Summary
Background
Understanding the duration of protection and risk of reinfection after natural infection is crucial to planning COVID-19 vaccination for at-risk groups, including care home residents, particularly with the emergence of more transmissible variants. We report on the duration, neutralising activity, and protection against the alpha variant of previous SARS-CoV-2 infection in care home residents and staff infected more than 6 months previously.
Methods
We did this prospective observational cohort surveillance in 13 care homes in Greater London, England. All staff and residents were included. Staff and residents had regular nose and throat screening for SARS-CoV-2 by RT-PCR according to national guidelines, with ad hoc testing of symptomatic individuals. From January, 2021, antigen lateral flow devices were also used, but positive tests still required RT-PCR confirmation. Staff members took the swab samples for themselves and the residents. The primary outcome was SARS-CoV-2 RT-PCR positive primary infection or reinfection in previously infected individuals, as determined by previous serological testing and screening or diagnostic RT-PCR results. Poisson regression and Cox proportional hazards models were used to estimate protective effectiveness of previous exposure. SARS-CoV-2 spike, nucleoprotein, and neutralising antibodies were assessed at multiple timepoints as part of the longitudinal follow-up.
Findings
Between April 10 and Aug 3, 2020, we recruited and tested 1625 individuals (933 staff and 692 residents). 248 participants were lost to follow-up (123 staff and 125 residents) and 1377 participants were included in the follow-up period to Jan 31, 2021 (810 staff and 567 residents). There were 23 reinfections (ten confirmed, eight probable, five possible) in 656 previously infected individuals (366 staff and 290 residents), compared with 165 primary infections in 721 susceptible individuals (444 staff and 277 residents). Those with confirmed reinfections had no or low neutralising antibody concentration before reinfection, with boosting of titres after reinfection. Kinetics of binding and neutralising antibodies were similar in older residents and younger staff.
Interpretation
SARS-CoV-2 reinfections were rare in older residents and younger staff. Protection from SARS-CoV-2 was sustained for longer than 9 months, including against the alpha variant. Reinfection was associated with no or low neutralising antibody before reinfection, but significant boosting occurred on reinfection.
Funding
Public Health England.
Introduction
The COVID-19 pandemic had a disproportionately high effect on care homes worldwide, with case fatality rates of up to 25% among the oldest and most frail residents.1, 2, 3, 4 Care home staff, although younger and healthier than residents, have among the highest SARS-CoV-2 infection and fatality rates of any occupation.5 Consequently, residents and staff in long-term care facilities are prioritised for COVID-19 vaccination in many countries.6, 7 Understanding immune responses and duration of protection after infection in this high-risk group, particularly in the context of new variants, is crucial for designing vaccination strategies.8 Highly transmissible alpha and delta variants have successively replaced early strains in the UK and elsewhere, and other variants such as beta and gamma that have less clear transmission advantages but more pronounced antigenic variation circulate in other parts of the world.
We investigated COVID-19 outbreaks in care homes in England early in the pandemic and found high rates of SARS-CoV-2 infection among residents and staff.2 We did a prospective longitudinal cohort of 13 London care homes in April, 2020, with regular sampling of staff and residents, including serum antibodies.9 Antibody seroprevalence varied markedly between care homes (from 10·7–84·0%), indicating marked heterogeneity of exposure during the first wave (peak in April, 2020).10 118 (89·4%) of 132 seropositive individuals had neutralising antibodies irrespective of age, sex, or symptom status.11
Research in context.
Evidence before this study
We searched PubMed with the terms “COVID-19” or “SARS-CoV-2”, “care home”, “nursing home”, “nursing facility” or “residential home”, and “reinfection” or “humoral immunity” to identify publications relating to SARS-CoV-2 reinfections and longevity of antibody responses to natural infection between Jan 1, 2020, and June 30, 2021. Protection from reinfection following natural infection was reported to last at least 6 months in healthy adults, mainly in longitudinal cohorts of health-care workers. Among older care home residents and staff, reports suggest protection for at least 10 months after primary infection.
Added value of this study
Antibody persistence was similar between care home residents and staff members, with reinfections occurring rarely in either group, indicating high protection for at least 9 months after previous infection, including against emerging SARS-CoV-2 variants. The few individuals with reinfection had low or undetectable neutralising antibody titres before reinfection.
Implications of all the available evidence
Given the high morbidity and mortality associated with COVID-19 outbreaks in care homes, our data provide evidence of high antibody persistence in older residents after primary infection and subsequent protection from reinfection. Further studies are needed to assess whether a single dose of vaccine might be sufficient to protect previously infected care home residents and staff in countries with poor access to vaccination. Immune evasion by circulating variant viruses and time since primary infection will need to be considered when evaluating the appropriateness of such an approach.
In England, the alpha variant was first identified in September, 2020, and increased rapidly from November, becoming responsible for more than 90% of community infections by the end of December, and more than 98% by the end of January, 2021.12 General population studies involving previously infected adults reported reinfection rates of less than 1%,13, 14, 15 but protection from reinfection decreased with age.13 We report on the duration, neutralising activity, and protection against the alpha variant of previous SARS-CoV-2 infection in care home residents and staff infected more than 6 months previously.
