Abstract
This review’s objective was to synthesize the literature on the repercussions of obstructive sleep apnea (OSA) in the retinal vascular system. Two independent investigators conducted a search using the MEDLINE/PubMed database using the following terms: sleep apnea syndrome, obstructive sleep apnea, retina, vascular tortuosity, central serous chorioretinopathy, diabetes mellitus, and subfoveal choroidal thickness. Patients with OSA present increased vascular tortuosity compared with patients without OSA, decreased parafoveal and peripapillary vessel density, and increased retinal vein occlusion incidence. In central serous chorioretinopathy patients and patients who are poor responders to intravitreal anti-VEGF (-vascular endothelial growth factor) treatment for macular edema, OSA is more frequent. Macular choroidal thickness alterations are controversial, and OSA may worsen diabetic maculopathy, thus being a risk factor for diabetic retinopathy, proliferative diabetic retinopathy, and macular edema. OSA is a prevalent syndrome with many systemic vascular changes. The retina and choroid are the most affected ocular structures, with primarily vascular changes. New noninvasive technologies such as optical coherence tomography and optical coherence tomography angiography could help to better understand retinal structures and help clarify the ophthalmological repercussions of OSA.
Citation:
Nakayama LF, Tempaku PF, Bergamo VC, et al. Obstructive sleep apnea and the retina: a review. J Clin Sleep Med. 2021;17(9):1947–1952.
Keywords: retina, sleep apnea syndrome, optical coherence tomography
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic sleep-related breathing disorder characterized by recurrent partial or complete cessation of airflow due to upper airway obstruction during sleep that results in sleep fragmentation, intermittent hypoxia, and hypercapnia leading to increased sympathetic nervous system activity.1 OSA is underdiagnosed; however, it has a prevalence reaching 32.8% of the adult population in São Paulo, Brazil, considering all OSA severities2.
Apnea-hypopnea index (AHI), respiratory events by sleep hour, determines OSA severity as mild when more than 5 events and less than 15 events, moderate when more than 15 events and less than 30 episodes, and severe when with more than 30 events/h.
Night-to-night AHI variability does not have a definitive explanation but is reported in polysomnography measurements and should be considered in treatment decisions.3,4
There are many features in the pathophysiology of OSA and include an anatomically compromised or collapsible upper airway, inadequate responsiveness of the upper airway dilator muscles during sleep, a low respiratory arousal threshold, and an oversensitive ventilatory control system (high loop gain).5 Patients with OSA may exhibit loud and chronic snoring, gasping episodes during sleep, sleepiness, obesity, and increased neck circumference.6 OSA is associated with physical examination alterations and systemic complaints, including daytime fatigue and impaired concentration.7–9 OSA is also an independent risk factor for arterial hypertension, stroke, ischemic heart disease, cardiac arrhythmia, and heart failure.1 OSA is associated with diabetes-related complications mediated by systemic hypertension secondary to intermittent hypoxemia, leading to systemic vasoconstriction and increased oxidative stress.10 Obesity is one of the most critical OSA covariates, with an increased risk of OSA in obese patients and increased weight gain in patients with OSA.11 Advanced age, alcohol use, smoking, and use of sedatives are also risk factors.9
Differential diagnoses of OSA include central sleep apnea, characterized by a lack of respiratory drive during sleep without respiratory effort during apnea, leading to insufficient ventilation and compromised gas exchange.12 Obesity hypoventilation syndrome occurs in obese patients with daytime hypercapnia and sleep breathing alterations without a known cause of hypercapnia and should also be considered an exclusionary diagnosis.13
Some known ophthalmological findings associated with OSA include palpebral laxity, dry eye, and posterior segment findings.14 The retinal and choroidal vasculature originate from the inner carotid arteries, and they represent a terminal microvascular system. OSA and vascular diseases such as diabetes mellitus and systemic arterial hypertension may lead to significant and permanent retinal anatomy changes.
