Abstract
Study Objectives:
Prior studies have suggested a benefit of yoga for alleviating sleep disturbance; however, many studies have had methodological limitations. This trial study aimed to extend that literature by including an active sleep hygiene comparison.
Methods:
Participants aged 25–59 years with a primary complaint of sleep onset insomnia lasting at least 6 months were block randomized to an 8-week Kundalini yoga or sleep hygiene intervention, both consisting of initial 60-minute instruction and weekly check-ins. Daily sleep diaries and questionnaires were collected at baseline, throughout the intervention, and at 6-month follow-up. Data were analyzed using linear mixed models (n = 20 in each group).
Results:
Participant ratings of the interventions did not significantly differ. Sleep hygiene improved several diary and questionnaire outcomes, however, yoga resulted in even greater improvements corresponding to medium-to-large between-group effect sizes. Total sleep time increased progressively across yoga treatment (d = 0.95, P = .002), concurrent with increased sleep efficiency (d = 1.36, P < .001) and decreased sleep onset latency (d = −1.16, P < .001), but without changes in pre-sleep arousal (d =−0.30, P = .59). Remission rates were also higher for yoga compared to sleep hygiene, with ≥ 80% of yoga participants reporting average sleep onset latency < 30 minutes and sleep efficiency > 80% at 6-month follow-up. For over 50% of yoga participants, the insomnia severity index decreased by at least 8 points at end of treatment and follow-up.
Conclusions
Yoga, taught in a self-care framework with minimal instructor burden, was associated with self-reported improvements above and beyond an active sleep hygiene comparison, sustained at 6-month follow-up. Follow-up studies are needed to assess actigraphy and polysomnography outcomes, as well as possible mechanisms of change.
Clinical Trial Registration: Registry: ClinicalTrials.gov; Name: Yoga as a Treatment for Insomnia; URL: https://clinicaltrials.gov/ct2/show/NCT00033865; Identifier: NCT00033865.
Citation:
Khalsa SBS, Goldstein MR. Treatment of chronic primary sleep onset insomnia with Kundalini yoga: a randomized controlled trial with active sleep hygiene comparison. J Clin Sleep Med. 2021;17(9):1841–1852.
Keywords: insomnia, yoga, meditation, sleep hygiene, behavior therapy, clinical trial
BRIEF SUMMARY
Current Knowledge/Study Rationale: We report the first randomized controlled trial of a yoga intervention for primary sleep-onset insomnia with an active comparison intervention (instructor-guided sleep hygiene with regular telephone follow-ups). Both interventions were 8 weeks, with comparable evaluation ratings by participants.
Study Impact: Sleep hygiene improved daily sleep diary outcomes and insomnia symptom severity on par with effect sizes observed from cognitive behavioral therapy for insomnia meta-analyses, while yoga showed improvements above and beyond that magnitude. Notably, alleviation of insomnia symptom complaints was concurrent with increased total sleep time, suggesting a mechanism of change not dependent on boosting homeostatic sleep drive. Similar to mindfulness interventions, yoga may serve as a useful complement to existing cognitive behavioral therapy for insomnia, either concurrently or as an entry point in a stepped care framework.
INTRODUCTION
Insomnia is the most common sleep complaint and a major public health concern, with prevalence estimates ranging from 4% to above 20% of the adult population.1–3 A number of contributory factors have been implicated in the etiology and maintenance of chronic insomnia, including psychological conditioning, constitutional predisposing factors, dysfunctional beliefs and attitudes, genetic factors, cognitive and physiological arousal, and stress reactivity.4 The observed elevated physiological arousal may be related to hyperactivation of the stress system in these patients and is the basis for a hyperarousal hypothesis of insomnia. In this hypothesis, insomnia is considered as a disorder of inappropriate arousal rather than a disorder of sleep,5,6 and this is consistent with evidence that interventions that address arousal and stress reactivity show efficacy as insomnia treatments.7
Given the hypothesis that insomnia is related to inappropriate levels of arousal, research has shown that behavioral treatments that are known to reduce psychophysiological arousal have shown efficacy, including physical exercise,8 mind-body practices,9 progressive relaxation,10,11 and meditation.10,12 Cognitive behavioral therapy for insomnia (CBT-I), which has included cognitive behavioral therapy, other sleep-specific strategies, and relaxation techniques, has been found to be superior to pharmacological treatments for long-term outcomes and is thus recommended as gold-standard treatment.4 It has been proposed that skills learned in CBT-I can be implemented long term, beyond discontinuation of CBT-I treatment, whereas medication use needs to continue to retain the benefit.13 It is further hypothesized that other behavioral treatments such as yoga, which incorporates multiple mind-body practices, would also be effective.
Yoga is a comprehensive system with the aim of achieving physical, psychological, and spiritual health and well-being that incorporates a wide variety of postures/exercises, breathing practices, relaxation, and meditation/mindfulness techniques.14 Yoga has also been used as a therapeutic treatment (“yoga therapy”), as it is believed that different techniques can produce unique psychophysiological effects and that this specificity can be used to target specific disorders.14–16 Basic research on yoga has suggested that it is effective in influencing psychophysiological, neuroendocrine, and autonomic parameters, and it has therefore been found to be a particularly effective intervention for addressing stress and stress reactivity.17,18 It is therefore logical to consider yoga as a therapeutic treatment for insomnia. This is supported by evidence of higher quality sleep in long-term yoga practitioners19,20 and a few prospective trials in relatively healthy populations.21–23
However, despite the currently high use (practiced by over 14% of US adults) and rapidly increasing popularity of yoga,24 perceived benefit,19 and particular use for sleep,25 there is only modest evidence of its clinical use in chronic primary insomnia.26,27 Research trials have been published on the effectiveness of meditation as an insomnia treatment either alone28–30 or, in a growing number of studies, as part of a multicomponent treatment.30,31 A few studies have also reported benefits of breath regulation techniques.32,33 Published studies evaluating a yoga treatment for primary insomnia include our preliminary single-arm trial,34 plus a few other small trials.35–38 Our pilot trial showed a within-group effect size of −0.94 for diary-based sleep onset latency (SOL) and 1.33 for sleep efficiency (SE). Otherwise, the best comparison to the present study is a randomized controlled trial for chronic insomnia in postmenopausal women aged 50 to 65 by Afonso et al,38 which showed a between-group effect size on the Insomnia Severity Index (ISI) of −0.62 compared to waitlist control (diary measures not evaluated).9 However, there have been a number of trials of yoga and other mind body practices for the treatment of insomnia secondary to other conditions such as cancer and in elderly populations, many of which have not used formal chronic insomnia diagnostic criteria.9,39,40 In a meta-analysis by Wang et al,9 yoga showed an overall effect size between treatment and control of −0.35 on the ISI (n = 4 studies) and −0.42 on the Pittsburgh Sleep Quality Index (PSQI; n = 9 studies). Baglioni et al40 grouped studies of meditative movement therapies together (n = 9, including yoga, mindfulness, Tai Chi) in a meta-analysis and reported overall effect sizes between active treatment and control groups of 0.15 for SOL, 1.22 for SE, and −0.86 for sleep problem severity (ISI/PSQI). Finally, Wang et al39 reported overall effect sizes between yoga and control groups (n = 16 yoga studies in women) of −0.13 for the ISI and −0.54 for the PSQI.
Theoretically, yoga can be classified as a “self-care” practice noted in the reviews of CBT-I treatment where “stepped care models” have been proposed.13,41 These stepped care models are commonly conceptualized as a pyramid, with the least intensive therapy (eg, readily accessible, lowest cost, least personal inconvenience, least specialist time) as entry point at the bottom and smaller numbers of patients with insomnia requiring intensive treatments further up the pyramid. Full CBT-I treatments have been the most widely researched treatment modality, with demonstrable efficacy and effectiveness across a variety of primary and comorbid clinical presentations,42–49 albeit requiring relatively high amount of time and provider training to administer. Sleep hygiene (SH) treatment has previously been used as a control treatment to strengthen the interpretation of CBT-I outcomes50 and can also be considered an entry level in stepped care models. However, more evidence is needed to determine the relative efficacy of SH vs yoga and other interventions requiring minimal clinician burden.