Methods
Study design and participants
We did this prospective observational cohort study in 13 care homes in London, England. The care homes included provide residential or nursing care, including specialist dementia care, for between 40 and 110 residents per home, aged from 40 to over 100 years.10
Eight of the included care homes underwent whole-home SARS-CoV-2 RT-PCR testing during the first wave (timepoint T0; weeks 15–17, 2020) because of a confirmed outbreak (≥2 cases within 14 days; six care homes),2 or a single case (two care homes). Five care homes reported no cases during the same period.10 All residents and staff were offered SARS-CoV-2 antibody testing more than 4 weeks after timepoint T0 (timepoint T1; weeks 20–30, 2020), irrespective of symptoms, and all patients and staff who remained in the care homes were followed up between Aug 4, 2020, and Jan 31, 2021, with no exclusion criteria.
Vaccination in care homes started on Dec 10, 2020. By Jan 31, 2021, 60% of residents had received the first dose, compared with less than 5% of staff. Because of the UK recommended 12-week interval between doses, no patient or member of staff received a second dose before the end of the follow-up period.
Care home managers obtained informed verbal consent from staff members and from residents who could give their own consent. Otherwise, next of kin provided informed verbal consent. The protocol was reviewed and approved by the Public Health England (PHE) Research Ethics and Governance Group (NR0204).
Procedures
Care home staff underwent regular nose and throat swab screening for SARS-CoV-2 RT-PCR according to national guidelines, with ad hoc testing of symptomatic individuals. Testing capacity was low at first but improved from the last quarter of 2020.16 Staff members swabbed themselves and the residents. Although national guidelines mandated testing of staff once every week and testing of residents once every 4 weeks, total numbers of tests were similar between the two groups in this cohort (appendix p 2). The swabs were initially tested in national testing laboratory networks and PHE Colindale, but RT-PCR testing was done only at PHE Colindale by the end of September, 2020, for 12 care homes, whereas the remaining care home continued testing through their local National Health Service laboratory. From January, 2021, national screening policy for care homes incorporated SARS-CoV-2 antigen lateral flow devices, although a positive test continued to require RT-PCR confirmation.
Symptom status for all RT-PCR positive individuals in the 2 weeks before and after the test was obtained through self-reporting questionnaires by staff. For residents this was recorded by staff caring for them. Typical symptoms (fever, shortness of breath, or cough) and atypical symptoms (delirium, fatigue, lethargy, diarrhoea, or reduced alertness) were recorded, with free-text options to include other symptoms.
As part of follow-up, care home staff and residents had blood sampling for antibody persistence 4 or more months after baseline T1 serology (timepoint T2, weeks 40–46, 2020). Individuals who were SARS-CoV-2 RT-PCR positive during the surveillance period were offered serological testing at least 28 days later. In care homes with a suspected COVID-19 outbreak, whole-home RT-PCR testing was done on days 0, 7, and 28, alongside serological testing on day 28.16
SARS-CoV-2 RT-PCR testing at PHE Colindale was done using RT-PCR assays targeting the Orf1ab and E genes.17, 18 RT-PCR testing done in other clinical laboratories used different commercial tests. Whole genome sequencing (WGS) for RT-PCR positive samples was done as previously described.10 Serological analysis included a native virus antigen lysate assay,11 receptor binding domain (RBD) assay,10 and a commercial nucleocapsid protein antibody assay (Abbott, Chicago IL, USA). Seropositivity was defined as reactivity above the defined assay cutoff on 2 or more assays. Neutralising antibody titres were assessed for a subset of individuals using a live virus neutralisation assay against the virus strain isolated from the second case detected in England (England.2) and alpha strains.19
Outcomes
The primary outcome was SARS-CoV-2 RT-PCR positivity during the follow-up period. This was defined as primary infection in those with no previous SARS-CoV-2 exposure (previous negative RT-PCR samples or negative serological tests). Suspected reinfection was defined as RT-PCR positivity in an individual who had been SARS-CoV-2 RT-PCR positive at least 90 days previously, or was seropositive. Reinfection was designated as confirmed if baseline serology was positive across 2 or more assays and RT-PCR positive respiratory material was available for confirmation at PHE Colindale. Reinfection was considered probable if the RT-PCR positive sample was not available for confirmation, whereas possible reinfections included patients with discordant baseline serology (contradictory results between serological assays) and without a swab for confirmation. Secondary outcomes were seroconversion in the absence of RT-PCR positivity, and antibody persistence.
Statistical analysis
The study included all staff or residents in care homes at the time of the original investigations in weeks 15–30, 2020; there were no exclusions.2, 10, 11 From Aug 4, 2020, staff or residents in the original cohort who were still in the home were followed up with repeat RT-PCRs and serology and included in the analysis. RT-PCR results in individuals associated with these care homes were obtained from national databases (Unified Single Dataset, Second Generation Surveillance System, and PHE Colindale Laboratory Information Management System). Data were analysed using R (v4.0.2) in R Studio (v1.3.1056) with the cowplot (v1.1.0), eeptools (v1.2.4), egg (v0.4.5), grid (v4.0.2), ISOweek (v0.6.2), lubridate (v1.7.9.2), plyr (v1.8.6), readxl (v1.3.1), scales (v1.1.1), stringdist (v0.9.6.3), survival (v3.2.7), survminer (v0.4.8), tidyverse (v1.3.0), tm (v0.7.8), and zoo (v1.8.8) packages. Continuous data that did not follow a normal distribution are presented as medians with IQRs and compared using the Mann–Whitney U test. Antibody results are presented as index values or titres with geometric mean titres (GMTs) and 95% CIs. Correlations for non-parametric data were assessed using Spearman's rank correlation with 95% CI. Data were analysed using GraphPad Prism.
