42; Fig 4).
Fig 4. Forest plot for comparison: High-flow nasal cannula versus conventional oxygen therapy.
Outcome: PaO2 at the end of bronchoscopy. HFNC, high-flow nasal cannula; COT, conventional oxygen therapy; CI, confidence interval; SD, standard deviation.
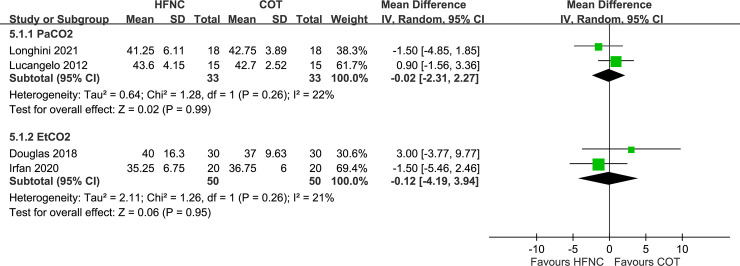
PaCO2 and EtCO2 at the end of the procedure
Two trials each measured PaCO2 [16, 17] and EtCO2 [14, 15] at the end of the procedure in patients undergoing bronchoscopy. We compared the HFNC group with a flow rate of 60 LPM with the conventional oxygen therapy group in Lucangelo’s study [17]. The unit was converted from kPa to mmHg. PaCO2 was higher among patients on conventional oxygen therapy than among those on HFNC (WMD, −0.02; 95% CI, −2.31–2.27). EtCO2 was also higher in the conventional oxygen therapy group than in the HFNC group (WMD, −0.12; 95% CI, −4.19–3.94). However, these differences were not significant (Fig 5).
Fig 5. Forest plot for comparison: High-flow nasal cannula versus conventional oxygen therapy.
Outcomes: PaCO2 and EtCO2 at the end of the procedure. HFNC, high-flow nasal cannula; COT, conventional oxygen therapy; CI, confidence interval; SD, standard deviation.
Incidence of intubation after the procedure
Two trials measured the incidence of intubation after bronchoscopy [14, 15]. No adverse events of endotracheal intubation were reported in either group. The incidence of intubation in both arms was 0.
GRADE evidence quality
The GRADE evidence quality for each main outcome is shown in Table 3. In the risk-of-bias domain, all items were rated as serious because of the risk of bias in allocation. In the inconsistency and indirectness domains, all items were rated as having low risk because heterogeneity was acceptable among the trials, and all used head-to-head comparison. In the imprecision domain, the incidence of interrupted bronchoscopy due to desaturation and PaO2, PaCO2, and EtCO2 at the end of the procedure were rated as serious due to imprecision attributable to an insufficient number of trials with a wide 95% confidence interval. No publication bias was observed. Thus, we obtained evidence of low quality for the incidence of interrupted bronchoscopy due to desaturation and PaO2, PaCO2, and EtCO2 at the end of the procedure and of moderate quality for the incidence of hypoxemic events (SpO2 < 90%) and lowest SpO2 during bronchoscopy.
Table 3. Summary of findings compiled using GRADE methodology.
| Outcome | No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Effect size | Certainty | Importance |
|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | ||||||||||
| Incidence of hypoxemic events (SpO2 < 90%) | 4 | RCT | Seriousa | Not serious | Not serious | Not serious | Undetected | RR: 0.25 | ⨁⨁⨁◯ | Critical |
| (0.14−0.42) | Moderate | |||||||||
| Incidence of interrupted bronchoscopy due to desaturation | 2 | RCT | Seriousa | Not serious | Not serious | Seriousb | Undetected | RR: 0.19 | ⨁⨁◯◯ | Important |
| (0.02−1.86) | Low | |||||||||
| Lowest SpO2 during bronchoscopy | 4 | RCT | Seriousa | Not serious | Not serious | Not serious | Undetected | WMD: 7.12 | ⨁⨁⨁◯ | Important |
| (5.39–8.84) | Moderate | |||||||||
| PaO2 at the end of bronchoscopy | 2 | RCT | Seriousa | Not serious | Not serious | Seriousb | Undetected | WMD: 20.36 | ⨁⨁◯◯ | Important |
| (0.30–40.42) | Low | |||||||||
| PaCO2 at the end of bronchoscopy | 2 | RCT | Seriousa | Not serious | Not serious | Seriousb | Undetected | WMD: −0.02 | ⨁⨁◯◯ | Important |
| (−2.31–2.27) | Low | |||||||||
| EtCO2 at the end of bronchoscopy | 2 | RCT | Seriousa | Not serious | Not serious | Seriousb | Undetected | WMD: −0.12 | ⨁⨁◯◯ | Important |
| (−4.19–3.94) | Low |
Abbreviations: CI, confidence interval; GRADE, quality of evidence grade; NI, no information; RCT, randomized controlled trial; RR, risk ratio; WMD, weighted mean difference; ⨁⨁⨁◯, moderate certainty; ⨁⨁◯◯, low certainty.