The purpose of this small trial study was to evaluate the suitability and efficacy of a simple set of yoga exercises requiring minimal training compared to active SH control, as previously done in CBT-I research. We hypothesized that SH would yield improvements in average diary-reported SOL, our primary outcome measure, with yoga demonstrating improvements above and beyond those of SH. We also hypothesized that a similar pattern of differential improvement would be observed across a number of secondary sleep diary and questionnaire measures.
METHODS
Study design
This yoga intervention study was a randomized controlled trial focusing on comparison of change against an active sleep hygiene comparison. Outcome measures were collected at baseline, throughout the 8-week treatment phase, and at 6-month follow-up. The study was conducted from April 2003 to January 2007. A study coordinator met individually with volunteers prior to enrollment to inform them about nature of the study, describe study procedures, and obtain written informed consent. Participants were remunerated for their participation. The experimental protocol was approved by the Institutional Review Board of Brigham and Women’s Hospital.
Participants were randomized and stratified by sex in blocks of 4 and 6, and assignment was accomplished by randomly selected slips of paper from an opaque envelope (blinded assignment). Assignments were completed just before the beginning of the first treatment session. Therefore, both instructor and participants did not know the treatment assignments until just before the first treatment session. The instructor (S.B.S.K.) met with participants for one 60-minute, in-person training session for both the yoga and SH treatments. Each participant was given paper instructions and asked to follow the treatment protocol. About 1 week after treatment start, the instructor met again in-person for up to 15 minutes to confirm the accuracy of the yoga and SH practices, answer any questions, address issues of adherence, and offer suggestions to address barriers to adherence. Thereafter, participants from each treatment group were followed-up via telephone by the instructor every 2 weeks. Each 5-minute to10-minute call addressed issues with adherence and/or barriers to the treatment protocol.
Participants
Participant recruitment
Participants aged 25 to 59 years with a complaint of difficulty initiating sleep (primary sleep-onset insomnia) were recruited primarily from advertisements in newspapers and posters. To exclude expectation and bias effects, all study recruitment materials and the consent form did not include any mention of yoga or meditation. Participants were informed they would receive 1 of 2 active and credible nonpharmaceutical behavioral treatments for insomnia, either a sleep hygiene treatment that included education and application of sleep habits or a relaxation treatment that included body positions in specific postures, a specified breathing pattern, and mental focus.
Participant screening
Participant screening included 2 phases: 1) telephone screening and written consent and 2) in-person 1-hour comprehensive sleep history interview. All participants meeting entry criteria through a preliminary telephone screening completed a written consent form. Participants underwent a comprehensive sleep history interview to determine the presence of chronic primary sleep-onset insomnia. This was then reviewed with the participant and a board-certified sleep specialist to verify the primary insomnia diagnosis and the appropriateness of the participant’s participation in the study. Data recorded in the sleep history interview included: 1) duration of the insomnia, 2) the potential relationship of its onset to prior life events, 3) severity over time, 4) history of prior attempts to treat the insomnia either pharmacologically or behaviorally, 5) participant’s habitual daily sleep-wake schedule, 6) typical/average SOL, 7) typical/average number and duration of midsleep awakenings, 8) nature of cognitive activity during the sleep onset period and during midsleep awakenings, 9) timing, frequency and duration of any daytime naps, 10) use of caffeine and other substances and medications, 11) presence and severity of daytime fatigue or sleepiness, 12) symptoms consistent with other sleep disorders (ie sleep apnea, narcolepsy, parasomnias, restless legs syndrome, periodic leg movements, etc.), and 13) a brief medical and psychiatric history.
The insomnia criteria were: 6-month minimum history of insomnia, at least 1 negative daytime complaint due to insomnia, and average SOL of greater than 30 minutes (based on daily sleep diary entries for 1 week). Of the 157 assessed for eligibility prior to randomization, 13 failed to meet this diary-based criterion and were excluded from the study. This study focused on sleep-onset insomnia due to the evidence that relaxation-based treatments have been more effective at improving SOL compared to sleep maintenance parameters,9,10 as well as the advantage of increasing homogeneity of the clinical sample in this small trial study. Exclusion criteria were: age < 25 years or > 59 years (due to concerns about ability and safety of performing the yoga intervention and increasing comparability of this small trial study with the existing literature); use of hypnotic medications within 2 weeks prior to enrollment (and participants were asked to refrain from use during study); any major medical disorders or conditions that were known to interfere with sleep or that would preclude the participant’s ability to carry out the experimental protocol; previously diagnosed or symptomatic evidence of sleep-disordered breathing, periodic leg movements, restless legs syndrome, or sleep disorder other than primary insomnia; current Axis I Diagnostic and Statistical Manual of Mental Disorders, fourth edition, diagnosis as per a structured clinical interview; psychotropic medications or any other concurrent nonpharmacological treatment for insomnia during the course of the protocol; and any anticipated life stressors (moving, divorce, etc.), shift work, or transcontinental travel. Participants had to report being physically and medically capable of practicing the techniques safely. Complaints of difficulty maintaining sleep (sleep maintenance insomnia, early morning awakenings) were not explicitly part of the inclusion/exclusion criteria. Because the study design included the blinding of the intervention to participants to not indicate the word “yoga” in the recruitment and enrollment process, there were no exclusion criteria applied specifically for an existing yoga and/or other ongoing mind-body practice. However, there was exclusion for any current/ongoing regular nonpharmacological treatment for insomnia, including mind-body practices as determined in the sleep history interview, and participants had to agree to not adopt any other treatments/practices for insomnia during the study.
Interventions
Yoga treatment
Participants assigned to the yoga treatment were informed that their treatment included yoga-based practices only after they were randomly assigned to treatment, which was instructed in a 1-hour training session. Participants were not instructed in or informed about any other behavioral treatment recommendations for insomnia (eg, stimulus control, SH, sleep restriction, etc.). The 45-minute daily session of yoga used practices from Kundalini yoga as taught by Yogi Bhajan, which is a safe and widely practiced style of yoga that emphasizes meditation, relaxation, and breathing techniques in addition to postures. The exercises chosen were selected because they were specifically recommended for improving sleep and were easy to learn and perform with minimal instruction. The same set of exercises was performed every day during the intervention. All exercises were completed in the seated posture, with instructions to maintain the spine erect, but relaxed, with all breathing through the nose, and with eyes closed unless otherwise specified. Special attention in the initial training session was devoted to specific instructions on the practice of long, slow abdominal breathing to ensure that participants understood the breathing pattern. Participants were instructed to breathe as slowly as was comfortable. The basic cognitive process of meditation was also described in detail. Participants were instructed to maintain a relaxed mental focus either on their breathing or a mantra (word or phrase of their choice), returning their attention to this focus in a relaxed manner when they found their thoughts wandering.
The full set of exercises included the following. 1) Arms extended upwards at a 60 degree angle with the palms flat and facing upwards with meditation on the breath for 1 to 3 minutes. 2) Arms extended horizontally to the sides with the wrists bent upwards and the palms facing away with meditation on the breath for 1 to 3 minutes. 3) Hands clasped together at the sternum with the arms pushing the palms together with meditation on the breath for 1 to 3 minutes. 4) A breathing meditation called “Shabad Kriya”. Palms are resting in the lap facing upward with right over left and the thumbs touching. Eyes are 1/10 open and gaze is downward past the tip of the nose. The inhale is in 4 segments or “sniffs”, followed by breath retention for 16 counts and an exhale in 2 segments, so that the ratio of inhale:hold:exhale is 4:16:2. During the inhale, the mantra “Sa, Ta, Na, Ma” is mentally recited with each segment. During the breath retention, this mantra is mentally repeated 4 times. During the exhale, the mantra “Wahe Guru” is mentally recited concurrently with each exhaled segment. Participants are encouraged to maintain the overall breathing frequency as slow as is comfortable, while maintaining the specified ratio of inhale:hold:exhale for 11 to 31 minutes.
