Abstract
Background
We aimed to determine the association of atrial fibrillation (AF) with 1-year outcome in ST-elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PCI).
Methods
Patients (n = 8830) enrolled in the Trial of Routine Aspiration Thrombectomy with PCI vs PCI Alone in Patients With STEMI (TOTAL) were followed for 1 year. The primary outcome was a composite of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or new or worsening class IV heart failure. The presence or absence of AF was determined from a single pre-PCI electrocardiogram.
Results
Patients with AF (n = 437; 4.9%) were older, and more often had a history of stroke, hypertension, or myocardial infarction. The rate of the primary outcome was higher in the AF group than in the sinus rhythm (SR) group (17.4% vs 7.4%, P < 0.001), as was the rate of cardiovascular death (9.8% vs 3.3%, P < 0.001). In multivariable analysis, AF was independently predictive of the primary outcome (adjusted hazard ratio [aHR] 1.68; 95% confidence interval [CI], 1.30-2.16, P < 0.001), cardiovascular death (aHR 1.69; 95% CI, 1.19-2.40, P = 0.003), all-cause mortality (aHR 1.63; 95% CI, 1.18-2.24, P = 0.003), and severe heart failure (aHR 1.96; 95% CI, 1.25-3.07, P = 0.003). Among patients who were in SR, the primary outcome occurred in 307 of 4252 (7.2%) in the thrombectomy group and 310 of 4141 (7.5%) in the PCI alone group, and among those with AF, these rates were respectively 42 of 218 (19.3%) and 34 of 219 (15.5%) (Pinteraction = 0.26).
Conclusions
In STEMI patients, AF on the pre-PCI electrocardiogram is associated with a higher risk of the primary composite cardiovascular outcome, all-cause and cardiovascular death, and severe heart failure during 1-year follow-up than it is in patients with SR.
Résumé
Contexte
Notre objectif était de déterminer le lien entre la fibrillation auriculaire (FA) et le résultat à un an de patients ayant subi un infarctus du myocarde avec élévation du segment ST (STEMI) puis une intervention coronarienne percutanée (ICP) primaire.
Méthodologie
Les patients (n = 8 830) admis à l’étude TOTAL (Trialof Routine AspirationThrombectomy with PCI vs PCIAlone in Patients With STEMI) ont été suivis pendant une année. Le principal critère d’évaluation était composé des décès d'origine cardiovasculaire, de l'infarctus du myocarde récurrent, du choc cardiogénique ou de l'apparition/aggravation d'une insuffisance cardiaque de classe IV. La présence ou l'absence de FA était établie à partir d'un seul électrocardiogramme effectué avant l'ICP.
Résultats
Les patients atteints de FA (n = 437; 4,9 %) étaient âgés, et la plupart avaient des antécédents d'AVC, d'hypertension ou d'infarctus du myocarde. La fréquence des manifestations liées au principal critère d’évaluation était plus élevée dans le groupe FA que dans le groupe en rythme sinusal (17,4 % vs 7,4 %, p < 0,001); il en était de même pour le taux de décès d'origine cardiovasculaire (9,8 % vs 3,3 %, p < 0,001). Dans une analyse multivariée, la FA était indépendamment prédictive des manifestations liées au principal critère d’évaluation (rapport des risques instantanés ajusté [RRIa] : 1,68; intervalle de confiance [IC] à 95 % : 1,30-2,16, p < 0,001), décès d'origine cardiovasculaire (RRIa : 1,69; IC à 95 % : 1,19-2,40, p = 0,003), mortalité toutes causes confondues (RRIa : 1,63; IC à 95 % : 1,18-2,24, p = 0,003) et insuffisance cardiaque grave (RRIa : 1,96; IC à 95 % : 1,25-3,07, p = 0,003). Parmi les patients en rythme sinusal, les manifestations du principal critère d’évaluation sont survenues chez 307 patients sur les 4 252 (7,2 %) du groupe ayant subi une thrombectomie, et chez 310 patients sur les 4 141 (7,5 %) du groupe ayant subi une ICP sans thrombectomie; parmi ceux atteints de FA, ces taux étaient respectivement de 42 sur 218 (19,3 %) et de 34 sur 219 (15,5 %) (pinteraction = 0,26).
Conclusions
Chez les patients ayant subi un STEMI, la détection d'une FA à l’électrocardiogramme réalisé avant l'ICP est associée à un risque accru de manifestation cardiovasculaire liée au principal critère d’évaluation composé, de décès toutes causes confondues et d'origine cardiovasculaire, et d'insuffisance cardiaque grave, pendant la première année de suivi comparativement aux patients en rythme sinusal.
Atrial fibrillation (AF) accompanies acute myocardial infarction (AMI) in 2.3% to 21% of cases.1,2 The reported prevalence of AF before percutaneous coronary intervention (PCI) in ST-elevation myocardial infarction (STEMI) is 3.0% to 4.7%.3, 4, 5, 6 Several recent studies have indicated a negative impact of AF on the long-term outcome of STEMI patients treated with primary PCI.3,4,6, 7, 8, 9, 10 Conversely, in other studies, AF on admission did not affect long-term outcome after STEMI.11, 12, 13 Thus, controversy remains regarding the long-term prognostic value of AF on the admission electrocardiogram (ECG) of STEMI patients undergoing primary PCI.
The aim of the present study, an ancillary study of the Trial of Routine Aspiration Thrombectomy with PCI vs PCI Alone in Patients With STEMI (TOTAL), was to define whether AF on the pre-procedural ECG in STEMI patients undergoing primary PCI is associated with the long-term outcome.
