Abstract
Background
Here, we investigated the relationship between clinical parameters, including the site of surgical anastomosis and radiation dose to the anastomotic region, and anastomotic complications in esophageal cancer patients treated with trimodality therapy.
Methods
Between 2007 and 2016, esophageal cancer patients treated with trimodality therapy at a tertiary academic cancer center were identified. Patient, treatment, and outcome parameters were collected. Radiation dose to the gastric regions were extracted. Anastomotic complication was defined as leak and/or stricture. We used Fisher's exact and Wilcoxon rank‐sum tests to compare the association between clinical parameters and anastomotic complications.
Results
Of 89 patients identified, the median age was 63 years, 82% (n = 73) were male, and 82% had distal (n = 47) or gastroesophageal junction (n = 26) tumors. Median follow‐up was 25.8 months. Esophagectomies were performed with cervical (65%, n = 58) or thoracic anastomoses (35%, n = 31). Anastomotic complications developed in 60% (n = 53). Cervical anastomosis was associated with anastomotic complications (83%, n = 44/53, p < 0.01). Radiation to any gastric substructure was not associated with anastomotic complications (p > 0.05). In the subset of patients with distal/gastroesophageal junction tumors undergoing esophagectomy with cervical anastomosis where radiation was delivered to the future neoesophagus, 80% (n = 35/44) developed anastomotic complications. In this high‐risk subgroup, radiation was not associated with anastomotic complications (p > 0.05).
Conclusions
Our analysis did not demonstrate an association between radiation dose to gastric substructures and anastomotic complications. However, it showed an association between esophagectomy with cervical anastomosis and anastomotic complications. Patients with distal/gastroesophageal junction tumors who undergo esophagectomy with cervical anastomosis have higher rates of anastomotic complications unrelated to radiation to gastric substructures.
Keywords: anastomotic leak, anastomotic stricture, esophageal cancer, neoadjuvant radiation, trimodality therapy
In trimodality therapy for esophageal cancer, radiation dose to any individual gastric substructure was not associated with anastomotic complications but anastomotic location (cervical vs. thoracic) is more closely associated with whether patients will experience anastomotic complications than neoadjuvant radiation treatment parameters. These data support the safety of neoadjuvant chemoradiation prior to esophagectomy for patients with esophageal cancers.
INTRODUCTION
In patients with locally advanced esophageal cancers, neoadjuvant chemoradiation followed by surgery (trimodality therapy [TMT]) has become the gold standard treatment. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 TMT compared with surgery alone improved overall survival and disease‐free survival. 3 , 4 , 5 , 6 , 7 Anastomotic leak and stricture remain the major anastomotic complications (AC) after esophagectomy. 9 There is debate over whether neoadjuvant therapy that includes radiation (RT) prior to esophagectomy results in an increased rate of AC. 10 , 11 Anastomotic leak incidence ranges between 9% and 26%. 6 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Stricture incidence was reported to be higher with a range between 22% to 45%. 12 , 13
The stomach is used to recreate a neoesophagus during an esophagectomy with an anastomosis created between the remnant proximal esophagus and the gastric fundus or body along the greater curvature. 18 These gastric substructures are often located in or adjacent to the preoperative RT field. Conflicting data evaluating the effect of RT on AC are available. 11 , 15 , 16 , 19 It is unclear whether extra efforts should be made to limit the RT doses delivered to the stomach and its substructures during the RT planning, especially to the gastric substructures that will be used to create the esophagogastric anastomosis.
Herein, we assessed associations between the clinical factors, especially surgical and RT treatment parameters, and the development of postoperative AC (leak and/or stricture) in esophageal patients who were treated with TMT at a tertiary academic institution.
METHODS
Patient data
This was a retrospective IRB approved (IRB00163262) study. Patients with esophageal cancer who underwent TMT were identified in a prospectively collected thoracic surgery database. Patients with available restored electronic RT plans between 2007 and 2016 were included in this analysis. Exclusion criteria included patients with cervical esophageal cancers, patients treated with radiation at outside institutions, and patients who died, or were otherwise lost to follow‐up within 90 days of surgery.
Baseline, treatment, and outcome characteristics were obtained from chart review. Follow‐up time was defined as the time from date of surgery to date of last patient interaction or death. Margin status was defined as margin involved with tumor (pathological margin positive) or with Barrett's esophagus when the above was specifically mentioned in the surgical pathology report. Given treatment over different staging eras, patients were restaged according to the seventh edition of the American Joint Committee on Cancer (AJCC, 2010) 20 staging system using pretreatment endoscopic, diagnostic positron emission tomography (PET) and computed tomography (CT) findings.
