ABSTRACT
Dengue is an important mosquito-borne viral disease in humans in tropical and subtropical countries. In 2019, a total of 6917 dengue cases were reported in Tanzania based on serological analysis. The aim of this study was to confirm the presence of dengue virus (DENV) and conduct its genetic characterization. A total of 191 serum samples were collected from the outpatients seeking care from health facilities in Kinondoni and Ilala districts between March and May 2019. All the samples were initially tested for the presence of non-structural protein 1 and anti-DENV immunoglobulin G (IgG) and IgM using a commercial OnSite Duo Dengue Ag-IgG/IgM rapid test. Of the 191 sera, 110 (57.6%) were DENV seropositive. The presence of DENV ribonucleic acid was confirmed in 18.2% of the seropositive sera by reverse transcription polymerase chain reaction (RT-PCR). The RT-PCR products were cleaned and partial sequences of DENV polyprotein gene determined using dideoxynucleotide cycle sequencing followed by phylogenetic analysis. We present the occurrence of DENV serotype 1 (DENV-1) during the 2019 outbreak in Tanzania. The DENV-1 strains reported in the present study are highly identical and cluster with Asian DENV-1 strains indicating the possibility of intercontinental spread of DENV through globalization. We advocate for the need for molecular surveillance of dengue viruses during outbreaks to provide rapid evidence of the disease to guide public health interventions.
KEYWORDS: Dengue virus, serotype 1, outbreak, Tanzania
Introduction
In recent years, dengue has become an important mosquito-borne viral disease affecting more than 100 countries in the tropical and subtropical regions of the world, with 50 to 100 million people infected annually [1]. In Africa, the disease is endemic in 34 countries [2], with the most recent outbreaks reported in the East African countries of the Comoros, Ethiopia, Kenya, Mauritius, Seychelles, and Tanzania that share common trade and transport networks [3,4]. In Tanzania, a dengue outbreak was reported for the first time in 1823 in the Islands of Zanzibar [5]. Over the last decade, five dengue outbreaks have occurred in 2010, 2013, 2014, 2018 and 2019 [4,6–8]. From January to October 2019, the total confirmed cases of dengue outbreak from the beginning of outbreak were 6917 cases and 13 deaths translating to 0.2% case fatality rate. The Dar es Salaam region accounted for the majority of cases during this phase of outbreak [4]. Other regions of the country affected included: Arusha Tanga, Dodoma, Kagera, Lindi, Morogoro, Pwani and Ruvuma [4]. Moreover, several studies have reported dengue to be prevalent in several regions of Tanzania including Iringa, Kilimanjaro, Manyara, Morogoro, Pemba and Zanzibar [9–12].
There are four antigenically distinct DENV serotypes; DENV-1, DENV-2, DENV-3, and DENV-4 that share 60–80% homology. Each serotype can cause mild febrile illness to severe forms of the disease known as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [13,14]. Since 1960, all four DENV serotypes have been reported in Africa, with DENV-2 epidemics dominating, followed by DENV-1 [2]. In Tanzania, DENV-2 and DENV-3 have been reported during previous outbreaks [6,7,15].
Although dengue is endemic in Africa, genetic characterization of circulating serotypes is not often conducted [16]. The increasing number of dengue outbreaks and improved access to pathogen genome sequencing tools have allowed defining the molecular epidemiology of the disease [17]. Molecular detection is useful for identification of specific pathogen causing epidemics and tracking the sources of outbreaks for appropriate intervention and response. In Tanzania, two studies have reported the evolutionary relationships of DENV [7,8]. During the 2013 and 2014 outbreaks, phylogenetic analysis of capsid pre-membrane nucleotide sequences of DENV indicated that DENV-2 was responsible for the outbreaks, that were genetically related to DENV-2 strains reported in China, Indonesia and Singapore [18]. The objective of this study was to confirm the presence of DENV during the 2019 outbreak in Dar es Salaam, Tanzania and conduct its genetic characterization.
Materials and methods
Ethics statement
The study was approved by the Medical Research Coordinating Committee of the National Institute for Medical Research in Tanzania (Ref. No. NIMR/HQ/R.8a/Vol.IX/2974). Written informed consent was obtained from all study participants. Consent was obtained from the parents/guardians for those aged 7–17 years. All the records were documented through tracking forms and handled anonymously.
Study area and design
This cross-sectional health facility-based study was conducted in the Kinondoni and Ilala districts of the Dar es Salaam region in Tanzania. The region usually experiences hot and humid climate throughout the year, with the primary dry season from June to September and short rainy season between October and December followed by long rainy periods between March and May. The average daily temperature is 26°C, and total annual rainfall averages 1110 mm, with a relative humidity of 100% and 60% during the night and daytime, respectively [19]. The study involved four health facilities, namely the International School of Tanganyika, Premier Care Hospital, Doctor’s Plaza Hospital, and Regency Medical Center. The facilities are located within Dar es Salaam city serving a diverse population comprising business people, diplomats, and others with high socio-economic interactions. These facilities were purposely selected because they provide routine dengue laboratory testing services. Furthermore, they also provide reference laboratory services for dengue-suspected cases from other health-care facilities within the city.
