Abstract
The local antiviral photodynamic inactivation (PDI) may prove to be a helpful tool reducing the viral load in the nose and throat area in the early phase of a Covid19 infection. Both the infectivity and the prognosis of SARS-CoV-2 infections in the early phase can depend on the viral load in this area. The aim of our study was to find a simplified PDI therapy option against corona viruses in this region with low dose methylene blue (MB) as photosensitizer and use of LED light instead of laser. As a substitute for SARS-CoV2 viruses we started with BCoV infected U373 cells first. We used an 810nm diode laser with 300mW/cm2 and 100J/cm2 light dose as well as a 590 nm LED and a broadband LED with irradiation intensity of 10,000 lx each (irradiation time 2.5 and 10 min) and concentrations of the sensitizer of 0.001% and 0.0001%. The 0.001% MB sensitizer experiments showed similar results with all exposures. The logarithmic reduction factor varied between ≥ 5.29 and ≥ 5.31, (0.001% MB sensitizer) and ≥ 4.6 and ≥ 5.31 (0.0001% MB) respectively. Extending the LED irradiation time from 2 to 5 and 10 minutes did not change these results. In contrast approaches of BCoV-infected cells in the dark, treated with 0.001% and 0.0001% MB sensitizer alone, a lot of residual viruses could be detected after 10 minutes of incubation (RF 0.9 and RF 1.23 for 0.001% MB and 0.0001% MB respectively) In our SARS-CoV-2 experiments with VERO E6 infected cells the irradiation time was reduced to 1, 2 and 3 minutes for both concentrations with increasing broadband LED radiation intensity from 20 to 50 and 100.000 lx. (RF 4.67 for 0.001% and 0.0001% respectively). This showed a minimum concentration of 0.0001%MB and a minimum radiation intensity of 20,000 lx leads to a 99.99% reduction of intracellular and extracellular viruses after one minute exposure.
Keywords: Phenothiazine dye in aPDT, Methylene blue, PDI (photodynamic inactivation), aPDT (antimicrobial photodynamic therapy), SARS-CoV-2 inactivation
1. Introduction
The Covid-19 pandemic crisis is a major challenge for national health systems, the world economy and social happiness. The existing vaccines protect largely against severe disease progression but their capacity to stimulate protective immunity against infection of the mucous membranes of the nose and throat area remains unknown. In this region the infection often starts by binding of the virus to ACE2 receptors and the upper respiratory tract is the location where the virus multiplies first [1]. The fundamental risk of infection cannot be ruled out with certainty, after surviving a Covid-19 infection and even complete vaccination protection does not confer sterilizing immunity. Recently there have been reports of post vaccination infections [2], [3], [4]. This is now becoming apparent in some countries in June 2021,where despite high vaccination rates, the incidences for variants B.1.617.2 and B.1.617.2.1 continue to rise. A lack of mucosal immunity (sterile immunity) can thus, as a retract for the virus, contribute the spread of the virus also by fully vaccinated people and the mucous membranes of the nasopharynx can thus contribute to the development of further mutations.
With the exception of vaccines, there are currently (June 2021) no drugs with monocausal effect but only with an adjuvant effect against the different variants of SARS-CoV2 viruses, depending on phase of infection, available e.g. Remdesivir [5], Lopinavir, Ritonavir, Chloroquine, Ribavirin [6] and many antiviral drugs seemed to be ineffective to stop disease progression [7,8].
Because the infection of Covid-19 often begins in the nasal, hypo and oropharynx, it should be possible to reduce the viral load locally in this region. The extent of viral load has been shown as a predictor of disease severity and progression [9]. Significant reduction of the viral load in this area, however, could both improve the prognosis of the course of the disease for those affected, and prevents the infection from spreading hereby reducing the risk of virus mutations [10].
A promising and inexpensive solution to this is known as antimicrobial photodynamic inactivation (PDI) as an alternative method for inactivation of viruses. The advantage of this method is that it is unlikely to cause resistance and is applicable for a great variety of viruses [11,12].
PDI requires the combined action of three elements: a light source, a special dye (photosensitizer (PS)) and free oxygen molecules. The mechanism depends on concentrations of molecular oxygen, photosensitizer and light properties used.
Key-element in this process is oxygen molecules in the first two excited singlet states, dissolved in a solvent (e. g. water). Despite oxygen in ground state, these excited molecules are highly reactive, thus being quickly reduced by organic molecules also dissolved in the solvent. Due to quantum mechanical selection rules, oxygen cannot be excited directly by electro-magnetic radiation, the transition is spin-forbidden and there is also an interdiction caused by parity of the electronic wave-functions [53]. These restrictions require an alternate way for producing singlet oxygen. A well-established method is the photo-activation of a sensitizer with subsequent energy transfer to the oxygen molecules [54].
Methylene blue (MB), a broad band absorbing dye, with energy bands located above or matching the sharp energy levels of oxygen, is a commonly used substance for this purpose [54]. If photon energy (wavelength) of the light irradiated fits into the transitions between ground state and the upper bands of the dye, free valence electrons of the dye undergo a transition to higher energy levels, (singlet 0 -singlet 1-triplett level).
The excitation energy will be transferred to the dissolved oxygen molecules, mostly by collisions, but also multipolar interactions are imaginable. This method of activating energy levels is a common procedure in several technical applications, e.g. the introduction of Ho³+ solid-state lasers in the mid-eighties of the last century by Huber et al. [55]. The oxygen is then reduced by organic molecules dissolved in water, and radicals are created for a short time and locally limited (reactive oxygen species (ROS) like hydroxyl‑ perhydroxyl-radicals, superoxide anions (OH-, HOH, O2-,) and singlet oxygen (1O2)). These ROS can promote the damage of virus targets. This happens when the dye binds to vital viral components (lipid envelope (where present) and core proteins, membrane lipids or nucleic acids) [12], [13], [14], [15].
The destruction of the virus targets due to energy transfer from dye to oxygen and resulting ROS is in this case a purely physical induced and not a pharmacological process. Neither viruses nor bacteria are able to defend themselves against this physical attack or develop resistance.
Since its discovery at the end of the 19th century methylene blue (MB) has made medical history and is still used successfully in numerous fields of medicine today. Depending on the type of application and dosage, the dye can act either physically as medical product or pharmacologically as a drug.
The dye is used for example systemic for the treatment of methaemoglobinemia [16], septic shock [17], as an antimalarial agent [18], as a neuroprotective drug [19], in local application in colonoscopy [20], for visualization of organ structures during surgery, for antimicrobial photodynamic therapy in dentistry [21] and for photo-deactivation of viruses in human fresh plasma [22,23].
With 1 micro molar concentration of methylene blue and one hour visible light irradiation MB succeeds for example to inactivate enveloped viruses like HIV1, HIV2, HBV, HBC, HPV, and Sindbis- and West Nile- viruses in 300 ml fresh human plasma. Latest studies also show the possibility of inactivation acute respiratory syndrome Coronavirus as well as the possibility to inactivate SARS-CoV2 viruses in human plasma [24,25].
Already 88 years ago Perdau et al. [26] demonstrated the broad spectrum effectiveness of methylene blue (MB) based photodynamic therapy in veterinary medicine on different virus strains e.g. vaccinia, herpes, foul plague, louping III, borna disease, and canine distemper viruses. The MB PD inactivated viruses, retained their antigenicity (immunogenicity) and reduced their infectivity. So the Perdau group used the MB PD inactivated viruses for the production of an effective vaccine [27]. T. Dempsey et al. [28] showed in 1934 already the vaccination with methylene blue PDI based vaccine led to protective antibody formation without an infection of the treated animals, and Galloway et al. [29] demonstrated the antigenic value of MB based PDI on fixed rabies viruses.
30 years later similar observations were made by S. Thurner et al. [30], by inducing antigenicity after methylene blue PDI of vaccinia viruses. They also could observe a pH depending dye binding to the virus and its strong affinity to nucleic acids. Methylene blue binds to nucleotides, polynucleotides and nucleic acids in aqueous solution [31], [32], [33]. It intercalates with guanine bases of nucleic acids and is able to induce strand breaks in the viral RNA/DNA after light activation. MB PDI generates reactive oxygen species (ROS) that cause the destruction of guanine residues thus preventing viral replication [34]. MB also can induce e.g. protein cross link of envelope glycoproteins forming spikes of the virus to enter into the host cell. [35] and viral proteins and genome were disrupted after MB PDI with simultaneous loss of infectivity of the virus [36].
