Abstract
Radiation-induced brain injury is a major adverse event in head and neck tumor treatment, influencing the quality of life for the more than 50% of patients who undergo radiation therapy and experience long-term survival. However, no effective treatments are available for these patients, and preventative drugs and effective drug-delivery methods must be developed. Based on our results, miR-122-5p was upregulated in the mouse radiation-induced brain injury (RBI) model and patients with nasopharyngeal carcinoma (NPC) who received radiation therapy. Intranasal administration of a single antagomiR-122-5p dose before irradiation effectively alleviated radiation-induced cognitive impairment, neuronal injury, and neuroinflammation in the mouse RBI model. Results further indicated that miR-122-5p inhibition in microglia reduced the levels of proinflammatory cytokines and enhanced the phagocytic function to protect against radiation-induced neuronal injury in cell models. Further, we profiled transcriptome data and verified that Tensin 1 (TNS1) may be the target of miR-122-5p in RBI. In summary, our results reveal a distinct role for miR-122-5p in regulating neuroinflammation in RBI, indicating that a non-invasive strategy for intranasal miR-122-5p administration may be an attractive therapeutic target in RBI, providing new insights for clinical trials. Further systematic safety assessment, optimization of drug administration, and clarity of mechanism will accelerate the process into clinical practice.
Keywords: miR-122-5p, nasal delivery, radiation-induced brain injury, microglia
Graphical abstract

Radiation-induced brain injury is a major adverse event in head and neck tumor treatment, with no effective treatments. Zhou et al. report a non-invasive method to prevent the side effects of radiotherapy by nasal administration targeting miRNA in mouse model, providing a new potential strategy for this kind of clinical treatment.
Introduction
Radiation therapy is an indispensable mainstay treatment for most brain tumors designed to overcome the obstacle contributing to chemotherapy resistance and the inability to deliver drugs.1,2 Improved anticancer therapies have resulted in the long-term survival of patients with brain tumors.3 However, the side effects of intracranial radiotherapy inevitably lead to permanent and substantial cognitive disability, which is called radiation-induced brain injury (RBI), affecting 50%–90% of long-term survivors.4, 5, 6 Unfortunately, the mechanisms underpinning RBI remain poorly understood; therefore, a treatment that ameliorates those adverse effects is unavailable.7 Thus, the identification of strategies that minimize neurotoxicity and promote central nervous system (CNS) repair after radiotherapy is urgently needed. Studies aiming to explore the pathogenesis and targets for the treatment of RBI and to optimize therapeutic or preventative solutions are crucial.
As a classic type of short, noncoding, single-stranded RNA, microRNAs (miRNAs) regulate multiple genes or interacting pathways, suggesting that they represent potential targets in the pathological processes of CNS diseases.8, 9, 10 Because they are endogenous and characteristically small molecules, miRNAs are suitable as therapeutic targets for brain diseases and have excellent potential clinical-application value.11 However, few studies on the intervention mediated by miRNAs and their mechanism in RBI have been conducted. Intranasal administration, an effective noninvasive route for the treatment of brain diseases, has received increasing attention in the clinic by virtue of its ability to partially circumvent the blood-brain barrier (BBB).12,13 A previous study from our laboratory evaluated the effect of intranasal administration of a miRNA on treating CNS diseases in a mouse model of Alzheimer disease.14
In the present study, we aimed to screen miRNAs that have roles in RBI and further prevent the progression of RBI by intranasally administering miRNA intervention. Here, we report miR-122-5p as a promising potential target for preventing RBI. The intranasal administration of a single antagomiR-122-5p dose before RBI was induced by irradiation effectively alleviated radiation-induced nerve injury in a mouse model of RBI. Our results reveal a promising-candidate miRNA target and a feasible new noninvasive strategy for intranasal miRNA administration to prevent RBI.
Results
Screening of candidate miRNAs in a mouse RBI model and in patients with nasopharyngeal carcinoma (NPC) who received radiotherapy
We first constructed a mouse RBI model to identify the potential miRNAs involved in RBI. Mice were exposed to radiation and were evaluated by performing behavioral tests and by pathological assays 1 week later, as shown in Figure 1A. The Morris water maze (MWM) trial showed that mice exposed to irradiation (30 Gy) suffered cognitive impairment compared with control mice (Figures 1B–1D). The results from H&E staining showed that the neuronal area in the hippocampus decreased in radiation-treated mice (Figure 1E). Thus, we successfully modeled RBI in mice. Then, hippocampal samples from the three RBI-model mice with the most significant cognitive impairment were analyzed using miRNA sequencing. Forty-five miRNAs were upregulated and 30 miRNAs were downregulated in the hippocampus after radiation exposure. We further screened miRNAs with homology between humans and mice and verified their expression response to radiation by quantitative reverse transcription PCR (qRT-PCR). Among them, miR-122-5p was the most significantly upregulated miRNA with high evolutionary conservation (Figures 1F and S1A). Considering the abovementioned factors, we chose miR-122-5p for further analysis (Figure 1H). We evaluated the association between the level of miR-122-5p and radiation in patients with NPC. Clinical blood samples were collected from 28 patients with NPC who received radiotherapy at different time points. The qRT-PCR results showed significant increases in the relative expression of miR-122-5p at 2 weeks, 4 weeks, and 1 month after radiotherapy compared with that of the time point before radiotherapy (Figures 1H and S1B). The results from the mouse model and humans suggest that miR-122-5p may be related to the occurrence and development of RBI. In addition, the levels of interleukin 1β (IL-1β) and S100 calcium-binding protein B (S100B) were significantly increased to a degree corresponding to that of the level of miR-122-5p (Figure S1C).
Figure 1.
miR-122-5p was significantly upregulated in a mouse model of RBI and in patients with NPC after radiotherapy
(A) Procedures used to establish and validate RBI-model mice. (B) The escape latency of mice from the control and radiation groups (1 week after radiation) in the MWM test. n = 5. (C) The trajectory in the water maze of mice from the control and radiation groups on the fifth day of the MWM test. (D) The distance traveled in the target quadrant (%), the time (s) spent in the target quadrant, and the times the platform was crossed by mice from the control and radiation groups. (E) Pathological damage assessed using H&E staining. The sum area and the number of nuclei in the hippocampus of mice from the control and radiation groups (1 week after radiation). n = 3. Scale bars represent 200 μm for original magnification 20× (left panel) and 60× (right panel). (F) Screening of miRNAs in the hippocampus from the control and RBI-model mice. Significantly upregulated (or downregulated, p < 0.05) miRNAs were highly homologous between humans and mice (6 weeks after radiation, top-10 miRNAs). (G) The relative expression of these miRNAs in the hippocampus from control and RBI-model mice was measured using qRT-PCR (6 weeks after radiation). Data were normalized to U6. (H) The relative expression of miR-122-5p in patients with different stages of NPC (the same patients, n = 28, were divided into different tumor stages). Data were normalized to U6. Data are presented as the means ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Prior nasal administration of antagomiR-122-5p prevents RBI-related cognitive impairment in the mouse model of RBI
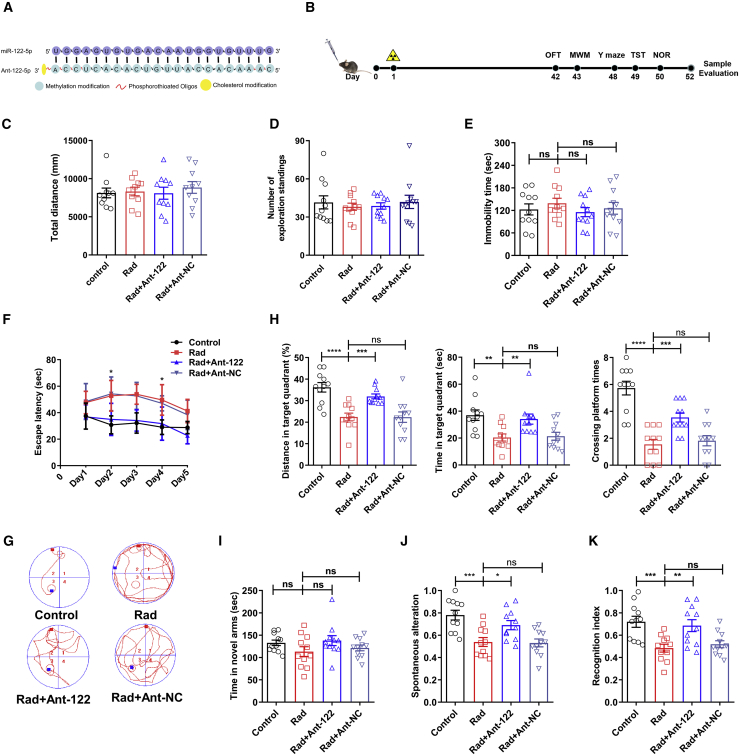
Considering the restrictive nature of the BBB, which provides an obstacle for most drug-delivery methods to the CNS, in this study, we adopted a non-invasive method of nasal administration to deliver a miR-122-5p inhibitor (antagomiR-122-5p) to the brain before radiation (Figure 2A). We expanded the evaluation to 6 weeks after radiation to further explore long-term survival and exclude other side effects of radiation in the long term. The timeline of the experiment is illustrated in Figure 2B. We labeled antagomiR-122-5p with Cy5 before delivery and detected its levels in the mouse olfactory bulb and hippocampus 24 h after delivery (Figure S2A). The inhibitory effect of antagomiR-122-5p on hippocampal and blood miR-122-5p expression after intranasal delivery was confirmed by qRT-PCR (Figure S2B).
