ABSTRACT
The aim was to assess neurological complications in children with an invasive neurological disease by dengue virus (DENV) and the time to resolve symptoms after hospital discharge. A prospective study was conducted at a referral hospital for infectious diseases in Brazil between March 2014 and July 2019. All children hospitalized with neurologic manifestations and DENV RNA detected by real-time reverse transcription-polymerase chain reaction (RT-qPCR) in cerebrospinal fluid (CSF) were followed up until complete resolution of neurological complications. On average, they were followed up for 16 months. Among 56 DENV-positive children, 39% had some neurologic complications after hospital discharge and found that 19.6% were discharged with anticonvulsants due to seizures, 10.7% developed motor complications (e.g. muscle weakness, paresis, ataxia, and walking disability), 5.4% had headaches, and 14.3% had sleep disorders. Among the 56 children, only three had a clinical diagnosis of dengue because the symptoms are nonspecific and 35% showed no change in cerebrospinal fluid (CSF). The average time to resolve complications was 5.9 months (ranging from 1 m to 32 m). These results should alert physicians to the difficulties of a clinical diagnosis of an infection that causes neurological complications after discharge in a significant number of children. RT-qPCR’s etiological diagnosis of DENV infection enabled better clinical follow-up for early intervention in children with neurological complications.
KEYWORDS: Dengue virus, encephalitis, meningitis, myelitis, neurologic complication, children
1. Introduction
Dengue fever (DF) is a mosquito-borne tropical disease caused by the dengue virus (DENV). DF is endemic in ≥ 100 countries, and the number of reported cases has been increasing every year [1]. DF has been the second most diagnosed febrile illness [2] and is spreading to new countries, with outbreaks occurring in Europe since 2010 [1,2].
Significant outbreaks of DF occurred worldwide in 2016 and 2019 [1]. Brazil reported 1.5 million cases of DF in 2016, which was a three-fold increase in the number of cases compared with that in 2014 [3]. In 2019, major outbreaks of DF occurred in Asia, Africa, Australia, South America, Central America, and the eastern Mediterranean [1]. About 500.000 cases of severe DF necessitating hospitalization occur each year, with 2.5% of those patients dying [1,3].
Neurologic manifestations such as meningitis, encephalitis, Guillain-Barre syndrome (GBS), acute demyelinating encephalopathy (ADEM), and myelitis have been described in association with a confirmed infection by the DENV in the central nervous system (CNS) [4–8]. In a recent review of neurologic manifestations in children with DF, 3.2% were diagnosed as DENV-positive by immunoglobulin-M, RT-qPCR, or viral isolation in cerebrospinal fluid (CSF) they overcome with death or neurological sequelae [9].
However, few studies have followed clinically children and adolescents with a neurological invasive disease by DENV until complete resolution of neurological complications [9].
We undertook a prospective study of children hospitalized with neurological manifestations and who had a diagnosis of DENV confirmed by detection of genomic RNA in CSF to assess the evolution of neurological complications.
2. Methods
2.1. Ethical approval of the study protocol
The Research Ethics Committee of Hospital Infantil João Paulo II (Belo Horizonte – MG, Brazil) approved the study protocol (132/2009), and Universidade Federal de Minas Gerais approved the protocol: CAE number: 09273012.9.0000.5149. All study participants provided written informed consent signed by their parents or legal guardians.
2.2. Design
This prospective study was conducted at a referral hospital for infectious diseases between March 2014 and July 2019.
2.3. Inclusion and exclusion criteria
All children hospitalized with neurologic manifestations and confirmed DENV infection within the nervous system were considered eligible. We excluded children (i) with previous neurological diseases or other morbidities, (ii) who were using immunosuppressive drugs, and (iii) with bacterial co-infection in the CNS confirmed by culture or RT-qPCR in CSF or blood.
2.4. Follow-up and outcomes
Patients were followed up in an outpatient clinic after hospital discharge until the complete resolution of neurologic complications. If they were discharged from the hospital without any sequelae, they were also followed up for at least six months after discharge to evaluate possible neurological complications that could appear later.