These observations maybe of great importance again today related to Covid-19 infections. The local photodynamic destruction of the virus’ envelope and RNA could not only hamper virus replication but theoretically trigger immunological effects at cellular level by photodynamically induced "unmasking" of the virus.
In our study we investigated in vitro whether a low dose aqueous solution of the organic dye methylene blue (0.0001% and 0.001%) can be used to inactivate intracellular Corona viruses of different strains (BCoV and SARS-CoV-2 wildtype) through excitation with different light sources in order to eventually figure out e.g. new therapy options for local photodynamic reduction of Corona and SARS-CoV-2 virus load in naso, hypo and oropharynx.
Depending on dye concentration, pH level and solvent [33], methylene blue shows a broadband absorption spectrum which extends from UV over the visible range to the near infrared region with two absorption maxima at 250 nm and 664 nm.
Thus the photosensitizer activation of methylene blue can be achieved with a variety of light sources e.g. non coherent (xenon arc, metal halide. quartz halogen, phosphor sodium lamps, LED lamps and even incandescent lamps) or coherent light sources with a variety of lasers.
Most PDI systems stimulate dyes in the resonance, i.e. in their absorption maximum. However the high absorption coefficient of the dye at this wavelength leads according to Lambert-Beer's law to a small penetration-depth, which means the optical energy is absorbed only in the upper layers of the dye solution. As a consequence the dye has to be rinsed off after superficial application, in order to get light to its target chromophores (close to bacteria or virus envelope). This additional dilution effect now reduces the number of possibly excited dye molecules, and so the transfer rate from the dye molecules to oxygen and of course the amount of ROS. Trying to compensate this effect at high absorption cross-sections by increasing the light output can lead to photo-bleaching of the dye [37].
To avoid this, we used different beam sources away from the MB resonance wavelength (broadband white LED, 590 nm LED, and 810 nm laser light (Fig. 1 )). In our experiments MB was chemically stable in the presence of all employed light sources and irradiation conditions we used. Main goal was to find out which beam source is most effective, inexpensive and best suited for this purpose, regarding a possible clinical application at a later date. For the broadband NiMh powered LED lamp we constructed a special voltage regulation to maintain a constant light flux over the lifetime of the battery.
Fig. 1.
Loading of a 48-well plate with U373 cells for irradiation.
In our study we tested the basic effectiveness and efficacy of an antiviral photodynamic treatment to inactivate different Corona strains (BCoV and SARS-CoV-2 wildtype) with methylene blue based PDI under different excitation wavelengths, dye concentrations, power densities and doses. The aim was to find out, which of the used parameter sets could be most suitable for a later clinical use. Further we wanted to find out whether the method is insensitive to possible Corona virus mutations and shows a possible broadband effect against different Corona strains. Our first attempts with bovine corona viruses served also as substitutes for human corona viruses. The results of the BCoV tests were used to optimize possible radiation sources and dye concentrations for the SARS-CoV-2 testing.
2. Material and methods
2.1. Virus and cells: BCoV study
The BCoV strain L9 was obtained by Dr. G. Zimmer, Institute of Virology at the School of Veterinary Medicine Hannover (Tierärzliche Hochschule.DE 30559 Hannover)
The U373 cells (passage 14) were as well obtained by Dr. G. Zimmer, Institute of Virology at the School of Veterinary Medicine Hannover.
The cells were inspected regularly for morphological alterations and for contamination by mycoplasmas. No morphological alterations of cells and no contamination by mycoplasmas could be detected.
2.2. Virus and cells: SARS-CoV-2 study
The SARS-CoV-2/Germany strain was derived from a patient isolate.
(HCoV-19/Germany/BY-Bochum-1/20) (B.1.1.70) (GISAID accession ID: EPI_ISL_1118929).
The Vero E6 cells were obtained from university Bern, Switzerland.
The cells were inspected regularly for morphological alterations and for contamination by mycoplasmas. No morphological alterations of cells and no contamination by mycoplasmas could be detected.
SARS-CoV-2 experiments were conducted in a biosafety level 3 laboratory after obtaining all certificates and permissions required for SARS-CoV-2 studies.
2.3. Methods BCoV study
To analyze the efficacy of the PDI with different light sources to inactivate the bovine coronavirus.
U373 cells were cultivated in 48-well plates and infected with BCoV before irradiation treatment. The following short flowchart provides an overview of the process: (Test procedure accredited to DIN EN ISO/ IEC 17025).
(Sub) culturing of U373 cells in 48-well plates.
► 150 µl cell suspension + 500 µl medium (EMEM 10% FKS).
(Day 1) ↓.
►Infection of the U373 cells with 200 µl of a virus-medium-mixture.
(Day 4) ↓.
►Pre-treatment and irradiation of the BCoV-infected cells.
(Day 5).
2.4. Preparation of test virus suspension BCoV
For preparation of test virus suspension, U373 cells were cultivated in 175 cm2 flask with in EMEM supplemented with l-glutamine, non-essential amino acids and sodium pyruvate an 10% fetal calf serum. Before virus infection, cells were washed two times with phosphate buffered saline (PBS), incubated for 3 h with EMEM without FCS and were washed once with EMEM supplemented with trypsin. For virus production, BCoV strain L9 was added to the prepared monolayer. After an incubation period of 24 to 48 h (cells showed a constant cytopathic effect), cells were lysed be a rapid freeze/thaw cycle. Cellular debris was removed by low speed centrifugation. After aliquotation of the supernatant, test virus suspension was stored at −80 °C.
2.5. Preparation of U373 cells for irradiation treatment
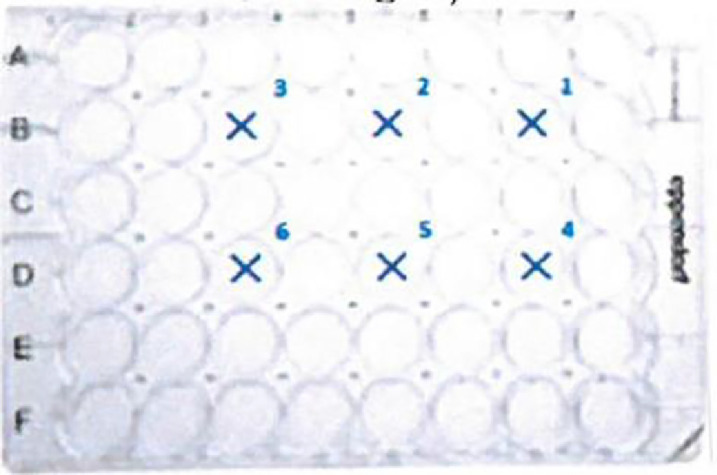
U373 cells of a 175 cm2 cell culture flask were detached enzymatically with Trypsin-EDTA solution and taken up in a total of 60 ml of EMEM medium with 10% fetal calf serum.150 µl each of this cell suspension were transferred into maximum six wells of a 48-well plate with a final volume of 650 µl (filled up with 500 µl EMEM 10% FCS (see Fig. 1).
48 well plates were selected, because the diameter of one well corresponds exactly to the diameter of the laser cone (light cone laser: approx.1 cm diameter; well of 48-well plate 1.04 cm), which ensures that the cells are treated evenly throughout the entire well during radiation. The beam-diameter of the other two non-coherent light sources was larger and therefore not a problem.
After three days of cultivation at 37 °C and 5% CO2, cells were washed two times with EMEM without FCS (2 × 200 µl per well) and incubated for further 3 h at 37 °C an 5% CO2. For virus infection, medium was removed from the individual wells and replaced by 200 µl virus medium mixture (500 µl BCoV virus suspension were mixed with 30 ml EMEM without FCS/with penicillin/streptomycin, and trypsin).
After an incubation period of 20–24 h BCoV-infected cells were used for irradiation treatment (see Table 1 ).
Table 1.
