Abstract
Background
We aimed to clarify the benefits of the addition of rh‐endostatin into concurrent chemoradiotherapy (CCRT) versus CCRT alone for locally advanced non‐small cell lung cancer (NSCLC) by a meta‐analysis.
Methods
PubMed, Embase, Cochrane Central Register of Controlled Trials, Wanfang and Chinese National Knowledge Infrastructure (CNKI) were systematically screened from inception to November 2020 using the prespecified terms. Prospective trials (evaluating or) comparing the efficacy of endostar combined with CCRT and CCRT for locally advanced NSCLC were included. The primary endpoints were risk ratios (RRs) for objective response rate (ORR) and disease control rate (DCR). The secondary endpoints were RRs for overall survival (OS) and adverse events (AEs).
Results
Ten studies with 716 patients were included in this meta‐analysis. Endostar combined with CCRT significantly improved ORR and DCR compared with CCRT. The RRs of ORR and DCR for endostar combined with CCRT versus CCRT were 1.263 (95% CI: 1.137–1.403, p < 0.001) and 1.274 (95% CI: 1.124–1.444, p < 0.001), respectively. Endostar combined with CCRT significantly improved one‐year survival rate compared with CCRT with pooled RR = 1.113 (95% CI: 1.006–1.231, p = 0.038). Endostar combination treatments had similar incidences of main adverse events compared with CCRT (p > 0.05).
Conclusion
Endostar combined with CCRT is associated with significantly higher ORR, DCR and survival rate than CCRT with similar incidences of main adverse events in NSCLC.
Keywords: chemoradiotherapy, endostar, meta‐analysis, NSCLC
In our meta‐analysis of 716 patients, endostar combined with CCRT significantly improved ORR and DCR compared with CCRT. Endostar combination treatments had similar incidences of main adverse events compared with CCRT (p > 0.05). Therefore, we concluded that endostar combined with CCRT is associated with significantly higher ORR and DCR than CCRT with similar incidences of main adverse events in NSCLC.

INTRODUCTION
With consolidation immunotherapy becoming a new standard of care in unresectable stage III non‐small cell lung cancer (NSCLC), the overall survival (OS) has been significantly improved, with 3‐year OS rate reaching up to 57%,according to the PACIFIC study. 1 However, this improvement was only observed in patients who responded well to upfront concurrent chemoradiotherapy (CCRT). Unfortunately, response rates of CCRT have reached a plateau over the decades. The clinical efficacy of CCRT is warranted to improve to further lengthen survival in the era of immunotherapy. There is therefore a great need to develop chemoradiosensitizer in combination with CCRT in order to enhance the treatment response.
Inducing angiogenesis is one of the hallmarks of cancer, 2 and therefore, tumor antiangiogenesis has made a promising field of current cancer research. In 1997, Folkman et al. first reported a new protein named endostatin, a 20 kD internal fragment of the carboxy terminus of collagen XVIII, in the conditioned media of hemangioendothelioma cells as an antiangiogenic molecule. 3 Endostar, a recombinant human endostatin (rh‐endostatin), was approved by National Medical Products Administration (NMPA) in China for the treatment of NSCLC in 2005. Several clinical trials and meta‐analysis have proven that the combination of endostar and platinum‐based chemotherapy can improve the treatment response rate. 4 , 5 , 6 Previous trials have also indicated better survival and local control with no severe adverse events resulting from the use of endostar in combination with CCRT in NSCLC. 7 , 8 However, high‐level evidence is lacking for the routine use of endostar concurrently with chemoradiotherapy in patients with locally advanced NSCLC. The aim of this meta‐analysis was to investigate the efficacy and safety of endostar combined with CCRT versus standard chemoradiotherapy alone for patients with locally advanced NSCLC.
METHODS
This meta‐analysis was performed following preferred reporting items for systematic reviews and meta‐analyses (PRISMA) (supplementary materials). The protocol was registered in the Prospective Register of Systematic Reviews (PROSPERO CRD42020203424).
Data sources and searches
PubMed, Embase, Cochrane Central Register of Controlled Trials, Wanfang and Chinese National Knowledge Infrastructure (CNKI) were systematically screened from inception to November 2020 using a combination of the main search terms “chemoradiotherapy” and “endostar” and “non‐small cell lung cancer” within the restriction of “clinical trial” (detailed search strategy in supplementary materials). Abstracts, letters, editorials and expert opinions, reviews without original data, and case reports were excluded. Manual searches from previous meta‐analyses were also performed.