The outcomes we analyzed were neurological complications that persisted as (i) use of anti-crisis medications, (ii) headache, (iii) sleep disturbance, and (iv) motor complications (e.g. neuropathic pain, walking disability, muscle weakness or paresis, paralysis, polyneuropathy or ataxia).
For cognitive assessment and psychomotor development by age, the following standardized scales were used for the Brazilian population: Ages & Stages Questionnaires _ 3rd edition (ASQ-3_ cognitive and developmental assessment from 0 to 66 months) and the Evolutionary Neurological Exam, ENE 3–7 years [10,11].
2.6. Study population and assessed variables
All children with neurological manifestations and suspected of having a nervous system infection (meningitis and encephalitis) were routinely submitted to lumbar puncture, and CSF was collected for viral detection by RT-PCR. During the study period, cerebrospinal fluids were collected from 505 children, and DENV was detected in 71 (14.1%). Thus, the sample for this study was not probabilistic.
All neurological manifestations evaluated in this study followed well-defined clinical criteria, and diagnoses were made by pediatric neurologists.
The neurological manifestations evaluated are as follows: encephalitis; meningitis; seizures; CNS focal impairment (e.g. focal seizure crisis or cranial nerve involvement); ataxia; paresis or paralysis secondary to encephalitis, acute-disseminated encephalomyelitis (ADEM) or acute viral myelitis; and pain/muscle weakness secondary to involvement of the peripheral nervous system (PNS).
The laboratory and imaging examinations evaluated are as follows: complete blood upon hospital admission; parameters of the collected CSF, alterations in electroencephalograms (EEG), alterations in images of the nervous system using tomography or nuclear magnetic resonance (MRI). Radiologists reported all imaging tests. The electroencephalogram tests were done in waking and spontaneous sleep or just in spontaneous sleep, and neurophysiologists reported them.
All children included in our study were diagnosed by conventional RT-qPCR in CSF. For RNA extraction, the QIAamp® Viral RNA Kit (QIAGEN®, USA.) was used. The viruses tested by PCR were DENV, Zika virus (ZIKV), Yellow Fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV), Sant Luis encephalitis virus (SLEV), Mayaro virus (MAYV), Oropouche virus (OROV), Chikungunya virus (CHKV), herpes virus (HHV-1, HHV-2, HHV-3, and HHV-4) and enterovirus (ENV). We achieved viral isolation on mosquitoes from 32% of DENV-positive samples by RT-qPCR. The molecular diagnosis and viral isolation were made at the Virus Laboratory at Universidade Federal de Minas Gerais.
2.7. Statistical analysis
A descriptive and comparative analysis of clinical and laboratory variables of the children, who had a positive diagnosis by RT-PCR for DENV, was performed, comparing demographic, clinical variables, and changes in CSF and brain imaging tests for defined neurological outcomes and death.
Categorical variables were quantified by frequency percentage, and continuous variables were described by the mean, standard deviation, or median, according to the normal or non-normal distribution, assessed by the Shapiro-Wilk normality test. In categorical variables, t-test was used for comparison of two means of continuous variables with normal distribution, Mann-Whitney for comparison of continuous variables of non-normal distribution, and Kruskal-Wallis to compare means of numerical variable, of non-normal distribution, for more than two groups.
3. Results
3.1. Neurological manifestations
DENV RNA was detected by RT-qPCR in the CSF of 71 children (14.1%) of the 505 CSF samples analyzed.
Six children were excluded from subsequent analysis because of co-infection of DENV subtype 1 (five children) and bacteria such as Streptococcus pneumoniae, Neisseria menigitidis or Haemophilus influenza; one child had DENV-2 and Streptococcus pneumoniae. Six children with previous neurological diseases were also excluded. Three children were lost to clinical follow-up (5%).
Fifty-six children presented with the DENV RNA in CSF were included in the cohort. The prevalence of detection ranged between 0% and 32% from 2014 to 2019, with the highest prevalence of detection in 2015 (16.6%), 2018 (17.5%) and 2019 (34%).
RT-qPCR detected DENV-1 RNA in the CSF of 16 children, DENV-2 RNA in 12 children, DENV-3 RNA in 18 children, and DENV-4 in three children. RT-qPCR was used for RNA detection in two children infected with DENV-1, −2, and −3, three with DENV-1 and −3, one child with DENV-1 and −2, and one child with DENV-2 and-3.