We used a cohort analysis to explore protection against reinfection. Poisson regression and Cox proportional hazards models were fitted with a new positive RT-PCR result as the outcome. Participants were censored at their last positive RT-PCR test date or Jan 31, 2021. Individuals were not censored at vaccination. For Cox proportional hazards, baseline hazard functions could vary by care home, and a random effect for care home was used for Poisson regression to take into account care-home specific outbreaks. Models included adjustments by staff or resident, sex, and age group (16–29 years [August–September], 16–29 years [October–January], 30–69 years, and ≥70 years). A simple age–time interaction was included for the youngest age group; infections between August and September, 2020, were more frequent among young adults (staff), in accordance with surveillance data.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
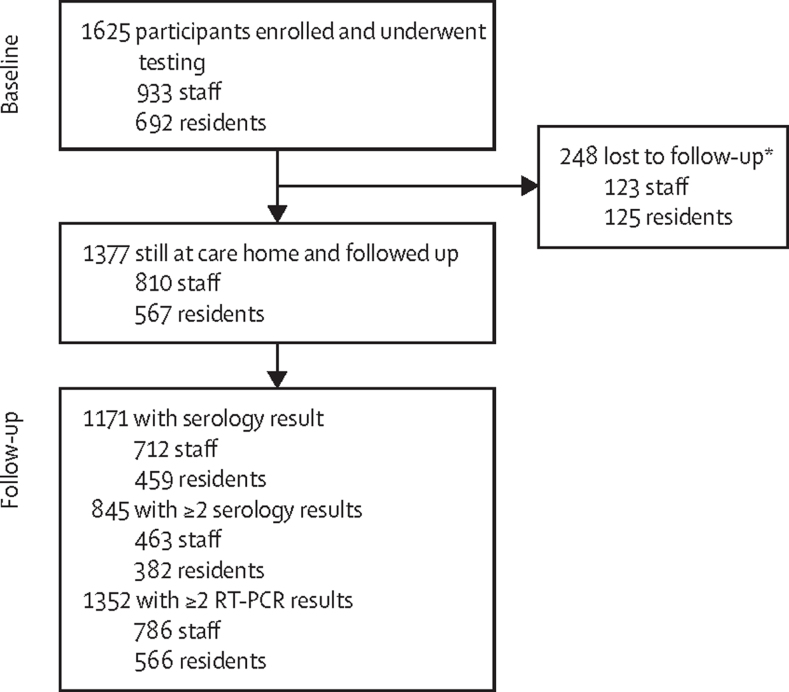
Between April 10 and Aug 3, 2020, 1625 individuals (933 staff and 692 residents) were enrolled for testing at baseline and 1377 (84·7%; 810 staff and 567 residents) with baseline RT-PCR, serology, or both were followed up, including 845 (463 staff and 382 residents) who had additional blood sampling for SARS-CoV-2 antibodies (figure 1). The 248 individuals who were lost to follow-up included residents who died or moved from the care home and staff who changed employment. Age and sex distributions were similar between the originally enrolled and follow-up cohorts (appendix p 4). The median age was 49 years (IQR 39–57) in staff, and 87 years (80–92) in residents (appendix p 4). 487 (70·4%) of 692 residents and 764 (81·9%) of 933 staff were female (appendix p 2). Staff and residents had similar numbers of RT-PCR and blood tests after sporadic infection and outbreak investigations (appendix p 2).
Figure 1.
Trial profile
*Including residents who died or moved from the care home and staff who changed employment.
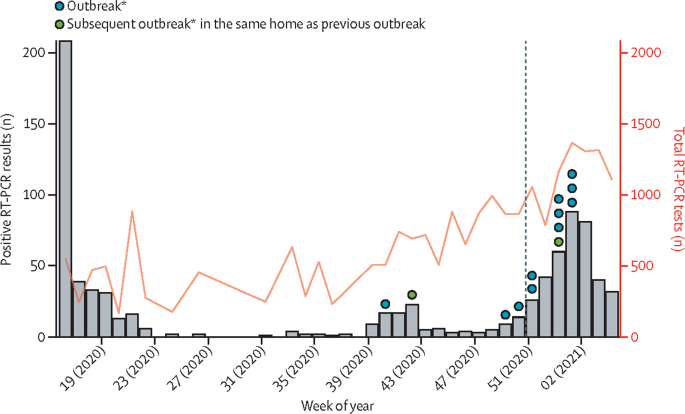
After the first peak of infections in April, 2020, (weeks 13–21), positive test results declined during summer, with occasional cases in susceptible staff and residents, but no outbreaks, before rising again during early autumn, with outbreaks in 12 homes (week 40, 2020, to week 2, 2021; figure 2). The alpha variant accounted for more than 90% of all cases in England by the end of January, 2021. Outbreaks before December, 2020, were exclusively caused by original pre-alpha viruses, and were predominantly caused by the alpha variant thereafter. The proportion of new infections caused by the alpha variant in care homes increased with the increasing frequency of circulating alpha variant in the community.