Overview of the selected conditions during the photodynamic treatments of BCoV-infected cells *illuminance: 10,000 lx light green: after addition of sensitizer plate was immediately wrapped with aluminum foil.
| BCoV infected U373 cells | Conc. Of sensitizer in 200 µl/well [%] | Irradiation time with the laser for 100 joule/cm² [min] | Irradiation time with the 590 nm diodes LED [min]* | Irradiation time with the flash-light LED [min]* | Incubation time in the dark [min] |
|---|---|---|---|---|---|
| Non treated (virus control) | – | – | – | – | – |
| treated | 0.001 | 5.46 | – | – | – |
| treated | 0.0001 | 5.46 | – | – | – |
| treated | 0.001 | – | 10 | – | – |
| treated | 0.001 | – | 5 | – | – |
| treated | 0.001 | – | 2 | – | – |
| treated | 0.0001 | – | 10 | – | – |
| treated | 0.0001 | – | 5 | – | – |
| treated | 0.0001 | – | 2 | – | – |
| treated | 0.001 | – | – | 10 | – |
| treated | 0.001 | – | – | 5 | – |
| treated | 0.001 | – | – | 2 | – |
| treated | 0.0001 | – | – | 10 | – |
| treated | 0.0001 | – | – | 5 | – |
| treated | 0.0001 | – | – | 2 | – |
| treated | 0,001 | – | – | – | 10 |
| treated | 0,0001 | – | – | – | 10 |
2.6. Preparation of sensitizer MB
The Methylene blue was used in aqueous solution in the following concentrations:
-
a)
0.001% (end concentration in 200 µl/well).
-
b)
0.0001% (end concentration in 200 µl/well).
For 0.001% methylene blue concentration (1:100 diluted) 18.68 µl sensitizer was added to 181.31 µl medium per well. For the further concentration of 0.0001% MB sensitizer was diluted 1:1000 in Aqua dest. and 18.68 µl of the dilution were added to 181.31 µl medium.
2.7. Manual PDI irradiation procedure with the different light sources
For photodynamic inactivation of the bovine coronavirus (BCoV), first the appropriately diluted methylene blue (18.69 µl per well) was added to the BCoV-infected cells. Right after, irradiation with Gigaa optronics 810 nm diode laser was performed in the following with an light dose of 100 joule/cm2 for 5.46 min under constant power of 0,3 W/cm2. Each well was treated separately with a maximum of six wells per plate (see Fig. 2 b). Additional, irradiation procedure was performed with the alternative 589 nm LEDs and the conventional flashlight LED in the same manner as the laser (see Fig. 2c-f).
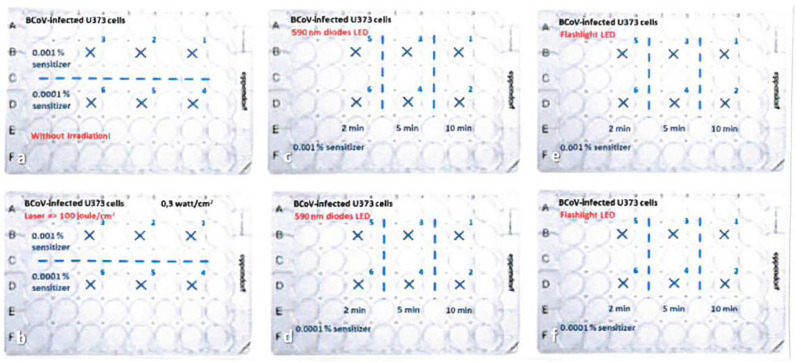
Fig. 2.
Schematic representation of the individual approaches per plate for the treatment of the BCoV-infected cells Treatment of the cells with sensitizer only (a), photodynamic treatment with laser (b), 589 nm LEDs (c, d) and flashlight (e, f).
After treatment of the respective last well of a culture plate, the entire plate was immediately stored and frozen at −80 °C (Fig. 3, Fig. 4, Fig. 5 ).
Fig. 3.
Determination of infectivity (virus recovery per well of a 48 well plate) from infected and treated U373-cells using the end point dilution process. Meaning, each virus suspension was immediately diluted, and dilutions were transferred to the permissive cells, respectively.
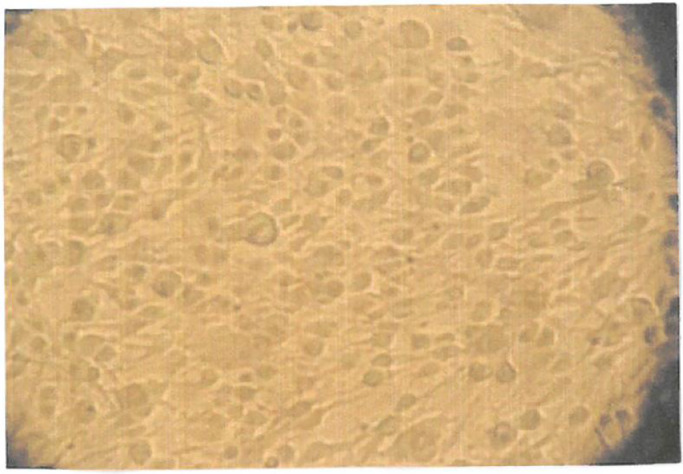
Fig. 4.
Shows the intracellular cytopathic effect of BCoV infected cells under the light microscope.
Fig. 5.
Shows the results after PDI treatment and recultivation. There is no more reinfection visible after 2 min irradiation with the flashlight LED and 0.001% MB sensitizer.
2.8. Recovery of the residual virus and determination of infectivity
For recovery of residual virus from the infected and treated cells, plates were subjected for a freeze/thawing procedure. This was followed by mixing of cell suspension in each well by pipetting up and down 15 times to re-suspend the virus. After that, series of ten-fold dilutions of the suspensions took place in ice-cold maintenance medium, respectively. Finally, 100 µl of each dilution were placed in eight wells of a sterile polystyrene flat-bottomed plate with a preformed U373 monolayer (see Fig. 6 ).
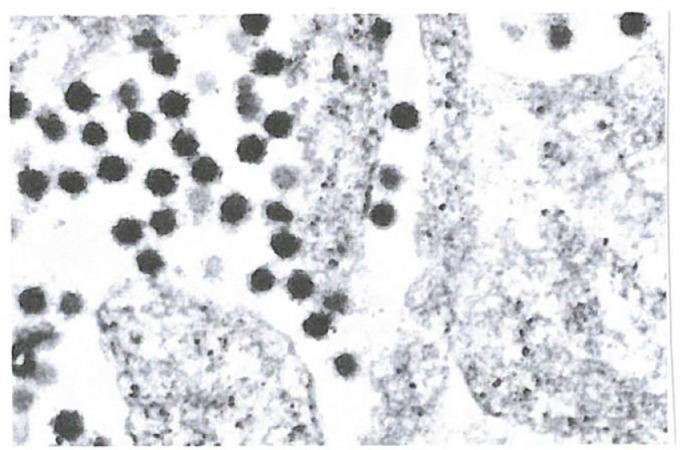
Fig. 6.
shows an electron microscope picture of our experiments with BCoV infected cells. Interestingly we could not see clear morphological changes before and after irradiation. But we fixed the samples immediately after irradiation. We suppose now, that it takes a few minutes for morphological changes to become visible under the electron microscope. (August 04.2020, University Ulm, Head of Z.E. Prof. Dr. Paul Walther).
Before addition of the dilutions, cells were washed twice with EMEM and incubated for 3 h with 100 µl EMEM with trypsin. The cells were incubated at 37 °C in a CO2-atmosphere (5.0% CO2-content).
After six days of incubation, cultures were observed for cytopathic effects. The infectious dose (TCID50) was calculated according to the method of Spearman [1] and Kälber [2].
2.9. Controls
2.9.1. Virus control (VC)
Virus recovery was performed from non-treated BCoV-infected U373-cells (no sensitizer and no irradiation) as described under 2.5. The mean virus titer was used as reference for calculation of the reduction factor (Fig. 7 ).
Fig. 7.

Picture of the flashlight by Ledlenser GmbH & Co KG Solingen, Germany Model Ledlenser P6, modified with voltage control, max 200 lm.
2.9.2. Treatment with MB- sensitizer (without radiation)
Another virus recovery was performed from infected U373-cells, treated with the MB sensitizer only. Thereby the culture plate was wrapped up with aluminum foil immediately after addition of the dye (without irradiation or light exposing), stored for 10 min in the dark and frozen until titration of the residual virus, as described in 2.5.
2.9.3. Cell culture control
Furthermore, a cell control (only addition of medium) was incorporated.
2.10. Calculation of effectiveness
The virucidal effectiveness of the MB PDI and the photodynamic inactivation properties using the non-coherent light sources was evaluated by calculating the decrease in titer of the treated and radiated culture in comparison with the control titration of the approaches without treatment (VC). The difference is given as reduction factor (RF).
Based on standard EN 14476, [40]a system is having a virucidal efficacy if the titer is reduced at least by 4 log10 steps [3]. This corresponds to an inactivation of ≥ 99.99%.