The mean age of children was 18 months (range from 1 month to 9 years). The highest prevalence of DENV was in children under four years of age (84%), and there was no significant difference between males (59%) and females (41%). The clinical manifestations presented by these patients are as follows: fever (93%), vomiting (45%), and rash (14%). Of the 21 children older than one year of age who could report pain, 36% suffered headaches and 16% complained of myalgia or arthralgia. Only one child had thrombocytopenia, 11.5% had leukopenia, and no child had a hematocrit above the reference value for the age group. As the symptoms presented by the children were very nonspecific and few laboratory alterations were observed, the clinical diagnosis of DF was made only in 3 (5,3%) of the children among the 56 evaluated. (Table 1).
Table 1.
Demographic characteristics, clinical symptoms, and laboratory examinations of patients with neurological manifestations diagnosed with DENV serotypes. (March 2014-July 2019)
| TOTAL | DENV-1 | DENV-2 | DENV-3 | DENV-4 | DENV-1/2/3 | |
|---|---|---|---|---|---|---|
| Age group % 1 m-3 m 4 m < 1 year 1y-4 years >4 years |
15.4 42.3 26.9 15.4 |
14.3 50 35.7 0 |
5 25 50 16.7 8.3 |
3 18.8 31.2 31.2 18.8 |
0 66.7 33.3 0 |
0 28.6 14.3 57.1 |
| Sex % Feminine Male |
40.4 59.6 |
42.3 57.7 |
58.3 41.7 |
11.1 88.9 |
100 0 |
42.9 57.1 |
| Fever % | 94.2 | 92.8 | 91.6 | 100 | 100 | 85.7 |
| Headache % | 40.9 | 14.3 | 0 | 62.5 | 0 | 40 |
| Vomit % | 46 | 57 | 25 | 68.8 | 33.3 | 14.3 |
| Myalgia % | 9.6 | 7.2 | 0 | 37.5 | 0 | 14.3 |
| Rash % | 13.5 | 7.2 | 8.3 | 12.5 | 33.3 | 28.6 |
| Hemoglobin* (g/dl) |
10.7 (6.9–13.7) |
10.5 (8.3–12.4) |
10.4 (6.9–13.2) |
11.2 (9–12.4) |
9.2 (6.5–11.1) |
12.2 (10.7–13.7) |
| Leucocytes* (x103/mm3) |
14.35 4–38.1 |
17.96 4–38.1 |
10.34 6.28–18.09 |
12.93 4.22–33.66 |
17.63 10.8–26.22 |
12.90 4.75–24.3 |
| Platelets* (x103/mm3) |
354.0 172–736 |
360.0 172–566 |
463.7 327–736 |
375.1 199–557 |
297.0 84–555 |
277.1 199 − 422 |
| CSF cells* (/mm3) |
79.7 (1–1640) |
45 (1–305) |
98.4 (1–760) |
184.3 (1–1640) |
46.3 (5–126) |
24.6 (8–70) |
| CSF glucose* (mg/dl) |
53 (38–88) |
55.4 (39–88) |
51.4 (40–65) |
53 (37–74) |
49.3 (44–54) |
56 (38–74) |
| CSF protein* (mg/dl) |
39.6 (6–237) |
40.4 (16–91) |
60 (6–237) |
37.9 (11–114) |
35.3 (9–77) |
24.4 (9–58) |
*Average, minimum and maximum value
The neurological manifestations are as follows: seizures (34%), encephalitis (26.8%), meningitis (21.4%), bulging fontanels (17.8%), ataxia (9%), focal signs (9%), and intracranial hypertension (3.5%). Paresis, paralysis, or walking disability secondary to encephalitis, myelitis, or ADEM was detected in 9% of children during hospitalization. Muscle weakness or neuropathic pain secondary to peripheral nervous system (PNS) involvement was observed in 5.4% of cases. The prevalence of neurologic manifestations according to the DENV serotype detected in CSF is shown in Table 2, and there were no clinical differences between groups.
Table 2.