Participants were instructed to perform the treatment in the evening, preferably just before bedtime. If, on occasion, the participant’s evening schedule made it difficult to incorporate the treatment, participants were to practice the treatment at another time of day.
During the in-person 1-week follow-up (lasting from 5 to 15 minutes), the instructor had participants in this treatment perform all exercises to ensure understanding of the breathing and meditation component of this yoga treatment. The instructor also addressed issues of adherence.
Sleep hygiene
The sleep hygiene treatment was adopted from Edinger et al.50,51 It was selected to be a weak treatment that would still be viewed as credible by participants but would not include any behavioral interventions such as cognitive behavioral therapy, stimulus control, or sleep restriction that are known to have substantial clinical benefit. The 1-hour SH treatment training session began with the reading of a ∼20-minute script of basic sleep education that included information on sleep physiology, sleep stages, function of sleep, sleep needs, dreaming, etc. followed by an opportunity for discussion. Participants were then instructed in the adoption of the following behavioral recommendations for improving sleep: 1) restriction of caffeinated foods and beverages to no more than 3 cups of coffee per day and no caffeine in the late afternoon or evening hours, 2) limit intake of alcohol in the evening or using alcohol as a sleep aid, 3) engage in regular moderate exercise (eg 3 days per week) such as walking, swimming, or biking, particularly in the late afternoon or early evening but to avoid exercise right before bed, 4) consuming a light bedtime snack that includes items such as cheese, milk, or peanut butter, 5) minimizing noise and light by wearing ear plugs, running a fan, using a “white noise” machine, minimizing the use of night lights and using dark shades over windows, and 6) keeping room temperature comfortable using an air conditioner if necessary. All of these recommendations were described in detail and the participant’s current and anticipated adherence was discussed. The investigator then worked with the participant to address plans for implementing these recommendations and addressing any problems or limitations. One week after the initial training session, the instructor addressed barriers to adherence and offered suggestions to each individual participant during a 5-minute to 15-minute in-person follow-up meeting.
Outcome measures
Therapy evaluation questionnaire
Treatment credibility was assessed via Likert ratings (range 1–7) of the 7-item Therapy Evaluation Questionnaire (TEQ).50 The first 5 questions of the TEQ assess perceived logic of and confidence in a treatment, willingness to repeat the treatment, and likelihood the treatment will help others. The final 2 items assess therapist warmth and competence. The TEQ was administered in the beginning and end session of the treatment protocol.
Sleep diaries
Participants completed daily sleep-wake diaries throughout the 2-week pretreatment baseline, the 8-week treatment phase, and during the 2-week long-term follow-up 6 months after the end of the treatment. They were instructed to complete the diaries shortly after awakening on a regular basis and to avoid completing them during the night. Participants recorded the time in and out of bed, SOL, the number and duration of all nocturnal awakenings, the timing of any daytime naps on the previous day, and the quality of nocturnal sleep and restedness on a 10-point numerical scale. During the treatment and long-term follow-up phases, the diaries included a daily adherence entry for the timing and duration of the yoga treatment or for the specific SH recommendations not adhered to. Completed diaries were brought in by the participants following the 2-week baseline and after the first week of the treatment and then mailed in every 2 weeks for the remainder of the treatment phase and for 6-month follow-up.
Sleep questionnaires
Participants completed the 7-item ISI,52 13-item Insomnia Symptom Questionnaire (ISQ),53 PSQI,54 9-item Self-Efficacy for Sleep scale (SES),55 and Pre-Sleep Arousal Scale (consisting of “Somatic” and “Cognitive” subscales).56 The SES and ISQ were collected with a modified visual analog scale format, consistent with procedures from Edinger et al.51 Each of these instruments have shown good psychometric properties (α ≥ 0.71) and ability to detect improvements in response to insomnia treatment.57 Questionnaire measures were collected once at baseline, end of intervention (week 8), and follow-up, with varying intervals during treatment phase: weekly for ISI and ISQ, biweekly for Pre-Sleep Arousal Scale, treatment midpoint (week 4) for SES, and no intermediate intervals for PSQI.
Statistical analyses
For daily sleep diaries, average values were calculated for the 2-week interval of the baseline, each of the 4 consecutive 2-week intervals in the treatment phase, and 2-week interval of the 6-month follow-up timepoint.
Data visualization was done in Matlab (Mathworks, Natick, MA), and statistical analysis was conducted in IBM SPSS 26.0 using Linear mixed models (LMM) and specified post hoc comparisons focusing on within-group change for yoga and SH at postintervention and follow-up compared to baseline. All available data from participants who completed at least 4 weeks of intervention were included for analysis. For each LMM, results are reported for main effects of Group (yoga, sleep hygiene) and Time (baseline, varying intervals of treatment phase depending on measure [see above], and 6-month follow-up), as well as Group × Time interaction. To balance type I and type II error with post hoc comparisons, a Bonferroni correction was applied by multiplying uncorrected P values by 4 (the number of post hoc comparisons per model), allowing alpha to remain at 0.05 for significance. Effect size estimates were calculated with Cohen’s d using paired difference scores of the raw data for within-group change, as well as between-group for direct comparison of 2 interventions.
Based on the results from a preceding open trial pilot study of this yoga intervention for insomnia,34 using an effect size of −0.94 for diary-based SOL as the primary outcome for the current study, power of .80, and alpha of .05, an estimated sample size of 11 participants per group would be needed to observe comparable effects for 2-tailed paired t tests of within-group change from pretreatment to posttreatment, and 19 participants per group for between-group effects of that same magnitude.
RESULTS
Participant characteristics
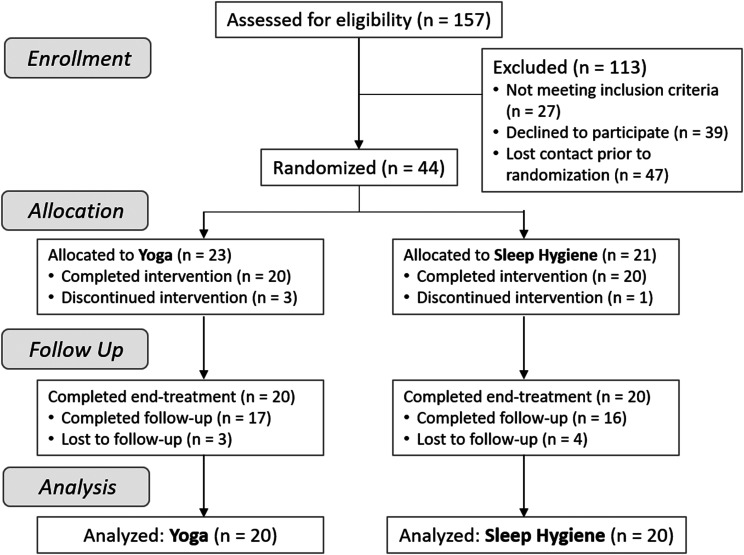
Of the 157 screened for eligibility, 44 participants were randomly assigned to either yoga (n = 23) or SH treatment (n = 21) and underwent a treatment training session (Figure 1). Four participants withdrew during the treatment phase, including 3 (13%) in the yoga group and 1 (5%) in the SH group (χ2 = 0.91 P = .340), yielding a sample size n = 20 in each group with at least 4 weeks of treatment data for analysis. Reasons for withdrawal included change in life circumstances precluding continued time for and/or commitment to the protocol (eg, illness, family emergency, moving, etc.), did not wish to continue committing time to the treatment, and dislike of the treatment. Of the 17 participants in yoga who completed follow-up questionnaires, all 17 also completed daily sleep diaries. For the SH group, notably higher data loss was observed, as 16 participants completed questionnaires at follow-up and only 11 completed sleep diaries. No adverse events were reported during the study.
