Abstract
In patients with moderate-to-severe COVID-19 pneumonia, an aberrant post-viral alveolitis with excessive inflammatory responses and immunothrombosis underpins use of immunomodulatory therapy (eg, corticosteroids and interleukin-6 receptor antagonism). By contrast, immunosuppression in individuals with mild COVID-19 who do not require oxygen therapy or in those with critical disease undergoing prolonged ventilation is of no proven benefit. Furthermore, a window of opportunity is thought to exist for timely immunosuppression in patients with moderate-to-severe COVID-19 pneumonia shortly after clinical presentation. In this Viewpoint, we explore the shortcomings of a universal immunosuppression approach in patients with moderate-to-severe COVID-19 due to disease heterogeneity related to ongoing SARS-CoV-2 replication, which can manifest as RNAaemia in some patients treated with immunotherapy. By contrast, immunomodulatory therapy has overall benefits in patients with rapid SARS-CoV-2 clearance, via blunting of multifaceted, excessive innate immune responses in the lungs, potentially uncontrolled T-cell responses, possible autoimmune responses, and immunothrombosis. We highlight this therapeutic dichotomy to better understand the immunopathology of moderate-to-severe COVID-19, particularly the role of RNAaemia, and to refine therapy choices.
Introduction
There is ongoing interest in understanding the link between SARS-CoV-2 infection and inappropriate or excessive immune responses, which might contribute substantially to mortality from COVID-19.1 These responses were first reported in early 2020, when cases of severe systemic inflammation with coagulopathy, which were superficially reminiscent of cytokine storm syndromes, were described.2, 3, 4 A common example of a cytokine storm syndrome is macrophage activation syndrome, which encompasses a consumptive bleeding diathesis termed disseminated intravascular coagulation.2, 3, 5 The observation of high levels of systemic inflammation with elevated concentrations of C-reactive protein, ferritin, and serum cytokines; cytopenias; and suspected cardiac immune toxicity, in conjunction with coagulopathy, in patients with severe COVID-19 also suggested a cytokine storm with macrophage activation syndrome.6, 7, 8 Detection of SARS-CoV-2 RNA in oropharyngeal swab tests for only 8–10 days following SARS-CoV-2 infection, as well as hyperinflammation with increased risk of mortality in the subsequent weeks, also supported the concept of a hyperactivation of the immune system in the post-viral replication phase, which could be modified therapeutically.9, 10 This apparent post-infectious inflammation is of considerable interest to rheumatologists, who are familiar with treating conditions such as post-infection inflammation and macrophage activation syndrome.
Data from open-label studies of patients with severe COVID-19 treated with tocilizumab, the first licenced interleukin (IL)-6 receptor blocker, and the efficacy of tocilizumab in treating cytokine storm syndrome associated with chimeric antigen receptor (CAR) T-cell therapy, invigorated a global trial agenda of clinical trials to test immunomodulatory therapy in patients with COVID-19 pneumonia.11, 12 As data from randomised controlled trials on immunomodulatory therapy in patients with COVID-19 have emerged, including corticosteroid13 and IL-6 receptor blockade strategies,12, 14 the benefits have proven to be modest compared with the benefit of these therapies in patients with genuine cytokine storm syndromes, in whom they can be curative.5 The most encouraging randomised controlled trial in patients with severe COVID-19 pneumonia showed an incremental benefit, with a 4% reduction in mortality when tocilizumab was added to corticosteroids—far lower than that seen with similar strategies for CAR T-cell therapy-associated cytokine storm syndromes.12 The benefits of corticosteroid or IL-6 receptor blockade in patients with COVID-19 have not been replicated in every study,15, 16 indicating an incomplete understanding of disease mechanisms and disease heterogeneity. Although tocilizumab has been widely heralded as efficacious in patients with COVID-19 on the basis of large, open-label platform trials, small randomised controlled trials have not shown a survival benefit of the drug.17 Beyond corticosteroids, IL-6 receptor blockade, and antagonism of the Janus kinase (JAK) pathway,17, 18 there is insufficient evidence for other immunomodulatory therapies (including IL-1 antagonism) due to the relative paucity of data from large, phase 3 clinical trials; therefore, we do not discuss immunomodulatory therapies further.
In this Viewpoint, we contend that much of the heterogeneity in benefit of immunotherapy is linked to ongoing SARS-CoV-2 replication in a subgroup of patients (figure 1 ). We focus on patients with moderate-to-severe COVID-19, rather than on those with mild disease who do not require oxygen therapy or those with critical disease undergoing prolonged ventilation, in whom immunotherapy can be less effective (figure 1). Whereas immunotherapy seems to show overall benefits at the population level, we argue that a window of opportunity for immunotherapy might not exist universally in patients with moderate-to-severe COVID-19, because unrestrained viral replication is still taking place in some. We frame our arguments through the established concept that SARS-CoV-2-related alveolitis triggers an intrapulmonary macrophage activation syndrome-like state with a distinctive immunothrombosis of the lung (termed pulmonary intravascular coagulopathy). This pathology has been supported by single-cell analysis of bronchoalveolar fluid from patients with severe COVID-19, and intrapulmonary macrophage activation has been confirmed.19, 20 We propose that immunotherapy would improve survival only in clinical settings in which excessive innate and adaptive immune responses (including autoimmune responses) occur in the context of rapid control of SARS-CoV-2 replication. We argue that distinguishing ongoing infection in the alveolar territory, which manifests as RNAaemia, from excessive inflammatory responses and immunothrombosis occurring after clearance of infection, could be useful to improve survival.
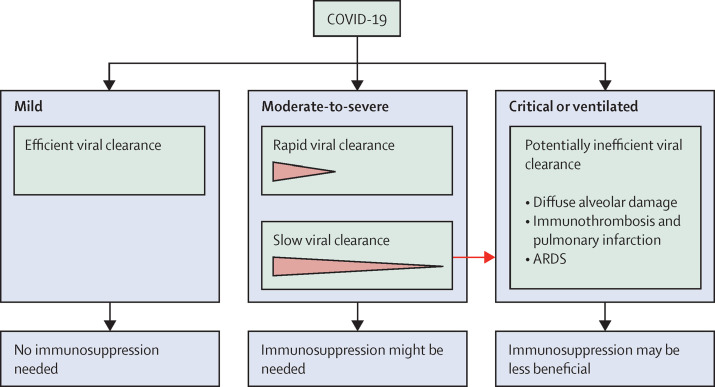
Figure 1.