Postoperative anastomotic complication was defined as an anastomotic leak and/or stricture, confirmed by a thoracic surgeon (SB). Anastomotic leak was determined by 1 a diagnostic barium swallowing study or CT scan demonstrating extravasation of oral contrast or 2 by endoscopic direct visualization of anastomotic dehiscence or fistula. 12 , 16 , 21 Anastomotic stricture was defined as 1 endoscopic confirmation of stenosis or 2 receipt of endoscopic dilatation(s) to relieve an anastomotic stenosis. 12 , 21
Radiation dosimetric data
The stomach and its gastric substructures were contoured on restored preoperative RT planning CT scans using Philips Pinnacle treatment planning system, version 9.10. The gastric substructures (fundus, the body which was further subdivided into the greater and lesser curvature, and the pylorus/antrum) were defined according to published radiographic definitions 22 , 23 and in consultation with two thoracic surgeons (SB, EB), a radiologist and a thoracic radiation oncologist (KRV) (See Figure 1). Dosimetric data included: mean dose, point maximum dose (Dmax), point minimum dose (Dmin), and dose volume histogram (DVH) parameters including the percent volume of the structure receiving 20 and 30 Gy, respectively (V20 and V30, intermediate RT dose‐volume parameters).
FIGURE 1.
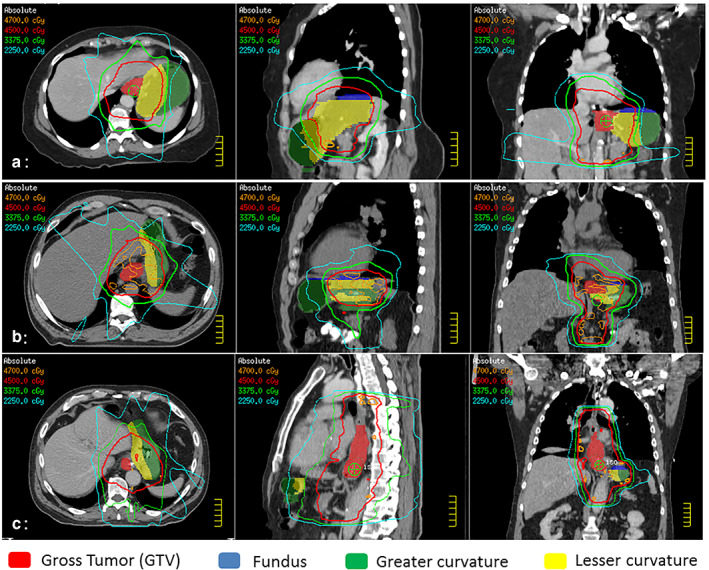
Representative images of the preoperative radiation planning scans of three patients with esophageal cancer treated with trimodality therapy. Radiation doses (colored lines) are overlaid on top of gastric substructures delineated in colorwash (red: gross tumor, yellow: lesser curvature, green: greater curvature, blue: fundus). (a) A patient with stage III (T2N2) gastroesophageal junction adenocarcinoma with postoperative anastomotic complications (both leak and stricture) after esophagectomy with cervical anastomosis. (b) A patient with stage III (T3N1) gastroesophageal junction adenocarcinoma without postoperative anastomotic complications after esophagectomy with cervical anastomosis. (c) A patient with stage III (T3N1) lower esophageal adenocarcinoma without postoperative anastomotic complications after esophagectomy with intrathoracic anastomosis
Surgical technique
Six to eight weeks after neoadjuvant chemoradiation, patients underwent transhiatal, three‐incision, or Ivor‐Lewis esophagectomies. Cervical anastomoses were performed with a manual approach and thoracic anastomosis were performed with a semi‐manual approach using an EEA circular stapler. For simplicity, we refer to the transhiatal esophagectomies and three‐incision as esophagectomies with cervical anastomoses (EC) and refer to the Ivor‐Lewis esophagectomies as esophagectomies performed with thoracic anastomosis (ET) throughout the manuscript. 24
Statistical analysis
The association of baseline clinical and treatment characteristics with postoperative AC was assessed using Chi‐squared (χ2) and Fisher's exact tests for categorical variables or Student's t‐test and Wilcoxon rank‐sum test for testing medians of continuous parameters. Statistical analysis was performed using STATA (version 14). p‐values <0.05 were considered statistically significant.
