Abstract
Background
Limited data are available on the long-term outcomes of drug-eluting stents (DES) vs bare-metal stents (BMS) in patients with left main coronary artery (LMCA) disease.
Methods
In this observational cohort of the Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty vs Surgical Revascularization (MAIN-COMPARE) registry, we evaluated patients with unprotected LMCA stenosis who received DES or BMS between January 2000 and June 2006. The primary outcome was a composite of all-cause death or myocardial infarction (MI) at 10 years. Adjusted outcomes were compared using propensity scores and inverse probability of treatment weighting.
Results
A total of 1102 patients underwent DES (n = 784) or BMS (n = 318) during the study period. At 10 years, the adjusted rate of the primary outcome was significantly lower in DES group than in the BMS group (27.9% vs 37.0%; hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.53-0.94; P = 0.02). The adjusted 10-year mortality rate was significantly lower in DES group than in the BMS group (20.6% vs 29.6%; HR, 0.65; 95% CI, 0.46-0.91; P = 0.01), whereas the 10-year rate of MI was similar between the 2 groups (9.9% vs 11.0%; HR, 0.93; 95% CI, 0.54-1.59; P = 0.78). DES use was associated with a significant reduction in the rate of target-lesion revascularization (10.2% vs 21.8%; HR, 0.41; 95% CI, 0.27-0.61; P < 0.001).
Conclusions
In this 10-year follow-up study in patients with LMCA disease, DES use was associated with a significant reduction in the rate of the composite of death or MI, mortality, and target-lesion revascularization, when compared with BMS.
Résumé
Contexte
On dispose de peu de données sur les résultats à long terme de la mise en place d'endoprothèses médicamentées par rapport aux endoprothèses non médicamentées chez les patients atteints d'une maladie de l'artère coronaire principale gauche.
Méthodologie
Dans cette cohorte d'observation du registre MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty vs Surgical Revascularization), nous avons évalué les patients présentant une sténose de l'artère coronaire principale gauche non protégée et ayant reçu une endoprothèse médicamentée ou une endoprothèse non médicamentée entre janvier 2000 et juin 2006. Le paramètre d’évaluation principal était composé de la mortalité toutes causes confondues et de l'infarctus du myocarde (IM) à 10 ans. Les résultats ajustés ont été comparés en utilisant des scores de propension et la pondération inverse sur la probabilité d’être traité.
Résultats
Au total, 1 102 patients ont reçu des endoprothèses médicamentées (n = 784) ou des endoprothèses non médicamentées (n = 318) pendant la période d’étude. À 10 ans, le taux ajusté de survenue du paramètre d’évaluation principal était nettement plus faible dans le groupe endoprothèses médicamentées que dans le groupe endoprothèses non médicamentées (27,9 % vs 37,0 %; rapport des risques instantanés [RRI] : 0,71; intervalle de confiance [IC] à 95 % : 0,53-0,94; p = 0,02). Le taux de mortalité ajusté à 10 ans était considérablement plus faible dans le groupe endoprothèses médicamentées que dans le groupe endoprothèses non médicamentées (20,6 % vs 29,6 %; RRI : 0,65; IC à 95 % : 0,46-0,91; p = 0,01), tandis que le taux d'IM à 10 ans était similaire dans les deux groupes (9,9 % vs 11,0 %; RRI : 0,93; IC à 95 % : 0,54-1,59; p = 0,78). L'utilisation d'endoprothèses médicamentées était associée à une diminution importante du taux de revascularisation de la lésion cible (10,2 % vs 21,8 %; RRI : 0,41; IC à 95 % : 0,27-0,61; p < 0,001).
Conclusions
Dans cette étude de suivi de 10 ans menée auprès de patients atteints d'une maladie de l'artère coronaire principale gauche, l'utilisation d'endoprothèses médicamentées a été associée à une diminution importante du taux des décès ou des IM regroupés, de la mortalité et du taux de revascularisation de la lésion cible, par rapport à l'utilisation d'endoprothèses non médicamentées.