Viral clearance and immunosuppression recommendations in patients with COVID-19
Patients with mild COVID-19 who do not need oxygen therapy might be immunocompetent and in an early phase of a self-limiting disease with minimal viral replication. Immunosuppression might increase viral replication and is of no benefit in these patients. In patients with moderate or severe COVID-19 who are not mechanically ventilated, some immunosuppression strategies might be beneficial, although optimal strategies await definition. Most of these patients clear the virus by day 8–11; however, some patients with ongoing viral replication might not benefit from immunosuppression. For critically ill patients with COVID-19 who are mechanically ventilated, post-mortem studies have reported extensive fibrosis, lung destruction, and associated pulmonary infarction; therefore, immunosuppression therapy might not be beneficial. ARDS=acute respiratory distress syndrome.
Ongoing active SARS-CoV-2 infection
At the beginning of the COVID-19 pandemic, studies from China indicated that the duration of viral shedding as detected by RT-PCR (not direct viral cultivation) was 7–11 days from illness onset (figure 2 ).10, 21 Consequently, the view emerged that a post-viral alveolitis and hyperinflammation state could be effectively targeted with immunomodulatory strategies.22, 23 However, some patients hospitalised with severe COVID-19 pneumonia had detectable viral RNA in the blood (termed RNAaemia) that persisted for several weeks after initial infection.6, 24, 25 Other studies have also corroborated viral RNA detection in the respiratory and gastrointestinal tract for over 20 days in some patients with severe COVID-19,26, 27 with increased viral shedding from the nasopharynx during the second week.27 A prospective study of 267 endotracheal aspirates from 90 patients with COVID-19 who required ventilation showed that persistent detection of SARS-CoV-2 by RT-PCR at 6 weeks after the onset of symptoms strongly equated with mortality;28 therefore, it is difficult to conceptualise a window of opportunity for early therapy initiation in this patient group.
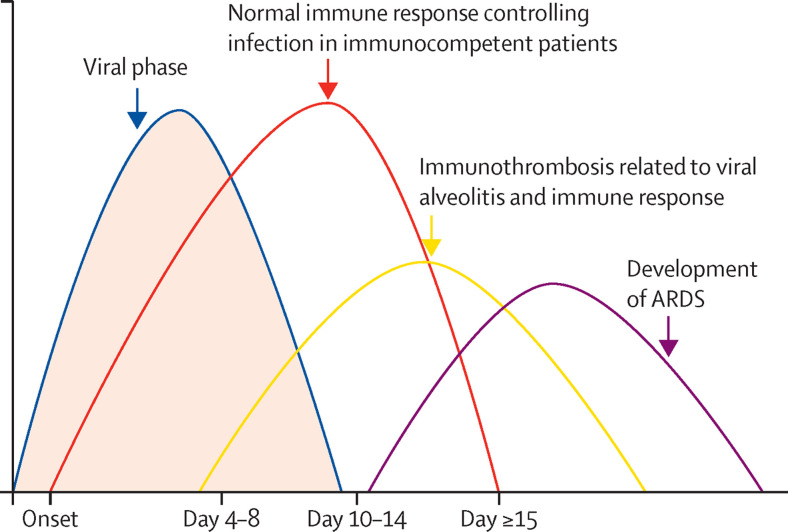
Figure 2.
Optimal trajectory of COVID-19 pneumonia
Typically, SARS-CoV-2 alveolar infection proceeds with a viral phase, an inflammatory phase, and an associated immunothrombotic phase. In severe disease, ARDS can develop. In patients who are otherwise immunocompetent, obesity and other cardiovascular risk factors can lead to cardiopulmonary system decompensation. ARDS=acute respiratory distress syndrome.
Viral RNA in respiratory tract secretions or in the blood, as detected by PCR, does not necessarily equate to active viral replication, and therapeutic suppression of innate immune responses to viral nucleic acids could be beneficial, provided viral replication is no longer occurring. Although this concept was not well understood at the outset of the COVID-19 pandemic, several studies have since directly investigated active viral replication of SARS-CoV-2 in individuals who are immunocompetent or immunosuppressed, using virological culture techniques that are considered to be the gold standard (table ). In one study, only 29% of RT-PCR-positive SARS-CoV-2 cases had cultivatable virus, although this did not last beyond 8 days, and most patients included in the study had mild COVID-19.29 The positivity rate in oropharyngeal cultures from otherwise healthy people with mild COVID-19 was 74% at 1 week and 20% at 2 weeks.29 In another study, viral cultivation was more difficult in patients with moderate-to-severe COVID-19 older than 41 years, perhaps indicating excessive immune responses following viral clearance in some cases.30 In that study, the risk of infection from respiratory secretions declined considerably to 6% in patients with mild-to-moderate disease after 10 days.30 In another study of 129 patients hospitalised with severe PCR-positive COVID-19, infectious viral particle shedding from the upper airways was detected by viral cultures in only 23 (18%) patients and correlated with RT-PCR viral loads of more than 10 million copies per mL. The median duration of infectious virus shedding was 8 days after symptom onset;31 the probability of cultivating infectious virual was below 5% after a duration of symptoms of 15 days. Increases in the number of anti-SARS-CoV-2 antibodies correlated with an inability to detect infectious virus.
Table.
