Summary
Neurons convert synaptic or sensory inputs into cellular outputs. It is not well understood how a single neuron senses, processes multiple stimuli, and generates distinct neuronal outcomes. Here we describe the mechanism by which the C. elegans PVD neurons sense two mechanical stimuli: external touch and proprioceptive body movement. These two stimuli are detected by distinct mechanosensitive DEG/ENaC/ASIC channels, which trigger distinct cellular outputs linked to mechanonociception and proprioception. Mechanonociception depends on DEGT-1 and activates PVD’s downstream command interneurons through its axon, while proprioception depends on DEL-1, UNC-8 and MEC-10 to induce local dendritic Ca2+ increase and dendritic release of a neuropeptide NLP-12. NLP-12 directly modulates neuromuscular junction activity through the Cholecystokinin receptor homolog on motor axons, setting muscle tone and movement vigor. Hence, the same neuron simultaneously uses both its axon and dendrites as output apparatus to drive distinct sensorimotor outcomes.
Introduction
Sensory neurons detect specific sensory modalities and deliver signals to interneurons and motoneurons in order to generate appropriate behavioral responses. Many sensory neurons can detect different types of stimuli. For example, in the mouse dorsal root ganglion, the majority of thermally sensitive neurons also respond to noxious mechanical stimulation (Chisholm et al., 2018). In C. elegans, the polymodal ASH neuron are activated by osmotic as well as mechanic stimulus, both of which trigger glutamate release from ASH but act through different postsynaptic receptors (Hart et al., 1995; Mellem et al., 2002). The best studied polymodal neurons is the class IV da neurons in Drosophila which can sense mechanical nociceptive, proprioceptive, light and thermal stimuli (Ainsley et al., 2003; Grueber et al., 2002; Hwang et al., 2007; Xiang et al., 2010). Understanding the sensory receptors for each modality and how they interact with each other in the same neuron is critical to develop a model for polymodal neurons. Not surprisingly, multiple sensory receptors have been discovered in the class IV da neurons. Both the thermal and light sensing are mediated by TRPA1 (Xiang et al., 2010; Zhong et al., 2012). Two DEG/ENaC/ASIC genes, ppk1 and ppk26 are required for both nociceptive touch and locomotion but not for thermal sensing (Ainsley et al., 2003; Gorczyca et al., 2014; Guo et al., 2014; Zhong et al., 2010), while dPiezo is only required for mechanonociception but not for locomotion (Gorczyca et al., 2014; Zhong et al., 2010). These results suggest that the class IV da neurons can process multiple signals in parallel fashion. However, how neurons process multiple sensory stimuli to generate distinct behavioral output is not fully understood.
Proprioceptive sensory systems provide feedback to motor circuits and are key for generating precise movements. C. elegans generate and modulate their body movements through sensory motor feedback. Several neuron classes have been implicated in proprioception in worms. The B type motor neurons have unusually long distal axons that might serve as stretch sensors (Chalfie et al., 1985; Wen et al., 2012). Additionally, two types of proprioceptive neurons, DVA and PVDs, regulate the extent of body bending (Albeg et al., 2011; Li et al., 2006). While DVA is a monopolar neuron with a single, unbranched neurite that does not directly interact with muscles, PVDs contain elaborate dendrites that innervate the entire body musculature. Cell ablation experiments support the notion that PVD has a proprioceptive function (Albeg et al., 2011). Interestingly, PVD has also been shown to mediate sensing harsh touch and cold, suggesting that PVD is a polymodal neuron encoding at least two types of mechanic sensory stimuli and temperature (Chatzigeorgiou et al., 2010; Way and Chalfie, 1989). PVD axon synapses onto the command interneurons, which drive the escape response triggered by the harsh touch (Albeg et al., 2011; Husson et al., 2012; White et al., 1986). Yet, the PVD axon does not directly synapses onto motoneurons and it is unclear how PVD modulates the extent of muscle contraction to provide proprioceptive regulation.
The DEG/ENaC/ASIC channels are known mechanosensitive channels and were first discovered from C. elegans mutants with a defective response to gentle touch (Árnadóttir and Chalfie, 2010). This family of transmembrane proteins assemble into trimeric, Na+-selective, non-voltage gated channels (Eastwood and Goodman, 2012). The worm genome encodes 30 DEG/ENaC/ASICs (Hobert, 2013). Two DEG/ENaC/ASIC proteins MEC-4 and MEC-10 both function in the touch receptor neurons to enable touch sensation (O'Hagan et al., 2005) and gain-of-function mutations in mec-4 and mec-10 cause neuronal degeneration (Driscoll and Chalfie, 1991; Huang and Chalfie, 1994). Heterologous expression of a gain-of-function form of MEC-4 (MEC-4d) in Xenopus oocytes generates sodium currents. Physiological studies suggest that MEC-4 forms a homomeric channel and can also form a heteromeric channel with MEC-10 (Goodman et al., 2002). Further, a recent single molecule imaging study showed that MEC-4 and MEC-10 can form heterotrimers (Chen et al., 2015), consistent with structural analyses of homologous trimeric ASIC channels (Gonzales et al., 2009; Jasti et al., 2007). Another DEG/ENaC/ASIC protein, DEG-1 functions as a mechanosensor for nose touch in ASH (Geffeney et al., 2011).
A number of studies suggest that DEG/ENaC/ASIC are also involved in proprioception in worms. In PVD neurons, the body-bend-induced Ca2+ signal depends on mec-10 (Albeg et al., 2011). Another DEG/ENaC/ASIC mutant unc-8 has also been reported to have defective locomotion with a dramatically reduced wavelength and amplitude (Tavernarakis et al., 1997). Expression of unc-8 was found in motor neurons, and it was suggested that unc-8 and another DEG/ENaC/ASIC, del-1 may be candidates for locomotion regulation (Tavernarakis et al., 1997). More recently, a large-scale characterization of movement phenotypes in 305 mutants determined that del-1 had subtly abnormal movements (Yemini et al., 2013). However, a large number of mutants exhibited similar or slightly different phenotypes compared with the del-1 mutants, precluding specific conclusions to be drawn.
Together these data suggest DEG/ENaC/ASIC channels and sensory neurons are critical for proprioceptive function. However, several key limitations hinder a clear interpretation. First, the cellular loci of these channel proteins are often not clear. Where are DEG/ENaC/ASIC channels present and active in sensory neurons? Second, the relationship between each of the putative channel proteins are not known. Third, the mutations often utilized to date are deletions which demonstrate the necessity of specific genes but do not directly probe the channel functions of these genes. Fourth, sensory cells like PVD have been shown to respond to multiple sensory modalities. It is not understood how neurons can process these information in parallel. Most importantly, for cells like PVD with a single unbranched axon innervating a small number of postsynaptic cells, it is not understood how different sensory modalities engage distinct neural circuit mechanisms to elicit distinct behavioral responses.
In this study, we identify the putative mechanosensitive channel that is responsible for the proprioceptive function in PVD. We find that MEC-10, DEL-1 and UNC-8 are required in PVD sensory neurons to regulate the amplitude and wavelength of C. elegans locomotion. This is distinct from the DEGT-1 channel in PVD which is responsible for sensing harsh touch (Chatzigeorgiou et al., 2010). While harsh touch activates PVD’s downstream command interneurons through its axon (Husson et al., 2012), we find that proprioceptive stimuli induce local dendritic Ca2+ increase and dendritic release of a neuropeptide NLP-12. NLP-12 directly modulates neuromuscular junction activity through its receptor on motor axon, setting muscle tone. Therefore, PVD processes mechanical nociceptive and proprioceptive information with the activation of axon and dendrite, respectively.
Results
PVD depends on the formation of menorah structures for proprioception
C. elegans generates sinusoidal movement by alternating dorsal and ventral body wall muscles. The amplitude and wavelength of the movements are regulated and reflect the strength of the muscle contraction. To directly test PVD’s role in proprioception, we used a chemogenetics approach based on PVD-specific expression of a histamine gated chloride channel (HisCl1) (Pokala et al., 2014; Tsalik et al., 2003). Treating these transgenic worms with histamine caused a reduction in the amplitude and normal wavelength of the sinusoidal movement (Figure S1A-C). Untreated transgenic animals or treated non-transgenic animals showed normal sinusoidal movements. Thus, PVD signaling supports the normal coordination of movement and functions as a proprioceptor.
To test if the elaborate dendrite of PVD is required for its role in proprioception, we examined a dma-1 mutant that completely lacks the “menorah” structure but retains a normal primary dendrite and axon (Figure 1A). dma-1 mutants exhibit reduced amplitude and wavelength movements, and this phenotype can be rescued by cell-autonomous expression of wild type dma-1 in PVD (Figure 1B-D). This defect is not specific to the dma-1 mutations since hpo-30, kpc-1 and mnr-1 mutants, which also lack the menorah structures (Dong et al., 2013; Salzberg et al., 2013; Salzberg et al., 2014; Smith et al., 2013), have similar locomotion defects (Fig. 1C and D). These results indicate that the menorah structure which innervates the body wall muscles is required for movement regulation.
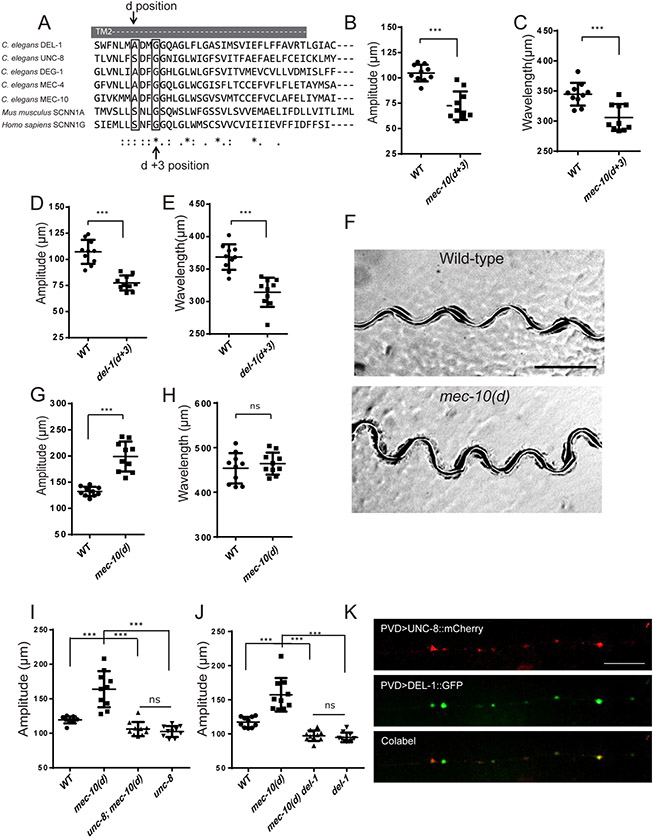
Figure 1. mec-10, unc-8 and del-1 are required for proprioceptive regulation.