Prevalence of neurological manifestations in children by the DENV serotypes detected in CSF
| Neurological manifestation (%) | DENV-1 | DENV-2 | DENV-3 | DENV-4 | DENV 1/2/3 | p-value* |
|---|---|---|---|---|---|---|
| Seizures | 31.2 | 41.6 | 27.8 | 33.3 | 42.8 | 0.921 |
| Encephalitis | 31.2 | 26.7 | 27.8 | 33.3 | 28.6 | 0.928 |
| Meningitis | 18.7 | 8.3 | 27.8 | 33.3 | 28.6 | 0.702 |
| Bulging fontanel | 18.7 | 16.7 | 22.2 | 33.3 | 0 | 0.687 |
| Motor symptoms secondary encephalitis, myelitis or ADEM | 6.2 | 26.7 | 11.1 | 0 | 0 | 0.715 |
| PNS involvement | 0 | 0 | 11,1 | 0 | 14.3 | 0.402 |
| Focal signs | 6.2 | 16.7 | 5.6 | 0 | 14.3 | 0.769 |
| Ataxia | 6.2 | 8.3 | 11.1 | 0 | 14.3 | 0.939 |
| Intracranial hypertension | 0 | 0 | 5.5 | 0 | 14.3 | 0.450 |
| SNC image was taken | 25 | 50 | 44.4 | 33.3 | 42.8 | 0.694 |
| SNC image was altered | 25 | 33.3 | 37.5 | 100 | 66.7 | 0.580 |
| EEG was taken | 12.5 | 16.7 | 22.2 | 33.3 | 28.6 | 0.849 |
| EEG was altered | 50 | 0 | 0 | 0 | 50 | 0.369 |
| NEUROLOGICAL COMPLICATIONS AFTER HOSPITAL DISCHARGE (%) | ||||||
| Discharge with some neurological complication | 37.5 | 50 | 27.8 | 66.7 | 28.7 | 0.582 |
| Use of anticonvulsant | 12.5 | 25 | 16.7 | 33.3 | 28.6 | 0.818 |
| Motor complications | 6.2 | 16.7 | 11.1 | 0 | 14.3 | 0.872 |
| Headache | 6.2 | 0 | 11.1 | 0 | 0 | 0.654 |
| Sleep disturbance | 25 | 16.7 | 5.6 | 33.3 | 0 | 0.316 |
*p-value = Z test of proportions
In the CSF analysis of these patients, it was evidenced that 35.7% did not present any change. CSF analysis revealed the average number of cells to be 87.7 (range, 1–1640), with a predominance of mononuclear cells in 87.5% of samples. In 100% of cases, the glucose in CSF was within the reference value (2/3 of blood glucose), and in 69.6%, the CSF protein was within the reference value according to age.
The average time between the onset of fever and collection of CSF in which RT-qPCR detected DENV RNA was 3.7 days, but some children underwent CSF puncture on the first day of fever and others 14 days from the onset of fever.
As some patients with neurological manifestations due to DENV evolved with persistent epileptic seizures, 19.6% were discharged with anti-crisis medication. During hospitalization, 28.6% received antibiotics for seven days or more due to the impossibility of excluding bacterial infection, and 9% were medicated with acyclovir for a period equal to or greater than 14 days. Two children were treated with human venous immunoglobulin due to the diagnosis of ADEM and another due to suspected Guillain-Barre syndrome (not confirmed by electroneuromyography). One child received pulse therapy with methylprednisolone, due to severe encephalitis, with Glasgow 3. None of the DENV positive children died during hospitalization or clinical follow-up.
3.2. Clinical follow-up
Among the 56 DENV-positive children, 39% had some neurologic complications after hospital discharge, 19.6% were medicated with anticonvulsants due to seizures, 10.7% developed motor complications (e.g. muscle weakness, paresis, ataxia, or walking disability), 5.4% had a headache, and 14.3% had a sleep disorder.
During hospitalization, eleven children with seizures, in which DENV was detected in CSF, underwent an electroencephalogram (EEG) due to seizures, and two children had an altered EEG. One child presented with EEG, slowing in the left frontal region with disorganized base rhythm, and the other child presented bilateral intermittent delta waves of greater amplitude to the left.