Sample size and inclusion criteria
The sample size was calculated using a formula described by Arya and Antonisamy [20], assuming 20.9% seroprevalence of DENV infection in Dar es Salaam region [8], and an error rate of 10%. A design effect of three was used to correct for the variability between study districts. The study districts were regarded as clusters and a design effect of three was chosen to obtain an effective sample size of 191 subjects adequate to detect the expected effect. The clinicians recruited patients with dengue-like illness and fever (temperature ≥ 38°C), presenting with at least one of the following clinical signs: retro-orbital pain, rash, arthralgia, malaise, signs of persistent vomiting, severe hemorrhage and organ failure. Febrile patients with bacterial infections and those who were unwilling to participate in the study were excluded. All dengue cases were categorized clinically either as dengue with/without warning signs and severe dengue according to the World Health Organization classification scheme [21].
Sample collection and experimental approach
Serum samples from dengue-suspected outpatients were obtained from selected health facilities from March to May 2019. All the samples were initially tested using OnSite Duo Dengue Ag-IgG/IgM rapid test (CTK BIOTECH Inc, CA, USA), according to the manufacturer’s instructions. The sera samples were placed into sterile cryotubes labeled with a unique identification and stored temporarily at −20°C. Thereafter, the samples were transported in dry-ice to the laboratory at Sokoine University of Agriculture in Morogoro, where they were stored at −80°C until analyzed. The sample flow and experimental approach of this study are shown in Figure 1.
Figure 1.
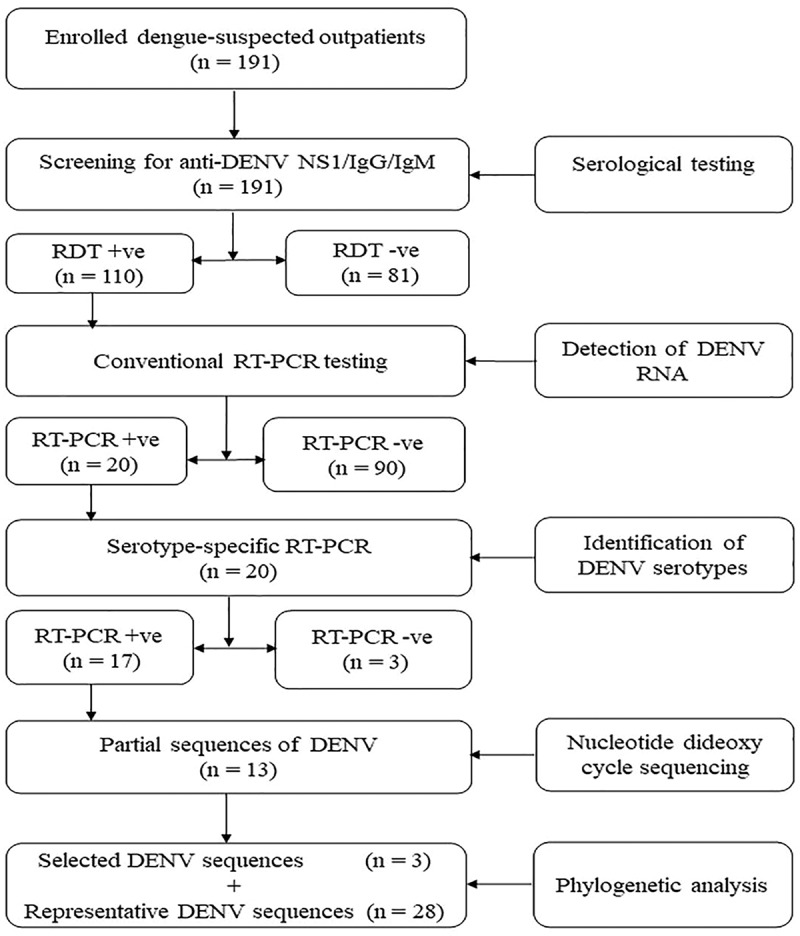
Sample flow and experimental approach used in this study. The chart illustrates the methods for screening non-structural protein 1 (NS1) and anti-DENV immunoglobulin G (IgG) and IgM using a commercial OnSite Duo Dengue Ag-IgG/IgM rapid test (CTK BIOTECH Inc, CA, USA), detection of DENV RNA by conventional reverse transcription polymerase chain reaction (RT-PCR) and genetic characterization after sequencing and phylogenetic analysis
Viral RNA extraction
RNA was extracted from 140 µL of serum using a QIAamp RNA mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The purified RNA was immediately stored in three aliquots each of 10 µL at −20°C until used in order to avoid freeze-thaw cycles that could damage viral RNA. The quality of RNA was determined using a NanoDrop ND1000 spectrophotometer at 260 and 280 absorbance units (GE Healthcare, Buckinghamshire, UK).