Material and Methods
Patients and randomization
The design of the TOTAL trial has been described previously.14 In brief, the TOTAL trial was an international, investigator-initiated, multicentre, prospective, randomized trial of routine upfront manual aspiration thrombectomy vs PCI alone.15 Between Aug 5, 2010 and July 25, 2014, a total of 10,732 STEMI patients were enrolled and randomly assigned to 1 of 2 treatment groups—manual aspiration thrombectomy followed by PCI, or PCI alone. The study protocol specified the recording of a pre-procedural ECG in all eligible patients, accompanied by a second recording 30-60 minutes after the index procedure. All clinical outcomes were treated as time-to-event data. All patients were followed for the prespecified study period or until death. Missing outcome data were handled by censoring in the time-to-event analysis. Follow-up was completed in 99% of patients at 1 year.
The TOTAL trial was approved by the ethics committees at each participating centre and by the national regulatory authorities in all countries where the trial was conducted.
All the ECGs from the TOTAL trial were analyzed manually in the ECG core laboratory at the Heart Hospital, Tampere University Hospital (Tampere, Finland), and all the readers were blinded to the treatment assignment, as well as to clinical and angiographic findings.
This electrocardiographic substudy was prespecified, including the statistical analysis. For this substudy, we selected all patients who had undergone the index PCI and had a baseline pre-procedural ECG recorded (n = 10,064) showing AF (n = 428), atrial flutter (n = 9), or sinus rhythm (SR; n = 8393). The exclusion criteria included missing data on pre-ECG rhythm in the case record form (n = 576), and rhythm other than AF, atrial flutter, or SR (n = 658). The final study population comprised 8830 patients.
Study outcomes
The primary outcome of this ancillary study was a composite of cardiovascular death, recurrent MI, cardiogenic shock, and new or worsening New York Heart Association (NYHA) class IV heart failure within 1 year of randomization. The components of the primary outcome were identical to those in the main TOTAL trial. A key secondary outcome was the primary outcome plus stent thrombosis or target-vessel revascularization. All-cause mortality and major bleeding were additional secondary outcomes. The primary safety outcome was stroke, and the key net-benefit outcome was the composite of the primary efficacy outcome plus the primary safety outcome.
Subgroup analysis
The comparative rates of the 1-year primary outcome were also determined in the following subgroups: initial thrombolysis in myocardial infarction (TIMI) thrombus grade (grade < 3 vs grade ≥ 3; and grade < 4 vs grade ≥ 4); symptom onset (< 6 hours vs 6-12 hours); initial TIMI flow 0-1 vs 2-3 (with a higher grade indicating better flow); site PCI volume (centres reported annual primary PCI volume and were then ranked and divided by thirds for subgroup analysis by centre volume); MI type (anterior vs non-anterior); age (≤ 65 years vs > 65 years); smoking (yes/no); diabetes (yes/no); bivalirudin usage (yes/no); glycoprotein IIb/IIIa usage (yes/no); proximal lesion (yes/no); gender (male/female); and prior anticoagulation (yes/no).
ECG analysis
ST elevation (STE) was measured from the J point, using the TP segment as the isoelectric line. As investigators were unaware of the patients’ age and gender, a modified cut-point of 0.2 mV for STE in leads V2-V3 was used. For all the other leads, except the augmented vector right (aVR) lead, the guideline-based cutoff of ≥ 0.1 mV in 2 or more contiguous leads was used. The STEMI location was defined based on the location of the STE, as anterior (leads V1-V4 [-V5]); inferior (leads II, III, aVF); lateral (leads I, augmented vector left [aVL], V5-V6); or other. The anterior and inferior MI locations were used in those patients in whom the cutoff values of STE were fulfilled for both locations; these patients were included in the lateral or other group. Pathologic Q waves were defined as any Q-wave duration of 0.02 seconds or more, or a QS complex in leads V2 and/or V3. Regarding other leads, a Q-wave duration of ≥ 0.03 seconds and depth ≥ 0.1 mV was considered a pathologic Q wave, if observed in ≥ 2 contiguous leads.16 Q waves in leads aVR, III, and V1 were not taken into account.
The rhythm was analyzed manually. AF and atrial flutter were combined because of the low number of ECGs with atrial flutter (n = 9), and therefore, in the study results, the designation “AF” includes atrial flutter.
Statistical analyses
Categorical variables were documented as counts and percentages; continuous variables were documented as means with standard deviations (SDs) or as medians with interquartile ranges (IQRs). Comparisons between AF and SR patients were made using the χ2 test for categorical variables, and a 2-sample t test or a Wilcoxon rank-sum test for normally and non-normally distributed continuous variables, respectively. The Cox proportional hazards regression model was used to assess the effect of AF on the risk of primary and secondary outcomes, adjusted for age, time from symptom onset, gender, location of MI, current smoking, previous stroke, previous hypertension, previous diabetes mellitus, previous MI, previous PCI, Killip class (≥ 2), and proximal lesion. The proportional hazards assumption of the Cox model and the linearity of continuous covariates were assessed using the Kolmogorov-type supremum test and restricted cubic spline plots, respectively. No violation was detected. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The adjusted cumulative incidence curves of the primary outcome and cardiovascular death for AF and SR patients were generated by the average of the predicted cumulative incidence curves for the study population based on the above-mentioned multivariable Cox models. Interactions between predefined subgroups and rhythm (AF vs SR) on the primary outcome were analyzed by likelihood ratio test of the interaction term in the unadjusted Cox regression model. A 2-tailed P-value of < 0.05 was considered statistically significant. All analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
AF on admission was observed in 437 (4.9%) of 8830 TOTAL STEMI patients with available data. The baseline characteristics of patients according to rhythm are presented in Table 1. Compared to patients with SR, patients with AF were older and more often female, had a higher heart rate, lower systolic and diastolic blood pressure, and a higher prevalence of Killip class ≥ 2. AF patients also more often presented with a medical history of previous stroke, MI, and hypertension. An inferior infarct location was more frequent in the AF group.
