Abstract
Objective
To evaluate the diagnostic performance of dynamic contrast‐enhanced magnetic resonance imaging (DCE‐MRI) for breast cancer identification.
Methods
A comprehensive electronic systematic searching of Medline, Ovid, EMBASE, Web of Science, CNK, and Cochrane Library databases was performed up to 2 August 2021. Clinical studies associated with DCE‐MRI for breast cancer detection were screened and inlcuded in the meta‐analysis. The data of true positive(tp), false positive(fp), false negative(fn) and true negative(tn) was extracted from includded studies. The sensitivity, specificity, diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic (SROC) were pooled under fixed or random effect models. Publication bias was evaluated by Deek's funnel plot.
Results
A final set of 15 studies with 1321 breast lesions were included in the present work. The pooled diagnostic sensitivity, specificity, and DOR were 0.87 (95% confidence interval [CI] 0.81–0.92), 0.74 (95% CI 0.68–0.80), and 18.83 (95% CI 9.07–36.54), respectively, and the area under the SROC was 0.86 (95% CI 0.82–0.88). Given a pretest probability of 50%, the positive post‐test probability was 77%, and the negative post‐test probability was 14%. Deek's funnel plot indicated low publication bias (p = 0.61).
Conclusion
DCE‐MRI is a noninvasive method of breast cancer diagnosis for suspected malignant breast lesions with relative high diagnostic sensitivity and specificity.
Keywords: breast cancer, DCE‐MRI, diagnosis, meta‐analysis
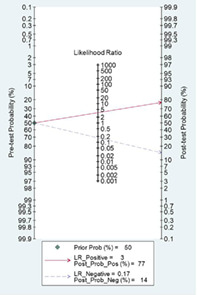
Likelihood ratio and post‐test probability are important indexes of diagnostic tests. In our meta‐analysis, both the positive and negative likelihood ratio and post‐test probability were moderate. Given a pretest probability of 50%, the positive post‐test probability is 77% and the negative post‐test probability is 14%.
INTRODUCTION
Breast cancer is one of the most common malignant tumors and leading cause of cancer‐associated death for females. It has been estimated that 284 200 new breast carcinomas and 44 130 associated deaths will occurred in 2021 in the United States. 1 In China, breast cancer is also a common carcinoma and one of the main causes of cancer‐related death for women. In recent years, the incidence of breast disease has increased and some of it is malignant. 2 Therefore, the ability to distinguish benign from malignant breast lesions is important and affects the treatment strategy. Breast ultrasonography and mammography are the main diagnostic tools for breast carcinoma. However, these two methods are generally unsatisfactory in distinguishing benign from malignant breast lesions, especially for dense fibroglandular tissue, and may lead to unnecessary breast lesion biopsy.
Magnetic resonance imaging (MRI) is widely used for medical diagnosis, staging, and follow‐up of disease. 3 , 4 , 5 With research conducted over two decades, breast MRI has become an established clinical imaging modality for management of breast diseases without invasion. Dynamic contrast‐enhanced MRI (DCE‐MRI) is a rapidly evolving imaging technique that is considered to be one of the major methods for breast MR imaging. Studies have evaluated the clinical diagnostic performance of DCE‐MRI in distinguishing benign from malignant breast lesions. 6 Most studies showed satisfactory findings with relative high diagnostic sensitivity and specificity. In the present work, we combined the open published data relevant to DCE‐MRI in breast cancer to provide more reliable evidence for DCE‐MRI application in breast carcinoma diagnosis.
MATERIAL AND METHODS
Search strategy
Study searching was based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 7 A comprehensive electronic systematic searching of the Medline, Ovid, EMBASE, Web of Science, CNK, and Cochrane Library databases was performed up to 2 August 2021. The electronic searching keywords were (breast neoplasms OR breast lesions OR breast cancer OR breast tumor OR mammary neoplasms OR mammary cancer OR mammary tumor) and (magnetic resonance OR MR) and (dynamic contrast enhanced OR DCE MR). Languages were limited to English and Chinese.
Inclusion and exclusion criteria
The following inclusion criteria for studies were applied:
(i) Prospective or retrospective clinical studies relevant to DCE‐MRI for breast cancer detection.
(ii) Enough data true positive (tp), false positive (fp), false negative (fn), and true negative (tn) can be extracted or calculated from the original studies.