Figure 2.
Numbers of SARS-CoV-2 RT-PCR tests, positive results, and outbreaks in care homes during the study period
*Outbreak was defined as two or more people with positive SARS-CoV-2 RT-PCR results within 14 days. The dashed line indicates commencement of vaccination in cohort.
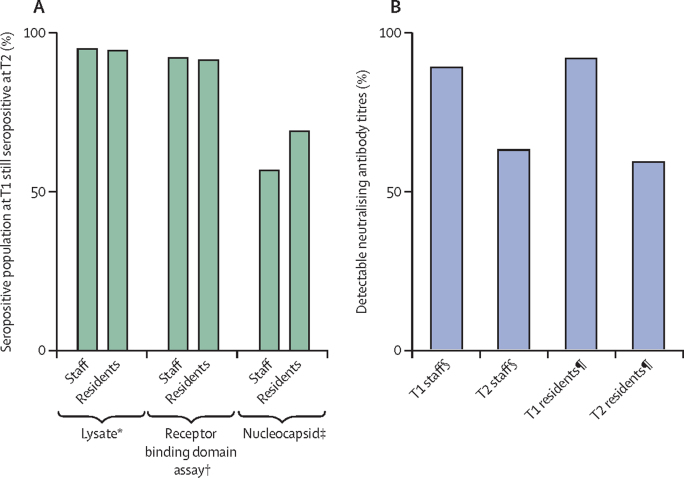
The mean interval between T1 and T2 blood sampling was 133 days (range 86–161 days). During this period, there was an outbreak in one home, which was excluded from antibody longevity assessments. In the remaining 12 homes, antibodies remained positive for more than 90% of individuals and at similar rates between staff and residents in native viral lysate antigen ELISA (330 [94·8%] of 348 participants] and spike protein RBD (308 [91·9%] of 335; figure 3A). By contrast, more than 30% of individuals lost detectable nucleocapsid (120 [38·0%] of 316 participants) and neutralising antibodies (42 [35·0%] of 120 participants) at T2 (figure 3). A similar proportion of staff and residents lost neutralising antibodies (12 [29%] of 41 staff and 30 [38%] of 79 residents; p=0·71; figure 3B), but a higher proportion of staff sero-reverted in the nucleocapsid assay (78 [43%] of 180 staff and 42 [31%] of 136 residents; p=0·026; figure 3A). For the participants who were seropositive at T1, there was a small but significant decline between T1 and T2 in both RBD antibody titres (1197 at T1 and 564 at T2; p<0·0001) and neutralising antibody titres (76·4 at T1 and 29·1 at T2; p<0·0001). Nine participants with RBD antibodies at T1 (2%; 3 residents and 6 staff [2%]) of 442 people with RBD antibodies at T1 had a more than 4-times increase in titre at T2 (1488 at T1 and 14022 at T2; p=0·039), associated with significant boosting of neutralising antibody titres (76·7 at T1 and 573·8 at T2; p=0·0039; figure 4).
Figure 3.
Antibody longevity between T1 and T2 timepoints
(A) Seropositive staff and residents by binding assays. (B) Seropositive staff and residents with detectable neutralising antibody titres to live virus (England.2). Timepoint T1 was May, June,or July, 2020, 4 weeks after the first set of testing. Timepoint T2 was September or October, 2020, 4 months after baseline T1 serology. *201 staff and 147 residents, p>0·9999. † 193 staff and 142 residents, p=0·84. ‡180 staff and 136 residents, p=0·026; Fisher's exact p<0·05. §46 staff, loss between T1 and T2 p=0·0063; Fisher's exact p<0·005. ¶86 residents, loss between T1 and T2 p<0·0001; Fisher's exact p<0·0001.
Figure 4.
RBD and neutralising antibody titres by previous infection status
(A) RBD titre to England.2 strain at T1 and T2 for all individuals with detectable RBD antibodies at T1. (B) Live virus neutralising antibody titre to England.2 virus at T1 and T2 for all individuals with detectable RBD antibodies at T1. Bars indicate geometric mean and 95% CI. Dashed line indicates assay threshold. Timepoint T1 was May, June, or July, 2020, 4 weeks after the first set of testing. Timepoint T2 was September or October, 2020, 4 months after baseline T1 serology. RBD=receptor binding domain assay. Statistical analysis using Wilcoxon matched pairs: *p<0·0001, † p=0·039. ‡p<0·0001, §p<0·0039.
Of the 1377 individuals followed up since Aug 4, 2020, 1171 (85·0%) had serological testing (712 staff and 459 residents) at T2. We recorded 23 potential reinfections, including ten confirmed reinfections (five staff and five residents; only one staff member was symptomatic with mild fever and cough), eight probable re-infections, and five possible re-infections among 656 previously infected individuals (366 staff and 290 residents). Of 618 (353 staff and 265 residents) individuals who were seropositive at T1, 20 (3%; 13 staff and seven residents) had a positive RT-PCR after T2 (appendix p 5). One resident who seroconverted between T1 and T2 was subsequently RT-PCR positive during an outbreak after T2. Two residents of 167 individuals (55 staff and 112 residents) who were RT-PCR positive during the first wave became SARS-CoV-2 RT-PCR positive again between December, 2020, and January, 2021 (appendix p 5).
