Abstract
Seventy-six, crossbred, porcine reproductive and respiratory syndrome virus (PRRSV)-free pigs were weaned at 12 days of age and randomly assigned to seven groups of 10 to 11 pigs each. Pigs in group 1 served as unchallenged controls. Pigs in groups 2 to 7 were challenged intranasally with 2 ml of high-virulence PRRSV isolate VR-2385 (104.47 50% tissue culture infective doses per 2 ml) on day 0 of the study (30 days of age). Seven days after PRRSV challenge, pigs in groups 2 to 7 were challenged intranasally with 2 ml of Streptococcus suis serotype 2 (108.30 CFU/2 ml). Group 2 pigs served as untreated positive controls. Antimicrobial treatments included daily intramuscular injection with 66,000 IU of procaine penicillin G per kg of body weight on days 8 to 10 (group 3), drinking water medication with 23.1 mg of tiamulin per kg during days 8 to 10 (group 4), and daily intramuscular injection of 5.0 mg of ceftiofur hydrochloride per kg on days 8 to 10 (group 5). Vaccination regimens included two intramuscular doses of an autogenous killed S. suis vaccine (group 6) prior to S. suis challenge or a single 2-ml intramuscular dose of an attenuated live PRRSV vaccine (group 7) 2 weeks prior to PRRSV challenge. Mortality was 0, 63, 45, 54, 9, 40, and 81% in groups 1 to 7, respectively. Ceftiofur treatment was the only regimen that significantly (P < 0.05) reduced mortality associated with PRRSV and S. suis coinfection. The other treatments and vaccinations were less effective. We conclude that ceftiofur administered by injection for three consecutive days following S. suis challenge was the most effective regimen for minimizing disease associated with PRRSV and S. suis coinfection.
Field evidence strongly suggests that PRRSV infection makes pigs more susceptible to bacterial diseases in nursery and grow-finish pigs (2). Combined porcine reproductive and respiratory syndrome virus (PRRSV) and Streptococcus suis infections are common (10, 14, 16) and can be especially problematic to control with conventional medication and vaccination regimens (1, 15). Models to study the pathogenesis and control of PRRSV and S. suis coinfection have recently been described (4, 14). Our coinfection model uses 2- to 4-week-old conventional pigs which are inoculated intranasally with PRRSV, followed 7 days later by intranasal inoculation with S. suis (14). In this model, we demonstrated that pigs infected with the high-virulence VR-2385 strain of PRRSV exhibit more frequent and severe clinical central nervous system (CNS) disease and lesions typical of S. suis infection, have more widespread tissue dissemination of S. suis, and experience significantly higher mortality than pigs infected with S. suis alone. We believe that the model mimics what occurs in the field, making it an ideal model to test the efficacy of control and treatment regimens. The objective of the study reported here was to measure the efficacies of several commonly used control and treatment protocols for minimizing losses associated with PRRSV and S. suis coinfection of nursery pigs.
MATERIALS AND METHODS
Experimental design.
The study was approved by the Iowa State University Committee on Animal Care and Use. Seventy-six, crossbred, PRRSV-free pigs were weaned at 12 days of age and moved to an isolated facility. The pigs were randomly assigned to seven groups of 10 to 11 pigs each (Table 1). Pigs in group 1 served as unchallenged negative controls. Pigs in the remaining groups (2 to 7) were challenged intranasally with 2 ml of high-virulence PRRSV isolate VR-2385 on day 0 of the study (30 days of age). Seven days after PRRSV challenge, pigs in groups 2 to 7 were challenged intranasally with 2 ml of S. suis serotype 2, isolate ISU VDL 40634/94.
TABLE 1.
Experimental design of control and treatment protocols for PRRSV and S. suis coinfection
| Group | No. of pigs | Challengea | Treatment (codeb) | Dose | Routec | Day(s) of treatment |
|---|---|---|---|---|---|---|
| 1 | 11 | None | None (NC) | |||
| 2 | 11 | PRRS and S. suis | None (PC) | |||
| 3 | 11 | PRRS and S. suis | Procaine penicillin G (Pfi-Pen G) (PEN) | 66,000 IU/kg | i.m. | 8, 9, 10 |
| 4 | 11 | PRRS and S. suis | Tiamulin (Denagard) (TIA) | 23.1 mg/kg | p.o. | 8, 9, 10 |
| 5 | 11 | PRRS and S. suis | Ceftiofur (Excenel) (CEF) | 5.0 mg/kg | i.m. | 8, 9, 10 |
| 6 | 10 | PRRS and S. suis | Autogenous S. suis vaccine (SS VX) | 2 ml | i.m. | −18, −4 |
| 7 | 11 | PRRS and S. suis | MLV PRRSV vaccine (RespPRRS/Repro) (PR VX) | 2 ml | i.m. | −14 |
PRRSV challenge on day 0, S. suis challenge on day 7.