Studies that evaluated EEG in patients with invasive neurological disease due to DENV described similar changes and detected resolution of these changes 15 days after hospital discharge [12,13]. In our cohort, patients underwent a control EEG only one year after hospitalization, and the new tests are normal. The electroencephalographic changes may occur only during the acute phase of the disease, but further studies are needed to prove these findings.
The mean duration of clinical follow-up for children in whom DENV RNA was detected in CSF using RT-qPCR was 16 months. Some children were followed up for ≥ 3 years because of neurologic complications. However, the average time required for neurologic complications to resolve was 5.9 months. By the end of our study, all children had recovered completely.
Three children were lost to clinical follow-up (5%). These three children were very well clinically without neurological symptoms and not using anti-crisis medications when they were discharged from the hospital. However, it was not possible to assess headache or sleep disorders in these children as a possible post-discharge complication.
4. Discussion
The results of the prevalence of neurological manifestations by DENV corroborate the results described in the literature [5,6,8]. Studies evaluating children with meningitis and encephalitis in Cambodia, China, and Brazil found a prevalence of DENV infection between 4.6 and 12% [5,6,8]. These results should alert pediatricians and neurologists to greater clinical suspicion of dengue infection in patients with invasive neurological disease, especially during outbreaks.
According to the Epidemiological Bulletin of diseases transmitted by Aedes, the four dengue serotypes in the State were identified, since 2011, with a predominance of DENV-1 until 2017 and DENV-2 from 2018 from Minas Gerais State [14]. In 2019, 3071 samples were analyzed by RT-PCR in the State, and the four dengue serotypes were identified, especially in the metropolitan region of Belo Horizonte, as can be seen in Figure 1 [14].
Figure 1.
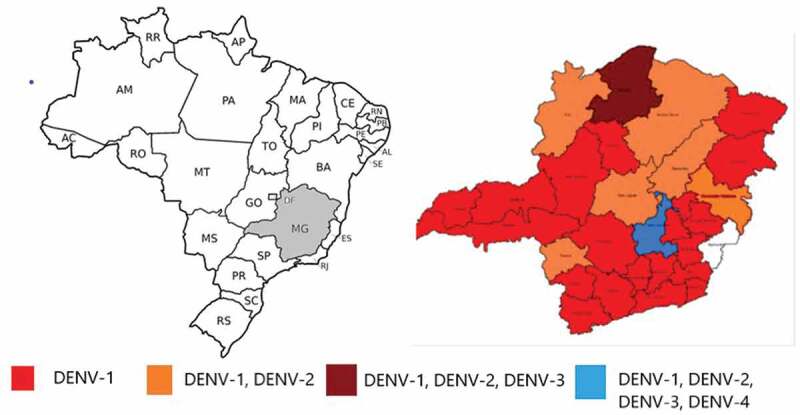
Map of Brazil, with the State of Minas Gerais in gray. Map of dengue virus (DENV) serotypes in Minas Gerais State. The city of Belo Horizonte is located in the central region of the state, represented in black, where the four DENV serotypes circulate
If we compare the data extracted from the Epidemiological Bulletin (Figure 2) with the detection of dengue serotypes in our cohort, it is possible to identify the importance of the DENV-2 and DENV-3 since 2014 in children with dengue neurology invasive diseases (Figure 3). These results suggest that even when these serotypes circulate to a lesser extent in the region, they can cause invasive neurological disease in susceptible children, reinforcing other studies that identified DENV-2 and 3 as more virulent [4,5,15,16].
Figure 2.
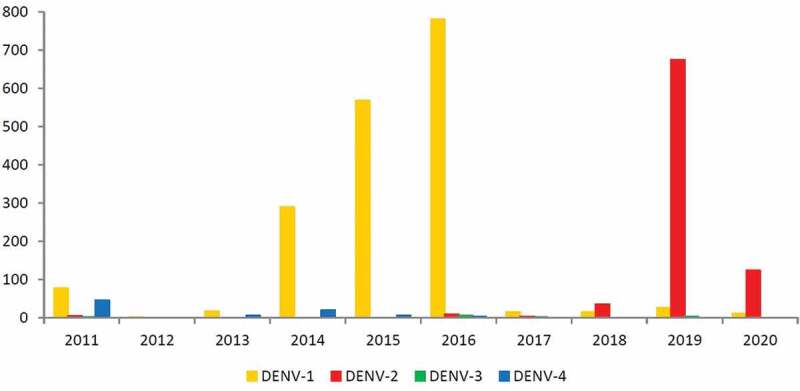
Detection of dengue virus serotypes (DENV 1–4) by RT-PCR in patients in Minas Gerais, 2011–2020
Figure 3.