Study selection
We included published studies that met the following criteria: (i) prospective clinical trials; (ii) trials that enrolled NSCLC patients, especially those with unresectble stage III disease; (iii) trials that evaluated the efficacy of endostar combined with CCRT or compared the efficacy of endostar combined with CCRT and CCRT for locally advanced NSCLC; (iv) trials that reported at least one of the following clinical endpoints: objective response rate (ORR), defined as the proportion of patients achieving an objective response; disease control rate (DCR), defined as the proportion of patients achieving an objective response and stable disease; OS, defined as the time from randomization to death; progression free survival (PFS), defined as the time from randomization to first progression (locoregional or distant); adverse events (AEs) defined and graded by the National Cancer Institute's common terminology criteria for adverse events; and (v) articles for which full text in English or Chinese was available were included. If multiple publications of the same trial were retrieved, the most recent and informative publication was included.
Data extraction
Two authors were responsible for screening the titles and abstracts of the retrieved references independently. The full texts of the included studies were assessed based on the aforementioned criteria by two review authors. Any discrepancies were settled by consensus and arbitration by a panel of group discussion. Data on general trial details (study ID, first author, publication year, number of patients, baseline characteristics of the study population) and treatments were extracted. For efficacy outcomes, ORR, DCR, survival rates were extracted. For safety profiles, counts of each specific AE were extracted.
Risk‐of‐bias assessment
The quality of each eligible randomized controlled trial was evaluated by the revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2) (August 22, 2019 version). The entire scale is constituted by the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. According to the detailed guidance of RoB 2, each domain could be judged as any of the three levels: low risk, high risk or unclear risk of bias.
Data synthesis and statistical analysis
To assess the efficacy and safety of endostar combined with CCRT versus CCRT for locally advanced NSCLC, two different meta‐analysis approaches were applied: the fixed effects and the random effects models. Statistical heterogeneity of each study was assessed by I2 with planned cutoff for significance of I2 = 50%. If I2 ≤ 50%, which indicates no significant heterogeneity existing between the included studies, a fixed effects model was adopted to combine the results; otherwise, a random effects model was employed. Pooled analysis was reported as risk ratios (RRs) with 95% confidence intervals (CIs). The statistical significance of the pooled RR was determined by the Z‐test. Publication bias was assessed using funnel plots, the Egger's and the Begg's tests. The meta‐analysis was performed with STATA version 12. All p‐values were two‐sided, and p < 0.05 was considered to indicate statistical significance.
RESULTS
Characteristics of included studies
Our systematic search identified 124 potentially relevant publications. After a full text review, 114 studies were excluded because of duplication, nonclinical or retrospective studies, case reports, review articles or insufficient data to calculate the outcomes of interest. Finally, 10 studies published between June 2009 and March 2020 were eligible and included in the meta‐analysis. 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 The 10 studies consisted of eight randomized controlled trials (RCTs), and two prospective single arm studies with their matched prospective controlled cohorts. 23 Figure 1 outlines the selection process flow. The total number of patients identified in these 10 trials was 716, including 305 patients treated with endostar combined with chemoradiotherapy, and 411 with chemoradiotherapy. The main characteristics of all studies are reported in Table 1.
FIGURE 1.
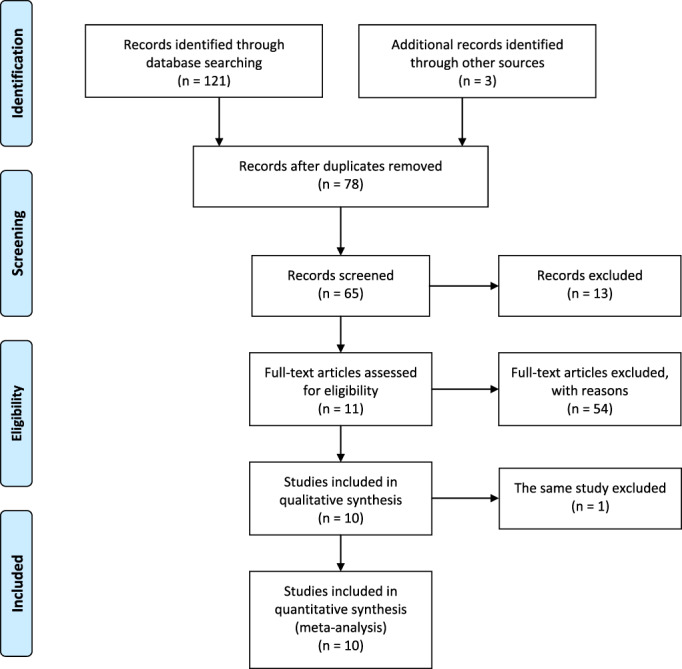
Literature search / PRISMA flow chart
TABLE 1.