Figure 2.
AntagomiR-122-5p improved the behavioral and cognitive impairment of RBI-model mice
(A) The concrete sequence and structure of antagomiR-122-5p and a schematic diagram of its combination with miR-122-5p. (B) General plan and timeline of the antagomiR-122-5p intervention and tests. The detailed phases and tests are listed in the Materials and methods (from days 0 to 52). (C and D) The total distance traveled (C) and exploratory rearing (D) behaviors of mice from different groups in the OFT. n = 11. (E) Immobility time in the TST. n = 11. (F and G) The escape latency (F) in the MWM test and the activity trajectory (G) of mice on the fifth day of the MWM test. (H) The distance traveled in the target quadrant (%), the time spent in the target quadrant, and the times the platform was crossed by mice on the sixth day. n = 11. (I and J) The time spent in the novel arm (I) and SAP (J) in the Y-maze. n = 11. (K) Recognition index in the NOR test. n = 11. Data are presented as means ± SEM, ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Four groups were established: control mice, RBI-model mice (Rad), RBI-model mice treated with antagomiR-122-5p (Rad + Ant-122), and RBI-model mice treated with antagomiR-122-5p negative control (Rad + Ant-NC).
First, we evaluated the growth of mice. The weight of the mice was not significantly influenced by administration during the 6-week observation period (Figure S2C). Irradiation caused severe hair loss on the head and neck of mice (Figure S2D). The results of the open field test did not reveal a noticeable difference in motor ability among the different groups (Figures 2C and 2D). In the tail-suspension test (TST), the immobility time was recorded to evaluate the anxiety and depression behavior of the mice, and no significant differences were observed among the groups (Figure 2E). Then, the MWM test was conducted to evaluate hippocampus-dependent spatial cognitive ability. Compared with the radiation group, the escape latency of the antagomiR-122-5p group decreased significantly on days 2 and 4–5 (Figures 2F and 2G). AntagomiR-122-treated mice traveled a greater distance (%), spent more time (s) in the target quadrant, and crossed the platform more times than did mice in the radiation group (Figure 2H). Moreover, the Y-maze test showed that the time spent exploring the novel arms of the maze and the spontaneous alternation percentage (SAP) were increased in the antagomiR-122-5p group compared with that of the radiation group (Figures 2I and 2J). In addition, the novel object recognition (NOR) test was used to evaluate cognitive status, and the results showed a greater recognition index for the antagomiR-122-5p group than that of the radiation group (Figure 2K). In summary, the changes in performance on these behavioral tests showed that cognitive memory disorders caused by radiation were significantly improved by the prior intra-nasal administration of antagomiR-122-5p.
Prior nasal administration of antagomiR-122-5p relieved neural injury in the hippocampus of RBI model mice
Radiation causes cellular injury in brain parenchyma. Hematoxylin and eosin (H&E) staining and βIII-tubulin immunofluorescence staining were used to evaluate neuronal damage in the mouse hippocampus. As shown in Figure 3A, the total area and number of nuclei in the hippocampus of mice in the radiation group were significantly decreased compared with those of control mice, whereas the miR-122-5p intervention reversed that reduction. βIII-tubulin staining was further used to specifically evaluate neurons, and staining was reduced after radiation, whereas miR-122-5p inhibition ameliorated the damage (Figure 3B). Then, we evaluated neuronal apoptosis and neurogenesis, two aspects of neuronal cell homeostasis. The dentate gyrus (DG) of the hippocampus is one of the primary sites of nerve regeneration. Therefore, the proliferation of neurons in the DG was detected by performing double-immunofluorescence staining for NeuN (labeled neurons) and bromodeoxyuridine (BrdU; labeled proliferating cells). As shown in Figure 3C, a greater number of double-stained, BrdU/NeuN-labeled neurons were detected in this area after the inhibition of miR-122-5p than in the radiation group (Figure 3C), indicating that miR-122-5p inhibition also promoted the regeneration of neurons in the RBI-model mice. TUNEL staining showed that miR-122-5p inhibition ameliorated neuronal apoptosis in RBI-model mice (Figure S3). In summary, the results described above showed that the antagomiR-122-5p intervention relieved neural injury in the hippocampus of the RBI-model mice, which ameliorates cognitive impairment.
Figure 3.
Neuronal damage induced by RBI was alleviated by the antagomiR-122-5p intervention
(A) H&E staining of the hippocampus from the different groups. The sum area and the number of nuclei in the hippocampus of mice (6 weeks after radiation). Scale bars represent 50 μm for original magnifications of 20× (top panel) and 40× (bottom panel). (B) βIII-tubulin-positive cells in the hippocampus and cortex from different groups (an important neuronal marker that is widely used in neuroscience to identify neurons). Scale bars represent 20 μm for original magnifications of 60×. (C) BrdU and NeuN double staining in neurons in the DG area of the hippocampus in the four groups. Scale bars represent 200 μm for original magnifications of 20×. Data are presented as means ± SEM, ns, not significant, ∗p < 0.05, ∗∗p < 0.01.
Prior nasal administration of antagomiR-122-5p mitigated glial activation and neuroinflammation in the hippocampus of RBI mice
Because RBI is associated with the neuroimmune response, we further examined the effect of antagomiR-122-5p on glial cells in the mouse model of RBI. As shown in Figure 4A, the microglia in the radiation group displayed an amoeboid morphology, which is characteristic of reactive microglia, whereas the branch length was increased in the miR-122-5p intervention group and accompanied by a small cell body compared with that of the radiation group. These morphological changes in microglia suggested that miR-122-5p inhibition alleviated the overactivation of microglia upon radiation exposure. In addition, we evaluated the morphology of astrocytes with glial fibrillary acidic protein (GFAP) immunolabeling, and a significantly higher integrated optical density (IOD) of GFAP and significantly greater astrocyte area were observed in the hippocampus after irradiation than in the control group. The miR-122-5p inhibition group also showed lower IOD values and a smaller astrocyte area than the radiation group showed (Figure 4B). Moreover, because glial activation is closely related to the level of neuroinflammation, enzyme-linked immunosorbent assays (ELISAs) were used to assess the levels of inflammatory cytokines in the hippocampus of each group. The levels of tumor necrosis factor alpha (TNF-α), IL-6, IL-1β, and S100B were significantly increased after irradiation but decreased upon miR-122-5p inhibition (Figures 4C–4F), consistent with the results showing that miR-122-5p inhibition rescued glial activation.
Figure 4.
