Abstract
The perturbation of gut health is a common yet unresolved problem in broiler chicken production. Antibiotics used as growth promoters have remarkably improved the broiler production industry with high feed conversion efficiency and reduced intestinal problems. However, the misuse of antibiotics has also led to the increase in the development of antibiotic resistance and antibiotic residues in the meat. Many countries have enacted laws prohibiting the use of antibiotics in livestock production because of the increasing concerns from the consumers and the public. Consequently, one of the most significant discussions in the poultry industry is currently antibiotic-free livestock production. However, the biggest challenge in animal husbandry globally is the complete removal of antibiotics. The necessity to venture into antibiotic-free production has led researchers to look for alternatives to antibiotics in broiler chicken production. Many strategies can be used to replace the use of antibiotics in broiler farming. In recent years, many studies have been conducted to identify functional feed additives with similar beneficial effects as antibiotic growth promoters. Attention has been focused on prebiotics, probiotics, organic acids, emulsifiers, enzymes, essential oils, tributyrin, and medium-chain fatty acids. In this review, we focused on recent discoveries on gut health maintenance through the use of these functional feed additives as alternatives to antibiotics in the past 10 years to provide novel insights into the design of antibiotic-free feeds.
Keywords: gut, health, antibiotic, antibiotic-free strategies, broilers
Introduction
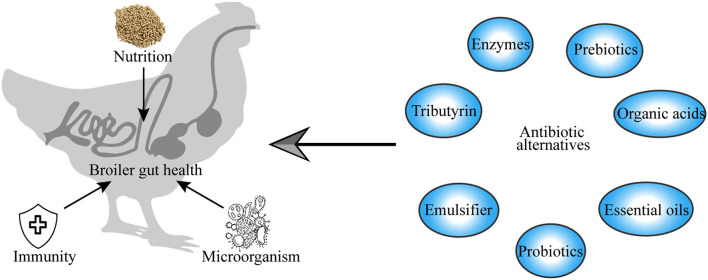
Gut health is an increasingly important topic in broiler chicken production. The rapid rise in the global human population has increased the demand for animal protein for human nutrition, which consequently led to the intensive production of broiler chickens to meet the demand for food, causing unintended gut health problems and performance impairment in broiler chickens. Intestinal diseases are associated with gut mucosal barrier leakage, inflammation, and gut microbiome dysbiosis. For a long time, broiler production has relied on the use of antibiotics, which have led to significant improvements in the growth performance of broiler chicken and have helped in the fight against bacterial infections (1–3). Antibiotics have demonstrated significant value in terms of the enhancement of health and productivity in broiler chickens. However, their misuse in intensive livestock production has led to public and consumer concerns about antibiotic residues in the meat and the development of antibiotic resistance among pathogenic bacteria, with serious implications for human and animal health and the environment. This has led to the enactment of legal regulations prohibiting the use of antibiotics in broiler production to alleviate its risks to human and animal health, as well as threats to the environment (4, 5). This has led to the need to identify alternatives to antibiotics that can be used to fight gut pathogens that cause intestinal diseases (Figure 1).
Figure 1.
Graphical abstract.
This review aims to identify the causes of gut health problems, show the reported results of various regulatory measures or alternative antibiotics, and analyze the feasibility of feeding without antibiotics.
Lessons From the Gut Health Impairment in Broilers
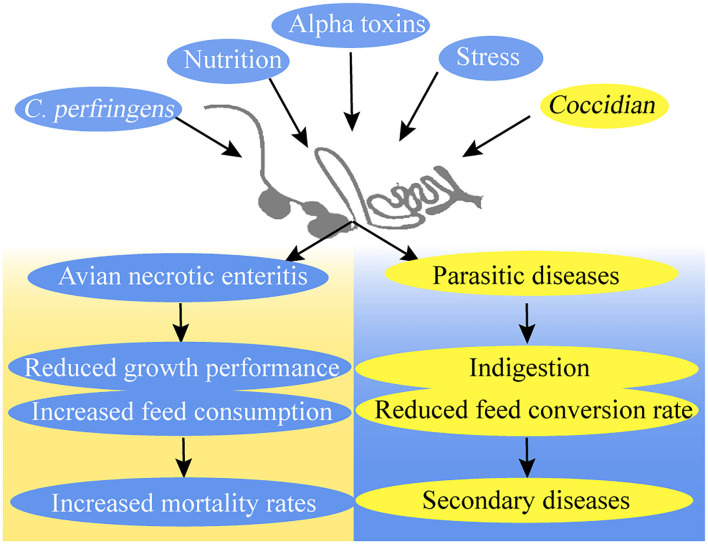
Poultry production losses caused by avian necrotic enteritis (NE) and parasitic diseases, such as coccidiosis, have become a global challenge for the poultry industry (6, 7). Since the ban on antibiotics in animal feed, the high prevalence of NE and coccidiosis has become a major cause of mortality in broilers (8–10) (Figure 2).
Figure 2.
Avian necrotic enteritis and parasitic diseases in broilers.
Necrotic enteritis increases feed consumption and mortality rates but reduces the growth performance of broiler chickens (11). Several reasons can be cited as to why broilers contract NE. Nutrition, stress, and coccidiosis are predisposing factors that influence the incidence of NE (8). Clostridium perfringens is a crucial factor for the development of NE in broiler chickens because of its negative influence on the epithelial barrier (12, 13). C. perfringens uses and releases more than 16 toxins that cause histotoxic and intestinal infections in animals. Different toxins may bring virulence flexibility to C. perfringens, thereby causing a series of diseases (14). C. perfringens is also one of the most common contaminants in feeds (15). Alpha toxins are C. perfringens type A product, which causes gas gangrene (16, 17). Early studies on NE showed that alpha-toxin is the main virulence factor for the development of the disease, but recent studies proposed that alpha-toxin is not an essential virulence factor in the pathogenesis of NE in poultry (8, 13, 18–20). NetB is identified in a C. perfringens strain isolated from NE in broilers and has considerable potential for novel vaccines against NE in broilers (19, 21–23).