(A) Confocal images showing PVD morphology of wt and dma-1(tm5159) mutants at L4 stage. PVD morphology: ser2prom3>myr-gfp. Scale bar: 50 μm. (B) Representative tracks of wt, dma-1(tm5159) and dma-1(tm5159); PVD>dma-1 animals. Expressing dma-1 in PVD (PVD>dma-1::GFP) fully rescued the sinusoidal locomotion defects of dma-1 mutants. Scale bar, 500 μm. (C-D) Quantification of amplitude and wavelength of tracks for the genotypes indicated (n=10 for each genotype, L4 stage worms). (E-J) Representative tracks of wt (E), mec-10(tm1552) (F), unc-8(e15lb145) (G), del-1(ok150) (H), degt-1(ok3307) (I) and unc-8(e15lb145); mec-10(tm1552) del-1(ok150) triple mutants (J), Scale bar: 500 μm. (K-L) Quantification of amplitude and wavelength of tracks for the indicated genotypes. (n=20 for wt, n=10 for other genotypes, L4 stage worms). For (C), (D), (K) and (L), One-way ANOVA with a Tukey correction was used for statistical analysis. Each dot represents a single worm. Data are represented as mean ± SD. ***p<0.001. ns: not significant. See also Figure S1.
PVD’s role in proprioception depends on the function of a trio of DEG/ENaC/ASIC genes
To identify the potential molecular sensors of body movement in PVD, we examined loss-of-function mutants in genes encoding putative mechanosensitive channels, including members of the DEG/ENaC/ASIC channel family and Piezo channels. While mec-4 and piezo-1 showed normal movements (data not shown), we identified three DEG/ENaC/ASIC mutants with abnormal movements: deletion alleles of mec-10, unc-8 and del-1 exhibit sinusoidal movements with a reduced amplitude and wavelength (Figure 1E-H, 1K-L). The bodies of all three single mutants were similar to wild-type (Table S2). Moreover, locomotion defects were not due to a loss of menorah structures since mec-10, unc-8 and del-1 mutants showed normal PVD morphology (Figure S1D-G). Taken together, with their homology to MEC-4, which functions as a pore-forming subunit of a mechanotransduction channel in C. elegans touch receptor neurons (O'Hagan et al., 2005), these data identify these three genes as putative mechanosensitive ion channels activated by body movement.
None of these three DEG/ENaC/ASIC proteins are expressed exclusively in the PVD neurons. mec-10 is also expressed in the touch receptor neurons linked to gentle touch behaviors and in the ASH nociceptors (Huang and Chalfie, 1994). unc-8 and del-1 are expressed in motor neurons(Tavernarakis et al., 1997). To determine if they function cell-autonomously in PVD to regulate movements, we created transgenes that express wild type copies of mec-10, unc-8 and del-1 from a PVD specific promoter in corresponding mutants. Since del-1 and unc-8 were reported to be expressed in VA and VB Motor Neurons (Tavernarakis et al., 1997), we also tested expression of these genes in the cholinergic motoneurons using a unc-17 promoter. PVD-specific expression of each wild-type del-1 and unc-8 in their respective loss-of-function backgrounds increased both the amplitude and the wavelength defects to their wild type levels, while expression of unc-8 and del-1 in motor neurons did not result in any rescue effects (Figure S2B-E). To study where del-1 is expressed, we used a transgene that includes both the promoter and cDNA of del-1 followed by a spliced leader SL2 and mCherry (Pdel-1>del-1::SL2::mCherry). This revealed DEL-1 is expressed in PVD in addition to its motor neuron expression (Figure S2A). mec-10 has been shown to be expressed in PVD (Huang and Chalfie, 1994). PVD expression of mec-10 rescued both the amplitude and the wavelength defects (Figure S2F-G). Together, these results argue that mec-10, unc-8 and del-1 function in PVD to process the proprioceptive information. Next, we asked if mec-10, unc-8 and del-1 function in the same genetic pathway to regulate movements by studying double and triple mutants. Deleting two or all three genes together led to movement phenotypes indistinguishable from single mutants, indicating that these three genes likely function together in PVD (Figure 1J-L).
To specifically test if these three DEG/ENaC/ASIC proteins function as channels, we designed or obtained pore mutants based on the alignment of selected paralogs and vertebrate homologs (Figure 2A). DEG/ENaC/ASIC proteins assemble into trimeric channels. The second transmembrane domain (TM2) of each subunit forms the channel pore (Eastwood and Goodman, 2012). Specific mutations in TM2 at the “d” position lead to prolonged open channel state and larger currents, while mutations in the “d+3” position lead to ion selectivity defects and diminished currents in analogous channels (Brown et al., 2007; Geffeney et al., 2011; O'Hagan et al., 2005). We first investigated the locomotion behavior of mec-10(u20) [G676R], an existing “d+3” mutation allele, which exhibited a reduced sodium permeability of mechanosensitive currents in touch receptor neurons (O'Hagan et al., 2005). Similar to the mec-10 null allele, mec-10(u20) mutants showed a striking locomotion defect (Figure 2B-C). Similarly, we introduced the “d+3” mutation into the endogenous locus of del-1 using CRISPR and found that del-1(d+3) [G636R] mutants also had reduced amplitude and wavelength similar to the full loss-of-function mutants with normal body length (Figure 2D-E, table S2). These “d+3” mutations, together with the deletion alleles of del-1 and mec-10 argue strongly that the channel activity is required to generate normal body movement.
Figure 2. MEC-10, DEL-1 and UNC-8 are interdependent and function together to regulate movement.
(A) Sequence alignment of the second transmembrane region (TM2) of mouse, human, and worm DEG/ENaC/ASICs channels. (B-E) Quantification of amplitude and wavelength of tracks in mec-10(d+3) (B and C) and del-1(d+3) (D and E) mutants. del-1(d+3) is del-1(wy1117). mec-10(d+3) is mec-10(u20). (F) Representative tracks of wt and mec-10(d) mutants. mec-10(d) is mec10(wy1112). Scale bar, 500 μm. (G-H) Quantification of amplitude and wavelength of tracks for wt and mec-10(d). (I-J) The increased amplitude of mec-10(d) was suppressed by unc-8(e15lb145) (I) or del-1(ok150) (J) mutants. For (B-E), (G-J), Data are represented as mean ± SD. Each dot represents a single worm. 10 animals were quantified for each genotype. Student’s T-test was used for statistical analysis. *** p<0.001. ns: not significant. L4 worms were used in (B-E). Day 1 young adult worms were used in (G-J). (K) Co-localization of UNC-8::mCherry (red) and DEL-1::GFP (green) in PVD dendritic puncta. The primary PVD dendrite is shown. n>9. Scale bar, 10 μm. See also Figure S2.
To ask whether enhanced channel activity modulates body movement, we introduced a mutation in the “d” position A673V in the endogenous mec-10 locus. We selected this particular missense mutation based on prior studies demonstrating that overexpression of MEC-10[A673V] in the touch receptor neurons triggers degeneration (Huang and Chalfie, 1994) and generates larger currents than wild-type MEC-10 in Xenopus oocytes (Goodman et al., 2002). Interestingly, endogenous mec-10(d) [A673V] did not cause a morphological phenotype in PVD dendrite (data not shown) or body length (table S2), enabling us to investigate its functional consequences. Remarkably, mec-10(d) exhibited larger amplitude movements with normal wavelengths compared to control animals, indicating that MEC-10 channel activity sets the amplitude of body bends (Figure 2F-H). The fact that these specific pore mutations generated the predicted loss-of-function and gain-of-function phenotypes strongly argues that mec-10, unc-8 and del-1 function as channel proteins in PVD to regulate movements.
Next, we asked whether these three proteins form one single channel or three different channels. The fact that triple mutants exhibited indistinguishable phenotypes compared with the single mutant is consistent with the notion that they form the same channel. To further test this, we took advantage of the enhanced movement phenotype of mec-10(d) mutants by creating double mutants between this mutation and unc-8 null and del-1 null mutants. We reasoned that if these three genes form the same channel, then the mec-10(d) phenotype should be completely dependent on the presence of the other two genes. If, however, these three genes form three independent channels, then the mec-10(d) phenotype should be still detectable when one of the other channels are absent. We found that null mutations in unc-8 or del-1 completely suppress the increased amplitude phenotype in the mec-10(d) worms. These results are consistent with the model that they might form a single channel (Figure 2I and J).
To understand more about the relationship between DEL-1 and UNC-8, we co-expressed mCherry tagged UNC-8 and DEL-1::GFP as transgenes in PVD. The transgenes also yielded puncta along the PVD processes similar to the endogenous DEL-1::GFP. In addition, we observed high degree of colocalization of these two proteins along the PVD primary dendrites (Figure 2K). MEC-10::GFP as transgenes or knockin showed no fluorescence in neurites in the PVD neurons, consistent with similar result in the TRNs (Chelur et al., 2002). These genetic interactions between these three genes and the colocalization data are consistent with the hypothesis that MEC-10, DEL-1 and UNC-8 form a heterotrimeric DEG/ENaC/ASIC channel in PVD and that this channel is needed for normal locomotion. However, they cannot exclude alternate models in which the proteins might also contribute to distinct channels but interact genetically in the PVD neurons. Future experiments will be needed to resolve this question.