3. Results
The effectiveness of the different photodynamic systems was determined after the irradiation treatment of two to three BCoV infected U373 cell cultures of a 48-well plate (corresponds to two to three wells) per concentration of the sensitizer, respectively. Results of examination are shown in Table 2 in the appendix.
Table 2.
Photodynamic inactivation of the bovine coronavirus (BCoV with MB-sensitizer under different irradiation conditions.
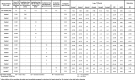 |
3.1. Diode laser 810 nm
With methylene blue and subsequent continuous wave diode laser irradiation sequences with constant power density (0.3 watt/cm2) and a light dose of 100 joule/cm2, no or only minimal amount of residual virus was found with 0.001% or 0.0001% concentrations of the dye. The mean reduction factor (RF) was ≥ 5.31 (0.001% sensitizer) and ≥ 5.19 (0.0001% sensitizer) respectively, which corresponds to an inactivation of the BCoV of 99.999%. See Table 2.
3.2. 590 nm Diode LED
After addition of 0.001% MB sensitizer to the infected cells and subsequent irradiation with 10,000 lx of the 590 nm LEDs no virus could be detected after 5 and 10 min exposition. A minimum amount of residual virus could still be detected in one approach after two minutes of exposition. The mean RF was ≥ 5.29 (irradiation time of two minutes) and ≥ 5.35 (irradiation for 5 and 10 min), respectively.
Similar results could be detected with the 590 nm LEDs and 0.0001% of the MB sensitizer.
The reduction factor varied from ≥ 5.17 after 2 and 5 min to ≥ 5.35 after 10 min. See Table 2.
3.3. Flashlight LED
When using flashlight LED and 0.001% of the dye no residual virus was found after an irradiation with 10,000 lx after 2, 5 and 10 min of exposition, which corresponds to a RF ≥ 5.35, respectively. After addition of 0.0001% of the sensitizer residual virus could be detected in all approaches. Nevertheless, mean reduction factors between 4.6 and 4.79 could be achieved Table 2.
In contrast, approaches of BCoV-infected cells, that were treated with 0.001% or 0.0001% of MB alone (without irradiation) and immediately wrapped in aluminum foil after addition of the sensitizer, a lot of residual virus could be detected after 10 min of incubation in the dark.(RF= 0,9 with 0.0001% sensitizer; RF=1.23 with 0.001% Table 2.
A methylene blue based photodynamic irradiation treatment with an 810 nm diode laser or alternative simple light sources was able to inactivate bovine coronavirus under the defined test conditions as follows:
All light sources used in this study (diode laser 810 nm, power density 0,3 W/cm2 CW, 598 nm LED, and conventional flashlight, both with a minimal illuminance of 10,000 lux) have proven suitable under the defined condition for the antiviral photodynamic treatment of BCoV infected cells. Based on our results on BCoV viruses, we used a conventional flashlight to activate the dye for the irradiation of SARS-CoV-2 viruses, in particular to test a simple and inexpensive beam source regarding a later clinical application. To achieve defined and reproducible radiation conditions (constant light output) the lamp was modified with a special voltage control circuitry including an acoustic signal if the battery is getting weak (Table 3 ).
Table 3.
Defined test conditions for inactivation bovine coronavirus.
| BCoV infected U373 cells | Conc. Of sensitizer in 200 µl/well [%] | Irradiation time with the laser for 100 joule/cm² [min] | Irradiation time with the 590 nm diodes LED [min]* | Irradiation time with the flash-light LED [min]* |
|---|---|---|---|---|
| treated | 0.001 | 5.46 | – | – |
| treated | 0.0001 | 5.46 | – | – |
| treated | 0.001 | – | 10 | – |
| treated | 0.001 | – | 5 | – |
| treated | 0.001 | – | 2 | – |
| treated | 0.0001 | – | 10 | – |
| treated | 0.0001 | – | 5 | – |
| treated | 0.0001 | – | 2 | – |
| treated | 0.001 | – | – | 10 |
| treated | 0.001 | – | – | 5 |
| treated | 0.001 | – | – | 2 |
| treated | 0.0001 | – | – | 10 |
| treated | 0.0001 | – | – | 5 |
| treated | 0.0001 | – | – | 2 |
| treated | 0,001 | – | – | – |
| treated | 0,0001 | – | – | – |
4. Methods SARS-CoV-2 study
To analyze the efficacy of MB based PD to inactivate the humane coronavirus, Vero E6 cells were cultivated in 24-well plates and infected with SARS-CoV-2 before irradiation treatment. The following short flowchart provides an overview of the process (test procedure accredited according to DIN EN ISO/IEC 17025).
►(Sub) culturing of Vero cells in 24-well plates.
1 × 105 cells /well-1 ml medium/well-1well/plate.
(Day 1) ↓.
► Infection of the Vero cells with SARS-CoV-2 (MOI 3).
500 µl virus suspension/well for 1 h-wash with PBS (1x) add 1 ml medium/well.
(Day 2) ↓.
► Pre-treatment/ irradiation of SARS-CoV-2-infected cells:
Pre-treatment with 0.001% or 0.0001% Sensitizer and irradiation for 1, 2, and 3 min.
(Immediately after treatment plates were wrapped in aluminum foil).
Harvest: Remove supernatant, wash 1x with PBS; add 500 µl PBS and freeze.
(Day 3) ↓.
Virus recovery (3x freeze/thaw procedure) and virus titration.
4.1. Preparation of test virus suspension
For virus production, 2 × 106 Vero E6 cells were cultivated in a 75 cm2 flask in DMEM supplemented with 1% l-Glut, NEAAs, and P/S and 10% FBS. One day after seeding, medium was changed to 10 ml fresh DMEM inoculated with 100 µl of SARS-CoV-2/Germany virus suspension. The supernatant was harvested after 3 days at 37 °C by centrifugation at 1500 rpm for 5 min to remove cell debris. The supernatant was aliquoted and stored at −80 °C. Viral titers were determined by plaque assay and endpoint dilution.
4.2. Preparation of Vero cells for irradiation treatment
Vero E6 cells of a cell culture flask were detached enzymatically with Trypsin-EDTA solution.1 × 105 cells were transferred into one well (B3) of a 24-well plate with final volume of 1000 µl cell culture medium (see Fig. 8 ).
Fig. 8.
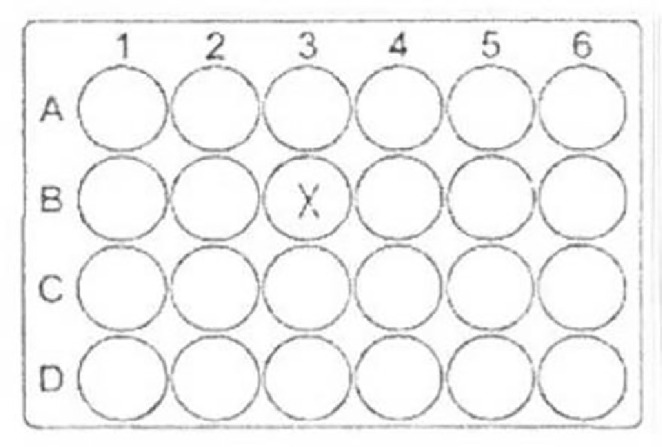
Loading of a 24-well plate with the Vero E6 cells for irradiation treatment.
After one day of cultivation at 37 °C and 5% CO2 medium was removed from the individual wells and cells were infected with SARS-CoV2 (500 µl virus suspension per well 2 × 200 µl per well: MOI 3).
After 1 h of incubation at 37 °C, inoculum was removed; cells were washed once with PBS and cultivated in 1 ml culture medium for further 20 to 24 h at 37 °C and 5% CO2. After that, SARS-CoV-2 infected cells were used for irradiation treatment (see 4.4).
4.3. Preparation of MB-sensitizer
The methylene blue was used in aqueous solution in the following concentrations:
-
a)
0.001% (end concentration in 1000 µl/well).
-
b)
0.0001% (end concentration in 1000 µl/well).
For the 0.001% methylene blue concentration 1 µl 1% MB was added to 1000 µl medium per well (plate swayed). For the further concentration of 0.0001% the MB sensitizer was diluted 1:10 in Aqua dest. and 1 µl of the dilution were added to 1000 µl medium.
4.4. Manual irradiation procedure with different intensities
For photodynamic inactivation of the humane coronavirus (SARS-CoV-2), first the appropriately diluted MB sensitizer (1 µl per well) was added to the SARS-CoV-2-infected cells. Right after, irradiation with the Ledlenser P6 was performed with intensities of 20,000 lx, 50,000 lx and 100,000 lx. The single well of each plate was treated separately.