Figure 1. CONSORT flow diagram of recruitment and participation.
CONSORT = Consolidated Standards of Reporting Trials.
The sample was predominantly middle aged (yoga: 43.5 ± 12.2 yr, SH: 40.8 ± 8.7 yr, P = .422) and majority female (yoga: 55%, SH: 70%, P = .340). Race did not significantly differ between groups (yoga: 20% Black, 0% Asian, 80% White; SH: 15% Black, 20% Asian, 65% White; P = .692) nor did self-identified Hispanic ethnicity (yoga: 10%, SH: 0%, P = .154).
The TEQ had high internal reliability for this sample (α = .866). Mean ratings did not significantly differ between groups or over time (scale range 1–7; yogafirst session: 5.5 ± 1.0, yogalast session: 5.5 ± 1.2, SHfirst session: 5.5 ± 1.2, SHlast session: 5.0 ± 1.2, P ≥ .347 for LMM main effects and Group × Time interaction).
Yoga practice characteristics
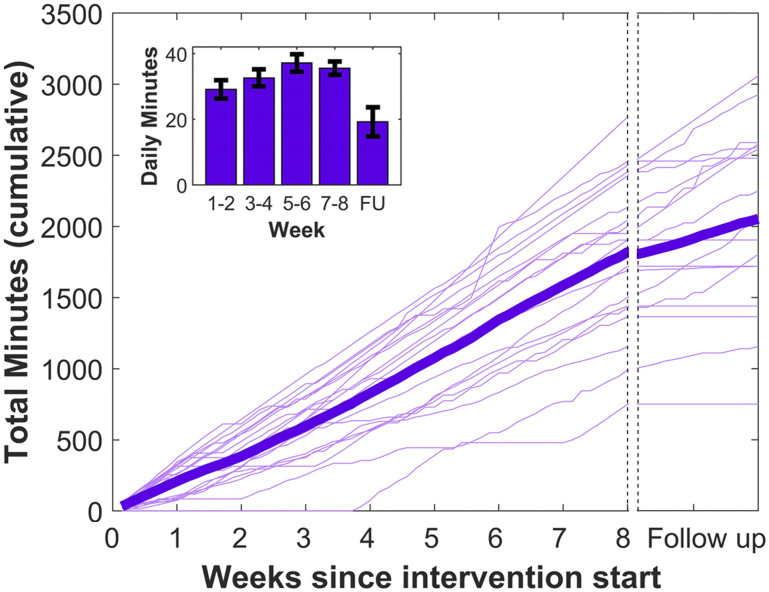
Figure 2 illustrates the trajectory and individual variability of reported practice duration across time. As depicted in the inset, average duration of daily practice increased over the first few weeks of intervention (from 29.1 ± 12.6 to 37.2 ± 12.1 min/day, P = .011), plateaued until the end of intervention, and then was significantly lower during the 2-week-long 6-month follow-up period (21.0 ± 18.5 min/day, P = .009), with approximately half of participants reporting no or minimal practice.
Figure 2. Yoga practice volume over time.
Inset depicts average daily practice minutes in 2-week intervals. Error bars reflect standard error of the mean. FU = follow-up.
Primary outcome
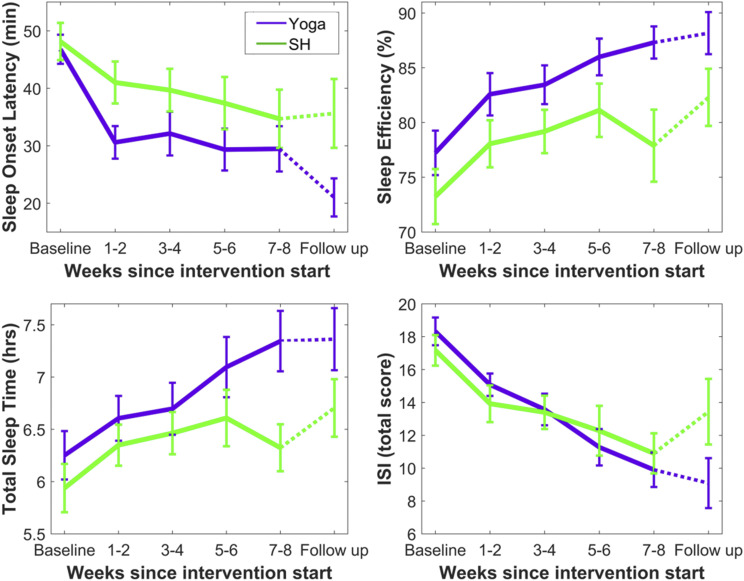
Given that this study specifically focused on individuals with complaints of difficulty initiating sleep, the primary outcome was average SOL from daily sleep diaries. Yoga and SH had comparable SOL at baseline (46.8 ± 11.6 minutes vs 48.1 ± 14.1 minutes, P = .748). LMM indicated significant main effects of Group (F1,182 = 11.65, P = .001) and Time (F5,61 = 7.21, P < .001) but not a Group × Time interaction (F5,61 = 0.76, P = .583). However, only for yoga were the within-group improvements in SOL significant after Bonferroni correction for multiple comparisons at end of intervention (yoga: P < .001, d = −1.16; SH: P = .203, d = −0.55) and follow-up (yoga: P < .001, d = −1.70; SH: P = .062, d = −0.53) (Table 1; Figure 3). The groups also significantly differed at the follow-up timepoint (P = .035; Figure 3).
Table 1.
Daily sleep diary and questionnaire outcomes.