Detection and serotyping of DENV by RT-PCR
A conventional one-step reverse transcription polymerase chain reaction (RT-PCR) was conducted on a Mastercyler nexus gradient thermocycler (Eppendorf, Hamburg, Germany) for the detection of DENV using Superscript III Platinum/Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The primers, previously described by Lanciotti and others [22], were used to detect the DENV polyprotein gene region that encodes capsid pre-membrane protein (CprM) followed by serotype identification using serotype-specific primers (Table 1). RT-PCR was performed in a 25 µL reaction mix containing 12.5 µL of 2x reaction mix, 1 µL of Superscript III RT/Platinum Taq mix, 0.5 µL of 10 μM sense primer (D1), 0.5 µL of 10 μM anti-sense primer (D2), 0.5 µL magnesium sulfate (MgS04), 4 µL of RNA template and 6 µL of nuclease-free water. Reverse-transcription reaction was performed in one cycle at 48°C for 30 minutes, followed by one cycle of initial denaturation and inactivation of RT-PCR at 94°C for 2 minutes. Amplification was conducted in 35 cycles, each consisting of a denaturation at 94°C for 15 seconds, annealing at 55°C for 30 seconds and elongation at 68°C for 60 seconds. A final extension was performed at 68°C for 5 minutes. Serotype-specific PCR was performed in a 25 µL reaction containing, 12.5 µL of 2x reaction mix, 1 µL of Superscript III RT/Platinum Taq mix, 0.5 µL of 10 μM forward primer (D1) and 0.5 µL of 10 μM of each reverse serotype-specific primer TS1, TS2, TS3 and TS4, 0.5 µL magnesium sulfate (MgS04), 2 µL of initial RT-PCR products (1:5 dilution) and 6.5 µL of nuclease-free water. Amplification was conducted with an initial denaturation at 94°C for 2 minutes followed by 35 cycles, each consisting of denaturation at 94°C for 15 seconds, annealing at 55°C for 30 seconds and elongation at 72°C for 60 seconds with a final extension at 72°C for 7 minutes. The PCR amplicons were separated on 1.5% agarose gel, stained with Gel Red (Phenix Research Products, Candler, NC, USA), visualized and imaged using a Gel Doc EZ Imager system (Bio-Rad Laboratories Inc, CA, USA).23
Table 1.
List of primers used in conventional RT-PCR
| Primer name | Sequence (‘5–3ʹ) | Serotype specificity | Genome position | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| D1 | TCAATATGCTGAAACGCGCGAGAAACCG | DENV-1-4 | 134–161 | 511 | [22] |
| D2 | TTGCACCAACAGTCAATGTCTTCAGGTTC | DENV-1-4 | 616–644 | 511 | [22] |
| TS1 | CGTCTCAGTGATCCGGGGG | DENV-1 | 581–599 | 482 | [22] |
| TS2 | CGCCACAAGGGCCATGAACAG | DENV-2 | 232–252 | 119 | [22] |
| TS3 | TAACATCATCATGAGACAGAGC | DENV-3 | 400–421 | 290 | [22] |
| TS4 | CTCTGTTGTCTTAAACAAGAGA | DENV-4 | 506–527 | 392 | [22] |
Sequencing and phylogenetic analysis
The partial genome sequences of DENV in the capsid pre-membrane junction region (CprM, 482 nucleotides) of the polyprotein gene were determined using dideoxynucleotide cycle sequencing using an ABI 3730 Genetic Analyzer (Applied Biosystems, MA, USA). The quality of nucleotide sequences was observed using a sequence scanner software (v2.0) (Applied Biosystems, MA, USA). Consensus nucleotide sequences from the forward and reverse nucleotide sequences were created in BioEdit software (v7.2) [19]. A comparison with homologous sequences available at the National Center for Biotechnology Information (NCBI) database was done using the BLAST nucleotide (BLASTn) online tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple sequence alignments were created using CLUSTAL W tool [24], involving 31 DENV nucleotide sequences selected on the basis of four inclusion criteria; (i) three representative DENV nucleotide sequences (n = 3) selected from 13 identical sequences (99.7% to 100% sequence identity) generated in this study as target sequences, (ii) closest match DENV nucleotide sequences (n = 8) from BLASTn search (99.7% to 100% nucleotide identity), (iii) DENV nucleotide sequences (n = 2) from previous outbreaks in Tanzania to indicate serotype shift, (iv) representative DENV nucleotide sequences (n = 18) from all the four DENV serotypes (DENV1-4) reported from countries in different continents including neighboring countries for global and regional comparison. The phylogenetic relationship was inferred by the Maximum Likelihood method using the General Time Reversible model with a gamma distribution and a fraction of invariant sites (GTR +G5 + I). The reliability of phylogenetic analysis was evaluated with 1000 bootstrap replicates. Furthermore, we computed genetic distances to estimate the evolutionary divergence between pairs of nucleotide sequences. All analyses were performed in Molecular Evolutionary Genetics Analysis software (MEGA v7) [25].
Data analysis
Socio-demographic and serological data were entered into Microsoft Excel spreadsheet (MS-Excel 2016, Microsoft Corp., and Redmond, WA, USA). Descriptive analysis was conducted and results were presented in tables and figures.
Results
Socio-demographic characteristics and serological results
The age of dengue patients ranged from 16 to 75 years (Median = 35 years, IQR = 18). Two-third (67.5%) of the patients were males, 63.9% had post-secondary education and 42.9% had formal employment. Of the 191 sera collected, 57.6% were positive for OnSite Duo Dengue Ag-IgG/IgM rapid test with a larger proportion tested positive for IgM and IgG (17.8%) followed by NS1 and IgM (14.7%) combined tests. High dengue incidence was observed among individuals aged 35 to 44 years, males, those with post-secondary education and those with a formal employment (Table 2).
Table 2.