We also evaluated the effect of daylight (light of the clean bench with ≈ 1500 lx).
Table 4 gives an overview of the selected conditions during the respective photodynamic treatment of SARS-CoV-2 infected cells and the individual approaches.
Table 4.
Overview of selected conditions of SARS-CoV-2 treatment approaches.
| SRS-CoV-2 infected Vero E6 cells | Conc. of sensitizer in 1000 µl/well [%] | Irradiation time with daylight* and about 1500 lx [min] | Irradiation time with the flashlight LED and 20,000 lx [min] | Irradiation time with the flashlight LED and 50,000 lx [min] | Irradiation time with the flashlight LED and 100,000 lx [min] |
|---|---|---|---|---|---|
| non treated (virus control) | – | – | – | – | – |
| treated (Tox 2) | 0.001 | – | – | – | – |
| treated (Tox 1) | 0.0001 | – | – | – | – |
| treated | 0.001 | 1 | 1 | 1 | 1 |
| treated | 0.001 | 2 | 2 | 2 | 2 |
| treated | 0.001 | 3 | 3 | 3 | 3 |
| treated | 0.0001 | – | 1 | – | – |
| treated | 0.0001 | – | 2 | – | – |
| treated | 0.0001 | – | 3 | – | – |
* Daylight = light of the clean bench (MaxiSafe 2030, ThermoFisher Scientific => ∼ 1500 lx).
After treatment, the entire plate was immediately wrapped with aluminum foil for max 1 min.
Afterwards, supernatant was removed, cells were washed once with 1000 µl PBS, overlaid with 500 µl PBS and stored at −80 °C.
4.5. Recovery of the residual virus and determination of infectivity
For recovery of residual virus from the infected and treated cells, plates were subjected to three freeze/thawing cycles. This was followed by mixing of cell suspension in each well by pipetting up and down 15 times to re-suspend the virus. After that, 22 µl of the virus-disinfectant solution was immediately added to the first row of Vero E6 cells (seeded at 1 × 104 cells/well in a 96 well plate one day prior the examination), followed by a serial endpoint dilution titration. After 3 days of incubation at 37 °C in a CO2-atmosphere (5% CO2-content) cultures were observed for cytopathic effects by crystal violet staining. The infectious dose (log10 TCID50/ml) was calculated according to the method of Spearman [38] and Kärber [39].
4.6. Controls
4.6.1. Virus control (VC)
Virus recovery was performed from non-treated SARS-CoV-2-infected Vero E6 cells (no sensitizer and no irradiation) as described under 2.5. The mean virus titer was used as reference for calculation of the reduction factor.
4.6.2. Treatment with methylene blue sensitizer (without irradiation)
Another virus recovery was performed from infected Vero E6 cells treated with the methylene blue sensitizer only for 3 min under the clean bench (light was off). After treatment, the culture plate was wrapped up with aluminum (without irradiation or light exposing), stored for a maximum of 1 min in the dark before harvest (remove supernatant, washing with PBS (1x), adding 500 µl PBS and frozen until virus recovery and titration and titration of the residual virus), as described in 2.5.
4.6.3. Treatment with methylene blue sensitizer and daylight
Infected Vero E6 cells were treated with the methylene blue sensitizer and incubated with daylight under the clean bench (Maxisafe 2030, ThermoFisher Scientific, light with about 1500lx) for 1, 2 or 3 min (each approach was performed once) Subsequently, harvest was performed as described in 2.5.
4.6.4. Cell culture control
Furthermore, a cell control (only addition of medium) was incorporated.
4.7. Calculation of effectiveness
The virucidal effectiveness of the MB based PDI System and the photodynamic inactivation properties with the Ledlenser P6 lamp was evaluated by calculating the decrease in titer of the treated and radiated culture compared to the control titration of the approaches without treatment (VC) The difference is given as reduction factor (RF).
5. Results
With methylene blue sensitizer and Ledlenser P6 irradiation no residual virus was found with 0.001% or 0.0001% dye concentrations and exposition to LED light after just 1 min of incubation, even with an irradiation of 20,000 lx and an average initial virus titer of 6.92 log10 TCID50/ml. The mean reduction factor (RF) was found ≥ 4.67 for 0.001% and 0.0001% of methylene blue sensitizer which corresponds to an inactivation of SARS-CoV-2 of 99.99%.
SARS-CoV-2-infected cells, treated with sensitizer concentrations of 0.001% or 0.0001% exposed to environment without irradiation for 3 min showed no (0.001%) or few residual viruses (0.0001%) (RF= ≥ 4.67 with 0.001% sensitizer, RF= 2.5 with 0.0001% sensitizer).
"Daylight" exposure (light of the clean bench) with about 1500 lx instead LED exposure and 0.001% sensitizer, resulted in a small amount of residual virus after 1 min of irradiation and no residual virus after 2 and 3 min of incubation. Because of the low initial virus titer of 4.03 log10 TCID50/ml in the test, a maximum RF of ≥ 1.83 was found after 2 min (Table 5 ).
Table 5.
Photodynamic inactivation of humane coronavirus (SARS-CoV-2) with MB sensitizer under different irradiation conditions.
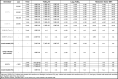 |
6. Conclusion
An irradiation treatment with methylene blue based PD treatment is able to inactivate humane coronavirus SARS-CoV-2 under the following defined test conditions:
Minimal concentration of methylene blue sensitizer 0.0001%.
Minimal illuminance of the flashlight 20,000 lx.
Minimal irradiation time 1 min.
It is remarkable, that similar reduction could be achieved with 0.001% sensitizer concentration alone after 3 min of incubation. Further, a significant reduction could already be detected with 0.001% concentration in combination with 'daylight' irradiation after an incubation time of 2 min.
7. Discussion
Our results show that all radiation sources and wavelengths used (590 nm LED, broadband LED, and 810 nm laser light) are suitable for the methylene blue based photodynamic inactivation of intracellular and extracellular corona viruses. Both intracellular and extracellular viruses were detected by three freezing and thawing steps and mixing of cell suspension to assess complete inactivation of the virus. For a potential local clinical application reducing virus load (e.g. naso -hypo-oropharynx) the use of a LED lamp with a wide emission spectrum seems to be most suitable. These lamps are widely used worldwide, cost effective and easy to use by non-medical personnel. Special training and protective measures for laser applications are no longer required. The lamps can also be used in countries with limited medical infrastructure. Non coherent short waved light sources (LED lamps) have more divergent beam properties in comparison to laser light, making them unsuitable for treating deeper tissues [41], but in case of superficial viral infections in the mucous membrane the small light penetration into biological tissue does not play an important role. In addition the irradiation times can be significantly shortened in comparison to laser irradiation.
Methylene blue, a thiazine dye is widely used as topically photo sensitizer not only for destruction of bacteria (e.g. in dentistry) but also to treat superficial lesions of mucous membranes (e.g. leukoplakia) in the field of dentistry and ENT [42]. MB it is water soluble and cannot pass easily through biological membranes, but dysplastic cell formations show a high affinity for the dye [43]. That is, why MB can be used for the early detection of potentially malignant lesions of oral mucosa and in contrast to other thiazine dyes, methylene blue shows very low toxicity to healthy human cells, one reason that it is suitable for vital staining [44]. (In contrast to toluidine blue, MB shows e.g. no toxicity to fibroblasts.) As already mentioned in the introduction MB was chemically stable in the presence of all employed light sources and irradiation conditions we used. Additionally, the dye shows an apparently high affinity to 'changed' (infected) cells in our experiments. The observed rapid penetration of the hydrophilic dye methylene blue into the infected cells could be an indication of a changed pH value of infected compared to uninfected cells, a fact that should be further investigated. (The irradiation took place immediately after addition of the sensitizer to the infected cells, approx. after 30 s = "drug to light").
In our experiments we used the dye methylene blue at a concentration of 0.001% = 31 µM. and 0.0001% = 3.1 µM. respectively. It is important to mention, that such low concentrations in the micromolar range do not indicate cytotoxicity, genotoxicity or carcinogenic effects on healthy body cells [36].