RESULTS
Patient cohort
Eighty‐nine patients were included in the final analysis. Of 89 patients, the median age was 63 years (IQR: 55–69), 82% (n = 73) were male, and 82% had distal (n = 47) or gastroesophageal junction (GEJ, n = 26) tumors. Median follow‐up was 25.8 months (IQR: 14–54). Patients received 45 Gy (69%, n = 61) or 50.4 Gy (31%, n = 28) with concurrent chemotherapy. Esophagectomies were performed with cervical (EC, 65%, n = 58) or thoracic anastomoses (ET, 35%, n = 31). The baseline characteristics are summarized in Table 1.
TABLE 1.
Baseline patient and treatment characteristics of all patients (n = 89)
| Anastomotic complication | p‐value | ||
|---|---|---|---|
| Yes | No | ||
| n = 53 (59.6%) | n = 36 (40.4%) | ||
| n, (%) | n, (%) | ||
| Age at diagnosis (median year, IQR) | 59 (55–69) | 64 (57–69.5) | 0.21 |
| Length of follow‐up (months, IQR) | 27.3 (14.0–65.1) | 25.7 (13.2–50.5) | 0.55 |
| Male sex | 46 (86.8) | 27 (75.0) | 0.16 |
| BMI (median kg/m2, IQR) | 25.6 (23.2–28.8) | 25.9 (22.4–31.0) | 0.98 |
| Diabetes | 14 (26.4) | 9 (25.0) | 1.00 |
| History of smoking | 45 (84.9) | 27 (75.0) | 0.28 |
| Histology type | 0.80 | ||
| Adenocarcinoma | 41 (77.4) | 29 (80.6) | |
| SCC | 12 (22.6) | 7 (19.4) | |
| Location of tumor | 0.82 | ||
| Proximal esophagus | 1 (1.9) | 2 (5.6) | |
| Middle esophagus | 8 (15.1) | 5 (13.9) | |
| Distal esophagus | 29 (54.7) | 18 (50.0) | |
| GEJ | 15 (28.3) | 11 (30.6) | |
| Group stage | 1.00 | ||
| II | 18 (34.0) | 13 (36.1) | |
| III | 35 (66.0) | 23 (63.9) | |
| Clinical T stage | 0.33 | ||
| cT1 | 0 (0.0) | 0 (0.0) | |
| cT2 | 13 (24.5) | 9 (25.0) | |
| cT3 | 36 (67.9) | 27 (75.0) | |
| cT4 | 4 (7.6) | 0 (0.0) | |
| Clinical N stage | 0.76 | ||
| cN0 | 14 (26.4) | 7 (19.4) | |
| cN1 | 33 (62.3) | 26 (72.2) | |
| cN2 | 5 (9.4) | 2 (5.6) | |
| cN3 | 1 (1.9) | 1 (2.8) | |
| Total RT (Gy) | 0.25 | ||
| 45.0 | 39 (73.6) | 22 (61.1) | |
| 50.4 | 14 (26.4) | 14 (38.9) | |
| Chemotherapy regimen | 0.06 | ||
| Platin/taxol | 17 (32.1) | 21 (58.3) | |
| Platin/fluorouracil | 28 (53.8) | 11 (30.6) | |
| Other | 8 (15.1) | 4 (11.1) | |
| Resection year (median, range) | 2010 (2008–2013) | 2013 (2010–2015) | 0.45 |
| Esophagectomy anastomotic location | <0.01 | ||
| Cervical | 44 (83.0) | 14 (38.9) | |
| Thorax | 9 (17.0) | 22 (61.1) | |
| Positive resection cancer margin | 2 (3.8) | 2 (5.6) | 1.00 |
| Distal | 2 (3.8) | 1 (2.8) | |
| Proximal | 0 (0.0) | 0 (0.0) | |
| Circumferential | 0 (0.0) | 1 (2.8) | |
| Positive Barrett's esophagus | 15 (28.3) | 12 (33.3) | 0.44 |
| Distal margin | 0 (0.0) | 0 (0.0) | |
| Proximal margin | 1 (1.9) | 0 (0.0) | |
| Interval last RT to surgery (median days, IQR) | 42 (36–52) | 47 (38–60) | 0.13 |
Abbreviations: BMI, body mass index; IQR, interquartile range; GEJ, gastroesophageal junction; Gy, gray; Platin, cisplatin or carboplatin; RT, radiation; SCC, squamous cell carcinoma.
Postoperative anastomotic complications
AC developed in 53 patients (60%). Of these 53 patients, n = 45 (51%) patients developed stricture and n = 14 (16%) developed leak. Among 45 and 14 patients, six patients (7%) developed both leak and stricture. The median time between esophagectomy and development of leak was 8 days (IQR: 7–11). The median time between esophagectomy and endoscopic visualization or treatment of stricture was 57 days (IQR: 43–137).