(iii) Pathology or cytology was applied as the gold reference diagnostic method.
The exclusion criteria were as follows:
(i) Duplicated publication or data.
(ii) Studies on animals.
(iii) Studies without enough data to calculated tp, fp, fn, and tn.
Data extraction
Two authors (D.H.H. and K.L.Q.) reviewed the full‐text manuscript and independently extracted the data from all the included studies. The following information and data were extracted from the included original publications:
(i) First author of the included studies.
(ii) Period of the study published.
(iii) Sample size and breast lesions.
(iv) Age of the included cases.
(v) MRI type and manufacturer.
(vi) tp, fp, fn, and tn distribution.
Statistical analysis
STATA12.0 (Stata Corp LP) statistical software was applied for data analysis. The heterogeneity tests were performed by Q test and I 2 statistics. I 2 > 50% was considered as statistically heterogeneity significant and data was combined by a random effect model or a fixed effects model. The publication bias was evaluated by Deek's funnel plot.
RESULTS
Literature searching and study screening
After systematically searching the relevant electronic databases and other sources, we initially identified 621 publications. After duplicated studies and data removing, 402 studies were further screened for title and abstract. After reviewing the titles and abstracts, 334 studies were excluded and 68 publications were reviewed for full‐text evaluation. Finally, 15 studies were deemed to fulfil the inclusion critera and were included in the meta‐analysis (Figure 1 ).
FIGURE 1.
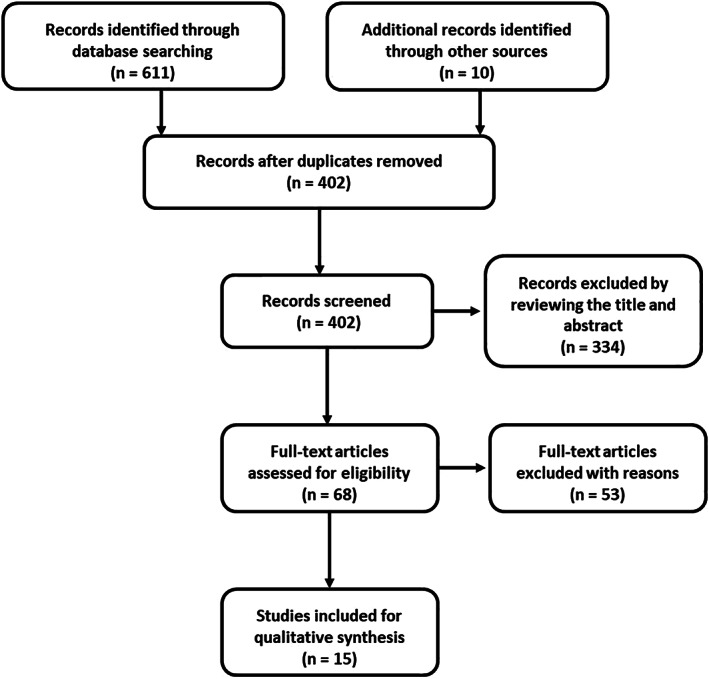
Flow chart of study identification according to inclusion and exclusion criteria
General characteristics of the included studies
A final set of 15 studies with 1321 breast lesions were included in the present work. The sample size of each study ranged from 13 to 205. All studies were published in English or Chinese. The age of the included cases ranged from 38.7 to 56.7 years. The MRI devices were purchased from GE, Simens, Philips or Bayer. Ten studies from China, two from Japan, one from the United States, one from Turkey, and one from Austria. The main features of the included studies are shown in Table 1.
TABLE 1.