Viral replication of SARS-CoV-2 in patients with COVID-19 pneumonia
| PCR at 1 week | PCR at 2 weeks | Viral culture | |
|---|---|---|---|
| Oropharynx | +++ | + | Detectable in <80% of cultures at week 1, but in only <6% after 10 days; might persist for weeks in immunosuppressed patients |
| Airways (endotrachael tube aspirate) | +++ | ++ | + |
| Blood | Negative in mild or moderate COVID-19; variably positive in severe COVID-19 (+ or ++) | Negative in mild or moderate COVID-19; variably positive in severe COVID-19 (+ or ++) | No culture attainable in any group; infection of endothelial cells in vitro not usually attainable |
+, ++, and +++ refer to the strength of the positivity of PCR. The magnitude of elevation by RT-PCR in the blood is 3–4 logs lower than in the oropharynx or airways. Viral detection by PCR at low-cycle thresholds is associated with SARS-CoV-2 replication, especially in the oropharynx and airways during the first week of infection. By contrast, little evidence exists for replication in the circulation.
At the population level, patients with moderate-to-severe COVID-19 are generally immunocompetent, unlike patients with cancer who have been treated with chemotherapy and are considered to be immunodeficient (figure 3 ). In patients with cancer receiving chemotherapy who are concomitantly infected with SARS-CoV-2, replication-competent virus was retrievable from the upper airways for 3–8 weeks after symptom onset.32, 33 Several other studies have provided evidence for ongoing SARS-CoV-2 replication in patients with severe COVID-19 pneumonia or in those receiving therapeutic immunosuppression. However, ongoing viral replication has generally been overlooked in therapy decisions for critically ill patients, in whom prompt immunosuppression is likely to worsen outcomes in the face of potentially rampant viral replication (figure 3).33, 34, 35
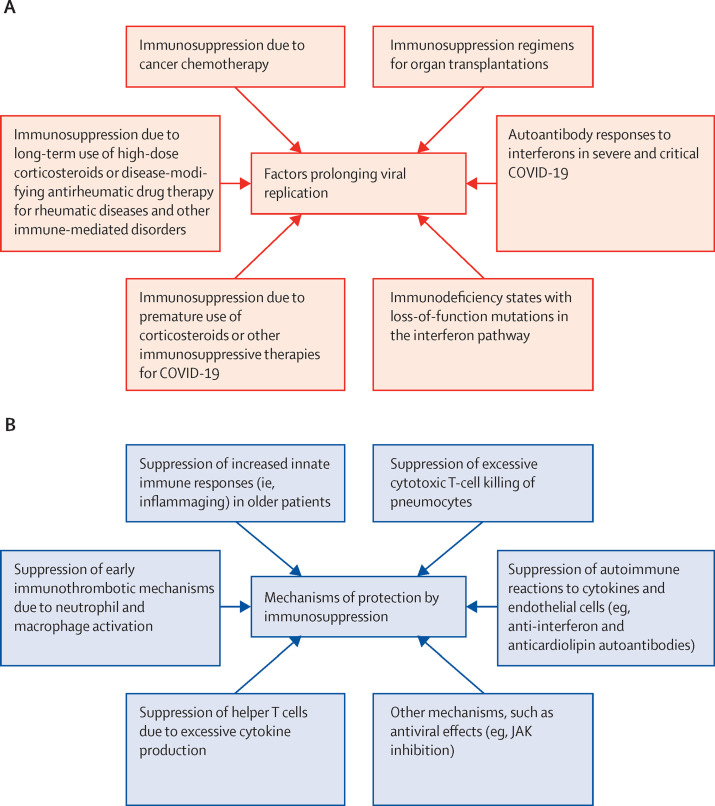
Figure 3.
Factors and mechanisms causing prolonged viral replication in the alveolar territory
(A) Factors leading to prolonged viral replication include pre-existent, unrecognised immunodeficiency states, such as type-1 interferon pathway defects, immunosuppression regimens in autoimmune diseases, organ transplantation settings, cancer-related immunosuppression or chemotherapy, or the administration of corticosteroid therapy in patients not requiring oxygen. (B) Potential mechanisms underlying benefit of immunosuppression in patients with controlled SARS-CoV-2 viral replication. Given that most mortality occurs 2 weeks from onset of infection, at a stage when viral replication might be less of a consideration, it has been suggested that helper T-cell cytokine responses in the pulmonary compartment might contribute to high local concentrations of proinflammatory cytokines, which drive macrophage and neutrophil activation, and immunothrombosis. Excessive cytotoxic CD8 T-cell responses, leading to alveolar pneumocyte killing and diffuse alveolar damage, have been suspected but not proven. Additionally, the effects of ageing, with a reduced robustness of adaptive immunity and an increased reliance on innate immune mechanisms via IL-6, IL-8, and IL-1, are considered to be important factors in severe pneumonia. Furthermore, post-viral cytokine storm scenarios might occasionally occur, although this is not well defined. IL=interleukin. JAK=Janus kinase.