Glial activation and neuroinflammation caused by irradiation were alleviated by the antagomiR-122-5p intervention
(A) The area and branch length of microglia in the hippocampus of different groups. Scale bars represent 50 μm for original magnifications of 20× (top panels), and scale bars represent 20 μm for original magnifications of 60× (bottom panels). The area and branch length were measured using Image-Pro Plus software. (B) The IOD of GFAP and the area of astrocytes in the hippocampus of mice from different groups. Scale bars represent 50 μm for original magnifications of 20× (top panels), and scale bars represent 10 μm for original magnifications of 60× (bottom panels). The area and IOD of astrocytes were measured using Image-Pro Plus software. (C–F) The levels of the inflammatory factors TNF-α (C), IL-1β (D), IL-6 (E), and S100B (F) in the hippocampus of RBI model mice at 6 weeks after intranasal administration. n = 6. Data are presented as means ± SEM, ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Inhibition of miR-122-5p polarized M1 microglia toward the M2 phenotype to alleviate radiation-induced neural injury
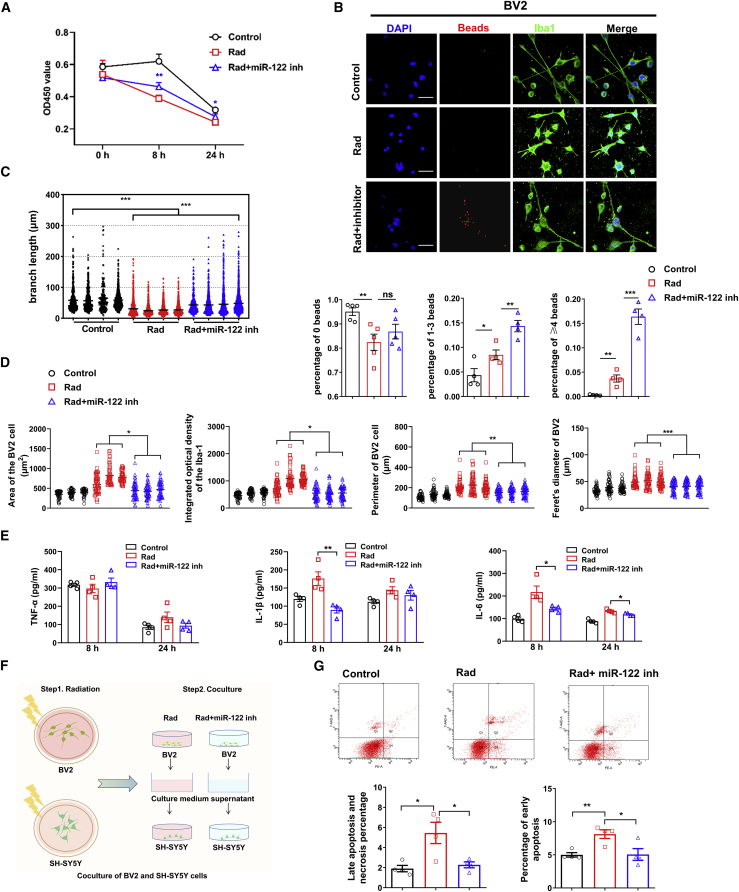
We examined neuroinflammation in a cellular model of radiation-induced damage to further understand the neuroprotective effect of miR-122-5p inhibition on radiation-induced injury. Through a preliminary experimental screen, an in vitro radiation-induced cell model was established by irradiation with 10 Gy (Figures 4S4A–S4D), and 100 nM miR-122 inhibitor was selected for subsequent in vitro experiments (Figure S4E). As shown in Figure 5, compared with cells in the control group, the viability of BV2 cells was significantly decreased at 8 h after irradiation, but miR-122-5p inhibition alleviated the decrease in cell viability caused by irradiation (Figure 5A). Morphological observations with ionized calcium-binding adaptor molecule-1 (Iba-1) fluorescence staining showed that the cell bodies of irradiation-induced BV2 cells became enlarged and the branches of the irradiated cells became shorter, whereas the area, IOD, perimeter, and Feret diameter were increased compared with those in the control cells (Figures 5B–5D). However, changes in morphology with an increased branch length and reduced area, IOD, perimeter, and Feret diameter of BV2 cells were observed after miR-122-5p inhibition compared with the radiation group (Figures 5B–5D). Microbead experiments showed that radiation eliminated the phagocytosis of BV2 cells and that miR-122-5p inhibition activated the phagocytosis of BV2 cells (Figure 5B). Based on these results, the miR-122-5p inhibitor promoted the return of BV2 cells to the phagocytic phenotype, which was changed by radiation. Changes in the levels of TNF-α, IL-6, and IL-1β were also ameliorated after miR-122-5p inhibition (Figures 5E and S4F). These changes in morphology, phagocytic activity, and the levels of inflammatory factors suggested that inhibition of miR-122-5p blocked microglial M1 polarization and promoted microglial polarization toward the M2 phenotype, which may exert neuroprotective effects on irradiation-induced damage in vitro.
Figure 5.
The radiation resistance of the cell model of radiation injury was induced by the inhibition of miR-122-5p
(A) The viability of BV2 cells at different time points (0 h, 8 h, and 24 h) in the control, Rad (irradiation with 10 Gy), and Rad + inhibitor groups (irradiation with 10 Gy + miR-122-5p inhibitor). (B and C) Immunofluorescence staining for Iba-1 in microglia, the different numbers of beads phagocytosed by microglia (BV2) (B) and the branch length of microglia in the three groups (C). Scale bars represent 20 μm for original magnifications of 60×. (D) The area of BV2 cells, IOD of Iba-1 staining, perimeter, and Feret diameter of microglia (BV2) in the different groups. The data were measured using Image-Pro Plus software. (E) The TNF-α, IL-1β, and IL-6 levels in the supernatant from microglia in different groups (BV2, 8 h and 24 h) are shown. (F and G) A diagram illustrating the coculture of neurons with the microglia supernatant (F). The percentages of early apoptotic, late apoptotic and necrotic neurons after coculture with different microglia (BV2) supernatants (G). Data are presented as means ± SEM, ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Then, we determined whether the shift in microglial polarization affected neuronal survival. We added medium from miR-122-inhibited BV2 cells to irradiation-stimulated SH-SY5Y cells to evaluate the rescue effect of miR-122-inhibited microglia supernatant on neuronal cell injury (Figure 5F). After irradiation, apoptosis was further increased in SH-SY5Y cells treated with the supernatant of medium from irradiated BV2 cells. Conversely, supernatant from the medium of BV2 cells in which miR-122-5p had been inhibited significantly ameliorated neuronal apoptosis after irradiation (Figure 5G), indicating that injury and neuronal apoptosis after irradiation were indeed related to microglia and their polarized subtypes. Furthermore, we also verified the in vitro results in another microglia cell line, HMC3 cells. The results showed that irradiation promotes the microglia switch from phagocytic phenotype to pro-inflammatory phenotype (Figure S5) and that miR-122-5p inhibition reduces pro-inflammatory factors release and neuron death (Figure S6).
Inhibition of miR-122-5p did not affect the proliferation or migration of NPC cells in vitro
RBI occurs in patients with tumors who undergo radiotherapy. Investigation is needed to determine whether an intervention administered before radiation influences the tumor. The inhibitory effect of miR-122-5p on human NPC cell lines (CNE-2 cells) was confirmed before the experiment (Figure 6A). Because of the sensitivity of NPC cells to radiotherapy, CNE-2 cells were irradiated at a dose 4 Gy less than that used to irradiate microglia. Cell viability was detected using the CCK-8 assay, and the viability of the CNE-2 cells was less than that of the healthy group at 12 h after irradiation, whereas the viability of cells treated with the miR-122-5p inhibitor was further reduced compared with that of the radiation group, suggesting that 4 Gy is a suitable dose for irradiation and inhibition of human NPC cell growth in vitro (Figure 6B). Clone formation and scratch-wound-migration assays are usually used to evaluate tumor cell proliferation and migration in vitro, which are closely related to tumor staging and clinical outcome. As shown in Figures 6C and 6D, compared with the control and irradiation groups, miR-122-5p inhibition had no significant effect on the number of clones, but the average colony area was decreased. Meanwhile, no significant difference in the average scratch width was observed among the different groups (Figure 6E). These preliminary data indicate that miR-122-5p inhibition did not affect the proliferation or migration of NPC cells in vitro.
Figure 6.
The miR-122-5p intervention did not affect the proliferation and migration of NPC cells in vitro
(A) The transfection efficiency of the miR-122-5p inhibitor (inhibitor) and scrambled inhibitor (SIH, inhibitor NC) in CNE-2 cells. (B) Cell viability at different time points in the control, scrambled inhibitor (SIH) without irradiation, miR-122-5p inhibitor (inhibitor) without irradiation, SIH with irradiation (Rad + SIH), and miR-122 IH with irradiation groups. (Rad + miR-122 inh). (C and D) Colonies formation of CNE-2 cells without exposure to irradiation (C) and exposed to irradiation (D). (E) Scratch-wound assay results and average scratch-wound width of CNE-2 cells from different groups at different time points. Data are presented as means ± SEM, ns, not significant, ∗p < 0.05, ∗∗p < 0.01.
Transcriptional characterization of the protective effects of miR-122-5p inhibition on RBI
Because miR-122-5p inhibition protected against RBI, a transcriptome analysis was performed to profile the downstream genes in the hippocampus that were affected by miR-122-5p inhibition. As shown in Figure 7, there were 237 genes significantly upregulated in the radiation group compared with the healthy group, whereas 124 genes were significantly downregulated. Compared with the radiation group, 698 genes were upregulated in the miR-122 intervention group, whereas 169 genes were downregulated (Figure 7A). Among those genes, we screened the genes that displayed a change in expression upon radiation exposure and were subsequently restored by the miR-122-5p intervention, and 57 node genes were identified (Figure 7B). The subsequent thermographic analysis showed that 15 genes were significantly downregulated and 42 genes were significantly upregulated after irradiation, and those changes were abrogated by miR-122-5p inhibition (Figures 7C and 7D). Furthermore, the pathways related to the downregulated and upregulated genes identified in the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis are shown in Figures 7E and 7F, respectively. The KEGG analysis revealed that the downregulated genes were mainly involved in extracellular matrix (ECM) receptor interaction, the Hippo signaling pathway, phototransmission, the p53 signaling pathway, and the arrhythmogenic right ventricular cardiovascular pathway (ARVC). The upregulated genes were mainly involved in ECM-receptor interaction, protein digestion and absorption, complement and coagulation cascades, cell adhesion molecules (CAMs), gastric acid secretion, cytokine-cytokine receptor interactions, the Wnt signaling pathway, and transcriptional misregulation, highlighting the multiple signaling pathways in which these genes are involved (Figure S7A). Moreover, the Gene Ontology (GO) enrichment analysis of the differentially expressed genes identified pathways related to cells and responses to stimuli and other cellular processes through which miR-122-5p inhibition may regulate RBI (Figure S7B).
Figure 7.