Socio-demographic characteristics of the participants and serological results
| Characteristic | No participants (%) | Seropositive cases* | ||||||
|---|---|---|---|---|---|---|---|---|
| Age group (Years) | NS1 | IgM | NS1+ IgM | IgG | IgM+IgG | NS1+ IgM+IgG | Total cases (%) | |
| < 18 | 17 (8.9) | 1 | 2 | 3 | 1 | 2 | 3 | 12 (6.3) |
| 18–24 | 22 (11.5) | 0 | 3 | 1 | 1 | 5 | 2 | 12 (6.3) |
| 25–34 | 38 (19.9) | 2 | 5 | 6 | 4 | 7 | 2 | 26 (13.6) |
| 35–44 | 65 (34.0) | 3 | 7 | 11 | 3 | 11 | 4 | 39 (20.4) |
| 45–54 | 23 (12.0) | 2 | 1 | 1 | 0 | 6 | 1 | 11 (5.8) |
| ≥ 55 | 26 (13.6) | 0 | 1 | 6 | 0 | 3 | 0 | 10 (5.2) |
| Gender | ||||||||
| Male | 129 (67.5) | 6 | 16 | 26 | 6 | 21 | 3 | 78 (40.8) |
| Female | 62 (32.5) | 2 | 3 | 2 | 3 | 13 | 9 | 32 (16.8) |
| Education | ||||||||
| None | 5 (2.6) | 0 | 0 | 0 | 3 | 1 | 0 | 4 (2.1) |
| Primary | 5 (2.6) | 1 | 0 | 0 | 1 | 3 | 0 | 5 (2.6) |
| Secondary | 59 (30.9) | 5 | 2 | 11 | 1 | 19 | 0 | 38 (19.9) |
| College/University | 122 (63.9) | 2 | 17 | 17 | 4 | 11 | 12 | 63 (32.9) |
| Occupation | ||||||||
| Self-employed | 57(29.5) | 2 | 8 | 13 | 2 | 9 | 1 | 35 (18.3) |
| Business | 16 (8.4) | 1 | 3 | 2 | 1 | 2 | 1 | 10 (5.2) |
| Formal employment | 82 (42.9) | 4 | 5 | 6 | 4 | 17 | 9 | 45 (23.6) |
| Students | 36 (18.8) | 1 | 3 | 7 | 2 | 6 | 1 | 20 (10.5) |
| Total (%) | 191 | 8 (4.2%) | 19 (9.9%) | 28 (14.7%) | 9 (4.7%) | 34 (17.8%) | 12 (6.3%) | 110 (57.6%) |
*NS1, DENV non-structural protein 1 antigen; IgM, anti-DENV immunoglobulin M; IgG, anti-DENV immunoglobulin IgG.
Detection of DENV RNA and serotypes
A total of 20 samples (18.2%, n = 110 seropositive) were positive for DENV RNA by conventional RT-PCR. Of the 20 DENV RNA positive samples, 17 were positive for DENV-1 and three were negative due to low RNA titers after serotype-specific RT-PCR test. The serotype-specific RT-PCR tests for DENV-2, DENV-3 and DENV-4 serotypes were all negative (Table 3).
Table 3.
The characteristics of RT-PCR positive sera samples
| Sample | District | Location* | Sex | Age | RNA quality | DENV RNA test | DENV-1 test | DENV-2 test | DENV-3 test | DENV-4 test |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Kinondoni | RMC | M | 16 | 2.83 | +a | DENV-1 | −b | −b | −b |
| P2 | Kinondoni | RMC | M | 48 | 2.25 | + | DENV-1 | − | − | − |
| P3 | Ilala | DPH | M | 45 | 1.54 | + | DENV-1 | − | − | − |
| P4 | Kinondoni | IST | F | 34 | 2.75 | + | DENV-1 | − | − | − |
| P5 | Kinondoni | DPH | F | 36 | 1.42 | + | −b | − | − | − |
| P6 | Ilala | RMC | M | 56 | 2.09 | + | DENV-1 | − | − | − |
| P7 | Ilala | RMC | M | 26 | 2.45 | + | DENV-1 | − | − | − |
| P8 | Kinondoni | DPH | M | 35 | 2.22 | + | DENV-1 | − | − | − |
| P9 | Kinondoni | DPH | M | 44 | 1.57 | + | DENV-1 | − | − | − |
| P10 | Ilala | PCH | M | 40 | 1.66 | + | DENV-1 | − | − | − |
| P11 | Ilala | PCH | M | 65 | 4.98 | + | DENV-1 | − | − | − |
| P12 | Kinondoni | IST | M | 29 | 5.39 | + | DENV-1 | − | − | − |
| P13 | Ilala | RMC | M | 22 | 1.69 | + | − | − | − | − |
| P14 | Kinondoni | DPH | M | 33 | 2.81 | + | DENV-1 | − | − | − |
| P15 | Kinondoni | DPH | M | 20 | 2.61 | + | DENV-1 | − | − | − |
| P16 | Kinondoni | PCH | M | 27 | 1.53 | + | − | − | − | − |
| P17 | Kinondoni | PCH | M | 21 | 1.95 | + | DENV-1 | − | − | − |
| P18 | Ilala | IST | F | 37 | 2.00 | + | DENV-1 | − | − | − |
| P19 | Kinondoni | RMC | M | 21 | 2.14 | + | DENV-1 | − | − | − |
| P20 | Kinondoni | RMC | F | 31 | 2.43 | + | DENV-1 | − | − | − |
*RMC, Regency Medical Center; DPH, Doctor’s Plaza Hospital; IST, International School of Tanganyika Clinic; PCH, Premier Care Hospital; a RT-PCR positive for universal DENV RNA test; b Serotype-specific RT-PCR negative test; DENV-1, dengue virus serotype 1; DENV-2, dengue virus serotype 2; DENV-3, dengue virus serotype 3; DENV-4, dengue virus serotype 4.