The viral reservoir in these patients is poorly understood; however, its persistence over weeks might be linked to permissive bronchial mucosal or upper airway environment, and an inability to kill virus-infected cells.35, 36 In addition to patients with COVID-19 receiving chemotherapy for cancer, studies using RT-PCR showed higher amounts of SARS-CoV-2 RNA in patients with acute respiratory distress syndrome (ARDS) treated with corticosteroids compared with those treated with standard of care.37 High viral loads are associated with immunosuppression, as well as extended stay in intensive care, and prolonged intubation, but the relationship between viral load and mortality remains controversial.38
Cytokine elevation in moderate-to-severe COVID-19 pneumonia and RNAaemia
The magnitude of elevation in serum IL-6 concentrations in patients with moderate-to-severe COVID-19 is in the range of 100 pg/mL or less, compared with approximately 10 000 pg/mL typically observed in cytokine storm syndromes in patients receiving CAR T-cell therapy. Low IL-6 concentrations have been used to stratify patients with COVID-19 with a good prognosis who do not require anti-cytokine therapy.39 However, high serum cytokine concentrations might be linked to ongoing SARS-CoV-2 viral replication.39, 40, 41 The concept that active viral replication might be detrimental in patients with severe COVID-19 is supported by the early termination of several studies on IL-6 receptor blockade, in which increased mortality was reported in the group receiving tocilizumab.42 Additionally, data from a meta-analysis of trials suggested that tocilizumab was less effective in patients requiring ventilation than in those not requiring ventilation (figure 3).43 One study reported that only seven (4%) of 168 patients with severe SARS-CoV-2 infection had elevations in serum cytokines indicative of cytokine storm, and that these elevations were of a lower magnitude than in patients with severe influenza infection, challenging the concept of a systemic cytokine storm and highlighting pulmonary-centred pathology in patients with severe COVID-19.44
A key observation in patients with severe COVID-19 is that RNAaemia or detectable SARS-CoV-2 RNA in the blood, but not proven cultivable virus, is linked to serum concentrations of IL-6 up to 10 times higher than in patients without RNAaemia.2 In a study of 192 patients with severe COVID-19, 71 (37%) had RNAaemia (defined as a positive result by real-time PCR for E, RdRp, or N genes in plasma samples at any timepoint), which was associated with increased risk of invasive mechanical ventilation support, admission into intensive care, multi-organ dysfunction, and death.45 Baseline disease severity, baseline corticosteroid use, and viral titre were also associated with risk of death in the same study.45 Furthermore, heavily infected secretory cells of the human airway epithelium expressed IL-6 abundantly.46 Modest RNAaemia (as compared with elevated RNA concentrations measured by PCR of respiratory secretions), has been associated with high IL-6 concentrations and mortality in critically ill patients with COVID-19 pneumonia (table 1).47, 48 Patients with COVID-19 and RNAaemia had higher viral loads in respiratory secretion samples than did those without RNAaemia,49 and high plasma RNAaemia has been associated with severe COVID-19 requiring admission to intensive care.50, 51
It is incompletely understood how RT-PCR positivity for SARS-CoV-2 in upper respiratory tract secretions aligns with cultivable virus in the alveolar territory, and this is a major consideration for therapy. Worryingly, ongoing SARS-CoV-2 replication in the alveolar compartment is expected to drive potentially lethal, diffuse alveolar damage with adjacent immunothrombosis, as well as development of ARDS (figure 3). Direct measurement of alveolar infection is not possible; however, RNAaemia in patients critically ill with COVID-19 could be a surrogate for severe alveolitis with damage to the alveolar–vascular barrier.4 This theory is supported by post-mortem reports describing SARS-CoV-2 in the damaged alveolar compartment by use of electron microscopy, RNAscope assays, and SARS-CoV-2 protein immunohistochemistry, which point to active alveolar viral replication in patients with late-stage COVID-19.52 However, the presence of viral protein or viral nucleic acid in post-mortem examinations does not necessarily equate to actual viral replication at the time of sampling.53 Nevertheless, bronchoalveolar lavage from patients with COVID-19 in intensive care showed viral particles inside mononuclear cells through electron transmission microscopy, confirmed by immunostaining of antiviral capsid and spike antibodies.54 Collectively, these findings support the idea that unrestrained viral replication is a credible factor that could account for the heterogeneity of outcomes in clinical trials of patients with severe COVID-19 pneumonia.
Immunodeficiency states and SARS-CoV-2 persistence
Autoinflammatory syndromes and autoimmune diseases might be intimately intertwined with primary immunodeficiency states.55 For example, genetic defects in the perforin pathway machinery in primary haemophagocytoic lymphohistiocytosis are linked to simultaneous immunodeficiency in CD8+ T cells and natural killer cells, immune dysregulation, and a hyperinflammatory state;56, 57 such scenarios are uncommon in COVID-19, although some studies have shown heterozygous mutations in the perforin pathway in patients with severe COVID-19.58, 59 Loss-of-function mutations in genes involved in interferon signalling pathways (including toll-like receptors) have also been reported.60, 61 Genome-wide association studies in patients with severe COVID-19 have also indicated dysregulation of the interferon pathway in critically ill patients, including single-nucleotide polymorphisms in OAS1, OAS2, and OAS3 genes, and adjacent to TYK2 and IFNAR2,62 although the precise functional correlates of these polymorphisms need further evaluation. In general, studies showing absence of measurable interferon in critical cases of COVID-19 attest to the multifaceted mechanisms in which SARS-CoV-2 can disable antiviral interferon responses.63
Acquired immunodeficiency states, such as those secondary to prolonged corticosteroid treatment, B-cell depleting therapy, or immunosuppressive drugs (eg, calcineurin inhibitors, mycophenolate, azathioprine), have marked effects on T-cell function in patients with rheumatological conditions, and these states have been associated with an increased risk of COVID-19-related mortality.64, 65 Likewise, pan-cytokine inhibition with corticosteroids in patients with mild COVID-19 was associated with increased mortality.13 Collectively, these factors are likely to contribute to ongoing viral replication, which might be a major challenge in selecting patients for immunosuppression and means that consideration of early immunomodulatory therapy in patients with moderate-to-severe COVID-19 pneumonia needs careful re-evaluation (figure 3). This challenge is already well recognised with respect to hepatitis B virus reactivation in patients receiving rituximab-containing regimens during lymphoma therapy.66 Although haematologists pay close attention to active viral infection when treating patients with primary haemophagocytic lymphohistiocytosis, scant consideration has been given to virus-induced immunodeficiency or underlying, unrecognised immunodeficiency in the context of moderate-to-severe COVID-19, in which a one-size-fits-all approach has been used (figure 3). By contrast, the history of cryoglobulinaemia related to hepatitis C virus has shown that immunosuppressive treatments are not deleterious, even in the presence of increased viral replication.67 In patients with moderate-to-severe COVID-19 pneumonia, the evidence to date suggests that immunosuppression in the context of active replication of SARS-CoV-2, which quickly damages the alveolar territory and causes rapidly developing diffuse alveolar damage, might be detrimental.
ARDS development during immunosuppression in COVID-19
Patients hospitalised with COVID-19 might require high-dependency respiratory support, given that many of these patients meet the ARDS Berlin Definition of diffuse pulmonary infiltrates and severe disease (PaO2 <100 mm Hg or FiO2 <100%) not explained by fluid overload.68 In patients with ARDS without COVID-19, the role of corticosteroid treatment remains controversial. Pre-emptive or late use of corticosteroids in these patients is not beneficial and could be harmful,69 with late use probably reflecting the ineffectiveness of immunosuppression in the setting of fibroproliferative or fibrotic ARDS.70 A meta-analysis of trials in patients with severe COVID-19 found that combination therapy with corticosteroids plus tocilizumab was not efficacious in patients on invasive mechanical ventilation,43 many of whom probably had ARDS.