Clinical and treatment factors associated with postoperative anastomotic complications
Analysis of associations between clinical factors and postoperative AC are summarized in Table 1. A total of 76% (n = 44 of 58) of patients who received EC experienced AC, a significantly higher rate than ET, where only 29% (n = 9 of 31) experienced AC (p < 0.01). There was no association between total neoadjuvant RT dose, neoadjuvant chemotherapy regimen, year of resection, resection margin status, nor interval from end of neoadjuvant therapy to surgery and AC (p > 0.05).
The risk of each respective complication (leak or stricture) with respect to the postoperative anastomotic location is shown in Table S1. Leaks occurred more commonly in patients who received EC as compared to ET (EC: 24%, n = 14/58 vs. ET: 0%, n = 0/31; p < 0.01). Strictures also occurred more commonly in patients who received EC versus ET (64%, n = 37/58 vs. 26%, n = 8/31; p < 0.01).
Dosimetric factors associated with postoperative anastomotic complications
The association of preoperative RT parameters to the entire stomach or gastric substructures with postoperative AC are summarized in Table 2. There was no association between RT dose parameters (mean dose, Dmin, Dmax, and intermediate RT dose‐volume parameters: V20, V30) exposed to the stomach nor the gastric substructures and AC (p > 0.05). Also, our results did not show an association (p > 0.05) between RT dose parameters with each individual complication (leak or stricture) as shown in Table S2.
TABLE 2.
Preoperative radiation dose delivered to the stomach and gastric substructures of all (n = 89) patients
| Anastomotic complication | p‐value | ||
|---|---|---|---|
| Yes | No | ||
| n = 53 (59.6%) | n = 36 (40.4%) | ||
| Median (IQR) | Median (IQR) | ||
| Entire stomach | |||
| Mean dose (Gy) | 27.24 (15.71–34.24) | 27.42 (21.00–34.75) | 0.88 |
| Maximum dose (Gy) | 48.21 (47.28–51.12) | 49.23 (47.39–53.54) | 0.21 |
| Minimum dose (Gy) | 3.21 (0.74–8.86) | 2.31 (0.80–6.29) | 0.54 |
| V20 (%) | 68 (31–91) | 63 (44–89) | 0.81 |
| V30 (%) | 40 (12–57) | 38 (25–61) | 0.66 |
| Fundus | |||
| Mean dose (Gy) | 36.17 (22.21–44.45) | 36.23 (28.23–45.07) | 0.36 |
| Maximum dose (Gy) | 47.56 (46.41–49.15) | 48.22 (46.69–53.20) | 0.15 |
| Minimum dose (Gy) | 14.75 (5.83–21.93) | 16.77 (7.10–28.61) | 0.33 |
| V20 (%) | 98 (49–100) | 98 (67–100) | 0.35 |
| V30 (%) | 66 (13–93) | 71 (41–100) | 0.23 |
| Greater curvature | |||
| Mean dose (Gy) | 23.34 (10.53–32.20) | 24.07 (16.33–33.88) | 0.88 |
| Maximum dose (Gy) | 47.63 (45.87–49.71) | 48.44 (46.31–52.96) | 0.17 |
| Minimum dose (Gy) | 3.54 (0.66–10.28) | 2.48 (0.93–8.73) | 0.61 |
| V20 (%) | 61 (13–89) | 54 (33–87) | 0.88 |
| V30 (%) | 28 (1–52) | 25 (12–61) | 0.70 |
| Lesser curvature | |||
| Mean dose (Gy) | 35.80 (25.42–40.96) | 36.28 (30.11–42.57) | 0.47 |
| Maximum dose (Gy) | 48.11 (47.22–50.03) | 49.10 (47.02–53.51) | 0.21 |
| Minimum dose (Gy) | 9.97 (1.33–16.57) | 6.25 (1.57–21.13) | 0.97 |
| V20 (%) | 90 (59–99) | 84 (69–100) | 0.85 |
| V30 (%) | 62 (37–82) | 63 (45–95) | 0.49 |
| Pylorus/antrum | |||
| Mean dose (Gy) | 16.94 (2.02–26.82) | 12.43 (1.92–29.97) | 0.82 |
| Maximum dose (Gy) | 36.37 (4.42–46.25) | 31.87 (4.87–44.21) | 0.73 |
| Minimum dose (Gy) | 4.86 (0.94–12.43) | 2.90 (0.99–10.89) | 0.71 |
| V20 (%) | 31 (0–83) | 9.5 (0–98) | 0.95 |
| V30 (%) | 1 (0–34) | 0 (0–49) | 0.80 |
Abbreviations: Gy, Gray; IQR, interquartile range; V20, the percent volume of the structure receiving at least 20 Gy; V30, the percent volume of the structure receiving at least 30 Gy.