Baseline characteristics of studies included in the meta‐analysis
| Author | Year | Design | No. of patients | Histology | TNM stage | RT schedule | Endostar dosage |
|---|---|---|---|---|---|---|---|
| Chen et al. 12 | 2013 | CCRT+E VS CCRT | 42 | NSCLC | IIIA, IIB (1997UICC) | 60 Gy/2 Gy | 7.5 mg/m2 (15 mg/day) |
| Jiang et al. 14 | 2011 | CCRT+E VS CCRT | 39 | NSCLC | IIIB, IV | 60–76 Gy/30–38 f | 15 mg/day |
| Liu et al. 17 | 2017 | CCRT+E VS CCRT | 60 | NSCLC | IIIB, IV | Mediastinal foci: 65–70 Gy, thoracic Nonmediastinal foci: 66–90 Gy, Lymph nodes: 65–70 Gy, intracranial Metastases:70–90 Gy, bone Metastases:40–55 Gy, other Metastases:40–60 Gy | 15 mg/day |
| Ma et al. 18 | 2009 | CCRT+E VS CCRT | 46 | NSCLC | IIIA, IIIB (1997 UICC) | 60–76 Gy/30–38 f | 15 mg/day |
| Xu et al. 20 | 2018 | CCRT+E VS CCRT | 78 | AC | III, IV | 45–60 Gy/1.8–2.0 Gy/25–30 f | 15 mg/day |
| Yao 21 | 2020 | CCRT+E VS CCRT | 96 | AC | Locally advanced | 45–60 Gy/1.8–2.0 Gy/25–30 f | 15 mg/day |
| Zhang et al. 22 | 2018 | CCRT+E VS CCRT | 50 | NSCLC | Locally advanced | 60 Gy/30 f | 7.5 mg/m2 (15 mg/day) |
| Ding et al. 13 | 2011 | CCRT+E VS CCRT | 28 | NSCLC | IIIA, IIIB (UICC sixth) | 50–60 Gy/25–30 f, residual disease: SBRT 18–27 Gy/3 Gy | 15 mg |
| Zhai et al. 7 | 2019 | CCRT+E VS CCRT | 67 (+95) | NSCLC | Inoperable stage III (AJCC seventh) | 60–66 Gy/2 Gy/30–33 f | 7.5 mg/m2/24 h |
| Sun et al. 10 | 2016 | CCRT+E VS CCRT | 19 (+96) | NSCLC | Unresectable stage III | 60–66 Gy/30–33 f | 7.5 mg/m2 |
Clinical efficacy
Comparison of ORR between endostar combined with CCRT and CCRT
Ten studies compared the ORR between endostar in combination with CCRT and CCRT. Figure 2a shows the ORR of the two groups. There was statistically significant benefit on ORR in the endostar combined with the CCRT group. No significant heterogeneity was detected among the included studies, so fixed effects model was adopted for analysis. The results of the fixed effects model showed that RR for endostar + CCRT compared with CCRT was 1.263 (95% CI: 1.137–1.403, p < 0.001). In the sensitivity analysis, exclusion of studies individually did not substantially alter the estimators, with a RR pool oscillating between 1.24 and 1.36. In a second sensitivity analysis, the studies by Jiang et al., Liu et al., and Xu et al. were removed from the meta‐analysis due to potential heterogeneous clinical features, as these three studies also included some stage IV patients. Results did not show deviations compared with the original ones, with RR for endostar + CCRT comparing with CCRT was 1.196 (95% CI: 1.067–1.340, p = 0.002).
FIGURE 2.
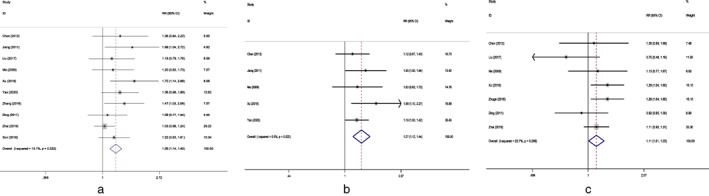
(a) Forest plot of objective response rate for endostar combined with CCRT versus CCRT. (b) Forest plot of disease control rate for endostar combined with CCRT versus CCRT. (c) Forest plot of 1‐year survival rate for endostar combined with CCRT versus CCRT. RR, risk ratio; CI, confidence interval; CCRT, concurrent chemoradiotherapy
Comparison of DCR between endostar combined with CCRT and CCRT
Five studies compared the DCR between endostar + CCRT and CCRT. Figure 2b shows the DCR of the two groups. No significant heterogeneity was observed among the included studies. DCR was significantly higher in the endostar + CCRT group with pooled RR = 1.274 (95% CI: 1.124–1.444, p < 0.001).