Screening and bioinformatics analysis of downstream target genes of the miR-122-5p that participate in RBI
(A) The significantly upregulated and downregulated genes identified in the transcriptome analysis of the hippocampus from the irradiation group, the control group, and the antagomiR-122-5p intervention group. (B) The gene set included 15 downregulated genes (Rad versus control) that were reversed by miR-122-5p inhibition (miR-122-5p versus Rad) and 42 upregulated genes (Rad versus control) that were reversed by miR-122-5p inhibition (miR-122-5p versus Rad). (C and D) Thermographic analysis of genes (15 and 42) exhibiting significant changes in expression after irradiation that were rescued by miR-122-5p inhibition (antagomiR-122-5p intervention). (E and F) KEGG pathway analysis of differentially expressed genes (15 and 42).
Tensin 1 (TNS1) identified as a potential target of miR-122-5p preventing RBI
We focused on the genes with a negative relationship with the change in miR-122-5p expression in the different experimental groups (Figure 7E). We then predicted the downstream target genes of miR-122-5p using miRWalk and TargetScan databases. Among the 15 genes, TNS1, kallikrein-related peptidase 12 (Klk12), and ANTXR cell adhesion molecule 1 (Antxr1) were predicted to contain plausible binding sites for miR-122-5p (Figure 8A). Among them, the most significantly upregulated gene, TNS1, was selected for further exploration, and the binding and regulatory relationships with miR-122-5p were verified. The prediction analysis showed that miR-122-5p may bind within the 3′ untranslated region (UTR) of the TNS1 mRNA at positions 2765–2771. Therefore, we verified the direct binding between TNS1 and miR-122-5p by performing a dual-luciferase assay; the results showed that miR-122-5p significantly decreased the fluorescence activity, and that effect was abrogated when the binding site was mutated (Figure 8B). Furthermore, the relative expression of the TNS1 mRNA and protein was decreased after treatment with miR-122-5p mimics but was significantly increased after treatment with the miR-122-5p inhibitor (Figures 8C–8E). However, the relative expressions of Klk12 and Antxr1 were not significantly increased after the inhibition of miR-122-5p (Figure S8). These results indicate that TNS1 may be the direct target of miR-122-5p and is negatively regulated by miR-122-5p.
Figure 8.
TNS1 may be related to the regulation of microglial polarization by the miR-122-5p intervention
(A) Screen of the miR-122-5p target gene by transcriptome data, TargetScan database, and miRwalk database. (B) Construction of the TNS1 dual-luciferase reporter gene and its potential sequence to which miR-122-5p binds. The relative luciferase activity of the miR-122-5p inhibitor and SIH groups expressing TNS1-WT and TNS1-MUT. (C and D) The relative expression of miR-122-5p and TNS1 in HMC3 cells (qRT-PCR) transfected with the miR-122-5p mimics (or scrambled mimics [SMs]) and miR-122-5p inhibitor (or scrambled inhibitor [SIH]). (E) The relative level of the TNS1 protein in different groups. Data were normalized to α-tubulin. Data are presented as means ± SEM, ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Discussion
In the present study, we report that miR-122-5p is a promising drug target for the clinical prevention of RBI. We first performed miRNA screening and confirmed that miR-122-5p was significantly upregulated in the hippocampus of a mouse model of RBI, consistent with the results obtained from blood samples of patients with NPC who received radiotherapy. In our subsequent evaluation of the ability of miR-122-5p to prevent RBI, antagomiR-122-5p significantly improved cognitive impairment, rescued neuronal injury, and increased inflammation levels after irradiation in RBI model mice compared with that of the irradiation group. We further verified that the inhibition of miR-122-5p switches microglia from a proinflammatory phenotype to a phagocytic phenotype to protect against neuronal injury in vitro. We also assayed the effect of miR-122-5p on NPC cell lines and found that it did not affect the NPC cell lines. Finally, we primarily screened the plausible target of miR-122-5p in the RBI model. Based on our results, intranasal administration of antagomiR-122-5p may be a promising treatment for RBI. In addition, miR-122-5p regulated the polarization of microglia from the M1 to M2 phenotype in vitro and reduced the apoptosis and necrosis of cocultured neurons, suggesting that miR-122-5p may be beneficial for increasing resistance to neuroinflammatory injury mediated by the M1 polarization of microglia in a cellular model of RBI.
Notably, miRNAs are involved in the regulation of various nervous system diseases. However, the role of miRNAs in regulating the occurrence and development of RBI has been less studied and is unclear. Notably, miR-122-5p, which was initially studied for its abundant expression in the liver and close relationship with liver diseases, has already shown clinical potential.15,16 In recent years, its role in the nervous system has been gradually explored; miR-122-5p is involved in regulating cerebral ischemia and the neuroinflammatory pathway after brain injury.17,18 Studies on the role of miR-122-5p in NPC have provided inconsistent results to date. According to one study, miR-122-5p exerts antiproliferative and apoptotic effects on NPC cells via the PI3K/AKT signaling pathway.19 However, several studies showed that miR-122-5p suppresses cell proliferation, migration, and invasion by targeting SATB1 in NPC,20 and it has also been reported to function as a tumor suppressor in NPC by targeting TRIM29 and blocking PI3K/AKT signaling in vitro.21 In our study, miR-122-5p inhibition did not influence the development of NPC in vitro. This result supports the feasibility of targeted miR-122-5p inhibition for the prevention of brain injury caused by radiotherapy in patients with NPC.
Nasal administration is a noninvasive method with a significant advantage over other methods because it focuses on the brain as the target site. Compared with drug delivery via the circulatory system, nasal delivery allows smaller amounts of the drug to reach the target brain area, largely eliminating the possible side effects caused by systemic administration.22,23 Moreover, this method of administration has better clinical operability especially for the one-time administration of a drug to prevent brain injury reported in this study. Additionally, the combined characteristic small size of miRNAs and highly efficient delivery of miRNAs also suggest that the intranasal administration of miRNA drugs is promising. The intracellular pathway involved in intranasal drug administration relies mainly on endocytosis by olfactory sensory cells; after which, the drug is transported to the synaptic cleft in the olfactory bulb through axons. Olfactory neurons repeat this transsynaptic transmission process and distribute drugs to other brain regions.24 Intranasal drug delivery has been pinpointed as a reliable and direct method to bypass the BBB.25,26 Given the mucosal injury observed in patients with NPC, some methods have been developed to overcome those drawbacks and increase delivery efficiency.27,28 Nanosystems have been reported as an excellent vehicle for the direct transport of drugs to the brain because they protect the drug from biological and chemical hazards and prevent efflux of the drug, thus increasing the drug concentration in the brain. The nasal delivery of some small molecules at different doses exerts a satisfactory effect.14,26,29, 30, 31 Delivery of glial-derived neurotrophic factor (GDNF) protects dopaminergic neurons and arrests disease progression in individuals with Parkinson's disease (PD).26 Nanoparticle/agomir complexes improve bone regeneration in vivo.32 Intranasal administration of a suitable chitosan-coated nanostructured lipid carrier (CS-NLC) formulation results in efficient brain delivery of the particles after intranasal administration.29 An miRNA (miR-146a)-modified analog slows the cognitive deterioration and pathological process of AD in mice.33 At present, research into the novel dosages of drugs is increasing. For example, the delivery of chitosan-rich nanoparticles rich in an anti-HTT small interfering RNA (siRNA) from the nose to brain34 and the delivery of an siRNA by a concentrated chitosan nanoparticle suspension to CNS tumors within a few hours after intranasal administration have been observed.35 Recently, it was reported that the systemic delivery of human neural stem cell (hNSC)-derived extracellular vesicles (EVs) with abundant miR-124 resolved radiation-induced neurocognitive decrements in a mouse model,36 supporting our research on radiation-induced brain injury, the miRNA target, and the microglia mechanism. Based on these studies, the administration of miRNA target or other small-molecule drugs via the nasal-brain pathway may be a promising solution for clinical RBI therapy.
The gene set of three upregulated genes included TNS1, Klk12, and Antxr1; among which, TNS1 is closely related to M2 macrophages, regulatory T cells, and other immune cells.37 It is an actin-binding protein that regulates cell motility and other important cellular functions.38 Klk12 was discovered to participate in lung cancer and breast cancer.39,40 Antxr1 is a highly conserved cell surface protein that is overexpressed in the tumor-infiltrating vascular system and is a functional biomarker that is enriched in stem cells relevant to breast cancer metastasis.41,42 Therefore, based on the close relationship between its function and microglial polarization, combined with the preliminary verification of its mRNA and protein levels, we preliminarily speculate that TNS1 may be a potential downstream target of miR-122-5p in RBI that regulates microglial polarization, and the detailed role of TNS1 in RBI as the target of miR-122-5p requires further verification.