WGS was obtained for seven of ten confirmed reinfections and showed various viral lineages, with six due to the alpha variant; virus was cultured from four of six patients (table 1). RT-PCR cycle threshold values were similar between primary and confirmed reinfections (Mann–Whitney test p=0·055; appendix p 6). One staff member who tested positive with a B.1.1.36.28 lineage in October, 2020, was reinfected before the emergence of the alpha variant (table 1, figure 5). Of the 13 probable and possible reinfections, genomic lineage information was available for only two possible cases (B.1.1.7 and B.1.177).
Table 1.
Characteristics of individuals with confirmed reinfection
| Role | Age group, years | Sex | Diagnosis of primary infection | Ct value | Virus isolate* | Lineage | Symptom status T0 | 2nd PCR + | Ct value | Lineage | Virus isolate | Symptom status reinfection | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Staff | 30–39 | F | Seropositive T1 | NA | NA | NA | Asymptomatic | Sept 30, 2020 | 33·65 | B.1.36.28 | N | Asymptomatic |
| 2 | Staff | 30–39 | F | PCR + T0 Seropositive T1 | 38·08 | ND | Failed sequencing* | Asymptomatic | Dec 17, 2020 | 21·49 | B.1.1.7 | Y | Asymptomatic |
| 3 | Staff | 20–29 | F | PCR + T0 Seropositive T1 | 35·56 | ND | B.40 | Asymptomatic | Jan 3, 2021 | 20·57 | B.1.1.7 | Y | Symptomatic |
| 4 | Staff | 20–29 | F | Seropositive T1 | NA | NA | NA | Asymptomatic | Jan 4, 2021 | 26·35 | B.1.1.7 | Y | Asymptomatic |
| 5 | Staff | 40–49 | M | Seropositive T1 | NA | NA | NA | Asymptomatic | Jan 13, 2021 | 26·61 | B.1.1.7 | Y | Asymptomatic |
| 6 | Resident | 80–89 | F | PCR + T0 | 34·98 | Y | B.1.1.162 | Asymptomatic | Jan 13, 2021 | 27·47 | B.1.1.7 | N | Asymptomatic |
| 7 | Resident | 90–99 | F | Seropositive T1 | NA | NA | NA | Asymptomatic | Jan 16, 2021 | 35·53 | Failed sequencing | N | Asymptomatic |
| 8 | Resident | 80–89 | F | Seropositive T1 | NA | NA | NA | Asymptomatic | Jan 16, 2021 | 36·25 | Failed sequencing† | N | Asymptomatic |
| 9 | Resident | 90–99 | F | PCR + T0 Seropositive T1 | 28·33 | ND | B | Asymptomatic | Jan 16, 2021 | 30·52 | B.1.1.7 | N | Asymptomatic |
| 10 | Resident | 70–80 | F | Seropositive T1 | NA | NA | NA | Asymptomatic | Jan 20, 2021 | 33·09 | Not sequenced‡ | N | Asymptomatic |
Ct=cycle threshold.
Y=live virus isolated in viral culture; N=live virus not isolated in viral culture; NA=not applicable; ND=not done.
Unacceptable level (>20%) of unresolvable nucleotides.
Insufficient volume of material remaining for sequencing. Timepoint T1 was May, June, or July, 2020, 4 weeks after the first set of testing. Timepoint T2 was September or October, 2020, 4 months after baseline T1 serology.
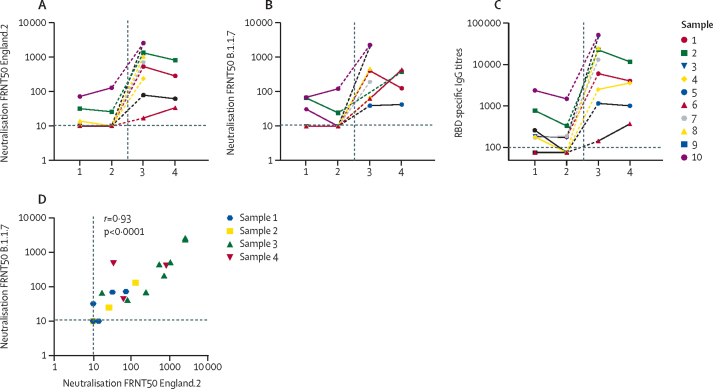
Figure 5.
Antibody titres of individuals with reinfection over time
(A) Live virus 50% reduction in neutralising antibody measured by Focus reduction (FRNT50) to England.2 virus. (B) Live virus FRNT50 to alpha (B.1.1.7). (C) RBD specific IgG titres over time for the 10 individuals with confirmed reinfection (identification numbers correspond to table 1). (D) Correlation between live virus FRNT50 to England.2 virus and live virus FRNT50 to alpha (B.1.1.7) virus. Statistical analysis using Spearman's rank correlation coefficient (r). X axes refer to timepoints of serological sampling in figures A–C. A minimum of two samples were available for nine individuals with the first sample in May or June, 2020 (at T1), and second sample in September or October (at T2). One individual only had one sample available before reinfection. Samples 1 and 2 represent samples taken from individuals before reinfection (T1 or T2) and samples 3 and 4 represent samples taken after reinfection. Post-reinfection serological samples were available for nine individuals who had a sample within 7–14 days of reinfection RT-PCR (sample 3); an additional five had a further sample (sample 4) taken 4–6 weeks after reinfection RT-PCR. RBD=receptor binding domain assay. FRNT50=focus reduction neutralisation test with 50% reduction of the virus control.