Anastomotic complications for the subset of distal esophageal tumors undergoing esophagectomies with cervical anastomosis
To evaluate the association of RT with AC in a high‐risk group, we limited the analysis to patients with distal esophageal or GEJ tumors undergoing chemoradiation followed by EC. In this high‐risk population, RT was delivered to or near the gastric substructures that would then be used to form the anastomosis in the neck and the neoesophagus. Of the 44 out of 89 patients who met these criteria: 80% (n = 35/44) developed AC (stricture: 73%, n = 32/44; leak: 25%, n = 11/44; both: 18%, n = 8/44).
Clinical characteristics and their association with postoperative cervical AC for this high‐risk subset are summarized in Table 3. In this subset, no significant associations were found between clinical nor treatment details and AC. There were also no associations between the RT dose parameters exposed to the stomach nor gastric substructures and subsequent post‐surgical AC (p > 0.05) (Table 4).
TABLE 3.
Baseline patient and treatment characteristics of the subset of patients with lower/GEJ tumors treated with esophagectomies with cervical anastomoses (n = 44)
| Anastomotic complication | p‐value | ||
|---|---|---|---|
| Yes | No | ||
| n = 35 (79.6%) | n = 9 (20.5%) | ||
| n, (%) | n, (%) | ||
| Age at diagnosis (median year, IQR) | 56 (53–65) | 59 (54–68) | 0.83 |
| Length of follow‐up (days, IQR) | 1071 (547–3046) | 1511 (904–2191) | 0.97 |
| Male sex | 32 (91.4) | 8 (88.9) | 1.00 |
| BMI (median kg/m2, IQR) | 26.8 (24.3–29.3) | 25.8 (20.0–30.3) | 0.39 |
| Diabetes | 9 (25.71) | 1 (11.1) | 0.66 |
| History of smoking | 30 (85.7) | 7 (77.8) | 0.62 |
| Histology type | 0.81 | ||
| Adenocarcinoma | 32 (91.4) | 8 (88.9) | |
| SCC | 3 (8.6) | 1 (11.1) | |
| Location of tumor | 1.00 | ||
| Distal esophagus | 21 (60.0) | 5 (55.6) | |
| GEJ | 14 (40.0) | 4 (44.4) | |
| Group stage | 0.23 | ||
| II | 13 (37.1) | 1 (11.1) | |
| III | 22 (62.9) | 8 (88.9) | |
| Clinical T stage | 0.31 | ||
| cT1 | 0 (0.0) | 0 (0.0) | |
| cT2 | 7 (20.0) | 0 (0.0) | |
| cT3 | 28 (80.0) | 9 (100) | |
| cT4 | 0 (0.0) | 0 (0.0) | |
| Clinical N stage | 0.37 | ||
| cN0 | 10 (28.6) | 1 (11.1) | |
| cN1 | 22 (62.9) | 8 (88.9) | |
| cN2 | 3 (8.6) | 0 (0.0) | |
| cN3 | 0 (0.0) | 0 (0.0) | |
| Total RT (Gy) | 1.00 | ||
| 45.0 | 27 (77.1) | 7 (77.8) | |
| 50.4 | 8 (22.9) | 2 (22.2) | |
| Chemotherapy regimen | 0.13 | ||
| Platin/Taxol | 8 (22.9) | 3 (33.3) | |
| Platin/Fluorouracil | 23 (65.7) | 3 (33.3) | |
| Other | 4 (11.4) | 3 (33.3) | |
| Resection year (median, range) | 2009 (2008–2010) | 2009 (2008–2012) | 0.94 |
| Positive resection cancer margin | 2 (5.7) | 0 (0.0) | 1.00 |
| Distal | 2 (5.7) | 0 (0.0) | |
| Proximal | 0 (0.0) | 0 (0.0) | |
| Circumferential | 0 (0.0) | 0 (0.0) | |
| Positive Barrett's esophagus | 13 (37.1) | 2 (22.2) | 0.66 |
| Distal margin | 0 (0.0) | 0 (0.0) | |
| Proximal margin | 1 (2.9) | 0 (0.0) | |
| Interval from last RT to surgery (median days, IQR) | 39 (33–44) | 34 (32–44) | 0.47 |
Abbreviations: BMI, body mass index; Gy, gray; IQR, interquartile range; GEJ, gastroesophageal junction; Platin, cisplatin or carboplatin; RT, radiation; SCC, squamous cell carcinoma.