Phylogenetic analysis of DENV
DENV partial genome sequences of the polyprotein gene obtained in this study were submitted to GenBank and assigned with accession numbers, MT835384, MT835385 and MT835386 (Table 4). Phylogenetic analysis showed that the Tanzanian DENV strains clustered into DENV-1 (Figure 2). The Tanzanian DENV strains had nucleotide identity of 99.7% with Indian DENV strains reported in 2015, 2016, 2017 and 2018, and had 100% nucleotide identity with 2019 Chinese DENV-1 strains (Table 5).
Table 4.
Representative DENV virus isolates/strains used for reconstruction of phylogenetic tree
| s/n | DENV isolate/strain | Country | Year | Serotype | Genetic distance* | Accession no | Reference | |
|---|---|---|---|---|---|---|---|---|
| 1 | DENV-1/TAN/DAR-S1/2019 | Tanzania | 2019 | DENV-1 | 0.000 | MT835384 | This study | |
| 2 | DENV-1/TAN/DAR-S2/2019 | Tanzania | 2019 | DENV-1 | 0.000 | MT835385 | This study | |
| 3 | DENV-1/TAN/DAR-S3/2019 | Tanzania | 2019 | DENV-1 | 0.004 | MT835386 | This study | |
| 4 | 19XN09422 | China | 2019 | DENV-1 | 0.000 | MN923102.1 | NCBI** | |
| 5 | 19XN08100 | China | 2019 | DENV-1 | 0.000 | MN923101.1 | NCBI | |
| 6 | 19XN51128 | China | 2019 | DENV-1 | 0.000 | MN923096.1 | NCBI | |
| 7 | 19XN25802 | China | 2019 | DENV-1 | 0.000 | MN923085.1 | NCBI | |
| 8 | NU1876 | India | 2018 | DENV-1 | 0.004 | MK796420.1 | NCBI | |
| 9 | STM1818 | India | 2017 | DENV-1 | 0.004 | MT126440.1 | NCBI | |
| 10 | STM1369 | India | 2016 | DENV-1 | 0.004 | MT126438.1 | NCBI | |
| 11 | STM20778 | India | 2015 | DENV-1 | 0.004 | MT126436.1 | NCBI | |
| 12 | 01/21,123 | Brazil | 2011 | DENV-1 | 0.042 | JN092559.1 | NCBI | |
| 13 | Comoros 04.329/93 | Comoros | 1993 | DENV-1 | 0.030 | DQ285562.1 | NCBI | |
| 14 | Angola_2013 | Angola | 2013 | DENV-1 | 0.090 | KF184975 | [33] | |
| 15 | 295arg00 | Argentina | 2002 | DENV-1 | 0.038 | AF514885.3 | [34] | |
| 16 | 19,492 | Singapore | 2016 | DENV-1 | 0.004 | MF033253. | [35] | |
| 17 | 2006Den-1 | India | 2006 | DENV-1 | 0.042 | EF080814.2 | [36] | |
| 18 | MG-2/2013 | Brazil | 2013 | DENV-1 | 0.054 | KM093798.1 | NCBI | |
| 19 | 22,125 | Singapore | 2015 | DENV-1 | 0.007 | MF033237.1 | [35] | |
| 20 | C | Brazil | 2010 | DENV-2 | 0.574 | JN086992.1 | [37] | |
| 21 | DENV2/TAN/Muhimbili/2014 | Tanzania | 2014 | DENV-2 | 0.550 | KM892496.1 | [18] | |
| 22 | DENV2/TAN/IFM/2013 | Tanzania | 2014 | DENV-2 | 0.550 | KM892493.1 | [18] | |
| 23 | MDU140 | India | 2017 | DENV-2 | 0.697 | LR595983.1 | NCBI | |
| 24 | 21/RMRC/Orissa/2011 | India | 2011 | DENV-3 | 0.412 | JQ717295.1 | [38] | |
| 25 | BH-1/2006 | Brazil | 2006 | DENV-3 | 0.404 | GQ330909.1 | [39] | |
| 26 | Strain 251,991 | Kenya | 1991 | DENV-3 | 0.404 | AF547239.1 | [40] | |
| 27 | 89-SriLan3 | Sri Lanka | 2016 | DENV-3 | 0.378 | AF547232.1 | [40] | |
| 28 | IND/JIPMER/2866 | India | 2017 | DENV-4 | 0.697 | MH431924.1 | NCBI | |
| 29 | PHR3C10D4/2014 | Philippines | 2014 | DENV-4 | 0.764 | KT070878.1 | NCBI | |
| 30 | B1008 | French Guiana | 2006 | DENV-4 | 0.735 | EU518596.1 | [41] | |
| 31 | Den-M18/2010 | India | 2010 | DENV-4 | 0.697 | KM507024.1 | NCBI | |
*The number of base substitutions per site from between sequences was estimated using Kimura-2 parameter model [42], **NCBI, National Center for Biotechnology Information nucleotide database available at https://www.ncbi.nlm.nih.gov/nuccore/
Figure 2.
