Abstract
T cell receptors (TCRs) are unique markers that define antigen specificity for a given T cell. With the evolution of sequencing and computational analysis technologies, TCRs are now prime candidates for the development of next-generation non-cell based T cell biomarkers, which provide a surrogate measure to assess the presence of antigen-specific T cells. Type 1 diabetes (T1D), the immune-mediated form of diabetes, is a prototypical organ specific autoimmune disease in which T cells play a pivotal role in targeting pancreatic insulin-producing beta cells. While the disease is now predictable by measuring autoantibodies in the peripheral blood directed to beta cell proteins, there is an urgent need to develop T cell markers that recapitulate T cell activity in the pancreas and can be a measure of disease activity. This review focuses on the potential and challenges of developing TCR biomarkers for T1D. We summarize current knowledge about TCR repertoires and clonotypes specific for T1D and discuss challenges that are unique for autoimmune diabetes. Ultimately, the integration of large TCR datasets produced from individuals with and without T1D along with computational ‘big data’ analysis will facilitate the development of TCRs as potentially powerful biomarkers in the development of T1D.
Keywords: T cells, TCR sequencing, autoimmunity, type 1 diabetes, HLA, MHC
Introduction
A T cell receptor (TCR) determines antigen specificity of T cells by interacting with a peptide-major histocompatibility complex (peptide-MHC), and signals received through the TCR along with the CD3 complex are the primary components that regulate function and fate of T cells. Individual T cells express unique TCRs, and therefore TCR sequences can be used as an identifier of T cells that are specific to particular antigens and involved in immune responses. In this review, we will focus on the potential use of TCR sequences as non-cell based T cell biomarkers for type 1 diabetes (T1D), a tissue-specific autoimmune disease targeting insulin-secreting pancreatic beta cells (1–3).
Several features of self-reactive T cells make it challenging to develop T cell biomarkers in diabetes (4). First, the frequency of autoreactive T cells is extremely low in the peripheral blood, estimated to be 1/105 – 1/106. Second, response to peptide-MHC by autoreactive T cells tends to be minimal compared to anti-cancer or anti-pathogen T cell responses (5, 6). Third, healthy individuals with T1D-risk MHC molecules can have autoreactive T cells that are quantitatively and functionally similar to those in T1D patients (7). TCR sequencing allows for the analysis of TCR clonotypes from tens of millions of T cells using nucleotide samples rather than living cell biospecimens and may overcome many of these challenges when appropriately utilized. Advantages provided by TCR biomarkers include (1) living T cells are not required for assays; (2) intra- and inter-assay variations due to cell conditions and operator performance are minimized; and (3) extremely infrequent and low-responding T cells are detectable by recently emerging high-throughput sequencing technologies. Here, we will review current knowledge about TCR repertoires and clonotypes specific for T1D and address the knowledge gaps to develop TCR biomarkers that can stratify individuals throughout the stages of T1D development.
Tri-Molecular Complex Consisting of TCR, Peptide, and MHC Molecules
TCRs expressed by classical T cells are composed of alpha and beta chains, both of which are formed by somatic recombination of the variable (V) and joining (J) segment genes (and diversity [D] for beta chains). In humans, 45 TRAV and 52 TRAJ genes have been identified as functional V and J segment genes for alpha chains (8, 9). Likewise, there are 49 TRBV, 2 TRBD, and 13 TRBJ functional V, D, and J segment genes in the beta chain locus (10, 11). During maturation in the thymus, individual T cells undergo rearrangement of segment genes, resulting in one V, one D (for beta), and one J segment genes assembled adjacent to each other. Since additional nucleotides are often inserted or deleted between the segments, billions of junction sequences with hundreds of different V, D, J combinations are possibly assembled (12–14). Experimentally, each adult person is expected to have over 100 million TCR clonotypes uniquely expressed by hundreds of billions of individual T cells in the body (15–19). There are three regions, called complementarity determining regions (CDR), that directly interact with peptide-MHC complexes, thereby crucial to determine antigen specificity (20–22). Two CDR regions, CDR1 and CDR2, are included in the V segment, and the CDR3 region is formed at the junction between V, D (for beta), and J segments. Amino acid residues in the CDR3 regions closely interact with peptide, and thus are considered to be important to determine antigen specificity and are often used as a property of each TCR clonotype for TCR repertoire analysis.
MHC Molecules in T1D
The major genetic determinant in susceptibility to most autoimmune diseases reside in the human MHC that contains the human leukocyte antigen (HLA) region. MHC molecules are heterodimers formed between alpha and beta chains that function to present peptides to TCRs on T cells. Class I molecules are on all nucleated cells and present antigens to CD8 T cells, while class II molecules are expressed by antigen presenting cells (e.g. B cells, dendritic cells, and macrophages) and present peptides to CD4 T cells. In T1D, specific HLA class I and II alleles are associated with increased risk (23, 24). Several HLA class I and II alleles confer risk for T1D and are associated with other autoimmune disorders ( Table 1 ) (25, 26). DR is in close linkage disequilibrium with DQ such that the DR4-DQ8 and DR3-DQ2 haplotypes confer the greatest risk for T1D development. Both the alpha and beta chains of DQ molecules are polymorphic, and have the ability to form mixed molecules in cis and trans. As an example, the alpha chain of DQ2 can pair with the beta chain of DQ8 to form DQ8-trans (DQA1*05:01-DQB1*03:02) when both DQ2 and DQ8 are in the genotype. DQ8-trans has an odds ratio of disease development for T1D at 35 (35 times more likely to develop diabetes compared to those without these alleles), compared to odds ratios of ~11 and ~4 for DQ8 and DQ2, respectively (27, 28). Interestingly, HLA-DQ6 (DQA1*01:02-DQB1*06:02) provides dominant protection for T1D development with an odds ratio of only 0.03 (29, 30). The stark dichotomy of risk between DQ molecules highlights the important role of antigen presentation to TCRs in T1D.
Table 1.
Common HLA alleles associated with type 1 diabetes risk.
| Name | Allele | Associated Autoimmune Diseases |
|---|---|---|
| HLA Class II | ||
| DQ8 | DQA1*03:01-DQB1*03:02 | Celiac disease, Addison’s disease |
| DQ2 | DQA1*05:01-DQB1*02:01 | Celiac disease, Addison’s disease |
| DQA1*02:01-DQB1*02:02 | Celiac disease, Addison’s disease | |
| DQ8-trans | DQA1*05:01-DQB1*03:02 | Celiac disease |
| DR4 | DRB1*04:01 | Rheumatoid Arthritis, Thyroid disease, Addison’s disease, Alopecia Areata |
| DRB1*04:02 | Rheumatoid Arthritis (protective), Thyroid disease, Addison’s disease | |
| DRB1*04:04 | Rheumatoid Arthritis, Thyroid disease, Addison’s disease | |
| DRB1*04:05 | Rheumatoid Arthritis, Thyroid disease, Addison’s disease | |
| DR3 | DRB1*03:01 | Systemic Lupus Erythematous (SLE), Neuromyelitis Optica (NMO), Myasthenia Gravis, Thyroid disease, Addison’s disease |
| HLA Class I | ||
| A2 | A*02:01 | Vitiligo |
| A24 | A*24:02 | unknown |
| B39 | B*39:06 | unknown |
| B18 | B*18:01 | unknown |
| B7 | B*07:05 | unknown |
Diversity of TCR Repertoires
Adults have approximately 108-1010 unique TCR clonotypes (15, 17, 18, 31). With an assumption that the TCR repertoire size may represent a capacity for responding to diverse antigens, the TCR repertoire diversity in the blood has been examined to determine whether it is associated with immune conditions. For example, having diverse TCR repertoires is associated with desirable responses to immune therapies in cancer (32–34). In T1D, it has been reported that TCR repertoires in peripheral blood of T1D patients are less diverse compared with those without T1D (35). Thus, there may be trends of TCR repertoire sizes that are preferred by a certain immune condition. However, it should be noted that the diversity of TCR repertoires cannot specify a certain disease.
Use of TCR Clonotypes as Surrogates to Quantify Antigen-Specific T Cells
TCR clonotypes determine antigen specificity, and therefore they can be utilized as a surrogate marker to evaluate the presence and prevalence of antigen-specific T cells in the blood. Frequencies of these antigen-specific TCR clonotypes can be quantified by high-throughput sequencing, which is expected to be more specific to individual diseases compared to surveying the broad TCR repertoire. Furthermore, once a panel of antigen-specific TCR clonotypes are determined, a single TCR sequencing assay allows for evaluating specificity to many antigens rather than needing to test specificity to each individual antigen. TCR sequencing has been done from different tissues in many disease states (36), including autoimmune disorders (37) and cancer (38–40). Remarkably, TCR sequencing has been shown to differentiate early-stage cancer patients from healthy individuals (41, 42). This strategy requires a list of TCR clonotypes beforehand that can be searched in blood samples, and such TCR clonotypes used as surrogate biomarkers need to satisfy three factors: (1) publicity (i.e. commonality and shared between individuals), (2) abundancy, and (3) disease specificity. Namely, T cells expressing the same or similar TCR clonotypes need to be commonly present in a number of people; frequency of such T cells in the blood of each person needs to be high enough for quantification; and presence or absence of such T cells needs to be associated with a disease state. In addition, with larger numbers of TCR clonotypes in a given panel, the more specific and sensitive an assay will become. Thus, identifying disease-specific TCR candidates is essential to establish a robust TCR sequencing assay that can discriminate a subset of individuals having a specific stage or feature of T1D such as those who have potential to respond to an interventional therapy.
There are several strategies to identify disease-specific TCR clonotypes. Since a significant portion of disease-specific TCRs are likely to recognize islet antigens, TCR clonotypes expressed by islet antigen-specific T cells are reasonable candidates for TCR biomarkers. Such T cell sources include peripheral blood T cells responding to islet antigen stimulation or enriched by staining with fluorescence-conjugated multimers consisting of an islet-derived peptide and a particular HLA molecule (43–45). Alternatively, TCR clonotypes identified in the target organ (i.e. pancreas or pancreatic islets) or draining lymph nodes may be also disease-specific. In any of these T cell sources, specificity (i.e. potential contamination of non-disease associated T cells) as well as sensitivity (i.e. missing a portion of antigen-specific T cells) needs to be carefully considered. For example, T cell samples enriched by antigen stimulation may contain only a few clonotypes that readily proliferate in response to the stimulation or could be non-specific T cells that proliferate due to “bystander effect.” Likewise, T cells in the pancreas and pancreatic lymph nodes may not necessarily be islet-reactive or disease-specific (46). On the other hand, T cell populations enriched by multimer staining may contain only those having high affinity to bind peptide-MHC complexes, and TCRs weakly binding to peptide-MHC may be missed. This possibility is likely important for autoreactive TCRs since T cell responsiveness to self-antigens tends to be low compared to pathogen T cell responses. Nevertheless, identifying TCR clonotypes from samples enriched with antigen-specific T cells is indispensable to identify disease-specific TCR candidates. These TCR clonotypes should then be assessed for frequency in peripheral blood of individuals with different stages of T1D to determine the ultimate association with disease status. The next subsections will summarize features of TCR clonotypes specific to islet-specific autoantigens as well as those potentially associated with T1D pathogenesis.
Lessons From Islet-Specific TCRs in T1D Animal Models
Non-diabetic (NOD) mice spontaneously develop autoimmune diabetes and represent many features of human T1D including a T1D-susceptible MHC allele (I-Ag7), homologous to HLA-DQ8, the development of insulin autoantibodies prior to diabetes onset, and insulitis. A number of T cell clones reacting with islet tissues have been isolated from pancreatic islets and spleens of NOD mice in the past few decades and further characterized for antigen specificity as well as TCR clonotypes (47). In the 1990’s, Santamaria and colleagues discovered that a large portion of CD8 T cells infiltrating NOD islets share an identical Valpha segment (i.e. TRAV16) along with a specific junction motif (i.e. MRD or MRE) (48), and subsequently identified a peptide derived from islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) as an epitope targeted by these CD8 T cells (49). Likewise, CD4 T cell clones as well as T-hybridoma cells that are reactive to insulin B-chain peptides have been established from NOD islets by a number of investigators using different methods (50–56). The majority of these T cells expresses TCRs containing specific Valpha and Jalpha segment motifs, TRAV5D-4 or TRAV10 along with TRAJ53 or TRAJ42. When mice are forced to have only T cells expressing TCRs containing TRAV5D-4, approximately one percent of CD4 T cells becomes specific to an insulin B chain 9-23 peptide (57), and the mice are susceptible to develop anti-insulin autoimmunity (58, 59). Alanine scanning and crystal structure analyses identified several amino acid residues in the TRAV5D-4 and TRAV10 CDR1 and CDR2 regions that are crucial to interact with the insulin peptide-MHC complex (59, 60). Also, among insulin B chain-specific CD4 T cells, those particularly recognize an insulin B chain 12-20 peptide prefer to express TCR beta chains containing a negatively charged amino acid (i.e. aspartic acid [D] or glutamic acid [E]) in the junction region (56). This observation is consistent with a notion that the I-Ag7 T1D-susceptible MHC class II molecule, which has a positively charged patch in the surface area near the p9 pocket due to the lack of a negatively charged amino acid residue at the beta 57 position, engage TCRs having a negatively charged residue when p9 of peptides is not negatively charged (the position 20 of insulin B chain is glycine). Thus, these studies provide a molecular elucidation of how TCR motif selection occurs by interaction with a particular peptide-MHC complex.