aData reported as downgraded because of some concerns of bias.
bData reported as downgraded because of wide CI or insufficient studies.
Discussion
Our findings indicate that in patients undergoing bronchoscopy, regardless of the sedative or anesthetic agents used, HFNC resulted in a lower incidence of hypoxemic events (SpO2 < 90%) when compared with conventional oxygen therapy, and it improved the values of the lowest SpO2 during bronchoscopy and PaO2 at the end of the procedure. The incidence of interrupted bronchoscopy, PaCO2, EtCO2, and the incidence of intubation did not differ significantly between groups. However, the evidence was limited by the low to moderate quality of these studies as assessed by the GRADE system.
Patients undergoing bronchoscopy are more vulnerable to desaturation resulting from hypoventilation [2–4]. To overcome the patients’ peak inspiratory demand flow and relieve their respiratory distress during bronchoscopy, a high-flow system can provide additional support [26, 27]. First, the heated humidification of inhaled gas may enhance bronchial hygiene and reduce bronchoconstriction [8]. Second, washout of the upper airway may reduce dead space [9]. Third, positive airway pressure may restore atelectatic lung regions [10]. Fourth, decreased entrainment of ambient air may increase oxygen supply [27]. Thus, HFNC may be a safe alternative to conventional oxygen therapy in patients undergoing bronchoscopy.
A reduced incidence of hazardous hypoxemic events (SpO2 < 90%) has been observed in both therapeutic and diagnostic bronchoscopy [13–16]. In our systematic review, Douglas et al. and Irfan et al. evaluated the efficacy of HFNC in patients undergoing endobronchial ultrasound procedure [14, 15], and Longhini et al. and Lucangelo et al. investigated the efficacy of HFNC in patients undergoing bronchoscopy for bronchoalveolar lavage (BAL) [16, 17]. The procedure duration varied among the trials and ranged from 11 min 30 s to 34 min. Regardless of the procedure performed, the lowest SpO2 during bronchoscopy and the PaO2 at the end of the procedure were higher in the HFNC group than in the conventional oxygen therapy group. Notably, with the exception of the trials by Douglas et al. and Lucangelo et al. [14, 17], all included trials showed that the lowest SpO2 values were less than 90% in the control groups. Thus, the pooled results indicated that HFNC may improve patient safety and provide clinical benefits to patients undergoing bronchoscopy.
Both low and high pulmonary risk patients undergoing bronchoscopy may benefit from HFNC according to our inclusion criteria, because desaturation may occur at any level of forced expiratory volume 1 (FEV1), even without sedation [28]. Among our included trials, Ben-Menachem et al. investigated the same issues in patients after lung transplantation [13], who were at significantly higher risk of hypoxemia or adverse events during bronchoscopy due to a higher incidence of hypoxemic events (SpO2 < 90%) than the other four trials (Fig 2), indicating the value of HFNC during invasive bronchoscopy in more vulnerable patients. Hence, a comprehensive algorithm for choosing HFNC or conventional oxygen therapy in patients undergoing bronchoscopy should be established, especially in patients with underlying severe lung disease. In the past, several studies have suggested that patients with (1) SpO2 on room air pre-procedure < 90% [28]; (2) moderate sedation [29]; (3) obstructive sleep apnea (OSA) [30]; or (4) requiring long-term oxygen therapy, such as for congestive heart failure (CHF) [31], chronic obstructive pulmonary disease (COPD) [32], or interstitial lung disease (ILD) [33], may benefit from HFNC treatment. However, this need to be validated by more evidence.