Percutaneous coronary intervention (PCI) with stent implantation has become one of the most frequently performed therapeutic procedures in patients with unprotected left main coronary artery (LMCA) disease.1 In particular, the use of drug-eluting stents (DES) is found to be more effective in the prevention of angiographic and clinical restenosis than the use of bare-metal stents (BMS).2 Several studies found that PCI with DES is a safe and effective modality for patients with LMCA disease and low-to-intermediate anatomic complexity when compared with coronary artery bypass grafting (CABG),3, 4, 5, 6 although some trials show conflicting results.7
Although DES have been found to perform better than BMS with regard to efficacy, there is increasing concern about the safety of DES with regard to an increased rate of very late stent thrombosis and a possible increase in the rate of death and myocardial infarction (MI).8, 9, 10 Previous studies of DES found better efficacy and similar safety when compared with BMS in patients with LMCA stenosis.1,11 Our previous study of patients with unprotected LMCA disease also found that compared with BMS, DES were associated with a reduced rate of repeat revascularization at 3 years, with no increase in the risk of death or MI.12 However, the evidence in favor of DES over BMS beyond 5 years may not be as strong, and the very long-term safety and efficacy of DES compared with BMS in patients with LMCA is still unknown. A recent 10-year study found that the annual risk of target-lesion revascularization (TLR) and stent thrombosis significantly decreased beyond 5 years after implantation of first-generation DES.13
This study aimed to evaluate the very long-term (10-year) risks and benefits of DES use vs BMS use, using extended follow-up data from the Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty vs Surgical Revascularization (MAIN-COMPARE) registry.14
Methods
Study design and population
The design and enrollment criteria of the MAIN-COMPARE registry have been published previously.15,16 Briefly, the MAIN-COMPARE study included consecutive patients with unprotected LMCA stenosis (defined as stenosis of > 50%), who underwent either PCI or CABG as the index procedure at 12 major cardiac centers in Korea between January 2000 and June 2006. Patients with prior CABG, concomitant valvular or aortic surgery, ST-segment elevation MI, or cardiogenic shock were excluded. The local ethics committees at each hospital approved the use of clinical data for this study, and all patients provided written informed consent. There was no industry involvement in the design, conduct, or analysis of the study.
Patients in the MAIN-COMPARE registry who underwent stent implantation for LMCA disease were divided into 2 groups: those who received BMS and those who received DES for the treatment of LMCA disease. Because of device availability, PCI was performed exclusively with BMS between January 2000 and May 2003 and exclusively with DES between May 2003 and June 2006. The final 10-year report of the MAIN-COMPARE study was published recently,14 and the current long-term analyses were based on these datasets.
PCI Procedures
PCI was considered for patients with significant unprotected LMCA stenosis who had suitable anatomy for stenting with disagreement with or contraindications for surgery.12 In our registry, first-generation DES, which were made of stainless steel, were used; sirolimus-eluting (Cypher, Cordis Corp, Johnson & Johnson, Miami Lakes, FL) or paclitaxel-eluting (Taxus, Boston Scientific, Natick, MA) stents. All PCI procedures were performed with standard interventional techniques. The decision to use glycoprotein IIb/IIIa inhibitors, intra-aortic balloon pump, or intravascular ultrasound scan was made at the operator's discretion. Antiplatelet therapy and periprocedural anticoagulation followed standard regimens. Before or during the procedure, patients were administered loading doses of aspirin (200 mg) and clopidogrel (300 or 600 mg) unless they had previously received antiplatelet medications. After the procedure, patients were maintained on aspirin (100 to 200 mg once daily) and clopidogrel (75 mg once daily) for at least 1 month after BMS and for at least 6 months after DES. A longer duration of clopidogrel treatment was administered at the operator's discretion. During the follow-up period, medical therapy for secondary prevention and management was administered in accordance with accepted guidelines and established standards of care.
Study endpoints and follow-up
The primary endpoint of this analysis was a composite incidence of death from any cause and MI at 10 years. Secondary outcomes included death, MI, or TLR. MI was defined when the patient had CK-MB levels > 3 times the upper limit of the normal after the procedure or CK-MB levels above normal with ischemic symptoms or signs during follow-up.12 TLR was defined as any repeat revascularization with PCI or CABG in the treated segment or within the adjacent 5 mm. Definite stent thrombosis was also assessed according to the Academic Research Consortium criteria.17 All clinical events were confirmed by source documentation collected at each hospital and centrally adjudicated by an independent group of clinicians unaware of stent type.