T1D-specific TCR repertoires in rat models have been extensively studied by the group of Mordes and Blankenhorn (61). Of note, diabetes-susceptible rat strains have a T1D risk MHC haplotype (RT1B/Du), which lacks a negatively charged amino acid residue at the beta chain 57 position and is homologous to HLA-DQ8 (61). In addition to the HLA gene locus, Iddm14, which contains the TCR beta chain genes, is a T1D-susceptible locus (62). The group identified a TCR Vbeta allele, Tcrb-V13S1A1, that is shared among T1D-susceptible rat strains but not with T1D-resistant ones (63), and demonstrated that genetic elimination of this allele or depletion of T cells expressing TCRs containing Vbeta13a (product of the Tcrb-V13S1A1 gene) abrogates diabetes development in T1D-susceptible rats (64–66). A series of these studies elegantly linked the genetic risk with a functional mechanism in which a particular TCR motif facilitates T1D development with a specific MHC molecule.
In sum, these animal studies demonstrate the presence of preferred TCR motifs in both germline-encoded and rearranged regions to recognize particular epitope sequences, which can be reasonably explained by molecular interaction between the TCR – peptide – MHC molecule. From a view of TCR biomarker development, TCR motifs shared by antigen-specific or disease-susceptible T cells can be utilized to enrich and classify TCR clonotypes that are distinctive of T1D.
TCR Repertoires in the Pancreas of Humans
Emerging sequencing technologies and increasing availability of human samples, in particular pancreas and peripheral immune tissues isolated from organ donors having T1D, facilitate identification of islet antigen-specific or T1D-associated T cells and TCR clonotypes (7, 67–74). In the 1990’s, two groups in Spain and Japan separately analyzed TCR repertoires in the pancreas and demonstrated clonal expansion of T cells with particular Vgene segment usage in individual patients (75, 76). Importantly, the same group in Spain demonstrated that a clonally expanding TCR in islet and pancreas samples was detected in the blood of the same individual, indicating that islet-residing TCR clonotypes are detectable in peripheral blood samples (77). More recently, Brusko and colleagues further corroborated this concept by studying a larger number of individuals using a next generation sequencing technology that allows to analyze much higher numbers of T cells (72, 78). This high resolution analysis discovered that CD8 TCR clonotypes in the pancreas and draining lymph nodes are detected in peripheral blood more frequently than those expressed by CD4 T cells and provided important insights about the depth of TCR sequencing to achieve quantitative measurement.
Another important concept is to consider commonality of TCR repertoires in the pancreas across patients. We recently determined thousands of TCR clonotypes expressed by T cells in the islets of organ donors with and without T1D (73, 74). Our analysis indicated clonal expansion in the pancreas of individual donors regardless of the disease, but also found that the frequency of TCR clonotypes shared between donors is limited. This low frequency of shared TCR clonotypes may be due to diverse HLA restrictions present in different individuals. Another reason could be the fact that T cells in the islets may not be necessarily islet-specific. Indeed, multiple studies analyzing islet T cell specificity found that over half of T cell clones and lines derived from the islets did not respond to preproinsulin and other known islet epitopes (46, 70, 71, 73, 74). However, it should be noted that collecting TCR clonotypes from a larger number of donors significantly increases the number of shared clonotypes and such large TCR repertoire information allows for identifying common motifs even when not sharing entire TCR sequences, which will be essential to precisely cluster TCRs recognizing the same epitope (see below regarding TCR clustering). Thus, continuing efforts to accumulate TCR sequence information from the target organ along with epitope identification is crucial to establish a sufficient list of TCR clonotypes that can be used for disease-associated TCR biomarkers.
Islet Antigen-Specific TCR Clonotypes in Humans
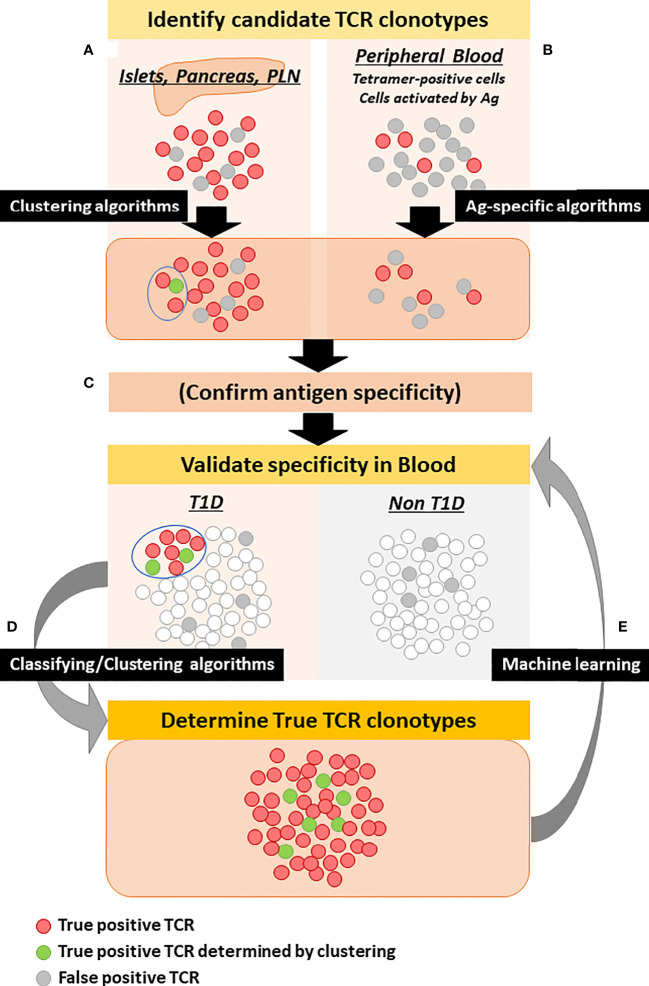
TCRs expressed by islet-reactive T cells may be another optimal source that can be used as clonotypes for T1D biomarkers, especially if they circulate in the peripheral blood. Such clonotypes could come from T cell clones, T cell lines, hybridomas, and transductant cells that have been confirmed to respond to islet antigens, cell subsets enriched by multimer staining, and those activated or proliferated by antigen stimulation. TCR clonotypes for which reactivity to epitopes has been confirmed at a single cell level would be the most reliable source. Here we summarize islet antigen-specific TCR clonotypes that were isolated from individuals having T1D ( Table 2 ). To date, over a hundred TCR alpha and beta paired sequences specific to common islet epitopes have been reported by a number of investigators, and it is notable that the majority of these TCRs were identified in the past several years (7, 45, 70, 73, 74, 79–99). However, hundreds of disease-associated TCR clonotypes are far too small to cover T1D patients having heterogeneous antigen specificity. With rapidly evolving sequencing technologies, future efforts to identify islet epitope-specific TCR clonotypes is essential to develop TCR biomarkers for T1D. In addition to TCR clonotypes listed in Table 2 , Bonifacio and colleagues reported hundreds of TCR sequences expressed by T cells that were stained with multimer composed of islet epitopes or those proliferated in response to islet antigens (44, 45). While it is necessary to carefully validate true reactivity to antigens, this type of analysis is an excellent resource to gain T1D-associated TCR clonotypes. Computational tools to decrease the “noise” (i.e. eliminating non-specific binding TCR clonotypes) may help to enrich truly antigen-specific clonotypes (100, 101). Further, these candidate TCR clonotypes could be validated for disease specificity using larger cohorts analyzed with whole blood TCR sequencing, and then clonotypes that were detected only in individuals having various stages of T1D could be assessed for functional reactivity ( Figures 1A–C ). Retro/lentiviral transduction systems, especially in a moderate to high throughput multiplex assay, will facilitate verifying reactivity to antigens (82, 98, 102, 103).
Table 2.
T cell receptors specific to islet epitopes.
| Clone/Sequence ID | Epitope | Epitope sequence | HLA# | TRAV | TRAJ | CDR3a | TRBV | TRBJ | CDR3b | Source of T cells | Method to confirm reactivity | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRI4.13 | GAD65:555-567 | NFFRMVISNPAAT | DR4 | TRAV19 | TRAJ44 | CALSENRGGTASKLTF | TRBV5-1 | TRBJ1-1 | CASSLVGGPSSEAFF | PBMC CD4 | Clone/Transgenic cells | (79–81) |
| BRI164 | GAD65:555-567 | NFFRMVISNPAAT | DR4 | TRAV19 | TRAJ56 | CALSEEGGGANSKLTF | TRBV5-1 | TRBJ1-6 | CASSLAGGANSPLHF | PBMC CD4 | Clone/Transgenic cells | (80, 82) |
| T1D2-1&2 | IGRP:305-324 | QLYHFLQIPTHEEHLFYVLS | DR4 | TRAV29 | TRAJ40 | CAATRTSGTYKYIF | TRBV6-6 | TRBJ2-3 | CASSPWGAGGTDTQYF | PBMC CD4 | Clone/TCR transductant | (81) |
| T1D4-3&4 | IGRP:305-324 | KWCANPDWIHIDTTPFAGLV | DR4 | TRAV2 | TRAJ15 | CAVEDLNQAGTALIF | TRBV5-1 | TRBJ2-1 | CASSLALGQGNQQFF | PBMC CD4 | Clone/TCR transductant | (81) |
| 23.G8 | PPI:36-50 | VEALYLVCGERGFFY | DR4 | TRAV39 | TRAJ56 | CAWRTGANSKLTF | TRBV24-1 | TRBJ2-2 | CATGLAANTGELFF | Islet CD4 | TCR transductant | (83) |
| SD52.c1 | PPI:72-90 | PGAGSLQPLALEGSLQKRG | DR4 | TRAV4 | TRAJ27 | CLVGDSLNTNAGKSTF | TRBV27 | TRBJ1-5 | CASSWSSIGNQPQHF | PBMC CD4 | Clone | (82, 84) |