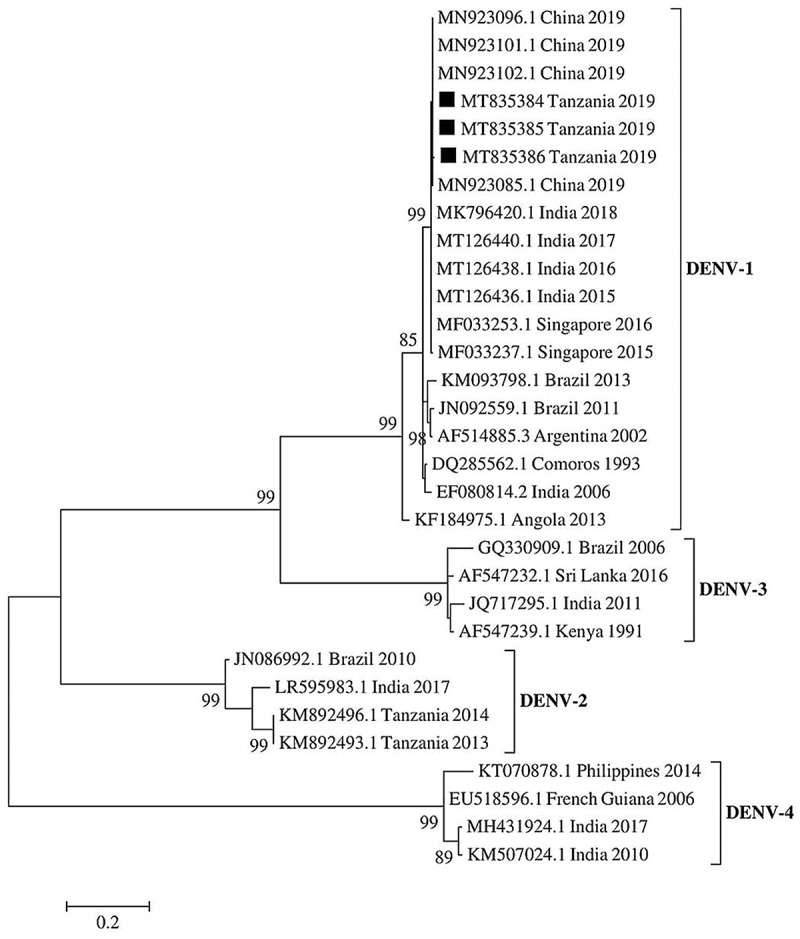
Phylogenetic tree of DENV partial polyprotein gene sequences at capsid pre-membrane junction region (CprM). DENV-1 strains detected during the 2019 outbreak in Tanzania are indicated in black squares. The evolutionary relationship was inferred by the Maximum likelihood method in 1000 bootstrap replicates. The bootstrap support values >80% are shown at the nodes. The scale bar indicates nucleotide substitutions per site
Table 5.
Basic local alignment search results for homologous DENV-1 sequences available at GenBank database
| s/n | Isolate | Country | Accession no | Collection date | Serotype | Nucleotide identity | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 19XN09422 | China | MN923102.1 | May 2019 | DENV-1 | 100% | NCBI* |
| 2 | 19XN08100 | China | MN923101.1 | Apr 2019 | DENV-1 | 100% | NCBI |
| 3 | 19XN51128 | China | MN923096.1 | Oct, 2019 | DENV-1 | 100% | NCBI |
| 4 | 19XN25802 | China | MN923085.1 | Oct 2019 | DENV-1 | 100% | NCBI |
| 5 | NU1876 | India | MK796420.1 | Jun 2018 | DENV-1 | 99.79% | NCBI |
| 6 | STM1818 | India | MT126440.1 | Oct 2017 | DENV-1 | 99.79% | NCBI |
| 7 | STM1369 | India | MT126438.1 | Oct 2016 | DENV-1 | 99.79% | NCBI |
| 8 | STM20778 | India | MT126436.1 | Nov 2015 | DENV-1 | 99.79% | NCBI |
*NCBI, National Center for Biotechnology Information nucleotide database available at https://www.ncbi.nlm.nih.gov/nuccore/
Discussion
Dengue has emerged as a significant public health problem in Tanzania that has evolved from small outbreaks previously seen to large outbreaks experienced in recent years. We performed serological rapid tests to screen for DENV and determined genetic characterization of DENV detected in serum samples obtained from outpatients who presented at health facilities in Kinondoni and Ilala districts of Tanzania.
Our results showed that the age-specific incidence was high among individuals aged 35 to 44 years. This observation indicates low endemicity of the virus in the study area. In highly endemic settings in South-East Asian region, dengue fever occurs mostly in children as adults have acquired immunity following sustained exposure over decades [26]. High vulnerability to DENV infection among this age group may be due to active involvement in socio-economic activities that increase the chances of exposure. These findings are consistent with the reports from other studies [27,28].
A large proportion of sera collected during the outbreak phase was seropositive for anti-DENV IgM and IgG combined tests followed by NS1 antigen and IgM combined tests. These results suggest that most patients presented at health-care facilities during the late acute phase of infection (≥5 days after the onset of fever). During this phase, both NS1 antigen and IgM/IgG antibodies against DENV can be detected in the blood of infected patients [27].