Our study still has some limitations that need to be addressed. First, we have not been able to evaluate the effect of miR-122-5p on mice with NPC after radiotherapy because a mouse NPC model is currently difficult to construct. Although our behavioral data showed an overall protective effect of intervention on RBI, our current data cannot confirm whether miR-122 intervention can protect or damage other nerve cells and the different brain tumors under the real clinical-irradiation treatment; more-detailed assessments are needed. Second, although we observed an increase in miR-122-5p levels in blood samples from patients who received clinical radiotherapy, the level of miR-122-5p in the nerve cells of patients undergoing radiotherapy was hard to evaluate; however, miR-122-5p expression was confirmed to be increased at the cellular level in blood samples and in the animal brain after irradiation. Third, in this study, we concluded that miR-122-5p inhibition mainly resists radiation injury by affecting microglial activation. However, because our administration method resulted in whole-brain distribution, the possibility that miR-122-5p may also be able to resist radiation-induced injury by affecting other brain cells, such as astrocytes, neurons, and endothelial cells, cannot yet be excluded (Figure S9). Meanwhile, in this study we mainly evaluated the pathological changes in the hippocampus after administration; therefore, data on pathological changes in the other regions of the brain is lacking. Further studies will focus on the effect of the miR-122-5p intervention on the interactions among glia, neurons, and other regions of the brain in patients with, or models of, RBI. Although the current dose of 30 Gy is suitable for mouse modeling and intervention, a higher dose should also be explored in combination with the radiotherapy plan of clinical patients with NPC. Fourth, in view of future clinical applications, pharmacological details, such as the delivery efficiency under various pathophysiological conditions, require further comprehensive and thorough exploration.
In conclusion, in the present study, we screened and verified miR-122-5p as a potential target in RBI with a promising preventative effect. The use of nasal administration of antagomiR-122-5p generated a noninvasive system with clinical application potential for the prevention of radiation encephalopathy that is preferable to other systems. In the future, we will continue to focus on how to improve the dosage forms to adapt to different pathological conditions of the nasal mucosa and different radiotherapy conditions of the patients. In view of the important role of miR-122-5p in regulating microglial polarization in RBI, we will also continue to explore the downstream targets and upstream regulators related to the miR-122-5p pathway, accelerating the clinical practice.
Materials and methods
Inclusion and exclusion criteria for the case-observation group
Inclusion criteria
The patients enrolled in this study were diagnosed with NPC based on pathology or radiology. They were all treated with radiotherapy, had ages between 18 and 80 years, had no restriction regarding sex, did not present with distant metastasis at initial treatment, and had no previous brain lesions.
Exclusion criteria
Patients with NPC metastasis that was discovered before receiving the first radiation therapy; patients with treatment abandonment or an interrupted course of treatment; patients suffering from other diseases, such as stroke, traumatic brain injury, CNS infections, severe cardiac disease, hepatic disease, or renal disease; and all pregnant or lactating women and women who were preparing for pregnancy during the study period were excluded from this study.
Data collection from the selected population
Basic clinical information was collected from the patients, including age, gender, pathological types of NPC, radiotherapy scheme, and pathological stage. Detailed clinical information is shown in Table S1.
Collection of blood samples from patients with NPC
Twenty-eight patients with NPC who were admitted to the Oncology Department of the Affiliated Hospital of Guangdong Medical University beginning in January 2020 were enrolled. They were aged 30 to 64 years, including 17 men and 11 women. We collected clinical blood samples from patients with NPC before radiotherapy (without radiotherapy), 2 weeks after radiotherapy (2 W), 4 weeks after radiotherapy (4 W), and 1 month after radiotherapy (1 M after Rad). The project was approved by the Medical Ethics Committee of the Affiliated Hospital of Guangdong Medical University and followed the ethical principles of medical research outlined in the Declaration of Helsinki (2008).
Experimental animals
The 57 specific-pathogen-free (SPF) male C57BL/6 mice, aged 7 weeks (weighing 20 ± 3 g), were purchased from Guangdong Medicine Laboratory Animal Center. All mice were housed at an appropriate temperature and humidity with a light/dark cycle similar to the day and natural changes at night and were provided free access to food and water. After adapting to the environment, 8-week-old mice were used in the experiment. The study protocol was conducted in accordance with the guidelines for the care and use of laboratory animals (Ministry of Science and Technology of China, 2006) and was approved by the Animal Ethics Committee of Guangdong Medical University.
Grouping and experimental procedures
Construction and screening of RBI-model mice
Ten C57BL/6 mice were randomly divided into a control group (without intervention, n = 5) and a radiation group (exposed to radiation, n = 5). The miRNAs were sequenced and screened after behavioral tests and an evaluation of H&E staining.
AntagomiR-122-5p intervention and evaluation
Forty-four mice were randomly divided into the following four groups: an unirradiated control group administered RNase-free water, designated as the control group (control, n = 11); an irradiated group, designated the radiation group (Rad, n = 11); irradiated mice (40 nmol/mL) administered antagomiR-122-5p, designated as the antagomiR-122-5p-intervention group (Rad + Ant-122, n = 11); and irradiated mice (40 nmol/mL) administered antagomiR negative control (NC), designated the antagomiR-NC intervention group (Rad + Ant-NC, n = 11). The drug was administered intranasally 24 h before irradiation, and the animals were anaesthetized for irradiation on day 1. For the treatment regimen, mice were administered 24 μL (40 nmol/mL) of antagomiR-122-5p, antagomiR-NC, or RNase-free water. Subsequently, the weight of every mouse was recorded from drug administration to 6 weeks (6 weeks) after radiation. As shown in the experimental plan presented in Figure 2, the following behavioral tests were performed on days 42, 43, 48, 49, and 50: the open field test (OFT), the MWM test, the Y-maze, the TST, and NOR test, respectively. BrdU was intraperitoneally injected from days 47 to 52. Mice were sacrificed on day 52, 6 weeks after the injection of antagomiR-122-5p, to investigate the effect of the miR-122-5p intervention on the RBI-model mice. Subsequently, the mice were randomly used for qRT-PCR, HE staining, immunofluorescence staining, and ELISAs.
Nasal administration of antagomiR-122-5p and detection of miR-122-5p relative expression
According to the manufacturer’s instructions, 40 nmol of antagomiR-122-5p (sequence: 5′-CAAACACCAUUGUCACACUCCA-3′; RiboBio, Guangzhou, China) or antagomiR-NC (the antagomir negative control, its antisense chain sequence: 5′-UCUACUCUUUCUAGGAGGUUGUGA-3′) was dissolved in 1 mL of RNase-free water. Referring to a previous study,43 mice were fixed in the supine position in a simple mouse fixator (Yuyan instruments, Shanghai, China). Then, a total of 24 μL of the solution (1 nmol per one mouse) was instilled with a pipette, alternately into the left and right nostrils (1 μL/time), with an interval of 3–5 min. The respiration of mice was not blocked, and other disorders were not observed; all mice in different groups survived. The same dosage and mode of administration were used for intranasal Cy5-antagomiR-122-5p administration (n = 3) to trace the distribution of antagomiR-122-5p after administration. We observed the olfactory bulb, hippocampus, and cortex using confocal microscopy at 0 h and 24 h after intranasal administration of Cy5-antagomiR-122-5p.
Irradiation
The animals were anaesthetized by an intraperitoneal (i.p.) injection of 5% chloral hydrate (5 mg/10 g). After sufficient anesthesia was induced, the mice were attached to a fixed plate, and irradiation was applied at locations ranging from 0.3 cm behind the eyes to the posterior part of both ears to ensure that the radiation surface did not involve the eyes, mouth, and spinal cord (to prevent the normal exercise ability of the mouse from being affected by irradiation). Mice were subjected to a single, full-dose, whole-brain irradiation (30 Gy, 6 electron volt [MeV], and β electron beam) using a medical linear accelerator (Elekta AB, Stockholm, Sweden), as previously described.4
Intraperitoneal administration of BrdU
Neurogenesis in the subgranular area of the dentate gyrus is closely related to spatial memory and behavioral cognition.44 BrdU (B9285, Sigma, USA) was i.p. administered at 100 mg/kg starting 7 days before sacrifice (day 47), once daily for 6 consecutive days,45 and then three times on day 52 (10 h, 6 h, and 2 h before sacrifice) to detect neurogenesis in the DG. The number of cells displaying double-immunofluorescence staining for NeuN and BrdU was observed and analyzed with Image-Pro Plus software.
qRT-PCR
Total RNA was isolated in an RNase-free environment using TRIzol reagent, chloroform, isopropanol, and 75% ethanol prepared in diethyl pyrocarbonate (DEPC)-treated H2O. Samples with a 260/280 absorbance ratio between 1.8 and 2.0 were considered qualified. Then, the RNA was manipulated using a Prime Script RT reagent kit with genomic DNA (gDNA) Eraser (RR047A, Takara) according to the manufacturer’s instructions. The cDNA template was then amplified using a LightCycler 96 (Roche Applied Science, Penzberg, Germany). The forward and reverse primers used in this study are shown in Table S2. The relative expression was determined using the 2−ΔΔCT method.
Behavioral analyses
OFT
All animals performed multiple behavior tests to evaluate spatial memory. In our study, no mortality was observed throughout the study; the survival rate of mice was 100%. An OFT was used to ensure the consistency of motor ability in the irradiation and other intervention groups, which is a necessary prerequisite for other behavioral tests designed to evaluate learning and cognition. The mice were placed in th test area (plastic chamber with a width of 40 cm, length of 40 cm. and height of 30 cm) for 10 min, and the total distance traveled and the number of exploratory rearing behaviors (standing with front feet suspended and climbing the walls) were recorded using a video-tracking system (Viewpoint).