| 95.A9-1 | PPI:87-101 | QKRGIVEQCCTSICS | DR4.4 | TRAV9-2 | TRAJ18 | CALRTDRGSTLGRLYF | TRBV11-2 | TRBJ1-6 | CASSLQSSYNSPLHF | Islet CD4 | TCR transductant | (83) |
| Mi.1 | Insulin A:1-15 (PPI: 90-104) | GIVEQCCTSICSLYQ | DR4 | TRAV8-3 | TRAJ44 | CAVGALAGTASKLTF | TRBV29-1 | TRBJ2-3 | CSVEATRADTQYF | PLN CD4 | Clone | (85) |
| Ba.14 | Insulin A:1-15 (PPI: 90-104) | GIVEQCCTSICSLYQ | DR4 | TRAV39 | TRAJ33 | CAVVNMDSNYQLIW | TRBV5-1 | TRBJ2-3 | CASSLATSGGGSDTQYF | PLN CD4 | Clone | (85) |
| Ba.11 | Insulin A:1-15 (PPI: 90-104) | GIVEQCCTSICSLYQ | DR4 | TRAV22 TRAV26-2 ## |
TRAJ52 TRAJ47 |
CADAGGTSYKLF CIPGSEEYGNKLVF |
TRBV5-1 | TRBJ2-3 | CASSLATSGGGSDTQYF | PLN CD4 | Clone | (85) |
| 6.H11 | PPI:94-108 | QCCTSICSLYQLENY | DR4.2 | TRAV26-1 | TRAJ13 | CIVRVYSGGYQKVTF | TRBV30 | TRBJ2-3 | CAWSARLAGGPRTQYF | Islet CD4 | TCR transductant | (83) |
| SD32.5 | PPI:94-110 | QCCTSICSLYQLENYCN | DR4 | TRAV26-1 | TRAJ23 | CIVRVSSAYYNQGGKLIF | TRBV27 | TRBJ2-3 | CASSPRANTDTQYF | PBMC CD4 | Clone | (82, 84) |
| B3.3 | Proinsulin:52-62 (PPI:76-86) | SLQPLALEGSL | DR4 | TRAV17 | TRAJ54 | CATGPIQGAQKLVF | TRBV6-5 | TRBJ1-1 | CASSYAWGRATEAFF | PBMC CD4 | Clone | (86) |
| K4.4/K6.4 | Proinsulin:54-63 (PPI:78-87) | QPLALEGSLQ | DR4 | TRAV10 | TRAJ17 | CVVSAKAAGNKLTF | TRBV7-8 | TRBJ2-7 | CASSLAGTDHYEQYF | PBMC CD4 | Clone | (86) |
| 23.F7 | PPI:24-38 | AFVNQHLCGSHLVEA | DR1 | TRAV8-2 | TRAJ29 | CAVIASGNTPLVF | TRBV19 | TRBJ2-3 | CASKGPGTVIRADTQYF | Islet CD4 | TCR transductant | (83) |
| 55.B3 | PPI:37-51 | EALYLVCGERGFFYT | DR9 | TRAV21 | TRAJ29 | CAVLPPTPLVF | TRBV18 | TRBJ1-1 | CASSYPGTGGARTEAFF | Islet CD4 | TCR transductant | (83) |
| 55.C10 | PPI:58-72 | AEDLQVGQVELGGGP | DR53 | TRAV26-1 | TRAJ26 | CIVRSHGQNFVF | TRBV20-1 | TRBJ2-7 | CSARPGTRNYEQYF | Islet CD4 | TCR transductant | (83) |
| Clone 5 | Insulin B:9-23 (PPI: 33-47) | SHLVEALYLVCGERG | DQ8 | TRAV21 | TRAJ6 | CAVKRTGGSYIPTF | TRBV11-2 | TRBJ2-2 | CASSSFWGSDTGELFF | PBMC CD4 | Clone/TCR transductant | (82, 87, 88) |
| GSE.6H9 | Insulin B:9-23 (PPI: 33-47) | SHLVEALYLVCGERG | DQ8, DQ8-trans | TRAV26-1 | TRAJ40 | CIVRVDSGTYKYIF | TRBV7-2 | TRBJ2-1 | CASSLTAGLASTYNEQFF | Islet CD4 | TCR transductant | (73, 83) |
| GSE.20D11 | Insulin B:9-23 (PPI: 33-47) | SHLVEALYLVCGERG | DQ8 | TRAV12-3 | TRAJ4 | CAILSGGYNKLIF | TRBV2 | TRBJ2-5 | CASSAETQYF | Islet CD4 | TCR transductant | (73, 83) |
| T1D#3 C8 | Insulin B:11-23 (PPI: 35-47)R22E | LVEALYLVCGEEG | DQ8 | TRAV17 | TRAJ23 | CATDAGYNQGGKLIF | TRBV5-1 | TBBJ1-3 | CASSAGNTIYF | PBMC CD4 | Clone | (82, 89) |
| T1D#3 C10 | Insulin B:11-23 (PPI: 35-47)R22E | LVEALYLVCGEEG | DQ8 | TRAV12-3 | TRAJ26 | CATAYGQNFVF | TRBV4-1 | TRBJ2-2 | CASSRGGGNTGELFF | PBMC CD4 | Clone | (82, 89) |
| 19.A4 | PPI:55-69 | RREAEDLQVGQVELG | DQ8 | TRAV8-6 | TRAJ32 | CAVRETGATNKLIF | TRBV20-1 | TRBJ2-7 | CSARPQGFSSYEQYF | Islet CD4 | TCR transductant | (83) |
| GSE.8E3 | PPI:72-87 hEL:C-peptide (HIP11) |
PGAGSLQPLALEGSLQ SLQPLALEAEDLQV |
DQ8, DQ8-trans | TRAV2 | TRAJ37 | CAVDGSGNTGKLIF | TRBV4-1 | TRBJ2-7 | CASSQDLAGVREQYF | Islet CD4 | TCR transductant | (73, 83) |
| 6.G4 | PPI:86-100 | LQKRGIVEQCCTSIC | DQ8, DQ8-trans | TRAV26-1 | TRAJ8 | CIVRVRNTGFQKLVF | TRBV27 | TRBJ1-1 | CASSPGPGNTEAFF | Islet CD4 | TCR transductant | (83) |
| 56.B1 | PPI:40-54 | YLVCGERGFFYTPKT | DQ2 | TRAV13-1 | TRAJ40 | CAVLSPSGTYKYIF | TRBV7-9 | TRBJ1-4 | CASSLMGNPHEKLFF | Islet CD4 | TCR transductant | (83) |
| 53.A4-1 | PPI:72-87 | PGAGSLQPLALEGSLQ | DQ2 | TRAV39 | TRAJ33 | CAVDPMDSNYQLIW | TRBV29-1 | TRBJ2-6 | CSVGTDPSGANVLTF | Islet CD4 | TCR transductant | (83) |
| 23.G6 | PPI:29-43 | HLCGSHLVEALYLVC | DP4 | TRAV9-2 | TRAJ6 | CALSISGGSYIPTF | TRBV5-1 | TRBJ2-5 | CASSFRQGEQETQYF | Islet CD4 | TCR transductant | (83, 90) |
| A4.13 | Proinsulin:41-51 (PPI:65-75) | QVELGGGPGAG | DQ8 | TRAV6 | TRAJ36 | CALKYGANNLFF | TRBV18 | TRBJ1-1 | CASSPTTGGDEAFF | Islet CD4 | Clone | (70) |
| A1.1 | Proinsulin:50-59 (PPI:74-83) | AGSLQPLALE | DQ8 | TRAV25 | TRAJ16 | CAGGFSDGQKLLF | TRBV20-1 | TRBJ2-7 | CSARTEAYEQYF | Islet CD4 | Clone | (70) |
| A1.2 | Proinsulin:50-58 (PPI:74-82) | AGSLQPLAL | DQ8 | TRAV20 | TRAJ58 | CAVIETSGSRLTF | TRBV20-1 | TRBJ2-3 | CSARDQQRVDTQYF | Islet CD4 | Clone | (70) |
| A2.4 | Proinsulin:52-62 (PPI:76-86) | SLQPLALEGSL | DQ8-trans | TRAV19 | TRAJ49 | CALSRAGTGNQFYF | TRBV5-1 | TRBJ2-4 | CASSLGLRGENIQYF | Islet CD4 | Clone | (70) |
| B3.1 | Proinsulin:48-59 (PPI:72-83) | PGAGSLQPLALE | DQ8 | TRAV12-1 | TRAJ9 | CVVKSTGGFKTIF | TRBV20-1 | TRBJ2-5 | CSAGGLAGASQETQYF | PBMC CD4 | Clone | (86) |
| K3.2/K9.5 | Proinsulin:54-62 (PPI:78-86) | QPLALEGSL | DQ2 | TRAV3 | TRAJ31 | CAVRGDNNARLMF | TRBV7-2 | TRBJ2-2 | CASSPIIWGTGELFF | PBMC CD4 | Clone | (86) |
| K6.2 | Proinsulin:49-58 (PPI:73-82) | GAGSLQPLAL | DQ8-trans | TRAV8-2/8-4 | TRAJ11 | CAVTPKSGYSTLTF | TRBV20-1 | TRBJ2-3 | CSARDLAIPDTQYF | PBMC CD4 | Clone | (86) |
| K9.6 | Proinsulin:41-51 (PPI:65-75) | QVELGGGPGAG | DQ8 | TRAV26-1 | TRAJ54 | CIVRVEIQGAQKLVF | TRBV3-2 | TRBJ2-1 | CASSSPGTEYNEQFF | PBMC CD4 | Clone | (86) |
| D1.1/D1.4 | Proinsulin:34-43 (PPI:58-67) | AEDLQVGQVE | DQ8 | TRAV13-1 | TRAJ38 | CAARNAGNNRKLIW | TRBV4-2 | TRBJ2-3 | CASSFRGLGGGTDTQYF | PBMC CD4 | Clone | (86) |
| T6.1 | Proinsulin:52-63 (PPI:76-87) | SLQPLALEGSLQ | DQ2, DQ2-trans | Functional alpha not detected | TRBV9 | TRBJ2-1 | CASSVDPGVYNEQFF | PBMC CD4 | Clone | (86) | ||
| T6.6 | Proinsulin:56-62 (PPI:80-86) | LALEGSL | DQ2 | TRAV35 | TRAJ28 | CAAALSGAGSYQLTF | TRBV19 | TRBJ2-3 | CASRLDPSTDTQYF | PBMC CD4 | Clone | (86) |
| T17.1 | Proinsulin:56-62 (PPI:80-86) | LALEGSL | DQ2, DQ2-trans | TRAV35 | TRAJ28 | CAAALSGAGSYQLTF | TRBV19 | TRBJ2-3 | CASRLDPSTDTQYF | PBMC CD4 | Clone | (86) |
| H3.3/H6.4 | Proinsulin:52-61 (PPI:76-85) | SLQPLALEGS | DQ8-trans | TRAV19 | TRAJ57 | CALSGRGSEKLVF | TRBV5-1 | TRBJ2-7 | CASSTRTGQGGNEQYF | PBMC CD4 | Clone | (86) |
| H3.7/H7.4/H8.5 | Proinsulin:50-58 (PPI:74-82) | AGSLQPLAL | DQ8 | TRAV12-1 | TRAJ20 | CVVNPTDDYKLSF | TRBV20-1 | TRBJ2-3 | CSARSLASGGPDTQYF | PBMC CD4 | Clone | (86) |
| H11.5 | Proinsulin:42-51 (PPI:66-75) | VELGGGPGAG | DQ8 | TRAV26-1 | TRAJ36 | CIVRVVTGANNLFF | TRBV5-1 | TRBJ2-5 | CASSLERETQYF | PBMC CD4 | Clone | (86) |
| E2.3 | Proinsulin:54-62 (PPI:78-86) | QPLALEGSL | DQ2 | TRAV30 | TRAJ37 | CGTEKPGSGNTGKLIF | TRBV20-1 | TRBJ1-4 | CSARDGARGEKLFF | PBMC CD4 | Clone | (86) |
| E2.5 | Proinsulin:35-46 (PPI:59-70) | EDLQVGQVELGG | DQ8 | TRAV12-3 | TRAJ5 | CVISPPGRRALTF | TRBV5-4 | TRBJ2-2 | CASSSGTSAGTGELFF | PBMC CD4 | Clone | (86) |
| A3.10 | hEGGG : IAPP2 (HIP6) | GQVELGGGNAVEVLK | DQ8 | TRAV38-1 | TRAJ54 | CAFFGQGAGKLVF | TRBV5-1 | TRBJ2-3 | CASSLSASGGATDTQYF | Islet CD4 | Clone | (70, 91, 92) |
| A1.9 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) |
VELGGGPGA GQVELGGGNAVEVLK |
DQ8 | TRAV20 | TRAJ7 | CAVQAGGNNRLAF | TRBV5-1 | TRBJ1-2 | CASSLERDGYTF | Islet CD4 | Clone | (70, 92) |
| A6.15/A5.8 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) |
VELGGGPGA GQVELGGGNAVEVLK |
DQ8 | TRAV26-1 | TRAJ21 | CIAIYNFNKFYF | TRBV5-1 | TRBJ1-6 | CASSLEASSYNSPLHF | Islet CD4 | Clone | (70, 92) |
| A2.13 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) |
VELGGGPGA GQVELGGGNAVEVLK |
DQ8 | TRAV26-1 | TRAJ39 | CIVSHNAGNMLTF | TRBV5-1 | TRBJ2-5 | CASSLERETQYF | Islet CD4 | Clone | (70, 92) |
| A5.5 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) |
VELGGGPGA GQVELGGGNAVEVLK |
DQ8 | TRAV26-1 | TRAJ54 | CIVRVEIQGAQKLVF | TRBV5-1 | TRBJ2-5 | CASSLGPGQRETQYF | Islet CD4 | Clone | (70, 92) |
| A2.11 | hEGGG : IAPP2 (HIP6) | GQVELGGGNAVEVLK | Not reported | TRAV38-1 | TRAJ54 | CAFMGAGAQKLVF | TRBV4-3 | TRBJ2-3 | CASSQILRGGPPDTQYF | Islet CD4 | Clone | (70, 91) |
| HIP14-G10/D3 | hEL : IAPP2 (HIP14) | SLQPLALNAVEVLK | DR | TRAV16 TRAV5 ## |
TRAJ37 TRAJ40 |
CARSHGSGNTGKLIF CAESIASGTYKYIF |
TRBV27 | TRBJ2-5 | CASSSGYGGETQYF | PBMC CD4 | Clone | (93) |