Coccidiosis is a recurring disease that endangers the intestinal health of broilers and causes economic losses in the chicken industry (24). The effects of coccidiosis include indigestion and increased feed conversion rate, weight gain, and susceptibility to secondary diseases in infected broilers (25). The main cause of coccidiosis outbreaks is the protozoan Eimeria species, and the infection route is through fecal–oral transmission (2, 9). The Eimeria species increase their environmental survival through their ability as oocysts and their drug resistance (9, 10). Eimeria induces plasma protein leakage by damaging epithelial cells in the intracellular phase, which includes mucus production enhancement and the secretion of collagenases and collagenolytic enzymes in the intestines (18, 20).
Impairment Factors of Gut Health in Broilers
Among the wide range of significant factors affecting broiler health, stress, diets, exogenous infection, and water are the most common indicators. Recently, more studies on the impairment factors of the intestinal health of broilers have focused on phytic acid, non-starch polysaccharides (NSPs), inhibitors of enzymes, lectins, and heat stress (Figure 3).
Figure 3.
Impairment factors of gut health in broilers.
Phytic Acid
Phytic acid is a natural antioxidant found naturally in the form of salts and is present in cereals, vegetables, nuts, and natural oils (26). For example, phytic acid forms insoluble salts with minerals, including phosphorus, calcium, zinc, magnesium, and copper. Phytic acid increases the mucin (MUC) excretion and endogenous nutrient losses, which are hazardous to intestinal health.
Early studies showed that phytic acid is considered an antinutritional component because of its ability to chelate with minerals, but recent studies proposed that phytic acid performs well in various pathological conditions, intoxication, and cancer (27, 28). Phytic acid protects the integrity of the cytoplasmic membrane of intestinal cells against the harmful effects of deoxynivalenol, which is related to NE (29).
Low-phytate pea affects iron bioavailability, physiological status, gut microbiota composition and metagenome, and intestinal function (30). Phytase optimizes the phosphate transporter gene expression and improves efficient dietary phosphorus utilization (31).
Non-Starch Polysaccharides
Non-starch polysaccharides, together with resistant starch and lignin from the dietary fiber, are found in plants especially in the endospermic cell wall of multiple kinds of seeds (32). Many viscous NSPs are present in the diet of chickens, leading to increased fermentation in the small intestine, which is harmful to the performance and gut health of poultry.
In the past, NSPs are considered an antinutritional factor because they increase the viscosity of digests and inhibit digestion. However, the beneficial effects of NSPs cannot be denied. NSPs can promote the immune system, reduce inflammation (33–37), and modulate the gut microbiota (38).
Inhibitors of Enzymes
The inhibitors of enzymes, including trypsin, chymotrypsin, carboxypeptidases, elastase, and α-amylase, are important naturally occurring antinutritional factors. Soybeans are a major source of trypsin inhibitors among food and feed products (39). Trypsin inhibitors cause the enlargement of the pancreas and decrease protein digestibility.
Toasted soya beans decrease the trypsin inhibitor activity. Nontoasted full-fat soya beans increase subclinical NE lesions in the gut compared with toasted full-fat soya beans (40). The high content of trypsin inhibitors negatively affects nutrient utilization in the diets of broiler chickens (41).
Lectins
Lectins, which can be subdivided into hololectins, merolectins, chimerolectins, and superlens, are widely distributed in all plant tissues.
Plant lectins have oral toxicity to higher animals because of the resistance of lectins to proteolysis (42). Tannins are naturally occurring water-soluble polyphenolic compounds with the ability to complex and precipitate proteins in aqueous solutions and are responsible for the astringent taste of many fruits and vegetables (43).
Heat Stress
Heat stress can adversely affect welfare and productivity by altering the activity of the neuroendocrine system of broilers (44). Heat stress induces perturbation in the gut microbiome of chicken (45, 46) and impairs nutrient transport and gut health by modulating oxidative stress and inflammation (47).
Antibiotic-Free Management Strategies in Broilers
Housing conditions, pathogen exposure, and dietary nutrients play major roles in moderating the gut health of broilers. Therefore, improving the gut health includes reducing stress, promoting precision nutrition, preventing exogenous infection, and having concern over how antibiotic-free management strategies are used and how the breeding environment can be improved. In this study, a review of antibiotic-free management strategies will be detailed and will be discussed from the feed quality control, feed additive enzymes, prebiotics, probiotics, organic acids, and plant extract (Figure 4).
Figure 4.
Antibiotic-free management strategies in broilers.
Feed Quality Control
Mycotoxins, including aflatoxins, ochratoxin A, fumonisins, trichothecenes, zearalenone, emerging Fusarium mycotoxins, ergot alkaloids, and patulin, are the secondary metabolites produced by fungi and can cause mycotoxicosis (48). The strategies for its prevention include the use of biological control agents, appropriate environmental factors, and favorable storage practices (49–52). Natural means, such as thermal insulation, radiation treatment, and low-temperature plasma; chemical methods, such as oxidation, reduction, hydrolysis, alcoholysis, and absorption; biological methods with the use of biological agents can eradicate mycotoxins (53). Prevention is the most important strategy in the fight against mycotoxins.
Fermentation is a new cheap way to improve the nutritional value of feed ingredients for broilers. Fermented poultry feeds use solid-state (SSF) and submerged (SmF) fermentation methods (54). Phytase is produced from fungal strains during SmF. Fermented feeds affect the growth performance, gastrointestinal tract microecology, gut morphology, immune system, and welfare of poultry.