Y-maze
The three arms were randomly designated the start, the novel, and the old arms; visual cues were placed on the edges of each arm. Animals were allowed to explore only two arms in the first trial (10 min). For the second trial (5 min), mice were placed back in the start arm but had free access to all arms of the maze. Trials were captured using a video-tracking system (Viewpoint). The SAP and time (s) spent in the novel arm were evaluated. The SAP was calculated as the number of alternations/(total number of times − 2). After placing the animals, Supermaze software was used to collect images and analyze the data. Before the next test, 75% alcohol was used to eliminate the odor in the Y-maze.
MWM
The maze consisted of a circular pool (diameter: 170 cm, depth: 50 cm) filled with clean water (23 ± 1°C; depth: 25 cm) and was divided into four quadrants (east, west, south, and north). A transparent escape platform (diameter: 14 cm, height: 30 cm) was placed in the same quadrant and was invisible to animals for 5 consecutive days. The experiment was recorded with the Viewpoint system. The number of platform crossings, distance traveled in the target quadrant, time spent in the target quadrant, and escape latency of every mouse within 90 s were recorded by Supermaze software to evaluate the learning and memory performance of the mice.46
NOR test
The NOR test took place in an open area (a plastic chamber with a width and length of 40 cm each). After a habituation period, animals were allowed to explore two identical (familiar) objects for 10 min. After 24 h, mice were returned to the area, but one of the familiar objects was replaced with a novel object (different shape and texture). After the animals were placed in the apparatus, Supermaze software was used to acquire images and analyze the data. A recognition index was calculated using the following formula: time spent exploring the novel object/(time spent exploring the novel object + time spent exploring the familiar object).47
TST
The mouse was fixed at the rear 1/3 of the tail with tape and hung on the bracket. The head was 15 cm away from the table. The camera background (white) was significantly different from the hair color of the mouse. The mice were stopped after 6 min. The immobility time of the mice in the last 4 min (3–6 min) was recorded.
H&E staining
The brain tissues from mice in different groups were fixed with 4% formaldehyde for 72 h. After decalcification in 20% ethylenediaminetetraacetic acid, the samples were dehydrated with gradient concentrations of ethanol and embedded in paraffin. Then, the brain tissue (5 μm) was rewarmed at room temperature for 1 h, dewaxed with xylene, and dehydrated by an incubation with a gradient of alcohol solutions. Afterward, the slices were washed with distilled water, stained with hematoxylin, differentiated with hydrochloric acid, rinsed with distilled water, and dried. After staining with eosin, the sections were washed with a gradient of alcohol solutions. The HE-stained sections were observed with a light microscope for morphological changes (BX51; Olympus, Tokyo, Japan). All experiments were repeated three times. The sum area of the nucleus and the number of nuclei in the hippocampus were counted using Image-Pro Plus software.
Immunohistochemistry
Mice were anaesthetized with 5% chloral hydrate (5 mg/10 g). After anesthesia, the chest of the mouse was exposed, the left ventricle was perfused with saline, and then 50 mL of 4% paraformaldehyde was rapidly perfused. The brain tissue was removed and soaked in polyoxymethylene overnight at 4°C and then dehydrated with 10%, 20%, and 30% sucrose at 4°C for 24 h. After embedding in OCT Compound (Tissue-Tek 4583, Sakura, USA), the brain slices were stored at −80°C and cut with cryostat. The thickness of the brain slices was 25 μm, and the slices were dried in a 37°C drying oven for 40 min and washed with PBS three times for 5 min each. The cells were thoroughly infiltrated with immunofluorescence-penetrating solution for 5 min and washed with PBS three times for 5 min each. The sections were blocked with 10% normal goat serum for 1 h and then incubated overnight at 4°C with the following primary antibodies: Iba-1 at 1:200 (ab178847, Abcam, UK), NeuN at 1:700 (ab177487, Abcam, UK), BrdU at 1:500 (ab6326, Abcam, UK), β-III-tubulin antibody at 1:500 (ab18207), GFAP at 1:300 (ab7260, Abcam, UK), CD31 (platelet/endothelial cell adhesion molecule-1 [PECAM-1], CST, 77699, USA). After incubating overnight with the primary antibody, sections were washed with PBS three times for 5 min each. Secondary antibodies—conjugated Alexa Fluor 488 (goat anti-rabbit immunoglobulin G [IgG], concentration 1:1,000; ab150077, Abcam, UK) and Alexa Fluor 555 (goat anti-rat IgG, concentration 1:1,000; ab150158, Abcam, UK)—were incubated at room temperature for 1 h. For the TUNEL apoptosis assay, the thickness of the brain slices was 15 μm, and the specific steps were completed according to the instructions in the TUNEL apoptosis assay kit (C1090, Beyotime, China). Finally, 4,6-diamidino-2-phenylindole (DAPI) was incubated with the sections for 3 min, which were then dried and sealed with an anti-fluorescence quencher.
Measurement of immunofluorescence staining
Sections were observed with an Olympus laser confocal microscope, and the corresponding excitation wavelengths of Alexa Fluor 488 and DAPI were set using the FV31S-DT imaging software. For the measurement, the target image was opened in Image-Pro Plus software, and then, the default ruler was set according to the image ruler. The IOD and area of astrocytes and branch length, area, IOD, perimeter, and Feret diameter of the microglia were recorded.
ELISAs of TNF-α, IL-6, IL-1β, and S100B
The hippocampus of each mouse was placed in lysis buffer and thoroughly ground with a tissue grinder. The lysis buffer was centrifuged, and the supernatant was collected for ELISAs. For the ELISAs of cells, the supernatant of culture medium was collected. The levels of TNF-α, IL-6, and IL-1β (mouse, C609-02, C604-02, and C601-02, GenStar, China; human, EK0525, EK0410, and EK0392, BOSTER, China, respectively) were analyzed. Because S100B is a specific marker of astrocytes, it bypasses the damaged BBB and is transferred to the peripheral blood after RBI. 48Therefore, the level of S100B (mouse, E-EL-M1033c, Elabscience, China; human, EH0543, FineTest, China) was also determined. The specific operation method was performed according to the manual provided with each kit.
Transcriptome analysis
Samples were collected from three groups: the control group, the irradiation group, and the Rad + Ant-122 (Rad + AntagomiR-122-5p). Then, sample processing and detection were performed by RiboBio (Guangzhou, China)
For mRNAs detected
The differentially expressed genes in the hippocampal samples from mice in the control group, Rad group, and Rad + Ant-122 group were further calculated. The GO and KEGG enrichment analyses were performed for the differentially expressed genes.
For the gene expression analysis
The differentially expressed genes among the samples were selected (|log2(fold change)| > 1 and p < 0.05). The control and Rad groups were compared to screen significantly differentially expressed genes (as group A). Group A was then compared with the antagomiR-122-5p intervention group to screen differentially expressed genes (as group B), which could be considered the candidate regulatory targets after the antagomiR-122-5p intervention
For predictions of miR-122-5p targets
Genes that were significantly downregulated after irradiation and significantly upregulated after the antagomiR-122-5p intervention were selected. The common targets to which miR-122-5p may bind were screened in the TargetScan and miRwalk databases. Three candidate genes were identified as candidate targets of the miR-122-5p intervention in RBI. Finally, the literature was searched, in combination with the preliminary miR-122-5p inhibitor intervention, to verify the relative expression levels of the candidate genes. We identified genes related to inflammation and glial cell regulation, which were potential targets of the miR-122-5p intervention to regulate RBI.
Cell culture
SH-SY5Y cells were cultured in high-glucose medium, BV2 cells (immortalized mouse microglia) were cultured in DMEM/F12, HMC3 (human microglia cells) were cultured in high-glucose medium, and CNE-2 cells were cultured in high-glucose RPMI 1640 medium. All media (HyClone, USA) used for cell cultures were supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS; GIBCO, USA). For cocultures of SH-SY5Y cells and BV2 cells, the supernatant of the media of BV2 cells cultured under different conditions was added to SH-SY5Y cells. The cells were digested and passaged in a sterile operating platform, and the medium was changed according to conventional culture methods. Cells were cultured in a 37°C incubator containing 5% CO2.
Cell irradiation and intervention
According to changes in the viability of BV2 (microglia) and SH-SY5Y (neurons) cells exposed to different radiation doses, we used a dose of 10 Gy in our study because it was the optimal radiation dose for microglial activation. Because CNE-2 cells (human nasopharyngeal carcinoma cells) are more sensitive to radiation, 4 Gy was selected for in vitro irradiation, and the tumor cells were evaluated in vitro after irradiation.49
Cell viability
Cells in the plates were incubated in the incubator for a suitable period (6 h, 12 h, or 24 h). The CCK-8 detection kit (Beyotime, China) was used according to the instructions. The CCK-8 solution (10–15 μL) was added to each well of 96-well plates, and the plates were incubated in the incubator for 1.5–2 h. The absorbance was measured at optical density 450 nm (OD450 value) by Epoch (Biotek, USA). The proliferation activity of different cells was analyzed at the corresponding time points.