| E2b | hEL:C-peptide (HIP11) | SLQPLALEAEDLQV | DQ2 | TRAV8-4 | TRAJ43 | CAVGATNNNDMRF | TRBV5-4 | TRBJ2-1 | CASSPIGASGGNEQFF | PBMC CD4 | Clone | (94) |
| ET650-2 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) HIPL11C |
VELGGGPGA GQVELGGGNAVEVLK GQVELGGGNAVEVCK |
DQ8 | TRAV26-1 | TRAJ39 | CIVRVGYNAGNMLTF | TRBV20-1 | TRBJ1-5 | CSAIAGPNQPQHF | PBMC CD4 | Clone | (92) |
| ET650-4 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) HIPL11C |
VELGGGPGA GQVELGGGNAVEVLK GQVELGGGNAVEVCK |
DQ8 | TRAV26-1 | TRAJ42 | CIVRVAIEGSQGNLIF | TRBV5-1 | TRBJ1-3 | CASSLRRGDTIYF | PBMC CD4 | Clone | (92) |
| ET650-5 | hEGGG : IAPP2 (HIP6) HIPL11C |
GQVELGGGNAVEVLK GQVELGGGNAVEVCK |
DQ8 | TRAV26-1 | TRAJ9 | CIVRLQSGGFKTIF | TRBV20-1 | TRBJ1-2 | CSAYSPGDRDFSNYGYTF | PBMC CD4 | Clone | (92) |
| ET672-1 | Proinsulin:42-50 (PPI:66-74) hEGGG : IAPP2 (HIP6) HIPL11C |
VELGGGPGA GQVELGGGNAVEVLK GQVELGGGNAVEVCK |
DQ8 | TRAV12-2 | TRAJ48 | CAVNHGNEKLTF | TRBV18 | TRBJ1-1 | CASSPWEGRMDTEAFF | PBMC CD4 | Clone | (92) |
| 1E6 | PPI:15-24 | ALWGPDPAAA | A*02:01 | TRAV12-3 | TRAJ12 | CAMRGDSSYKLIF | TRBV12-4 | TRBJ2-4 | CASSLWEKLAKNIQYF | PBMC CD8 | Clone | (82, 95) |
| 1D5/1D10/2B3/4C6/3E7 | PPI:3-11 | LWMRLLPLL | A*24:02 | TRAV5 | TRAJ37 | CAEPSGNTGKLIF | TRBV7–9 | TRBJ2-7 | CASSLHHEQYF | PBMC CD8 | Clone | (96) |
| Clone 7 | IGRP:265-273 | VLFGLGFAI | A*02:01 | TRAV41 | TRAJ48 | CAVTSNFGNEKLTF | TRBV6-2/6-3 | TRBJ2-7 | CASSSRFVGEGLFRYGYEQYF | PBMC CD8 | Clone/Transgenic cells | (97, 98) |
| Clone 32 | IGRP:265-273 | VLFGLGFAI | A*02:01 | TRAV12-1 | TRAJ48 | CVVNILSNFGNEKLTF | TRBV20-1 | TRBJ2-1 | CSASRQGWVNEQFF | PBMC CD8 | Clone/Transgenic cells | (97, 98) |
| Clone 16/17 | IGRP:265-273 | VLFGLGFAI | A*02:01 | TRAV25 | TRAJ53 | CAGLGDSGGSNYKLTF | TRBV3-1 | TRBJ2-4 | CASSQDRWDVMSKNIQYF | PBMC CD8 | Clone | (45) |
| Clone 22/27 | IGRP:265-273 | VLFGLGFAI | A*02:01 | TRAV29/DV5 | TRAJ53 | CAASGGSNYKLTF | TRBV10-3 | TRBJ1-2 | CAISDRFMREGMTYGYTF | PBMC CD8 | Clone | (45) |
| Clone#1 | INS-DRiP:1-9 | MLYQHLLPL | A*02:01 | TRAV12-2 | TRAJ34 | CAVNKTDKLIF | TRBV6-1 | TRBJ1-2 | CASSVTGNGYTF | PBMC CD8 | Clone | (99) |
| Clone#2 | INS-DRiP:1-9 | MLYQHLLPL | A*02:01 | TRAV10 | TRAJ8 | CVVNMNTGFQKLVF | TRBV12-3/12-4 | TRBJ2-2 | CASSPPQGGNTGELFF | PBMC CD8 | Clone | (99) |
| 1.C1 | INS-DRiP:1-9 | MLYQHLLPL | A*02:01 | TRAV12-1 | TRAJ39 | CGENNAGNMLTF | TRBV27 | TRBJ2-5 | CASSLQPPGTSTETQYF | Islet CD8 | TCR transductant | (74) |
| 96.A9 | INS-DRiP:1-9 | MLYQHLLPL | B*08:01 | TRAV12-2 | TRAJ39 | CAVNVYNAGNMLTF | TRBV30 | TRBJ1-1 | CAWSVRGGSYMNTEAFF | Islet CD8 | TCR transductant | (74) |
| D222D Clones 2 | ZNT8:186-194 | VAANIVLTV | A*02:01 | TRAV17 | TRAJ36 | CAVTGANNLFF | TRBV19 | TRBJ2-2 | CASSIEGPTGELF | PBMC CD8 | Clone | (7) |
| D010R clone 1E2 | ZNT8:186-194 | VAANIVLTV | A*02:01 | TRAV35 | TRAJ36 | CAGTRNNLFF | TRBV19 | TRBJ2-7 | CASGGSSYEQYF | PBMC CD8 | Clone | (7) |
| D010R clone 1D3 | ZNT8:186-194 | VAANIVLTV | A*02:01 | TRAV25 | TRAJ20 | CAGGSNDYKLSF | TRBV6-1 | TRBJ2-3 | CASSSVGVDTQYF | PBMC CD8 | Clone | (7) |
| D267T 33B8 | ZNT8:186-194 | VAANIVLTV | A*02:01 | TRAV19 | TRAJ23 | CALSEATYNQGGKLIF | TRBV19 | TRBJ1-3 | CASSIFPNPGNTIYF | PBMC CD8 | Clone | (7) |
| D349D 178B9 | ZNT8:186-194 | VAANIVLTV | A*02:01 | TRAV14/DV4 | TRAJ9 | CAMREGLTGGFKTIF | TRBV11-2 | TRBJ1-1 | CASSPFLTGSNTEAFF | PBMC CD8 | Clone | (7) |
| D351D 188D3 | ZNT8:186-194 | VAANIVLTV | A*02:01 | TRAV19 | TRAJ20 | CALSPAETSDYKLSF | TRBV19 | TRBJ1-1 | CASTLTGFAEAFF | PBMC CD8 | Clone | (7) |
| 23.F9 | PPI:1-11 | MALWMRLLPLL | C*03:04 | TRAV12-3 | TRAJ48 | CAMSALGNFGNEKLTF | TRBV19 | TRBJ2-1 | CASSIAGGNEQFF | Islet CD8 | TCR transductant | (74) |
| 19.A1 | PPI:1-11 | MALWMRLLPLL | C*03:04 | TRAV8-4 | TRAJ11 | CAVSDQGSGYSTLTF | TRBV28 | TRBJ1-5 | CASSWTANQPQHF | Islet CD8 | TCR transductant | (74) |
| 20.E5 | PPI:1-11 | MALWMRLLPLL | C*03:04 | TRAV14/DV4 | TRAJ52 | CAMSNAGGTSYGKLTF | TRBV28 | TRBJ1-4 | CASSLARYNEKLFF | Islet CD8 | TCR transductant | (74) |
| 20.F1 | PPI:1-11 | MALWMRLLPLL | C*03:04 | TRAV14/DV4 | TRAJ43 | CAMRLHNNNDMRF | TRBV28 | TRBJ1-5 | CASIASRYNQPQHF | Islet CD8 | TCR transductant | (74) |
| 22.A10 | PPI:1-11 | MALWMRLLPLL | C*03:04 | TRAV8-1 | TRAJ13 | CAVNAAGGYQKVTF | TRBV28 | TRBJ2-1 | CASIPDRYNEQFF | Islet CD8 | TCR transductant | (74) |
| 1.C8 | PPI:1-11/2-12/2-10 | MALWMRLLPLL ALWMRLLPLLA ALWMRLLPL |
A*02:01 | TRAV24 | TRAJ58 | CAFKRETSGSRLTF | TRBV13 | TRBJ2-1 | CASSTRLAGDEQFF | Islet CD8 | TCR transductant | (74) |
| 1.F3 | PPI:2-12 | ALWMRLLPLLA | A*02:01 | TRAV39 | TRAJ39 | CAVENAGNMLTF | TRBV10-2 | TRBJ2-1 | CASWTVSYNEQFF | Islet CD8 | TCR transductant | (74) |
| 96.F5 | PPI:3-11 | LWMRLLPLL | A*02:01 | TRAV8-6 | TRAJ48 | CAVSDISNFGNEKLTF | TRBV9 | TRBJ2-3 | CASSVVGLGTDTQYF | Islet CD8 | TCR transductant | (74) |
| 23.H5 | PPI:3-13 | LWMRLLPLLAL | A*02:01 | TRAV38-2/DV8 | TRAJ22 | CAYRSPARQLTF | TRBV6-1 | TRBJ2-7 | CASSEGWGVPSYEQYF | Islet CD8 | TCR transductant | (74) |
| 1.B10-1 | PPI:15-23 | ALWGPDPAA | A*02:01 | TRAV8-3 | TRAJ33 | CAVVADSNYQLIW | TRBV4-2/4-3 | TRBJ2-2 | CASSQTKGTGELFF | Islet CD8 | TCR transductant | (74) |
| 1.F1 | PPI:15-24 | ALWGPDPAAA | A*02:01 | TRAV39 | TRAJ41 | CAVSNSGYALNF | TRBV29-1 | TRBJ2-5 | CSVFHRGETQYF | Islet CD8 | TCR transductant | (74) |
| 23.C12 | PPI:15-24/15-25 | ALWGPDPAAA ALWGPDPAAAF |
A*02:01 | TRAV41 | TRAJ42 | CAVSGGSQGNLIF | TRBV28 | TRBJ1-2 | CASSPPTGWGGYTF | Islet CD8 | TCR transductant | (74) |
| 93.D1 | PPI:15-25 | ALWGPDPAAAF | A*02:01 | TRAV5 | TRAJ8 | CAVTKDTGFQKLVF | TRBV20-1 | TRBJ2-1 | CSARDHFGGSGYEQFF | Islet CD8 | TCR transductant | (74) |
| 10.C6-1 | PPI:23-32 | AAFVNQHLCG | C*12:03 | TRAV19 | TRAJ39 | CALSGALNNAGNMLTF | TRBV27 | TRBJ2-5 | CASSLFGYRQETQYF | Islet CD8 | TCR transductant | (74) |
| 28.D3 | PPI:31-41/34-41 | CGSHLVEALYL HLVEALYL |
A*02:01 | TRAV26-2 | TRAJ26 | CILTDNYGQNFVF | TRBV27 | TRBJ1-1 | CASSLIGLNTEAFF | Islet CD8 | TCR transductant | (74) |
| 28.E6 | PPI:46-54/47-54 | RGFFYTPKT GFFYTPKT |
A*29:02 | TRAV19 | TRAJ28 | CALSEAGAGSYQLTF | TRBV2 | TRBJ2-5 | CASSPSGTSSQETQYF | Islet CD8 | TCR transductant | (74) |
| 20.G1 | PPI:69-77/69-79 | GGGPGAGSL GGGPGAGSLQP |
C*03:04 | TRAV1-2 | TRAJ8 | CAVRMNTGFQKLVF | TRBV9 | TRBJ2-1 | CASSVGMDPGLGYNEQFF | Islet CD8 | TCR transductant | (74) |
| 96.B4 | PPI:91-99 | IVEQCCTSI | C*05:01 | TRAV12-2 | TRAJ31 | CAVNNARLMF | TRBV6-5 | TRBJ2-1 | CASRPTSGGYNEQFF | Islet CD8 | TCR transductant | (74) |
| 86.C1 | PPI:91-100/92-100/92-102 | IVEQCCTSIC VEQCCTSIC VEQCCTSICSL |
B*41:02 | TRAV19 | TRAJ16 | CALSEAGFSDGQKLLF | TRBV19 | TRBJ2-1 | CASSIQFSYNEQFF | Islet CD8 | TCR transductant | (74) |
| 84.D9 | PPI:91-100/92-100/92-102 | IVEQCCTSIC VEQCCTSIC VEQCCTSICSL |
B*41:02 | TRAV29/DV5 | TRAJ43 | CAASNSNDMRF | TRBV7-9 | TRBJ2-1 | CASSLAQREQFF | Islet CD8 | TCR transductant | (74) |
| 28.E11 | PPI:91-100 | IVEQCCTSIC | B*18:01 | TRAV12-2 | TRAJ49 | CAVSMNTGNQFYF | TRBV29-1 | TRBJ2-1 | CSVQVYNEQFF | Islet CD8 | TCR transductant | (74) |
| 1.E9-1 | PPI:92-99 | VEQCCTSI | B*50:01 | TRAV12-2 | TRAJ34 | CAVNIRYNTDKLIF | TRBV6-2/6-3 | TRBJ1-5 | CASSSIQGSGSGQPQHF | Islet CD8 | TCR transductant | (74) |
| 86.G3-2 | PPI:94-102 | QCCTSICSL | B*35:01 | TRAV8-6 | TRAJ33 | CAVSDGYQLIW | TRBV6-1 | TRBJ2-7 | CASSGREAPYEQYF | Islet CD8 | TCR transductant | (74) |
| 54.F1 | PPI:96-103 | CTSICSLY | A*01:01 | TRAV3 | TRAJ26 | CAVPDNYGQNFVF | TRBV7-2 | TRBJ2-2 | CASSLVVELFF | Islet CD8 | TCR transductant | (74) |
#HLA class II alleles: DR4 (DRB1*04:01); DR4.4 (DRB1*04:04); DR4.2 (DRB1*04:02); DR1 (DRB1*01:01); DR9 (DRB1*09:01); DR53 (DRB4*01:01); DQ8 (DQA1*03:01-DQB1*03:02); DQ8-trans (DQA1*05:01-DQB1*03:02); DQ2 (DQA1*05:01-DQB1*02:01/02:02), DQ2-trans (DQA1*03:01-DQB1*02:01); DP4 (DPA1*01:03-DPB1*04:01).
##Two in-frame alpha chains detected. Functional alpha not determined.
Figure 1.