Fermented feeds can improve growth performance, immune function, and antioxidant capacity in chickens. Fermented-feed diets for chickens reduce the antinutritional factors process that modulates host T-cell proliferation, T helper type 1, and T helper type 2 cytokine production; many antioxidation are associated with nuclear factor-κB (NF-κB) activation (55).
Feed Additive Enzymes
Fungal and bacterial fermentation methods produce feed additive enzymes that maximize feed conversion efficiency. Enzymes facilitate component, protein, phytate, and glucan degradation and improve diet digestion. The phytase (500 FTU per kg) enzyme increases the villus width and decreases the crypt depth, which improves the average daily gain (ADG) (56). Ply3626 lysine (enzyme) may be exploited as an antibacterial for the treatment of C. perfringens infection and is proposed as a biocontrol agent in poultry production (57). Feed additive enzymes ameliorate the deleterious effects of coccidiosis on the gut health and function of broiler chickens (58).
Phytase (500 and 1,500 units per kg) supplements improve phosphorous availability and have high plasma concentrations of kynurenine and creatinine, and low concentrations of histamine and cis-4-hydroxyproline (59). The NSP enzyme (150 mg per kg) affects the digestive function, serum cholecystokinin, pancreatic lipase, and amylase enzyme activities, and mRNA expression in broilers (60). Exogenous multienzyme complexes increase the intestinal peptide transporter 1, facilitative glucose transporter (GLUTs; e.g., GLUT2), acetyl-CoA carboxylase, and interleukin (IL)-2 expression levels, which improve the absorption of micronutrients and enhance the growth performance of broiler chickens (61).
Prebiotics
Prebiotics stimulate the growth and activity of bacteria in the intestines through their fermentable properties, benefitting the health of broilers. Prebiotics are composed of short-chain polysaccharides and oligosaccharides. Prebiotics cannot be digested by broilers but can be metabolized by gut microbes to produce short-chain fatty acids (62). Prebiotics have been shown to reduce Campylobacter relative to its abundance in cecal contents and other intestinal sections of the gut. Prebiotics, such as propionic, acetic, and butyric acids, have a positive effect on the performance of broilers, contribute to their gut health, and can be a good alternative to antibiotics. Prebiotics change the composition of cecal microbes in the gut, leading to changes in the Proteobacteria and the genus and family of bacteria and improving the performance of broilers (56).
The addition of a product rich in mannose, mannoproteins, and mannanoligosaccharide (0.2 and.5%) in feeds significantly increases the number of intestinal villus cells, and mannanoligosaccharide confers gut health benefits over antibiotics through the reduction of pathogenic bacteria, morphological development, and increased colonization by beneficial bacteria (63, 64). The ingestion of in ovo prebiotics in the chicken embryo is an effective practice and alternative to antibiotic growth promoters in broilers (65).
Curcumin (50 and 100 mg per kg) supplementation induces the expression of nuclear factor E2-related factor 2 (Nrf2) and Nrf2-mediated phase II detoxifying enzyme genes and increases the glutathione content and glutathione-related enzyme activities (66). Yeast cell wall supplementation modulates the intestinal glutathione pathway, proteolytic enzyme activity, nutrient transport, and messenger RNA (mRNA) expression levels of neutral, cationic, and oligopeptide transporters (67). Nanoparticles of Fe3O4 (50 mg per kg) prevent the invasion of Salmonella enteritidis through the regulation of phosphatidylinositol-3-kinase (PI3K)/protein kinase (Akt)/mammalian target of rapamycin signaling pathways (68, 69).
Probiotics
Probiotics, as a live microorganism feed supplementation, improve growth, feed efficiency, and intestinal health (70). Enterococcus faecium (5 ×108 or 5 × 109 cfu per kg feed), Streptomyces sp., and Bacillus subtilis (5 × 108 cfu/kg feed) have antibacterial effects on the bacterial microflora in the small intestine. Beneficial microflora contributes to the development of the intestinal immune system and helps in the activation of innate and adaptive immune responses (71, 72).
In terms of coccidiosis, probiotics may also have an anticoccidial role. A study found a protecting effect in probiotic preparations that help reduce the negative effects of coccidiosis (70). Probiotics help minimize the risk of coccidiosis spread and maintain gut health.
Probiotics stimulate endogenous enzymes, reduce metabolic reactions, and promote vitamin or antimicrobial substance production. Bacteriocins are antimicrobial peptides of bacterial origin and have antimicrobial activities that inhibit the production of toxins and the adhesion of pathogens (73).
Probiotics induce IFN-γ, MUC2, transforming growth factor-beta 4 cytokine expression patterns, and the relative abundance of specific bacterial taxon changes in the cecal microbiota (74). Virginiamycin supplementation enhances the epithelial barrier integrity and increases the expression levels of IL-2 and INF-γ, and B. subtilis supplementation improves the growth performance, intestinal immunity, and epithelial barrier integrity. The expression levels of IL-2 and INF-γ are downregulated (75). E. faecium supplementation upregulates the expression of intestinal-type IIb sodium-dependent phosphate cotransporter mRNA, increases the concentration of serum alkaline phosphatase, changes the gut microbiota populations, and increases the utilization of phosphorus (76). Bacillus subtilis BYS2 supplementation improves the production performance, immunity, and disease resistance; promotes innate immune response, increases the expression levels of interferon (IFN)-stimulated genes and β-defensins, and upregulates inflammatory cytokines (77). Lactobacillus supplementation increases the expression of sugar transporter genes (e.g., GLUT2, GLUT5, sodium-glucose cotransporter [SGLT] 1, and SGLT4) and improves the bacterial population of cecal contents (78).