Phagocytosis evaluation
Plates were first coated with poly-d-lysine (PDL) in an incubator overnight. On the next day, PDL was discarded, and the plate was exposed to UV light for at least 1 h to sterilize it. The density of microglia added to the plate was approximately 30%. Fluorescent beads (1 μm in diameter, MKCG4343, Sigma) were added to complete DMEM containing FBS. The final concentration of beads was maintained at 0.01% (v/v, 1 μg/mL) and mixed thoroughly. The beads were incubated at 37°C for 1 h. Afterward, the original medium of microglia was replaced, and cells were incubated in a cell incubator for 1–2 h. Then, the microglia were washed with PBS three times for 5 min each, fixed with paraformaldehyde, immunolabeled with Iba-1, stained with DAPI for 1 min, and observed under an Olympus confocal microscope. The percentage of microglia with different beads (0, 1–3, and ≥4 beads) was counted using Image-Pro Plus software and was analyzed statistically to evaluate the phagocytic ability of microglia in different states.
Detection of apoptosis using flow cytometry
Neuronal cells were treated with 0.25% trypsin without EDTA (HyClone, USA) after apoptosis induction and intervention according to the experimental scheme. The specific operation was performed according to the instructions of the Annexin V-PE/7-aminoactinomycin D (AAD) apoptosis detection kit (40310es60, Yeasen, Shanghai, China). Apoptosis was detected using flow cytometry. Flow cytometry results showed that Annexin V-PE single-positive cells were early apoptotic cells, and Annexin V-PE and 7-AAD double-positive cells were necrotic or late apoptotic cells. The numbers of early and late apoptotic cells in different groups were counted.
Luciferase reporter assay
HMC3 cells were cultured on a 12-well plate and transfected with miR-122-5p mimics, miR-122-5p inhibitor, mimics NC, inhibitor NC, psicheck2-TNS1-MUT, and psicheck2-TNS1-WT plasmids (IGE, Guangzhou, China) using LipoIMax (13778030, Invitrogen, USA), according to the instructions provided with the miRNA products. After 24 h, the cells were digested with 0.25% trypsin (+EDTA, HyClone, USA); relative luciferase activity was measured using a dual luciferase assay, and the activity of firefly luciferase was normalized to that of Renilla luciferase as an internal control, according to the instructions of the dual-luciferase detection kit (RG027, Beyotime, China). Then, the relative fluorescence intensity of the different groups was compared.
Clone formation assay
CNE-2 cells were digested with 0.25% trypsin (+EDTA, HyClone, USA). Then, the cells were suspended in RPMI 1640 medium containing 10% FBS. Generally, 100 cells were added to the plate and incubated at 37°C with 5% CO2. When visible clones appeared in the culture dish (after 6 days), the supernatant was discarded, and the cells were rinsed twice with PBS. The cells were fixed with 1 mL of 4% paraformaldehyde or methanol for 15 min. Then, 3 mL of crystal violet solution was added, incubated for 10–30 min, and washed slowly with PBS. The number of clones was counted, and the clone area was analyzed.
Cell scratch-wound assay
Appropriate amounts of cells were added to the wells of the plate to ensure that the density of the cells would be about 100% after growth overnight. Then, the 200 μL sterile pipette tip was used to produce the same scratch wounds in the monolayer in each well of the plate. Next, the cells were washed with PBS three times to remove the floated cells. Serum-free medium was added, and the cells were cultured in a 5% CO2 incubator at 37°C. At 0 h, 4 h, 8 h. and 12 h after washing, the scratch width was measured using ImageJ software.
Immunocytochemistry
BV2 cells were cultured in confocal dishes and treated with 100 nM miR-122-5p inhibitor at 24 h after irradiation. The cells were fixed with 4% paraformaldehyde for 30 min and blocked with 2% goat serum for 30 min. Then, the cells were incubated overnight with an anti-Iba-1 (1:100; Abcam) antibody. After washing with PBS, the secondary antibody and the antifluorescence quencher were added. As mentioned above, fluorescence images were obtained and analyzed.
Western blotting
Total proteins were electrophoretically separated on 15% alkyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (microporous) using an electrophoretic transfer apparatus. The membrane was placed on a shaker platform and incubated with a TNS1 antibody (ab233133, 1:1,000, UK) and α-Tubulin antibody (2144, 1:1,000, CST, USA) at 4°C overnight. After washing with Tris-buffered saline with 0.1% Tween (TBST), membranes were incubated with the corresponding rabbit secondary antibody and exposed using a C600 chemiluminescence detector. ImageJ software was used to analyze the relative gray values.
Heatmap analysis
The differentially expressed mRNAs identified in the transcriptome analysis were analyzed by the software Morpheus (https://software.broadinstitute.org/morpheus).
Statistical analysis
The results of the experiments were statistically analyzed using SPSS 15.0 statistical software, Image-Pro Pro Plus (IpWin 60), FT-SW and FT-DT (3000), ImageJ 6.0, and GraphPad Prism 6.0 software. At least three independent experiments were performed. The data were analyzed using one-way analysis of variance (ANOVA) or a Student’s t test. All data are presented as the means of each treatment group ± SEM.
The study protocol was conducted in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Animal Ethics Committee of Guangdong Medical University (GDY1902051). The clinical project was approved by the Medical Ethics Committee of the Affiliated Hospital of Guangdong Medical University (PJ-2019-004) and followed the ethical principles of medical research outlined in the Declaration of Helsinki (2008).
Acknowledgments
We would like to thank Dr. Wang Yan of Guangdong Key Laboratory of Age-related Cardiac and Cerebral Diseases, Guangdong Medical University, for her substantive revisions and helpful comments on this article. This work was funded by the National Natural Science Foundation of China (82071475 and 81671181), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017), and Guangdong Province: Special Support Plan for High-Level Talents Grant (2016).
Author contributions
L.C. and Y.C designed the study and provided the conceptualization. L.C., Y.C., H.Z., F.S., and M.O., finished the original draft. H.Z., F.S., M.O., Y.Z., H.L., M.L., and Y.Y. collected the data and samples. H.Z., F.S., M.O., H.L., M.L., Y.Y., H.X., M.L., K.Z., X.W., Y.S., and W.F. did the investigation and statistical analysis. H.X., M.L., K.Z., X.W., and C.L. revised the investigation and methodology.
Declaration of interests
The authors declare no competing interests
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.06.019.
Contributor Information
Yujie Cai, Email: musejie163@163.com.
Lili Cui, Email: cuilili@gdmu.edu.cn.