Angelika Rück et al. [45] investigated 1997 the dark and photo-toxicity of methylene blue in the 1 µM range on BKEz-7 endothelial cells. Even after 24 h of incubation in a 1 µM methylene blue solution no cell damage could be found in the dark and an irradiation dose in resonance (662 nm laser light) up to 30 J/cm2 induced no photo-toxicity. But it is important to know, that methylene blue can induce cytotoxicity in human brain tumor cells (U373 human astrocytoma cells in a dose dependent manner) [46]. We were also able to observe this effect of dose-dependent cytotoxicity of methylene blue in our experiments on BCoV infected U373 cells. In some experiments of the tissue culture infectious dose 50 tests, we observed a cytotoxic effect of methylene blue on the culture medium (U373 cells) in the first series of the end point dilution process. Afterwards no more cytopathic effect could be evaluated. But the cytotoxic effect usually appears later than the cytopathic effect of the virus infection (visible under a light microscope). Interestingly, before the cytotoxic effect occurred, no infection was found in the first dilution stage, while single viruses were still detectable in the second dilution stage. Further studies should clarify whether these observations contain any form of scientific evidence. It would be conceivable e.g. that the dye in certain concentrations protects the cell from infection of the virus.
Hideki Abe et al. [47] described for example, a MB based decline in infectivity of vesicular stomatitis virus (VSV) and an inhibited fusion of the virus envelope to Vero cells.
Bojadzic et al. showed in 2020 [48] that methylene blue inhibits in vitro the SARS-CoV-2 spike-ACE2 protein-protein interaction. This is a mechanism that can contribute to its antiviral activity without irradiation, which could confirm our observation in the first dilution stage of the TCID50 tests. In a possible clinical application of the dye, this could be an addition to the intracellular photodynamic virus inactivation - an effect usable for local infection prophylaxis after application of MB on the mucous membranes of naso-, oro-, and hypopharynx.
Recent studies have shown that methylene blue develops a 50% cytotoxic concentration on Vero E6 cells when the concentration exceeds 100 µM. This was no problem in our SARS-CoV-2 experiments because the highest MB dye concentration we used was only 31 µM [49].
G.S. Thurner et al. [30] demonstrated in their experiments on vaccinia viruses that a concentration of 0.001% (30 µM) MB and irradiation with a 40 W Philips Photolita incandescent lamp leads to complete photodynamic inactivation of the viruses after 6 min. They also showed the influence of pH level on rate of photo inactivation. The rate of inactivation could almost be tripled in a strongly basic environment (pH5 vs. pH9). In these experiments they showed that the viruses lose their infectivity but not their antigenicity through photodynamic therapy. Therefore, these photo dynamically inactivated viruses could be used to develop an effective vaccine for rabbits against vaccinia infection.
In our SARS-CoV-2 experiments we reduced the viral load of intracellular viruses with 0.0001% MB (3.1 µM) in combination with 20,000 lx broadband LED beam by 99.99% after 1 min exposure.
Higher dye concentration of 0.001% (31 µM) instead of 0.0001% (3.1 µM) with the same beam strength of 20,000 lx did not affect the reduction of viral load (99.99%). The same applies to the use of higher beam strengths 50,000 lx and 100,000 lx respectively and/or doubling or tripling the exposure time. (2 and 3 min instead of 1 min).This results also correspond with our BCoV experiments, where an extension of the exposure time with the LED irradiation with 10,000 lx from 2 to 5 or 10 min did not influence the high reduction factor of 5,35 log10.
However, if the light output is significantly reduced, an extension of exposure time is necessary in order to achieve the same virucidal effect (doubling of the irradiation time when irradiated with.
1500 lx "daylight" instead of 20,000 lx LED broadband light). Basically photodynamic virus reduction is e.g. a dye concentration, light dose and time depending process.
Tak-Wah Wong et al. [36] for example showed that the light dose, power density of the light source and dye concentration plays an important role for the inactivation process of viruses. For their experiments they used a red-light LED lamp with 632 nm+/- 20 nm wavelength, MB concentration of 0.0016% (0.05 mM) and a power density of 100 mW/cm² and an exposure time of 16.6 min. A light dose 100 J/cm2 leads to 1.5 log10 reduction factor (RF) on enterovirus 71 (EV71). After increasing the power density to 150 mW using the same dye concentration (0.05 mM) and irradiation time the reduction factor increased to 3log10. Doubling the dye concentration (0.1 mM = 0.08% MB), power density: 200 mW/cm2 and a corresponding light dose of 200 J/cm2, PDI provided a killing rate of nearly 8 orders of magnitude on RV71 viruses. Methylene blue showed no effect in the dark in these experiments (dark control).
For comparison: in our BCov experiments with a similar dye concentration (0.001%) and light dose (100 J/cm²) but different light source (810 nm coherent laser light) and triple power density (300 mW/cm²) we reached a ≥ 5.31 log-fold killing rate of BCoV viruses. Also in the dark, without irradiation the results were similar to those in this study. In our approaches of BCoV infected cells in the dark, a lot of residual virus could be detected after 10 min of incubation (RF= 0.90 with 0.0001% MB, RF=1.23 with 0.001% MB). These results on BCov infected cells in the dark differ to our results with SARS-CoV-2 infected cells in the dark. Here we observed the same effect after 3 min of dark incubation (without extra irradiation) as after 1 min of irradiation (RF≥ 4.67).The results in the dark showed a concentration dependence on dye concentration and showed a halving at 10 fold dilution (with 0.0001%). Irradiated samples did not show this concentration dependent reduction. The reduction factors remained constant for both concentrations (RF≥ 4.67). Valeria Cagno et al. [50] presented in vitro data demonstrating a potent antiviral activity of MB without light after 2 and 4 h of incubation with the virus. They observed that the antiviral effect of MB relies on multiple mechanisms of interaction. They reported that destruction of the viral genome seems depend on the effect of light. In the absence of light the antiviral effect should base on other mechanisms. Their experiments further highlighted MB is also effective when added on cells that were already infected, an effect corresponding to our experiments. In their experiments Cagno et al. used different concentrations of MB. With 0.0001% = 3.1 µM (one of our concentrations) they found a reduction of the virus load of SARS-CoV-2 in the dark of 3 log10 steps 4 h after incubation.
As in our experiments, a concentration dependency was shown here but in a much longer time interval (4 h vs. 3 min of incubation in the dark in our experiments) In their work the log of viral RNA copies/ml did only change under action of light (about 1 order of magnitude after 2 h and about 3 orders of magnitude after 20 h) with a 0.0002% MB concentration (measured on human H1N1 virus). However it is important to emphasize that no particular beam source was used for these results, so these results under light are not directly comparable to our experiments. In summary Cagno et al. emphasize, that MB might inactivate viruses limiting viral replication and spread beyond the upper respiratory tract in a clinical application.
Mathieu Gendrot et al. [49] also researched the MB effect in vitro on SARS-CoV-2 infected Vero E6 cells without extra light exposure. They added MB in different concentrations 4 h before infection and 48 h post infection the replication was estimated by RT-PCR (real time polymerase chain reaction). With 0.3 µM (0.00001% MB) a 50% inhibitory concentration (defined as the compound concentration producing 50% inhibition of the virus replication (IC50)) could be reached and the 90% inhibitory concentration (IC90) was about 0.75 µM (0.000024% MB). These IC50 and IC90 values were lower than those obtained for hydroxychloroquine and azithromycin, Remdesivir, lopanivir or Ritonavir, so the authors recommended clinical tests to use the dye systemically in case of Covid-19 infection. Svyatchenko et al. [51] also demonstrated significant antiviral activity of MB even in the absence of light. In this study they observed an IC50 with a concentration of about 0.00002% MB (0.62 µM) added immediately after SARS-CoV-2 infection of Vero E 6 cells and increased threefold (0.00006%) when the sensitizer was added 3.5 h post infection. Interestingly Svyatchenko et al. used the same sensitizer concentrations in their PDI experiments as we did, (0.001% and 0.0001% methylene blue). They used 662 nm laser irradiation instead of a wide band LED lamp, we used in our experiments. The output power of their laser was 350 mW, resulting in an irradiation dose of 16 J/cm2 and 40 J/cm2, respectively. The result of this study was that MB assisted PDI effectively inhibits viral replication and completely protects cellular mono layers from viral infection. They assume that MB can completely block viral replication within infected cells during the phase of active viral synthesis or may directly inactivate viral particles when they egress from previously infected cells.