Although there is substantial effort to detect the hypoxemic events in patients undergoing bronchoscopy, PaCO2 or EtCO2 are also important for clinicians to assess the lung condition. One previous study indicated the effectiveness of HFNC for CO2 removal [27]; however, our systematic review showed that HFNC was not as effective as expected. All the trials presented lower PaCO2 or EtCO2 in the control group than in the HFNC group, except for the trial by Douglas et al. [14]. There are several reasons for this discrepancy. First, the number of trials was small. Second, these trials enrolled patients without severe pulmonary illnesses. A conclusive result cannot be obtained because the severity of pulmonary disease may influence the extent of CO2 clearance. Hence, more evidence is required to evaluate the effectiveness of HFNC for CO2 removal.
Several clinical factors across the trials induced heterogeneity in our meta-analysis. First, the approaches to intervention varied in terms of flow intensity and FiO2. Second, different devices were used for the control groups, making exact FiO2 measurement difficult, because it was influenced by the peak respiratory flow and the design of each low-flow system. Third, the study population in the trial by Ben-Menachem et al. differed from that of the other trials [13]. Fourth, the use of sedative or anesthetic agents varied among the trials. To take such diversity into account, we utilized sensitivity analysis, with results shown in the S4 Appendix.
Our study has several limitations. First, the trials had small sample sizes for each group. Hence, the certainty of imprecision was downgraded accordingly. Second, the number of RCTs in patients with HFNC undergoing bronchoscopy was insufficient for conducting comprehensive analyses. Third, there is still debate over the routine use of oxygen supplementation during bronchoscopy [28]. Not all centers gave oxygen to patients, unless desaturation occurs. Fourth, we did not obtain PaCO2 and EtCO2 values during bronchoscopy, which prevented us from performing a more precise analysis. Fifth, according to the inclusion criteria, no suspected cancer patients or patients at high specified pulmonary risk were included in our systematic review, limiting the external validity of the results.
Conclusion
Our meta-analysis revealed that HFNC may provide consistent oxygenation (SpO2 ≥ 90%) and safer invasive procedures in patients undergoing bronchoscopy when compared with conventional oxygen therapy.
Supporting information
(DOCX)
(DOCX)
(TIF)
(DOCX)
Acknowledgments
We acknowledge Editage Academic Editing for editing this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors have received no specific funding for this work.
References
- 1.Lo YL, Wu HT, Lin YT, Kuo HP, Lin TY. Hypoventilation patterns during bronchoscopic sedation and their clinical relevance based on capnographic and respiratory impedance analysis. J Clin Monit Comput. 2020;34(1): 171–179. doi: 10.1007/s10877-019-00269-0 [DOI] [PubMed] [Google Scholar]
- 2.Sheahan CG, Mathews DM. Monitoring and delivery of sedation. Br J Anaesth. 2014;113 suppl 2: ii37–ii47. doi: 10.1093/bja/aeu378 [DOI] [PubMed] [Google Scholar]
- 3.Vaschetto R, Cammarota G, Colombo D, Longhini F, Grossi F, Giovanniello A, et al. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2014;42(1): 74–82. doi: 10.1097/CCM.0b013e31829e53dc [DOI] [PubMed] [Google Scholar]
- 4.Costa R, Navalesi P, Cammarota G, Longhini F, Spinazzola G, Cipriani F, et al. Remifentanil effects on respiratory drive and timing during pressure support ventilation and neurally adjusted ventilatory assist. Respir Physiol Neurobiol. 2017;244: 10–16. doi: 10.1016/j.resp.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 5.D’Cruz RF, Hart N, Kaltsakas G. High-flow therapy: physiological effects and clinical applications. Breathe (Sheffield, England). 2020;16(4): 200224. doi: 10.1183/20734735.0224-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake MG. High-flow nasal cannula oxygen in adults: An evidence-based assessment. Ann Am Thorac Soc. 2018;15(2): 145–155. doi: 10.1513/AnnalsATS.201707-548FR [DOI] [PubMed] [Google Scholar]
- 7.Service JA, Bain JS, Gardner CP, McNarry AF. Prospective experience of high-flow nasal oxygen during bronchoscopy in 182 patients: A feasibility study. J Bronchology Interv Pulmonol. 2019;26(1): 66–70. doi: 10.1097/LBR.0000000000000533 [DOI] [PubMed] [Google Scholar]
- 8.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1): 253–261. doi: 10.1378/chest.14-2871 [DOI] [PubMed] [Google Scholar]