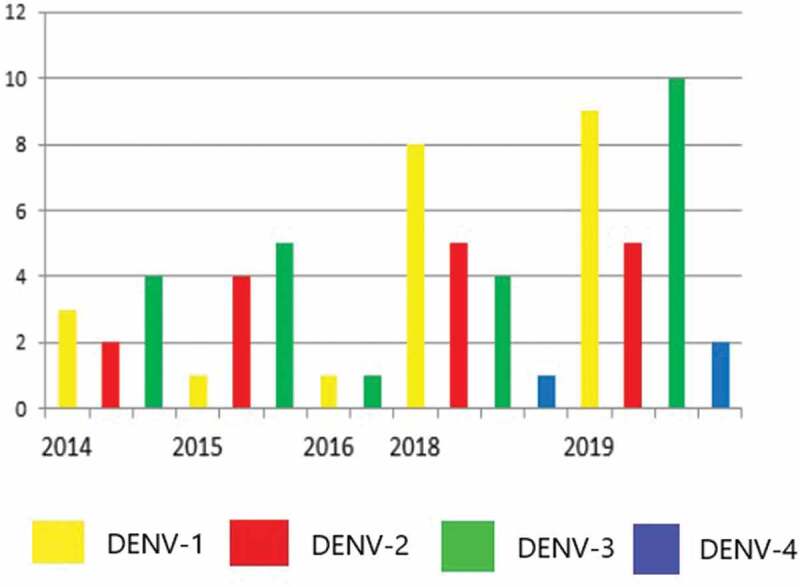
Detection of dengue virus serotypes by RT-PCR in the cerebrospinal fluid of children admitted to the João Paulo II Hospital (March 2014-July 2019)
Among patients with neurological manifestations, 84% of DENV-positive samples were from children ≤ 4 years. Our study was not designed to determine whether the neurological manifestations presented were associated with primary infection or reinfections. Several studies have shown that in reinfections due to different DENV serotypes, hemorrhagic manifestations occur more frequently with thrombocytopenia and hemoconcentration [17–19]. Only one child had ≤ 150.000 platelets in our cohort, and nobody had hematocrit above the reference value for the age group.
The neuropathogenic and immune-response mechanisms that lead to neurological manifestations in primary DF may be different from those after severe DF [17–19]. Immune-response studies are needed to test this hypothesis. However, the sub-neutralizing antibodies produced after primary infection would maintain a more prolonged viremia with a low degree of inflammatory response. This low inflammation would facilitate viral invasion in the CNS and explain the persistence of neurological symptoms for months [18–22].
Studies in animal and cellular models have reported that the DENV can infect microglia and endothelial cells in the blood-brain barrier that release cytokines. The latter attract leukocytes and monocytes that may also be infected by the DENV, thereby allowing viral invasion [20–22]. In the nervous system, a virus can infect neurons, which leads to apoptosis, cell death, and inflammation [20–22]. The latter, in turn, increases the permeability of the blood barrier and amplifies viral invasion [20].
Interestingly, persistence of sleep disorders for months after detection of DENV RNA in CSF by RT-qPCR was noted in the present study. This was a complication found in 14.3% of DENV-positive patients.
Sleep disorders have been studied increasingly in animal models, epidemiology studies, and human volunteers who undergo sleep deprivation [23]. In 2019, Besedovsky, Lange, and Haack published an extensive review in which they described the interactions between sleep and nervous, immune, and endocrinology systems [23]. They cited various scientific evidence that some pathogens can maintain a low degree of inflammation or unresolved inflammation that triggers chronic immune activation with increased secretion of cytokines such as interleukin-6, interleukin-1, and interferon-alpha to activate neuroendocrine messengers that lead to sleep disorders [23]. Sleep deprivation also changes the secretion of several pro-inflammatory cytokines and neurotransmitters by feeding a cycle of immune and neuroendocrine activation [23].