Although our understanding of the lung pathology in ARDS is based on post-mortem samples rather than on ante-mortem studies, the data suggest that ARDS is linked to diffuse alveolar damage in most cases.71, 72 Another major pathological finding is that the diffuse immunothrombosis seen in severe COVID-19 pneumonia is completely distinctive to that seen in disseminated intravascular coagulation.73 Regardless of nuanced pathological differences, most patients who die from severe COVID-19 respiratory failure ultimately show loss of lung compliance and the pathology of ARDS, suggesting that regardless of whether the genesis is viral pneumonia or vascular thrombosis associated with evolving ARDS, the final pathway is similar. Unremitting viral alveolitis secondary to immunodeficiency or immunosuppression might also fuel the associated development of ARDS (figure 3).
Treating immune responses when SARS-CoV-2 viral replication is contained
Despite the tentative link between corticosteroid use and viral replication, as measured by RNAaemia and elevated concentrations of IL-6, some types of immunotherapy seem to improve survival from severe COVID-19 at the population level. Having addressed ongoing active viral replication, it is worth discussing the scenarios in which viral replication is controlled or restricted, yet an exaggerated immune response occurs and manifests with immunothrombosis, which helps to contain dissemination of viral nucleic acids (figure 3). Pulmonary immunothrombosis is evident in over 90% of severe COVID-19 cases,74 occurs independent of disease duration, and is more prevalent in COVID-19 than in influenza.71, 75 This pulmonary immunopathology has been well described and termed as pulmonary intravascular coagulopathy, as opposed to overt disseminated intravascular coagulation.73, 76
Multiple molecular mechanisms have been shown to connect inflammation and coagulation under the integrated umbrella of immunothrombosis,77 which include dynamic bidirectional crosstalk between coagulation and inflammation in vivo. Conversely, activated coagulation proteases are able to cleave cell-surface receptors and can thereby trigger proinflammatory signalling pathways in various cell types, including macrophages, endothelial cells, and platelets. Besides endotheliopathy, which appears to occur independent of direct viral infection, several processes might contribute to pulmonary intravascular coagulopathy, including platelet activation, formation of neutrophil extracellular traps (NETosis), complement activation, and downregulation of fibrinolysis. Consistent with this multifactorial process, distinctive clot morphologies have been characterised in patients with severe COVID-19, including the presence of abundant necrotic neutrophils, free DNA, and platelet-rich areas with elevated megakaryocyte numbers.78 Megakaryocytes typically occur in the bone marrow and lungs under physiological conditions; however, the number of CD61-positive megakaryocytes was reported to be significantly higher in the lungs of patients with COVID-19 pneumonia than in patients with ARDS without COVID-19, suggesting active platelet production, aggregation, and consumption.79 Independently, peripheral blood neutrophilia and systemic neutrophil activation have also been consistently linked to poor prognosis in cases of severe COVID-19.80, 81 This dysregulated, local immunothrombosis seems to occur independent of disease duration and has specific deleterious effects on the monolayer lining of endothelial cells within the pulmonary microvasculature.71, 75
A key consideration is that infection might be compartmentalised to the alveolar compartment, with endothelial and associated vascular thrombosis occurring independent of infection and compartmentalisation between sites.4 Electron microscope studies claiming direct viral infection of the pulmonary endothelium have been strongly challenged, with some suggesting that the morphology of cellular organelles was misinterpretated as SARS-CoV-2.82 The general inability to culture SARS-CoV-2 in endothelial cells raises the possibility that the endothelial orchestration of vascular damage might occur solely via severe inflammation and multifaceted, injurious immune mechanisms.83, 84, 85 The effect of ACE2 receptor expression on the severity of COVID-19 pneumonia remains unclear, but it is probably not linked to the endothelium given the resistance of the endothelium to SARS-CoV-2 infection.85, 86 RNAaemia does not seem to occur in mild COVID-19 due to sparing of the alveolar territory or possibly because of viral containment by immunothrombosis.73, 87 The presence of SARS-CoV-2 RNA in the plasma of severely ill, but not mildly ill, patients supports the idea that the alveolar–vascular barrier breaks down, resulting in viral RNA dissemination and systemic immunothrombosis (figure 4 ).51, 88
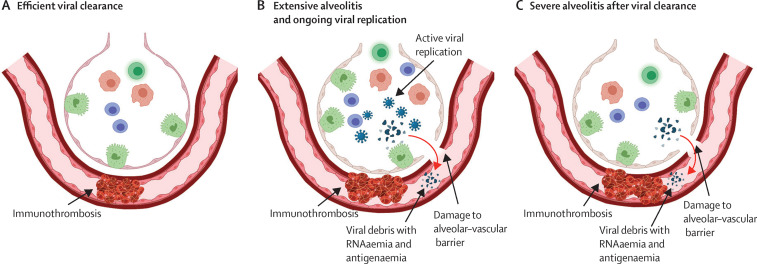
Figure 4.
RNAaemia and therapy considerations
(A) In patients who rapidly clear the virus, the ongoing immune response might contribute to alveolar hypoxaemia and adjacent immunothrombosis. This group is marked by an absence of both viral replication in the airways and of RNAaemia, and these patients are likely to respond better to therapy than are those with ongoing viral replication. (B) In patients with extensive ongoing alveolitis, severe damage to the alveolar–vascular barrier and associated immune responses occur, mediated by the virus. This group has RNAaemia due to the combination of viral replication and barrier disruption, and viral material in the systemic circulation might contribute directly to other organ damage via distant immunothrombosis and further activation of the immune system. RNAaemia might be especially high due to viral replication, and this group might respond badly to further immunosuppression. (C) In patients with severe alveolitis, the alveolar–vascular barrier is extensively damaged and viral debris, including RNA, is released into the systemic circulation. It is possible that this group has detectable RNAaemia, which is not sustained, with RNAaemia decreasing or disappearing on serial measurements. This group might benefit from immunosuppression because RNAaemia represents released viral debris via the damaged alveolar capillary barrier following the active replication phase.