TABLE 4.
Preoperative radiation dose delivered to the subset of patients with distal/GEJ tumors treated with esophagectomies with cervical anastomoses (n = 44)
| Anastomotic complication | p‐value | ||
|---|---|---|---|
| Yes | No | ||
| n = 35 (79.6%) | n = 9 (20.5%) | ||
| Median (IQR) | Median (IQR) | ||
| Entire stomach | |||
| Mean dose (Gy) | 27.00 (11.46–34.57) | 25.61 (21.42–31.75) | 0.94 |
| Maximum dose (Gy) | 48.21 (47.22–51.07) | 48.62 (47.58–49.73) | 0.57 |
| Minimum dose (Gy) | 3.33 (0.66–8.86) | 1.95 (1.07–5.71) | 0.83 |
| V20 (%) | 68 (26–91) | 53 (51–84) | 1.00 |
| V30 (%) | 34 (9–66) | 33 (24–56) | 0.78 |
| Fundus | |||
| Mean dose (Gy) | 34.19 (21.07–44.45) | 33.26 (31.29–36.24) | 0.87 |
| Maximum dose (Gy) | 47.57 (46.17–48.78) | 48.04 (47.05–48.62) | 0.36 |
| Minimum dose (Gy) | 13.51 (5.83–21.19) | 14.43 (8.74–18.34) | 0.94 |
| V20 (%) | 96 (47–100) | 92 (73–100) | 0.79 |
| V30 (%) | 64 (11–93) | 56 (44–84) | 0.57 |
| Greater curvature | |||
| Mean dose (Gy) | 22.76 (7.83–32.55) | 22.39 (17.74–24.86) | 0.85 |
| Maximum dose (Gy) | 47.30 (42.12–48.86) | 47.00 (45.07–49.32) | 0.65 |
| Minimum dose (Gy) | 4.16 (0.51–10.28) | 1.99 (1.07–6.32) | 0.65 |
| V20 (%) | 59 (11–92) | 50 (44–91) | 0.88 |
| V30 (%) | 26 (1–58) | 20 (18–24) | 0.95 |
| Lesser curvature | |||
| Mean dose (Gy) | 35.45 (16.97–42.63) | 32.96 (28.83–40.68) | 0.94 |
| Maximum dose (Gy) | 48.09 (47.11–49.93) | 48.18 (46.66–49.73) | 0.78 |
| Minimum dose (Gy) | 10.57 (1.33–16.57) | 6.66 (2.73–12.80) | 0.94 |
| V20 (%) | 93 (29–100) | 82 (75–100) | 0.83 |
| V30 (%) | 62 (24–87) | 63 (37–87) | 0.72 |
| Pylorus/antrum | |||
| Mean dose (Gy) | 16.94 (1.52–27.89) | 12.84 (2.52–33.38) | 0.83 |
| Maximum dose (Gy) | 36.80 (2.29–46.51) | 26.37 (11.58–44.00) | 0.92 |
| Minimum dose (Gy) | 5.04 (0.85–12.43) | 3.67 (1.90–12.02) | 0.78 |
| V20 (%) | 31 (0–74) | 4 (0–98) | 0.91 |
| V30 (%) | 3 (0–34) | 0 (0–81) | 0.94 |
Abbreviations: Gy, Gray; V20, the percent volume of the structure receiving at least 20 Gy; IQR, interquartile range; V30, the percent volume of the structure receiving at least 30 Gy.
DISCUSSION
Our study demonstrated that RT dose exposures to the stomach and its substructures which are used to create the future neoesophagus are not associated with the development of postoperative AC. However, our study did demonstrate an increased risk of AC in the patients who underwent EC as compared to ET. No other clinical factors were identified as a significant risk for higher incidence of AC in our overall cohort.