NC, negative controls; PC, positive controls; PEN, penicillin; TIA, tiamulin; CEF, ceftiofur; SS VX, S. suis vaccine; PR VX, PRRSV vaccine.
i.m., intramuscular injection; p.o., per os via drinking water.
Pigs in group 2 served as untreated, dually inoculated controls. Pigs in group 3 were treated by intramuscular injection with 66,000 IU of procain penicillin G (Pfi-Pen G; Pfizer Animal Health, New York, N.Y.) per kg of body weight on days 8, 9, and 10. Pigs in group 4 received 23.1 mg of tiamulin (Denegard; Boehringer Ingelheim Animal Health, Inc., St. Joseph, Mo.) per kg per pig per day in the drinking water on days 8, 9, and 10. Pigs in group 5 were treated by intramuscular injection with 5.0 mg of ceftiofur hydrochloride (Excenel; Pharmacia & Upjohn, Kalamazoo, Mich.) per kg on days 8, 9, and 10. Pigs in group 6 received two intramuscular doses of a commercially prepared autogenous S. suis vaccine prior to S. suis challenge. The S. suis vaccine was prepared from the same isolate used for challenge, was formaldehyde inactivated, and was in Emulsigen and aluminum hydroxide adjuvants (MVP Laboratories Inc., Ralston, Nebr.). Pigs in group 7 received a single 2-ml intramuscular dose of attenuated live PRRSV vaccine (RespPRRS/Repro; Boehringer Ingelheim Animal Health, Inc.) 2 weeks prior to PRRSV challenge.
Inoculum preparation.
PRRS challenge virus isolate VR-2385 was propagated on MARC-145 cells and titrated by serial 10-fold dilutions in a 96-well microtiter plate. The challenge virus was at the seventh passage in cell culture and had a titer of 104.47 50% tissue culture infective doses/ 2 ml.
S. suis serotype 2, isolate ISU VDL 40634/94, was originally cultured from the meninges of a nursery pig that was naturally infected with S. suis. The isolate was passed by intravenous inoculation into a 3-week-old pig, which was euthanized when it exhibited signs of CNS disease. The brain and meninges were collected and homogenized, and aliquots of the homogenate were frozen at −70°C. The bacterial challenge inoculum for this study was prepared by growing an aliquot of the brain and meninges homogenate on bovine blood agar plates (BAPs) overnight and then in Todd-Hewitt broth with 5% fetal calf serum for 7.25 h. The bacteria and growth media were diluted 1:10 with Hanks' balanced salt solution and given intranasally to pigs. Pigs were administered 108.30 CFU/2-ml dose intranasally. The inoculum was checked for purity by streaking onto a BAP and incubating at 37°C in 5% CO2 air.
The susceptibility of the challenge inoculum to the three antimicrobials was determined by a microbroth dilution breakpoint susceptibility test (Sensititre; Trek Diagnostic Systems, Inc., Westlake, Ohio) and by determination of the MICs by microbroth dilution (Trek Diagnostic Systems, Inc.). The isolate was susceptible to all of the antimicrobials used in this experiment at levels at or below the lowest dilution tested. The isolate was found to be susceptible to ceftiofur at <1.0 μg/ml, tiamulin at <8.0 μg/ml, and penicillin at <0.03 μg/ml by the microbroth dilution breakpoint susceptibility test. The isolate was found to be susceptible to ceftiofur at <0.50 μg/ml, tiamulin at <4.0 μg/ml, and penicillin at <0.12 μg/ml by the MIC susceptibility test.
Clinical evaluation.
Daily clinical respiratory disease scores, ranging from 0 to 6 (0 = normal, 6 = severe), were recorded on days 0 to 28 postchallenge with PRRSV as previously described (5, 6). Other clinical observations, including rectal temperatures, inappetence, lethargy, CNS signs, and swollen joints and lameness (0 = normal, 1 = mild, 2 = moderate, and 3 = severe) were recorded daily.
Gross and microscopic pathology examination.
Pigs exhibiting severe CNS disease (ataxia, prostration, or opisthotonus) or severe joint swelling and lameness resulting in recumbence were euthanized immediately and necropsied. Complete necropsies were performed on all remaining pigs on day 28 (28 days after PRRSV and 21 days after S. suis challenge). An estimated percentage of the lung with grossly visible pneumonia was recorded for each pig based on a previously described PRRS lung lesion scoring scheme (5, 6). Sections for histopathologic examination were taken from nasal turbinate, lung, heart, brain, lymph nodes, tonsil, thymus, liver, spleen, joints, and kidney. Sections of lung were blindly examined microscopically and given a score for severity of interstitial pneumonia (0 = normal, 1 = mild, 2 = moderate, and 3 = severe).