Supplemental information
References
- 1.Owonikoko T.K., Arbiser J., Zelnak A., Shu H.K., Shim H., Robin A.M., Kalkanis S.N., Whitsett T.G., Salhia B., Tran N.L., et al. Current approaches to the treatment of metastatic brain tumours. Nat. Rev. Clin. Oncol. 2014;11:203–222. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown P.D., Ahluwalia M.S., Khan O.H., Asher A.L., Wefel J.S., Gondi V. Whole-brain radiotherapy for brain metastases: evolution or revolution? J. Clin. Oncol. 2018;36:483–491. doi: 10.1200/JCO.2017.75.9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D., Zhou W., Lam T.T., Weng C., Bronk L., Ma D., Wang Q., Duman J.G., Dougherty P.M., Grosshans D.R. Radiation induces age-dependent deficits in cortical synaptic plasticity. Neuro-oncol. 2018;20:1207–1214. doi: 10.1093/neuonc/noy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosi S., Andres-Mach M., Fishman K.M., Levy W., Ferguson R.A., Fike J.R. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68:9763–9770. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene-Schloesser D., Moore E., Robbins M.E. Molecular pathways: radiation-induced cognitive impairment. Clin. Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makale M.T., McDonald C.R., Hattangadi-Gluth J.A., Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017;13:52–64. doi: 10.1038/nrneurol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilke C., Grosshans D., Duman J., Brown P., Li J. Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults. Neuro-oncol. 2018;20:597–607. doi: 10.1093/neuonc/nox195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourhanifeh M.H., Mahjoubin-Tehran M., Karimzadeh M.R., Mirzaei H.R., Razavi Z.S., Sahebkar A., Hosseini N., Mirzaei H., Hamblin M.R. Autophagy in cancers including brain tumors: role of microRNAs. Cell Commun. Signal. 2020;18:88. doi: 10.1186/s12964-020-00587-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yapijakis C. Regulatory role of microRNAs in brain development and function. Adv. Exp. Med. Biol. 2020;1195:237–247. doi: 10.1007/978-3-030-32633-3_32. [DOI] [PubMed] [Google Scholar]
- 10.Korotkov A., Puhakka N., Gupta S.D., Vuokila N., Broekaart D.W.M., Anink J.J., Heiskanen M., Karttunen J., van Scheppingen J., Huitinga I., et al. Increased expression of miR142 and miR155 in glial and immune cells after traumatic brain injury may contribute to neuroinflammation via astrocyte activation. Brain Pathol. 2020;30:897–912. doi: 10.1111/bpa.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang J.H., Hwang Y.H., Lee D.J., Kim D.H., Park J.M., Wu H.G., Kim I.A. MicroRNA-203 modulates the radiation sensitivity of human malignant glioma cells. Int. J. Radiat. Oncol. Biol. Phys. 2016;94:412–420. doi: 10.1016/j.ijrobp.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Xiong G., Tsang W.C., Schätzlein A.G., Uchegbu I.F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019;370:593–601. doi: 10.1124/jpet.119.258152. [DOI] [PubMed] [Google Scholar]
- 13.Gonçalves J., Bicker J., Gouveia F., Liberal J., Oliveira R.C., Alves G., Falcão A., Fortuna A. Nose-to-brain delivery of levetiracetam after intranasal administration to mice. Int. J. Pharm. 2019;564:329–339. doi: 10.1016/j.ijpharm.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Mai H., Fan W., Wang Y., Cai Y., Li X., Chen F., Chen X., Yang J., Tang P., Chen H., et al. Intranasal administration of miR-146a agomir rescued the pathological process and cognitive impairment in an AD mouse model. Mol. Ther. Nucleic Acids. 2019;18:681–695. doi: 10.1016/j.omtn.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandiera S., Pfeffer S., Baumert T.F., Zeisel M.B. miR-122--a key factor and therapeutic target in liver disease. J. Hepatol. 2015;62:448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta D., Cassel T., Teng K.Y., Aljuhani M., Chowdhary V.K., Hu P., Zhang X., Fan T.W., Ghoshal K. Regulation of hepatic glutamine metabolism by miR-122. Mol. Metab. 2020;34:174–186. doi: 10.1016/j.molmet.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo D., Ma J., Li T., Yan L. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp. Cell Res. 2018;369:34–42. doi: 10.1016/j.yexcr.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Kong Y., Li S., Cheng X., Ren H., Zhang B., Ma H., Li M., Zhang X.A. Brain ischemia significantly alters microRNA expression in human peripheral blood natural killer cells. Front. Immunol. 2020;11:759. doi: 10.3389/fimmu.2020.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B., Dasgupta C., Huang L., Meng X., Zhang L. MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell. Mol. Immunol. 2020;17:976–991. doi: 10.1038/s41423-019-0257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng C., Xiaohua W., Ning J., Dan Z., Chengyun Y., Lijun Z., Li Y., Shengfu H., Hong J., He X. MiR-122 exerts anti-proliferative and apoptotic effects on nasopharyngeal carcinoma cells via the PI3K/AKT signaling pathway. Cell. Mol. Biol. (Noisy-le-grand) 2018;64:21–25. [PubMed] [Google Scholar]
- 21.Yang Y., Li Q., Guo L. MicroRNA-122 acts as tumor suppressor by targeting TRIM29 and blocking the activity of PI3K/AKT signaling in nasopharyngeal carcinoma in vitro. Mol. Med. Rep. 2018;17:8244–8252. doi: 10.3892/mmr.2018.8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdő F., Bors L.A., Farkas D., Bajza Á., Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Bors L.A., Bajza Á., Mándoki M., Tasi B.J., Cserey G., Imre T., Szabó P., Erdő F. Modulation of nose-to-brain delivery of a p-glycoprotein (MDR1) substrate model drug (quinidine) in rats. Brain Res. Bull. 2020;160:65–73. doi: 10.1016/j.brainresbull.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Mittal D., Ali A., Md S., Baboota S., Sahni J.K., Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21:75–86. doi: 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- 25.Crowe T.P., Greenlee M.H.W., Kanthasamy A.G., Hsu W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Bender T.S., Migliore M.M., Campbell R.B., John Gatley S., Waszczak B.L. Intranasal administration of glial-derived neurotrophic factor (GDNF) rapidly and significantly increases whole-brain GDNF level in rats. Neuroscience. 2015;303:569–576. doi: 10.1016/j.neuroscience.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Alam M.I., Baboota S., Ahuja A., Ali M., Ali J., Sahni J.K., Bhatnagar A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014;470:99–106. doi: 10.1016/j.ijpharm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Khan A.R., Liu M., Khan M.W., Zhai G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release. 2017;268:364–389. doi: 10.1016/j.jconrel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Gartziandia O., Herran E., Pedraz J.L., Carro E., Igartua M., Hernandez R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces. 2015;134:304–313. doi: 10.1016/j.colsurfb.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 30.van Woensel M., Wauthoz N., Rosière R., Amighi K., Mathieu V., Lefranc F., van Gool S.W., de Vleeschouwer S. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers (Basel) 2013;5:1020–1048. doi: 10.3390/cancers5031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliani A., Balducci A.G., Zironi E., Colombo G., Bortolotti F., Lorenzini L., Galligioni V., Pagliuca G., Scagliarini A., Calzà L., Sonvico F. In vivo nose-to-brain delivery of the hydrophilic antiviral ribavirin by microparticle agglomerates. Drug Deliv. 2018;25:376–387. doi: 10.1080/10717544.2018.1428242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X., Gu S., Chen B.F., Shen W.L., Yin Z., Xu G.W., Hu J.J., Zhu T., Li G., Wan C., et al. Nanoparticle delivery of stable miR-199a-5p agomir improves the osteogenesis of human mesenchymal stem cells via the HIF1a pathway. Biomaterials. 2015;53:239–250. doi: 10.1016/j.biomaterials.2015.02.071. [DOI] [PubMed] [Google Scholar]
- 33.Liang C., Zou T., Zhang M., Fan W., Zhang T., Jiang Y., Cai Y., Chen F., Chen X., Sun Y., et al. MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer’s disease. Theranostics. 2021;11:4103–4121. doi: 10.7150/thno.53418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sava V., Fihurka O., Khvorova A., Sanchez-Ramos J. Enriched chitosan nanoparticles loaded with siRNA are effective in lowering Huntington’s disease gene expression following intranasal administration. Nanomedicine (Lond.) 2020;24:102119. doi: 10.1016/j.nano.2019.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Woensel M., Wauthoz N., Rosière R., Mathieu V., Kiss R., Lefranc F., Steelant B., Dilissen E., Van Gool S.W., Mathivet T., Gerhardt H., et al. Development of siRNA-loaded chitosan nanoparticles targeting galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release. 2016;227:71–81. doi: 10.1016/j.jconrel.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Leavitt R.J., Acharya M.M., Baulch J.E., Limoli C.L. Extracellular vesicle-derived miR-124 resolves radiation-induced brain injury. Cancer Res. 2020;80:4266–4277. doi: 10.1158/0008-5472.CAN-20-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mi B., Li Q., Li T., Liu G., Sai J. High miR-31-5p expression promotes colon adenocarcinoma progression by targeting TNS1. Aging (Albany NY) 2020;12:7480–7490. doi: 10.18632/aging.103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou H., Zhang Y., Wu L., Xie W., Li L., Yuan Y., Chen Y., Lin Y., He X. Elevated transgelin/TNS1 expression is a potential biomarker in human colorectal cancer. Oncotarget. 2017;9:1107–1113. doi: 10.18632/oncotarget.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai Y., Zhang G., Cheng R., Yang R., Chu H. CASC15 contributes to proliferation and invasion through regulating miR-766-5p/ KLK12 axis in lung cancer. Cell Cycle. 2019;18:2323–2331. doi: 10.1080/15384101.2019.1646562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousef G.M., Magklara A., Diamandis E.P. KLK12 is a novel serine protease and a new member of the human kallikrein gene family-differential expression in breast cancer. Genomics. 2000;69:331–341. doi: 10.1006/geno.2000.6346. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhary A., Hilton M.B., Seaman S., Haines D.C., Stevenson S., Lemotte P.K., Tschantz W.R., Zhang X.M., Saha S., Fleming T., St Croix B. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21:212–226. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D., Bhat-Nakshatri P., Goswami C., Badve S., Nakshatri H. ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Cancer Res. 2013;73:5821–5833. doi: 10.1158/0008-5472.CAN-13-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y., Xiao S., Liu J., Zhou H., Liu Z., Xin Y., Suo W.Z. An experimental study of acute radiation-induced cognitive dysfunction in a young rat model. AJNR Am. J. Neuroradiol. 2010;31:383–387. doi: 10.3174/ajnr.A1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu P., Xu Y., Hu B., Wang J., Pan R., Murugan M., Wu L.J., Tang Y. Extracellular ATP enhances radiation-induced brain injury through microglial activation and paracrine signaling via P2X7 receptor. Brain Behav. Immun. 2015;50:87–100. doi: 10.1016/j.bbi.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yardan T., Erenler A.K., Baydin A., Aydin K., Cokluk C. Usefulness of S100B protein in neurological disorders. J. Pak. Med. Assoc. 2011;61:276–281. [PubMed] [Google Scholar]
- 49.Huang Y., Dong Y., Zhao J., Zhang L., Kong L., Lu J.J. Comparison of the effects of photon, proton and carbon-ion radiation on the ecto-calreticulin exposure in various tumor cell lines. Ann. Transl. Med. 2019;7:542. doi: 10.21037/atm.2019.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.