Organic Acids
Short-chain fatty acids, medium-chain fatty acids, and other organic acids are used as an alternative to antibiotics to reduce the pathogenic bacteria in the gut based on their antimicrobial activity outside the gut. The antibacterial effect of organic acids is specific to species (79). The addition of organic acids causes a decrease in Escherichia coli, Salmonella, Campylobacter, and other potentially pathogenic bacteria, which result in a beneficial effect on the gut health of broilers (80).
Growth, feed conversion rate, and feed utilization can be promoted by adding organic acids (0.06% Galliacid, 0.1% Biacid, or 0.02% Eneramycin) to the feed or drinking water at appropriate times (81). Butyric acid improves the growth performance of feed proteins of low digestible sources in chickens (82). Butyric acid is an energy source of Intestinal epithelial cells (IECs) that stimulate proliferation and differentiation. Thus, improving the feed efficiency of diet supplementation with organic acids (formic, propionic, and acetic acid) can positively affect the cecal microbiota composition and ileal microbial glycolytic enzyme activity (83). L-theanine is available as a dietary supplement, used as the best natural feed additive, and can improve the growth performance, immunity, intestinal morphology, and antioxidant status of chickens (84).
Taurine supplementation alleviates fat synthesis by suppressing the liver X receptor alpha pathway and decreasing lipid accumulation in the liver (85). Glutamine inducing the Nrf2–Keap1 pathway modulates the muscle glutamine level and improves the resistance of heat-stressed broiler muscles to oxidative damage (86). Diets supplemented with a blend of organic acids prime the immune cells, and boost the immune system of chicks. Heterophils have high expression levels of IL10, IL1β, and C-X-C Motif Chemokine Ligand 8 mRNA (87). Taurine improves immunity by regulating the PI3K–Akt signaling pathway (88).
Plant Extract
Plant extracts are phytogenic feed additives that can be divided into phenolics, nitrogen-containing alkaloids, sulfur-containing compounds, and terpenoids based on their biosynthetic origin. Ginger and oregano are suitable for poultry feed rather than garlic and rosemary because they appear to be less sensitive to odor (89, 90). Similarly, essential oils are promising alternatives to growth promoter antibiotics. Essential oils can play preventive and curative roles in NE in broilers (91). Adding oregano essential oil (300 and 600 mg per kg) in broiler chicken feed increases the ADG (92). Guduchi (T. cordifolia) has a positive effect on poultry growth performance, enhances the immune function in birds, and is used as a potent immunomodulator and an active antimicrobial agent in poultry (93).
Essential oils interfere with the modulation of immune responses and inflammation (5, 94). The antibacterial effects of essential oils disrupt the structure of the membrane and inner cell structures via their lipophilic characteristics and related ability to penetrate through the cell wall and cytoplasmic membrane (95). The antioxidant effects of essential oils are observed to be connected to the reduction of tumor cell proliferation either by apoptosis or necrotic effects (96). Ginger oil and carvacrol can influence the digestibility and speed of feed passage through the digestive tract, increase the secretion of saliva, bile, and mucus, and enhance enzyme activity. Essential oils with saponins can promote the growth performance of broilers and increase the protein digestibility and absorption of dietary nutrients that are related to intestinal development and protease activity (97).
Grape seed proanthocyanidin ameliorates aflatoxin B1-induced immunotoxicity and oxidative damage by modulating the NF-κB and activating the Nrf2 signaling pathways (98). Leonurine hydrochloride supplementation improves intestinal mucosal disruption by regulating the expression of tight junction (TJ) proteins and inhibiting the activation of the NF-κB/mitogen-activated protein kinase (MAPK) signaling pathway (99). Genistein ameliorates the growth performance of chicks with intestinal injury and prevents the Lipopolysaccharides (LPS)-induced NF-κB-dependent cytokine and MAPK cascade signaling (100). Epigallocatechin-3-gallate increases the antioxidant activity, regulates the MAPK/Nrf2 signaling pathway, and upregulates the P-38MAPK, Nrf2, and heme oxygenase 1 expression levels (101). Quercetin supplementation decreases the expression of NF-κB inhibitor-alpha mRNA and increases the expression levels of TNF-α, TNF receptor-associated factor-2, TNF receptor superfamily member 1B, NF-κB p65 subunit, and IFN-γ mRNA, thereby improving the immune function via the NF-κB signaling pathway triggered by TNF-α (102).
Conclusion
The livestock industry has paid considerable attention to the issues of antibiotics, and topics on intestinal health have become the spotlight. The purpose of antibiotic alternatives is to keep the environment and consumers healthy and maintain low mortality and high animal production. Identifying a single “ideal” solution within the wealth of options for gut health control is difficult. Several measures and alternatives to antibiotics can be used in conjunction with one another to achieve the perfect gut health.
In recent years, feed additive enzymes, prebiotics, probiotics,organic acids, and plant extracts play an increasingly important role in fighting infectious diseases and stimulating poultry growth. The use of antibiotic alternatives has addressed the problem of antibiotic resistance and residues in the food and environment, which can promote gut health. In addition, this strategy maintains favorable sanitary conditions and ensures high-quality feed ingredients. Precise nutrition is also critical.
Author Contributions
QZ wrote the original manuscript and contributed much to the revised figures. PS wrote the manuscript and revised it critically for important intellectual content. BZ edited the manuscript. LK and CX drawed the original figures. ZS designed the profiles of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MC170), the National Key R&D Program of China (2018YFD0501401-3), and the Shandong Province Agricultural Industry Technology (SDAIT-11-08).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Mr. Li Xianlei for his contribution to the writing of the primitive manuscript.