The general features of the 15 studies included in this meta‐analysis
| Author | Year | Country | Sample/lesion | Age (year) | MRI | Company |
|---|---|---|---|---|---|---|
| Guan 8 | 2017 | China | 13/21 | 56.7 | 3.0 T | GE |
| Yang 9 | 2017 | China | 85/85 | 48.7 | 1.5 T | GE |
| Peng 10 | 2016 | China | 78/83 | 50.7 | 1.5 | SIEMENS |
| He 11 | 2019 | China | 69/69 | 48.2 | 3.0 T | NA |
| Jiang 12 | 2017 | China | 80/80 | 38.7 | 1.5 T | PHILIPS |
| Zhang 13 | 2018 | China | 82/94 | 40.5 | 3.0 T | SIEMENS |
| Zhang 14 | 2018 | China | 60/60 | 44.3 | 1.5/3.0 T | GE |
| Jiang 15 | 2018 | China | 205/275 | NA | 1.5 T | GE |
| Kim 16 | 2016 | USA | 115/139 | 51.0 | 3.0 T | BAYER |
| Kul 17 | 2011 | Turkey | 26/29 | NA | 1.5 T | SIEMENS |
| Yabuuchi 18 | 2008 | Japan | 71/75 | NA | 1.5 T | PHILIPS |
| Pinker 19 | 2014 | Austria | 113/113 | 52.0 | 30.T | SIEMENS |
| Imamura 20 | 2010 | Japan | NA/27 | NA | 1.5 | PHILIPS |
| Luo 21 | 2013 | China | 95/95 | 51.0 | 3.0 | PHILIPS |
| Tang 22 | 2008 | China | 48/70 | 51.0 | 1.5 T | SIEMENS |
Statistical heterogeneity across the studies
Significant statistical heterogeneity was identified in diagnostic sensitivity (I 2 = 80.34, p < 0.01), specificity (I 2 = 60.95, p < 0.01), and diagnostic odds ratio (DOR, I 2 = 72.70, p < 0.01). The data was pooled in a random effect model.
Results of meta‐analysis
Fifteen studies were included and data pooling carried out under random effect model. The pooled diagnostic sensitivity, specificity, and DOR were 0.87 (95% confidence interval [CI] 0.81–0.92; Figure 2), 0.74 (95% CI 0.68–0.80; Figure 2), and 18.83 (95% CI 9.07–36.54; Figure 3), respectively, with an area under the summary receiver operating characteristic (SROC) of 0.86 (95% CI 0.82–0.88; Figure 4).
FIGURE 2.
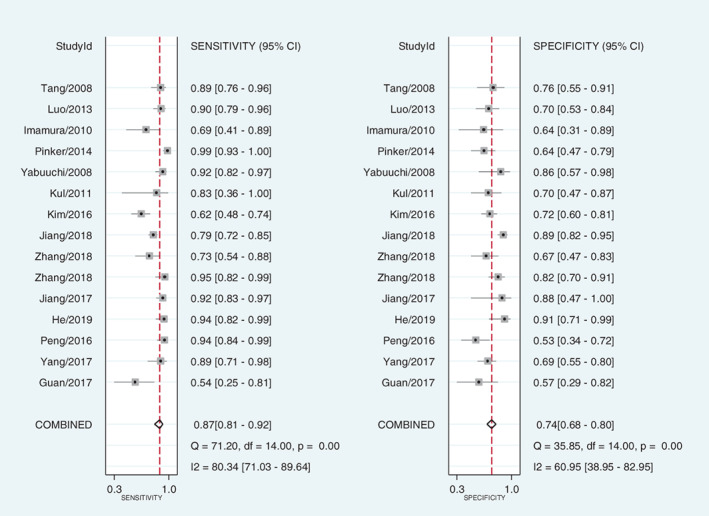
Forest plot of the diagnostic sensitivity and specificity of dynamic contrast‐enhanced magnetic resonance imaging for breast cancer detection
FIGURE 3.
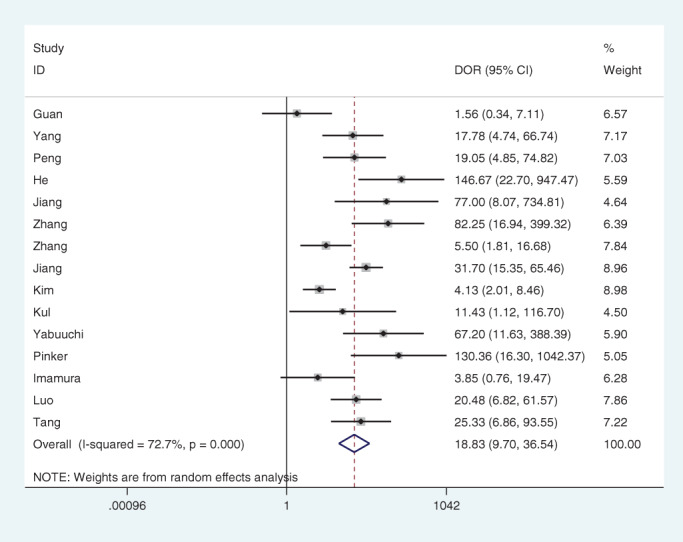
Forest plot of the diagnostic odds ratio (DOR) of dynamic contrast‐enhanced magnetic resonance imaging for breast cancer detection
FIGURE 4.