Clinical follow-up was recommended at 1 month, 6 months, 1 year, and annually thereafter. In this report, the follow-up period was extended to December 31, 2016 to ensure that all patients had the opportunity for ≥10-year follow-up evaluation. During the extended follow-up period, if a patient was unwilling or unable to return to the enrolling center, follow-up was maintained by the enrolling investigator through telephone contact or medical records obtained from other hospitals as necessary. To obtain a complete follow-up dataset, the MAIN-COMPARE database was merged with other national population registries. The population vital statistics and date of death were obtained through to December 31, 2016 from the National Population Registry of the Korea National Statistical Office on the basis of the unique 13 digit personal identification number assigned to all Korean citizens.
Statistical analysis
Differences in baseline clinical, angiographic, and procedural characteristics between the DES and BMS groups were compared using the t test or Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher Exact test for categorical variables, as appropriate. Cumulative incidence rates of individual and composite outcomes were estimated by the Kaplan-Meier method and compared by the log-rank test. To ensure that the clinical follow-up of the DES and BMS cohorts was comparable and to remove follow-up bias, clinical outcomes were censored at 10 years in both groups.
To reduce the impact of selection bias and potential confounding factors inherent in an observational study, we performed rigorous adjustment for baseline differences using the weighted Cox proportional hazards regression model, using the inverse probability of treatment weighting (IPTW) method.18,19 With this technique, weights for patients receiving BMS were the inverse of 1-propensity score, and weights for patients receiving DES were the inverse of the propensity score. The propensity scores were estimated by multiple logistic-regression analysis. All prespecified covariates were included in the full nonparsimonious models for treatment with DES vs BMS (Table 1). To create the propensity score, multiple imputation with the Markov chain Monte Carlo method was used to fill out incomplete baseline variables with the assumption that data were missing at random.20 In addition, a second Cox model was created to enable more rigorous adjustment and to avoid selection bias and profile effects. The model was created with taking into account IPTW weights, treatment effect (DES or BMS), and important other risk covariates, which had significant effects (P < 0.1) on the clinical outcomes. All the reported P values are 2 sided, and all the statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC) and the R programming language.
Table 1.
Baseline patient characteristics
| Unadjusted data |
Data adjusted using inverseprobability weighting |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | DES (N = 784) | BMS (N = 318) | P value | Standardizeddifference, % | DES (N = 784) | BMS (N = 318) | Standardizeddifference, % |
| Age, y | 62.5 ± 11.1 | 58.6 ± 12.6 | < 0.001 | 32.7 | 61.3 ± 11.4 | 61.7 ± 12.4 | 3.4 |