Table 1.
Baseline characteristics of patients according to the rhythm on the pre-percutaneous coronary intervention (PCI) electrocardiogram*
| Characteristics | All (N = 8830) | AF/Flutter (n = 437) | Sinus rhythm (n = 8393) | P† |
|---|---|---|---|---|
| Age, y | 60.8 ± 11.8 | 67.7 ± 11.8 | 60.4 ± 11.7 | < 0.001 |
| Age > 75 y | 1090 (12.3) | 118 (27.0) | 972 (11.6) | < 0.001 |
| Gender, male | 6881 (77.9) | 318 (72.8) | 6563 (78.2) | 0.008 |
| Heart rate, bpm | 77.0 ± 17.1 | 86.8 ± 26.5 | 76.5 ± 16.3 | < 0.001 |
| Systolic BP, mm Hg | 135.9 ± 26.3 | 128.1 ± 28.1 | 136.3 ± 26.2 | < 0.001 |
| Diastolic BP, mm Hg | 82.6 ± 16.4 | 80.2 ± 18.3 | 82.8 ± 16.2 | 0.004 |
| BMI, kg/m2 | 27.6 ± 4.6 | 27.7 ± 4.9 | 27.6 ± 4.6 | 0.870 |
| Killip class ≥ 2 | 365 (4.1) | 34 (7.8) | 331 (3.9) | < 0.001 |
| Location of MI | 0.006 | |||
| Anterior | 3660 (41.4) | 149 (34.1) | 3511 (41.8) | |
| Inferior | 4699 (53.2) | 268 (61.3) | 4431 (52.8) | |
| Lateral or other | 465 (5.3) | 20 (4.6) | 445 (5.3) | |
| Medical history | ||||
| Previous stroke | 261 (3.0) | 35 (8.0) | 226 (2.7) | < 0.001 |
| Hypertension | 4398 (49.8) | 267 (61.1) | 4131 (49.2) | < 0.001 |
| Diabetes | 1595 (18.1) | 91 (20.8) | 1504 (17.9) | 0.124 |
| Previous MI | 817 (9.3) | 61 (14.0) | 756 (9.0) | < 0.001 |
| Previous PCI | 750 (8.5) | 46 (10.5) | 704 (8.4) | 0.118 |
| Peripheral arterial disease | 203 (2.3) | 23 (5.3) | 180 (2.1) | < 0.001 |
| Current smoker | 4037 (45.7) | 159 (36.4) | 3878 (46.2) | < 0.001 |
Values are n (%) or mean ± standard deviation, unless otherwise indicated.
AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; bpm, beats per minute; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The analysis population was defined as STEMI patients with sinus rhythm or AF/Atrial flutter in the pre-PCI ECG.
P value is from χ2 test for categorical variables, and 2-sample t test for continuous variables.
Procedure-related characteristics of the 2 study groups are summarized in Table 2. Patients with AF were more likely to have had a totally occluded infarct-related artery (TIMI flow 0) before the index PCI and to undergo bailout thrombectomy than those with SR. Drug-eluting stents were more often used in patients with SR compared with patients who had AF. Oral anticoagulant use prior to hospitalization and at hospital discharge was more frequent in the AF than in the SR group (15.6% vs 0.9%, P < 0.001 and 27.5% vs 4.8%, P < 0.001, respectively). Sheath size did not differ between the 2 groups.
Table 2.
Procedure characteristics of patients according to the rhythm on the pre-percutaneous coronary intervention (PCI) electrocardiogram
| Procedure | All (N = 8830) | AF/flutter (n = 437) | Sinus rhythm (n = 8393) | P* |
|---|---|---|---|---|
| Transported by ambulance | 5802 (65.7) | 307 (70.3) | 5495 (65.5) | 0.042 |
| Initial PCI procedure | ||||
| Onset to hospital, min | 122.0 (72.0–220.0) | 122.0 (76.0–215.0) | 122.0 (71.0–220.0) | 0.671 |
| Hospital to procedure, min | 53.0 (23.0–90.0) | 46.0 (22.0–90.0) | 53.0 (23.0–90.0) | 0.278 |
| Radial access | 6017 (68.1) | 278 (63.6) | 5739 (68.4) | 0.037 |
| Sheath size, French | 0.813 | |||
| ≤ 5 | 153 (1.7) | 9 (2.1) | 144 (1.7) | |
| 6 | 8462 (95.8) | 415 (95.0) | 8047 (95.9) | |
| 7 | 202 (2.3) | 12 (2.7) | 190 (2.3) | |
| Medication use | ||||
| Unfractionated heparin | 7259 (82.2) | 356 (81.5) | 6903 (82.2) | 0.677 |
| Bivalirudin | 1679 (19.0) | 85 (19.5) | 1594 (19.0) | 0.812 |
| Enoxaparin | 764 (8.7) | 29 (6.6) | 735 (8.8) | 0.124 |
| Glycoprotein IIb/IIIa inhibitor | ||||
| Upfront | 2194 (24.8) | 80 (18.3) | 2114 (25.2) | 0.001 |
| Bailout | 1337 (15.1) | 60 (13.7) | 1277 (15.2) | 0.398 |