References
- 1.Khodambashi Emami N, Samie A, Rahmani HR, Ruiz-Feria CA. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim Feed Sci Technol. (2012) 175:57–64. 10.1016/j.anifeedsci.2012.04.001 [DOI] [Google Scholar]
- 2.Lee KW, Ho Hong Y, Lee SH, Jang SI, Park MS, Bautista DA, et al. Effects of anticoccidial and antibiotic growth promoter programs on broiler performance and immune status. Res Vet Sci. (2012) 93:721–8. 10.1016/j.rvsc.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay MK. Use of antibiotics as feed additives: a burning question. Front Microbiol. (2014) 5:334. 10.3389/fmicb.2014.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez Ronquillo M, Angeles Hernandez JC. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control. (2017) 72:255–67. 10.1016/j.foodcont.2016.03.001 [DOI] [Google Scholar]
- 5.Mehdi Y, Letourneau-Montminy MP, Gaucher ML, Chorfi Y, Suresh G, Rouissi T, et al. Use of antibiotics in broiler production: Global impacts and alternatives. Anim Nutr. (2018) 4:170–8. 10.1016/j.aninu.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grass JE, Gould LH, Mahon BE. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog Dis. (2013) 10:131–6. 10.1089/fpd.2012.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawal JR, Jajere SM, Ibrahim UI, Geidam YA, Gulani IA, Musa G, et al. Prevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria. Vet World. (2016) 9:653–9. 10.14202/vetworld.2016.653-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. (2009) 17:32–6. 10.1016/j.tim.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 9.Blake DP, Tomley FM. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. (2014) 30:12–9. 10.1016/j.pt.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Fatoba AJ, Adeleke MA. Transgenic Eimeria parasite: A potential control strategy for chicken coccidiosis. Acta Trop. (2020) 205:105417. 10.1016/j.actatropica.2020.105417 [DOI] [PubMed] [Google Scholar]
- 11.Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. (2017) 49:e338. 10.1038/emm.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin TG, Smyth JA. The ability of disease and non-disease producing strains of Clostridium perfringens from chickens to adhere to extracellular matrix molecules and Caco-2 cells. Anaerobe. (2010) 16:533–9. 10.1016/j.anaerobe.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 13.Antonissen G, Van Immerseel F, Pasmans F, Ducatelle R, Haesebrouck F, Timbermont L, et al. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE. (2014) 9:e108775. 10.1371/journal.pone.0108775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, et al. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. (2014) 9:361–77. 10.2217/fmb.13.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. (2011) 40:341–7. 10.1080/03079457.2011.590967 [DOI] [PubMed] [Google Scholar]
- 16.Nauerby B, Pedersen K, Madsen M. Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens. Vet Microbiol. (2003) 94:257–66. 10.1016/s0378-1135(03)00118-4 [DOI] [PubMed] [Google Scholar]
- 17.Gholamiandekhordi AR, Ducatelle R, Heyndrickx M, Haesebrouck F, Van Immerseel F. Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status. Vet Microbiol. (2006) 113:143–52. 10.1016/j.vetmic.2005.10.023 [DOI] [PubMed] [Google Scholar]
- 18.Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, et al. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. (2006) 74:6496–500. 10.1128/IAI.00806-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. (2008) 4:e26. 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timbermont L, Lanckriet A, Gholamiandehkordi AR, Pasmans F, Martel A, Haesebrouck F, et al. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp Immunol Microbiol Infect Dis. (2009) 32:503–12. 10.1016/j.cimid.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 21.Savva CG, Fernandes da Costa SP, Bokori-Brown M, Naylor CE, Cole AR, Moss DS, et al. Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens. J Biol Chem. (2013) 288:3512–3522. 10.1074/jbc.M112.430223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzal FA, McClane BA, Cheung JK, Theoret J, Garcia JP, Moore RJ, et al. Animal models to study the pathogenesis of human and animal Clostridium perfringens infections. Vet Microbiol. (2015) 179:23–33. 10.1016/j.vetmic.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro MA, McClane BA, Uzal FA. Mechanisms of Action and Cell Death Associated with Clostridium perfringens Toxins. Toxins (Basel). (2018) 10:212. 10.3390/toxins10050212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatas J, Adeleke MA. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res. (2019) 118:1701–10. 10.1007/s00436-019-06338-2 [DOI] [PubMed] [Google Scholar]
- 25.Chengat Prakashbabu B, Thenmozhi V, Limon G, Kundu K, Kumar S, Garg R, et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol. (2017) 233:62–72. 10.1016/j.vetpar.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva EO, Bracarense AP. Phytic Acid: From Antinutritional to Multiple Protection Factor of Organic Systems. J Food Sci. (2016) 81:R1357–1362. 10.1111/1750-3841.13320 [DOI] [PubMed] [Google Scholar]
- 27.Zhao Z, Liu W, Su Y, Zhu J, Zheng G, Luo Q, et al. Evaluation of biodistribution and safety of adenovirus vector containing MDR1 in mice. J Exp Clin Cancer Res. (2010) 29:1. 10.1186/1756-9966-29-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Silva E. O, Gerez J. R., do Carmo Drape T., Bracarense A.. (2014). Phytic acid decreases deoxynivalenol and fumonisin B1-induced changes on swine jejunal explants. Toxicol Rep 1, 284–292. 10.1016/j.toxrep.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacheco GD, Silva CA, Pinton P, Oswald IP, Bracarense AP. Phytic acid protects porcine intestinal epithelial cells from deoxynivalenol (DON) cytotoxicity. Exp Toxicol Pathol. (2012) 64:345–7. 10.1016/j.etp.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 30.Warkentin T, Kolba N, Tako E. Low Phytate Peas (Pisum sativum L) Improve Iron Status, Gut Microbiome, and Brush Border Membrane Functionality In Vivo (Gallus gallus). Nutrients. (2020) 12:2563. 10.3390/nu12092563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olukosi OA, Kong C, Fru-Nji F, Ajuwon KM, Adeola O. Assessment of a bacterial 6-phytase in the diets of broiler chickens. Poult Sci. (2013) 92:2101–8. 10.3382/ps.2012-03005. [DOI] [PubMed] [Google Scholar]