Serology and virus isolation.
Blood was collected from all pigs at necropsy and from all remaining live pigs on days 0, 7, 14, 21, and 28 postinoculation. Serum antibodies to PRRSV were measured using the Herd Check PRRSV enzyme-linked immunosorbent assay (IDEXX Laboratories, Westbrook, Mass.). Bronchoalveolar lavage (BAL) was performed aseptically at necropsy using 50 ml of lavage fluid consisting of minimal essential medium with antibiotics (9 μg of gentamicin/ml, 100 U of penicillin G/ml, and 100 μg of streptomycin/ml). Lavage fluid was gently dispensed and aspirated several times into the lungs. The BAL fluid was kept at −70°C until PRRSV isolation was attempted on a confluent monolayer of MARC-145 cells (11–13). Viral cytopathic effect was confirmed by an indirect immunofluorescence assay (8). Monolayers were stained with anti-PRRSV monoclonal antibody SDOW-17 (9) and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (Sigma, St. Louis, Mo.) and then viewed with a fluorescence microscope for evidence of specific viral antigens. If cytopathic effect was not observed within 7 days, the cultures were frozen and thawed and blindly passaged two more times before they were considered negative.
Bacteriology.
Whole blood was collected in EDTA tubes and cultured on BAPs and in Todd-Hewitt broth on days 7, 8, 9, and 10 from six randomly selected pigs in each group. The upper respiratory tract (nasal cavity and trachea), lungs, mandibular lymph node, pericardium, peritoneum, pleura, spleen, liver, CNS (brain and meninges), and joints were swabbed and cultured for S. suis serotype 2 at necropsy. A blood sample was taken from each animal at necropsy and cultured for S. suis serotype 2. Swabs obtained at necropsy were immediately streaked onto BAPs. All cultures were incubated at 37°C in 5% CO2 for 24 to 48 h. Alpha-hemolytic streptococcus-like colonies were tested for growth in 6.5% NaCl and production of amylase (3). Representative colonies that did not grow in NaCl and were positive for production of amylase were checked by coagglutination to determine if they were S. suis serotype 2 (7).
Statistical analysis.
Mortality and organism isolation data were analyzed by Fisher's exact test using a P of ≤0.05 as the level of significance for comparison. Clinical scores and macroscopic and microscopic lesion scores were evaluated by analysis of variance (ANOVA) using a completely randomized design with the pig as the experimental unit. If the overall ANOVA result was significant (P ≤ 0.05), pairwise comparisons were performed by least-significant-difference analysis.
RESULTS
Clinical evaluation.
Respiratory disease, lameness, and CNS disease scores are summarized in Table 2. Unchallenged, untreated (negative) control pigs remained normal throughout the experiment. Between 3 and 7 days postinoculation (DPI) with PRRSV, pigs in the PRRSV-challenged groups (2 to 7) developed fevers (40.5 to 42°C) and exhibited respiratory disease characterized by rapid and labored respiration. At 7 DPI, groups 2 to 7 were challenged with S. suis. Within 24 h of S. suis challenge, at least 1 pig in each of groups 2 to 7 exhibited CNS disease signs such as head tilt, nystagmus, tremors, ataxia, prostration, and opisthotonus. Antibiotic treatment was initiated in groups 3 to 5 at 24 h after S. suis challenge.
TABLE 2.
Rectal temperature data and respiratory disease, CNS disease, and lameness scoresc
| Group (coded) | No. of days with mean rectal temperatures over 40°C ± SD
|
Mean respiratory disease scorea
|
Mean CNS disease scoreb
|
Mean lameness scoreb
|
||||
|---|---|---|---|---|---|---|---|---|
| 1–11 DPI | 12–28 DPI | 1–11 DPI | 12–28 DPI | 1–11 DPI | 12–28 DPI | 1–11 DPI | 12–28 DPI | |
| 1 (NC) | 0.8 ± 1.2 C | 3.9 ± 2.0 A | 0.0 ± 0.0 C | 0.1 ± 0.1 D | 0.00 ± 0.00 C | 0.00 ± 0.00 A | 0.00 ± 0.00 C | 0.01 ± 0.02 B |
| 2 (PC) | 7.0 ± 3.9 A | 1.5 ± 1.3 A | 2.1 ± 0.4 A | 1.4 ± 0.1 B | 0.14 ± 0.18 A, B | 0.00 ± 0.00 A | 0.49 ± 0.42 A | 0.63 ± 0.16 A |
| 3 (PEN) | 3.7 ± 3.1 B | 3.7 ± 2.8 A | 2.2 ± 0.3 A | 1.4 ± 0.1 B | 0.10 ± 0.17 B, C | 0.01 ± 0.03 A | 0.38 ± 0.34 A, B | 0.71 ± 0.28 A |
| 4 (TIA) | 4.5 ± 1.7 B | 2.6 ± 2.2 A | 2.1 ± 0.2 A | 1.5 ± 0.2 B | 0.12 ± 0.13 A, B | 0.01 ± 0.03 A | 0.25 ± 0.23 B | 0.37 ± 0.26 A |
| 5 (CEF) | 4.8 ± 2.9 A, B | 4.5 ± 4.0 A | 1.8 ± 0.3 B | 1.0 ± 0.5 C | 0.02 ± 0.04 B, C | 0.01 ± 0.02 A | 0.28 ± 0.21 A, B | 0.50 ± 0.58 A |
| 6 (SS VX) | 4.6 ± 1.7 B | 5.2 ± 4.4 A | 2.0 ± 0.2 A | 1.9 ± 0.5 A | 0.08 ± 0.10 B, C | 0.02 ± 0.04 A | 0.27 ± 0.20 A, B | 0.37 ± 0.11 A |
| 7 (PR VX) | 3.6 ± 2.5 B | 0.0 ± 0.0 A | 2.0 ± 0.2 A, B | 2.1 ± 0.2 A | 0.22 ± 0.20 A | 0.00 ± 0.0 A | 0.45 ± 0.16 A, B | 0.32 ± 0.15 A, B |
Respiratory disease scores (0 to 6; 0 = normal, 6 = severe) are reported as group means ± standard deviations.