We have argued that this complex and prognostically relevant immunothrombosis without viral replication, especially in the vascular compartment, could partially explain the benefits of immunomodulatory therapy when used in the post-viral replication phase in patients with severe COVID-19 pneumonia.4 Unlike with immunomodulatory therapy, a therapeutic dose of anticoagulation with heparin did not improve outcomes in patients with severe or critical COVID-19, and their risk of major bleeding was increased (3·7% in treated patients vs 1·8% in controls),89 which supports the pre-eminence of immunothrombosis. However, in patients with mild COVID-19, full-dose anticoagulation conferred a small survival advantage.89 This scenario is similar to Behçet's disease, whereby thromboinflammation requires immunotherapy rather than anticoagulation.90
Excessive adaptive and innate immune responses in COVID-19
In patients with COVID-19 in whom active virus replication is controlled, various mechanisms have been suggested to explain how immunotherapy controls immunothrombosis. With respect to humoral immunity directed by B cells, production of a multiplicity of anti-cytokine, anti-endothelial, and other autoantibodies has been reported.91, 92 Extensive pulmonary infarction and tissue necrosis in the presence of abundant viral RNA and other adjuvants is likely to result in temporary failure of immunological tolerance against many self-proteins, with emergence of multiple autoantibodies.92 Such a transient autoimmune process is well recognised following tissue infarction in other settings, such as myocardial infarction or stroke.93, 94 Likewise, extensive pulmonary infarction, viral infection, and tissue necrosis might be key factors in the secondary production of anti-interferon and other autoantibodies that characterise moderate-to-severe COVID-19. If this is the case, immunosuppression of the secondary autoantibody responses might be of little value in moderate-to-severe COVID-19. However, the presence of anti-endothelial cell autoantibodies in patients with severe COVID-19 was shown to trigger NETosis and facilitate venous thrombosis in murine models.95 In another COVID-19 mouse model, such autoantibodies were shown to be immunosuppressive and exacerbate disease (figure 3).96 It is therefore possible that suppression of some of the plethora of autoantibodies reported in severe COVID-19 might be a factor in the beneficial effects of immunotherapy.
In cases of COVID-19 in which antiviral T-cell responses become detrimental, it is probable that immunosuppression blunts this response (figure 3). An attractive theory—based on the timing of viral clearance from day 10 onwards in many patients with COVID-19, which broadly correlates with the timing of emerging T–cell responses—is that the potential success of corticosteroid therapy is linked to the taming of overzealous CD4+ and CD8+ T-cell responses. Therefore, the excessive production of T-helper (Th)-1 cytokines in the lungs and CD8+ T-cell cytotoxicity could be driving immunopathology.97 One study, in which single-cell RNA sequences were analysed from the airways of patients with COVID-19, reported cytotoxic T cells with perforin and granzyme production in the vicinity of stressed pulmonary epithelial cells, suggesting a link between epithelial cell infectivity and adjacent lymphocyte toxicity.98 An obvious question is whether or not suppression of T-cell killing of infected pneumocytes might be beneficial in this scenario (figure 4). This notion is relevant because, experimentally, a low level of infection of type 2 pneumocytes with influenza A virus has not been shown to be detrimental to the functional activity of the cells (including cell division).99 Consequently, excessive cytotoxic T cell-mediated elimination of type 2 pneumocytes, which are stem cells for the alveolus, might be detrimental and could contribute to diffuse alveolar damage with ARDS development.
Older patients (aged >80 years) are at increased risk of poor outcomes from COVID-19 pneumonia and, in general, do not have robust T-cell and B-cell responses. This patient group also shows immunosenescence and an increased magnitude of inflammation driven by the innate immune system (also known as inflammaging), which probably underscores pathogen–host interactions that drive tissue damage and immunothrombosis (figure 3). Functional assays of samples from patients with severe COVID-19 showed reduced production of T-cell-derived cytokines (eg, IFNγ, IL-17, and IL-22) and prominent T-cell exhaustion in critically ill patients, whereas innate immune responses were intact or increased.100
Future directions and conclusions
In this Viewpoint, we have focused on patients with moderate-to-severe COVID-19 pneumonia who are thought to represent the best target group for immunotherapy, given that patients with mild COVID-19 or those who are critically ill and mechanically ventilated with extensive tissue destruction might not respond to these agents. Timing treatment with respect to the clinical phase of the disease, and not delaying such treatment for too long, are considered to be key potential factors in the selection of patients who are most likely to respond to immunotherapy. However, a roadmap for therapy stratification needs to be thoroughly evaluated, especially because some patients with rapidly progressing disease can have unrestrained viral replication. This patient group, who have poor prognosis related to ongoing viral replication, seems to be hidden among the larger subgroup of patients with COVID-19 who are responsive to immunotherapy, a factor that might be important in blunting immunotherapy responses, rendering them modest, incremental, or even futile.
We have reviewed the evidence suggesting that RNAaemia in patients with comparatively high elevations in cytokine concentrations probably reflects ongoing viral replication, and it is unlikely that these patients will respond to immunosuppression. Strategies to differentiate this patient group and to establish the PCR cycle threshold for detection of viral RNA or identify viral antigenaemia could resolve the question of whether immunosuppression should be completely avoided in these patients and anti-SARS-CoV-2 antibody cocktails and antiviral therapies would be more appropriate. Given that our understanding of the immunopathogenesis of COVID-19 has improved greatly over the past 18 months, we advocate for a careful, simultaneous evaluation of blood inflammatory markers and the magnitude and persistence of RNAaemia to formally identify which patients might optimally respond to therapy (figure 4). High initial RNAaemia or persistent RNAaemia might be indicators to exclusively pursue standard-of-care with antiviral therapy strategies, including anti-spike antibody cocktails and RNA transcriptase antagonism.