- 32.Lovegrove A, Edwards CH, De Noni I, Patel H, El SN, Grassby T, et al. Role of polysaccharides in food, digestion, and health. Crit Rev Food Sci Nutr. (2017) 57:237–53. 10.1080/10408398.2014.939263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Yan H, Zhang X. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J Agric Food Chem. (2012) 60:11560–6. 10.1021/jf304364c [DOI] [PubMed] [Google Scholar]
- 34.Berner VK, duPre SA, Redelman D, Hunter KW. Microparticulate β-glucan vaccine conjugates phagocytized by dendritic cells activate both naïve CD4 and CD8 T cells in vitro. Cell Immunol. (2015) 298:104–114. 10.1016/j.cellimm.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Nie S. The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. (2015) 6:3205–17. 10.1039/c5fo00678c [DOI] [PubMed] [Google Scholar]
- 36.Kang GD, Lim S, Kim DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-kappaB signaling pathway. Int Immunopharmacol. (2015) 29:393–400. 10.1016/j.intimp.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 37.Li, Oosting M, Smeekens SP, Jaeger M, Aguirre-Gamboa R, Le KTT, et al. A functional genomics approach to understand variation in cytokine production in humans. Cell. (2016) 167:1099–110 e1014. 10.1016/j.cell.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 38.Li, Pavuluri S, Bruggeman K, Long BM, Parnell AJ, Martel A, et al. Coassembled nanostructured bioscaffold reduces the expression of proinflammatory cytokines to induce apoptosis in epithelial cancer cells. Nanomed. (2016) 12:1397–407. 10.1016/j.nano.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 39.Sarwar Gilani G, Wu Xiao C, Cockell KA. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br J Nutr. (2012) 108:S315–32. 10.1017/s0007114512002371 [DOI] [PubMed] [Google Scholar]
- 40.Palliyeguru MWCD, Rose SP, Mackenzie AM. Effect of trypsin inhibitor activity in soya bean on growth performance, protein digestibility and incidence of sub-clinical necrotic enteritis in broiler chicken flocks. Br Poult Sci. (2011) 52:359–67. 10.1080/00071668.2011.577054 [DOI] [PubMed] [Google Scholar]
- 41.Aderibigbe A, Cowieson AJ, Sorbara JO, Pappenberger G, Adeola O. Growth performance and amino acid digestibility responses of broiler chickens fed diets containing purified soybean trypsin inhibitor and supplemented with a monocomponent protease. Poult Sci. (2020) 99:5007–17. 10.1016/j.psj.2020.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhutia SK, Panda PK, Sinha N, Praharaj PP, Bhol CS, Panigrahi DP, et al. Plant lectins in cancer therapeutics: Targeting apoptosis and autophagy-dependent cell death. Pharmacol Res. (2019) 144:8–18. 10.1016/j.phrs.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 43.Smeriglio A, Barreca D, Bellocco E, Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol. (2017) 174:1244–62. 10.1111/bph.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lara LJ, Rostagno MH. Impact of heat stress on poultry production. Animals (Basel). (2013) 3:356–69. 10.3390/ani3020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi D, Bai L, Qu Q, Zhou S, Yang M, Guo S, et al. Impact of gut microbiota structure in heat-stressed broilers. Poult Sci. (2019) 98:2405–13. 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Liao R, Wu N, Zhu G, Yang C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl Microbiol Biotechnol. (2019) 103:461–72. 10.1007/s00253-018-9465-8 [DOI] [PubMed] [Google Scholar]
- 47.Santos RR, Awati A, Roubos-van den Hil PJ, van Kempen TATG, Tersteeg-Zijderveld MHG, Koolmees PA, et al. Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathol. (2019) 48:582–601. 10.1080/03079457.2019.1648750 [DOI] [PubMed] [Google Scholar]
- 48.Liew WP, Mohd-Redzwan S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front Cell Infect Microbiol. (2018) 8:60. 10.3389/fcimb.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Y, Liu X, Li J. Updating techniques on controlling mycotoxins - A review. Food Control. (2018) 89:123–32. 10.1016/j.foodcont.2018.01.016. [DOI] [Google Scholar]
- 50.Sarrocco S, Vannacci G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Prot. (2018) 110:160–70. 10.1016/j.cropro.2017.11.013. [DOI] [Google Scholar]
- 51.Xiao Y, Zhang S, Tong H, Shi S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother Res. (2018) 32:384–94. 10.1002/ptr.5966 [DOI] [PubMed] [Google Scholar]
- 52.Sarrocco S, Mauro A., Battilani P.. (2019). Use of Competitive Filamentous Fungi as an Alternative Approach for Mycotoxin Risk Reduction in Staple Cereals: State of Art and Future Perspectives. Toxins (Basel) 11(12). 10.3390/toxins11120701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: prevention and detoxification in foods. Foods. (2020) 9:137. 10.3390/foods9020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugiharto S, Ranjitkar S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Animal Nutrition (Zhongguo xu mu shou yi xue hui). (2019) 5:1–10. 10.1016/j.aninu.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu F, Zhang B, Li J, Zhu L. Effects of fermented feed on growth performance, immune response, and antioxidant capacity in laying hen chicks and the underlying molecular mechanism involving nuclear factor-κB. Poult Sci. (2020) 99:2573–80. 10.1016/j.psj.2019.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohammadagheri N, Najafi R, Najafi G. Effects of dietary supplementation of organic acids and phytase on performance and intestinal histomorphology of broilers. Vet Res Forum. (2016) 7:189–95. [PMC free article] [PubMed] [Google Scholar]