| Baseline | Post | Post: Effect Size (d) | 6-Month | 6-Month: Effect Size (d) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Within | Between | Mean (SD) | Within | Between | |
| SOL (min) | |||||||
| Yoga | 46.8 (11.6) | 29.5 (18.1) | −1.16* | −0.24 | 21.0 (14.1) | −1.70* | −0.34 |
| Sleep Hygiene | 48.1 (14.1) | 34.7 (22.8) | −0.55 | 35.6 (20.8) | −0.53 | ||
| Awakenings (#) | |||||||
| Yoga | 1.5 (1.0) | 1.2 (1.0) | −0.44 | −0.06 | 1.1 (0.6) | −0.51 | 0.37 |
| Sleep Hygiene | 1.7 (1.1) | 1.5 (1.1) | −0.52 | 1.4 (0.8) | −0.48 | ||
| WASO (min) | |||||||
| Yoga | 31.3 (23.5) | 15.7 (14.5) | −0.68* | −0.21 | 15.1 (10.6) | −0.69* | 0.16 |
| Sleep Hygiene | 48.9 (45.9) | 37.9 (42.9) | −0.68* | 29.4 (20.3) | −1.16* | ||
| TWT (min) | |||||||
| Yoga | 108.6 (48.2) | 64.1 (35.1) | −1.18* | −0.55 | 58.7 (38.4) | −1.14* | −0.15 |
| Sleep Hygiene | 131.1 (61.5) | 113.0 (87.8) | −0.31 | 85.3 (43.5) | −1.08* | ||
| TST (min) | |||||||
| Yoga | 375.1 (63.8) | 440.6 (79.6) | 0.95* | 0.73* | 441.8 (75.8) | 0.81* | 0.56 |
| Sleep Hygiene | 356.3 (60.6) | 379.4 (60.4) | 0.59 | 402.3 (57.5) | 0.97 | ||
| SE (%) | |||||||
| Yoga | 77.2 (9.3) | 87.3 (6.7) | 1.36* | 0.60 | 88.2 (8.2) | 1.15* | 0.33 |
| Sleep Hygiene | 73.2 (11.0) | 77.9 (14.7) | 0.44 | 82.3 (9.1) | 1.18* | ||
| Sleep quality | |||||||
| Yoga | 4.4 (1.1) | 5.5 (1.6) | 0.68* | −0.08 | 6.3 (1.9) | 0.83* | −0.02 |
| Sleep Hygiene | 4.4 (1.4) | 5.5 (1.6) | 0.69* | 5.8 (2.7) | 0.52 | ||
| Restedness | |||||||
| Yoga | 4.2 (1.3) | 5.5 (1.8) | 0.70* | −0.04 | 6.2 (2.1) | 0.88* | 0.21 |
| Sleep Hygiene | 4.4 (1.3) | 5.5 (1.8) | 0.72* | 5.5 (2.6) | 0.46 | ||
| ISI | |||||||
| Yoga | 18.3 (3.9) | 9.9 (4.8) | −1.43 | −0.36 | 9.1 (6.5) | −1.17 | −0.60 |
| Sleep Hygiene | 17.2 (4.3) | 10.9 (5.6) | −1.03 | 13.4 (8.2) | −0.40 | ||
| ISQ | |||||||
| Yoga | 56.3 (18.0) | 30.3 (17.4) | −1.25 | −0.74* | 33.2 (19.5) | −0.90 | −0.86* |
| Sleep Hygiene | 46.8 (10.6) | 34.4 (15.3) | −0.80 | 45.8 (23.3) | −0.04 | ||
| PSQI | |||||||
| Yoga | 11.6 (3.2) | 8.7 (3.7) | −0.85* | −0.44 | 7.5 (3.9) | −0.78 | −0.42 |
| Sleep Hygiene | 10.3 (2.5) | 8.9 (4.1) | −0.32 | 8.8 (4.9) | −0.29 | ||
| SES | |||||||
| Yoga | 40.3 (17.5) | 59.5 (19.0) | 1.16* | 0.98* | 59.1 (23.4) | 0.84* | 0.57 |
| Sleep Hygiene | 41.6 (12.0) | 47.3 (22.3) | 0.32 | 46.1 (25.1) | 0.18 | ||
| PSAS-Somatic | |||||||
| Yoga | 13.0 (4.1) | 11.8 (3.6) | −0.31 | −0.25 | 12.2 (4.8) | −0.13 | −0.40 |
| Sleep Hygiene | 13.4 (4.1) | 12.8 (5.4) | −0.16 | 15.3 (7.2) | 0.44 | ||
| PSAS-Cognitive | |||||||
| Yoga | 14.1 (4.5) | 12.6 (4.3) | −0.30 | −0.41 | 13.8 (5.8) | −0.07 | −0.35 |
| Sleep Hygiene | 13.2 (3.3) | 13.7 (5.4) | 0.13 | 15.8 (6.3) | 0.58 | ||
Effect sizes are computed as Cohen’s d, reflecting the magnitude of change from baseline to posttreatment and 6-month follow-up within each group, as well as between groups (higher values indicate greater change for yoga compared to sleep hygiene). No significant differences were observed between groups at baseline. *P < .05, Bonferroni-corrected. ISI, Insomnia Severity Index, ISQ, Insomnia Symptom Questionnaire, PSAS, Pre-Sleep Arousal Scale, PSQI, Pittsburgh Sleep Quality Index, SD, standard deviation, SE, sleep efficiency, SES, Self-Efficacy for Sleep, SOL, sleep onset latency, TST, total sleep time, TWT, total wake time, WASO, Wake After Sleep Onset.
Figure 3. Changes in main self-reported outcomes across groups and time, including 6-month follow-up.
Daily sleep-wake diary variables were averaged across 2-week intervals. Error bars reflect standard error of the mean. See Figure S3 (918.6KB, pdf) and Discussion for further visualization and commentary regarding data loss at the 6-month follow-up. ISI = Insomnia Severity Index, SH = sleep hygiene.
Secondary outcomes
To further characterize changes in sleep between groups, a number of additional sleep diary variables and relevant questionnaires were evaluated (Table 1). Data for SE, total sleep time (TST), and ISI are plotted for visualization alongside SOL in Figure 3 and in further detail of individual-level change in Figure S1 (918.6KB, pdf) in the supplemental material. In addition, a more detailed time course of change in sleep diary variables SOL, SE, and TST is visualized in Figure S2 (918.6KB, pdf) in the supplemental material. No baseline differences between groups were observed for any secondary sleep diary or questionnaire measure (uncorrected P values: .086−.864).
Daily sleep diaries
LMM demonstrated a main effect of Time for total wake time, TST, SE, sleep quality, and restedness (P values ≤ .011), reflecting patterns of improvement across both groups. Although no Group × Time interactions were observed, a main effect of Group was observed for wake after sleep onset, total wake time, TST, and SE (P values ≤ .001). This effect of Group appears to be driven by between-group differences in each of these 4 measures at the end-intervention timepoint (P values ≤ .035; Figure 3 for SE and TST), as well as significantly greater increases (difference scores) in TST from baseline to end of intervention for yoga compared to SH (within-group d values: 0.95 and 0.59, between-group d = 0.73, P = .036).
Questionnaires
All questionnaires demonstrated high internal reliability for this sample (α: .802−.914). LMM demonstrated a main effect of Time for ISI, ISQ, and SES (P values ≤ .021), reflecting patterns of improvement across both groups. Similar to sleep diary measures, although no Group × Time interactions were observed, a main effect of Group was observed for SES (P = .021). The magnitude of change (difference scores) from baseline was also significantly greater for yoga compared to SH at end of intervention for SES (between-group d = 0.90) and at both end-intervention and follow-up for ISQ (between-group d values: −0.74 and −0.78; Table 1). No effects were observed for either Somatic or Cognitive subscales of the Pre-Sleep Arousal Scale.
Clinical significance
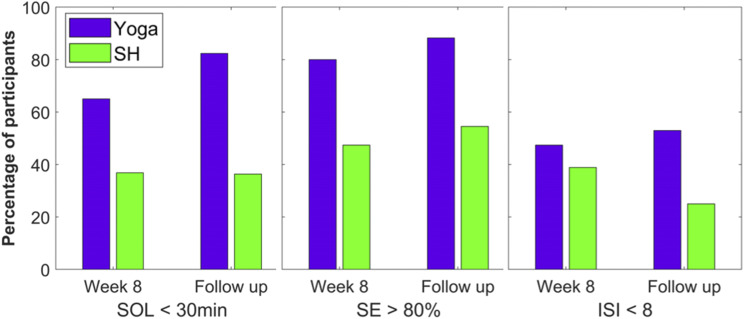
Clinical efficacy was assessed by comparing remission and response rates at end-intervention and follow-up. Following literature recommendations for cutoffs, remission was defined as < 30 minutes for SOL,50,58 > 80% for SE,59,60 and < 8 for ISI score.61 As illustrated in Figure 4, the majority of participants randomized to the yoga group, but not SH, achieved remission by the end of intervention for SOL (65.0% vs 36.8%) and SE (80.0% vs 47.4%). These patterns remained at follow-up for both SOL (82.4% vs 36.4%) and SE (88.2% vs 54.5%). Between-group differences were smaller for ISI remission (post: 47.4% vs 38.9%; follow-up: 52.9% vs 25.0%).
Figure 4. Clinical significance of main outcomes.
Clinical significance (remission rates) of main outcomes at postintervention and 6-month follow up for each group, using recommended cutoffs of less than 30 minutes for SOL, greater than 80% for SE, and less than 8 for ISI total score. ISI = Insomnia Severity Index, SE = sleep efficiency, SH = sleep hygiene, SOL = sleep onset latency.
Treatment response was defined as a reduction from baseline in ISI total score of at least 8 points. A similar pattern to ISI remission was observed, with relatively higher rates for yoga compared to SH (post: 52.6% vs 38.9%; follow-up: 58.8% vs 31.3%).