CNS signs and lameness scores (0 to 3; 0 = normal, 3 = severe) are reported as means ± standard deviations.
Within each column, values followed by letters (A, B, C, and D) are significantly different from other values followed by a different letter(s) (P < 0.05).
See Table 1 for an explanation of codes.
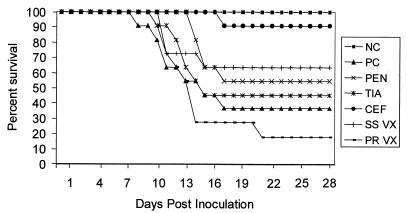
Pigs exhibiting severe CNS disease (ataxia, prostration, or opisthotonous) or severe joint swelling and lameness resulting in recumbency were euthanized and recorded as mortalities. Overall mortality and time of death or euthanasia are summarized in Fig. 1. Pigs from the untreated positive control group (group 2) died or were euthanized on days 8 (1 pig), 10 (1 pig), 11 (2 pigs), 13 (1 pig), 15 (1 pig), and 17 (1 pig), for an overall mortality of 63%. Respiratory disease in group 2 was moderate to severe from 8 to 16 DPI and mostly resolved by 28 DPI. The majority of the pigs in this group exhibited mild-to-severe lameness associated with swollen joints in the rear and front legs from 8 to 20 DPI.
FIG. 1.
Survivability of PRRSV- and S. suis-coinfected pigs after vaccination or antimicrobial treatment. See Table 1 for an explanation of the abbreviations.
Levels of clinical disease severity and progression in the penicillin (group 3)- and tiamulin (group 4)-treated groups were similar. Respiratory disease was moderate to severe between 8 and 17 DPI and mostly resolved by 28 DPI. Three pigs in each of groups 3 and 4 exhibited mild ataxia between 8 and 9 DPI. Joint swelling was present in the majority of the pigs in these groups by 10 DPI. All of the pigs except one pig in group 4 clinically improved during the period of antibiotic administration from 8 to 10 DPI. By 12 DPI, 48 h after antibiotic treatments ceased, the incidence and severity of CNS disease and lameness increased. Five pigs in each of groups 3 and 4 died or were euthanized between 12 and 17 DPI. Mild joint swelling and lameness persisted in several pigs in both of these groups through 28 DPI. Overall mortality was 45 and 54% in the penicillin and tiamulin groups, respectively.
Ceftiofur treatment (group 5) was the only regimen that significantly (P < 0.05) reduced mortality. Pigs in the ceftiofur-treated group remained the healthiest of the coinfected groups. Respiratory disease severity and progression was similar to those features of other PRRSV-infected groups. Ataxia and head tilt were observed in 2 pigs between 8 and 9 DPI. At 17 DPI, one pig was found in opisthotonus and was euthanized. This was the only pig in the ceftiofur group that had to be euthanized (overall mortality, 9%) or died prior to the scheduled necropsy at 28 DPI. Joint swelling and lameness were evident in the majority of the pigs by 10 DPI and became less severe and prevalent following the antibiotic treatment. Mild transient joint swelling and lameness recurred in several of the pigs between 21 and 28 DPI.
Respiratory disease severity and progression in the S. suis-vaccinated group (group 6) were similar to those of groups 2 to 5. Mild-to-severe joint swelling was observed in 9 of 11 pigs, and CNS disease characterized by ataxia and head tilt was observed in 5 of 11 pigs by 11 DPI. Three pigs were euthanized at 11 DPI and one was euthanized at 15 DPI because of CNS disease and/or severe lameness. Overall mortality was 40%. Lameness resolved in the remaining pigs by 28 DPI.