- 57.Fenton M, Ross P, McAuliffe O, O'Mahony J, Coffey A. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs. (2010) 1:9–16. 10.4161/bbug.1.1.9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiarie EG, Leung H, Akbari Moghaddam Kakhki R, Patterson R, Barta JR. Utility of Feed Enzymes and Yeast Derivatives in Ameliorating Deleterious Effects of Coccidiosis on Intestinal Health and Function in Broiler Chickens. Front Vet Sci. (2019) 6:473. 10.3389/fvets.2019.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez-Uarquin F, Kenéz Á, Rodehutscord M, Huber K. Dietary phytase and myo-inositol supplementation are associated with distinct plasma metabolome profile in broiler chickens. Animal. (2020) 14:549–59. 10.1017/S1751731119002337. [DOI] [PubMed] [Google Scholar]
- 60.Yuan L, Wang M, Zhang X, Wang Z. Effects of protease and non-starch polysaccharide enzyme on performance, digestive function, activity and gene expression of endogenous enzyme of broilers. PLoS ONE. (2017) 12:e0173941–e0173941. 10.1371/journal.pone.0173941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saleh AA, El-Far AH, Abdel-Latif MA, Emam MA, Ghanem R, Abd El-Hamid HS. Exogenous dietary enzyme formulations improve growth performance of broiler chickens fed a low-energy diet targeting the intestinal nutrient transporter genes. PLoS ONE. (2018) 13:e0198085–e0198085. 10.1371/journal.pone.0198085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SA, Jang MJ, Kim SY, Yang Y, Pavlidis HO, Ricke SC. Potential for Prebiotics as Feed Additives to Limit Foodborne Campylobacter Establishment in the Poultry Gastrointestinal Tract. Front Microbiol. (2019) 10:91. 10.3389/fmicb.2019.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baurhoo B, Phillip L, Ruiz-Feria CA. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult Sci. (2007) 86:1070–8. 10.1093/ps/86.6.1070 [DOI] [PubMed] [Google Scholar]
- 64.Baurhoo B, Ferket PR, Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult Sci. (2009) 88:2262–72. 10.3382/ps.2008-00562 [DOI] [PubMed] [Google Scholar]
- 65.Park SH, Kim SA, Lee SI, Rubinelli PM, Roto SM, Pavlidis HO, et al. Original XPC(TM) effect on salmonella typhimurium and cecal microbiota from three different ages of broiler chickens when incubated in an anaerobic in vitro culture system. Front Microbiol. (2017) 8:1070. 10.3389/fmicb.2017.01070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang JF, Bai KW, Su WP, Wang AA, Zhang LL, Huang KH, et al. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult Sci. (2018) 97:1209–19. 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- 67.Liu N, Wang J, Liu Z, Wang Y, Wang J. Effect of supplemental yeast cell walls on growth performance, gut mucosal glutathione pathway, proteolytic enzymes and transporters in growing broiler chickens. J Anim Sci. (2018) 96:1330–7. 10.1093/jas/sky046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi S, Wu S, Shen Y, Zhang S, Xiao Y, He X, et al. Iron oxide nanozyme suppresses intracellular Salmonella Enteritidis growth and alleviates infection in vivo. Theranostics. (2018) 8:6149–62. 10.7150/thno.29303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Y, Xiao Y, Zhang S, Wu S, Gao L, Shi S. Fe(3)O(4) Nanoparticles attenuated salmonella infection in chicken liver through reactive oxygen and autophagy via PI3K/Akt/mTOR signaling. Front Physiol. (2020) 10:1580–1580. 10.3389/fphys.2019.01580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giannenas I, Papadopoulos E, Tsalie E, Triantafillou E, Henikl S, Teichmann K, et al. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol. (2012) 188:31–40. 10.1016/j.vetpar.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 71.Adil S, Magray SN. Impact and manipulation of gut microflora in poultry: a review. J Vet Intern Med. (2012) 11:873–7. 10.3923/javaa.2012.873.877 [DOI] [Google Scholar]
- 72.Wu S, Shen Y, Zhang S, Xiao Y, Shi S. Salmonella interacts with autophagy to offense or defense. Front Microbiol. (2020) 11:721–721. 10.3389/fmicb.2020.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan D, Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. (2014) 5:108–19. 10.4161/gmic.26945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang CH, Teng PY, Lee TT, Yu B. The effects of the supplementation of multi-strain probiotics on intestinal microbiota, metabolites and inflammation of young SPF chickens challenged with Salmonella enterica subsp. enterica. Anim Sci J. (2019) 90:737–46. 10.1111/asj.13205 [DOI] [PubMed] [Google Scholar]
- 75.Park I, Lee Y, Goo D, Zimmerman NP, Smith AH, Rehberger T, et al. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult Sci. (2020) 99:725–33. 10.1016/j.psj.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang W, Cai H, Zhang A, Chen Z, Chang W, Liu G, et al. Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals. (2020) 10:1232. 10.3390/ani10071232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong Y, Li R, Liu Y, Ma L, Zha J, Qiao X, et al. Benefit of dietary supplementation with bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiotics Antimicrob Proteins. (2020) 12:1385–97. 10.1007/s12602-020-09643-w [DOI] [PubMed] [Google Scholar]
- 78.Faseleh Jahromi M, Wesam Altaher Y, Shokryazdan P, Ebrahimi R, Ebrahimi M, Idrus Z, et al. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int J Biometeorol. (2016) 60:1099–110. 10.1007/s00484-015-1103-x [DOI] [PubMed] [Google Scholar]