No scholars have assessed links between DENV infections and sleep disorders. Some studies have correlated infection with the human immunodeficiency virus and hepatitis C virus with worsened sleep quality, even in patients using antiretroviral therapy and after adjustment for mood, stress, and disease status [24–26].
DENV and Zika virus (ZIKV) are neurotropic and neurovirulent flavivirus and have similar neurological manifestations. Although more studies are needed to follow clinically and for more extended periods children with postnatal infection by DENV and ZIKV, the evidence published so far reports important differences between these two flaviviruses [27]. Damage of cognitive functions such as memory and learning, vision and hearing disorders is reported in postnatal ZIKV infections [27,28], but they were not described for DENV, as we did not find in our cohort.
The main limitation of this study was the sample size, which is still small to have predictive power to assess outcomes and verify statistical differences between different DENV serotypes. Thus, it was not possible to correlate each serotype with the evaluated outcomes or correlate the viral load with the severity of the neurological manifestations presented by the children.
5. Conclusion
Our study followed clinically 56 children with DENV confirmed by CSF RT-PCR until complete resolution of neurological complications. We performed RT-PCR on the CSF of 505 children and ruled out bacterial co-infections and performed a differential diagnosis with other viruses that cause neurological manifestations.
There was no CSF change in 35.7% of children with DENV. These results are also of great relevance in clinical practice, as they alert physicians to the possibility of this diagnosis in children with neurological manifestations with or without rash, normal blood count, low reactive C protein, and CSF with or without alteration.
This study’s central question sought to answer whether the neurological complications after DENV infection in children were permanent. In our cohort, neurological complications in some children persisted for three years, but on average, they resolved in 5.9 months.
Acknowledgments
We especially thank the children and their families who participated in this research. We thank colleagues from the Hospital Infantil João Paulo II for helping collect CSF from children with neurological manifestations. We thank all the team of Laboratório de Vírus for their excellent technical support.
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq - 440911/2015 and 2058/2016], Departamento de Ciência e Tecnologia do Ministério da Saúde do Brasil (DECIT) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) finance code 001 and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). EGK is a fellow from CNPq and FAPEMIG.
Conflicts of interest
No potential conflict of interest was reported by the author(s).
Author contributions
All authors have made substantial contributions. Professors Aline A Bentes, Roberta MC Romanelli and Erna G. Kroon conceived and designed the study, performed analysis and interpretation of data, drafted the article and revised it critically. Ana Paula C Crispim, Paula EM Marinho and Karina S Loutfi performed analysis and interpretation of data and revised it critically. Isabela, Sara, Luciana, Alice and Marcele, medical students, contributed to acquisition of data. All authors approved the final version to be submitted.
References
- [1].dev.m, Pan American Health Organization / World Health Organization. Epidemiological Update: Dengue . 23 March 2020, Washington, D.C. PAHO / WHO. 2020 [Google Scholar]
- [2].Beckham JD, Tyler KL.. Arbovirus Infections. Contin Lifelong Learn Neurol. 2015;21:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ramos-Castañeda J, Barreto dos Santos F, Martínez-Vega R, et al. Dengue in Latin America: systematic review of molecular epidemiological trends. PLoS Negl Trop Dis. 2017;11(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Soares CN, Faria LC, Peralta JM, et al. Dengue infection: neurological manifestations and cerebrospinal fluid (CSF) analysis. J Neurol Sci. 2006;249(1):19–24. [DOI] [PubMed] [Google Scholar]
- [5].de Oliveira DB, Candiani TM, Franco-Luiz APM, et al. Etiological agents of viral meningitis in children from a dengue-endemic area, Southeast region of Brazil. J Neurol Sci. 2017;375:390–394. [DOI] [PubMed] [Google Scholar]