The PRRSV-vaccinated pigs (group 7) remained clinically normal prior to PRRSV challenge. Respiratory disease severity and progression subsequent to PRRSV challenge were similar to those of unvaccinated groups 2 to 6. Nine pigs exhibited tremors, ataxia, head tilt, and/or opisthotonus by 14 DPI. Lameness associated with mild-to-severe joint swelling was observed in 11 of 11 pigs by 11 DPI. Eight pigs were euthanized due to CNS disease or recumbency associated with lameness between 10 and 14 DPI. The nineth pig was euthanized at 21 DPI. Overall mortality was 81%. The CNS signs and lameness resolved by 23 DPI in the remaining two pigs in this group.
Gross and microscopic lesions.
PRRSV-induced gross lesions were characterized by mottled-tan, firm lungs and enlarged, tan lymph nodes. PRRSV-induced gross lung lesions were well developed by 10 DPI when the first pigs in groups 2, 4, and 7 died. Gross lung lesions were not present or were in the resolving stages by 28 DPI. Microscopic examination revealed mild-to-severe, multifocal, proliferative interstitial pneumonia characteristic of PRRSV infection (5, 6). The onset, severity and progression of the microscopic lung lesions were similar in groups 2 to 7. Fibrinosuppurative pleuritis was observed in two pigs in group 2, one pig in group 3, and one pig in group 6. Mild-to-moderate necrotizing and lymphoplasmacytic pulmonary arteritis was observed in 9 of 11 pigs in group 7. This lesion was not observed in any other group.
Fibrinosuppurative meningitis, synovitis, peritonitis, pericarditis, and/or lymphadenitis typical of S. suis infection was observed in a portion of the pigs in all PRRSV and S. suis dually inoculated groups. Results are summarized in Table 3. Pigs in group 2 (untreated positive controls) and group 7 (PRRSV vaccinated) had the highest incidence (7 of 11 pigs) of suppurative meningitis. Mild nonsuppurative encephalitis and myocarditis were observed in the majority of PRRSV-infected pigs (data not shown).
TABLE 3.
Microscopic lesion summaryc
| Group | No. of pigs with microscopic lesionsa/ total no. of pigs in the group
|
|||
|---|---|---|---|---|
| Meningitis | Synovitis | Pl, Pt, Pcb | Lymphadenitis | |
| 1 | 0/11 A | 0/11 A | 0/11 A | 0/11 A |
| 2 | 7/11 C | 6/11 B, C | 3/11 A | 3/11 A, B |
| 3 | 5/11 B, C | 7/11 C | 2/11 A | 4/11 B |
| 4 | 4/11 B, C | 5/11 B, C | 1/11 A | 4/11 B |
| 5 | 1/11 A, B | 2/11 A, B | 2/11 A | 2/11 A, B |
| 6 | 1/10 A, B | 5/10 B, C | 1/11 A | 4/10 B |
| 7 | 7/11 C | 6/11 B, C | 0/11 A | 4/11 B |
Suppurative inflammation consistent with S. suis infection.
Pl, Pt, and Pc, pleuritis, peritonitis, and pericarditis, respectively.
Within each column, values followed by letters (A, B, C, and D) are significantly different from other values followed by a different letter(s) (P < 0.05).
Serology and virus isolation.
PRRSV isolation results from serum and BAL specimens are summarized in Table 4. All of the PRRSV-challenged pigs, except one pig in group 4, were viremic by 7 DPI. By 14 DPI, viremia was confirmed in all PRRSV-challenged pigs. No treatment differences were observed in the onset or incidence of viremia at 7 and 14 DPI. Viremia was still present at 28 DPI in 0 of 11, 2 of 4, 1 of 6, 3 of 5, 0 of 10, 3 of 6, and 0 of 2 pigs in groups 1 to 7, respectively. All of the PRRSV-challenged pigs became positive (S/P ratio, >0.4) for PRRSV serum antibodies by 14 DPI (data not shown).
TABLE 4.
PRRSV isolation from BAL and serum specimensc
| Group | No. of pigs from which PRRSV was isolated from BALa specimens/total no. of pigs | No. of pigs from which PRRSV was isolated from serum specimensb at indicated times/total no. of pigs
|
|||
|---|---|---|---|---|---|
| 0 DPI | 7 DPI | 14 DPI | 28 DPI | ||
| 1 | 0/11 A | 0/11 A | 0/11 A | 0/11 A | 0/11 A |
| 2 | 8/11 C | 0/11 A | 11/11 B | 4/4 B | 2/4 A, B |
| 3 | 7/11 C | 0/11 A | 11/11 B | 11/11 B | 1/6 A, B |
| 4 | 9/11 C | 0/11 A | 10/11 B | 6/7 B | 3/5 B |
| 5 | 2/11 A, B | 0/11 A | 11/11 B | 9/11 B | 0/10 A |
| 6 | 4/10 B, C | 0/10 A | 10/10 B | 7/7 B | 3/6 B |
| 7 | 8/11 C | 0/11 A | 11/11 B | 5/6 B | 0/2 C |
Bronchoalveolar lavage fluid collected at necropsy.