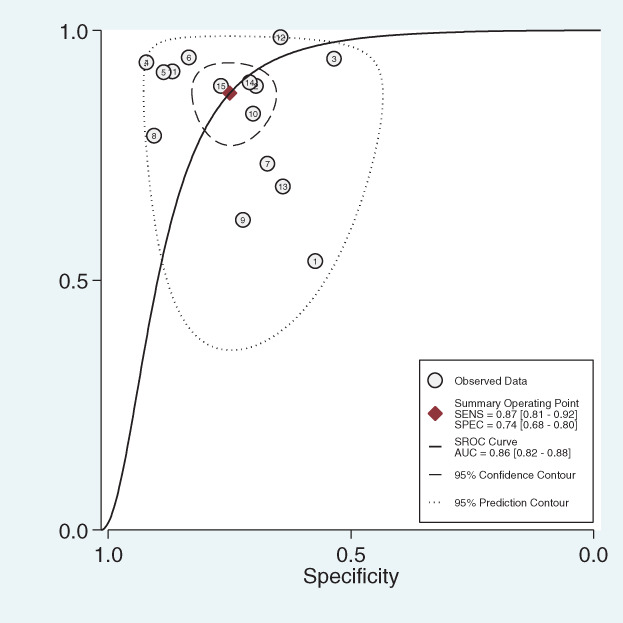
Summary receiver operating characteristic (SROC) of dynamic contrast‐enhanced magnetic resonance imaging for breast cancer detection. AUC, area under the summary receiver operating characteristic; SENS, sensitivity; SPEC, specificity
Predictive values
Likelihood ratio and post‐test probability were important indexes of the diagnostic tests. In our meta‐analysis, both the positive and negative likelihood ratio and post‐test probability were moderate (Figure 5). Given a pretest probability of 50%, the positive post‐test probability is 77% and the negative post‐test probability is 14%.
FIGURE 5.
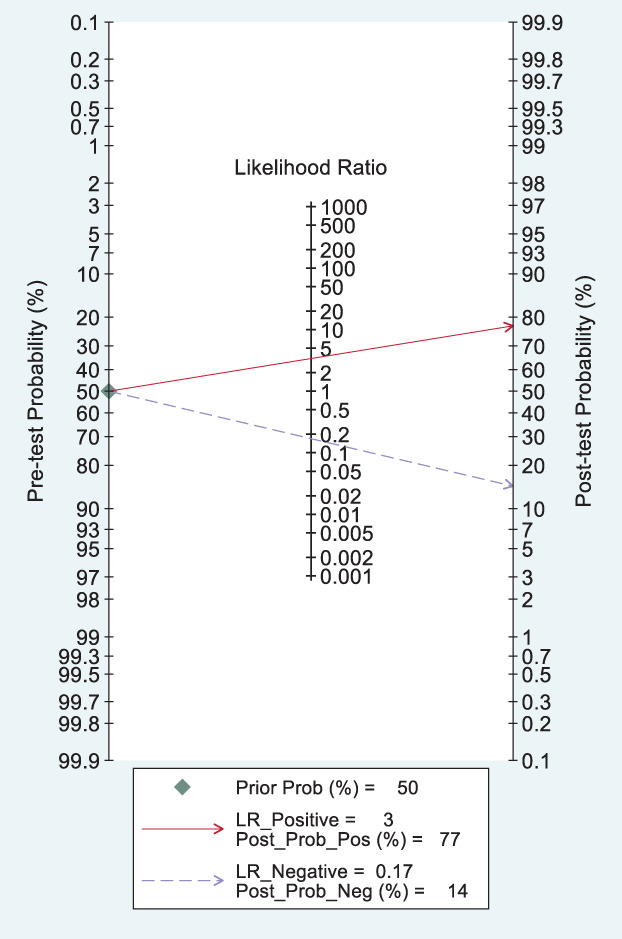
Fagan's plot for the post‐test probability of breast cancer after a positive result (upper line) or a negative result (lower line) from a dynamic contrast‐enhanced magnetic resonance imaging examination. LR, likelihood ratio
Publication bias
The shape of the Deek's funnel plot was generally symmetrical, which indicates low publication bias (p = 0.61; Figure 6).