Comparison of survival rates between endostar combined with CCRT and CCRT
There were seven studies reporting the survival rates of endostar + CCRT and CCRT groups. Figure 2c shows the 1‐year survival rates of the two groups. No significant heterogeneity was detected among the studies. Pooled analysis with the fixed effects model showed that the 1‐year survival rate was significantly improved in the endostar + CCRT group compared with the CCRT group with pooled RR = 1.113 (95% CI: 1.006–1.231, p = 0.038).
Adverse events
The most common AEs reported in the included trials were radiation‐induced pneumonitis (RIP), radiation‐induced esophagitis, leukopenia, nausea and vomiting. Six studies compared the incidence of RIP and radiation‐induced esophagitis, respectively. Pooled analysis showed that there was no difference in the incidence of RIP (grades 1–5) and radiation‐induced esophagitis (grades 1–5) between two arms with pooled RR = 0.913 (95% CI: 0.445–1.877, p = 0.805) and 1.070 (95% CI: 0.946–1.210, p = 0.282) (Figure 3a,b). Eight studies reported the incidence rates of leukopenia. The endostar combination arm had a similar incidence rate of leukopenia to the CCRT arm (RR = 0.920, 95% CI: 0.832–1.016, p = 0.101). (Figure 3c).
FIGURE 3.

(a) Forest plot of radiation‐induced pneumonitis for endostar combined with CCRT versus CCRT. (b) Forest plot of radiation esophagitis for endostar combined with CCRT versus CCRT. (c) Forest plot of leukopenia for endostar combined with CCRT versus CCRT. RR, risk ratio; CI, confidence interval; CCRT, concurrent chemoradiotherapy
Study quality assessment
Detailed risk‐of‐bias evaluation is given for each study (supplementary materials). There was no eligible RCT deemed at high risk of bias. Due to the nature of treatments, especially radiotherapy, blinding of participants was not possible in clinical settings, and also information of blinding of participants was hardly given in the articles. However, we believed it was unlikely that deviations would arise due to this in the results, and a “low risk” score was therefore given when appropriate.
Publication bias
In terms of publication bias for the ORR of endostar + CCRT versus CCRT, the funnel plot did not indicate any evident risk of publication bias due to the symmetrical distribution (Figure 4a,b). The results of the Begg's test were z = 1.43 (p = 0.152) and z = 1.53 (p = 0.125), and that of the Egger's test were t = 3.51 (p = 0.008) and t = 2.89 (p = 0.014).
FIGURE 4.
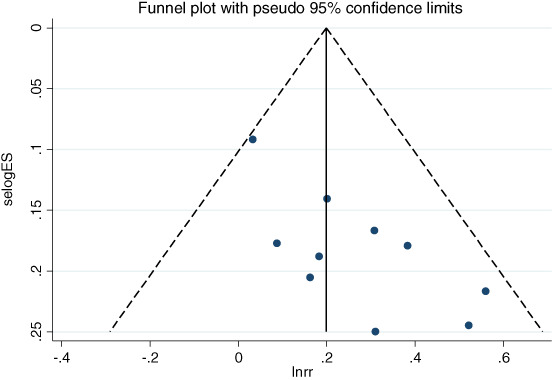
Funnel plot of the objective response rate for endostar combined with CCRT versus CCRT. CCRT, concurrent chemoradiotherapy
DISCUSSION
This is the first systematic review and meta‐analysis to compare the efficacy and safety of endostar (rh‐endostatin) combined with CCRT versus CCRT for NSCLC with modern RT techniques (IMRT/3DCRT: 100%). In this meta‐analysis of 716 patients, endostar combined with CCRT was demonstrated to significantly improve the clinical efficacy compared with CCRT, and with similar incidences of main AEs in NSCLC.