| Initial TIMI thrombus grade | 0.064 | |||
| 0 | 237 (2.7) | 11 (2.5) | 226 (2.7) | |
| 1 | 461 (5.2) | 9 (2.1) | 452 (5.4) | |
| 2 | 250 (2.8) | 13 (3.0) | 237 (2.8) | |
| 3 | 951 (10.8) | 40 (9.2) | 911 (10.9) | |
| 4 | 1235 (14.0) | 68 (15.6) | 1167 (13.9) | |
| 5 | 5690 (64.4) | 296 (67.7) | 5394 (64.3) | |
| TIMI 0 flow before PCI | 5842 (66.2) | 309 (70.7) | 5533 (65.9) | 0.039 |
| Upfront manual thrombectomy | 4327 (49.0) | 213 (48.7) | 4114 (49.0) | 0.911 |
| Bailout thrombectomy | 310 (3.5) | 35 (8.0) | 275 (3.3) | < 0.001 |
| Use of stenting | ||||
| Direct stenting | 2646 (30.0) | 110 (25.2) | 2536 (30.2) | 0.025 |
| Type of stent | ||||
| Bare metal | 4594 (52.0) | 266 (60.9) | 4328 (51.6) | < 0.001 |
| Drug-eluting | 3979 (45.1) | 145 (33.2) | 3834 (45.7) | < 0.001 |
| No. of stents | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.4 ± 0.7 | 0.742 |
| Total stent length, mm | 21.3 ± 6.5 | 20.9 ± 6.3 | 21.3 ± 6.5 | 0.141 |
| Stent diameter, mm | 3.1 ± 0.5 | 3.2 ± 0.5 | 3.1 ± 0.5 | 0.011 |
| Median PCI procedure time, min | 37.0 (27.0–50.0) | 40.0 (29.0–55.0) | 37.0 (27.0–50.0) | 0.003 |
| Concomitant medications within 7 d prior to hospitalization | ||||
| ASA | 1353 (15.3) | 82 (18.8) | 1271 (15.1) | 0.040 |
| Clopidogrel | 210 (2.4) | 11 (2.5) | 199 (2.4) | 0.845 |
| Prasugrel | 18 (0.2) | 2 (0.5) | 16 (0.2) | 0.223 |
| Ticagrelor | 33 (0.4) | 3 (0.7) | 30 (0.4) | 0.223 |
| Oral anticoagulants | 140 (1.6) | 68 (15.6) | 72 (0.9) | < 0.001 |
| Concomitant medications during initial hospitalization | ||||
| ASA | 8640 (97.8) | 426 (97.5) | 8214 (97.9) | 0.589 |
| Clopidogrel | 6236 (70.6) | 329 (75.3) | 5907 (70.4) | 0.028 |
| Prasugrel | 1298 (14.7) | 47 (10.8) | 1251 (14.9) | 0.017 |
| Ticagrelor | 2198 (24.9) | 95 (21.7) | 2103 (25.1) | 0.118 |
| Oral anticoagulants | 522 (5.9) | 112 (25.6) | 410 (4.9) | < 0.001 |
| Concomitant medications at discharge | ||||
| ASA | 8605 (97.5) | 398 (91.1) | 8207 (97.8) | < 0.001 |
| Clopidogrel | 5387 (61.0) | 287 (65.7) | 5100 (60.8) | 0.040 |
| Prasugrel | 1090 (12.3) | 28 (6.4) | 1062 (12.7) | < 0.001 |
| Ticagrelor | 1920 (21.7) | 69 (15.8) | 1851 (22.1) | 0.002 |
| Oral anticoagulants | 524 (5.9) | 120 (27.5) | 404 (4.8) | < 0.001 |
Values are n (%), median (IQR), or mean ± standard deviation, unless otherwise indicated. Wilcoxon test was used for non-normally distributed variables.
ASA, acetylsalicylic acid; IQR, interquartile range; TIMI, thrombolysis in myocardial infarction.
P value is from χ2 test for categorical variables, and 2-sample t test for normally distributed variables.
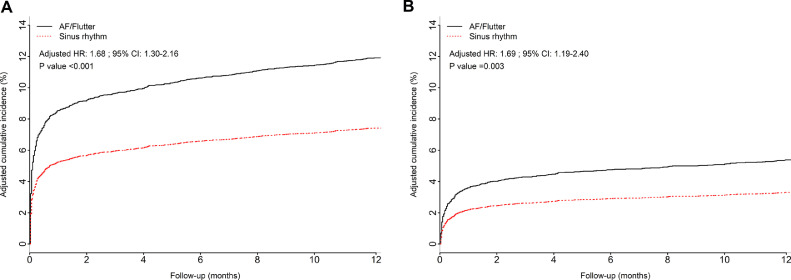
Table 3 shows the unadjusted and adjusted analyses for the primary and secondary outcomes. The rate of the primary outcome was higher in the AF group than in the sinus rhythm (SR) group (17.4% vs 7.4%, P < 0.001), as was the rate of cardiovascular death (9.8% vs 3.3%, P < 0.001). In the multivariable analysis, AF in the pre-PCI ECG independently predicted a higher rate of primary outcome (adjusted HR 1.68; 95% CI, 1.30-2.16, P < 0.001; Fig. 1A), key secondary outcome (adjusted HR 1.56; 95% CI, 1.24-1.95, P < 0.001), and cardiovascular death (adjusted HR 1.69; 95% CI, 1.19-2.40, P = 0.003; Fig. 1B) compared to the rate in patients with SR within the 1-year follow-up period. AF was also independently associated with a higher risk of all-cause mortality (adjusted HR 1.63; 95% CI, 1.18-2.24, P = 0.003), severe heart failure (adjusted HR 1.96; 95% CI, 1.25-3.07, P = 0.003), and net risk–benefit risk outcome (adjusted HR 1.62; 95% CI, 1.26-2.08, P < 0.001). Stroke did not maintain its statistical significance after adjustments for confounding factors.