The cellular and molecular mechanisms underscoring the beneficial effects of immunotherapy are likely to be multifaceted, including innate and adaptive immune mechanisms that remain incompletely understood. However, it is probable that the beneficial effects of immunotherapy are limited to the post-viral alveolitis phase of COVID-19 and that identifying the patient group with ongoing viral replication in the alveolar territory should help to optimise use of immunosuppressive therapy. It is possible that immunosuppression in patients with moderate-to-severe COVID-19 and marked inflammatory responses might facilitate viral replication and medium-term and long-term lung damage; long-term survival studies in such patients are needed.
Search strategy and selection criteria
We searched PubMed using the search terms “COVID-19”, “SARS-CoV-2”, “RNAaemia”, “Viral replication”, “Immunosuppression”, “Immunothrombosis”, “ARDS”, and “Lung tissue injury”. We also searched “IL-6”, “IL-1”, “Tocilizumab”, “Anakinra“, “Dexamethasone”, and “Baricitinib” in a separate search, and reviewed publications that reported data on these terms. We limited our search to articles that were published in English between Dec 22, 2019, and July 30, 2021.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
Acknowledgments
MFK is an employee of the National Institutes of Health who is funded in part by the National Institutes of Health and in part by intramural funds from the National Cancer Institute.
Acknowledgments
Contributors
DM developed the initial concepts for this paper. All authors contributed to the first draft writing, the literature review, critical revision, and editing. All authors have participated sufficiently in this work, take public responsibility for the content, and have made substantial contributions to this research. All authors approved the final version and had final responsibility for the decision to submit for publication.
References
- 1.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 2.McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3:e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGonagle D, Plein S, O'Donnell JS, Sharif K, Bridgewood C. Increased cardiovascular mortality in African Americans with COVID-19. Lancet Respir Med. 2020;8:649–651. doi: 10.1016/S2213-2600(20)30244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGonagle D, Ramanan AV, Bridgewood C. Immune cartography of macrophage activation syndrome in the COVID-19 era. Nat Rev Rheumatol. 2021;17:145–157. doi: 10.1038/s41584-020-00571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Cui P, Zeng S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 9.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li TZ, Cao ZH, Chen Y, et al. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2021;93:506–512. doi: 10.1002/jmv.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JW, Yang L, Luo RG, Xu JF. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. 2020;26:1171–1177. doi: 10.1016/j.cmi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li J, Ke J, et al. Adverse outcomes associated with corticosteroid use in critical COVID-19: a retrospective multicenter cohort study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.604263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam W, Bizri AR. Efficacy of tocilizumab in COVID-19: a review of the current evidence. Sci Prog. 2021;104 doi: 10.1177/00368504211030372. 368504211030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;385:406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 20.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs JL, Mellors JW. Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in blood of patients with coronavirus disease 2019 (COVID-19): what does it mean? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1316. published online Sept 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munker D, Osterman A, Stubbe H, et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur Respir J. 2021;58 doi: 10.1183/13993003.02724-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buetti N, Wicky P-H, Le Hingrat Q, et al. SARS-CoV-2 detection in the lower respiratory tract of invasively ventilated ARDS patients. Crit Care. 2020;24:610. doi: 10.1186/s13054-020-03323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camprubí D, Gaya A, Marcos MA, et al. Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir. Int J Infect Dis. 2021;104:379–381. doi: 10.1016/j.ijid.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901. doi: 10.1016/j.cell.2020.10.049. 12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hensley MK, Bain WG, Jacobs J, et al. Intractable COVID-19 and prolonged SARS-CoV-2 replication in a CAR-T-cell therapy recipient: a case study. Clin Infect Dis. 2021;73:e815–e821. doi: 10.1093/cid/ciab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima Y, Ogai A, Furukawa K, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27:387–389. doi: 10.1016/j.jiac.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarhini H, Recoing A, Bridier-Nahmias A, et al. Long term SARS-CoV-2 infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223:1522–1527. doi: 10.1093/infdis/jiab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthay MA, Wick KD. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Invest. 2020;130:6218–6221. doi: 10.1172/JCI143331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maltezou HC, Raftopoulos V, Vorou R, et al. Association between upper respiratory tract viral load, comorbidities, disease severity and outcome of patients with SARS-CoV-2 infection. J Infect Dis. 2021;223:1132–1138. doi: 10.1093/infdis/jiaa804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quartuccio L, Sonaglia A, McGonagle D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utrero-Rico A, Ruiz-Hornillos J, González-Cuadrado C, et al. IL-6-based mortality prediction model for COVID-19: validation and update in multicenter and second wave cohorts. J Allergy Clin Immunol. 2021;147:1652–1661. doi: 10.1016/j.jaci.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quartuccio L, Fabris M, Sonaglia A, et al. Interleukin 6, soluble interleukin 2 receptor alpha (CD25), monocyte colony-stimulating factor, and hepatocyte growth factor linked with systemic hyperinflammation, innate immunity hyperactivation, and organ damage in COVID-19 pneumonia. Cytokine. 2021;140 doi: 10.1016/j.cyto.2021.155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mudd PA, Crawford JC, Turner JS, et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv. 2020;6 doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Gu X, Li H, et al. Risk factors of viral rnaaemia and its association with clinical prognosis among patients with severe COVID-19. Chest. 2021;159:1382–1386. doi: 10.1016/j.chest.2020.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiege JK, Thiede JM, Nanda HA, et al. Single cell resolution of SARS-CoV-2 tropism, antiviral responses, and susceptibility to therapies in primary human airway epithelium. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Vicente M, Almansa R, Martínez I, et al. Absent or insufficient anti-SARS-CoV-2 S antibodies at ICU admission are associated to higher viral loads in plasma, antigenemia and mortality in COVID-19 patients. medRxiv. 2021 doi: 10.1101/2021.03.08.21253121. published online March 8. (preprint). [DOI] [Google Scholar]