During tumor progression, an “angiogenic switch” is almost always activated and remains on, causing normally quiescent vasculature to continually sprout new vessels that help sustain expanding neoplastic growths. 24 The blood vessels produced within tumors by chronically activated angiogenesis and an unbalanced mix of proangiogenic signals are typically aberrant: tumor neovasculature is marked by precocious capillary sprouting, convoluted and excessive vessel branching, distorted and enlarged vessels, erratic blood flow, microhemorrhaging, leakiness, and abnormal levels of endothelial cell proliferation and apoptosis. 25 , 26 Studies in the 1990s revealed that type XVIII collagen (endostatin) could act as endogenous inhibitors of angiogenesis. 27 When the circulating levels of an endogenous inhibitor are genetically increased, tumor growth is impaired, 28 , 29 suggesting that such endogenous angiogenesis inhibitors might act as intrinsic barriers to induction and/or persistence of angiogenesis by incipient neoplasias. Endostar, a recombinant human endostatin, can specifically inhibit the activity of vascular endothelial growth factor to block angiogenesis as well as induce cancer cell apoptosis. 30 A preclinical study has demonstrated that endostar could improve antitumor efficacy of chemotherapy via modulation of the balance between vascular endothelial growth factor (VEGF)‐A and thrombospondin‐1 in Lewis lung carcinoma. 31 Endostar has also been shown to be efficient and safe in the treatment of NSCLC in clinical trials and has been approved by NMPA in China for the treatment of NSCLC. 32 Some previous meta‐analyses have also demonstrated that endostar combined with chemotherapy could improve the response rate and prognosis of patients with advanced NSCLC without increasing the risk of toxicity. 5 , 6
Zhang and colleagues previously reported that endostar was found to downregulate hypoxia‐inducible factor‐1α(HIF‐1α) and VEGF expression, and enhance the radioresponse to human lung adenocarcinoma cancer cells. 33 The study by Zheng et al. 34 suggested that endostar is involved in the regulation of metabolism and tumor microenvironment hypoxia, which may be responsible for the enhanced antitumor effect of endostar in combination with radiotherapy. The study by Meng et al. 35 indicated decreased hypoxia in animals and patients upon endostar treatment, which also enhanced the radioresponse within the vasculature‐remodeling period. Upon these and other preclinical findings, several clinical trials have been carried out to demonstrate the efficacy of endostar in combination with radiotherapy and chemoradiotherapy in NSCLC. The preliminary clinical study of Jiang et al. reported that the total effective rates (CR + PR) in the endostar + radiotherapy group were 80%, which was significantly improved compared with the radiotherapy alone group (44%, χ2 = 6.87, p = 0.009). Results from the phase II HELPER study, which sought to evaluate the efficacy and toxicity of the addition of endostar to concurrent etoposide, cisplatin (EP) and radiotherapy for treatment of patients with NSCLC, indicated a prolonged median survival time of 34.7 months compared with results from historical studies which treated patients with concurrent EP and radiotherapy alone. 7 , 36 , 37 , 38 The 2‐ and 3‐year OS rates (59.9% and 47.7%) in the HELPER study were also superior to previous studies. RR and CR rates in the HELPER study (76.1% and 19.1%) were better than those reported in RTOG 9410, SWOG 9504, NPC95‐01 and PROCLAIM. 36 , 38 , 39 , 40 However, the number of patients in every single trial is too limited to achieve a definite conclusion. Accordingly, high‐level evidence is still lacking for routine use of endostar concurrent with chemoradiotherapy in NSCLC. Hence, we conducted this meta‐analysis to confirm the efficacy and safety of endostar in combination with CCRT in locally advanced NSCLC.
When we analyzed the 10 prospective clinical trials of endostar combined with CCRT, a significant benefit of endostar combined with CCRT versus CCRT in ORR was found (RR = 1.263, 95% CI: 1.137–1.403, p < 0.001). DCR was also significantly increased by combining endostar and CCRT (RR = 1.274, 95% CI: 1.124–1.444, p < 0.001). As for the survival, seven studies reported 1‐year survival rates, and only one reported 3‐year survival rates. Our meta‐analysis showed that the 1‐year survival rate was significantly improved in the endostar + CCRT group compared with the CCRT group (RR = 1.113, 95% CI: 1.006–1.231, p = 0.038). The data of 3‐year survival were relatively limited and insufficient to reach a decisive conclusion. Therefore, more high‐quality prospective clinical trials are warranted to evaluate the long‐term efficacy of this combination treatment.
The AEs found in our systematic review were mainly RIP, radiation‐induced esophagitis, leukopenia, nausea and vomiting, most of which were grade 1 or 2 and well tolerated. This meta‐analysis showed that there was no difference in the incidence of main AEs between endostar + CCRT and CCRT. In this review, we found that the risk of grade ≥ 2 RIP (22.4%) in the study by Zhai et al. 7 of endostar combination treatment was lower than that (76.8%) of the study by Liang et al, 23 a group of which adopted the same CCRT regimen. In the preclinical mice model of Zhang et al., endostar administration was demonstrated to effectively attenuate the magnitude of the increase in inflammatory cells as well as the elevation of TGF‐β1 expression in lung tissues after radiation‐induced lung injury (RILI), suggesting that endostar may be a novel protective agent against RILI. 41 Whether an endostar combination can relieve the AEs associated with treatment should be further evaluated and followed up in future studies.