| Male sex | 556 (70.9) | 223 (70.1) | 0.79 | 1.7 | 553 (70.5) | 219 (68.7) | 3.9 |
| Body mass index, kg/m2 | 24.5 ± 2.9 | 24.6 ± 3.1 | 0.71 | 2.4 | 24.5 ± 2.9 | 24.2 ± 3.1 | 8.8 |
| Diabetes mellitus | |||||||
| Any type | 251 (32.0) | 76 (23.9) | 0.008 | 18.2 | 234 (29.9) | 101 (31.8) | 4.1 |
| Insulin treated | 64 (8.2) | 11 (3.5) | 0.005 | 20.2 | 54 (6.8) | 19 (5.9) | 4.0 |
| Hypertension | 418 (53.3) | 128 (40.3) | < 0.001 | 26.4 | 388 (49.5) | 150 (47.0) | 4.8 |
| Hyperlipidemia | 241 (30.7) | 74 (23.3) | 0.01 | 16.9 | 223 (28.5) | 79 (24.9) | 8.0 |
| Current smoker | 193 (24.6) | 89 (28.0) | 0.25 | 7.7 | 199 (25.3) | 75 (23.7) | 3.8 |
| Family history of CAD | 56 (7.1) | 25 (7.9) | 0.68 | 2.7 | 58 (7.4) | 25 (7.7) | 1.2 |
| Previous MI | 63 (8.0) | 26 (8.2) | 0.94 | 0.5 | 62 (7.9) | 24 (7.5) | 1.4 |
| Previous PCI | 160 (20.4) | 40 (12.6) | 0.002 | 21.2 | 144 (18.4) | 62 (19.5) | 2.9 |
| Previous CHF | 20 (2.6) | 7 (2.2) | 0.73 | 2.3 | 18 (2.3) | 5 (1.6) | 4.6 |
| Peripheral vascular disease | 14 (1.8) | 2 (0.6) | 0.18 | 10.6 | 11 (1.4) | 3 (1.0) | 4.3 |
| Chronic lung disease | 20 (2.6) | 2 (0.6) | 0.04 | 15.4 | 16 (2.0) | 3 (0.9) | 8.9 |
| Chronic kidney disease | 26 (3.3) | 4 (1.3) | 0.06 | 13.8 | 21 (2.7) | 10 (3.0) | 1.7 |
| Atrial fibrillation | 18 (2.3) | 4 (1.3) | 0.26 | 7.9 | 15 (1.9) | 4 (1.2) | 5.8 |
| LVEF, % | 60 ± 11 | 61 ± 10 | 0.045 | 10.2 | 61 ± 11 | 61 ± 12 | 1.2 |
| Clinical indication for PCI | 0.002 | ||||||
| Silent ischemia | 27 (3.4) | 6 (1.9) | 25.8 | 23 (2.9) | 6 (1.7) | 8.3 | |
| Stable angina | 267 (34.1) | 86 (27.0) | 251 (32.0) | 100 (31.4) | |||
| Unstable angina | 405 (51.7) | 203 (63.8) | 435 (55.5) | 183 (57.5) | |||
| NSTEMI | 85 (10.8) | 23 (7.2) | 75 (9.6) | 30 (9.5) | |||
| LMCA lesion location | < 0.001 | ||||||
| Ostium and/or shaft | 339 (43.2) | 218 (68.6) | 52.7 | 395 (50.4) | 166 (52.2) | 3.6 | |
| Distal bifurcation | 445 (56.8) | 100 (31.5) | 389 (49.6) | 152 (47.8) | |||
| Extent of diseased vessel | < 0.001 | ||||||
| LMCA only | 145 (18.5) | 133 (41.8) | 66.3 | 196 (25.1) | 82 (25.9) | 9.5 | |
| LMCA plus 1-vessel disease | 182 (23.2) | 82 (25.8) | 188 (24.0) | 78 (24.7) | |||
| LMCA plus 2-vessel disease | 217 (27.7) | 70 (22.0) | 205 (26.2) | 91 (28.5) | |||
| LMCA plus 3-vessel disease | 240 (30.6) | 33 (10.4) | 194 (24.8) | 66 (20.9) | |||
| RCA disease | 333 (42.5) | 63 (19.8) | < 0.001 | 50.5 | 282 (36.0) | 103 (32.4) | 7.7 |
| De novo lesion | 757 (96.6) | 313 (98.4) | 0.09 | 12.0 | 761 (97.1) | 310 (97.4) | 1.8 |
| Use of glycoprotein IIb/IIIa inhibitor | 46 (5.9) | 19 (6.0) | 0.95 | 0.5 | 45 (5.7) | 22 (7.0) | 5.5 |
| Use of IABP | 26 (3.3) | 18 (5.7) | 0.07 | 11.3 | 29 (3.7) | 11 (3.4) | 1.6 |
| Guidance of IVUS | 600 (76.5) | 240 (75.5) | 0.71 | 2.5 | 600 (76.5) | 239 (75.2) | 3.0 |
| Complex stenting at bifurcation (≥2 stents) | 184 (23.5) | 24 (7.6) | < 0.001 | 45.1 | 148 (18.9) | 49 (15.4) | 9.3 |
Values are presented as number (percentages) or mean ± SD.
BMS, bare-metal stent; CAD, coronary artery disease; CHF, congestive heart failure; DES, drug-eluting stent; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound scan; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery.