FIGURE 6.
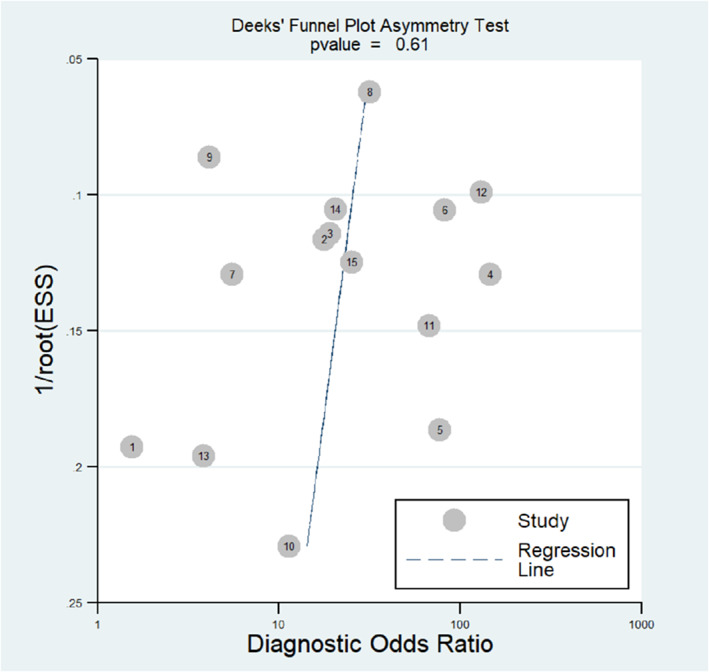
Deeks' funnel plot in evaluation of the publication bias of dynamic contrast‐enhanced magnetic resonance imaging for breast cancer detection. ESS, effect sample size
DISCUSSION
This comprehensive update meta‐analysis aggregated 15 open published studies and 1321 breast lesions to further investigate the clinical diagnostic performance of DCE‐MRI for breast carcinoma identification. The findings of the present work show that the combined diagnostic sensitivity, specificity, and DOR are 0.87 (95% CI 0.81–0.92), 0.74 (95% CI 0.68–0.80), and 18.83 (95% CI 9.07–36.54), respectively, with the area under the SROC 0.86 (95% CI 0.82–0.88). Both the combined sensitivity and specificity were relatively high and provide good diagnostic performance of breast carcinoma. In addition, the work also found that given a pretest probability of 50%, the positive post‐test probability is 77% and the negative post‐test probability is 14%. Our meta‐analysis demonstrates that DCE‐MRI is a noninvasive method of breast cancer diagnosis for suspected malignant breast lesions with relative high diagnostic sensitivity and specificity.
Mammography was applied in detection or screening of breast lesions in most of the countries. 23 , 24 However, its clinical application was limited to the density of the breast tissue. 25 In young women and Asian females, the breast tissue is general dense and mammography may provide limited information to distinguish benign from malignant lesions. However, MRI quality is not affected by the density of the breast and can provide high discrimination power from malignant to benign lesion. MRI was therefore was applied for breast disease examination and studies confirmed that MRI can identify lesions that are not detected by mammography or ultrasound in high‐risk women. In March 2007, the American Cancer Society in the United States issued a guideline recommending annual MRI screening for women who have greater than 20–25% lifetime risk of developing breast cancer. 26
Neoplasma, particularly the more aggressive carcinoma, needs angiogenesis and more nutrition to support rapid cancer cells growth. The new angiogenic vessels have a wider endothelial junction, which allows contrast agents to quickly leak from vascular space into the interstitial space and back‐diffuse to the vascular space to be cleared. 27 , 28 DCE‐MRI was applied to measure the transport kinetics of contrast agents in the tissue, allowing for analysis of transfer rates associated with vascular perfusion and permeability. Despite this advantage, the motivation for performing DCE‐MRI for diagnosis of breast cancer is mainly problem solving to detect the lesion without being obscured by strong background tissue enhancement, as well as to differentiate between malignant and benign lesions. 29
In 2016, Zhang et al. 6 performed a meta‐analysis of combined dynamic contrast‐enhanced magnetic resonance imaging and diffusion‐weighted imaging for breast cancer detection. The authors found that the pooled sensitivity and specificity of DCE‐MRI were 93.2% and 71.1%. The area under the SROC curve was 0.85. Our meta‐analysis found the combined diagnostic sensitivity, specificity, and AUC‐SROC were 0.87, 0.74, and 0.86, respectively, which is in accordance with Zhang's findings. Furthermore, our work also investigated the pre‐ and post‐test probability. Given a pretest probability of 50%, the positive post‐test probability is 77% and the negative post‐test probability is 14%. The diagnostic accuracy of malignant or benign lesions was moderately improved after DCE‐MRI examination. The improved diagnostic accuracy may also reduce the need for breast biopsy.