- 49.Colagrossi L, Antonello M, Renica S, et al. SARS-CoV-2 RNA in plasma samples of COVID-19 affected individuals: a cross-sectional proof-of-concept study. BMC Infect Dis. 2021;21:184. doi: 10.1186/s12879-021-05886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang K, Wu L, Luo Y, Gong B. Quantitative assessment of SARS-CoV-2 RNAemia and outcome in patients with coronavirus disease 2019. J Med Virol. 2021;93:3165–3175. doi: 10.1002/jmv.26876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bermejo-Martin JF, González-Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24:691. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deshmukh V, Motwani R, Kumar A, Kumari C, Raza K. Histopathological observations in COVID-19: a systematic review. J Clin Pathol. 2021;74:76–83. doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 53.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandolfi L, Fossali T, Frangipane V, et al. Broncho-alveolar inflammation in COVID-19 patients: a correlation with clinical outcome. BMC Pulm Med. 2020;20:301. doi: 10.1186/s12890-020-01343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoyos-Bachiloglu R, Chou J. Autoimmunity and immunodeficiency. Curr Opin Rheumatol. 2020;32:168–174. doi: 10.1097/BOR.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 56.Opoka-Winiarska V, Grywalska E, Roliński J. Could hemophagocytic lymphohistiocytosis be the core issue of severe COVID-19 cases? BMC Med. 2020;18:214. doi: 10.1186/s12916-020-01682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cron RQ, Behrens EM, Shakoory B, Ramanan AV, Chatham WW. Does viral hemorrhagic fever represent reactive hemophagocytic syndrome? J Rheumatol. 2015;42:1078–1080. doi: 10.3899/jrheum.150108. [DOI] [PubMed] [Google Scholar]
- 58.Cabrera-Marante O, Rodríguez de Frías E, Pleguezuelo DE, et al. Perforin gene variant A91V in young patients with severe COVID-19. Haematologica. 2020;105:2844–2846. doi: 10.3324/haematol.2020.260307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo H, Liu D, Liu W, et al. Germline variants in UNC13D and AP3B1 are enriched in COVID-19 patients experiencing severe cytokine storms. Eur J Hum Genet. 2021;29:1312–1315. doi: 10.1038/s41431-021-00886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Made CI, Simons A, Schuurs-Hoeijmakers J, et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 63.Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. published online Oct 13. [DOI] [PubMed] [Google Scholar]
- 65.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521–2530. doi: 10.1001/jama.2014.15704. [DOI] [PubMed] [Google Scholar]
- 67.Pietrogrande M, De Vita S, Zignego AL, et al. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun Rev. 2011;10:444–454. doi: 10.1016/j.autrev.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Ranieri V, Rubenfeld G, Thompson B, et al. ARDS definition task force. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 69.Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 71.Mauad T, Duarte-Neto AN, da Silva LFF, et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir Res. 2021;22:32. doi: 10.1186/s12931-021-01628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polak SB, Van Gool IC, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33:2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Pulmonary intravascular coagulopathy in COVID-19 pneumonia–Authors' reply. Lancet Rheumatol. 2020;2:e460–e461. doi: 10.1016/S2665-9913(20)30174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGonagle D, Sharif K, O'Regan A, Bridgewood C, Bridgewood CJAr. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foley JH, Conway EM, Conway EMJCr. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 78.Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine24: 100434. [DOI] [PMC free article] [PubMed]
- 79.Dwiputra Hernugrahanto K, Novembri Utomo D, Hariman H, et al. Thromboembolic involvement and its possible pathogenesis in COVID-19 mortality: lesson from post-mortem reports. Eur Rev Med Pharmacol Sci. 2021;25:1670–1679. doi: 10.26355/eurrev_202102_24878. [DOI] [PubMed] [Google Scholar]
- 80.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Busch MH, Timmermans SAMEG, Nagy M, et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020;142:1787–1790. doi: 10.1161/CIRCULATIONAHA.120.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395:e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmetaj-Shala B, Peacock TP, Baillon L, et al. Resistance of endothelial cells to SARS-CoV-2 infection in vitro. bioRxiv. 2020 doi: 10.1101/2020.11.08.372581. published online Nov 9. (preprint). [DOI] [Google Scholar]
- 84.McCracken IR, Saginc G, He L, et al. Lack of evidence of angiotensin-converting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells. Circulation. 2021;143:865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahl K, Bräsen JH, Hoeper MM, David S. Absence of SARS-CoV-2 RNA in COVID-19-associated intestinal endothelialitis. Intensive Care Med. 2021;47:359–360. doi: 10.1007/s00134-020-06326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCracken IR, Saginc G, He L, et al. Lack of evidence of angiotensin-converting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells. Circulation. 2021;143:865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 88.Berastegui-Cabrera J, Salto-Alejandre S, Valerio M, et al. SARS-CoV-2 RNAemia is associated with severe chronic underlying diseases but not with nasopharyngeal viral load. J Infect. 2021;82:e38–e41. doi: 10.1016/j.jinf.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sholzberg M, Tang GH, Rahhal H, et al. Heparin for moderately ill patients with COVID-19. medRxiv. 2021 doi: 10.1101/2021.07.08.21259351. published online July 12. (preprint). [DOI] [Google Scholar]
- 90.Hatemi G, Christensen R, Bang D, et al. 2018 update of the EULAR recommendations for the management of Behçet's syndrome. Ann Rheum Dis. 2018;77:808–818. doi: 10.1136/annrheumdis-2018-213225. [DOI] [PubMed] [Google Scholar]
- 91.Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Javidi E, Magnus T. Autoimmunity after ischemic stroke and brain injury. Front Immunol. 2019;10:686. doi: 10.3389/fimmu.2019.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao Y-H, Cheng X. Autoimmunity in myocardial infarction. Int J Cardiol. 2006;112:21–26. doi: 10.1016/j.ijcard.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 97.Altmann DM, Boyton RJ. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd6160. [DOI] [PubMed] [Google Scholar]
- 98.Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 99.Weinheimer VK, Becher A, Tönnies M, et al. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis. 2012;206:1685–1694. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Janssen NAF, Grondman I, de Nooijer AH, et al. Dysregulated innate and adaptive immune responses discriminate disease severity in COVID-19. J Infect Dis. 2021;223:1322–1333. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]