Strategy to determine disease-specific TCR clonotypes. Red and gray circles represent true and false disease-specific TCR clonotypes, respectively. Green circles are true disease-specific clonotypes determined by clustering with known disease-specific TCR clonotypes. (A) TCRs detected in the islets, pancreata, and pancreatic lymph nodes, in particular those for which antigen specificity has been determined as well as those that are clustered with known disease-specific TCRs, can be the initial source for disease-specific TCR candidates. (B) TCRs detected from peripheral blood T cells enriched by antigen stimulation or peptide-MHC-conjugated multimers are also an initial source. Antigen-specific algorithms can enrich TCR clonotypes that are truly specific to antigens. (C) Candidate TCR clonotypes may be assessed for specificity to islet tissues, proteins, and peptides. (D) Using classifying algorithms, candidate TCR clonotypes are assessed for frequency in the blood of individuals with and without T1D to determine disease specificity. Simultaneously, clustering algorithms can select additional clonotypes that are clustered with known disease-specific TCR clonotypes. (E) TCR clonotypes selected by classifying and clustering algorithms are used for machine learning of antigen-specific algorithms to further determine true disease-specificity.
Identification of Disease-Specific TCR Clonotypes Using Big Data
Big data analysis, which seeks to classify TCR repertoires in a specific condition using a large number of TCR samples, is an emerging strategy to identify disease-associated TCR clonotypes. A major advantage of this approach is the capability to identify disease-associated TCR clonotypes without knowing antigen specificity, thereby allowing one to include TCRs that are potentially disease-associated but not islet-specific and also those having low affinity to antigens. Indeed, specificities of large proportions of T cells in the islets are unknown (46, 70–74). Virus infections such as enterovirus and coxsackie B virus (CVB) are suggested to be involved in T1D development (104–106), and TCRs specific to these viruses could be identified by big data analysis by comparing TCR repertoires of individuals having or not having different stages of T1D. Although it has been demonstrated in infectious diseases that big data analyses can identify pathogen-specific TCR clonotypes, it has not yet been successful at identifying T1D-associated TCR clonotypes using PBMC samples from individuals with or without different stages of T1D. This could be explained by several possibilities: (1) the frequency of T1D-associated T cells may be lower than that of pathogen-specific T cells; (2) antigens involved in T1D pathogenesis, especially those at different stages of T1D, may be more heterogeneous than those in infectious diseases; (3) autoreactive TCRs could be more private (i.e. not common between patients) than those of conventional T cells; and (4) sample sizes studied to date have not been large enough. However, having large TCR data sets produced by next generation sequencing will enable machine learning algorithms to cluster and classify TCR clonotypes. Using these newly developed techniques, even infrequent disease-specific TCRs having less publicity (i.e. commonality) between people may be identified from relatively small numbers of samples. Indeed, some computational TCR classifying methods are now capable of identifying cancer patients responding to immune checkpoint inhibitors (40), and also early stages of cancer can be differentiated from healthy individuals using this type of technique (107, 108). In the next section, we will discuss how to take advantages of the latest TCR clustering/classifying techniques for T1D TCR biomarkers.
Clustering and Classification of TCR Clonotypes
TCR clonotypes recognizing the same peptide-MHC complex often share similar motifs and features. For example, influenza-specific TCRs prefer to use TRAV38-1/TRAJ52/TRBV19/TRBJ1-2 (109–111), and melanoma (MART-1)-specific TCRs often contain an alpha chain with TRAV12-2 (112). Likewise, several features common for islet antigen-specific TCRs have been reported. We discovered that insulin B-chain-specific TCRs tend to use TRAV38-1/38-2 and other Valpha segments having similar motifs in the CDR1 and CDR2 regions (113). Also, it has been shown that a specific motif “SGGSNYKLTF” is contained in the CDR3 region of alpha chains specific to an IGRP peptide (45). More recently, crystal structure analysis of TCRs specific to a hybrid insulin peptide composed of proinsulin and islet amyloid polypeptide (IAPP) demonstrated that motifs in the TRBV5-1 segment commonly interact with amino acid residues in IAPP (92). Our work also indicates that T cell responses to hybrid insulin peptides precede clinical T1D onset (114), making these TCR clonotypes excellent candidates for biomarkers. Thus, autoreactive TCRs share commonalities and similarities, which provide clues to cluster TCRs and stratify those specific to a certain condition.
A number of algorithms to cluster or classify TCR clonotypes have been developed. Each algorithm has advantages and disadvantages as reviewed by others (115, 116), but in respect to TCR biomarker development for T1D, the algorithms can be divided to two groups. First, those that clusters TCRs by assessing similarities of TCR sequences with each other in datasets. Second, those that seek to classify TCRs by identifying similar to known antigen-specific or disease-specific TCR clonotypes. The former algorithms such as TCRdist (111), GLIPH/GLIPH2 (117, 118), ClusTCR (119), and GIANA (108) do not need information about T1D-specific epitopes and TCR sequences beforehand, and thus can be used to predict disease-specific TCR clonotypes that are specifically detected in T1D patients but not in non-diabetic subjects. On the other hand, machine learning-based algorithms that assess similarities to known antigen-specific TCR datasets to predict epitopes, such as DeepTCR (101), DeepCAT (107), TCRmatch (120), and TCRAI (100) need prior information about disease-specific TCR sequences. These algorithms show excellent performance when classifying TCRs specific to the same epitopes that were used to develop the machine learning algorithm but not for those having different specificities. Therefore, large sets of disease-specific TCR sequence information for machine training are necessary to achieve high specificity and sensitivity. Typically these types of algorithms show better performance to detect antigen-specific TCR clonotypes than the clustering-based algorithms, thereby being useful to validate TCR clonotypes once epitopes or disease-specificity are determined. Alternatively, they can be also used to ‘clean up’ (i.e. eliminate non-specific TCR clonotypes) TCR datasets that are obtained from multimer-stained T cells or those activated by antigen stimulation ( Figure 1B ).
In any case, it is essential to prepare TCR datasets from a large number of individuals with and without T1D at multiple time points to elicit the best performance by machine learning and clustering algorithms. Typically, diverse datasets rather than large data but from a limited number of samples improve learning efficiency (100). In addition, it is also important to prepare accurate TCR clonotype information to differentiate T1D patients from healthy subjects. There are now several TCR databases available, which accumulate and curate information about TCR sequences along with target peptide-MHC complexes, such as VDJbase (121, 122), IEDB (123), VDJdb (124), iReceptor (125), and McPAS-TCR (126). While these are incredibly useful resources, a proportion of islet-specific clonotypes is still very small, accounting for only ~100 out of tens of thousands of clonotypes, the majority of which are specific to viruses and tumor antigens. Assuming that self-reactive TCR clonotypes are more heterogeneous and rarer compared to pathogen-specific ones, there is a need for higher numbers of clonotypes specific to T1D. Thus, identifying a large set of accurate disease-specific TCR clonotypes will be a key component to achieve successful big data analysis, which will ultimately lead us to establish TCR biomarkers in T1D ( Figure 1 ).
Perspective
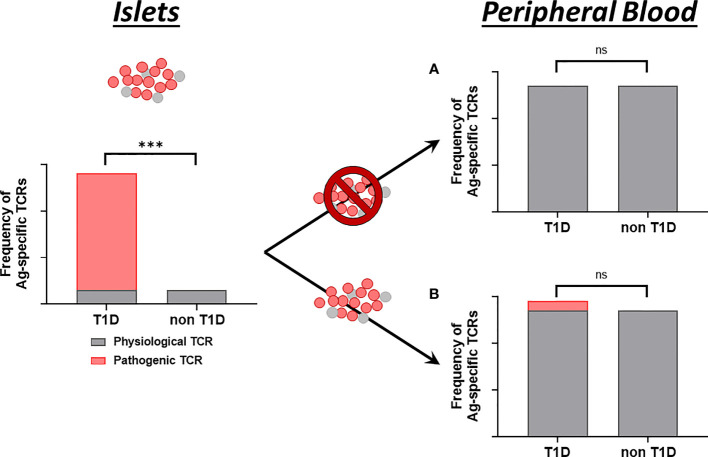
It is still controversial whether T1D patients have distinct islet antigen-specific T cell subsets in the blood compared to healthy individuals. Even in the pancreas, non-diabetic organ donors have preproinsulin-specific T cells in the exocrine compartment, but such antigen-specific T cells accumulate into the islets over the course of T1D progression (127). In the islets, we recently demonstrated that only T1D donors have CD8 T cells highly reactive to preproinsulin (74). Mallone and colleagues also reported that pancreata of T1D donors have a higher number of zinc transporter-8-specific T cells than non-diabetic controls (7). Thus, multiple studies demonstrate that islets of T1D individuals have distinct T cell repertoires from those without diabetes. However, a number of studies indicate that healthy individuals have islet-antigen specific T cells in the blood (7, 113, 128–131), and depending on cell subsets examined, some studies including those looking into pathogenic T cells show that T1D patients have higher numbers of islet-specific T cells, whereas others do not detect differential islet-specific T cells in T1D patients. This controversy could be explained by either (1) detectable numbers of pathogenic T cells in the islets do not leak into the peripheral blood ( Figure 2A ); or (2) pathogenic T cells in the islets do indeed circulate, but because there are already a number of islet-specific (but not harmful) T cells in the circulation, the total numbers of islet-specific T cells (i.e. pathogenic T cells leaked from the islets plus non-pathogenic T cells) are not differentiated enough in the blood of T1D patients from healthy individuals ( Figure 2B ). Given evidence that a portion of T cell repertoires are shared between pancreas, pancreatic lymph nodes, and peripheral blood cells (72), and that TCR repertoires in the islets of T1D organ donors are clonally distinct from those of non-diabetic donors (74), if the latter hypothesis ( Figure 2B ) is correct, islet-derived TCR sequences will be a powerful marker to discriminate pathogenic from physiological T cells, thereby capable of stratifying individuals with active insulitis prior to clinical T1D onset.
Figure 2.
Proposed models of islet-specific T cell detection in the blood. While pancreatic islets contain a certain amount of T cells regardless of disease status (gray bars), only islets of individuals with T1D contain T cells that are highly reactive to islet antigens (red bar). Model (A) Leak of T cells from the islets to peripheral blood is limited. T cell repertoires specific to islet antigens are not different between individuals with and without T1D. Model (B) There are substantial amounts of islet-specific but not disease-specific T cells in the blood regardless of disease status (gray bars). T cells in the islets do circulate in the blood (red bar), but the total numbers of islet-specific T cells are not different between individuals with and without T1D. Enumerating only T cells derived from the islets can identify individuals having T1D. TCR clonotypes are a distinct property to identify islet-derived T cells. *** significantly different. ns, not significant.
To develop practical TCR biomarkers in T1D, a number of obstacles need to be overcome, some of which may be unique to autoimmune diseases. These challenges can be considered from the view of (1) publicity, (2) abundancy, and (3) disease-specificity.
-
Publicity
It will be important to understand the frequencies of public vs private TCR clonotypes that are specific to the T1D disease state, and these likely fluctuate over time during T1D development. Given the genetic risk associated with HLA class II genes, heterogeneity provided by HLA diversity could be smaller than other diseases for TCR clonotypes expressed by CD4 T cells. However, autoreactive T cells, which often bind to peptide-MHC complexes with low affinity, may have a larger TCR repertoire than conventional anti-pathogen T cells, resulting in less commonality. Therefore, frequency of public T1D-specific TCR clonotypes may be low. Strategies that compare TCR repertoires in each individual such as pre and post treatment (40) do not need to consider publicity of clonotypes, and therefore may be more easily applicable to T1D immune intervention studies.
-
Abundancy
Theoretically, 1015-1016 diverse TCR clonotypes can be assembled (12–14); however, a practical TCR repertoire size is estimated to be about 108-1010 per person (15, 17, 18). This indicates that the frequency of target clonotypes is extremely low. However, there is evidence that identical clonotypes are persistently detected from the same individuals over time (44, 81, 93, 132). We believe quantitative resolution of TCRs will need to be increased. This could be achieved by enriching samples before sequencing (e.g. beads enrichment by antigen-specific multimers). Another very attractive approach is to target sequencing to TCRs containing a preferred Vgene segment of interest, thus greatly enhancing the depth of sequencing by analyzing clonotypes that can be obtained for a specific V allele. Blood sample volume needed to quantitatively evaluate frequency of disease-associated TCR clonotypes is another important consideration, which will need to be addressed given that the T1D disease process does begin in young children.
-
Disease-Specificity
Identification of disease-specific TCR clonotypes is an essential component to develop robust T1D TCR biomarkers. A larger number of TCR clonotypes with higher specificity to the disease that are in place will allow for more sensitive and specific assays. Therefore, the key is how to select such truly disease-specific TCR clonotypes. As illustrated in Figure 1 , both accumulation of actual TCR datasets produced from individuals with and without T1D and computational big data analysis will facilitate the development of biomarkers. While the majority of TCR big data analysis currently uses only CDR3-beta sequences, it has been demonstrated that inclusion of entire sequence information such as V and J segments, in particular CDR1 and CDR2 sequences, increases accuracy of classifying TCR clonotypes (100, 120). While the number of T1D-specific clonotypes that have been determined so far is low, evolutions in both TCR sequencing technologies and computational analysis strategies will dramatically impact this effort.