PVD functions as both a mechanical nociceptor and a proprioceptor
As PVD neurons have also been implicated in harsh touch response, we asked how it sensed both the proprioceptive and harsh touch stimuli. DEGT-1, another DEG/ENaC/ASIC, was shown to mediate the harsh touch (Chatzigeorgiou et al., 2010). Interestingly, degt-1 mutants did not show any movement defects as mec-10, del-1 or unc-8 mutant (Figure 1I, K and L), indicating that DEGT-1 is dispensable for the proprioceptive regulation. PVD morphology of degt-1 mutants were also normal (Figure S1H). We next measured the behavioral responses to harsh touch in degt-1, del-1, mec-10 and unc-8 mutants. While degt-1 mutants showed reduced response as expected, del-1, mec-10 and unc-8 single mutants showed normal response to harsh touch, suggesting that they are dispensable (Figure 3A).
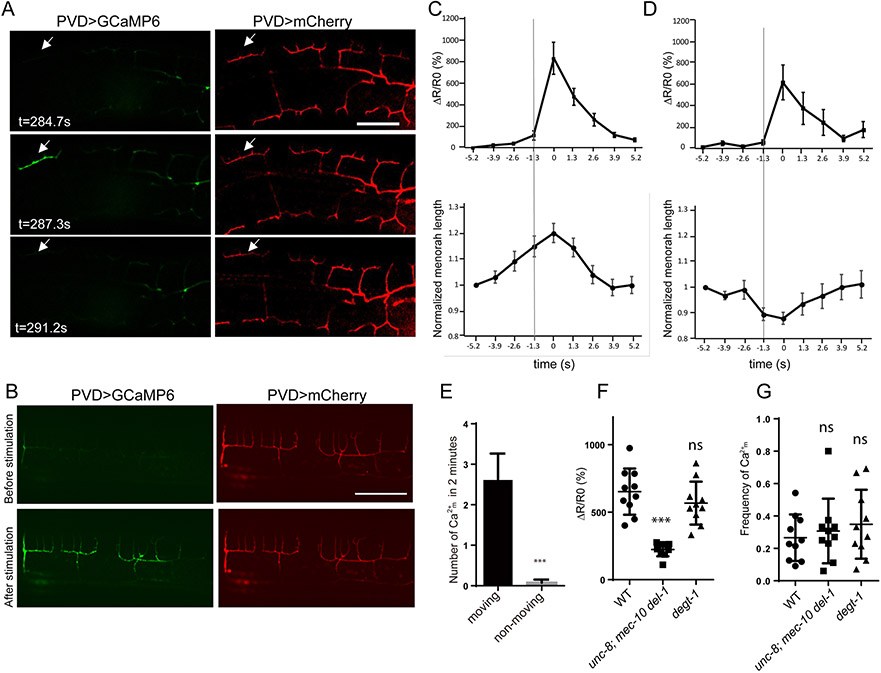
Figure 3. PVD neurons sense harsh touch and proprioceptive stimuli with different DEG/ENaC/ASICs channels.
(A) behavior assay showing that del-1(ok150), mec-10(tm1552), unc-8(e15lb145) mutants respond normally to harsh touch, while degt-1(ok3307) mutants have harsh touch defects. Student’s T-test was used for statistical analysis. ** p<0.01, *** p<0.001. ns: not significant. Error bars: SEM. n=20 for control and degt-1. n=10 for the other genotypes. (B) Fluorescent images showing GCaMP6 fluorescence (green) in PVD and PVD morphology (red) before and after harsh touch stimulation in wt, unc-8; mec-10 del-1 triple mutants and degt-1(wy1250) mutants, scale bar: 50μm. Harsh touch assay was done in a microfluidic device. (C) Sample traces of Ca2+m response (ΔR/R0) in quaternary dendrites during harsh touch stimuli in wt, degt-1(wy1250) and unc-8(e15lb145); mec-10(tm1552) del-1(ok150) mutants. R= GCaMP6/mCherry ratio. R0 is the baseline ratio. (D-E) Quantification of Calcium response (ΔR/R0) after harsh touch stimulation in the indicated genotypes in tertiary dendrites (D) and quaternary dendrites (E). One-way ANOVA with a Tukey correction was used for statistical analysis. ***p<0.001. ns: not significant. Error bars: SEM. wt (n=9), degt-1(wy1250) (n=10) and unc-8(e15lb145); mec-10(tm1552) del-1(ok150) triple mutants (n=9). (F) Confocal images of worms co-expression DEL-1::GFP (green) and DEGT-1::mCherry (red) in PVD primary dendrite. n>10. Scale bar, 10 μm. See also Figure S3, Video S1-2.
To further study the difference between DEGT-1-dependent and the UNC-8/MEC-10/DEL-1-depedent channels, we measured Ca2+ indicator GCaMP signals in PVD when defined harsh touch stimuli were applied to worms in a microfluidic device (Cho et al., 2017). Different from previous studies where Ca2+ in the PVD soma were measured, this device allowed us to visualize the PVD dendritic branches. In wild type animals, harsh touch induced a Ca2+ increase throughout the entire dendritic arbor across different menorahs (Fig. 3B, top row and Video S1). In the unc-8; mec-10 del-1 triple mutants, the harsh touch induced Ca2+ increase is indistinguishable from control (Figure 3B middle row, Figure 3C-E). Interestingly, in degt-1 mutant, the Ca2+ increase in the 4° branches are almost completely absent while the response along the 3° and 2° branches appear to be normal (Figure 3B bottom row, Figure 3C-E and Video S2). We next examined the subcellular localization of DEGT-1 using a single copy transgene and found that DEGT-1 formed puncta along all levels of dendritic branches including the 4° dendrites in PVD (Figure S3B). The endogenous DEL-1 is present as puncta along 1-3° dendrites but not 4° dendrites (Figure S3A), suggesting that the DEGT-1 channel’s presence in 4° dendrites is required for generating Ca2+ at terminal dendritic arbor and harsh touch induced behavioral responses. In the literature, mec-10’s involvement in harsh touch response has been controversial. One study reported that mec-10 is required for harsh touch mechanosensation in PVD (Chatzigeorgiou et al., 2010), while another study did not find abnormal harsh touch-induced electrophysiological response in PVD(Li et al., 2011). Both of our behavior and Ca2+ imaging experiments showed that mec-10 appeared to be dispensable for harsh touch induced response.
To further probe whether harsh touch and proprioceptive responses are dependent on different levels of dendritic branches, we examined the dma-1(wy908) mutants, a weak loss-of-function allele which has normal numbers of 1°, 2° and 3° dendrites but lack of 4° dendrites (Figure S3C-D). dma-1(wy908) mutants showed a decrease response of harsh touch, similar to degt-1 and dma-1 null mutants (Figure S3E). However, there was no movement defects detected in dma-1(wy908), suggesting that the 4° dendrites of PVD are only required for harsh touch but not for proprioception (Figure S3F-G).
Together, these results argue that the DEGT-1 channel and DEL-1/UNC-8/MEC-10 proteins function parallel to each other. We simultaneously labeled both DEL-1 and DEGT-1 in PVD to compare their localization in PVD. Interestingly, DEGT-1::mCherry and DEL-1::GFP puncta showed no colocalization along the primary dendrite (Figure 3F). This is in striking contrast with the high degree of colocalization between UNC-8 and DEL-1 (Figure 2K). Hence, the DEGT-1 and DEL-1 form distinct channels and sense harsh touch and proprioceptive stimuli, respectively.
Movement induces calcium transients in PVD dendrites
To learn more about how PVD detects body movements, we used GCaMP to monitor PVD activity in freely moving animals. Since all PVD dendrites and the axon converge on the soma, we reason that somatic Ca2+ activity should reflect the integrated signal from the dendritic arbor before reaching the axon. Indeed, during locomotion, we observed somatic Ca2+ transient signals that lasted for over 10 seconds (Figure S4A and D). These Ca2+ transients are unlikely caused by visualization artifacts from movements because similar recordings using a PVD>GFP strain yielded no transients (Figure S4B). Simultaneous recording of animal movement and PVD Ca2+ signal revealed that animals tend to increase their movement speed immediately before the onset of the Ca2+ transients (Figure S4C). This observation suggests that somatic calcium signals cause acceleration, consistent with the finding that photostimulation of PVD using ChR2 results in escape behaviors similar to those evoked by harsh body touch (Albeg et al., 2011; Husson et al., 2012).
The frequency of these events is too low to account for PVD’s role in proprioception. Consistent with this idea, the frequency of these somatic events was essentially unaffected in mec-10, del-1, unc-8 single mutants and triple mutants, and the amplitude of somatic calcium transients were only mildly reduced in mec-10, del-1, unc-8 single mutants and triple mutants. These events do appear to require an intact dendritic arbor, however, since somatic calcium transients were both less frequent and smaller in dma-1 mutants (Figure S4D-F). The sparse somatic activity we observed is counter-intuitive and conflicts with our observation that silencing PVD modulates all sinusoidal movements. This discrepancy prompted us to consider the possibility that PVD might rely on local signals in dendrites to regulate movement. To address this question, we sought to visualize PVD dendritic Ca2+ signal during movement. Attempts to visualize these signals in freely-moving animals were unsuccessful, due to the difficulty of tracking animals and monitoring GCaMP fluorescence in fine dendrites. As an alternative, we partially restrained animals by gluing their tails on an agarose-covered slide and limiting movement to the focal plane with a coverslip. This method allowed us to obtain high resolution images of local dendritic Ca2+ signal during sinusoidal movements.