A first MB-PDI based clinical study was published by Schikora et al. [52]. They used a 660 nm red light laser with 240 mW power with a dosage of about 72 J/cm² for excitation in combination with 1% MB sensitizer. The sensitizer was applied by flushing and gargling and by spraying in the nasal cavity. The study shows that a sufficient reduction of the SARS-CoV-2 virus load in the naso- and oral cavity can be achieved in the early stage of infection, leading to a significant decrease in morbidity and reduced mortality. It shows also maintaining the ability of the body to initiate immune response. No adverse effect could be observed even in the group of patients with co-morbidities. In contrast to the exposure times, light source and dye doses used in this study, our results show the theoretical possibility of a considerable reduction in both the irradiation time and dye concentration. A reduction of the dye concentration by a factor 10,000 (0.0001% instead of 1% MB) and irradiation time by a fifth (1 min instead of 5 min) should now be possible. The results of our studies show a promising inexpensive and easy method for new treatment options in the early phase of a Covid-19 infection with combination of a white LED lamp and low concentration of MB sensitizer. The use of a simple flashlight with internationally common AA-cell accumulators instead of a laser and simplifies clinical application and worldwide distribution considerably. Also the learning curve of potential users is dramatically flattened. Clinical studies on this are in preparation.
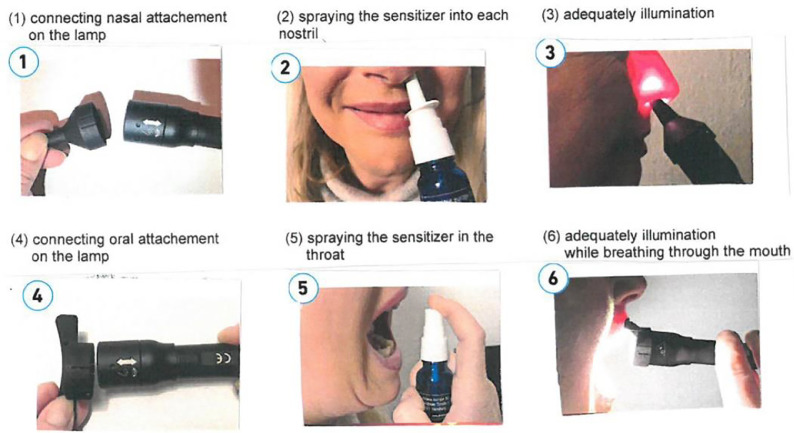
Possible clinical application
Figure 9 shows possible clinical application of the sensitizer and subsequent irradiation with LED lamp.
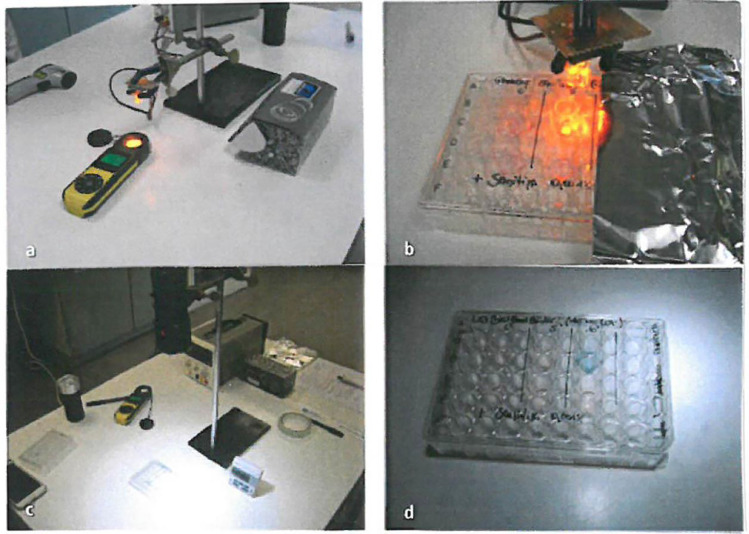
Fig. 10.
Pictures of the Diode Laser (a) Complete experimental setup, (b) standard display of the laser and (c) flat top
Fig. 11.
Experimental setup with the 590nm diodes LED before (a) and during irradiation (b) and pictures of the experimental setup with the conventional flashlight 12 XML-T6 (c, d)
Fig. 12.

Picture of the modified flashlight by Ledlenser GmbH & Co KG
Fig. 9.
The figure above show a possible clinical application of the sensitizer and subsequent irradiation with LED lamp
Acknowledgments
We thank Angelika Rück, Leader of Core Facility Confocal and Multiphoton Microscopy at Ulm university for kind support and Paul Walther, Central Facility for Electron Microscopy, Ulm university for help with electron microscopy, and Peter Pastors, PEP-Elektronik GmbH, Hamburg for development of the electronic current control of the lamps.
Our special thanks go to Hans Albrecht, Munich Germany for his tireless commitment and financial support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.pdpdt.2021.102642.
Appendix. Supplementary materials
References
- 1.Perrotta F., Matera M.G., et al. Severe respiratory SARS-CoV2 infection does ACE2 receptor matter? Respir. Med. 2020;168 doi: 10.1016/j.rmed.2020.105996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman J.D., Wang K., Roltgen K., Nielsen S.C.A., Roach J.C., Naccache S.N., et al. medRxiv; 2020. Reinfection With SARS-CoV-2 and Failure of Humoral Immunity: A Case Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson D., Brodniak S.L., Voegtly L.J., Cer R.Z., Glang L.A., Malagon F.J., et al. A case of early reinfection with SARS-CoV2. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postvaccination SARS-CoV-2 . 2021. Infections Among Skilled Nursing Facility Residents and Staff Members-Chicago, Illinois. December 2020-March. [Google Scholar]
- 5.Spinner C.D., Gottlieb R.L., et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate Covid 19 a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majunder J., Minko T. Recent development on therapeutic and diagnostic approaches for Covid 19. AAPS J. 2021;23 doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello A., Ferrara F. Remdesivir versus ritonavir /lopinavir in Covid 19 patients. Ir. J. Med. Sci. 2020;(1971) doi: 10.1007/s11845-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lian N., Xie H., Lin S. Umifenovir treatment is not associated with improved outcomes in patients with Corona virus disease a retrospective study. Clin. Microbiol. Infect. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fajnzylber J., Regan J. SARS-CoV-2 viral load is associated with increased disease severity and mortality, The Massachusetts Consortium for Pathogen Readiness. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epidemiology and Global Health Microbiology and Infectious Disease, Viral load and contact heterogeneity predict SARS-CoV-2 transmission and super-spreading events. Ashish Goyal. 2021 doi: 10.7554/eLife.63537. Feb.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cieplik F., et al. Antimicrobial photodynamic therapy-what we know and what we don’t. Crit. Rev. Microbiol. 2018;44(5):571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 12.Felber T.D., Smith E.B., Knox J.M., Wallis C., Melnick J.L. Photodynamic inactivation of herpes simplex: report of a clinical trial. J. Am. Med. Assoc. 1973;92:223–289. [Google Scholar]
- 13.Müller Breitkreuz K., Mohr H. epatitis C and human immunodeficiency virus RNA degradation by, Methylene blue/light treatment of human plasma. J. Med. Virol. 1998;56:239–245. doi: 10.1002/(sici)1096-9071(199811)56:3<239::aid-jmv11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Arno Wiehe Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019;18(11):2562–2612. doi: 10.1039/c9pp00211a. [DOI] [PubMed] [Google Scholar]
- 15.Jockush S., Lee D., Turro N.J., Leonard E.F. Photoinduced inactivation of viruses: adsorption of methylene blue, thionine and thiopyronineon Qß bacteriophage. Proc. Natl. Acad. Sci. U.S.A. 1996;93:7446–7451. doi: 10.1073/pnas.93.15.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradberry S.M. Occupational methaemoglobinaemia., Mechanism of production, features, diagnosis and management, including the use of methylene blue. Toxicol. Rev. 2003;22(1):13–27. doi: 10.2165/00139709-200322010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Kwok E.S.H., et al. Use of methylene blue in sepsis: a systemic review. J. Intensiv. Care Med. 2006;21(6):359–363. doi: 10.1177/0885066606290671. [DOI] [PubMed] [Google Scholar]
- 18.Lu G., Jahn A. Frank Peter Mockenhaupt, How worthwhile is methylene blue as a treatment of malaria? Expert Rev. Anti Infect. Ther. 2019;17(7):471–473. doi: 10.1080/14787210.2019.1634545. [DOI] [PubMed] [Google Scholar]
- 19.Zulian G.B., Tullen E., Maton B. Methylene Blue for ifosfamid associated encephalopathy. N. Engl. J. Med. 1995;332(18):1239–1240. doi: 10.1056/NEJM199505043321817. [DOI] [PubMed] [Google Scholar]
- 20.Repici A., Wallace M.B., et al. Efficacy of per-oral methylene blue formulation for screening colonoscopy. Gastroenterology. 2019;156(8):2198–2207. doi: 10.1053/j.gastro.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Rosa E.P., et al. Efficacy of photodynamic therapy and periodontal treatment in patients with gingivitis, and fixed orthodontic appliances: protocol of randomized,controlled double blind study. Medicine. 2020;(14):e19429. doi: 10.1097/MD.0000000000019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira A. Methylene blue photoinactivated plasma and its contribution to blood safety. Transfusion. 2004;44(6):948–950. doi: 10.1111/j.0041-1132.2004.359_6.x. [DOI] [PubMed] [Google Scholar]