To the best of our knowledge, this study reports DENV-1 for the first time in Tanzania, and thus provides evidence of active DENV transmission and identified serotypes circulating in the country. DENV-1 infections have increased persistently across the globe in the past four decades [29]. In Africa, DENV-1 is among the serotypes that cause frequent epidemics [2]. Phylogenetic analysis results from this study show that viruses from Tanzania had a close evolutionary relationship with the DENV-1 strains from China and India, indicating a possibility for intercontinental spread. In comparison, a recent study in Japan has also reported DENV-1 strain isolated from the patient who had traveled to Tanzania during May 2019 [30]. Subsequent phylogenetic analysis indicated that the Japanese DENV-1 strain (Accession no LC485151) was genetically related to 22,125 Singapore 2015 strain (Accession no MF033237.1). These findings suggest that DENV-1 strains that caused an epidemic during the 2019 in Tanzania are co-circulating in Asia.
Tanzania interacts with many countries for trading, social and economic activities, thus putting it at a risk of introducing DENV from one country to another. Extensive international travel, unplanned urbanization, infrastructure connectivity and global trade networks are significant factors in facilitating the spread of DENV infection between the affected and non-affected areas [29,31]. The recent spread of DENV-1 cases in the East African region is a growing public health threat. In 2019, WHO reported sporadic DENV-1 cases in the Comoros, Mauritius and Seychelles with evidence of importation from other countries [4]. Since circulation of DENV-2 and DENV-3 serotypes has been previously reported in Tanzania [6,7], occurrence of DENV-1 during the 2019 outbreak underscores the need for continuous molecular surveillance of dengue viruses in Tanzania. In endemic countries, the presence of multiple dengue viruses is associated with an increased risk of severe dengue infections [32]. These observations advocate the importance of global, regional and local surveillance of dengue viruses to inform public health interventions.
It is important to note that under the health facility-based study settings, the subjects may not represent the entire infected population, suggesting that the results should be generalized with caution. As dengue becomes endemic in Tanzania, health-care providers are increasingly becoming aware of the need to detect the infection and provide prompt appropriate clinical care to patients. It is important that rapid diagnostic kits are made available at both public and private health facilities to ensure people readily get the required services on time.
In conclusion, confirmatory laboratory diagnosis using RT-PCR facilitated the detection of DENV-1 circulation in Tanzania during the 2019 outbreak. Circulation of new or multiple serotypes is likely to lead to increased risk of severe dengue. There is, therefore, a need to establish a continuous dengue surveillance program to detect outbreaks, monitor the spread of serotypes, and determine dengue burden in the country, in space and time. Future complete genome sequencing of circulating dengue viruses in Tanzania will provide a better understanding of the molecular epidemiology of dengue infection and guide strategies for interventions.
Acknowledgments
The authors are grateful to the management of Regency Medical Centre, Doctor’s Plaza Hospital, International School of Tanganyika Clinic and Premier Care Hospitals for providing sera samples used in this study. We are thankful to the Lancet Laboratories Tanzania Limited for providing laboratory technical assistance in processing the blood samples. The authors wish to acknowledge the SACIDS Africa Centre of Excellence for Infectious Diseases of Humans and Animals in East and Southern Africa for providing doctoral fellowships to G.O.M.and S.R.
Funding Statement
This study was supported by the Government of the United Republic of Tanzania, through the World Bank [WB-ACE II Grant PAD1436, IDA credit 5799-TZ] to the SACIDS Africa Centre of Excellence for Infectious Diseases of Humans and Animals in East and Southern Africa.
Disclosure statements
The authors declare the absence of any competing interests.
References
- [1].Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amarasinghe A, Kuritsky JN, Letson GW, et al. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17(8):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nyaruaba R, Mwaliko C, Mwau M, et al. Arboviruses in the East African community partner states: a review of medically important mosquito-borne Arboviruses. Pathog Glob Health. 2019;113(5):209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weekly bulletins on outbreaks and other emergencies . In: WHO | regional Office for Africa [Internet]. [cited 2019 Jul 23]. Available: https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates
- [5].Christie J. Remarks on “Kidinga Pepo”: a peculiar form of exanthematous disease. Br Med J. 1872;1(596):577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gautret P, Simon F, Askling HH, et al. Dengue type 3 virus infections in European travellers returning from the Comoros and Zanzibar, February-April 2010. Eurosurveillance. 2010;15:19541. [PubMed] [Google Scholar]
- [7].Mboera LEG, Mweya CN, Rumisha SF, et al. The Risk of Dengue Virus Transmission in Dar es Salaam, Tanzania during an Epidemic Period of 2014. PLoS Negl Trop Dis. 2016;10(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vairo F, Mboera LEG, De Nardo P, et al. Clinical, virologic, and epidemiologic characteristics of dengue outbreak, Dar es Salaam, Tanzania, 2014. Emerg Infect Dis. 2016;22(5):895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Faustine NL, Sabuni EJ, Ndaro AJ, et al. Chikungunya, dengue and west Nile virus infections in northern Tanzania. J Adv Med Res. 2017;24(4):1–7. [Google Scholar]