DISCUSSION
The aim of the current study was to evaluate the efficacy of an 8-week Kundalini yoga intervention vs an active SH comparison. Both intervention groups were blinded to their randomly assigned intervention until the first session, and subsequently reported moderately high ratings of the treatments (TEQ averages > 5 on 7-point Likert scale, not significantly different between groups). Yoga demonstrated greater overall improvements in SOL, the primary outcome measure, as well as a number of additional sleep diary and questionnaire measures. These improvements corresponded to medium-to-large effect sizes and a majority of participants achieving clinical remission in terms of SOL < 30 minutes, SE > 80%, and ISI < 8.
Although missing data were minimal from baseline to the end of intervention and also minimal for questionnaires at follow-up, it was higher for daily sleep diaries at follow-up, specifically for the SH group (n = 5 completed questionnaires but not sleep logs). Further examination of the data revealed that the SH participants who completed daily sleep diaries at follow-up were those who tended also to report improvements during the treatment phase, whereas those who only completed follow-up questionnaires but not sleep diaries, or dropped out during the 6-month follow-up period entirely, tended to report initial improvements with SH but a return toward baseline by the end of the treatment phase (Figure S3 (918.6KB, pdf) in the supplemental material). This pattern likely reflects a self-selection bias, such that the participants who experienced benefit were much more likely to complete all study procedures. Therefore, it appears likely that the sleep diary measures may be an underestimate of between-group differences at follow-up.
Recent meta-analyses enable a helpful comparison of these results with the large existing literature on CBT-I, the current gold-standard treatment for chronic insomnia. At end of intervention as well as 6-month follow-up, within-group effect sizes for SH are on par with average CBT-I outcomes42,43 for SOL and ISI, with yoga demonstrating larger effects. Given that CBT-I has been superior to SH in prior studies,48,50 the effect sizes in the current study are likely overestimates, such that improvements with yoga in subsequent replication studies may be closer to those observed with CBT-I rather than superior to this current gold-standard. Additional limitations of this method of comparison are the specific focus of this study on sleep onset insomnia and the self-selection bias described above that likely inflates the effect sizes for sleep hygiene on SE and TST at follow-up. While acknowledging these caveats, one particularly notable comparison to highlight further is the increased TST associated with yoga at both end of intervention and follow-up. Decreasing sleep time (via time-in-bed restriction) is a key mechanism of CBT-I to increase homeostatic drive, and therefore improvements in TST are limited following CBT-I.4,62 The results of the current study suggest that yoga may serve as an adjunct to CBT-I or even an initial treatment option within the context of a stepped care approach41 involving minimal burden of instructor time or required insomnia treatment expertise to improve sleep efficiency while simultaneously increasing sleep time.
There is also substantial overlap between the meditative component of the yoga practice used in the current study and mindfulness-based interventions for insomnia, including Mindfulness-Based Therapy for Insomnia that has been developed as a formally integrated intervention with CBT-I.63 Compared to a trial study of Mindfulness-Based Therapy for Insomnia,61 effect sizes in the current study are slightly larger for total wake time and SE, much larger for TST (consistent with the key mechanism of CBT-I described above), but slightly smaller for ISI. One potentially meaningful difference between this yoga intervention and mindfulness-based interventions is the explicit inclusion of and focus on body posture, movements, and breath regulation, which may offer some individuals greater access to alleviating sleep-related worries by naturally shifting attention to physical sensations.64 An intriguing recent observation related to mindfulness-based interventions is an increase in beta/gamma non-rapid eye movement electroencephalography,65 conventionally interpreted as a marker of “hyperarousal” in insomnia.4,66,67 In other words, it would be expected that a process of de-arousal would correspond to a decrease in this electroencephalography marker, rather than the increase that was observed. One aspect of the current findings related to the hyperarousal framework of insomnia is the lack of any effects on either subscale of the Pre-Sleep Arousal Scale, indicating that a process of de-arousal was not concurrent with the medium-to-large effect size improvements in sleep parameters or insomnia symptom complaints. These findings suggest that the originally hypothesized mechanism of yoga (ie, relaxation) may need to be refined and better contextualized with physiological data. For example, Britton and colleagues68 highlighted that meditation practices often aim to cultivate a balance of relaxation and alertness, supported by data from multiple domains of arousal as a function of meditation training. It would be useful for future studies to examine whether similar findings with sleep electroencephalography data and self-reported arousal are observed in other contemplative interventions with body movement and postural components, such as yoga or Tai Chi,69,70 and whether these domains of arousal correlate with inflammation, autonomic regulation, or other domains relevant to downstream physical health outcomes.
Limitations of the current study merit discussion. The sample size was relatively small and consisted of primarily White females. The inclusion criteria focused on a complaint of difficulty falling asleep, thus the results may be most representative of chronic primary sleep-onset insomnia and not generalizable to other forms of insomnia. Due to logistical constraints, the first author and study lead (S.B.S.K.) also served as the instructor for both groups; however, to minimize potential bias with data analysis, the second author (M.R.G.) conducted independent statistics that are reported here. As discussed above, there was notable data loss for the daily sleep diaries at follow-up, primarily for the SH group. Linear mixed models were implemented to estimate overall treatment effects between groups while accounting for missing data. Although further analysis of the data suggested a self-selection bias that may have underestimated the relative strength of yoga over SH in this sample at follow-up, the results need to be replicated with a larger sample and improved data retention. Furthermore, the outcomes for this study were restricted to self-report daily sleep diaries and questionnaires. Although self-reported sleep complaints captured by these measures are indeed the information that drive standard clinical diagnosis and treatment, it would be valuable to concurrently collect actigraphic sleep and physiological data in future studies to better understand mechanisms of change. Although “yoga” was not included in the advertising material for this study, prior experience with yoga or related practices was not systematically measured, and, therefore, there may be interactions between prior experience and treatment received in this study that were not able to be identified here.
In conclusion, the findings from this small trial study of an 8-week Kundalini yoga intervention compared to an active SH control support the existing data in the literature that yoga may be a valuable addition to CBT-I in the treatment of insomnia. This study is the first to implement an active comparator against a yoga intervention for primary insomnia, and this area of research would benefit by continued progress in methodological rigor.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital. This study was funded by National Institutes of Health grants (NCCAM/NCCIH 5K01AT000066 and 1R21AT000266 to S.S.K.; NHLBI 5T32HL007901-22 to M.R.G.). S.B.S.K. is a certified teacher of Kundalini yoga as taught by Yogi Bhajan and currently serves as director of research for the Kundalini Research Institute, which serves to educate and promote the practice of Kundalini yoga as taught by Yogi Bhajan. S.B.S.K. receives grant and consultant funding from the Kundalini Research Institute.
ACKNOWLEDGMENTS
The authors acknowledge Yogi Bhajan, a master of Kundalini yoga, who originally taught the techniques employed in this study, and Gurucharan Singh Khalsa for their assistance with and consultation on the yoga intervention. The authors are also grateful to Jack Edinger and John Winkelman, who provided consultation on the study design and data analysis and on the manuscript. Ricardo Grandt and Ian Nagus provided technical assistance.