Our reported postoperative AC for this limited cohort including anastomotic leak (16%) and stricture rates (51%) are within the range of those published in prior studies (leak 9%–26% and stricture 45%). 6 , 11 , 12 , 13 , 14 , 15 , 16 , 21 , 24 The finding of a higher risk of AC after EC as compared to ET is conflicting. As in our study, an analysis of The Society of Thoracic Surgeons (STS) database reported significantly higher anastomotic leak rate for patients with EC (12%, 213 of 1720) compared with 9% with ET (145 of 1559) which was attributed to longer course of blood supply travel to reach neck anastomosis compared with shorter course with an intrathoracic anastomosis. 25 A meta‐analysis similarly showed higher rates of anastomotic leaks and anastomotic strictures in EC as compare with ET. 13 In contrast, another meta‐analysis concluded no significant difference between transhiatal and transthoracic esophagectomies on postoperative anastomotic leak. 17 Also, a recent analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) found no difference in anastomotic leak between patients who underwent ET versus EC after neoadjuvant chemoradiation. 24
The influence of the RT dose to the stomach on postoperative AC was also investigated prior with inconsistent findings. A retrospective study of 54 patients with distal esophageal tumors who received TMT with transthoracic anastomosis identified a higher D50 (median RT dose to 50% of the target volume) to the gastric fundus as a significant risk factor for both anastomotic leak and stricture. 15 On the other hand, a larger single institution retrospective study of 285 patients who predominantly had distal esophageal tumors and ET (Ivor‐Lewis) surgeries noted a protective effect of higher mean RT dose to the fundus on anastomotic complications. 19 The authors of this study attributed this paradoxical effect to the location of tumors, proposing a higher anastomotic leakage rate in upper and middle esophageal tumors due to an “in‐field” anastomosis which took place in a previously irradiated mediastinum. 19 Our series had a different population, where 82% of patients had distal or GEJ tumors and 66% underwent EC. In the overall cohort and in a higher risk cohort of patients with distal/GEJ tumors who received EC, there was no association between preoperative RT dose parameters and postoperative AC.
Our findings are in keeping with both CROSS and NEOCRTEC5010 clinical trials that showed no significant increase of postoperative AC with neoadjuvant chemoradiation therapy.3,6 A recent meta‐analysis of 19 trials similarly showed no difference of anastomotic leakage between low dose RT (biologically effective dose [BED] ≤48.85 Gy10) versus high dose RT (BED >48.85 Gy10) in patients treated with TMT. 11 Furthermore, the STS database analysis showed that preoperative RT did not carry on an inherited risk on anastomotic leak frequency (10%) compared to the baseline (11%) in patients who did not receive preoperative RT. The authors of this analysis expressed a level of ambiguity of these data regarding the accurate safety of preoperative radiation therapy due to lacking details about delivered RT dose. 25
Therefore, our reported data, with detailed RT dose parameters to the stomach and its substructures that are used for the future neoesophagus, does represent a clinically meaningful evidence that further support the safety of neoadjuvant chemoradiation prior to esophagectomy for patients with esophageal cancers.
The variation in AC risk associated with esophagectomy type and with RT parameters may be explained to some extent by variations in patient populations, tumor locations and definition of postoperative AC. 12 An ongoing phase III multicenter randomized control rail (ESOPEC), which is comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation therapy (CROSS protocol) and aiming to investigate postoperative complications among other endpoints, will potentially improve our understanding for postoperative AC risk in the future. 26
Several points deserve consideration. This study was limited by small sample size and retrospective nature of the study. We did not evaluate surgical nuances other than location of anastomotic location either in the neck or thorax. This study however was unique in that the actual delivered electronic plans were recovered for all patients, and the gastric substructures were delineated consistently among all patients with the input of thoracic specialists. But as such, this reduced the sample size of the patients to include those with available restored digital RT plans and may have altered ability to determine characteristics that may have been associated with AC.
Despite these limitations, our data suggests that the esophagectomy type with cervical anastomotic location has a much stronger association with development of AC than neoadjuvant RT dose to the gastric substructures. Further analysis in a larger pooled study population is needed to clarify associations between RT dosimetry parameters, surgical approach with anastomotic location, and postoperative AC.
CONFLICT OF INTEREST
No pertinent conflict of interest to this manuscript.
Supporting information
Table S1. Anastomotic complication type in relation to anastomotic location
Table S2. Preoperative radiation dose delivered to the stomach and gastric substructures per anastomotic complication type.
ACKNOWLEDGMENTS
The authors would like to thank Scott P. Robertson, PhD, for his assistance with this manuscript.