Sera was collected from the remaining live pigs at 0, 7, 14, and 28 DPI.
Within each column, values followed by letters (A, B, C, and D) are significantly different from other values followed by a different letter(s) (P < 0.05).
Bacteriology.
Table 5 summarizes the isolation of S. suis from blood and tissues. S. suis was isolated from the blood and/or internal tissues from all of the pigs in groups 2 to 6 that were euthanized or died prior to the 28-DPI necropsy. We were unable to recover S. suis from one pig in group 7 (RespPRRS/Repro modified live-virus vaccine) that died at 13 DPI (6 days postinoculation with S. suis). This pig had suppurative lymphadenitis suggestive of bacterial infection; however, the cause of death may have been associated with PRRSV infection based on microscopic lesions consistent with severe PRRSV-induced disease (nonsuppurative encephalitis, interstitial pneumonia, and nonsuppurative myocarditis). S. suis serotype 2 was isolated from the blood of 26 of 65, CNS specimens (cerebrospinal fluid or meninges) of 24 of 65, pleura or peritoneum or pericardial surfaces of 20 of 65, joints of 16 of 65, upper respiratory tracts of 11 of 65, and lungs of 11 of 65 of the pigs dually challenged with PRRSV and S. suis. S. suis serotype 2 was recovered from the cerebrospinal fluid of one control pig. Based on the lack of meningitis or other lesions consistent with S. suis infection of this pig, we believe that this isolate most likely is a procedural contaminant.
TABLE 5.
Isolation of S. suis type 2 from tissues and bloode
| Group | No. of pigs from which S. suis type 2 was isolated at indicated site/total no. of pigs
|
||||||
|---|---|---|---|---|---|---|---|
| Tissuesa
|
Bloodb
|
||||||
| CNS | URTc | Lung | Joint | Pl, Pt, and/or Pcd | Days 1–3 | Necropsy | |
| 1 | 1/11 A | 1/11 A | 0/11 A | 0/11 A | 0/11 A | 0/6 A | 0/11 A |
| 2 | 5/11 A, B | 2/11 A | 1/11 A | 3/11 A, B | 3/11 A, B | 1/6 A | 6/11 C |
| 3 | 4/11 A, B | 2/11 A | 2/11 A | 5/11 B | 4/11 A, B | 2/6 A | 4/11 A, B, C |
| 4 | 4/11 A, B | 2/11 A | 3/11 A | 2/11 A, B | 5/11 B | 0/6 A | 5/11 A, B, C |
| 5 | 1/11 A | 0/11 A | 1/11 A | 1/11 A, B | 1/11 A, B | 1/6 A | 1/11 A, B |
| 6 | 3/10 A, B | 2/10 A | 1/10 A | 2/10 A, B | 3/10 A, B | 2/6 A | 3/10 A, B |
| 7 | 7/11 B | 3/11 A | 3/11 A | 3/11 A, B | 4/11 A, B | 3/6 A | 7/11 C |
Tissues collected at the time of necropsy.
Blood collected on days 1, 2, and 3 after S. suis challenge and at necropsy.
URT, upper respiratory tract (turbinate or trachea).
Pl, Pt, and Pc, pleura, peritoneum, and pericardium, respectively.
Within each column, values followed by letters (A, B, C, and D) are significantly different from other values followed by a different letter(s) (P < 0.05).
DISCUSSION
Coinfection of nursery pigs with PRRSV and S. suis is common and can be especially problematic to control with conventional medication and vaccination protocols. Modern production technologies such as segregated early weaning have failed to control losses associated with PRRSV and S. suis coinfection in many herds. In order to address this problem we developed a model which mimics field cases of PRRSV and S. suis coinfection. The coinfection model has allowed us to test several control and treatment protocols that are commonly used in the field. In this study, we found that intramuscular injection of ceftiofur hydrochloride was the only protocol among the five tested that significantly reduced mortality and clinical disease associated with PRRSV and S. suis coinfection.