There are other antiangiogenic agents in addition to endostar. Bevacizumab has also been evaluated in phase I/II trials in combination with chemoradiotherapy, but has been found to induce high rates of pneumonitis and pulmonary hemorrhage, especially in patients with squamous cell carcinoma. 42 , 43 , 44 However, in our meta‐analysis, the addition of endostar did not increase the rate of pneumonitis and pulmonary hemorrhage. Preclinical studies have reported that endostar could induce apoptosis in cardiomyocytes, resulting in cardiotoxicity. 45 However, few cases of endostar‐associated cardiotoxicity have been reported in the clinical trials, which may indicate that endostar is actually safe in clinical practice. However, it may also be due to the fact that it takes longer for patients to develop cardiovascular toxicity, and during the relatively limited follow‐up, no associated toxicity was observed. In addition, in NSCLC, the antiangiogenic action in combination with esophageal or tracheal injury might cause tracheoesophageal or tracheomediastinal fistula, which have previously been reported in bevacizumab combination therapy. 46 , 47 However, such severe adverse events have not been reported in patients treated with endostar.
Several considerations should be mentioned when interpreting the results of our meta‐analysis. First, most of the trials included in this meta‐analysis involved Chinese patients which may have led to patient selection bias. Second, only a few studies reported long‐term survival, and therefore, the long‐term efficacy of endostar combined with CCRT requires further evaluation by high‐quality randomized controlled trials. Third, in three studies, some stage IV NSCLC patients were also included, which to some extent might decline the homogeneity. Therefore, we excluded these three studies in the sensitivity analysis and it showed similar results to the primary analysis, which indicated that the results were robust and consistent. Additionally, we cannot conclude about the optimal time window of endostar, and the preferable CT regimen or RT scheme in combination. However, a platinum‐based CT and a total RT dose of 60–66 Gy for locally advanced NSCLC as administered in most trials may be reasonable options.
In conclusion, for locally advanced NSCLC, endostar combined with CCRT is associated with significantly higher ORR and DCR without increased risk of main adverse events compared with CCRT. More randomized controlled trials are needed to confirm the long‐term survival benefits of endostar combination treatments, especially in this era of immunotherapy.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Supporting information
Table S1. PRISMA checklist
Figure S1. Summary of risk‐of‐bias assessment
ACKNOWLEDGMENTS
This work was supported by the National key research and development program (2017YFC1311000, 1311002), Beijing Hope Run Special Fund of Cancer Foundation of China (no. LC2016L03), CAMS Innovation Fund for Medical Sciences (no. 2016‐I2M‐1‐011), Clinical Application Project of Beijing Municipal Commission of Science and Technology (Z171100001017114), CAMS Innovation Fund for Medical Sciences (CIFMS: 2020‐I2M‐C&T‐B‐074) and CAMS Key Laboratory of Translational Research on Lung Cancer (2018PT31035).
Yuan M, Zhai Y, Men Y, Wang J, Deng L, Wang W, et al. Endostar (rh‐endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non‐small cell lung cancer: A systematic review and meta‐analysis . Thorac Cancer. 2021;12:3208–3215. 10.1111/1759-7714.14188
Funding information National key research and development program 2017YFC1311000 1311002; Beijing Hope Run Special Fund of Cancer Foundation of China, Grant/Award Number: LC2016L03; CAMS Innovation Fund for Medical Sciences, Grant/Award Number: 2016‐I2M‐1‐011; CAMS Key Lab of Translational Research on Lung Cancer, Grant/Award Number: 2018PT31035; Clinical Application Project of Beijing Municipal Commission of Science and Technology, Grant/Award Number: Z171100001017114; CAMS Innovation Fund for Medical Sciences (CIFMS), Grant/Award Number: 2020‐I2M‐CT‐B‐074
REFERENCES
- 1. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three‐year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC‐update from PACIFIC. J Thorac Onco. 2020;15:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 3. O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. [DOI] [PubMed] [Google Scholar]