This work was subject to two main limitations. (i) The clinical heterogeneity between included studies was obvious, such as the subject's age, race, breast lesion diameter, etc., and may affect the results and conclusions. Another clinical heterogeneity may arise from the radiology. The professional experience of the radiologists in each study was different, and this may affect the diagnostic accuracy of the DCE‐MIR. (ii) The statistical heterogeneity between the included studies was also significant and may reduce the statistical power of the combined effect size.
In conclusion, this meta‐analysis combined 15 studies and found DCE‐MRI was a noninvasive method of breast cancer diagnosis for suspected malignant breast lesions with relatively high diagnostic sensitivity and specificity. However, due to the two main limitations described, the conclusion needs further validation by well‐designed large sample size prospective diagnostic studies.
CONFLICT OF INTEREST
Not reported.
Dong H, Kang L, Cheng S, Zhang R. Diagnostic performance of dynamic contrast‐enhanced magnetic resonance imaging for breast cancer detection: An update meta‐analysis. Thorac Cancer. 2021;12:3201–3207. 10.1111/1759-7714.14187
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- 3. Thompson JL, Wright GP. The role of breast MRI in newly diagnosed breast cancer: an evidence‐based review. Am J Surg. 2021;221:525–8. [DOI] [PubMed] [Google Scholar]
- 4. Newman LA. Role of preoperative MRI in the management of newly diagnosed breast cancer patients. J Am Coll Surg. 2020;230:331–9. [DOI] [PubMed] [Google Scholar]
- 5. Partovi S, Sin D, Lu Z, Sieck L, Marshall H, Pham R, et al. Fast MRI breast cancer screening ‐ ready for prime time. Clin Imaging. 2020;60:160–8. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of combined dynamic contrast‐enhanced magnetic resonance imaging and diffusion‐weighted imaging for breast cancer detection: a meta‐analysis. Acta Radiol. 2016;57:651–60. [DOI] [PubMed] [Google Scholar]
- 7. Arya S, Kaji AH, Boermeester MA. PRISMA reporting guidelines for meta‐analyses and systematic reviews. JAMA Surg. 2021;156:789–90. [DOI] [PubMed] [Google Scholar]
- 8. Guan L. Diagnostic value of MR dynamic enhancement combined with diffusion weighted imaging in breast diseases. China Pract Med. 2017;12:88–90. [Google Scholar]
- 9. Yang HL. Application value of dynamic contrast‐enhanced MRI in the diagnosis of breast diseases. Henan Med Res. 2017;26:2571–2. [Google Scholar]
- 10. Peng JB, Li WW, Fan XT, Jiang KP, Li CM. The application value of dynamic enhanced MRI and diffusion weighted imaging in the diagnosis of breast cancer. Hebei Med. 2016;22:1626–9. [Google Scholar]
- 11. He GY, Shen D. The role of dynamic enhanced MRI in the diagnosis of breast cancer. Mod Med Imagel. 2019;28:1798–9. [Google Scholar]
- 12. Jiang ZY. Analysis of value of dynamic enhancement MRI in diagnosis of breast cancer. China Foreign Med Treat. 2017;36:185–6. +189. [Google Scholar]
- 13. Zhang L. Comparison of the value of dynamic enhanced MRI and molybdenum target in the diagnosis of breast cancer. Chin Commun Doctors. 2018;34:122–3. [Google Scholar]
- 14. Zhang JA, Chen HJ, Wang SS. Clinical value of diffusion‐weighted imaging combined with dynamic contrast‐enhanced MRI in the diagnosis of atypical breast cancer. Chin J Med Device. 2018;31:42–3. [Google Scholar]