Longitudinal serological responses in the ten people with confirmed reinfection showed that none had detectable neutralising antibodies to England.2 (representative of early circulating virus) or the alpha variant at the last sampling timepoint before reinfection; seven of ten did not have neutralising antibody titres at any stage before reinfection following primary infection. One asymptomatic resident did not seroconvert by any assay after first confirmed infection (figure 5A, B). Only six of ten had RBD IgG antibodies before reinfection (figure 5C).
After confirmed reinfection, follow-up serology was available for nine of ten individuals who all had boosting of RBD and neutralising antibodies against England.2 and alpha variant viruses, including six people who had confirmed reinfection with alpha (figure 5; table 1). There was a significant correlation between neutralising titres to the England.2 virus and alpha variants (r=0·93, 95% CI 0·86–0·97; p<0·0001, figure 5D), indicating a close antigenic relationship between the two variants. Individuals reinfected with the alpha variant had both an alpha variant antibody response and boosting of their original neutralising antibody response to England.2. RBD antibody titres in reinfected individuals had significantly positive correlation for neutralising antibody titres to both England.2 (r=0·91, 0·82–0·96; p<0·0001) and alpha (r=0·84, 0·69–0·92; p<0·0001) viruses across all timepoints (data not shown).
After excluding individuals with probable or possible reinfection and those with boosting of titres between T1 and T2, RBD antibody GMTs (n=10) before reinfection were significantly lower in individuals with confirmed reinfection compared with the most recent RBD titre in the rest of the cohort (152·4 [95% CI 76·4–304·1] vs 564·2 [508·7–625·8], p=0·0002), as were neutralising antibody GMTs (14·21 [95% CI 7·84–25·75] vs 29·12 [24·01–35·32], p=0·03; figure 4). RBD titres of eight of ten reinfected individuals before reinfection were in the lowest quartile of titres (<251·0) of individuals without reinfection.
Ten confirmed reinfections were recorded during follow-up of 103 557 person-days, compared with 165 primary infections in individuals with no infection history over 87 131 person-days. The estimated relative risk (RR) using adjusted Poisson regression analysis was 0·06 (95% CI 0·03–0·12, p<0·0001) with protective effectiveness estimate using RR from a comparison of proportions (100 × [1–RR]) of 94·1% (95% CI 88·2–97·0) against reinfection (table 2). Protective effectiveness was similar when calculated by estimated hazard ratio (HR) using adjusted Cox proportional hazards model of 0·05 (95% CI 0·03–0·11, p<0·0001) and protective effectiveness estimate using HR from a comparison of proportions (100 × [1–HR]) of 94·8% (95% CI 89·4–97·4). Separate models were fitted for residents and staff, both yielding high protective effectiveness (table 2; appendix p 3). If probable and possible reinfections were included, protective effectiveness from previous exposure calculated by Poisson regression was 85·5% (95% CI 76·1–91·1, table 2), with no differences when analysing residents and staff separately. The proportional hazards assumption was met (appendix pp 3, 7).
Table 2.
Protective effectiveness of previous SARS-CoV-2 infection
| RR (95% CI) | Protective effectiveness*(95% CI) | |
|---|---|---|
| Whole cohort: confirmed reinfections | 0·06 (0·03–0·12); p<0·001 | 94·1% (88·2–97·0) |
| Residents: confirmed reinfections | 0·04 (0·02–0·11); p<0·001 | 95·9% (88·9–98·5) |
| Staff: confirmed reinfections | 0·08 (0·03–0·21); p<0·001 | 91·8% (78·6–96·8) |
| Whole cohort: all suspected reinfections | 0·15 (0·09–0·24); p<0·001 | 85·5% (76·1–91·1) |
RR=relative risk.
Calculation of protective effectiveness of previous natural infection calculated using Poisson regression, 100x (1–RR).
Discussion
We found very low rates of reinfection among residents and staff, even in the high-risk, closed environment of care homes and after the emergence of the alpha variant, consistent with our previous observations and those of others.15, 20, 21 We report that individuals with reinfection either never made an antibody response, made a poor antibody response, or sero-reverted after primary infection and before reinfection. Importantly, protective effectiveness from previous infection, estimated using multiple statistical methods, was not significantly different between older residents and younger staff, even when separate models were fitted for residents and staff.
Reinfection with SARS-CoV-2 is well documented,22, 23, 24 but the correlates of protection against reinfection remain poorly defined.15, 25 Both older residents and younger staff are equally represented among confirmed and probable reinfections, despite similar numbers of RT-PCR tests done in both cohorts, indicating that age is not a major determinant of susceptibility to reinfection. Although vaccination for residents began in December, 2020, this coincided with the peak in community infection rates and outbreaks due to the alpha variant in these homes. Although the vaccine programme was still in its early phases, it is notable that there were no reinfections in vaccinated individuals after their first dose during the 46 days between the earliest vaccine dose and the end of the investigation period.