- 79.Van Immerseel F, Russell JB, Flythe MD, Gantois I, Timbermont L, Pasmans F, et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. (2006) 35:182–8. 10.1080/03079450600711045 [DOI] [PubMed] [Google Scholar]
- 80.Hu Y, Wang L, Shao D, Wang Q, Wu Y, Han Y, et al. Selectived and reshaped early dominant microbial community in the cecum with similar proportions and better homogenization and species diversity due to organic acids as AGP alternatives mediate their effects on broilers growth. Front Microbiol. (2020) 10:2948–2948. 10.3389/fmicb.2019.02948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hassan HMA, Mohamed MA, Youssef AW, Hassan ER. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian-Australas J Anim Sci. (2010) 23:1348–53. 10.5713/ajas.2010.10085 [DOI] [Google Scholar]
- 82.Qaisrani SN, van Krimpen MM, Kwakkel RP, Verstegen MW, Hendriks WH. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poult Sci. (2015) 94:2152–64. 10.3382/ps/pev003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palamidi I, Mountzouris KC. Diet supplementation with an organic acids-based formulation affects gut microbiota and expression of gut barrier genes in broilers. Anim Nutr. (2018) 4:367–77. 10.1016/j.aninu.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saeed M, Khan MS, Kamboh AA, Alagawany M, Khafaga AF, Noreldin AE, et al. L-theanine: an astounding sui generis amino acid in poultry nutrition. Poult Sci. (2020) 99:5625–36. 10.1016/j.psj.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu Z, He X, Ma B, Zhang L, Li J, Jiang Y, et al. Dietary taurine supplementation decreases fat synthesis by suppressing the liver X receptor α pathway and alleviates lipid accumulation in the liver of chronic heat-stressed broilers. J Sci Food Agric. (2019) 99:5631–7. 10.1002/jsfa.9817. [DOI] [PubMed] [Google Scholar]
- 86.Hu H, Dai S, Li J, Wen A, Bai X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult Sci. (2020) 99:1454–61. 10.1016/j.psj.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swaggerty CL, He H, Genovese KJ, Callaway TR, Kogut MH, Piva A, et al. A microencapsulated feed additive containing organic acids, thymol, and vanillin increases in vitro functional activity of peripheral blood leukocytes from broiler chicks. Poult Sci. (2020) 99:3428–36. 10.1016/j.psj.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han L, Yang J, Jing L, Li G, Li L, Ji M, et al. Tau-TCHF Inhibits Spleenic Apoptosis via PI3K-Akt Signaling Pathway in Chickens. In: J. Hu, F. Piao, S.W. Schaffer, A. El Idrissi & J.-Y. Wu, editors. Taurine 11. Singapore: Springer. (2019). p. 555–563. [DOI] [PubMed] [Google Scholar]
- 89.Janz JA, Morel PC, Wilkinson BH, Purchas RW. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. (2007) 75:350–5. 10.1016/j.meatsci.2006.06.027 [DOI] [PubMed] [Google Scholar]
- 90.Omonijo FA, Ni L, Gong J, Wang Q, Lahaye L, Yang C. Essential oils as alternatives to antibiotics in swine production. Anim Nutr. (2018) 4:126–36. 10.1016/j.aninu.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jerzsele A, Szeker K, Csizinszky R, Gere E, Jakab C, Mallo JJ, et al. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult Sci. (2012) 91:837–43. 10.3382/ps.2011-01853 [DOI] [PubMed] [Google Scholar]
- 92.Peng QY, Li JD, Li Z, Duan ZY, Wu YP. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim Feed Sci Technol. (2016) 214:148–53. 10.1016/j.anifeedsci.2016.02.010 [DOI] [Google Scholar]
- 93.Saeed M, Naveed M, Leskovec J, Ali Kamboh A, Kakar I, Ullah K, et al. Using Guduchi (Tinospora cordifolia) as an eco-friendly feed supplement in human and poultry nutrition. Poult Sci. (2020) 99:801–11. 10.1016/j.psj.2019.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khattak F, Ronchi A, Castelli P, Sparks N. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult Sci. (2014) 93:132–7. 10.3382/ps.2013-03387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burt S. Essential oils: their antibacterial properties and potential applications in foods–a review. Int J Food Microbiol. (2004) 94:223–53. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 96.Shen SC, Ko CH, Tseng SW, Tsai SH, Chen YC. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol Appl Pharmacol. (2004) 197:84–95. 10.1016/j.taap.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 97.Youssef IMI, Männer K, Zentek J. Effect of essential oils or saponins alone or in combination on productive performance, intestinal morphology and digestive enzymes' activity of broiler chickens. J Anim Physiol Anim Nutr. (2021) 105:99–107. 10.1111/jpn.13431 [DOI] [PubMed] [Google Scholar]
- 98.Rajput SA, Sun L, Zhang N-Y, Khalil MM, Ling Z, Chong L, et al. Grape seed proanthocyanidin extract alleviates AflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins. (2019) 11:23. 10.3390/toxins11010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang L, Liu G, Lian K, Qiao Y, Zhang B, Zhu X, et al. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers. J Anim Sci. (2019) 97:1679–92. 10.1093/jas/skz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lv Z, Dai H, Wei Q, Jin S, Wang J, Wei X, et al. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult Sci. (2020) 99:3411–27. 10.1016/j.psj.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J, Jia R, Celi P, Ding X, Bai S, Zeng Q, et al. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs. Food Funct. (2020) 11:534–43. 10.1039/C9FO02157D [DOI] [PubMed] [Google Scholar]
- 102.Yang JX, Maria TC, Zhou B, Xiao FL, Wang M, Mao YJ, et al. Quercetin improves immune function in Arbor Acre broilers through activation of NF-κB signaling pathway. Poult Sci. (2020) 99:906–13. 10.1016/j.psj.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]