Alfaifi S, Chu R, Hui X, Broderick S, Hooker C, Brock M, et al. Trimodality therapy for esophageal cancer: The role of surgical and radiation treatment parameters in the development of anastomotic complications. Thorac Cancer. 2021;12:3121–3129. 10.1111/1759-7714.14130
REFERENCES
- 1. van Heijl M, van Lanschot JJB, Koppert LB, van Berge Henegouwen MI, Muller K, Steyerberg EW, et al. Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS). BMC Surg. 2008;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Heer EC, Hulshoff JB, Klerk D, Burgerhof JGM, de Groot DJA, Plukker JTM, et al. Effect of extending the original eligibility criteria for the CROSS neoadjuvant chemoradiotherapy on toxicity and survival in esophageal cancer. Ann Surg Oncol. 2017;24(7):1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MCCM, Van Lanschot JJB, Steyerberg EW, Henegouwen MV, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. [DOI] [PubMed] [Google Scholar]
- 5. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten‐year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39:1995–2004. [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol. 2018;36:2796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long‐term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. 2021;156:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leng XF, Daiko H, Han YT, Mao YS. Optimal preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma. Ann N Y Acad Sci. 2020;1482:213–24. [DOI] [PubMed] [Google Scholar]
- 9. Parekh K, Iannettoni MD. Complications of esophageal resection and reconstruction. Semin Thorac Cardiovasc Surg. 2007;19:79–88. [DOI] [PubMed] [Google Scholar]
- 10. Ryan CE, Paniccia A, Meguid RA, McCarter MD. Transthoracic anastomotic leak after esophagectomy: current trends. Ann Surg Oncol. 2016;24:281–90. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Liu H, Sun C, Yin X, Tong J, Zhang X, et al. Comparison of clinical efficacy of neoadjuvant chemoradiation therapy between lower and higher radiation doses for carcinoma of the esophagus and gastroesophageal junction: a systematic review. Int J Radiat Oncol Biol Phys. 2021. 10.1016/j.ijrobp.2021.04.031 [DOI] [PubMed] [Google Scholar]
- 12. Koeter M, Van Der Sangen MJC, Hurkmans CW, Luyer MDP, Rutten HJT, Nieuwenhuijzen GAP. Radiation dose does not influence anastomotic complications in patients with esophageal cancer treated with neoadjuvant chemoradiation and transhiatal esophagectomy. Eur J Cancer. 2015;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer. Ann Surg. 2011;254:894–906. [DOI] [PubMed] [Google Scholar]
- 14. Merritt RE, Whyte RI, D'Arcy NT, Hoang CD, Shrager JB. Morbidity and mortality after esophagectomy following neoadjuvant chemoradiation. Ann Thorac Surg. 2011;92:2034–40. [DOI] [PubMed] [Google Scholar]
- 15. Vande Walle C, Ceelen WP, Boterberg T, Vande Putte D, Van Nieuwenhove Y, Varin O, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys. 2012;82:e513–9. [DOI] [PubMed] [Google Scholar]
- 16. Goense L, van Rossum PSN, Ruurda JP, van Vulpen M, Mook S, Meijer GJ, et al. Radiation to the gastric fundus increases the risk of anastomotic leakage after esophagectomy. Ann Thorac Surg. 2016;102:1798–804. [DOI] [PubMed] [Google Scholar]
- 17. Wei MT, Zhang YC, Deng XB, Yang TH, He YZ, Wang ZQ. Transthoracic vs transhiatal surgery for cancer of the esophagogastric junction: a meta‐analysis. World J Gastroenterol. 2014;20:10183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooke DT, Calhoun RF, Kuderer V, David EA. A defined esophagectomy perioperative clinical care process can improve outcomes and costs. Am Surg. 2017;83:103–11. [PubMed] [Google Scholar]
- 19. Juloori A, Tucker SL, Komaki R, Liao Z, Correa AM, Swisher SG, et al. Influence of preoperative radiation field on postoperative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol. 2014;9:534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- 21. Nederlof N, Tilanus HW, Tran TCK, Hop WCJ, Wijnhoven BPL, de Jonge J. End‐to‐end versus end‐to‐side esophagogastrostomy after esophageal cancer resection a prospective randomized study. Ann Surg. 2011;254:226–33. [DOI] [PubMed] [Google Scholar]
- 22. Mahadevan V. Anatomy of the stomach. Surgery (United Kingdom). 2014;32:571–4. [Google Scholar]
- 23. Richardson M. Gastrointestinal tract Part 3: the stomach. Nurs Times. 2006;21(8):24–5. [PubMed] [Google Scholar]
- 24. Chidi AP, Etchill EW, Ha JS, Bush EL, Yang SC, Battafarano RJ, et al. Effect of thoracic versus cervical anastomosis on anastomotic leak among patients who undergo esophagectomy after neoadjuvant chemoradiation. J Thorac Cardiovasc Surg. 2020;160:1088–95. [DOI] [PubMed] [Google Scholar]
- 25. Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919–26. [DOI] [PubMed] [Google Scholar]
- 26. Hoeppner J, Lordick F, Brunner T, Glatz T, Bronsert P, Röthling N, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer. 2016;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Anastomotic complication type in relation to anastomotic location
Table S2. Preoperative radiation dose delivered to the stomach and gastric substructures per anastomotic complication type.