Results
Baseline characteristics
Among 2240 patients enrolled on the MAIN-COMPARE registry, 1102 patients who underwent PCI with stent implantation were included in the analysis: 318 patients received BMS and 784 received DES (607 [77%] sirolimus-eluting stents, 171 [22%] paclitaxel-eluting stents, and 6 [1%] both sirolimus- and paclitaxel-eluting stents). The baseline demographic and clinical, angiographic, and procedural characteristics of the 2 groups are summarized in Table 1. When compared with patients treated with BMS, those treated with DES were significantly older and were more likely to have diabetes, hypertension, hyperlipidemia, prior PCI, chronic lung disease, and chronic kidney disease. Also, patients in the DES group had a lower ejection fraction and were more likely to present with stable angina or non–ST-segment elevation MI. With regard to anatomic and procedural characteristics, patients in the DES group had more complex angiographic features (ie, distal bifurcation and more advanced extent of coronary artery disease); thus, patients in the DES group received a higher proportion and underwent complex 2-stenting techniques compared with the BMS group.
Ten-year clinical outcomes
The median duration of follow-up among all patients was 11.5 years (interquartile range, 6.9-12.8 years). Unadjusted 10-year rates of clinical outcomes after DES and BMS are shown in Supplemental Figure S1 and Supplemental Table S1. Crude 10-year rate of the primary composite endpoint of death or MI was similar in the DES and BMS groups. Observed rates of death or MI were numerically lower in the BMS group without statistical significance. However, observed 10-year rate of TLR was significantly lower in the DES group than in the BMS group.
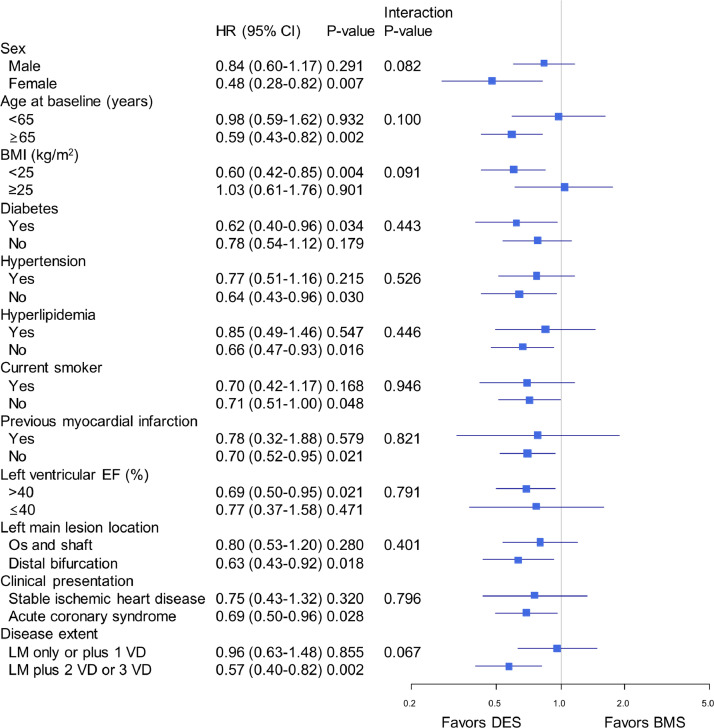
The IPTW-weighted Kaplan-Meier curves and risks for clinical outcomes are shown in Figure 1 and Table 2. The adjusted 10-year rate of the primary composite endpoint was significantly lower in patients who received DES than in those who received BMS (27.9% vs 37.0%, respectively; hazard ratio [HR] 0.71; 95% confidence interval [CI], 0.53-0.94; P = 0.02) (Fig 1). The adjusted 10-year mortality rate was also significantly lower in the DES group than in the BMS group (20.6% vs 29.6%, respectively; HR, 0.65; 95% CI, 0.46-0.91; P = 0.01), whereas the 10-year rate of MI was similar in the 2 groups (9.9% and 11.0%, respectively; HR, 0.93; 95% CI, 0.54-1.59; P = 0.78). The adjusted 10-year rate of TLR was also significantly lower after DES than after BMS (10.2% vs 21.8%, respectively; HR, 0.41; 95% CI, 0.27-0.61; P < 0.001). The results of subgroup analyses using the IPTW adjustment reflected the broad consistency of the relative effect of DES and BMS on the primary composite endpoint (Fig 2). There were no significant interactions between treatment (DES vs BMS) and key clinical or angiographic subgroups with respect to the primary outcome of death or MI at 10 years. At 10 years, there were 14 cases with definite stent thrombosis in the DES group and 1 case in the BMS group (1.9% vs 0.3%; P = 0.06) (Supplemental Figure S2). Among them, very late stent thrombosis after 1 year occurred in 7 patients receiving DES and none receiving BMS.