Using this approach, we detected local Ca2+ transients in PVD dendrites. These events, which we call Ca2+m involve an increase in calcium that is limited to an individual menorah does not appear to spread to neighboring menorahs or propagate to the soma (Figure 4A and Video S3). This is in striking contrast to the global Ca2+ signal induced by harsh touch, which spreads to all menorahs (Figure 4B and Video S1). Ca2+m has rapid onset, slower decay and lasts for a few seconds (t1/2 = 1.7 s for the falling phase) and only occurs during movements (Figure 4E). The contraction of dorsal and ventral muscles leads to alternating compression and stretching of PVD 3° branches. We found that Ca2+m increases occurred immediately after the onset of mechanical strain in the 3° branches, suggesting that the Ca2+m is triggered by these locomotion-induced movements (Figure 4C and D). Ca2+m is trigged by both stretching (Figure 4C) and compressing (Figure 4D) of the 3° branches. The amplitude of Ca2+m is reduced in the unc-8; mec-10 del-1 triple mutants (Figure 4F and Video S4), but unaffected in degt-1 mutants. Their frequency is unaffected in either the triple mutants and the degt-1 mutant (Figure 4G). These effects of the triple mutant on Ca2+m events are consistent with the reduced sinusoidal amplitude and wavelength of the triple mutants (Figure 1K and L). The normal responses in the degt-1 mutants further support the idea that the proprioceptive function of PVD is distinct from its degt-1-dependent role as a nociceptor.
Figure 4. Local dendritic Ca2+ transients are triggered during movement.
(A) Individual video frames of GCaMP6 (green) and mCherry (red) in PVD showing the local Ca2+ increase in individual menorah (Ca2+m) in moving worms on the slide. Arrows: activated individual menorah (B) Global Ca2+ increase under harsh touch stimuli. (C-D) Averaged traces of Ca2+m response (ΔR/R0) and menorah length change in moving animals. Ca2+m is trigged by both stretching (C) and compressing (D) of individual menorah. The line indicated the time right before Ca2+m. R=GCaMP6/mCherry ratio. R0 is the baseline ratio. The peak of Ca2+m response is aligned at the 0 second mark. error bar is SEM. (E) Quantification of the number of Ca2+m events within 2 mins for the moving (n=10) and non-moving wt animals (n=8). Error bar: mean ± SEM. Student’s T-test was used for statistical analysis. *** p<0.001. (F) Quantification of Ca2+m response (ΔR/R0) in wt, unc-8; mec-10 del-1 and degt-1 mutants. (G) Ca2+m frequencies (number of events per bending) in wt, unc-8; mec-10 del-1 and degt-1 mutants. Each dot represents a single worm. Data are represented as mean ± SD. 10 animals were quantified for each genotype. One-way ANOVA with a Tukey correction was used for statistical analysis. ***p<0.001. ns: not significant. See also Figure S4, S5 and Video S3-4.
To further test if local Ca2+m is only triggered by body movements but not harsh touch, we measured dendritic Ca2+ elicited by different intensity of harsh touch stimuli. At 5 psi and 15 psi, no detectable Ca2+ increase was detected. At 25 psi stimuli, global Ca2+ increase across all menorahs was observed (Figure S5). This result suggests that harsh touch is unlikely to trigger menorah specific Ca2+ signal, instead the proprioceptive stimuli during worm movement might be particularly appropriate to elicit this type of activation of PVD dendrite. We hypothesized that Ca2+m induces the feedback activation to increase muscle contraction.
Because Ca2+m transients are restricted to individual menorahs and do not propagate to the soma, it is unlikely that these events trigger synaptic vesicle release from the PVD axon. To further test if the PVD axon and its presynaptic terminals are required for movement and harsh touch, we used a cell specific RNAi construct to knockdown unc-104/kinesin-3 in PVD. Consistent with previous reports that UNC-104/KIF1A is essential for synaptic vesicle transport (Hall and Hedgecock, 1991), this manipulation dramatically reduced expression of the synaptic vesicle marker RAB-3 in the PVD axon (Figure S6A and B). We found that unc-104 PVD specific RNAi worms were defective in sensing harsh touch (Figure S6C), but retained wild-sinusoidal movements (Figure S6D-F). Thus, PVD-mediated mechanonociception, but not proprioception requires proper synaptic vesicle trafficking.
The neuropeptide NLP-12 is required for proprioception
We investigated the possibility that local Ca2+m in PVD dendrites might affect muscle contraction by a local dendritic mechanism. We first tested if such a signal might depend on neuropeptide secretion from PVD, turning once again to a cell-type specific RNAi strategy (Esposito et al., 2007) to knockdown UNC-31/CAPS. This protein is essential for dense core vesicle release (Renden et al., 2001), specifically in PVD. Among the two independent knockdown lines we tested, one had both reduced amplitude and wavelength of the sinusoidal movements. The other knockdown line showed only an amplitude reduction, which may reflect a lower efficiency of RNAi-mediated knockdown in this line (Figure 5A-C). Together, these data indicate that unc-31 contribute to PVD’s role in proprioception.
Figure 5. The neuropeptide NLP-12 functions in PVD to regulate normal sinusoidal locomotion.
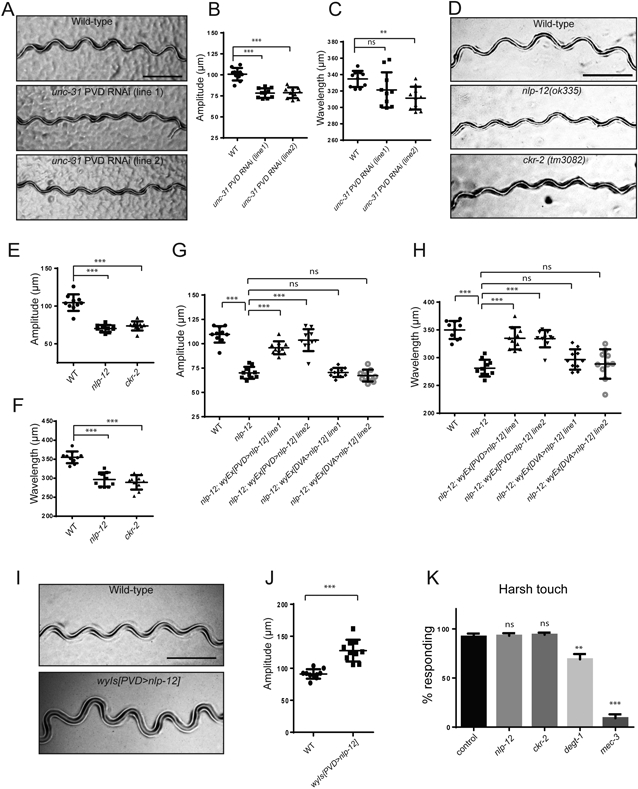
(A) Representative tracks of wt and unc-31 PVD specific RNAi transgenic worms. Scale bar, 500 μm. (B-C) Quantification of amplitude (B) and wavelength (C) of tracks for wt and unc-31 PVD-specific RNAi transgenic worms. (D) Representative tracks of wt, nlp-12(ok335), and ckr-2(tm3082) mutants. Scale bar, 500 μm. (E-F) Quantification of amplitude (E) and wavelength (F) of wt, nlp-12(ok335), and ckr-2(tm3082). (G-H) Expressing nip-12 in PVD but not in DVA completely rescued the reduction of both amplitude (G) and wavelength (H). (I) Representative tracks of wt and PVD>NLP-12 overexpression transgenic worms. Scale bar, 500 μm. (J) Quantification of amplitude of tracks for wt and PVD>NLP-12 overexpression transgenic worms. For (B-C, E-H and J), each dot represents a single worm. Data are represented as mean ± SD. 10 L4 animals were quantified for each genotype. One-way ANOVA with a Tukey correction was used for statistical analysis. ***p<0.001. **p<0.01. ns: not significant. (K) nlp-12 and ckr-2 mutants did not show significant defects in harsh touch response. Student’s T-test was used for statistical analysis. ** p<0.01, *** p<0.001. ns: not significant. Error bars: SEM. n=10 for each genotype. See also Figure S6 and Figure S7.
Next, we searched for neuropeptides expressed in PVD that might depend on unc-31 for release. Previous studies showed that FLP-4, FLP-22, and NLP-11 are expressed in PVD (Kim and Li, 2004; Nathoo et al., 2001). Since there are no mutant alleles available for nlp-11, and flp-22 mutant are homozygous lethal, we examined mutants of flp-4, npr-4 (a predicted receptor of FLP-4), and npr-22 (a predicted receptor of FLP-22) (Li and Kim, 2014). However, mutants for these genes showed normal amplitude and wavelength, indicating that they are not required to regulate these aspects of locomotion (data not shown). We next considered, NLP-12, which is required for aldicarb-induced potentiation of acetylcholine release from the neuromuscular junctions by activating the CKR-2 receptor on motor axons (Hu et al., 2011). Interestingly, we found that both nlp-12 and ckr-2 mutants exhibited reduced movement amplitude and wavelength but with normal body length, similar to the phenotypes of in the DEG/ENaC/ASIC mutants (Figure 5D-F, Table S2). Since NLP-12 is expressed in DVA, we asked whether the movement phenotypes of nlp-12 is due to its function in DVA or PVD. Although expressing wild-type NLP-12 in DVA failed to rescue the locomotion phenotypes, expressing NLP-12 in PVD completely rescued the movement phenotypes of nlp-12 mutants (Figure 5G and H). To directly detect if NLP-12 is transcribed in PVD, we FACS sorted PVD labeled with GFP from adult worms and performed RT-PCR experiments. This experiment showed that NLP-12 transcript is enriched in PVD (GFP +) from total cells (GFP −) (Figure S7A). Furthermore, overexpression of PVD>nlp-12 in the wild type background caused an increased movement amplitude (Figure 5I and J), suggesting that expressing NLP-12 from the PVD is sufficient to potentiate body musculature contraction and generate deep body bends. Overexpressing NLP-12 from its endogenous promoter also resulted in increased amplitude, an effect that was partially suppressed in dma-1 mutants lacking PVD menorah structures (Figure S7B-F). The partial suppression is consistent with an additional role for NLP-12 in another neuron, such as the DVA neurons previously known to contribute to locomotion via NLP-12-dependent (Bhattacharya et al., 2014; Hu et al., 2011).