In conclusion, the antigen receptor on disease specific T cells holds promise for a non-cell based biomarker of not only the presence of T1D but disease activity as well. Efforts to define the TCR repertoire within the human pancreas of T1D and non-T1D organ donors is underway with a need to define the antigen specificity and HLA restriction of these identified clonotypes. Those clonotypes that are shared between individuals with T1D, frequent, and circulate from the pancreas and pancreatic lymph nodes to the peripheral blood are prime candidates for deep sequencing and clustering of TCRs using developed computational analyses.
Author Contributions
MN and AM wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (R01DK099317, R01DK032083, R01DK108868, DP3DK110845, P30DK116073, Juvenile Diabetes Research Foundation (2018-480-S-B, 2020-911-A-N, 2018-557-Q-R), the Leona M. & Harry B. Helmsley Charitable Trust (2103-05093), and the Culshaw Family Junior Investigator Award.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 Diabetes. Lancet (London England) (2014) 383:69–82. doi: 10.1016/S0140-6736(13)60591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 Diabetes Mellitus. Nat Rev Dis Primers (2017) 3:17016. doi: 10.1038/nrdp.2017.16 [DOI] [PubMed] [Google Scholar]
- 3. Bluestone JA, Buckner JH, Herold KC. Immunotherapy: Building a Bridge to a Cure for Type 1 Diabetes. Sci (New York NY) (2021) 373:510–6. doi: 10.1126/science.abh1654 [DOI] [PubMed] [Google Scholar]
- 4. Ahmed S, Cerosaletti K, James E, Long SA, Mannering S, Speake C, et al. Standardizing T-Cell Biomarkers in Type 1 Diabetes: Challenges and Recent Advances. Diabetes (2019) 68:1366–79. doi: 10.2337/db19-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, et al. Human TCR-Binding Affinity Is Governed by MHC Class Restriction. J Immunol (Baltimore Md. 1950) (2007) 178:5727–34. doi: 10.4049/jimmunol.178.9.5727 [DOI] [PubMed] [Google Scholar]
- 6. Unger WW, Velthuis J, Abreu JR, Laban S, Quinten E, Kester MG, et al. Discovery of Low-Affinity Preproinsulin Epitopes and Detection of Autoreactive CD8 T-Cells Using Combinatorial MHC Multimers. J Autoimmun (2011) 37:151–9. doi: 10.1016/j.jaut.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 7. Culina S, Lalanne AI, Afonso G, Cerosaletti K, Pinto S, Sebastiani G, et al. Islet-Reactive CD8(+) T Cell Frequencies in the Pancreas, But Not in Blood, Distinguish Type 1 Diabetic Patients From Healthy Donors. Sci Immunol (2018) 3(20):eaao4013. doi: 10.1126/sciimmunol.aao4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scaviner D, Lefranc MP. The Human T Cell Receptor Alpha Variable (TRAV) Genes. Exp Clin Immunogenetics (2000) 17:83–96. doi: 10.1159/000019128 [DOI] [PubMed] [Google Scholar]
- 9. Scaviner D, Lefranc MP. The Human T Cell Receptor Alpha Joining (TRAJ) Genes. Exp Clin Immunogenetics (2000) 17:97–106. doi: 10.1159/000019129 [DOI] [PubMed] [Google Scholar]
- 10. Folch G, Lefranc MP. The Human T Cell Receptor Beta Variable (TRBV) Genes. Exp Clin Immunogenetics (2000) 17:42–54. doi: 10.1159/000019123 [DOI] [PubMed] [Google Scholar]
- 11. Folch G, Lefranc MP. The Human T Cell Receptor Beta Diversity (TRBD) and Beta Joining (TRBJ) Genes. Exp Clin Immunogenetics (2000) 17:107–14. doi: 10.1159/000019130 [DOI] [PubMed] [Google Scholar]
- 12. Davis MM, Bjorkman PJ. T-Cell Antigen Receptor Genes and T-Cell Recognition. Nature (1988) 334:395–402. doi: 10.1038/334395a0 [DOI] [PubMed] [Google Scholar]
- 13. Elhanati Y, Marcou Q, Mora T, Walczak AM. repgenHMM: A Dynamic Programming Tool to Infer the Rules of Immune Receptor Generation From Sequence Data. Bioinf (Oxford England) (2016) 32:1943–51. doi: 10.1093/bioinformatics/btw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zarnitsyna VI, Evavold BD, Schoettle LN, Blattman JN, Antia R. Estimating the Diversity, Completeness, and Cross-Reactivity of the T Cell Repertoire. Front Immunol (2013) 4:485. doi: 10.3389/fimmu.2013.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive Assessment of T-Cell Receptor Beta-Chain Diversity in Alphabeta T Cells. Blood (2009) 114:4099–107. doi: 10.1182/blood-2009-04-217604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and Clonal Selection in the Human T-Cell Repertoire. Proc Natl Acad Sci USA (2014) 111:13139–44. doi: 10.1073/pnas.1409155111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farber DL, Yudanin NA, Restifo NP. Human Memory T Cells: Generation, Compartmentalization and Homeostasis. Nat Rev Immunol (2014) 14:24–35. doi: 10.1038/nri3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lythe G, Callard RE, Hoare RL, Molina-París C. How Many TCR Clonotypes Does a Body Maintain? J Theor Biol (2016) 389:214–24. doi: 10.1016/j.jtbi.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar BV, Connors TJ, Farber DL, Development HTC. Localization, and Function Throughout Life. Immunity (2018) 48:202–13. doi: 10.1016/j.immuni.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily Conserved Amino Acids That Control TCR-MHC Interaction. Annu Rev Immunol (2008) 26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T Cell Antigen Receptor Recognition of Antigen-Presenting Molecules. Annu Rev Immunol (2015) 33:169–200. doi: 10.1146/annurev-immunol-032414-112334 [DOI] [PubMed] [Google Scholar]
- 22. La Gruta NL, Gras S, Daley SR, Thomas PG, Rossjohn J. Understanding the Drivers of MHC Restriction of T Cell Receptors. Nat Rev Immunol (2018) 18:467–78. doi: 10.1038/s41577-018-0007-5 [DOI] [PubMed] [Google Scholar]
- 23. Concannon P, Rich SS, Nepom GT. Genetics of Type 1A Diabetes. New Engl J Med (2009) 360:1646–54. doi: 10.1056/NEJMra0808284 [DOI] [PubMed] [Google Scholar]
- 24. Robertson CC, Rich SS. Genetics of Type 1 Diabetes. Curr Opin Genet Dev (2018) 50:7–16. doi: 10.1016/j.gde.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 25. Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, et al. Localization of Type 1 Diabetes Susceptibility to the MHC Class I Genes HLA-B and HLA-A. Nature (2007) 450:887–92. doi: 10.1038/nature06406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noble JA, Valdes AM, Varney MD, Carlson JA, Moonsamy P, Fear AL, et al. And Genetic Susceptibility to Type 1 Diabetes: Results From the Type 1 Diabetes Genetics Consortium. Diabetes (2010) 59:2972–9. doi: 10.2337/db10-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. And Genotypes and Type 1 Diabetes Risk: Analysis of the Type 1 Diabetes Genetics Consortium Families. Diabetes (2008) 57:1084–92. doi: 10.2337/db07-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM, et al. Additive and Interaction Effects at Three Amino Acid Positions in HLA-DQ and HLA-DR Molecules Drive Type 1 Diabetes Risk. Nat Genet (2015) 47:898–905. doi: 10.1038/ng.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pugliese A, Boulware D, Yu L, Babu S, Steck AK, Becker D, et al. HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 Haplotype Protects Autoantibody-Positive Relatives From Type 1 Diabetes Throughout the Stages of Disease Progression. Diabetes (2016) 65:1109–19. doi: 10.2337/db15-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pecher SA, de Castro GB, Borrás MR. Anemophilus Fungi in the Brazilian-Colombian Border. Rev da Sociedade Bras Medicina Trop (1988) 21:63–6. doi: 10.1590/S0037-86821988000200006 [DOI] [PubMed] [Google Scholar]
- 31. Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the Composition of the Preimmune Repertoire of T Cells Specific for Peptide-Major Histocompatibility Complex Ligands. Annu Rev Immunol (2010) 28:275–94. doi: 10.1146/annurev-immunol-030409-101253 [DOI] [PubMed] [Google Scholar]
- 32. Snyder A, Nathanson T, Funt SA, Ahuja A, Buros Novik J, Hellmann MD, et al. Contribution of Systemic and Somatic Factors to Clinical Response and Resistance to PD-L1 Blockade in Urothelial Cancer: An Exploratory Multi-Omic Analysis. PloS Med (2017) 14:e1002309. doi: 10.1371/journal.pmed.1002309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature (2014) 515:568–71. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheikh N, Cham J, Zhang L, DeVries T, Letarte S, Pufnock J, et al. Clonotypic Diversification of Intratumoral T Cells Following Sipuleucel-T Treatment in Prostate Cancer Subjects. Cancer Res (2016) 76:3711–8. doi: 10.1158/0008-5472.CAN-15-3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tong Y, Li Z, Zhang H, Xia L, Zhang M, Xu Y, et al. T Cell Repertoire Diversity Is Decreased in Type 1 Diabetes Patients. Genomics Proteomics Bioinf (2016) 14:338–48. doi: 10.1016/j.gpb.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pai JA, Satpathy AT. High-Throughput and Single-Cell T Cell Receptor Sequencing Technologies. Nat Methods (2021) 18:881–92. doi: 10.1038/s41592-021-01201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitchell AM, Michels AW. T Cell Receptor Sequencing in Autoimmunity. J Life Sci (Westlake Village Calif.) (2020) 2:38–58. doi: 10.36069/jols/20201203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li N, Yuan J, Tian W, Meng L, Liu Y. T-Cell Receptor Repertoire Analysis for the Diagnosis and Treatment of Solid Tumor: A Methodology and Clinical Applications. Cancer Commun (London England) (2020) 40:473–83. doi: 10.1002/cac2.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joshi K, Milighetti M, Chain BM. Application of T Cell Receptor (TCR) Repertoire Analysis for the Advancement of Cancer Immunotherapy. Curr Opin Immunol (2021) 74:1–8. doi: 10.1016/j.coi.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 40. Fairfax BP, Taylor CA, Watson RA, Nassiri I, Danielli S, Fang H, et al. Peripheral CD8(+) T Cell Characteristics Associated With Durable Responses to Immune Checkpoint Blockade in Patients With Metastatic Melanoma. Nat Med (2020) 26:193–9. doi: 10.1038/s41591-019-0734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beausang JF, Wheeler AJ, Chan NH, Hanft VR, Dirbas FM, Jeffrey SS, et al. T Cell Receptor Sequencing of Early-Stage Breast Cancer Tumors Identifies Altered Clonal Structure of the T Cell Repertoire. Proc Natl Acad Sci USA (2017) 114:E10409–e10417. doi: 10.1073/pnas.1713863114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang H, Liu L, Zhang J, Chen J, Ye J, Shukla S, et al. Investigation of Antigen-Specific T-Cell Receptor Clusters in Human Cancers. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26:1359–71. doi: 10.1158/1078-0432.CCR-19-3249 [DOI] [PubMed] [Google Scholar]
- 43. Estorninho M, Gibson VB, Kronenberg-Versteeg D, Liu YF, Ni C, Cerosaletti K, et al. A Novel Approach to Tracking Antigen-Experienced CD4 T Cells Into Functional Compartments via Tandem Deep and Shallow TCR Clonotyping. J Immunol (Baltimore Md. 1950) (2013) 191:5430–40. doi: 10.4049/jimmunol.1300622 [DOI] [PubMed] [Google Scholar]
- 44. Eugster A, Lindner A, Catani M, Heninger AK, Dahl A, Klemroth S, et al. High Diversity in the TCR Repertoire of GAD65 Autoantigen-Specific Human CD4+ T Cells. J Immunol (Baltimore Md. 1950) (2015) 194:2531–8. doi: 10.4049/jimmunol.1403031 [DOI] [PubMed] [Google Scholar]
- 45. Fuchs YF, Eugster A, Dietz S, Sebelefsky C, Kühn D, Wilhelm C, et al. CD8(+) T Cells Specific for the Islet Autoantigen IGRP Are Restricted in Their T Cell Receptor Chain Usage. Sci Rep (2017) 7:44661. doi: 10.1038/srep44661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez-Calvo T, Christoffersson G, Bender C, von Herrath MG, Mallone R, Kent SC, et al. Means, Motive, and Opportunity: Do Non-Islet-Reactive Infiltrating T Cells Contribute to Autoimmunity in Type 1 Diabetes? Front Immunol (2021) 12:683091. doi: 10.3389/fimmu.2021.683091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haskins K. Pathogenic T-Cell Clones in Autoimmune Diabetes: More Lessons From the NOD Mouse. Adv Immunol (2005) 87:123–62. doi: 10.1016/S0065-2776(05)87004-X [DOI] [PubMed] [Google Scholar]