Table 3.
Primary and secondary outcomes at 1 year, according to the rhythm on the pre-percutaneous coronary intervention electrocardiogram
| Unadjusted |
Adjusted* |
|||||
|---|---|---|---|---|---|---|
| Outcomes | AF/flutter, n (%) (n = 437) | Sinus rhythm, n (%) (n = 8393) | HR (95% CI) | P | HR (95% CI) | P |
| Primary and secondary outcomes | ||||||
| Primary outcome | 76 (17.4) | 617 (7.4) | 2.51 (1.97–3.19) | < 0.001 | 1.68 (1.30–2.16) | < 0.001 |
| Cardiovascular death | 43 (9.8) | 274 (3.3) | 3.12 (2.26–4.31) | < 0.001 | 1.69 (1.19–2.40) | 0.003 |
| Recurrent MI | 12 (2.7) | 204 (2.4) | 1.17 (0.66–2.10) | 0.591 | 0.99 (0.55–1.79) | 0.965 |
| Cardiogenic shock | 16 (3.7) | 153 (1.8) | 2.05 (1.22–3.45) | 0.006 | 1.40 (0.80–2.48) | 0.242 |
| Class IV heart failure | 24 (5.5) | 157 (1.9) | 3.08 (2.01–4.74) | < 0.001 | 1.96 (1.25–3.07) | 0.003 |
| CVD, MI, CardShock, HF, stent thrombosis, TVR | 94 (21.5) | 909 (10.8) | 2.13 (1.72–2.64) | < 0.001 | 1.56 (1.24–1.95) | < 0.001 |
| All-cause mortality | 50 (11.4) | 327 (3.9) | 3.06 (2.27–4.12) | < 0.001 | 1.63 (1.18–2.24) | 0.003 |
| Stent thrombosis | 5 (1.1) | 166 (2.0) | 0.60 (0.24–1.45) | 0.255 | 0.47 (0.19–1.15) | 0.099 |
| Definite stent thrombosis | 4 (0.9) | 117 (1.4) | 0.67 (0.25–1.82) | 0.434 | 0.59 (0.22–1.62) | 0.307 |
| Target vessel revascularization | 23 (5.3) | 450 (5.4) | 1.03 (0.67–1.56) | 0.906 | 0.93 (0.61–1.43) | 0.753 |
| Major bleeding | 14 (3.2) | 154 (1.8) | 1.81 (1.05–3.13) | 0.033 | 1.32 (0.75–2.31) | 0.336 |
| Safety outcome | ||||||
| Stroke | 12 (2.7) | 75 (0.9) | 3.21 (1.75–5.91) | < 0.001 | 1.84 (0.95–3.58) | 0.071 |
| TIA | 1 (0.2) | 19 (0.2) | 1.06 (0.14–7.88) | 0.958 | 0.78 (0.10–5.97) | 0.808 |
| Stroke/TIA | 13 (3.0) | 93 (1.1) | 2.80 (1.57–5.01) | < 0.001 | 1.69 (0.90–3.16) | 0.102 |
| Net risk–benefit risk outcome | ||||||
| CVD, MI, CardShock, HF, stroke | 80 (18.3) | 663 (7.9) | 2.46 (1.95–3.11) | < 0.001 | 1.62 (1.26–2.08) | < 0.001 |
AF, atrial fibrillation; CardShock, cardiogenic shock; CI, confidence interval; CVD, cardiovascular disease; HF, heart failure; HR,hazard ratio; MI, myocardial infarction; TVR, target vessel revascularization; TIA, transient ischemic attack.
Adjusted for age, symptom onset (6-12 h vs < 6 h), gender, MI type, current smoking, previous hypertension, previous diabetes, previous MI, previous PCI, proximal lesion, previous stroke, and Killip class.
Figure 1.
Adjusted cumulative incidence of (A) the primary outcome (death from cardiovascular causes, recurrent myocardial infarction, cardiogenic shock, new or worsening New York Heart Association class IV heart failure) and (B) cardiovascular death, constructed with multivariable Cox models in patients with atrial fibrillation (AF) and sinus rhythm within 1-year of follow-up, along with the corresponding adjusted hazard ratios (HRs) from the multivariable analysis. CI, confidence interval.
Similar analyses for the primary and secondary outcomes reported above were conducted with exclusion of all patients with known oral anticoagulant use prior to hospitalization (Supplemental Table S1). The results for primary outcome, cardiovascular death, all-cause mortality, key secondary outcome, and net risk–benefit risk outcome all retained their statistical significance after multivariable analysis, and the results were fairly similar to those from analyses conducted without patient exclusions, as presented in Table 3.