Since nlp-12 and ckr-2 are required for the proprioceptive response, we asked if nlp-12 and ckr-2 are also required for DEGT-1 elicited response, we subjected nlp-12 and ckr-2 mutants to harsh touch stimuli and found that both mutants could still respond to harsh touch (Figure 5K), suggesting that nlp-12 is only required for regulating sinusoidal movements. Together, these results revealed NLP-12’s function in PVD and suggest NLP-12 is secreted from PVD to activate its receptor CKR-2 on the motor neuron axon to increase movement amplitude and wavelength.
NLP-12 is secreted by PVD dendrites in an UNC-8/MEC-10/DEL-1 dependent manner
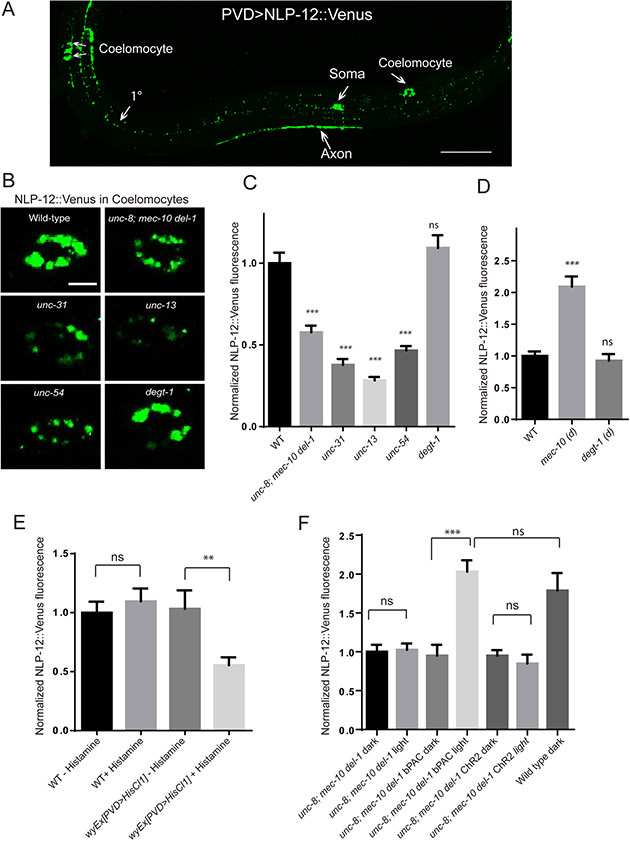
To directly test the hypothesis that NLP-12 is secreted from PVD, we expressed Venus tagged NLP-12 in PVD. This transgene yielded punctate fluorescence signals throughout the dendrites and axon of PVD (Figure 6A). We were able to consistently detect Venus fluorescence in coelomocytes, cells that can provide a readout of neuropeptide secretion due to their scavenging function (Ch'ng et al., 2008; Sieburth et al., 2007), indicating that NLP-12 can be secreted by PVD (Figure 6A). Next, we investigated the molecular mechanisms of NLP-12 secretion by crossing this transgene into several mutant backgrounds. A mutation in unc-31 strongly suppressed the fluorescence intensity in the coelomocytes, indicating that NLP-12 secretion relies on dense core vesicle release machinery (Figure 6B and C). Furthermore, coelomocyte fluorescence was also suppressed by mutations in unc-13 and unc-54/muscle myosin, suggesting that synaptic transmission and muscle contraction are needed for proper NLP-12 secretion from PVD (Figure 6B and C). Finally, triple unc-8; mec-10 del-1 mutants also showed reduced NLP-12 secretion from PVD, while the degt-1 mutants showed normal NLP-12 secretion (Figure 6B and C). Additionally, we examined the NLP-12 secretion in mec-10 (d) mutants, a constitutive active allele of mec-10 that exhibited larger amplitude movements (Figure 2F and G). We found that NLP-12 secretion was significantly increased in mec-10(d) mutants compare to wild type. On the contrary, a putative constitutive active allele degt-1 (d) [A813V] mutants did not show increase NLP-12 secretion (Figure 6D). This result suggests that the proprioceptive stimuli but not harsh touch likely activate secretion of NLP-12 from PVD.
Figure 6. NLP-12 secretion from PVD requires DEG/ENaC/ASICs and muscle function.
(A) A confocal image of NLP-12::Venus fluorescence expressed in the PVD neuron in wt animals. Scale bar, 50 μm. (B) Representative images of a single coelomocyte of wt, unc-8(e15lb145); mec-10(tm1552) del-1(ok150) triple, unc-31(e928), unc-13(s69), unc-54 (e190) and degt-1(ok3307) mutants expressing NLP-12::Venus in PVD neurons. Scale bar, 5 μm. (C) Quantification of fluorescent intensity (normalized to wt) measured from the posterior dorsal coelomocytes of wt (n=42), unc-8(e15lb145); mec-10(tm1552) del-1(ok150) triple(n=31), unc-31(e928) (n=32), unc-13(s69) (n=30), unc-54(e190) (n=30) and degt-1(ok3307) (n=37) mutants expressing NLP-12::Venus in PVD neurons. (D) Quantification of coelomocytes NLP-12::Venus fluorescent intensity (normalized to wt) in wt (n=39), mec-10(d) (n=21)and degt-1(d) (n=19) mutants. mec-10(d) is mec10(wy1112). degt-1 (d) is degt-1(wy1283). (E) Quantification of coelomocytes NLP-12::Venus fluorescent intensity (normalized to wt without histamine) in wt and wyEx[PVD>HisCl1] transgenic worms treated with histamine or without histamine. n>12 for each genotype. (F) Quantification of coelomocytes NLP-12::Venus fluorescent intensity under dark or photostimulated conditions in unc-8; mec-10 del-1 mutants with bPAC or ChR2 expressing in PVD and wt animals. Fluorescence was normalized to dark condition of unc-8; mec-10 del-1 mutants. n>10 for each condition. For (C-F), One-way ANOVA with a Tukey correction was used for statistical analysis. ***p<0.001. Data are represented as mean ±SEM. ns: not significant.
To test if the NLP-12 secretion is modulated by PVD activity, we used the histamine gated channel (HisCl1) to silence PVD. We found that HisCl1 reduced NLP-12 secretion by about 50% only in the presence but not in the absence of histamine (Figure 6E), linking PVD activity to peptide secretion. To test if activating PVD is sufficient to secret NLP-12, we used blue light activation on PVD>ChR2 worms. ChR2 stimulation in PVD was shown to elicit a forward locomotion response, similar to harsh touch, by activate PVD axon and its synaptic partners which are command neurons (Husson et al., 2012; Li et al., 2011). Interesting, we found that activation of ChR2 in the unc-8; mec-10 del-1 triple mutants were not sufficient to increase NLP-12 secretion (Figure 6F). On the contrary, stimulation of transgenic worms expressing PVD>bPAC (Beggiatoa-photoactivated adenylyl cyclase) in the same triple mutant rescued the NLP-12 secretion to wild type level (Figure 6F). bPAC stimulation in C. elegans neurons stimulates dense core vesicle release of neuropeptides from motor neurons in addition to enhanced synaptic vesicle fusion (Costa et al., 2017). Together, these results suggest that NLP-12 secretion from PVD is regulated by neuronal activity and possibly through cAMP as a second messenger. In addition, while harsh touch triggers global Ca2+ increase and activation of PVD axon, it is incapable of efficiently stimulate NLP-12 release, suggesting that NLP-12 might be secreted locally from dendrites.
Next we asked where in PVD NLP-12 is secreted. NLP-12::Venus is enriched in PVD axon but also localized to the dendrites (Figure 6A). To test whether the NLP-12 is released from axon or dendrites, we pursued two strategies. First, we examined the localization of NLP-12 in the unc-104 PVD specific RNAi worms. This manipulation almost eliminated the axonal NLP-12 in PVD without affecting dendritic NLP-12 puncta (Figure S7G and H). Consistent with the dendritic secretion model, the NLP-12::Venus fluorescence in the coelomocytes is unchanged from wildtype controls suggesting that dendritic secretion likely accounts for much of the secreted NLP-12 (Figure S7I). These data suggest that NLP-12 is unlikely to be released the axon and implicate a local dendritic release mechanism.
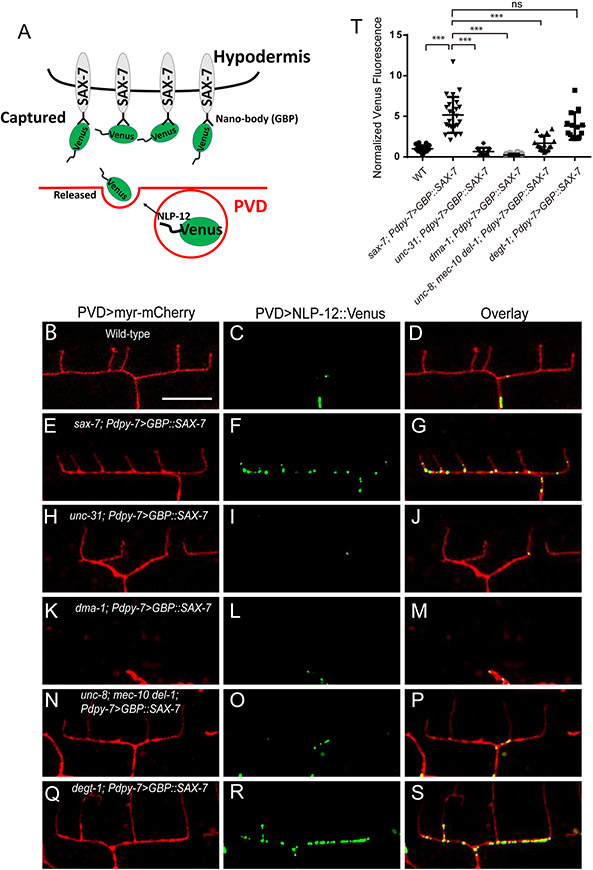
To directly visualize the release of NLP-12 from PVD dendrites, we designed an extracellular “trap” to detect locally secreted NLP-12::Venus by fusing a GFP nanobody to the extracellular domain of SAX-7 (GBP::SAX-7) and expressing this construct in the epidermal cells (Figure 7A). SAX-7 is localized to stripes that aligns the 1°, 3° and 4° PVD dendrites (Dong et al., 2013; Liang et al., 2015; Salzberg et al., 2013). GBP::SAX-7 rescued PVD morphology defects in the sax-7 mutants, indicating the construct is expressed and localized correctly. When we combined GBP::SAX-7 and PVD::NLP-12::Venus, we found that the GBP::SAX-7 transgene changed the localization pattern of NLP-12::Venus in PVD dendrites. While NLP-12::Venus alone showed punctate staining along the 1° dendrite, very few puncta were found in the 3° branches, which likely reflects the dense core vesicle distribution within PVD (Figure 7B-D). In the presence of GBP::SAX-7, numerous puncta appears along the 3° dendrites (Figure 7E-G and T). The presence of these puncta are completely dependent on unc-31/CAPS and dma-1, indicating that they are extracellular puncta representing secreted and captured NLP-12::Venus (Figure 7H-M and T). Since the PVD axon is localized ventrally, we postulated that if the axon is the main source of secreted NLP-12, a ventral-high, dorsal-low gradient would be observed. However, such a gradient was not present and instead extracellular NLP-12 precisely colocalization to 3° dendrite of PVD. We therefore conclude NLP-12 is likely secreted locally by the 3° dendrites. Then we crossed this GBP::SAX-7 transgene into unc-8; mec-10 del-1 triple mutants and degt-1 mutants. We found that the secreted and captured NLP-12 ::Venus signal in 3° branches is reduced in the triple mutant, but not in degt-1 mutants (Figure 7N-T). Collectively, this local trapping strategy shows that NLP-12 is released from 3° dendrites and suggests that UNC-8, DEL-1 and MEC-10, but not DEGT-1, are required for local NLP-12 secretion.