- [6].Horwood PF, Duong V, Laurent D, et al. Aetiology of acute meningoencephalitis in Cambodian children, 2010-2013. Emerg Microbes Infect. 2017;6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carod-Artal FJ, Wichmann O, Farrar J, et al. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–919. [DOI] [PubMed] [Google Scholar]
- [8].Lohitharajah J, Malavige N, Arambepola C, et al. Viral aetiologies of acute encephalitis in a hospital-based South Asian population. BMC Infect Dis. 2017;17(1). DOI: 10.1186/s12879-017-2403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Almeida Bentes A, Kroon EG, Romanelli RMC. Neurological manifestations of pediatric arboviral infections in the Americas. J Clin Virol. 2019;116:49–57. [DOI] [PubMed] [Google Scholar]
- [10].Squires J, Bricker D, Twonbly E. Ages e stages questionnaires, third edition (ASQ3): user’s guide. San Antonio: Paul H. Brookes Publishing Co.; 2009. [Google Scholar]
- [11].Lefévre AFB. Exame neurológico evolutivo. 2nd ed. São Paulo: Sarvier; 1976. [Google Scholar]
- [12].Kalita J, Misra UK. EEG in dengue virus infection with neurological manifestations: a clinical e CT/MRI correlation. Clin Neurophysiol. 2006;117(10):2252–2256. [DOI] [PubMed] [Google Scholar]
- [13].Wasay M, Channa R, Jumani M, et al. Encephalitis and myelitis associated with dengue viral infection clinical and neuroimaging features. Clin Neurol Neurosurg. 2008;110(6):635–640. [DOI] [PubMed] [Google Scholar]
- [14].SES-MG . Subsecretaria de vigilância em saúde. boletim epidemiológico das doenças transmitidas pelo aedes: dengue, chikungunya e zika. Belo Horizonte, MG; 2020, p.1–20.
- [15].Solbrig MV, Perng GC. Current neurological observations and complications of dengue virus infection. Curr Neurol Neurosci Rep. 2015;15(6):1–8 [DOI] [PubMed] [Google Scholar]
- [16].Salazar MI, Pérez-García M, Terreros-Tinoco M, et al. Dengue virus type 2: protein binding and active replication in human central nervous system cells. Sci World J. 2013;2013:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Castro-Bonilla L, Coronel-Ruiz C, Parra-Alvarez S, et al. Factors associated with dengue virus infection and reinfection in asymptomatic children in two Colombian municipalities. Am J Trop Med Hyg. 2018;99(6):1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wong R, Bhattacharya D. Basics of memory B-cell responses: lessons from and for the real world. Immunology. 2019;156(2):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Forshey BM, Reiner RC, Olkowski S, et al. Incomplete protection against dengue virus type 2 re-infection in Peru. PLoS Negl Trop Dis. 2016;10(2):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mustafá YM, Meuren LM, Coelho SVA, et al. Pathways exploited by flaviviruses to counteract the blood-brain barrier and invade the central nervous system. Front Microbiol. 2019;10. DOI: 10.3389/fmicb.2019.00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Puccioni-Sohler M, Soares CN, Papaiz-Alvarenga R, et al. Neurologic dengue manifestations associated with intrathecal specific immune response. Neurology. 2009;73(17):1413–1417. [DOI] [PubMed] [Google Scholar]
- [22].Silva Marinho PE, Kroon EG. Flaviviruses as agents of childhood central nervous system infections in Brazil. New Microbes New Infect. 2019;30:100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Monaco S, Mariotto S, Ferrari S, et al. Hepatitis C virus-associated neurocognitive and neuropsychiatric disorders. World J Gastroenterol. 2015;21(42):11974–11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thea DM, Porat R, Nagimbi K, et al. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124(8):757–762. [DOI] [PubMed] [Google Scholar]
- [26].Manzar MD, Sony P, Salahuddin M, et al. Electrolyte imbalance and sleep problems during anti-retroviral therapy: an under-recognized problem. Sleep Sci. 2017;10(2):64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Raper J, Chahroudi A. Clinical and preclinical evidence for adverse neurodevelopment after postnatal zika virus infection. Trop Med Infect Dis. 2021;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pacheco O, Newton SM, Daza M, et al. Neurodevelopmental findings in children 20–30 months of age with postnatal Zika infection at 1–12 months of age, Colombia, September–November 2017. Paediatr. Perinat. Epidemiol. 2021;35(1):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