- 4. Rong B, Yang S, Li W, et al. Systematic review and meta‐analysis of Endostar (rh‐endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non‐small cell lung cancer. World J Surg Oncol. 2012;10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ge W, Cao DD, Wang HM, Jie FF, Zheng YF, Chen Y. Endostar combined with chemotherapy versus chemotherapy alone for advanced NSCLCs: a meta‐analysis. Asian Pac J Cancer Prev. 2011;12:2705–11. [PubMed] [Google Scholar]
- 6. An J, Lv W. Endostar (rh‐endostatin) versus placebo in combination with vinorelbine plus cisplatin chemotherapy regimen in treatment of advanced non‐small cell lung cancer: a meta‐analysis. Thorac Cancer. 2018;9:606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang J, et al. HELPER study: a phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non‐small‐cell lung cancer. Radiother Oncol. 2019;131:27–34. [DOI] [PubMed] [Google Scholar]
- 8. Ma HL, Hui ZG, Zhao LJ, et al. Continuous intravenous pumping (CIP) of recombinant human endostatin (Endostar) combined with concurrent radiochemotherapy in patients with unresectable stage III non‐small‐cell lung cancer: preliminary data of a prospective multicenter phaseIIclinical trial. Chin J Radiat Oncol. 2016;25(2):114–8. [Google Scholar]
- 9. Jiang XD, Qiao Y, Dai P, Wu J, Song DA, Li SQ, et al. Preliminary clinical study of weekly recombinant human endostatin as a hypoxic tumour cell radiosensitiser combined with radiotherapy in the treatment of NSCLC. Clin Transl Oncol. 2012;14:465–70. [DOI] [PubMed] [Google Scholar]
- 10. Sun XJ, Deng QH, Yu XM, et al. A phase II study of Endostatin in combination with paclitaxel, carboplatin, and radiotherapy in patients with unresectable locally advanced non‐small cell lung cancer. BMC Cancer. 2016;16:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Q, Shi Q, Xie Q. Preliminary study of recombinant human endostatin(Endostar) combined with concurrent intensity‐modulated radiation therapy for inoperable local advanced non‐small cell lung cancer in elderly patients. Chin J Oncol Prev Treat. 2017;9:142–5. [Google Scholar]
- 12. Chen XJ, Wang JH, Liu YQ, et al. Clinical study of Rh‐endostatin combined with chemotherapy followed by concurrent radiotherapy for the treatment of locally advanced non‐small cell lung cancer. Henan Med Res. 2013;22:337–9. [Google Scholar]
- 13. Ding Y, Wang XC, Yang F, et al. Recombinant human endostatin combined with concurrent chemoradiotherapy in treatment of locally advanced non‐small cell lung cancer. Acad J Guangdong Coll Pharm. 2011;27:202–6. [Google Scholar]
- 14. Jiang ZG, KS LI, Yue WB, et al. Endostar combined with concurrent chemoradiotherapy in treatment of local advanced non‐small cell lung cancer in elderly patients. Chin J Pract Med. 2011;18:55–8. [Google Scholar]
- 15. JS LI, Wu S. Efficacy of Endostar combined with radiotherapy in 20 cases of locally advanced non‐small cell lung cance. J Aerosp Med. 2011;22:563–4. [Google Scholar]
- 16. Lin Y, An ZL, Zhang JH, et al. Clinical study on there‐dimensional conformal radiotherapy later‐course combined with Endostar in treatment of elderly patients with non‐small cell lung Cance. J Inner Mongolia Med Univ. 2016;38:564–7. [Google Scholar]
- 17. Liu L, Li T, Lang JY, et al. Study on concomitant radiotherapy and chemotherapy Combinating with Endostatin for IIIB and IV stage non‐small cell lung cancer. J Cancer Control Treat. 2017;30:265–70. [Google Scholar]
- 18. Ma JB, Jl Y, Zl L, et al. A randomized clinical trial of endostar combined with concurrent chemoradiotherapy in treatment of local advanced non‐small cell lung cancer. J Clin Med Pract. 2009;13:20–4. [Google Scholar]
- 19. Wen LC, Zhang LZ. The observation of short‐term effects of YH‐16 combined with concurrent three dimensional conformal radiotherapy on locally advanced non‐small cell lung Cance. J Basic Clin Oncol. 2009;22:218–20. [Google Scholar]
- 20. Xu H, Qin WJ, Guo RX, et al. Concurrent radiotherapy and chemotherapy combined with Endostar for locally middle and advanced non‐small cell lung cancer. Pract J Cancer. 2018;33:450–3. [Google Scholar]
- 21. Yao H. Concurrent chemoradiotherapy combined with Endostar in the treatment of locally advanced non‐small cell lung cancer. China Health Vision. 2020;5:13. [Google Scholar]
- 22. Zhang JY, Liu ZF, Ge YL, et al. Clinical effect of Endostar combined with concurrent chemoradiotherapy in the treatment of locally advanced non‐small cell lung cancer. China Health Care Nutr. 2018;28:97–8. [Google Scholar]
- 23. Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non‐small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol. 2017;28:777–83. [DOI] [PubMed] [Google Scholar]