Figure 7. NLP-12 is secreted by 3° dendrites of PVD in an UNC-8/MEC-10/DEL-1 dependent manner.
(A) A cartoon showing the design of using GFP nanobody tagged SAX-7(GBP::SAX-7) as a extracellular “trap” to detect secreted NLP-12::Venus from PVD. (B-D) Confocal images showing the localization of NLP-12::Venus in PVD ‘menorah’ structure. Expressing GBP::SAX-7 in hypodermal cells increased of NLP-12::Venus puncta along 3° dendrites (E-G), but not in unc-31(e928) (H-J) or dma-1 (tm5159) mutants (K-M). The increased NLP-12::Venus puncta along 3° dendrites is partially dependent on unc-8, del-1 and mec-10 (N-P), but not degt-1 (Q-S). (T) Quantification of NLP-12::Venus fluorescence intensity in sublateral line (normalized to wt) in the indicated genotypes.. Student’s T-test was used for statistical analysis. *** p<0.001. Error bars: SD. wt (n=21), sax-7; Pdpy-7>GBP::SAX-7 (n=24), unc-31; Pdpy-7> GBP::SAX-7 (n=12), dma-1; Pdpy-7>GBP::SAX-7 (n=12), unc-8; mec-10 del-1; Pdpy-7>GBP::SAX-7 (n=16) and degt-1; Pdpy-7>GBP::SAX-7 (n=14). See also Figure S7.
Discussion
In this study, we confirm previous studies that the PVD sensory neuron functions as dual mechanoreceptor neuron sensing both noxious external stimuli (harsh touch) and self-generated movement. We provide evidence that the former stimuli are linked to avoidance behaviors via axon-derived signals and the latter are linked to proprioception via local release of the NLP-12 neuropeptide thought to act directly on motor neurons. Several lines of evidence support this parallel processing model. First, responses to the two types of mechanical stimuli depend on distinct putative mechanosensitive ion channel genes. Specifically, harsh touch sensation depends on degt-1 and proprioception relies on a trio of DEG/ENaC/ASIC ion channel genes: unc-8, del-1, and mec-10. Triple mutants lacking UNC-8, DEL-1, and MEC-10 have wild-type responses to harsh touch and animals lacking the DEGT-1 protein have no clear movement defects. Second, calcium imaging reveals that the two mechanical stimuli generate distinct cellular responses: harsh touch evokes global increases in calcium that spreads throughout the dendritic arbor, while body movement (local bending) is associated with local Ca2+ transients in 3° dendrites. Loss of DEGT-1 decreases harsh touch-evoked signaling, but not the local dendritic calcium transients that occur during movement. Reciprocally, triple mutants lacking UNC-8, DEL-1, and MEC-10 have reduced movement-evoked calcium signals (Ca2+m) and wild-type responses to harsh touch. Third, responses to harsh touch and body movement depend on axonal and local dendritic signaling, respectively. Knocking down UNC-104/kinesin-3 needed for proper axonal trafficking of synaptic vesicles disrupted mechanonociception, but not proprioception. Finally, the neuropeptide, NLP-12, is trafficked to dendrites and released from this location and appears to regulate the amplitude of sinusoidal body movements by acting on motor neurons.
Is the dual and parallel mechanosensory function of PVD evolutionarily conserved? There are striking similarities between PVD and the type IV da sensory neurons in Drosophila larvae. Like PVD, the type IV da neuron also senses nociceptive mechanical and proprioceptive information (Ainsley et al., 2003; Hwang et al., 2007; Xiang et al., 2010). Although not emphasized in this study, both PVD and type IV da neurons also function as noxious thermoreceptors (Chatzigeorgiou et al., 2010; Zhong et al., 2012). Both neurons have extended, highly branched, and stereotyped dendritic arbors that are interpolated between the skin and muscles (Albeg et al., 2011; Grueber et al., 2002), positioning these neurons to sense both external touch and body motion. As in PVD, distinct ion channels, including members of the DEG/ENaC/ASIC family, are linked to mechanonociception and locomotion in the type IV da neurons (Gorczyca et al., 2014; Guo et al., 2014). In both cell types, single mutants have partial deficits in behavioral responses to these stimuli, indicating that harsh touch sensation depends on more than one putative mechanosensitive ion channel gene. For example, mutations in degt-1 reduced the harsh touch response by about 30% (Figure 3A and Figure S3E). Similarly, mutations in ppk1 and ppk26 reduced harsh touch response by about 25% (Gorczyca et al., 2014). These similarities suggest that the combination of nociceptive touch and proprioceptive processing at the level of the sensory neuron might be evolutionarily conserved.
We identified three DEG/ENaC/ASICs proteins that are expressed by PVD and act in PVD to control the amplitude of sinusoidal locomotion. We propose that DEL-1, UNC-8 and MEC-10 co-assemble to form a single heterotrimeric channel and that this channel is likely to be mechanosensitive channel. Genetic interactions support this idea, since single mutants have the same phenotype as double and triple mutants (Figure 1K and L). Introducing a gain-of-function mutation in the endogenous mec-10 locus encoding MEC-10[A673V] increased movement amplitude and this effect is suppressed by the loss of unc-8 or del-1 (Figure 2I and J). DEL-1 and UNC-8 colocalize at puncta along the PVD dendrites (Figure 2K). Both wild-type UNC-8 and a gain of function UNC-8 mutant protein [G387E] form calcium- and sodium-permeable channels in Xenopus oocytes (Wang et al., 2013), indicating that this protein has the capacity form functional channels on its own. By contrast, neither wild-type MEC-10 nor MEC-10[A673V] is able to form an ion channel on its own (Goodman et al., 2002). These vivo results are consistent with the finding that the effects of MEC-10[A673V] on PVD depend on UNC-8 expression. The capacity of DEL-1 to form homomeric channels is not known. Additional studies are needed to determine how these three proteins might co-assemble into a single channel that is localized to 3° dendrites and to directly examine the possibility that such a channel might be mechanosensitive.
While axons are the classical output device of neurons, it is well established that certain neuronal types can also use dendrites to release neurotransmitters. For example, the amacrine cells in the vertebrate retina release synaptic vesicles at distal dendrite domains (Famiglietti, 1991). Dendrites of hypothalamic and midbrain neurons are also known to release neuromodulators including neural peptides and dopamine (Ludwig et al., 2011). In this paper, we presented evidence that PVD can use both axon and dendrites as outputs for distinct behaviors. We propose that body movements activate the DEL-1 sensor which is selectively coupled with the dendritic release mechanism while harsh touch induces escape response by activating PVD axon. The parallel processing of two stimuli with distinct subcellular outputs allows this neuron to multitask and increase the processing capacity of the nervous system.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Worm strains and plasmids generated in this study are available upon request. Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kang Shen (kangshen@stanford.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
C. elegans strains
All strains were grown on nematode growth medium (NGM) plates seeded with OP50 E. coli. at 20°C or room temperature. N2 Bristol was used as wild-type strain. Mutant alleles and transgenes used in this study are listed in Table S1.
METHOD DETAILS
Plasmids
Plasmid constructs for C. elegans expression were generated in pSM delta vector, a derivative of pPD49,26. The ser2prom3 (PVD), Punc-17 (motor neurons), and Ptwk-16 (DVA) promoters were used for cell-specific expression. HisCl1 cDNA was kindly provided by Dr. Cornelia I. Bargmann. unc-8 cDNA was amplified from a mix-stage worm cDNA library and the codon optimized mec-10 cDNA and degt-1 cDNA are synthesized by Genescript. The codon optimized del-1 cDNA was directly synthesized by IDT (Integrated DNA Technologies. Venus coding sequence was amplified from KP#1383 (a plasmid kindly provided by Dr. Joshua M Kaplan). bPAC coding sequence was kindly provided by Dr. Alexander Gottschalk.
C. elegans Transformation
Transgenic extrachromosomal arrays were generated using a standard microinjection method (Mello and Fire, 1995). The DNA construct and co-injection marker were mixed and injected into the distal arm of the gonad. The details of the transgenic arrays are listed in Table S1.