The treatment protocols selected for testing in this study were based on protocols used by practicing veterinarians who regularly submit swine cases to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL). Our results confirmed that two of the widely used antimicrobial treatment regimens were not adequate. Based on case reports from submission to the ISU-VDL, penicillin is the most common drug used for treatment of S. suis-associated diseases. Mortality in the group treated with penicillin was 46% compared to 63% in the untreated controls (P > 0.05). Most of the penicillin-treated pigs improved in health status considerably during antibiotic treatment; however, within 48 h after antibiotic treatment ceased, the incidence and severity of CNS disease and lameness increased and death losses continued. The mortality in the tiamulin-water-medicated group was 54% compared to 63% for untreated controls (P > 0.05). Tiamulin is approved for use in treatment of swine dysentery associated with Brachyspira hyodysenteriae and for the treatment of pneumonia due to Actinobacillus pleuropneumoniae. Tiamulin is not approved for use in treatment of S. suis-induced disease; however, case reports indicate that it is sometimes used for this purpose. The convenience of water medication, compared to injections, often facilitates better compliance with recommended treatment protocols. There are no approved water medications for treatment of S. suis infections; however, amoxicillin and cephalexin are other medications that reportedly are sometimes used in the water by practitioners in an extralabel fashion for reduction of losses associated with S. suis. These drugs were not evaluated in this study.
Ceftiofur hydrochloride (Excenel) injections were the most effective in controlling mortality associated with PRRSV and S. suis coinfection. Mortality was reduced from 63% in positive controls to 9% in the ceftiofur group (P < 0.05). Ceftiofur is approved for treatment of swine bacterial respiratory disease associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis, and S. suis serotype 2. We used the recommended treatment protocol of three consecutive daily injections and the highest recommended dose (5 mg/kg). Although ceftiofur hydrochloride was clearly the most effective treatment, it may be difficult to get pig producers to comply with administering three consecutive daily injections of the drug, and the cost of Excenel may be prohibitive.
The challenge isolate used in this experiment was susceptible to all the antimicrobials used based on results of breakpoint susceptibility tests and microbroth dilution determinations of MICs. The health of the pigs improved considerably during treatment with all the antimicrobials; however, recrudescence of disease shortly after cessation of treatment was observed in many of the pigs in the penicillin and tiamulin treatment groups. This suggests that these antimicrobials did not effectively clear the S. suis infection from all pigs in these groups. The increased survivability observed in the group treated with ceftiofur might simply be attributed to better efficacy of clearance of S. suis from blood, internal tissues, and mucosal surfaces. S. suis type 2 was recovered from only one pig in the ceftiofur group, and that was the only pig in the group that died prior to the termination of the study. It is also possible that the penicillin and tiamulin treatment protocols selected for S. suis isolates that were less susceptible to those antimicrobials and the more resistant isolates subsequently induced disease and mortality. Unfortunately, the isolates recovered from the pigs at necropsy were later discarded, so posttreatment antimicrobial susceptibility profiles could not be obtained.
The mortality in the group vaccinated with the autogenous S. suis vaccine was 40% compared to 63% in the untreated positive controls (P > 0.05). Results were not significantly better than those with penicillin, tiamulin, or RespPRRS/Repro modified live-virus vaccination. The use of autogenous bacterins for the control of S. suis-associated disease is common; however, the efficacy of the products remains controversial. In the diagnostic laboratory at Iowa State University, we routinely forward S. suis isolates from field cases to commercial laboratories at the request of referring veterinarians for production of autogenous vaccines. Based on the results from this study, the use of autogenous S. suis bacterins may not be an effective approach for controlling S. suis in pigs coinfected with PRRSV. It is possible that an S. suis autogenous bacterin may have better efficacy in a natural-exposure field situation than in the artificial high-dose challenge exposure used in this experiment.
Mortality in the group vaccinated with RespPRRS/Repro modified live-virus vaccine was 81% compared to 63% in the untreated positive controls (P > 0.05). The data from our previous model development experiments (14) suggest that intranasal administration of RespPRRS/Repro modified live-virus vaccine may exacerbate S. suis-induced disease and increase susceptibility to S. suis challenge. The data from the current experiment further support our previous observations. Veterinarians should carefully evaluate the safety and efficacy of using modified live-virus vaccines in swine production systems where S. suis-associated disease is endemic. It is possible that the RespPRRS/Repro vaccine may have better efficacy with a different strain of challenge virus or in a natural-exposure situation rather than in the artificial high-dose challenge exposure used in this experiment. A longer time between PRRSV vaccination and PRRSV challenge may increase the efficacy as well. However, challenge dose, strain, and timing often cannot be controlled or predicted under field conditions.
Of the regimens tested in this model, intramuscular administration of ceftiofur hydrochloride appears to be the best option for minimizing disease associated with PRRSV and S. suis coinfection. This work should be extended to test the efficacy of different treatment intervals of the above-named drugs and to test additional antibiotics commonly used in the field such as ampicillin, amoxicillin, and cefalexin. Other commercial and experimental PRRSV and S. suis vaccines can also be tested with this model.