Longitudinal studies have reported persistent antibodies26, 27, 28, 29 and protection against reinfection for longer than 6 months in healthy adults.15, 29, 30 In our previous investigation of this cohort, we found no differences in seropositivity rate, antibody concentration, or neutralising antibody titre between residents and staff, irrespective of sex or symptom status in the first few months after primary infection.10 These observations are now extended up to 9 months. Neutralising antibodies are important for protection against reinfection, as shown in animal models31 and case reports of human infections.32, 33 Studies based on modelling predictions and synthesis of observational studies suggest that neutralising antibody titres can be predicted at which there is 50% protection against infection, and that this titre lies at approximately 20% (95% CI 4·4–28·4) of mean convalescent titres of neutralising antibodies of 1:10 to 1:30 in most studies.34 This empirical observation is consistent with our observed differences in antibody titres between those with and without confirmed reinfection. In those people with confirmed reinfection, neutralising antibodies were undetectable before reinfection, eight of ten had RBD antibody titres in the lowest quartile, and both increased significantly after reinfection.
The observation that reinfection rates were similar among residents and staff is important because of concerns about immunosenescence and higher fatality among residents,2, 35 and the potential for immune evasion by new variants.36 Before the alpha variant emerged, we estimated previous infection to be 96·2% protective against reinfection with previously circulating strains.20 Our estimates, with more cases and longer follow-up than in previous studies and our previous observations indicate that previous infection remains highly protective against reinfection, including against the alpha variant.
The close concordance of neutralising antibodies between prototype older variants and the alpha variant is also consistent with previous studies,37 but cannot be extrapolated to more antigenically diverse variants, such as beta. The emergence and rapid spread of the alpha variant, which is known to cause more severe disease and higher fatality than previously circulating strains,8, 38 was associated with large outbreaks in care homes across England, including among the care homes under investigation. This variant is characterised by multiple mutations across the viral genome separating it from its closest predecessors. Nine mutations occur in the spike protein, a key immunogen for human antibody response, including RBD mutations, which can affect binding with ACE2, the host cell receptor.39, 40
WGS identified multiple separate introductions of the virus into the care homes, consistent with our previous findings,2, 10 but low transmission within individual care homes (data not shown), most likely because of the extensive infection controls and acquired immunity compared with the first pandemic wave. The largest care home outbreaks occurred in those with the lowest seroprevalence after the first wave (data not shown).
Our study has some limitations. The participating care homes were all in greater London and, therefore, might have higher staff turnover and greater dependency on temporary staff than elsewhere, which could in turn increase the propensity to introduce infection in these homes. Resident turnover is related to life expectancy and is crudely estimated at 16% between Aug 4, 2020, and Jan 31, 2021.41 The level of care provided by individual homes is not controlled for in this study, and individual staff turnover varies greatly between homes (<5% to >20%). Our follow-up cohort only includes survivors of the first pandemic wave and might not be representative of all care home residents.10, 11 Immunological studies suggest that older adults with severe or fatal COVID-19 might have a defect in at least one protective immunological pathway compared with survivors.35 Another limitation is that screening swabs were initially sent to different national testing sites and could therefore not be verified, sequenced, or cultured. The overlap between vaccination and our investigation period was 46 days and, given the differential rate of single-dose vaccine uptake among residents and staff, and different testing regimens, it is difficult to realistically assess bias, when there was simultaneously high community prevalence and multiple outbreaks in care homes.
Natural infection with SARS-CoV-2 protects adults of all ages against antigenically similar variants, including the alpha variant, up to 9 months later. Reinfections are rare and associated with low or no neutralising antibody response after primary infection, followed by boosting of antibody responses after reinfection, indicating a strong correlation between susceptibility to infection and humoral antibody titre, and recognising that the role of cellular immunity in protection remains to be fully established. Ensuring that high concentration of neutralising antibody is maintained following vaccination campaigns will be an important contributor to overall protection from emerging variants across age groups.
On Dec 8, 2020, the UK became the first country to vaccinate against COVID-19 with a fully tested vaccine. The vaccination programme has been highly successful in preventing hospital admissions and deaths among vaccinated individuals including older adults,42 who develop robust antibody and cellular responses even after a single dose, especially if previously infected.43, 44 Further studies are needed to establish the breadth of protection provided by vaccines and previous infection against new variants, and whether neutralising antibody titres can serve as proxy correlates of protection.
Data sharing
The investigation was done as Public Health England's duty to manage outbreaks in response the COVID-19 outbreak. There are no additional data for the Care Home Investigation in addition to what we have already reported.
Declaration of interests
We declare no competing interests. The authors are all employed by Public Health England, which is a public body and an executive agency of the Department of Health and Social Care.
Acknowledgments
Acknowledgments
We would like to thank the care home managers, staff, and residents for their continued support and engagement; without them this work would not have been possible. In addition, the authors are grateful for the support for this work from staff across multiple Public Health England departments, including the London Coronavirus Response Cell, Virus Reference Department, Immunisation and Countermeasures, and Field Services.