- 24. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. [DOI] [PubMed] [Google Scholar]
- 25. Nagy JA, Chang SH, Shih SC, Dvorak A, Dvorak H. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–11. [DOI] [PubMed] [Google Scholar]
- 27. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. [DOI] [PubMed] [Google Scholar]
- 28. Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre‐malignant conditions. Eur J Cancer. 2009;45:1924–34. [DOI] [PubMed] [Google Scholar]
- 29. Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–79. [DOI] [PubMed] [Google Scholar]
- 30. Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF‐induced tyrosine phosphorylation of KDR/Flk‐1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. [DOI] [PubMed] [Google Scholar]
- 31. Huang G, Chen L. Recombinant human endostatin improves anti‐tumor efficacy of paclitaxel by normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res Clin Oncol. 2010;136:1201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang L, Wang JW. Sun Y, et al: [randomized phase II trial on escalated doses of Rh‐endostatin (YH‐16) for advanced non‐small cell lung cancer]. Zhonghua Zhong Liu Za Zhi. 2006;28:138–41. [PubMed] [Google Scholar]
- 33. Zhang L, Ge W, Hu K, Zhang YY, Li CH, Xu XM, et al. Endostar down‐regulates HIF‐1 and VEGF expression and enhances the radioresponse to human lung adenocarcinoma cancer cells. Mol Biol Rep. 2012;39:89–95. [DOI] [PubMed] [Google Scholar]
- 34. Zheng YF, Ge W, Xu HL, et al. Endostar enhances the antitumor effects of radiation by affecting energy metabolism and alleviating the tumor microenvironment in a Lewis lung carcinoma mouse model. Oncol Lett. 2015;10:3067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meng MB, Jiang XD, Deng L, Na FF, He JZ, Xue JX, et al. Enhanced radioresponse with a novel recombinant human endostatin protein via tumor vasculature remodeling: experimental and clinical evidence. Radiother Oncol. 2013;106:130–7. [DOI] [PubMed] [Google Scholar]
- 36. Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 study. J Clin Oncol. 2005;23:5910–7. [DOI] [PubMed] [Google Scholar]
- 37. Albain KS, Crowley JJ, Turrisi AT 3rd, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non‐small‐cell lung cancer: a southwest oncology group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454–60. [DOI] [PubMed] [Google Scholar]
- 38. Gandara DR, Chansky K, Albain KS, Gaspar LE, Lara PN Jr, Kelly K, et al. Long‐term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non‐small‐cell lung cancer: a phase II Southwest Oncology Group Study (S9504). Clin Lung Cancer. 2006;8:116–21. [DOI] [PubMed] [Google Scholar]
- 39. Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non‐small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: randomized phase III trial of Pemetrexed‐cisplatin or etoposide‐cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2016;34:953–62. [DOI] [PubMed] [Google Scholar]
- 41. Zhang K, Yang S, Zhu Y, et al. Protection against acute radiation‐induced lung injury: a novel role for the anti‐angiogenic agent Endostar. Mol Med Rep. 2012;6:309–15. [DOI] [PubMed] [Google Scholar]
- 42. Lind JSW, Senan S, Smit EF. Pulmonary toxicity after bevacizumab and concurrent thoracic radiotherapy observed in a phase I study for inoperable stage III non‐small‐cell lung cancer. J Clin Oncol. 2012;30:e104–8. [DOI] [PubMed] [Google Scholar]
- 43. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol. 2004;22:2184–91. [DOI] [PubMed] [Google Scholar]
- 44. Wozniak AJ, Moon J, Thomas CR, et al. A pilot trial of cisplatin/etoposide/radiotherapy followed by consolidation docetaxel and the combination of bevacizumab (NSC‐704865) in patients with inoperable locally advanced stage III non–small‐cell lung cancer: SWOG S0533. Clin Lung Cancer. 2015;16:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cao F, Cui J. Research progress of Endostar in the treatment of non‐small cell lung cancer. Hainan Med J. 2015;26(7):1016–8. [Google Scholar]
- 46. Nishie K, Yasuo M, Kitaguchi Y, Kobayashi N, Tateishi K, Ushiki A, et al. Bevacizumab‐induced tracheoesophageal fistula in a patient suffering from lung cancer with bulky subcarinal lymph node: a case report. Nagoya J Med Sci. 2018;80:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thawani R, Thomas A, Thakur K. Tracheomediastinal fistula: rare complication of treatment with bevacizumab. Cureus. 2018;10:e2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA checklist
Figure S1. Summary of risk‐of‐bias assessment