- 23.Lambrecht B., Mohr H., Hopf J.K. Photoinactivation of viruses in human fresh plasma by phenothiazine dyes in combination with visible light. Vox Sang. 1991;60:207–213. doi: 10.1111/j.1423-0410.1991.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 24.Jun C., Yu B. Methylene blue photochemical treatment as a reliable SARS-CoV2 plasma, virus inactivation method for blood saftey and convalescent plasma therapy, of Covid 19. BMC Infect Dis. 2021;21:357. doi: 10.1186/s12879-021-05993-0. April 16 published online. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.2021. Inactivation of Three Emerging Viruses-Severe Acute Respiratory Syndrom coronavirus, Crimean-Congo Haemorrhagig Fever Virus and Nipah virus in Platelet Concentrations By UVc, Light and in Plasma By Methylene Blue Plus Visible Light. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perdau J.R., Todd The photodynamic action of methylene blue on certain viruses. Proc. R. Soc. B. 1933;112:288. [Google Scholar]
- 27.Perdau J.R., Todd C. From the national Institut for Medical Research Hampstead London N.W.3; 1933. Canine Distemper The high Antigenetic Value of the Virus After Inactivation by Methylene Blue. [Google Scholar]
- 28.Dempsy T.F., Mayer V. Two experiments with vaccine prepared by photodynamic action of methylene blue. J. Comp. Path. Ther. 1934;46:78. [Google Scholar]
- 29.Galloway A., et al. The fixed virus of rabbies -The antigenic value of the virus inactivated by photodynamic action of methylene blue. Br. J. Exp. Path. 1934;15:97. [Google Scholar]
- 30.C.Kaplan S.T. Observations on photodynamic inactivation of vaccinia virus and its effect on immunogenicity. J. Hyg. Camb. 1965;63:395. doi: 10.1017/s0022172400045289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enescu M., Lindqvist L. Excited-state deactivation mechanism in methylene blue-nucleotide complexes: a picosecond transient absorption study. J. Phys. Chem. 1995;99:8405–8411. [Google Scholar]
- 32.Vardevanyan P.O., Antonyan A.P., Parsadanyan M.A., et al. Mechanisms for Binding between Methylene Blue and DNA. J. Appl. Spectrosc. 2021;80(4):595–599. [Google Scholar]
- 33.Singhai G.S., Rabinowitch E. September 1 1967, Changes in the absorption spectrum of methylene blue with pH. J. Phys. Chem. 1967;71(10):3347–3349. [Google Scholar]
- 34.Wainwright M. The use of methylene blue derivates in blood product disinfection. Int. J. Antimicrob. Agents. 2000;16:381–394. doi: 10.1016/s0924-8579(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 35.Bachmann B., et al. Target structures for HIV-1 inactivation by methylene blue and light. J. Med. Virol. 1995;47(2):172–178. doi: 10.1002/jmv.1890470211. [DOI] [PubMed] [Google Scholar]
- 36.Wong T.-.W., Huang H.-.J., Wang Y.-.F., Lee Y.-.P., Huang C.-.C., Yu C.-.K. Methylene blue mediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J. Antimicrob. Chemother. 2010;65:2176–2182. doi: 10.1093/jac/dkq301. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet R., Martinez G. Photobleaching of sensitizeres used in photodynamic therapy. Tetrahedron. 2001;57(47):9513–9547. [Google Scholar]
- 38.Spearman C. The method of ` right or wrong cases` (constant stimuli) without Gauss formular. Br. J. Psychol. 1908;2:227–242. [Google Scholar]
- 39.Kärber G. Beitrag Zur Kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Path Pharmak. 1931;162:480–487. [Google Scholar]
- 40.EN 14476: 2013+A2 . 2019. Chemical Disinfectants and Antiseptics- Quantitative Suspension Test For the Evaluation of Virucidal Activity of Chemicals Disinfectans and Antiseptics in Human Medicine Test-Test Method and Requirements. (phase 2, step 1) [Google Scholar]
- 41.Mallidi S., Anbil S. Beyond the barriers of light penetration: strategies, perspectives and possibilities for photodynamic therapy. Theranostics. 2016;6(13):2458–2487. doi: 10.7150/thno.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savarimuthu W.P., et al. Photodynamic therapy of oral leukoplakia and oral lichen planus using methylene blue: a pilot study. J. Innov. Opt. Health Sci. 2014;(1540005):811. [Google Scholar]
- 43.Lejoy A., Arpita R., Krishna B., Venkatesh N. Methylene blue as a diagnostic aid in the early detection of potentially malignant and malignant lesions of oral mucosa. Ethiop. J. Health Sci. 2016;26(3):201–208. doi: 10.4314/ejhs.v26i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginimuge PR., Jyothi S.D. Methylene blue:revisited. J. Anaesthesiol. Clin. Pharmacol. 2010;26(4):517–520. [PMC free article] [PubMed] [Google Scholar]
- 45.Rück A., Heckelsmiller K., Akgün N., Beck G., –Rapp K.K., Schick E., Steiner R. Nonlinear dynamics of intracellular methylene blue during light activation of cell cultures. Photochem. Photobiol. 1997;66(6):837–841. doi: 10.1111/j.1751-1097.1997.tb03234.x. [DOI] [PubMed] [Google Scholar]
- 46.Lee Y.S., et al. Methylene blue induces cytotoxicity in human brain tumor cells. Cancer Lett. 1995;88(2):141–145. doi: 10.1016/0304-3835(94)03629-w. [DOI] [PubMed] [Google Scholar]
- 47.Abe H., Wagner SJ. Analysis of viral DNA, protein and envelope damage after methylene blue, phtalocyanine derivate, merocyanin 549 photosensitization. Photochem. Photobiol. 2021;61(4):402–409. doi: 10.1111/j.1751-1097.1995.tb08630.x. [DOI] [PubMed] [Google Scholar]
- 48.Bojadzic D., Alcazar O., Buchwald P. bioRxiv; 2020. Methylene Blue Inhibits in Vitro the SARS-CoV-2 Spike-ACE2 Protein-Protein Interaction: A Mechanism That Can Contribute to Its Antiviral Activity Against COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gendrot M., Andreani J., et al. Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents. 2020;56(6) doi: 10.1016/j.ijantimicag.2020.106202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cagno V., Medaglia C., et al. Methylene Blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-92481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Svyatchenko VA., Nikonov SD., Mayorov AP., Gelfond Ml., et al. Antiviral photodynamic therapy: inactivation and inhibition of SARS-CoV-2 in vitro, using methylene blue and radahlorin, photodiagnosis and photodynamic therapy. J. Pre-Proof. 2021 doi: 10.1016/j.pdpdt.2020.102112. PII: S1572-1000(20)30466-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schikora D., Hepburn J., R.Plavin S. Reduction of the viral load by non-invasive photodynamic therapy in early stages of covid 19 infection. Am. J. Virol. Dis. 2020;2(1):01–05. [Google Scholar]
- 53.Engel T. Pearson Benjamin Cummings; 2006. Philip Reid ISBN 978-0-8053-3842-3, Physical Chemistry; p. 580. [Google Scholar]
- 54.Photosensitized generation of singlet oxygen. Photochem. Photobiol. 2006;82(5):1161–1177. doi: 10.1562/2006-03-03-IR-833. R. Schmidt DOI:10.1562/2006-03-03-IR-833. [DOI] [PubMed] [Google Scholar]
- 55.Huber G., Duczynski E.W., Mitzscherlich P., Teichmann H.O., Lumma D. Room-temperature 2-µm Ho: YAG and 3-µm Er: YAG lasers. J. Phys. Coll. 1987;48(C7) pp C7-347-C7-34910.1051/jphyscol:1987783 jpa-00227087. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.