Figure 1.
Adjusted 10-year event rates using inverse probability treatment weighting in patients who received drug-eluting stents or bare-metal stents. The Kaplan–Meier comparison of treatment with drug-eluting stents vs bare-metal stents. (A) Primary endpoint of death from any cause or myocardial infarction. (B) All-cause mortality. (C) Myocardial infarction. (D) Target-lesion revascularization. CI, confidence interval; HR, hazard ratio.
Table 2.
Adjusted 10-year rates and hazard ratios for clinical outcomes with DES vs BMS*
| Outcomes | Adjusted event rates at 10 years, % |
Adjusted by IPTW |
Adjusted by IPTW and covariates† |
|||
|---|---|---|---|---|---|---|
| DES | BMS | HR (95% CI) | P value | HR (95% CI) | P value | |
| Death or MI | 27.9 | 37.0 | 0.71 (0.53-0.94) | 0.02 | 0.70 (0.54-0.91) | 0.007 |
| Death | 20.6 | 29.6 | 0.65 (0.46-0.91) | 0.01 | 0.65 (0.47-0.88) | 0.006 |
| MI | 9.9 | 11.0 | 0.93 (0.54-1.59) | 0.78 | 0.91 (0.53-1.56) | 0.74 |
| Target-lesion revascularization | 10.2 | 21.8 | 0.41 (0.27-0.61) | < 0.001 | 0.38 (0.25-0.58) | < 0.001 |
BMS, bare-metal stent; CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; IPTW, inverse probability treatment weighting; MI, myocardial infarction.
Hazard ratios are for the DES group as compared with the BMS group.
Adjustment was performed using IPTW and significant covariates influencing outcomes.
Figure 2.
Adjusted hazard ratio (HR) for the primary composite outcome of death or myocardial infarction in major clinical and anatomical subgroups. HRs are for the DES group vs the BMS group. BMI, body mass index; BMS, bare-metal stents; CI, confidence interval; DES, drug-eluting stents; EF, ejection fraction; HR, hazard ration; VD, vessel disease.
Discussion
This analysis of 10-year follow-up data on the safety and effectiveness of DES and BMS for the treatment of LMCA disease showed these key findings: (1) when compared with BMS, the use of DES was associated with a significant reduction in the adjusted rate of the primary composite of death or MI; (2) the adjusted 10-year mortality rate was also significantly lower in the DES group than in the BMS group, but the 10-year rate of MI was similar in both; (3) DES was associated with a significant reduction in the 10-year rate of TLR; and (4) although the number of definite stent thrombosis was quite low, the incidence of thrombotic events was numerically higher in the DES group. Our study includes the longest period of follow-up to date and will help clinicians understand the very long-term performance of DES for complex patients with unprotected LMCA disease.
Our study confirms that DES are more effective than BMS in reducing the need for TLR, which is likely to be the main reason why a DES is now the default device for the treatment of LMCA or multivessel disease in contemporary PCI practice.21,22 Although the benefit of DES in reducing the need for repeat revascularization has been confirmed in several studies,1,23 there is great concern expressed about the potential for this benefit to be outweighed by the risk of late stent thrombosis or a late increase in mortality or MI.8, 9, 10,24 However, our results suggest that the adjusted 10-year rate of the primary composite of death or MI is significantly lower in patients treated with DES than in those receiving BMS. In particular, although DES patients were substantially older and had higher risk profiles for clinical and anatomic characteristics, the adjusted 10-year all-cause mortality rate was also lower in the DES group than in the BMS group. Prior large population-based data show that the 3-year adjusted mortality rate was significantly lower in patients treated with DES than in those treated with BMS, without a significant difference in MI rates,25 which was similar to that seen in this study. By contrast, a recent large-scale randomized clinical trial (RCT) found no significant differences in the 6-year rate of death from any cause or of nonfatal spontaneous MI between those receiving contemporary DES and those receiving contemporary BMS.26 These conflicting results might be in part explained by a different study design, a different duration of follow-up, a paradigm shift in PCI and medical care practice over time, a differential risk of patient and anatomic characteristics, and unmeasured confounding factors.