ACKNOWLEDGMENTS
Funding for this project was provided by Pork Check-Off dollars from the National Pork Producers Council on behalf of the National Pork Board and by a grant from the Iowa Livestock Health Advisory Council.
We thank Prem S. Paul for the use of laboratory equipment and technical advice, Cameron Schmitt and Ryan Royer for technical assistance and manuscript review, and Jeremy D. Bruna for animal care and monitoring.
REFERENCES
- 1.Amass S F, Clark L K, Wu C C. Source and timing of Streptococcus suis infection in neonatal pigs: implications for early weaning procedures. Swine Health Prod. 1995;3(5):189–193. [Google Scholar]
- 2.Dee S A, Joo H S, Polson D D, Marsh W E. Evaluation of the effects of nursery depopulation on the persistence of porcine reproductive and respiratory syndrome virus and the productivity of 34 farms. Vet Rec. 1997;140:247–248. doi: 10.1136/vr.140.10.247. [DOI] [PubMed] [Google Scholar]
- 3.Devriese L A, Ceyssens K, Hommez J, Kilpper-Balz R, Schleifer K H. Characteristics of different Streptococcus suis ecovars and description of a simplified identification method. Vet Microbiol. 1991;26:141–150. doi: 10.1016/0378-1135(91)90050-p. [DOI] [PubMed] [Google Scholar]
- 4.Galina L, Pijoan C, Sitjar M, Christianson W T, Rossow K, Collins J E. Interaction between Streptococcus suis serotype 2 and porcine reproductive and respiratory syndrome virus in specific pathogen-free piglets. Vet Rec. 1994;134:60–64. doi: 10.1136/vr.134.3.60. [DOI] [PubMed] [Google Scholar]
- 5.Halbur P G, Paul P S, Frey M L, Landgraf J, Eernisse K, Meng X-J, Lum M A, Andrews J J, Rathje J A. Comparison of the pathogenicity of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 6.Halbur P G, Paul P S, Meng X-J, Lum M A, Andrews J J, Rathje J A. Comparative pathogenicity of nine US porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a five-week-old cesarean-derived, colostrum-deprived pig model. J Vet Diagn Investig. 1996;8:11–20. doi: 10.1177/104063879600800103. [DOI] [PubMed] [Google Scholar]
- 7.Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Investig. 1990;2:249–252. doi: 10.1177/104063879000200324. [DOI] [PubMed] [Google Scholar]
- 8.Meng X-J, Paul P S, Halbur P G, Lum M A. Characterization of a high-virulence US isolate of porcine reproductive and respiratory syndrome virus in a continuous cell line, ATCC CRL11171. J Vet Diagn Investig. 1996;8:374–381. doi: 10.1177/104063879600800317. [DOI] [PubMed] [Google Scholar]
- 9.Nelson E A, Christopher-Henning J, Drew T, Wensvoort G, Collins J E, Benfield D A. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1993;31:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossow K D. Porcine reproductive and respiratory syndrome. Vet Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- 11.Thanawongnuwech R, Thacker E L, Halbur P G. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) (isolate VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): in vitro comparisons with pulmonary alveolar macrophages (PAMs) Vet Immunol Immunopathol. 1997;59:323–335. doi: 10.1016/s0165-2427(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Thanawongnuwech R, Halbur P G, Ackermann M R, Thacker E L, Royer R L. Effects of low (modified-live virus vaccine) and high (VR-2385)-virulence strains of porcine reproductive and respiratory syndrome virus on pulmonary clearance of copper particles in pigs. Vet Pathol. 1998;35:398–406. doi: 10.1177/030098589803500509. [DOI] [PubMed] [Google Scholar]
- 13.Thanawongnuwech R, Thacker E L, Halbur P G. Influence of pig age on virus titer and bactericidal activity of porcine reproductive and respiratory syndrome virus (PRRSV)-infected porcine intravascular macrophages (PIMs) Vet Microbiol. 1998;63:177–187. doi: 10.1016/s0378-1135(98)00245-4. [DOI] [PubMed] [Google Scholar]
- 14.Thanawongnuwech, R., G. B. Brown, P. G. Halbur, J. A. Roth, R. L. Royer, and B. J. Thacker. Pathogenesis of porcine reproductive and respiratory syndrome virus (PRRSV)-induced increased susceptibility to Streptococcus suis infection. Vet. Pathol. in press. [DOI] [PubMed]
- 15.Torremorell M, Pijoan C, Trigo E. Vaccination against Streptococcus suis: effect on nursery mortality. Swine Health Prod. 1997;5:139–143. [Google Scholar]
- 16.Zeman D. Concurrent infections in 221 cases of PRRS virus pneumonia: 1992–1994. Swine Health Prod. 1996;4:143–145. [Google Scholar]