- 15. Jiang X, Xie F, Liu L, Peng Y, Cai H, Li L. Discrimination of malignant and benign breast masses using automatic segmentation and features extracted from dynamic contrast‐enhanced and diffusion‐weighted MRI. Oncol Lett. 2018;16:1521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SG, Freed M, Leite A, Zhang J, Seuss C, Moy L. Separation of benign and malignant breast lesions using dynamic contrast enhanced MRI in a biopsy cohort. J Magn Reson Imaging. 2017;45:1385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kul S, Cansu A, Alhan E, Dinc H, Gunes G, Reis A. Contribution of diffusion‐weighted imaging to dynamic contrast‐enhanced MRI in the characterization of breast tumors. Am J Roentgenol. 2011;196:210–7. [DOI] [PubMed] [Google Scholar]
- 18. Yabuuchi H, Matsuo Y, Okafuji T, Kamitani T, Soeda H, Setoguchi T, et al. Enhanced mass on contrast‐enhanced breast MR imaging: lesion characterization using combination of dynamic contrast‐enhanced and diffusion‐weighted MR images. J Magn Reson Imaging. 2008;28:1157–65. [DOI] [PubMed] [Google Scholar]
- 19. Pinker K, Bogner W, Baltzer P, Gruber S, Bickel H, Brueck B, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast‐enhanced magnetic resonance imaging, diffusion‐weighted imaging, and 3‐dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol. 2014;49:421–30. [DOI] [PubMed] [Google Scholar]
- 20. Imamura T, Isomoto I, Sueyoshi E, Yano H, Uga T, Abe K, et al. Diagnostic performance of ADC for non‐mass‐like breast lesions on MR imaging. Magn Reson Med Sci. 2010;9:217–25. [DOI] [PubMed] [Google Scholar]
- 21. Yi L, Jianqun Y, Dongdong C, Zhongzi X, Hanjiang Z. The actions of diffusion weighted imaging (DWI) and dynamic contrast enhanced MRI in differentiating breast tumors. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013;30:1219–23. [PubMed] [Google Scholar]
- 22. Tang JH, Yan FH, Zhou ML, Ye F, Xu PJ. Comparative study of diffusion weighted imaging and dynamic contrast enhanced MRI for the detection of small breast cancers. Chin J Radiol. 2008;42:152–6. [Google Scholar]
- 23. Dibden A, Offman J, Duffy SW, Gabe R. Worldwide review and meta‐analysis of cohort studies measuring the effect of mammography screening Programmes on incidence‐based breast cancer mortality. Cancers (Basel). 2020;12:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broeders M, Allgood P, Duffy SW, Hofvind S, Nagtegaal ID, Paci E, et al. The impact of mammography screening programmes on incidence of advanced breast cancer in Europe: a literature review. BMC Cancer. 2018;18:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Wu Z, Chen L, George F, Chen Z, Salem A, et al. Breast tissue density and CAD cancer detection in digital mammography. Conf Proc IEEE Eng Med Biol Soc. 2005;2005:3253–6. [DOI] [PubMed] [Google Scholar]
- 26. Tillman GF, Orel SG, Schnall MD, Schultz DJ, Tan JE, Solin LJ. Effect of breast magnetic resonance imaging on the clinical management of women with early‐stage breast carcinoma. J Clin Oncol. 2002;20:3413–23. [DOI] [PubMed] [Google Scholar]
- 27. Daldrup‐Link HE, Okuhata Y, Wolfe A, Srivastav S, Øie S, Ferrara N, et al. Decrease in tumor apparent permeability‐surface area product to a MRI macromolecular contrast medium following angiogenesis inhibition with correlations to cytotoxic drug accumulation. Microcirculation. 2004;11:387–96. [DOI] [PubMed] [Google Scholar]
- 28. Raatschen HJ, Simon GH, Fu Y, Sennino B, Shames DM, Wendland MF, et al. Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats. Radiology. 2008;247:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J. Analysis of DCE‐MRI for diagnosis and neoadjuvant chemotherapy monitoring of breast cancer. Chin J Magn Reson Imaging. 2012;3:84–97. [Google Scholar]