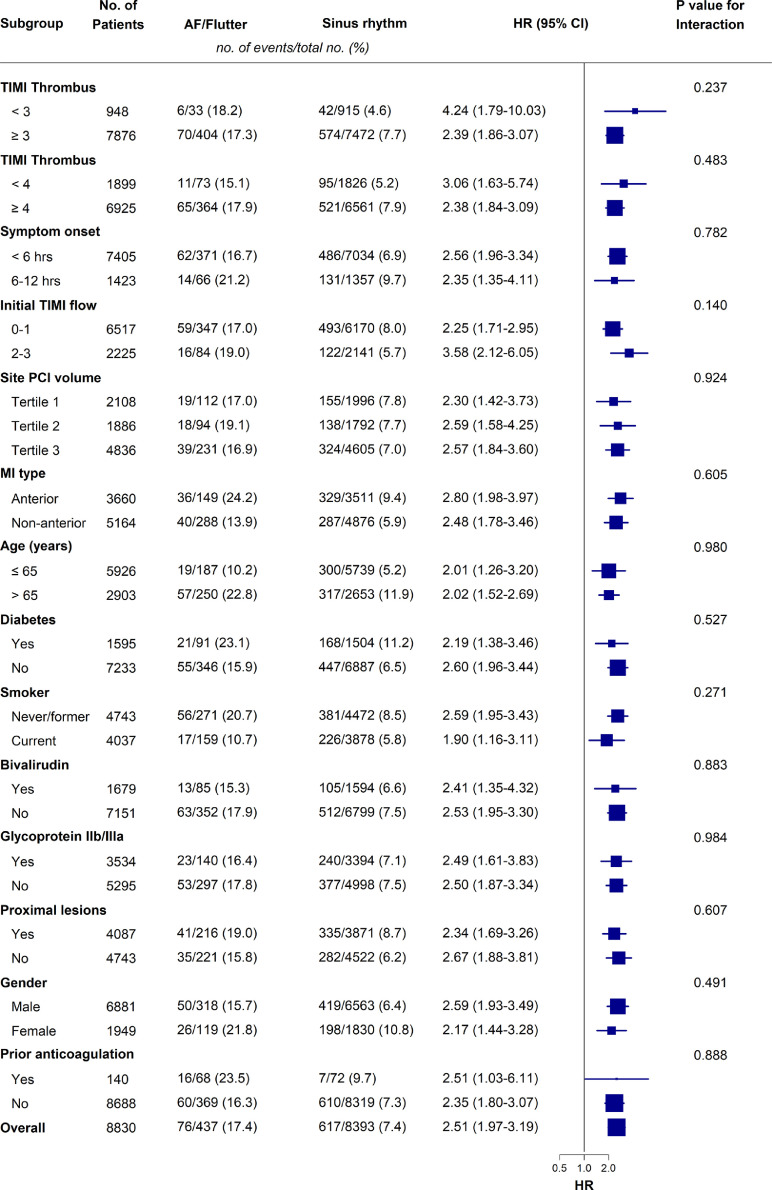
No significant difference in the effect of AF on the primary outcome among the different subgroups was detected (Fig. 2). The effect of routine thrombectomy compared with PCI alone on the primary outcome and its components was explored in the AF and SR groups, and we did not detect any statistically significant difference between the 2 rhythm groups (Supplemental Table S2).
Figure 2.
Subgroup analysis for the 1-year primary outcome (composite of cardiovascular death, recurrent myocardial infarction (MI), cardiogenic shock, new or worsening New York Heart Association (NYHA) class IV heart failure). AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
The 437 AF patients were divided into 2 groups according to antithrombotic medication at discharge. The differences for primary and secondary outcomes between the groups are shown in Supplemental Table S3. At discharge, 120 AF patients (27.5%) were on oral anticoagulant (OAC) therapy, and 293 AF patients (67%) were discharged with only antiplatelet medication (one or more drugs). In 24 patients (5.5%), data regarding the discharge medication were missing. The primary outcome occurred in 21 patients (17.5%) in the OAC group, and 32 patients (10.9%) in the antiplatelet group. Major bleeding was more frequent in the OAC group (6.7% vs 1.7%, P = 0.015). The incidence of ischemic/hemorrhagic stroke was also higher in the OAC group (7.5% vs 0.7%, P = 0.002).
Discussion
In this study, we have demonstrated an increased risk of the composite outcome of cardiovascular death, recurrent MI, cardiogenic shock, or new or worsening heart failure, in patients undergoing primary PCI for STEMI who had AF on presentation. Although patients with AF were older and had more unfavorable baseline characteristics than those with SR, the main study results were concordant across the studied subgroups as well as in the adjusted multivariable analysis.