- 48. Santamaria P, Utsugi T, Park BJ, Averill N, Kawazu S, Yoon JW. Beta-Cell-Cytotoxic CD8+ T Cells From Nonobese Diabetic Mice Use Highly Homologous T Cell Receptor Alpha-Chain CDR3 Sequences. J Immunol (Baltimore Md. 1950) (1995) 154:2494–503. [PubMed] [Google Scholar]
- 49. Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, et al. Identification of the Beta Cell Antigen Targeted by a Prevalent Population of Pathogenic CD8+ T Cells in Autoimmune Diabetes. Proc Natl Acad Sci USA (2003) 100:8384–8. doi: 10.1073/pnas.0932778100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daniel D, Gill RG, Schloot N, Wegmann D. Epitope Specificity, Cytokine Production Profile and Diabetogenic Activity of Insulin-Specific T Cell Clones Isolated From NOD Mice. Eur J Immunol (1995) 25:1056–62. doi: 10.1002/eji.1830250430 [DOI] [PubMed] [Google Scholar]
- 51. Simone E, Daniel D, Schloot N, Gottlieb P, Babu S, Kawasaki E, et al. T Cell Receptor Restriction of Diabetogenic Autoimmune NOD T Cells. Proc Natl Acad Sci USA (1997) 94:2518–21. doi: 10.1073/pnas.94.6.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abiru N, Wegmann D, Kawasaki E, Gottlieb P, Simone E, Eisenbarth GS. Dual Overlapping Peptides Recognized by Insulin Peptide B:9-23 T Cell Receptor AV13S3 T Cell Clones of the NOD Mouse. J Autoimmun (2000) 14:231–7. doi: 10.1006/jaut.2000.0369 [DOI] [PubMed] [Google Scholar]
- 53. Levisetti MG, Suri A, Petzold SJ, Unanue ER. The Insulin-Specific T Cells of Nonobese Diabetic Mice Recognize a Weak MHC-Binding Segment in More Than One Form. J Immunol (Baltimore Md. 1950) (2007) 178:6051–7. doi: 10.4049/jimmunol.178.10.6051 [DOI] [PubMed] [Google Scholar]
- 54. Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique Autoreactive T Cells Recognize Insulin Peptides Generated Within the Islets of Langerhans in Autoimmune Diabetes. Nat Immunol (2010) 11:350–4. doi: 10.1038/ni.1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4⁺ T Cells Recognizing an Unstable Peptide of Insulin Are Directly Recruited Into Islets Bypassing Local Lymph Nodes. J Exp Med (2013) 210:2403–14. doi: 10.1084/jem.20130582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gioia L, Holt M, Costanzo A, Sharma S, Abe B, Kain L, et al. Position β57 of I-A(g7) Controls Early Anti-Insulin Responses in NOD Mice, Linking an MHC Susceptibility Allele to Type 1 Diabetes Onset. Sci Immunol (2019) 4(38):eaaw6329. doi: 10.1126/sciimmunol.aaw6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang L, Jasinski JM, Kobayashi M, Davenport B, Johnson K, Davidson H, et al. Analysis of T Cell Receptor Beta Chains That Combine With Dominant Conserved TRAV5D-4*04 Anti-Insulin B:9-23 Alpha Chains. J Autoimmun (2009) 33:42–9. doi: 10.1016/j.jaut.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kobayashi M, Jasinski J, Liu E, Li M, Miao D, Zhang L, et al. Conserved T Cell Receptor Alpha-Chain Induces Insulin Autoantibodies. Proc Natl Acad Sci USA (2008) 105:10090–4. doi: 10.1073/pnas.0801648105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakayama M, Castoe T, Sosinowski T, He X, Johnson K, Haskins K, et al. Germline TRAV5D-4 T-Cell Receptor Sequence Targets a Primary Insulin Peptide of NOD Mice. Diabetes (2012) 61:857–65. doi: 10.2337/db11-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Y, Sosinowski T, Novikov A, Crawford F, White J, Jin N, et al. How C-Terminal Additions to Insulin B-Chain Fragments Create Superagonists for T Cells in Mouse and Human Type 1 Diabetes. Sci Immunol (2019) 4(34):eaav7517. doi: 10.1126/sciimmunol.aav7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat Models of Type 1 Diabetes: Genetics, Environment, and Autoimmunity. ILAR J (2004) 45:278–91. doi: 10.1093/ilar.45.3.278 [DOI] [PubMed] [Google Scholar]
- 62. Mordes JP, Cort L, Norowski E, Leif J, Fuller JM, Lernmark A, et al. Analysis of the Rat Iddm14 Diabetes Susceptibility Locus in Multiple Rat Strains: Identification of a Susceptibility Haplotype in the Tcrb-V Locus. Mamm Genome Off J Int Mamm Genome Soc (2009) 20:162–9. doi: 10.1007/s00335-009-9172-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stienekemeier M, Hofmann K, Gold R, Herrmann T. A Polymorphism of the Rat T-Cell Receptor Beta-Chain Variable Gene 13 (BV13S1) Correlates With the Frequency of BV13S1-Positive CD4 Cells. Immunogenetics (2000) 51:296–305. doi: 10.1007/s002510050623 [DOI] [PubMed] [Google Scholar]
- 64. Liu Z, Cort L, Eberwine R, Herrmann T, Leif JH, Greiner DL, et al. Prevention of Type 1 Diabetes in the Rat With an Allele-Specific Anti-T-Cell Receptor Antibody: Vβ13 as a Therapeutic Target and Biomarker. Diabetes (2012) 61:1160–8. doi: 10.2337/db11-0867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bogdani M, Faxius L, Fex M, Ramelius A, Wernersson A, Mordes JP, et al. The Vbeta13 T Cell Receptor Monoclonal Antibody Reduces Hyaluronan and CD68+, CD3+, and CD8+ Cell Infiltrations to Delay Diabetes in Congenic BB DRLyp/Lyp Rats. Front Endocrinol (2021) 12:629242. doi: 10.3389/fendo.2021.629242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mordes JP, Cort L, Liu Z, Eberwine R, Blankenhorn EP, Pierce BG, et al. Cell Receptor Genotype and Ubash3a Determine Susceptibility to Rat Autoimmune Diabetes. Genes (2021) 12(6):852. doi: 10.3390/genes12060852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O'Neill A, Staeva T, Nierras C, et al. Network for Pancreatic Organ Donors With Diabetes (nPOD): Developing a Tissue Biobank for Type 1 Diabetes. Diabetes/Metabolism Res Rev (2012) 28:608–17. doi: 10.1002/dmrr.2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kaddis JS, Pugliese A, Atkinson MA. A Run on the Biobank: What Have We Learned About Type 1 Diabetes From the nPOD Tissue Repository? Curr Opin Endocrinol Diabetes Obes (2015) 22:290–5. doi: 10.1097/MED.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 69. Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of Islet-Autoreactive CD8 T Cells in Insulitic Lesions From Recent Onset and Long-Term Type 1 Diabetes Patients. J Exp Med (2012) 209:51–60. doi: 10.1084/jem.20111187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pathiraja V, Kuehlich JP, Campbell PD, Krishnamurthy B, Loudovaris T, Coates PT, et al. Proinsulin-Specific, HLA-DQ8, and HLA-DQ8-Transdimer-Restricted CD4+ T Cells Infiltrate Islets in Type 1 Diabetes. Diabetes (2015) 64:172–82. doi: 10.2337/db14-0858 [DOI] [PubMed] [Google Scholar]
- 71. Babon JA, DeNicola ME, Blodgett DM, Crèvecoeur I, Buttrick TS, Maehr R, et al. Analysis of Self-Antigen Specificity of Islet-Infiltrating T Cells From Human Donors With Type 1 Diabetes. Nat Med (2016) 22:1482–7. doi: 10.1038/nm.4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seay HR, Yusko E, Rothweiler SJ, Zhang L, Posgai AL, Campbell-Thompson M, et al. Tissue Distribution and Clonal Diversity of the T and B Cell Repertoire in Type 1 Diabetes. JCI Insight (2016) 1:e88242. doi: 10.1172/jci.insight.88242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michels AW, Landry LG, McDaniel KA, Yu L, Campbell-Thompson M, Kwok WW, et al. Islet-Derived CD4 T Cells Targeting Proinsulin in Human Autoimmune Diabetes. Diabetes (2017) 66:722–34. doi: 10.2337/db16-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anderson AM, Landry LG, Alkanani AA, Pyle L, Powers AC, Atkinson MA, et al. Human Islet T Cells Are Highly Reactive to Preproinsulin in Type 1 Diabetes. Proc Natl Acad Sci USA (2021) 118(41):e2107208118. doi: 10.1073/pnas.2107208118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Somoza N, Vargas F, Roura-Mir C, Vives-Pi M, Fernández-Figueras MT, Ariza A, et al. Pancreas in Recent Onset Insulin-Dependent Diabetes Mellitus. Changes in HLA, Adhesion Molecules and Autoantigens, Restricted T Cell Receptor V Beta Usage, and Cytokine Profile. J Immunol (Baltimore Md. 1950) (1994) 153:1360–77. [PubMed] [Google Scholar]
- 76. Yamagata K, Nakajima H, Tomita K, Itoh N, Miyagawa J, Hamaguchi T, et al. Dominant TCR Alpha-Chain Clonotypes and Interferon-Gamma Are Expressed in the Pancreas of Patients With Recent-Onset Insulin-Dependent Diabetes Mellitus. Diabetes Res Clin Pract (1996) 34:37–46. doi: 10.1016/S0168-8227(96)01328-9 [DOI] [PubMed] [Google Scholar]
- 77. Codina-Busqueta E, Scholz E, Muñoz-Torres PM, Roura-Mir C, Costa M, Xufré C, et al. TCR Bias of In Vivo Expanded T Cells in Pancreatic Islets and Spleen at the Onset in Human Type 1 Diabetes. J Immunol (Baltimore Md. 1950) (2011) 186:3787–97. doi: 10.4049/jimmunol.1002423 [DOI] [PubMed] [Google Scholar]
- 78. Jacobsen LM, Posgai A, Seay HR, Haller MJ, Brusko TM. T Cell Receptor Profiling in Type 1 Diabetes. Curr Diabetes Rep (2017) 17:118. doi: 10.1007/s11892-017-0946-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, et al. GAD65-Specific CD4+ T-Cells With High Antigen Avidity Are Prevalent in Peripheral Blood of Patients With Type 1 Diabetes. Diabetes (2004) 53:1987–94. doi: 10.2337/diabetes.53.8.1987 [DOI] [PubMed] [Google Scholar]
- 80. Gebe JA, Yue BB, Unrath KA, Falk BA, Nepom GT. Restricted Autoantigen Recognition Associated With Deletional and Adaptive Regulatory Mechanisms. J Immunol (Baltimore Md. 1950) (2009) 183:59–65. doi: 10.4049/jimmunol.0804046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cerosaletti K, Barahmand-Pour-Whitman F, Yang J, DeBerg HA, Dufort MJ, Murray SA, et al. Single-Cell RNA Sequencing Reveals Expanded Clones of Islet Antigen-Reactive CD4(+) T Cells in Peripheral Blood of Subjects With Type 1 Diabetes. J Immunol (Baltimore Md. 1950) (2017) 199:323–35. doi: 10.4049/jimmunol.1700172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yeh WI, Seay HR, Newby B, Posgai AL, Moniz FB, Michels A, et al. Avidity and Bystander Suppressive Capacity of Human Regulatory T Cells Expressing De Novo Autoreactive T-Cell Receptors in Type 1 Diabetes. Front Immunol (2017) 8:1313. doi: 10.3389/fimmu.2017.01313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Landry LG, Anderson AM, Russ HA, Yu L, Kent SC, Atkinson MA, et al. Proinsulin-Reactive CD4 T Cells in the Islets of Type 1 Diabetes Organ Donors. Front Endocrinol (2021) 12:622647. doi: 10.3389/fendo.2021.622647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Endl J, Rosinger S, Schwarz B, Friedrich SO, Rothe G, Karges W, et al. Coexpression of CD25 and OX40 (CD134) Receptors Delineates Autoreactive T-Cells in Type 1 Diabetes. Diabetes (2006) 55:50–60. doi: 10.2337/diabetes.55.01.06.db05-0387 [DOI] [PubMed] [Google Scholar]
- 85. Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, et al. Expanded T Cells From Pancreatic Lymph Nodes of Type 1 Diabetic Subjects Recognize an Insulin Epitope. Nature (2005) 435:224–8. doi: 10.1038/nature03625 [DOI] [PubMed] [Google Scholar]
- 86. So M, Elso CM, Tresoldi E, Pakusch M, Pathiraja V, Wentworth JM, et al. Proinsulin C-Peptide Is an Autoantigen in People With Type 1 Diabetes. Proc Natl Acad Sci USA (2018) 115:10732–7. doi: 10.1073/pnas.1809208115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Eerligh P, van Lummel M, Zaldumbide A, Moustakas AK, Duinkerken G, Bondinas G, et al. Functional Consequences of HLA-DQ8 Homozygosity Versus Heterozygosity for Islet Autoimmunity in Type 1 Diabetes. Genes Immun (2011) 12:415–27. doi: 10.1038/gene.2011.24 [DOI] [PubMed] [Google Scholar]