Genome editing
mec-10(wy1112), del-1(wy1117) were generated by CRISPR/Cas9 as described (Arribere et al., 2014). Plasmids providing target sgRNA were made by replacing the target sequence in pBHC1084 (kindly provided by Dr. Baohui Chen) using a Quick-change method. pJW1259 (Peft-3>cas9, kindly provided by Dr. Jordan Ward) was used for Cas9 expression (Ward, 2015). The oligo containing the point mutations and homology arms was used as a repair template. dpy-10(cn64) was used as a coconversion marker (Arribere et al., 2014). To insert a GFP into the endogenous locus of del-1, the plasmid containing homology arms (1.4 kb upstream and 1.1 kb downstream of del-1 stop codon) +GFP (floxed Cbr-unc-119) was used as a PCR template to amplify the repair template. The construct of sgRNA, PCR products of repair template, pJW1259 and co-injection markers (Pmyo-2>mcherry and Podr-1>rfp) were injected into unc-119(ed4) strain. del-1(wy1149) was isolated based on 100% non-unc and no red fluorescence protein expression. degt-1(wy1250) and degt-1(wy1283) were generated by CRISPR/Cas9 using another method (Paix et al., 2015). crRNA, tracrRNA,Cas9 protein and the repair oligo were order from IDT, mixed with co-injection marker (rol-4) and injected into the worms. The sequence of sgRNA and repair templates for each gene are listed in Table S3.
Microscopy
L4 stage hermaphrodite worms were immobilized on 2% agar pads using 10 mM levamisole in M9 buffer. Images were taken by a Zeiss LSM710 confocal microscope with a Plan-Apochromat 40x/1.3 NA objective for PVD morphology, and 63x/1.4 NA objective for coelomocyte imaging. Z stacks were collected and maximum-intensity projections were obtained for additional analysis. For coelomocyte imaging, the posterior dorsal coelomocyte was imaged in L4 stage worms. The integrated density arising from NLP-12::Venus signal in each coelomocyte was determined in ImageJ (Wayne Rasband). The coelomocyte fluorescent values of mutants were then normalized to the wild-type average For PVD silence experiment, worms expressing HisCl1 in PVD were cultured on the seeded plate with or without 10mM histamine from L1 stage, and L4 worms were used for imaging. For PVD activation experiment, worms expressing Chr2 were cultured on the plates with ATR (all-trans-retinal). L4 stage worms expressing Ch2 or bPAC in PVD were either kept in dark or illuminated for 15 mins with a 470 nm LED, 400uW/mm2 right before imaging. For analyzing co-localization of UNC-8::mCherry and DEL-1::GFP, a spinning disk confocal microscope with a 63x/1.4 NA objective was used for imaging.
Locomotion assay
A locomotion assay was performed following the protocol as previously described (Tavernarakis et al., 1997) with some variations. Worms were grown at 20°C or room temperature on NGM plates seeded with E. coli strain OP50. Individual mid-L4 stage worms (except in Figure 3b-f which used young adult worms) were transferred to a fresh seeded plate for 10-20 min to allow them cut tracks on the plates. Pictures of the tracks were taken by a Zeiss Axioplan microscope with 2.5X (0.075NA) Plan-Neofluar objective. The amplitude (the distance between opposite peaks) and wavelength (the distance between two successive peaks) of the tracks were measured using ImageJ (Schindelin et al., 2012). For each strain, 10 worms are used for the locomotion assay. And for each worm, 50 measurements were randomly picked to calculate the average amplitude or wavelength. If there are less than 50 measurements per worm, all the measurements were used to calculate the average value. At least 35 measurements were used for each worm. Values were then tested for statistical significance with a one-way ANOVA with a Tukey correction or Student’s T-test. We have systematically measured the body length of worms of various genotypes used for the locomotion assays and found that the none of the mutant strains showed body length abnormalities compared to the wild type (Table S2).
For PVD silencing experiment, array-containing L4 stage worms or the control worms were first transferred to food-free plate to allow the animals to crawl away from carried-over food. Then 10 animals were picked from the food-free plate to a seeded plate with or without 10mM histamine for 30 min to allow the histamine to take effect. Individual worms were then transferred to a new seeded plate (with 10mM histamine or without 10mM histamine) to record the tracks.
Calcium imaging of free moving worms
Calcium imaging was performed on freely behaving worms using the CARIBN system as previously described (Piggott et al., 2011). Day 1 adult worms were used for imaging under the standard laboratory condition on unseeded nematode growth media (NGM) plates (20°C, 30% humidity). All imaging experiments were performed on lite-1 (ce314) mutated worms that do not show phototaxis response (Liu et al., 2010). Worms are imaged 1 min after transferred to the imaging plates. Ratiometric imaging was conducted on worms co-expressing GCaMP6f and mCherry and ΔR/R was used to quantify fluorescence changes. For single spike analysis, traces are lined up at the initiation of calcium spikes.
Dendritic calcium imaging of tail-glued worms
The tails of individual young adult worms were glued with Histoacryl® Blue to 2% agarose pads in M9 buffer with coverslips. Worms were imaged 5 min after gluing. Images were taken by a spinning disk confocal microscope with a 40x/1.3 NA objective. We manually subtracted the background value by measuring the average intensity of the neighboring region. Fluorescence intensity was measured use GCaMP6/mCherry ratio (R). ΔR/R0 was used to quantify fluorescence changes. R0 is the baseline ratio.
Harsh Touch Experiments performed in a microfluidic device
Harsh touch experiments were performed using a microfluidics system as described in Cho et al (Cho et al., 2017)..Animals loosely fit in a trapping channel and were stimulated by indentation via PDMS membranes actuated with air pressure. All imaging experiments were performed using a PerkinElmer UltraVIEW VoX spinning-disk microscope with a 40× oil objective (N.A. 1.3). Video sequences were captured using a Hamamatsu EM-CCD camera with 20 ms exposure time for each color (GCaMP6 and mCherry). Harsh touch emulating mechanical stimuli were delivered using a custom automated pressure delivery system, applying pressure of 30psi, unless otherwise stated. The width of each PDMS membrane is 150μm. In these experiments, animals were stimulated either 100μm or 450μm from the most anterior part of the trapped animal. For all trials, the baseline activity was recorded or 10 s, followed by stimulus with a duration of 0.5 s. Animals were age-synchronized and imaged as day 2 adults. Fluorescence intensities for each frame were obtained using ImageJ (Wayne Rasband). The background intensity was manually measured using the average intensity of the neighboring region. Fluorescence intensity was measured use GCaMP6/mCherry ratio (R). And ΔR/R0 was used to quantify fluorescence changes. R0 was computed as the mean fluorescence prior to stimulus delivery.
Harsh Touch escape assay Experiments
Harsh touch escape assay was performed by prodding day 1 adult worms with a platinum wire pick in the midsection of the body. The tip of the pick was flattened and cut into 20 μm thick and 30 μm wide. 10 worms were tested for each genotype, and each worm was tested ten times with a 2–10 min interval. Both forward and reverse responses were recorded. All the worms used for this assay were in mec-4(e1611) light touch mutant background, except mec-3(e1338), which was used as a negative control (Way and Chalfie, 1989). These experiments were performed blind to genotypes.
QUANTIFICATION AND STATISTICAL ANALYSIS
All quantification is explained in the relevant sections of the STAR Methods. Statistics analysis is presented in the figure legends.
DATA AND CODE AVAILABILITY
This study did not generate large-scale datasets or new code.
Supplementary Material
Example movie of global Ca2+ transients trigged by harsh touch in wild-type worms. Green channel is PVD>GcaMP6, red channel is PVD>mCherry. Scale bar: 30μm (Related to Figure 3).
Example movie of global Ca2+ transients trigged by harsh touch in degt-1 mutants. Green channel is PVD>GcaMP6, red channel is PVD>mCherry. Scale bar: 30μm (Related to Figure 3).
Example movie of dendritic local Ca2+ transients in wild-type animals during movement. Scale bar: 30μm (Related to Figure 4).