Contributors
SNL, JYC, and MZb conceived the study. AJ-S, NI, SNL, JYC, and MZb oversaw the study. MZb oversaw laboratory work. AJ-S, NI, SVW, RGi, LT, MZa, FA, KB, MER, SNL, JYC, and MZb developed the protocol. AJ-S, TAJR, NI, SVW, RGi, LT, MZv, FA, and JYC collected data. AJ-S, NI, SVW, RGi, LT, MZv, FA, SNL, JYC, and MZb were responsible for operational conduct. AJ-S, TAJR, HW, NI, SVW, KB, MER, RGo, JYC, SNL, and MZb prepared the manuscript. MP, RGo, KH, and KB did serological analysis. MP and RGo were responsible for virus isolation. JE did PCR detection work. AL did laboratory genomics. HW did statistical modelling. AJ-S, TAJR, HW, RGo, JYC, SNL, and MZb were responsible for data analysis. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2008–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladhani SN, Chow JY, Janarthanan R, et al. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennelly SP, Dyer AH, Noonan C, et al. Asymptomatic carriage rates and case fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing. 2021;50:49–54. doi: 10.1093/ageing/afaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashan MR, Smoll N, King C, et al. Epidemiology and clinical features of COVID-19 outbreaks in aged care facilities: a systematic review and meta-analysis. EClinicalMedicine. 2021;33 doi: 10.1016/j.eclinm.2021.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Office for National Statistics Coronavirus (COVID-19) related deaths by occupation, England and Wales: deaths registered between 9 March and 28 December 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/coronaviruscovid19relateddeathsbyoccupationenglandandwales/deathsregisteredbetween9marchand28december2020
- 6.Department for Health and Social Care Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI. 30 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020
- 7.Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices' interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladhani SN, Chow JY, Atkin S, et al. Regular mass screening for SARS-CoV-2 infection in care homes already affected by COVID-19 outbreaks: implications of false positive test results. J Infect. 2020;0 doi: 10.1016/j.jinf.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffery-Smith A, Dun-Campbell K, Janarthanan R, et al. Infection and transmission of SARS-CoV-2 in London care homes reporting no cases or outbreaks of COVID-19: prospective observational cohort study, England 2020. Lancet Reg Health Eur. 2021;3 doi: 10.1016/j.lanepe.2021.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladhani SN, Jeffery-Smith A, Patel M, et al. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: prospective cohort study, England. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Health England Investigation of novel SARS-CoV-2 variants of concern: technical briefings. https://www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201
- 13.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey RA, Rassen JA, Kabelac CA, et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021;181:672–679. doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Service Care Home COVID-19 Testing Guidance. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1021342/care-home-testing-guidance-england.pdf
- 17.Niu P, Lu R, Zhao L, et al. Three novel real-time RT-PCR assays for detection of COVID-19 virus. China CDC Wkly. 2020;2:453–457. doi: 10.46234/ccdcw2020.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvala H, Mehew J, Robb ML, et al. Convalescent plasma treatment for SARS-CoV-2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.28.2001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffery-Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.5.2100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krutikov M, Palmer T, Tut G, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study. Lancet Heal Longev. 2021;2:e362–e370. doi: 10.1016/S2666-7568(21)00093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS-CoV-2 reinfection—six possible cases. J Infect. 2021;82:282–327. doi: 10.1016/j.jinf.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Elslande J, Vermeersch P, Vandervoort K, et al. Symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2021;73:354–356. doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overbaugh J. Understanding protection from SARS-CoV-2 by studying reinfection. Nat Med. 2020;26:1680–1681. doi: 10.1038/s41591-020-1121-z. [DOI] [PubMed] [Google Scholar]
- 26.Yamayoshi S, Yasuhara A, Ito M, et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223:197–205. doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueiredo-Campos P, Blankenhaus B, Mota C, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020;50:2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breathnach AS, Riley PA, Cotter MP, Houston AC, Habibi MS, Planche TD. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J Infect. 2021;82:e11–e12. doi: 10.1016/j.jinf.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor H, Wall W, Ross D, et al. Cross sectional investigation of a COVID-19 outbreak at a London Army barracks: neutralising antibodies and virus isolation. Lancet Reg Health Eur. 2021;2 doi: 10.1016/j.lanepe.2020.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 35.Mueller AL, Mcnamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 37.Rees-Spear C, Muir L, Griffith SA, et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volz E, Mishra S, Chand M, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593:266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 39.Brouwer PJM, Caniels TG, van der Straten K, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. Virological. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563
- 41.Office for National Statistics Deaths involving COVID-19 in the care sector, England and Wales: deaths registered between week ending 20 March 2020 and week ending 2 April 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/deathsinvolvingcovid19inthecaresectorenglandandwales/deathsregisteredbetweenweekending20march2020andweekending2april2021
- 42.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subbarao S, Warrener LA, Hoschler K, et al. Robust antibody responses in 70-80-year-olds 3 weeks after the first or second doses of Pfizer/BioNTech COVID-19 vaccine, United Kingdom, January to February 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.12.2100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parry HM, Bruton R, Tut G, et al. Immunogenicity of single vaccination with BNT162b2 or ChAdOx1 nCoV-19 at 5–6 weeks post vaccine in participants aged 80 years or older: an exploratory analysis. Lancet Healthy Longev. 2021;2:e554–e560. doi: 10.1016/S2666-7568(21)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The investigation was done as Public Health England's duty to manage outbreaks in response the COVID-19 outbreak. There are no additional data for the Care Home Investigation in addition to what we have already reported.