- 88. Tan S, Li Y, Xia J, Jin CH, Hu Z, Duinkerken G, et al. Type 1 Diabetes Induction in Humanized Mice. Proc Natl Acad Sci USA (2017) 114:10954–9. doi: 10.1073/pnas.1710415114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang J, Chow IT, Sosinowski T, Torres-Chinn N, Greenbaum CJ, James EA, et al. Autoreactive T Cells Specific for Insulin B:11-23 Recognize a Low-Affinity Peptide Register in Human Subjects With Autoimmune Diabetes. Proc Natl Acad Sci USA (2014) 111:14840–5. doi: 10.1073/pnas.1416864111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Williams T, Krovi HS, Landry LG, Crawford F, Jin N, Hohenstein A, et al. Development of T Cell Lines Sensitive to Antigen Stimulation. J Immunol Methods (2018) 462:65–73. doi: 10.1016/j.jim.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, et al. Pathogenic CD4 T Cells in Type 1 Diabetes Recognize Epitopes Formed by Peptide Fusion. Sci (New York NY) (2016) 351:711–4. doi: 10.1126/science.aad2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tran MT, Faridi P, Lim JJ, Ting YT, Onwukwe G, Bhattacharjee P, et al. T Cell Receptor Recognition of Hybrid Insulin Peptides Bound to HLA-Dq8. Nat Commun (2021) 12:5110. doi: 10.1038/s41467-021-25404-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Baker RL, Rihanek M, Hohenstein AC, Nakayama M, Michels A, Gottlieb PA, et al. Hybrid Insulin Peptides Are Autoantigens in Type 1 Diabetes. Diabetes (2019) 68:1830–40. doi: 10.2337/db19-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wiles TA, Hohenstein A, Landry LG, Dang M, Powell R, Guyer P, et al. Characterization of Human CD4 T Cells Specific for a C-Peptide/C-Peptide Hybrid Insulin Peptide. Front Immunol (2021) 12:668680. doi: 10.3389/fimmu.2021.668680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Skowera A, Ellis RJ, Varela-Calviño R, Arif S, Huang GC, Van-Krinks C, et al. CTLs Are Targeted to Kill Beta Cells in Patients With Type 1 Diabetes Through Recognition of a Glucose-Regulated Preproinsulin Epitope. J Clin Invest (2008) 118:3390–402. doi: 10.1172/JCI35449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kronenberg D, Knight RR, Estorninho M, Ellis RJ, Kester MG, de Ru A, et al. Circulating Preproinsulin Signal Peptide-Specific CD8 T Cells Restricted by the Susceptibility Molecule HLA-A24 Are Expanded at Onset of Type 1 Diabetes and Kill β-Cells. Diabetes (2012) 61:1752–9. doi: 10.2337/db11-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Unger WW, Pearson T, Abreu JR, Laban S, van der Slik AR, der Kracht SM, et al. Islet-Specific CTL Cloned From a Type 1 Diabetes Patient Cause Beta-Cell Destruction After Engraftment Into HLA-A2 Transgenic NOD/scid/IL2RG Null Mice. PloS One (2012) 7:e49213. doi: 10.1371/journal.pone.0049213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Babad J, Mukherjee G, Follenzi A, Ali R, Roep BO, Shultz LD, et al. Generation of β Cell-Specific Human Cytotoxic T Cells by Lentiviral Transduction and Their Survival in Immunodeficient Human Leucocyte Antigen-Transgenic Mice. Clin Exp Immunol (2015) 179:398–413. doi: 10.1111/cei.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kracht MJ, van Lummel M, Nikolic T, Joosten AM, Laban S, van der Slik AR, et al. Autoimmunity Against a Defective Ribosomal Insulin Gene Product in Type 1 Diabetes. Nat Med (2017) 23:501–7. doi: 10.1038/nm.4289 [DOI] [PubMed] [Google Scholar]
- 100. Zhang W, Hawkins PG, He J, Gupta NT, Liu J, Choonoo G, et al. A Framework for Highly Multiplexed Dextramer Mapping and Prediction of T Cell Receptor Sequences to Antigen Specificity. Sci Adv (2021) 7(20):eabf5835. doi: 10.1126/sciadv.abf5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sidhom JW, Larman HB, Pardoll DM, Baras AS. DeepTCR Is a Deep Learning Framework for Revealing Sequence Concepts Within T-Cell Repertoires. Nat Commun (2021) 12:1605. doi: 10.1038/s41467-021-21879-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mann SE, Zhou Z, Landry LG, Anderson AM, Alkanani AK, Fischer J, et al. Multiplex T Cell Stimulation Assay Utilizing a T Cell Activation Reporter-Based Detection System. Front Immunol (2020) 11:633. doi: 10.3389/fimmu.2020.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Landry LG, Mann SE, Anderson AM, Nakayama M. Multiplex T-Cell Stimulation Assay Utilizing a T-Cell Activation Reporter-Based Detection System. Bio-Protocol (2021) 11:e3883. doi: 10.21769/BioProtoc.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stene LC, Oikarinen S, Hyöty H, Barriga KJ, Norris JM, Klingensmith G, et al. Enterovirus Infection and Progression From Islet Autoimmunity to Type 1 Diabetes: The Diabetes and Autoimmunity Study in the Young (DAISY). Diabetes (2010) 59:3174–80. doi: 10.2337/db10-0866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory Infections in Early Life and the Development of Islet Autoimmunity in Children at Increased Type 1 Diabetes Risk: Evidence From the BABYDIET Study. JAMA Pediatr (2013) 167:800–7. doi: 10.1001/jamapediatrics.2013.158 [DOI] [PubMed] [Google Scholar]
- 106. Ashton MP, Eugster A, Walther D, Daehling N, Riethausen S, Kuehn D, et al. Incomplete Immune Response to Coxsackie B Viruses Associates With Early Autoimmunity Against Insulin. Sci Rep (2016) 6:32899. doi: 10.1038/srep32899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Beshnova D, Ye J, Onabolu O, Moon B, Zheng W, Fu YX, et al. De Novo Prediction of Cancer-Associated T Cell Receptors for Noninvasive Cancer Detection. Sci Trans Med (2020) 12(557):eaaz3738. doi: 10.1126/scitranslmed.aaz3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang H, Zhan X, Li B. GIANA Allows Computationally-Efficient TCR Clustering and Multi-Disease Repertoire Classification by Isometric Transformation. Nat Commun (2021) 12:4699. doi: 10.1038/s41467-021-25006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ishizuka J, Stewart-Jones GB, van der Merwe A, Bell JI, McMichael AJ, Jones EY. The Structural Dynamics and Energetics of an Immunodominant T Cell Receptor Are Programmed by Its Vbeta Domain. Immunity (2008) 28:171–82. doi: 10.1016/j.immuni.2007.12.018 [DOI] [PubMed] [Google Scholar]
- 110. Song I, Gil A, Mishra R, Ghersi D, Selin LK, Stern LJ. Broad TCR Repertoire and Diverse Structural Solutions for Recognition of an Immunodominant CD8(+) T Cell Epitope. Nat Struct Mol Biol (2017) 24:395–406. doi: 10.1038/nsmb.3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable Predictive Features Define Epitope-Specific T Cell Receptor Repertoires. Nature (2017) 547:89–93. doi: 10.1038/nature22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cole DK, Yuan F, Rizkallah PJ, Miles JJ, Gostick E, Price DA, et al. Germ Line-Governed Recognition of a Cancer Epitope by an Immunodominant Human T-Cell Receptor. J Biol Chem (2009) 284:27281–9. doi: 10.1074/jbc.M109.022509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nakayama M, McDaniel K, Fitzgerald-Miller L, Kiekhaefer C, Snell-Bergeon JK, Davidson HW, et al. Regulatory vs. Inflammatory Cytokine T-Cell Responses to Mutated Insulin Peptides in Healthy and Type 1 Diabetic Subjects. Proc Natl Acad Sci USA (2015) 112:4429–34. doi: 10.1073/pnas.1502967112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mitchell AM, Alkanani AA, McDaniel KA, Pyle L, Waugh K, Steck AK, et al. T-Cell Responses to Hybrid Insulin Peptides Prior to Type 1 Diabetes Development. Proc Natl Acad Sci USA (2021) 118(6):e2019129118. doi: 10.1073/pnas.2019129118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee CH, Salio M, Napolitani G, Ogg G, Simmons A, Koohy H. Predicting Cross-Reactivity and Antigen Specificity of T Cell Receptors. Front Immunol (2020) 11:565096. doi: 10.3389/fimmu.2020.565096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vujovic M, Degn KF, Marin FI, Schaap-Johansen AL, Chain B, Andresen TL, et al. T Cell Receptor Sequence Clustering and Antigen Specificity. Comput Struct Biotechnol J (2020) 18:2166–73. doi: 10.1016/j.csbj.2020.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying Specificity Groups in the T Cell Receptor Repertoire. Nature (2017) 547:94–8. doi: 10.1038/nature22976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huang H, Wang C, Rubelt F, Scriba TJ, Davis MM. Analyzing the Mycobacterium Tuberculosis Immune Response by T-Cell Receptor Clustering With GLIPH2 and Genome-Wide Antigen Screening. Nat Biotechnol (2020) 38:1194–202. doi: 10.1038/s41587-020-0505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Valkiers S, Van Houcke M, Laukens K, Meysman P. ClusTCR: A Python Interface for Rapid Clustering of Large Sets of CDR3 Sequences With Unknown Antigen Specificity. Bioinf (Oxford England) (2021) btab446. doi: 10.1101/2021.02.22.432291 [DOI] [PubMed] [Google Scholar]
- 120. Chronister WD, Crinklaw A, Mahajan S, Vita R, Koşaloğlu-Yalçın Z, Yan Z, et al. TCRMatch: Predicting T-Cell Receptor Specificity Based on Sequence Similarity to Previously Characterized Receptors. Front Immunol (2021) 12:640725. doi: 10.3389/fimmu.2021.640725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shugay M, Bagaev DV, Zvyagin IV, Vroomans RM, Crawford JC, Dolton G, et al. VDJdb: A Curated Database of T-Cell Receptor Sequences With Known Antigen Specificity. Nucleic Acids Res (2018) 46:D419–d427. doi: 10.1093/nar/gkx760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Omer A, Shemesh O, Peres A, Polak P, Shepherd AJ, Watson CT, et al. VDJbase: An Adaptive Immune Receptor Genotype and Haplotype Database. Nucleic Acids Res (2020) 48:D1051–6. doi: 10.1093/nar/gkz872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, et al. The Immune Epitope Database (IEDB): 2018 Update. Nucleic Acids Res (2019) 47:D339–d343. doi: 10.1093/nar/gky1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bagaev DV, Vroomans RMA, Samir J, Stervbo U, Rius C, Dolton G, et al. VDJdb in 2019: Database Extension, New Analysis Infrastructure and a T-Cell Receptor Motif Compendium. Nucleic Acids Res (2020) 48:D1057–d1062. doi: 10.1093/nar/gkz874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Corrie BD, Marthandan N, Zimonja B, Jaglale J, Zhou Y, Barr E, et al. Ireceptor: A Platform for Querying and Analyzing Antibody/B-Cell and T-Cell Receptor Repertoire Data Across Federated Repositories. Immunol Rev (2018) 284:24–41. doi: 10.1111/imr.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tickotsky N, Sagiv T, Prilusky J, Shifrut E, Friedman N. McPAS-TCR: A Manually Curated Catalogue of Pathology-Associated T Cell Receptor Sequences. Bioinf (Oxford England) (2017) 33:2924–9. doi: 10.1093/bioinformatics/btx286 [DOI] [PubMed] [Google Scholar]
- 127. Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT, von Herrath MG. The Healthy Exocrine Pancreas Contains Preproinsulin-Specific CD8 T Cells That Attack Islets in Type 1 Diabetes. Sci Adv (2020) 6(42):eabc5586. doi: 10.1126/sciadv.abc5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective Suppressor Function in CD4(+)CD25(+) T-Cells From Patients With Type 1 Diabetes. Diabetes (2005) 54:92–9. doi: 10.2337/diabetes.54.1.92 [DOI] [PubMed] [Google Scholar]
- 129. Tree TI, Lawson J, Edwards H, Skowera A, Arif S, Roep BO, et al. Naturally Arising Human CD4 T-Cells That Recognize Islet Autoantigens and Secrete Interleukin-10 Regulate Proinflammatory T-Cell Responses via Linked Suppression. Diabetes (2010) 59:1451–60. doi: 10.2337/db09-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chadt A, Immisch A, de Wendt C, Springer C, Zhou Z, Stermann T, et al. “Deletion of Both Rab-GTPase–Activating Proteins TBC1D1 and TBC1D4 in Mice Eliminates Insulin- and AICAR-Stimulated Glucose Transport [Corrected]. Diabetes (2015) 64:746–59. doi: 10.2337/db14-0368 [DOI] [PubMed] [Google Scholar]
- 131. Skowera A, Ladell K, McLaren JE, Dolton G, Matthews KK, Gostick E, et al. β-Cell-Specific CD8 T Cell Phenotype in Type 1 Diabetes Reflects Chronic Autoantigen Exposure. Diabetes (2015) 64:916–25. doi: 10.2337/db14-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Risnes LF, Christophersen A, Dahal-Koirala S, Neumann RS, Sandve GK, Sarna VK, et al. Disease-Driving CD4+ T Cell Clonotypes Persist for Decades in Celiac Disease. J Clin Invest (2018) 128:2642–50. doi: 10.1172/JCI98819 [DOI] [PMC free article] [PubMed] [Google Scholar]