The fact that patients with AF were more likely to have suffered a stroke during follow-up is not surprising, highlighting the importance of effective anticoagulation therapy.17 Only 15.6% of the AF patients were already using oral anticoagulation prior to hospitalization, and a total of 27.5% of the AF patients were discharged with an oral anticoagulant. The low rate of OAC usage prior to hospitalization might suggest that the majority of the AFs were new onset. However, even a known history of AF does not necessarily correlate with a high rate of OAC use. In the secondary analysis of the Prognosis and Risk of ACS in Sweden (PRACSIS) study, Poçi et al. showed that only 18% of patients with permanent AF, and 41% of patients with known persistent or paroxysmal AF, were taking warfarin at the time of hospital admission.18 At the time this study was conducted, the treatment options regarding antithrombotic therapy of patients with acute coronary syndrome with concurrent AF were heterogeneous; thus, the proportion of AF patients discharged on OAC reflects the true rate at that time period. Nowadays, the decision regarding antiplatelet/antithrombotic therapy is based on practical guidelines, by assessing each patient's individual risk for bleeding, ischemic stroke, and future coronary events.19,20
Given that precedent or subsequent ECG recordings were unavailable for this study, the information on whether AF was truly preexisting vs new-onset could not be reliably determined. An attempt to distinguish the most representative group of new-onset AF patients was carried out by excluding all patients with known prior OAC use before hospitalization. Although no remarkable differences were observed for the outcomes, compared to the results with no exclusions, half of the strokes (n = 6) occurred in this small, excluded group of 68 AF patients. AF patients discharged on OAC therapy had a higher rate of the study outcome parameters than did those on antiplatelet medication. However, with the small number of patients and no adjustments for confounding factors, these results are prone to bias. In addition, the proportion of AF patients in the antiplatelet group who might have received OACs later on during the 1-year follow-up could not be assessed. Some data related to this subject can be derived from an observational study by Guenancia et al., in which AMI patients presenting with new-onset paroxysmal AF, within the first 2 days after AMI, had an AF recurrence rate of 13%-24% after discharge, at a median follow-up time of 1037 days.21
Drug-eluting stents were less frequently used in patients with AF than in those with SR. The probable reason for a higher rate of use of bare metal stents in AF patients stems from the fact that a longer duration of anti-platelet therapy has been recommended with drug-eluting stents, at least at the time of the study; this increased duration could result in more bleeding complications. However, Kiviniemi et al. showed that outcomes of treatment with bare metal stents and drug-eluting stents are comparable, and that concerns regarding complications of bare metal stent use might be overemphasized.22
Numerous studies have explored the association of AF with STEMI outcome. Hwang et al. found that AF on admission was an independent risk factor for all-cause mortality during 1-year follow-up in STEMI patients undergoing primary PCI.4 According to Topaz et al., new-onset and preexisting AF showed a trend for increased mortality, but only preexisting AF was an independent risk factor associated with a 5-fold increase for both short- and long-term mortality.3 In a massive AMI patient cohort (n = 155,071) of the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, AF was associated with worse outcome regardless of the temporal subtype of AF and of the MI category (STEMI vs non-STEMI).6 Other studies found no independent prognostic value of preexisting AF or AF recorded in the acute phase for the long-term outcome of STEMI patients. A study conducted by Beukema et al. investigated the prognostic value of AF present before or after a primary PCI procedure in patients with STEMI. Although both of the AF categories were associated with increased long-term mortality (mean follow-up 481 days), compared to that for patients with SR, only AF that was diagnosed after the primary PCI procedure retained its statistical significance after multivariable analysis.11 In a cohort study by Consuegra-Sánchez et al., neither preexisting nor new-onset AF independently predicted long-term outcome in STEMI patients within a median follow-up time of 7.2 years, although new-onset AF independently predicted higher in-hospital mortality.12 El-Omar et al. concluded that AF is a marker of unfavorable short- and long-term outcome after PCI in AMI, but this was due to the associated risk factors, rather than to the AF itself.13 Interestingly, although in our study, the patients with AF were relatively younger than those in other studies with similar outcome results, the prevalence of AF on admission was 4.9%, in line with the percentage in previous studies.3, 4, 5, 6, 7,23 Differences in inclusion criteria and patient characteristics could account for some of the observed outcome differences between the previous and the present study.
In the main TOTAL study, routine manual thrombectomy, as compared with PCI alone, did not reduce the risk of primary outcome.15 Similar results were found in both the AF and SR groups. This similarity is important, as embolic MI due to AF may be different pathophysiologically and so could respond better to thrombus aspiration. Angiographic core lab assessment for embolic vs other cause was not available for this study.
Our study shows that although STEMI patients presenting with AF before PCI tend to be older and bear more unfavorable baseline characteristics than do patients with SR, the dysrhythmia is also independently associated with impaired long-term outcome. The data reinforce the basis for the latest guidelines on the importance of notifying patients of AF in the acute phase and tailoring the treatment according to individual patient characteristics. This patient group might also benefit from a closer follow-up after discharge, with repeated evaluation and management of risk factors for thromboembolism and bleeding. Future studies to assess ways to reduce the impact of AF on long-term prognosis are required.
Strengths and limitations
A clear strength of this study is the large study population. This was a prespecified ancillary analysis of the TOTAL trial, which was an international, investigator-initiated, multicentre, prospective, randomized trial. Most previous studies related to the topic have been either retrospective, or prospective single-centre cohort registries.
There are limitations of our study. Of the original 10,732 patients, a total of 8830 were included in the final study. As some patients were missing important data, and the rhythm exclusion was based on a single ECG recording, the possibility of selection bias exists.
We had no explicit data on whether AF was preexisting or new-onset. Hence, we were unable to differentiate among previously diagnosed paroxysmal, persistent, and permanent AF in our patients. However, it should be noted that the group of patients using OAC before admission to the hospital was most likely composed mainly of patients with preexisting AF, since AF is the most common indication for this type of medication. The low rate of AF patients discharged on an OAC is also a limitation, as the values presented in our study do not fully reflect the clinical practice at present.
Also, the rhythm was not analyzed from the post-PCI ECG, but SR in the post-PCI ECG would not have excluded the possibility of AF later during the hospital stay or during the follow-up period. Although we accounted for confounding factors in adjusted multivariable analysis, residual confounding due to unmeasured variables may still exist.
Conclusions
AF on the ECG recorded in the acute phase of STEMI before primary PCI is an independent risk factor for unfavorable long-term outcomes. In STEMI patients, AF was associated with a higher risk of the primary composite cardiovascular outcome, all-cause mortality, cardiovascular death, and severe heart failure during 1-year follow-up than in patients with SR. No statistically significant difference in the effect of routine manual thrombectomy compared with PCI alone on the risk of primary outcome was detected between patients with AF and those with SR.
Acknowledgments
Acknowledgements
The authors thank our research personnel and everybody who contributed to the TOTAL project.
Funding Sources
This work was supported by the Canadian Institutes of Health Research, Canadian Network and Centre for Trials Internationally (CANNeCTIN), and Medtronic Inc. (ClinicalTrials.gov number NCT01149044).