- 9.Möller W, Feng S, Domanski U, Franke KJ, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol (1985). 2017;122(1): 191–197. doi: 10.1152/japplphysiol.00584.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015;60(10): 1397–1403. doi: 10.4187/respcare.04028 [DOI] [PubMed] [Google Scholar]
- 11.Chung SM, Choi JW, Lee YS, Choi JH, Oh JY, Min KH, et al. Clinical effectiveness of high-flow nasal cannula in hypoxaemic patients during bronchoscopic procedures. Tuberc Respir Dis (Seoul). 2019;82(1): 81–85. doi: 10.4046/trd.2017.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42(9): 1336–1349. doi: 10.1007/s00134-016-4277-8 [DOI] [PubMed] [Google Scholar]
- 13.Ben-Menachem E, McKenzie J, O’Sullivan C, Havryk AP. High-flow nasal oxygen versus standard oxygen during flexible bronchoscopy in lung transplant patients: A randomized controlled trial. J Bronchology Interv Pulmonol. 2020;27(4): 259–265. doi: 10.1097/LBR.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 14.Douglas N, Ng I, Nazeem F, Lee K, Mezzavia P, Krieser R, et al. A randomised controlled trial comparing high-flow nasal oxygen with standard management for conscious sedation during bronchoscopy. Anaesthesia. 2018;73(2): 169–176. doi: 10.1111/anae.14156 [DOI] [PubMed] [Google Scholar]
- 15.Irfan M, Ahmed M, Breen D. Assessment of high flow nasal cannula oxygenation in endobronchial ultrasound bronchoscopy: A randomized controlled trial. J Bronchology Interv Pulmonol. 2021; Volume 28: 130–137. doi: 10.1097/LBR.0000000000000719 [DOI] [PubMed] [Google Scholar]
- 16.Longhini F, Pelaia C, Garofalo E, Bruni A, Placida R, Iaquinta C, et al. High-flow nasal cannula oxygen therapy for outpatients undergoing flexible bronchoscopy: A randomised controlled trial. Thorax. 2021. doi: 10.1136/thoraxjnl-2021-217116 [DOI] [PubMed] [Google Scholar]
- 17.Lucangelo U, Vassallo FG, Marras E, Ferluga M, Beziza E, Comuzzi L, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract. 2012;2012: 506382. doi: 10.1155/2012/506382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J. TJC. Handbook for systematic reviews of interventions version 6; updated 2019. Available: https://training.cochrane.org/handbook/current. The Cochrane Collaboration. [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ (Clin Res Ed). 2008;336(7652): 1049–1051. doi: 10.1136/bmj.39493.646875.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6(7): e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14: 135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3): 177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11): 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 24.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1): 64–75. doi: 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 25.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8): 763–769. doi: 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 26.Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care. 2015;3(1): 15. doi: 10.1186/s40560-015-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goligher EC, Slutsky AS. Not just oxygen? Mechanisms of benefit from high-flow nasal cannula in hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9): 1128–1131. doi: 10.1164/rccm.201701-0006ED [DOI] [PubMed] [Google Scholar]
- 28.Jones AM, O’Driscoll R. Do all patients require supplemental oxygen during flexible bronchoscopy? Chest. 2001;119(6): 1906–1909. doi: 10.1378/chest.119.6.1906 [DOI] [PubMed] [Google Scholar]
- 29.José RJ, Shaefi S, Navani N. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev. 2013;22(128): 106–116. doi: 10.1183/09059180.00006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darie AM, Schumann DM, Laures M, Strobel W, Jahn K, Pflimlin E, et al. Oxygen desaturation during flexible bronchoscopy with propofol sedation is associated with sleep apnea: the PROSA-Study. Respir Res. 2020;21(1): 306. doi: 10.1186/s12931-020-01573-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68 suppl 1: i1–i44. doi: 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 32.Bellinger CR, Khan I, Chatterjee AB, Haponik EF. Bronchoscopy safety in patients with chronic obstructive lung disease. J Bronchology Interv Pulmonol. 2017;24(2): 98–103. doi: 10.1097/LBR.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 33.Kebbe J, Abdo T. Interstitial lung disease: the diagnostic role of bronchoscopy. J Thorac Dis. 2017;9(suppl 10): S996–S1010. doi: 10.21037/jtd.2017.06.39 [DOI] [PMC free article] [PubMed] [Google Scholar]