A relatively small RCT, registry data, and meta-analysis found that the efficacy of DES for the treatment of unprotected LMCA stenosis was superior to that of BMS, without increased safety risks.11,12,27 On the basis of these promising data, consecutive RCTs comparing first- and second-generation DES and standard CABG were performed, showing that DES implantation was a good alternative for selected patients with LMCA disease.6,7,28 However, the follow-up period of such studies was limited (less than 5 years’ duration). To the best of our knowledge, this study represents the first investigation of the very long-term outcomes (beyond 10 years) associated with DES and BMS use in patients with unprotected LMCA stenosis. The use of data from a large national registry of consecutive patients recruited at multiple centers may be a good indicator of real-world outcomes.
Until recently, very long-term data on the occurrence of stent thrombosis in this critical portion of LMCA-PCI have been limited. In our study, although the number of stent thromboses was quite low, an incidence of definite stent thrombosis (especially, very late thrombosis) was numerally higher in the DES group compared with the BMS group. Considering the catastrophic consequence of thrombotic events in the LMCA, this observation warrants further evaluation through extended follow-up of the Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) and Nordic-Baltic-British Left Main Revascularization Study (NOBLE) trials using contemporary DES.6,7 However, in the clinical viewpoint, our findings support the argument that the benefits in terms of restenosis or TLR, which may manifest as angina or MI, may outweigh the risks associated with a slight increase in the rate of late stent thrombosis in patients undergoing LMCA-PCI.29
Our study has several limitations. First, the nonrandomized, observational study design may have introduced selection bias or ascertainment bias. Although rigorous adjustment of baseline differences was performed using propensity-score analyses, the effect of unmeasured confounders may remain. Second, patients receiving DES later in the study period could have benefited from improvements in procedural skills and more advanced cardioactive medications than patients receiving BMS at an earlier timepoint. In addition, long-term medication use and compliance with guideline-directed medical management after PCI varied substantially. Third, there were only a few cases of stent thrombosis in this analysis. This small number of events does not allow for a sophisticated statistical adjustment for comparison of DES and BMS. Fourth, intravascular ultrasound-guided PCI was performed in approximately three-quarters of patients with unprotected LMCA stenosis, which may limit the generalizability and reproducibility of our results in other settings. Finally, our registry only evaluated first-generation DES; as suggested by a recent meta-analysis comparing new-generation DES and BMS,30 our findings should be confirmed or refuted by further studies using contemporary DES in patients receiving LMCA-PCI.
Conclusions
In this 10-year follow-up study of patients undergoing PCI with stenting for unprotected LMCA disease, DES use was associated with a significant reduction in the rate of the serious composite outcome of death or MI when compared with patients receiving BMS. The rates of all-cause mortality and TLR were also significantly lower after DES implantation. Although the 10-year rate of definite stent thrombosis was quite low, a higher incidence was seen in the DES group, and this observation warrants further investigation.
Funding Sources
This work was partly supported by grants from the Cardiovascular Research Foundation of South Korea.
Disclosures
All authors have reported that they have no relationships to disclose that are relevant to the content of this paper.
Footnotes
Ethics Statement: This study was conducted in compliance with the Declaration of Helsinki and approved by the institutional review board of Asna Medical Hospital.
Clinical Trial Registration:http://ClinicalTrials.gov (Identifier: NCT02791412)
See page 1205 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.05.009.