Disclosures
S.S.J. reports grants from Medtronic, during the conduct of the study; and grants from Boston Scientific, outside the submitted work. V.D. reports research grants from Astra Zeneca during the conduct of the TOTAL trial. J.A.C. reports research grants from Medtronic, Boston Scientific, and Astra Zeneca during the conduct of the TOTAL trial. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The TOTAL trial was approved by the ethics committees at each participating centre and by the national regulatory authorities in all countries where the trial was conducted.
See page 1228 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at doi:10.1016/j.cjco.2021.06.001.
Appendix. Supplementary materials
References
- 1.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 2.Jabre P, Roger VL, Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topaz G, Flint N, Steinvil A, et al. Long term prognosis of atrial fibrillation in ST-elevation myocardial infarction patients undergoing percutaneous coronary intervention. Int J Cardiol. 2017;240:228–233. doi: 10.1016/j.ijcard.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 4.Hwang K-K, Eom S-Y, Lee SY, et al. Atrial fibrillation on admission is related with higher mortality in ST-segment elevation myocardial infarction patients. Int Heart J. 2017;58:486–494. doi: 10.1536/ihj.16-286. [DOI] [PubMed] [Google Scholar]
- 5.De Luca L, Casella G, Rubboli A, et al. Recent trends in management and outcome of patients with acute coronary syndromes and atrial fibrillation. Int J Cardiol. 2017;248:369–375. doi: 10.1016/j.ijcard.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Batra G, Svennblad B, Held C, et al. All types of atrial fibrillation in the setting of myocardial infarction are associated with impaired outcome. Heart. 2016;102:926–933. doi: 10.1136/heartjnl-2015-308678. [DOI] [PubMed] [Google Scholar]
- 7.Braga CG, Ramos V, Martins J, et al. Impact of atrial fibrillation type during acute coronary syndromes: clinical features and prognosis. Rev Port Cardiol. 2015;34:403–410. doi: 10.1016/j.repc.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Lin C-J, Liu C-F, Kung C-T, et al. The prognostic value of atrial fibrillation on 30-day clinical outcome in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int Heart J. 2011;52:153–158. doi: 10.1536/ihj.52.153. [DOI] [PubMed] [Google Scholar]
- 9.Jabre P, Jouven X, Adnet F, et al. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123:2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podolecki T, Lenarczyk R, Kowalczyk J, et al. Effect of type of atrial fibrillation on prognosis in acute myocardial infarction treated invasively. Am J Cardiol. 2012;109:1689–1693. doi: 10.1016/j.amjcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Beukema RJ, Elvan A, Ottervanger JP, et al. Atrial fibrillation after but not before primary angioplasty for ST-segment elevation myocardial infarction of prognostic importance. Neth Heart J. 2012;20:155–160. doi: 10.1007/s12471-012-0242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Consuegra-Sánchez L, Melgarejo-Moreno A, Galcerá-Tomás J, et al. Short- and long-term prognosis of previous and new-onset atrial fibrillation in ST-segment elevation acute myocardial infarction. Rev Esp Cardiol (Engl Ed) 2015;68:31–38. doi: 10.1016/j.rec.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 13.El-Omar MM, Dangas G, Mehran R, et al. Usefulness of atrial fibrillation as a marker of outcome after percutaneous coronary intervention. Am J Cardiol. 2003;91:232–234. doi: 10.1016/s0002-9149(02)03114-4. [DOI] [PubMed] [Google Scholar]
- 14.Jolly SS, Cairns J, Yusuf S, et al. Design and rationale of the TOTAL trial: a randomized trial of routine aspiration ThrOmbecTomy with percutaneous coronary intervention (PCI) versus PCI ALone in patients with ST-elevation myocardial infarction undergoing primary PCI. Am Heart J. 2014;167 doi: 10.1016/j.ahj.2013.12.002. 315-21.e1. [DOI] [PubMed] [Google Scholar]
- 15.Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–1398. doi: 10.1056/NEJMoa1415098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 17.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 18.Poçi D, Hartford M, Karlsson T, Edvarsson N, Caidalh K. Effect of new versus known versus no atrial fibrillation on 30-day and 10-year mortality in patients with acute coronary syndrome. Am J Cardiol. 2012;110 doi: 10.1016/j.amjcard.2012.03.018. 217-12. [DOI] [PubMed] [Google Scholar]
- 19.Lip GY, Collet J-P, Haude M, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA) Europace. 2019;21:192–193. doi: 10.1093/europace/euy174. [DOI] [PubMed] [Google Scholar]
- 20.Mehta SR, Bainey KR, Cantor WJ, et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi: 10.1016/j.cjca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Guenancia C, Toucas C, Fauchier L, et al. High rate of recurrence at long-term follow-up after new-onset atrial fibrillation during acute myocardial infarction. Europace. 2018;20:e179–e188. doi: 10.1093/europace/euy168. [DOI] [PubMed] [Google Scholar]
- 22.Kiviniemi T, Puurunen M, Schlitt A, et al. Bare-metal vs drug-eluting stents in patients with atrial fibrillation undergoing percutaneous coronary intervention. Circ J. 2014;78:2674–2681. doi: 10.1253/circj.cj-14-0792. [DOI] [PubMed] [Google Scholar]
- 23.González-Pacheco H, Márquez MF, Arias-Mendoza A, et al. Clinical features and in-hospital mortality associated with different types of atrial fibrillation in patients with acute coronary syndrome with and without ST elevation. J Cardiol. 2015;66:148–154. doi: 10.1016/j.jjcc.2014.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.