- [10].Hertz JT, Munishi OM, Ooi EE, et al. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86(1):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vairo F, Nicastri E, Meschi S, et al. Seroprevalence of dengue infection: a cross-sectional survey in mainland Tanzania and on Pemba Island, Zanzibar. Int J Infect Dis. 2012;16(1):2011–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vairo F, Nicastri E, Yussuf SM, et al. IgG against dengue virus in healthy blood donors, Zanzibar, Tanzania. Emerg Infect Dis. 2014;20(3):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bos S, Gadea G, Despres P. Dengue: a growing threat requiring vaccine development for disease prevention. Pathog Glob Health. 2018;112(6):294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nimmagadda SS, Mahabala C, Boloor A, et al. Atypical manifestations of Dengue Fever (DF) – where do we stand today? J Clin Diagn Res JCDR. 2014;8(1):71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moi ML, Takasaki T, Kotaki A, et al. Importation of dengue virus type 3 to Japan from Tanzania and Côte d’Ivoire. Emerg Infect Dis. 2010;16(11):1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sharp TM, Moreira R, Soares MJ, et al. Underrecognition of dengue during 2013 epidemic in Luanda, Angola. Emerg Infect Dis. 2015;21(8):1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jaenisch T, Junghanss T, Wills B, et al. Dengue expansion in Africa—not recognized or not happening? Emerg Infect Dis. 2014;20(10). DOI: 10.3201/eid2010.140487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jaswant G Detection and genetic characterisation of dengue virus among patients in Dar es salaam, Tanzania during the 2013-2014 outbreak. PhD Thesis, Sokoine University of Agriculture. 2015. [Google Scholar]
- [19].Trpis M. Seasonal changes in the larval populations of Aedes aegypti in two biotopes in Dar es Salaam, Tanzania. Bull World Health Organ. 1972;47:245. [PMC free article] [PubMed] [Google Scholar]
- [20].Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Peadiatric. 2015;79(11):1482–1488. [DOI] [PubMed] [Google Scholar]
- [21].World Health Organization . Dengue: guidelines for diagnosis, treatment, prevention, and control. Spec Programme Res Train Trop Dis. 2009. 147. [PubMed] [Google Scholar]
- [22].Lanciotti RS, Calisher CH, Gubler DJ, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30(3):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hall T, Biosciences I, Carlsbad C. BioEdit: an important software for molecular biology. GERF Bull Biosci. 2011;2:60–61 [Google Scholar]
- [24].Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bhatia R, Dash AP, Sunyoto T. Changing epidemiology of dengue in South-East Asia. WHO South-East Asia J Public Health. 2013;2(1):23. [DOI] [PubMed] [Google Scholar]
- [27].Murhekar MV, Kamaraj P, Kumar MS, et al. Burden of dengue infection in India, 2017: a cross-sectional population-based serosurvey. Lancet Glob Health. 2019;7(8):e1065–e1073. [DOI] [PubMed] [Google Scholar]
- [28].Eldigail MH, Abubaker HA, Khalid FA, et al. Recent transmission of dengue virus and associated risk factors among residents of Kassala state, eastern Sudan. BMC Public Health. 2020;20(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Messina JP, Brady OJ, Scott TW, et al. Global spread of dengue virus types: mapping the 70-year history. Trends Microbiol. 2014;22(3):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Okada K, Morita R, Egawa K, et al. Dengue virus type 1 infection in traveler returning from Tanzania to Japan, 2019. Emerg Infect Dis. 2019;25(9):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39(4SUPPLEMENT):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Soo K-M, Khalid B, Ching S-M, et al. Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. Huy NT, editor. PLoS One. 2016;11(5):e0154760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sessions OM, Khan K, Hou Y, et al. Exploring the origin and potential for spread of the 2013 dengue outbreak in Luanda, Angola. Glob Health Action. 2013;6(1):21822. .v6i0.21822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Avilés G, Rowe J, Meer J, et al. Phylogenetic relationships of dengue-1 viruses from Argentina and Paraguay. Arch Virol. 2002;147(11):2075–2087. [DOI] [PubMed] [Google Scholar]
- [35].Koo C, Tien WP, Xu H, et al. Highly selective transmission success of dengue virus type 1 lineages in a dynamic virus population: an evolutionary and fitness perspective. iScience. 2018;6:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kukreti H, Chaudhary A, Rautela RS, et al. Emergence of an independent lineage of dengue virus type 1 (DENV-1) and its co-circulation with predominant DENV-3 during the 2006 dengue fever outbreak in Delhi. Int J Infect Dis. 2008;12(5):542–549. [DOI] [PubMed] [Google Scholar]
- [37].Bona ACD, Twerdochlib AL, Navarro-Silva MA. Genetic diversity of dengue virus serotypes 1 and 2 in the state of Paraná, Brazil, based on a fragment of the capsid/premembrane junction region. Rev Soc Bras Med Trop. 2012;45(3):297–300. [DOI] [PubMed] [Google Scholar]
- [38].Das B, Das M, Dwibedi B, et al. Molecular investigations of dengue virus during outbreaks in Orissa state, Eastern India from 2010 to 2011. Infect Genet Evol. 2013;16:401–410. [DOI] [PubMed] [Google Scholar]
- [39].Vilela APP, Figueiredo LB, Dos Santos JR, et al. Dengue virus 3 genotype I in Aedes aegypti mosquitoes and eggs, Brazil, 2005–2006. Emerg Infect Dis. 2010;16:989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Messer WB, Gubler DJ, Harris E, et al. Emergence and global spread of a dengue serotype 3, Subtype III virus. Emerg Infect Dis. 2003;9(7):800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].De Thoisy B, Lacoste V, Germain A, et al. Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis Larchmt N. 2009;9(2):157–170. [DOI] [PubMed] [Google Scholar]
- [42].Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–120. [DOI] [PubMed] [Google Scholar]