ABBREVIATIONS
- CBT-I,
cognitive behavioral therapy for insomnia
- ISI,
Insomnia Severity Index
- ISQ,
Insomnia Symptom Questionnaire
- LMM,
linear mixed models
- PSQI,
Pittsburgh Sleep Quality Index
- SE,
sleep efficiency
- SES,
Self-Efficacy for Sleep scale
- SH,
sleep hygiene
- SOL,
sleep onset latency
- TEQ,
Therapy Evaluation Questionnaire
- TST,
total sleep time
REFERENCES
- 1. Roth T , Coulouvrat C , Hajak G , et al . Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders . Biol Psychiatry. 2011. ; 69 ( 6 ): 592 – 600 . [DOI] [PubMed] [Google Scholar]
- 2. Ford ES , Cunningham TJ , Giles WH , Croft JB . Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012 . Sleep Med. 2015. ; 16 ( 3 ): 372 – 378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartescu I , Morgan K . Regular physical activity and insomnia: an international perspective . J Sleep Res. 2019. ; 28 ( 2 ): e12745 . [DOI] [PubMed] [Google Scholar]
- 4. Morin CM , Drake CL , Harvey AG , et al . Insomnia disorder . Nat Rev Dis Primers. 2015. ; 1 ( 1 ): 15026 . [DOI] [PubMed] [Google Scholar]
- 5. Bonnet MH , Burton GG , Arand DL . Physiological and medical findings in insomnia: implications for diagnosis and care . Sleep Med Rev. 2014. ; 18 ( 2 ): 111 – 122 . [DOI] [PubMed] [Google Scholar]
- 6. Kalmbach DA , Buysse DJ , Cheng P , Roth T , Yang A , Drake CL . Nocturnal cognitive arousal is associated with objective sleep disturbance and indicators of physiologic hyperarousal in good sleepers and individuals with insomnia disorder . Sleep Med. 2020. ; 71 : 151 – 160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kudesia RS , Bianchi MT . Decreased nocturnal awakenings in young adults performing bikram yoga: a low-constraint home sleep monitoring study . ISRN Neurol. 2012. ; 2012 : 153745 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowe H , Haddock G , Mulligan LD , et al . Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal . Clin Psychol Rev. 2019. ; 68 ( 68 ): 1 – 12 . [DOI] [PubMed] [Google Scholar]
- 9. Wang X , Li P , Pan C , Dai L , Wu Y , Deng Y . The effect of mind-body therapies on insomnia: A systematic review and meta-analysis . Evid Based Complement Alternat Med. 2019. ; 2019 : 9359807 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morin CM , Culbert JP , Schwartz SM . Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy . Am J Psychiatry. 1994. ; 151 ( 8 ): 1172 – 1180 . [DOI] [PubMed] [Google Scholar]
- 11. Richmond J , Berman BM , Docherty JP , et al. ; NIH Technology Assessment Panel on Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia . Integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia . JAMA. 1996. ; 276 ( 4 ): 313 – 318 . [DOI] [PubMed] [Google Scholar]
- 12. Rusch HL , Rosario M , Levison LM , et al . The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials . Ann N Y Acad Sci. 2019. ; 1445 ( 1 ): 5 – 16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siebern AT , Manber R . New developments in cognitive behavioral therapy as the first-line treatment of insomnia . Psychol Res Behav Manag. 2011. ; 4 : 21 – 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khalsa S , Cohen L , McCall T , Telles S . Principles and Practice of Yoga in Health Care. Handspring Publishing Limited; : Pencaitland, UK: ; 2016. . https://www.handspringpublishing.com/product/principles-practice-yoga-health-care/ . Accessed on June 29, 2020 [Google Scholar]
- 15. Cramer H , Lauche R , Dobos G . Characteristics of randomized controlled trials of yoga: a bibliometric analysis . BMC Complement Altern Med. 2014. ; 14 ( 1 ): 328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephens I . Medical yoga therapy . Children (Basel). 2017. ; 4 ( 2 ): 12 . [Google Scholar]
- 17. Wang F , Szabo A . Effects of yoga on stress among healthy adults: a systematic review . Altern Ther Health Med. 2020. ; 26 ( 4 ): AT6214 . [PubMed] [Google Scholar]
- 18. Pascoe MC , Thompson DR , Ski CF . Yoga, mindfulness-based stress reduction and stress-related physiological measures: aA meta-analysis . Psychoneuroendocrinology. 2017. ; 86 : 152 – 168 . [DOI] [PubMed] [Google Scholar]
- 19. Ross A , Friedmann E , Bevans M , Thomas S . National survey of yoga practitioners: mental and physical health benefits . Complement Ther Med. 2013. ; 21 ( 4 ): 313 – 323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vera FM , Manzaneque JM , Maldonado EF , et al . Subjective sleep quality and hormonal modulation in long-term yoga practitioners . Biol Psychol. 2009. ; 81 ( 3 ): 164 – 168 . [DOI] [PubMed] [Google Scholar]
- 21. Manjunath NK , Telles S . Influence of yoga and Ayurveda on self-rated sleep in a geriatric population . Indian J Med Res. 2005. ; 121 ( 5 ): 683 – 690 . [PubMed] [Google Scholar]
- 22. Hariprasad VR , Sivakumar PT , Koparde V , et al . Effects of yoga intervention on sleep and quality-of-life in elderly: a randomized controlled trial . Indian J Psychiatry. 2013. ; 55 , 7, Suppl 3 : S364 – S368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang R , Li X . A regular yoga intervention for staff nurse sleep quality and work stress: a randomised controlled trial . J Clin Nurs. 2015. ; 24 ( 23-24 ): 3374 – 3379 . [DOI] [PubMed] [Google Scholar]
- 24. Clarke TC , Barnes PM , Black LI , Stussman BJ , Nahin RL . Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over . NCHS Data Brief. 2018. ; 325 : 1 – 8 . [PubMed] [Google Scholar]
- 25. Voiß P , Höxtermann MD , Dobos G , Cramer H . The use of mind-body medicine among US individuals with sleep problems: analysis of the 2017 National Health Interview Survey data . Sleep Med. 2019. ; 56 : 151 – 156 . [DOI] [PubMed] [Google Scholar]
- 26. Kozasa EH , Hachul H , Monson C , et al . Mind-body interventions for the treatment of insomnia: a review . Br J Psychiatry. 2010. ; 32 ( 4 ): 437 – 443 . [DOI] [PubMed] [Google Scholar]
- 27. Zhou ES , Gardiner P , Bertisch SM . Integrative medicine for insomnia . Med Clin North Am. 2017. ; 101 ( 5 ): 865 – 879 . [DOI] [PubMed] [Google Scholar]
- 28. Carr-Kaffashan L , Woolfolk RL . Active and placebo effects in treatment of moderate and severe insomnia . J Consult Clin Psychol. 1979. ; 47 ( 6 ): 1072 – 1080 . [DOI] [PubMed] [Google Scholar]
- 29. Gong H , Ni C-X , Liu Y-Z , et al . Mindfulness meditation for insomnia: a meta-analysis of randomized controlled trials . J Psychosom Res. 2016. ; 89 : 1 – 6 . [DOI] [PubMed] [Google Scholar]
- 30. Ong JC , Moore C . What do we really know about mindfulness and sleep health? Curr Opin Psychol. 2020. ; 34 : 18 – 22 . [DOI] [PubMed] [Google Scholar]
- 31. Jacobs GD , Benson H , Friedman R . Perceived benefits in a behavioral-medicine insomnia program: a clinical report . Am J Med. 1996. ; 100 ( 2 ): 212 – 216 . [DOI] [PubMed] [Google Scholar]
- 32. Chóliz M . A breathing-retraining procedure in treatment of sleep-onset insomnia: theoretical basis and experimental findings . Percept Mot Skills. 1995. ; 80 ( 2 ): 507 – 513 . [DOI] [PubMed] [Google Scholar]
- 33. Tsai HJ , Kuo TBJ , Lee GS , Yang CCH . Efficacy of paced breathing for insomnia: enhances vagal activity and improves sleep quality . Psychophysiology. 2015. ; 52 ( 3 ): 388 – 396 . [DOI] [PubMed] [Google Scholar]
- 34. Khalsa SBS . Treatment of chronic insomnia with yoga: a preliminary study with sleep-wake diaries . Appl Psychophysiol Biofeedback. 2004. ; 29 ( 4 ): 269 – 278 . [DOI] [PubMed] [Google Scholar]