Example movie of dendritic local Ca2+ transients in unc-8; mec-10 del-1 animals during movement. Scale bar: 30μm (Related to Figure 4).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| E. coli OP50 | Caenorhabditis Genetics Center | https://cgc.umn.edu/strain/OP50 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Histamine dihydrochloride | Sigma-Aldrich | Cat# H7250-5G |
| levamisole | Sigma-Aldrich | Cat#1359302 |
| all trans-Retinal | Sigma-Aldrich | Cat# R2500 |
| Alt-R® S.p.Cas9 Nuclease V3 | IDT | Cat#1081059 |
| Histoacryl® Blue | B∣Braun | Cat# TS1050044FP-SD |
| Critical Commercial Assays | ||
| Phusion High-Fidelity DNA polymerase | New England | Cat # M0530S |
| Gibson Assembly® Master Mix | New England | Cat# E2611S |
| Q5® Site-Directed Mutagenesis Kit | New England | Cat# E0554S |
| Experimental Models: Organisms/Strains | ||
| C. elegans strains | This paper | see Table S1 |
| Oligonucleotides | ||
| SgRNA sequences and CRISPR repair templates | This paper | see Table S3 |
| Alt-R® CRISPR-Cas9 tracrRNA | IDT | Cat# 1072532 |
| Recombinant DNA | ||
| Punc-17::UNC-8 cDNA | This paper | N/A |
| Pser2prom3::UNC-8 cDNA | This paper | N/A |
| Punc-17::DEL-1 cDNA | This paper | N/A |
| Pser2prom3::DEL-1 cDNA | This paper | N/A |
| Pser2prom3::MEC-10 cDNA | This paper | N/A |
| Pser2prom3::NLP-12 cDNA::SL2::mcherry | This paper | N/A |
| Ptwk-16::NLP-12 cDNA::sl2::mcherry | This paper | N/A |
| Pser2prom3::UNC-8 cDNA::mcherry | This paper | N/A |
| Pser2prom3::DEL-1 cDNA::GFP | This paper | N/A |
| Pser2prom3::DEGT-1::cDNA::mcherry | This paper | N/A |
| pBHC1084 | N/A | N/A |
| pJW1259 | Ward, 2015 | Cat# 61251 |
| Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Prism 6 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
Acknowledgements
This work was supported by the Howard Hughes Medical Institute and the National Institute of Neurological Disorders and Stroke (1R01NS082208) to K.S., NIH (R01GM088333) to H.L., NIH (R35NS105092) to M.B.G., and NIGMS to X.Z.X. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, and Johnson WA (2003). Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Current biology 13, 1557–1563. [DOI] [PubMed] [Google Scholar]
- Albeg A, Smith CJ, Chatzigeorgiou M, Feitelson DG, Hall DH, Schafer WR, Miller DM, and Treinin M (2011). C. elegans multi-dendritic sensory neurons: morphology and function. Molecular and Cellular Neuroscience 46, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Árnadóttir J, and Chalfie M (2010). Eukaryotic mechanosensitive channels. Annual review of biophysics 39, 111–137. [DOI] [PubMed] [Google Scholar]
- Arribere JA, Bell RT, Fu BX, Artiles KL, Hartman PS, and Fire AZ (2014). Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics, 114.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R, Touroutine D, Barbagallo B, Climer J, Lambert CM, Clark CM, Alkema MJ, and Francis MM (2014). A Conserved Dopamine-Cholecystokinin Signaling Pathway Shapes Context–Dependent Caenorhabditis elegans Behavior. PLoS genetics 10, e1004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Fernandez-Illescas SM, Liao Z, and Goodman MB (2007). Gain-of-function mutations in the MEC-4 DEG/ENaC sensory mechanotransduction channel alter gating and drug blockade. The Journal of general physiology 129, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng Q, Sieburth D, and Kaplan JM (2008). Profiling synaptic proteins identifies regulators of insulin secretion and lifespan. PLoS genetics 4, e1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, and Brenner S (1985). The neural circuit for touch sensitivity in Caenorhabditis elegans. Journal of Neuroscience 5, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee W-H, Spencer WC, Kindt KS, Hwang SW, Miller III DM, Treinin M, and Driscoll M (2010). Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nature neuroscience 13, 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, O'Hagan R, and Chalfie M (2002). The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature 420, 669. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bharill S, Isacoff EY, and Chalfie M (2015). Subunit composition of a DEG/ENaC mechanosensory channel of Caenorhabditis elegans. Proceedings of the National Academy of Sciences 112, 11690–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm KI, Khovanov N, Lopes DM, La Russa F, and McMahon SB (2018). Large scale in vivo recording of sensory neuron activity with GCaMP6. eNeuro, ENEURO. 0417–0417.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Porto DA, Hwang H, Grundy LJ, Schafer WR, and Lu H (2017). Automated and controlled mechanical stimulation and functional imaging in vivo in C. elegans. Lab on a Chip 17, 2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa WS, Yu S.-c., Liewald JF, and Gottschalk A (2017). Fast cAMP modulation of neurotransmission via neuropeptide signals and vesicle loading. Current biology 27, 495–507. [DOI] [PubMed] [Google Scholar]
- Dong X, Liu OW, Howell AS, and Shen K (2013). An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell 155, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, and Chalfie M (1991). The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349, 588. [DOI] [PubMed] [Google Scholar]
- Eastwood AL, and Goodman MB (2012). Insight into DEG/ENaC channel gating from genetics and structure. Physiology 27, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Di Schiavi E, Bergamasco C, and Bazzicalupo P (2007). Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395, 170–176. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV (1991). Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. Journal of Comparative Neurology 309, 40–70. [DOI] [PubMed] [Google Scholar]
- Geffeney SL, Cueva JG, Glauser DA, Doll JC, Lee TH-C, Montoya M, Karania S, Garakani AM, Pruitt BL, and Goodman MB (2011). DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron 71, 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales EB, Kawate T, and Gouaux E (2009). Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'hagan R, Yao CA, and Chalfie M (2002). MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415, 1039. [DOI] [PubMed] [Google Scholar]
- Gorczyca DA, Younger S, Meltzer S, Kim SE, Cheng L, Song W, Lee HY, Jan LY, and Jan YN (2014). Identification of Ppk26, a DEG/ENaC channel functioning with Ppk1 in a mutually dependent manner to guide locomotion behavior in Drosophila. Cell reports 9, 1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, and Jan YN (2002). Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129, 2867–2878. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Wang Q, and Wang Z (2014). The role of PPK26 in Drosophila larval mechanical nociception. Cell reports 9, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Hall DH, and Hedgecock EM (1991). Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65, 837–847. [DOI] [PubMed] [Google Scholar]
- Hart AC, Sims S, and Kaplan JM (1995). Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378, 82. [DOI] [PubMed] [Google Scholar]
- Hobert O (2013). The neuronal genome of Caenorhabditis elegans. Wormbook, 1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Pym EC, Babu K, Murray ABV, and Kaplan JM (2011). A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron 71, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, and Chalfie M (1994). Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367, 467. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Costa WS, Wabnig S, Stirman JN, Watson JD, Spencer WC, Akerboom J, Looger LL, Treinin M, Miller DM, et al. (2012). Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Current biology 22, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, and Tracey WD (2007). Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Current Biology 17, 2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, and Gouaux E (2007). Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316. [DOI] [PubMed] [Google Scholar]
- Kim K, and Li C (2004). Expression and regulation of an FMRFamide - related neuropeptide gene family in Caenorhabditis elegans. Journal of Comparative Neurology 475, 540–550. [DOI] [PubMed] [Google Scholar]
- Li C, and Kim K (2014). Family of FLP peptides in Caenorhabditis elegans and related nematodes. Frontiers in endocrinology 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, and Xu XS (2006). A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kang L, Piggott BJ, Feng Z, and Xu XS (2011). The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nature communications 2, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Dong X, Moerman DG, Shen K, and Wang X (2015). Sarcomeres pattern proprioceptive sensory dendritic endings through UNC-52/Perlecan in C. elegans. Developmental cell 33, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ward A, Gao J, Dong Y, Nishio N, Inada H, Kang L, Yu Y, Ma D, and Xu T (2010). C. elegans phototransduction requires a G protein–dependent cGMP pathway and a taste receptor homolog. Nature neuroscience 13, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Apps D, Menzies J, Patel JC, and Rice ME (2011). Dendritic release of neurotransmitters. Comprehensive Physiology 7, 235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, and Maricq AV (2002). Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron 36, 933–944. [DOI] [PubMed] [Google Scholar]
- Mello C, and Fire A (1995). DNA transformation. In Methods in cell biology (Elsevier; ), pp. 451–482. [PubMed] [Google Scholar]
- Nathoo AN, Moeller RA, Westlund BA, and Hart AC (2001). Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proceedings of the National Academy of Sciences 98, 14000–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan R, Chalfie M, and Goodman MB (2005). The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature neuroscience 8, 43. [DOI] [PubMed] [Google Scholar]
- Paix A, Folkmann A, Rasoloson D, and Seydoux G (2015). High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics 201, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott BJ, Liu J, Feng Z, Wescott SA, and Xu XS (2011). The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell 147, 922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokala N, Liu Q, Gordus A, and Bargmann CI (2014). Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proceedings of the National Academy of Sciences 111, 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, Berwin B, Davis W, Ann K, Chin C-T, Kreber R, Ganetzky B, Martin TF, and Broadie K (2001). Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron 31, 421–437. [DOI] [PubMed] [Google Scholar]
- Salzberg Y, Díaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, Kaprielian Z, and Bülow HE (2013). Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell 155, 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg Y, Ramirez-Suarez NJ, and Bülow HE (2014). The proprotein convertase KPC-1/furin controls branching and self-avoidance of sensory dendrites in Caenorhabditis elegans. PLoS genetics 10, e1004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, and Schmid B (2012). Fiji: an open-source platform for biological-image analysis. Nature methods 9, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, and Kaplan JM (2007). PKC-1 regulates secretion of neuropeptides. Nature neuroscience 10, 49. [DOI] [PubMed] [Google Scholar]
- Smith CJ, O’Brien T, Chatzigeorgiou M, Spencer WC, Feingold-Link E, Husson SJ, Hori S, Mitani S, Gottschalk A, and Schafer WR (2013). Sensory neuron fates are distinguished by a transcriptional switch that regulates dendrite branch stabilization. Neuron 79, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Shreffler W, Wang S, and Driscoll M (1997). unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron 18, 107–119. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, and Hobert O (2003). LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Developmental biology 263, 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Matthewman C, Han L, Miller T, Miller DM, and Bianchi L (2013). Neurotoxic unc-8 mutants encode constitutively active DEG/ENaC channels that are blocked by divalent cations. The Journal of general physiology 142, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JD (2015). Rapid and precise engineering of the Caenorhabditis elegans genome with lethal mutation co-conversion and inactivation of NHEJ repair. Genetics 199, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way JC, and Chalfie M (1989). The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes & development 3, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Wen Q, Po MD, Hulme E, Chen S, Liu X, Kwok SW, Gershow M, Leifer AM, Butler V, and Fang-Yen C (2012). Proprioceptive coupling within motor neurons drives C. elegans forward locomotion. Neuron 76, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, and Brenner S (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314, 1–340. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, and Jan YN (2010). Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemini E, Jucikas T, Grundy LJ, Brown AE, and Schafer WR (2013). A database of Caenorhabditis elegans behavioral phenotypes. Nature methods 10, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, Pitt GS, and Tracey WD (2012). Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell reports 1, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, and Tracey WD (2010). Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Current Biology 20, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example movie of global Ca2+ transients trigged by harsh touch in wild-type worms. Green channel is PVD>GcaMP6, red channel is PVD>mCherry. Scale bar: 30μm (Related to Figure 3).
Example movie of global Ca2+ transients trigged by harsh touch in degt-1 mutants. Green channel is PVD>GcaMP6, red channel is PVD>mCherry. Scale bar: 30μm (Related to Figure 3).
Example movie of dendritic local Ca2+ transients in wild-type animals during movement. Scale bar: 30μm (Related to Figure 4).
Example movie of dendritic local Ca2+ transients in unc-8; mec-10 del-1 animals during movement. Scale bar: 30μm (Related to Figure 4).
Data Availability Statement
This study did not generate large-scale datasets or new code.