Appendix. Supplementary materials
References
- 1.Lee PH, Ahn JM, Chang M, et al. Left main coronary artery disease: secular trends in patient characteristics, treatments, and outcomes. J Am Coll Cardiol. 2016;68:1233–1246. doi: 10.1016/j.jacc.2016.05.089. [DOI] [PubMed] [Google Scholar]
- 2.Stefanini GG, Holmes DR., Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcante R, Sotomi Y, Lee CW, et al. Outcomes after percutaneous coronary intervention or bypass surgery in patients with unprotected left main disease. J Am Coll Cardiol. 2016;68:999–1009. doi: 10.1016/j.jacc.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Palmerini T, Serruys P, Kappetein AP, et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: a meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. 2017;190:54–63. doi: 10.1016/j.ahj.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391:939–948. doi: 10.1016/S0140-6736(18)30423-9. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Sabik JF, Serruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. doi: 10.1056/NEJMoa1610227. [DOI] [PubMed] [Google Scholar]
- 7.Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743–2752. doi: 10.1016/S0140-6736(16)32052-9. [DOI] [PubMed] [Google Scholar]
- 8.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [discussion 1455] [DOI] [PubMed] [Google Scholar]
- 9.Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 10.Bavry AA, Kumbhani DJ, Helton TJ, et al. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med. 2006;119:1056–1061. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Pandya SB, Kim Y-H, Meyers SN, et al. Drug-eluting versus bare-metal stents in unprotected left main coronary artery stenosis: a meta-analysis. JACC: Cardiovasc Interv. 2010;3:602–611. doi: 10.1016/j.jcin.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YH, Park DW, Lee SW, et al. Long-term safety and effectiveness of unprotected left main coronary stenting with drug-eluting stents compared with bare-metal stents. Circulation. 2009;120:400–407. doi: 10.1161/CIRCULATIONAHA.108.800805. [DOI] [PubMed] [Google Scholar]
- 13.Yamaji K, Räber L, Zanchin T, et al. Ten-year clinical outcomes of first-generation drug-eluting stents: the Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) VERY LATE trial. Eur Heart J. 2016;37:3386–3395. doi: 10.1093/eurheartj/ehw343. [DOI] [PubMed] [Google Scholar]
- 14.Park DW, Ahn JM, Yun SC, et al. 10-year outcomes of stents versus coronary artery bypass grafting for left main coronary artery disease. J Am Coll Cardiol. 2018;72:2813–2822. doi: 10.1016/j.jacc.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Seung KB, Park DW, Kim YH, et al. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781–1792. doi: 10.1056/NEJMoa0801441. [DOI] [PubMed] [Google Scholar]
- 16.Park DW, Seung KB, Kim YH, et al. Long-term safety and efficacy of stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease: 5-year results from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) registry. J Am Coll Cardiol. 2010;56:117–124. doi: 10.1016/j.jacc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 18.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass) 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Therneau T G, P. Springer-Verlag; New York, NY: 2000. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- 20.Rubin DB. John Wiley & Sons; New York, NY: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 21.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001-2008. Jama. 2011;305:1769–1776. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiomi H, Morimoto T, Makiyama T, et al. Evolution in practice patterns and long-term outcomes of coronary revascularization from bare-metal stent era to drug-eluting stent era in Japan. Am J Cardiol. 2014;113:1652–1659. doi: 10.1016/j.amjcard.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 23.James SK, Stenestrand U, Lindback J, et al. Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N Engl J Med. 2009;360:1933–1945. doi: 10.1056/NEJMoa0809902. [DOI] [PubMed] [Google Scholar]
- 24.Farb A, Boam AB. Stent thrombosis redux—the FDA perspective. N Engl J Med. 2007;356:984–987. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 25.Tu JV, Bowen J, Chiu M, et al. Effectiveness and safety of drug-eluting stents in Ontario. N Engl J Med. 2007;357:1393–1402. doi: 10.1056/NEJMoa071076. [DOI] [PubMed] [Google Scholar]
- 26.Bonaa KH, Mannsverk J, Wiseth R, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–1252. doi: 10.1056/NEJMoa1607991. [DOI] [PubMed] [Google Scholar]
- 27.Erglis A, Narbute I, Kumsars I, et al. A randomized comparison of paclitaxel-eluting stents versus bare-metal stents for treatment of unprotected left main coronary artery stenosis. J Am Coll Cardiol. 2007;50:491–497. doi: 10.1016/j.jacc.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 28.Park S-J, Kim Y-H, Park D-W, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–1727. doi: 10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 29.Chen MS, John JM, Chew DP, et al. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151:1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Piccolo R, Bonaa KH, Efthimiou O, et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393:2503–2510. doi: 10.1016/S0140-6736(19)30474-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.