- 35. Joshi KS . Yogic treatment of insomnia: an experimental study . Yoga Mimamsa. 1992. ; 30 ( 4 ): 24 – 26 . [Google Scholar]
- 36. Halpern J , Cohen M , Kennedy G , Reece J , Cahan C , Baharav A . Yoga for improving sleep quality and quality of life for older adults . Altern Ther Health Med. 2014. ; 20 ( 3 ): 37 – 46 . [PubMed] [Google Scholar]
- 37. Sobana R , Parthasarathy S , Duraisamy, Jaiganesh K , Vadivel S . The effect of yoga therapy on selected psychological variables among male patients with insomnia . J Clin Diagn Res. 2013. ; 7 ( 1 ): 55 – 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Afonso RF , Hachul H , Kozasa EH , et al . Yoga decreases insomnia in postmenopausal women: a randomized clinical trial . Menopause. 2012. ; 19 ( 2 ): 186 – 193 . [DOI] [PubMed] [Google Scholar]
- 39. Wang WL , Chen KH , Pan YC , Yang SN , Chan YY . The effect of yoga on sleep quality and insomnia in women with sleep problems: a systematic review and meta-analysis . BMC Psychiatry. 2020. ; 20 ( 1 ): 195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baglioni C , Bostanova Z , Bacaro V , et al . A systematic review and network meta-analysis of randomized controlled trials evaluating the evidence base of melatonin, light exposure, exercise, and complementary and alternative medicine for patients with insomnia disorder . J Clin Med. 2020. ; 9 ( 6 ): 1949 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Espie CA . “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment . Sleep. 2009. ; 32 ( 12 ): 1549 – 1558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Straten A , van der Zweerde T , Kleiboer A , Cuijpers P , Morin CM , Lancee J . Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis . Sleep Med Rev. 2018. ; 38 : 3 – 16 . [DOI] [PubMed] [Google Scholar]
- 43. van der Zweerde T , Bisdounis L , Kyle SD , Lancee J , van Straten A . Cognitive behavioral therapy for insomnia: a meta-analysis of long-term effects in controlled studies . Sleep Med Rev. 2019. ; 48 : 101208 . [DOI] [PubMed] [Google Scholar]
- 44. Zachariae R , Lyby MS , Ritterband LM , O’Toole MS . Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials . Sleep Med Rev. 2016. ; 30 : 1 – 10 . [DOI] [PubMed] [Google Scholar]
- 45. Seyffert M , Lagisetty P , Landgraf J , et al . Internet-delivered cognitive behavioral therapy to treat insomnia: A systematic review and meta-analysis . PLoS One. 2016. ; 11 ( 2 ): e0149139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ho FYY , Chung KF , Yeung WF , et al . Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials . Sleep Med Rev. 2015. ; 19 : 17 – 28 . [DOI] [PubMed] [Google Scholar]
- 47. Geiger-Brown JM , Rogers VE , Liu W , Ludeman EM , Downton KD , Diaz-Abad M . Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis . Sleep Med Rev. 2015. ; 23 : 54 – 67 . [DOI] [PubMed] [Google Scholar]
- 48. Trauer JM , Qian MY , Doyle JS , Rajaratnam SMW , Cunnington D . Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis . Ann Intern Med. 2015. ; 163 ( 3 ): 191 – 204 . [DOI] [PubMed] [Google Scholar]
- 49. Wu JQ , Appleman ER , Salazar RD , Ong JC . Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions a meta-analysis . JAMA Intern Med. 2015. ; 175 ( 9 ): 1461 – 1472 . [DOI] [PubMed] [Google Scholar]
- 50. Edinger JD , Olsen MK , Stechuchak KM , et al . Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial . Sleep. 2009. ; 32 ( 4 ): 499 – 510 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edinger JD , Sampson WS . A primary care “friendly” cognitive behavioral insomnia therapy . Sleep. 2003. ; 26 ( 2 ): 177 – 182 . [DOI] [PubMed] [Google Scholar]
- 52. Bastien CH , Vallières A , Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research . Sleep Med. 2001. ; 2 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 53. Okun ML , Kravitz HM , Sowers MF , Moul DE , Buysse DJ , Hall M . Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia . J Clin Sleep Med. 2009. ; 5 ( 1 ): 41 – 51 . [PMC free article] [PubMed] [Google Scholar]
- 54. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 55. Lacks P . Behavioral Treatment for Persistent Insomnia. Pergamon Press; : New York: ; 1987. . [Google Scholar]
- 56. Nicassio PM , Mendlowitz DR , Fussell JJ , Petras L . The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale . Behav Res Ther. 1985. ; 23 ( 3 ): 263 – 271 . [DOI] [PubMed] [Google Scholar]
- 57. Edinger JD , Wohlgemuth WK , Radtke RA , Coffman CJ , Carney CE . Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial . Sleep. 2007. ; 30 ( 2 ): 203 – 212 . [DOI] [PubMed] [Google Scholar]
- 58. Lichstein KL , Durrence HH , Taylor DJ , Bush AJ , Riedel BW . Quantitative criteria for insomnia . Behav Res Ther. 2003. ; 41 ( 4 ): 427 – 445 . [DOI] [PubMed] [Google Scholar]
- 59. Bathgate CJ , Edinger JD , Krystal AD . Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for Insomnia . Sleep. 2017. ; 40 ( 1 ): zsw012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Desjardins S , Lapierre S , Hudon C , Desgagné A . Factors involved in sleep efficiency: a population-based study of community-dwelling elderly persons . Sleep. 2019. ; 42 ( 5 ): 1 – 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ong JC , Manber R , Segal Z , Xia Y , Shapiro S , Wyatt JK . A randomized controlled trial of mindfulness meditation for chronic insomnia . Sleep. 2014. ; 37 ( 9 ): 1553 – 1563 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maurer LF , Espie CA , Omlin X , et al . Isolating the role of time in bed restriction in the treatment of insomnia: a randomized, controlled, dismantling trial comparing sleep restriction therapy with time in bed regularization . Sleep. 2020. ; 43 ( 11 ): zsaa096 . [DOI] [PubMed] [Google Scholar]
- 63. Ong JC . Mindfulness-Based Therapy for Insomnia. Washington, DC: : American Psychological Association; ; 2017. . [Google Scholar]
- 64. Schmalzl L , Crane-Godreau MA , Payne P . Movement-based embodied contemplative practices: definitions and paradigms . Front Hum Neurosci. 2014. ; 8 ( 1 Apr ): 1 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goldstein MR , Turner AD , Dawson SC , et al . Increased high-frequency NREM EEG power associated with mindfulness-based interventions for chronic insomnia: Preliminary findings from spectral analysis . J Psychosom Res. 2019. ; 120 : 12 – 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riemann D , Spiegelhalder K , Feige B , et al . The hyperarousal model of insomnia: a review of the concept and its evidence . Sleep Med Rev. 2010. ; 14 ( 1 ): 19 – 31 . [DOI] [PubMed] [Google Scholar]
- 67. Bonnet MH , Arand DL . Hyperarousal and insomnia: state of the science . Sleep Med Rev. 2010. ; 14 ( 1 ): 9 – 15 . [DOI] [PubMed] [Google Scholar]
- 68. Britton WB , Lindahl JR , Cahn BR , Davis JH , Goldman RE . Awakening is not a metaphor: the effects of Buddhist meditation practices on basic wakefulness . Ann N Y Acad Sci. 2014. ; 1307 ( 1 ): 64 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Irwin MR , Olmstead R , Carrillo C , et al . Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial . Sleep. 2014. ; 37 ( 9 ): 1543 – 1552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Irwin MR , Olmstead R , Breen EC , et al . Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial . Biol Psychiatry. 2015. ; 78 ( 10 ): 721 – 729 . [DOI] [PMC free article] [PubMed] [Google Scholar]