Techniques for studying retinal structures have been developed in recent years, including optical coherence tomography (OCT) and OCT angiography (OCT-A), both of which enable rapid in vivo histological analysis of retinal and vascular structures without requiring intravenous contrast15,16. Studies of the retinal anatomy and vasculature details might provide new insights into retinal involvement in OSA.
This study’s objective was to review the literature on the sequelae of OSA related to the retinal vascular system.
METHODS
São Paulo Federal University (UNIFESP) Ethics Institutional Review Board number: 83653718.4.000.5505–0189/2018.
Literature search
For this comprehensive review, a search was conducted by 2 independent investigators using the MEDLINE/PubMed database for articles up to December 2019 using the following terms: sleep apnea syndrome, obstructive sleep apnea, retina, vascular tortuosity, central serous chorioretinopathy, diabetes mellitus, and subfoveal choroidal thickness. We gathered articles referring to ophthalmological retinal structures in human adults. No language or date restrictions were imposed. Reference lists of primary reports and review articles were also searched. We excluded articles about glaucoma and optic neuritis, duplicate articles, articles with no abstract available, responses to other articles, and case reports. Based on the inclusion and exclusion criteria, 24 articles were selected for this review and were read entirely by the researchers. No contact was made with the authors of the included articles.
RESULTS
Vascular morphological changes
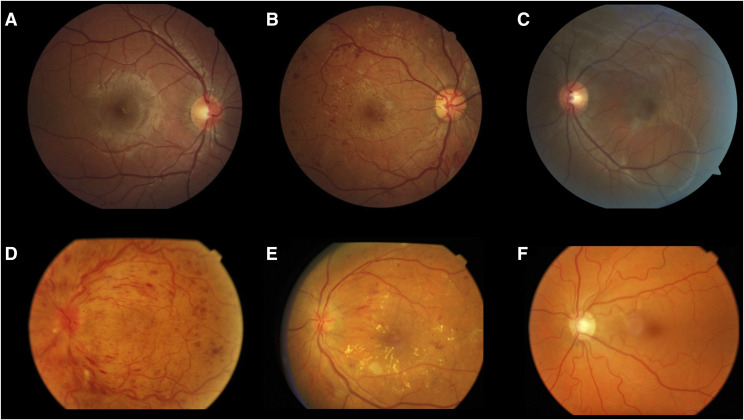
Pathological increased retinal vascular tortuosity may occur in several conditions, including diabetes mellitus, inflammatory bowel disease, hypoxia, older age, hypertension, and elevated body mass index (Figure 1).17 Because OSA is a systemic disease that affects the vascular system, pathological vascular changes are expected. Mohsenin et al17 reported significantly increased vascular tortuosity in OSA by comparing 9 patients with OSA with 7 controls without OSA. In OSA hypoxemia and hypercapnia occur secondary to breathing cessation, leading to hyperlactatemia, lactic acidosis, and subsequent decrease in arterial oxygen and increased arterial carbon dioxide levels.13
Figure 1. Retinal images.
(A) Normal retina: normal optic disc, vessels, macula, and attached retina. (B) Diabetic retinopathy: proliferative diabetic retinopathy with retinal hemorrhages and neovessels. (C) Central serous choroidopathy: an area of circular retinal detachment in inferior retinal arcades. (D) Central vein occlusion: retinal hemorrhages in all quadrants and blurred optic disc. (E) Nonproliferative diabetic retinopathy and macular edema: retinal hemorrhages and hard exudates in the macular area. (F) Increased vascular tortuosity: diffusely increased venular and arteriolar tortuosity.
The authors proposed that multifactorial alterations to the retinal vascular pattern were attributable to endothelial dysfunction, intermittent arterial blood pressure surges, increased venular pressure, increased intracranial pressure, and disrupted cerebral autoregulation.
This increased vascular tortuosity retinal repercussions remain unclear, with a possible association with occlusive and thromboembolic events. More studies are necessary to determine the exact pathophysiological mechanism linking OSA severity with increased retinal vascular tortuosity.
Fraser et al18 evaluated 127 patients with OSA and 88 controls in 2013. They found a significantly greater prevalence of arteriolar changes similar to those seen in mild hypertensive retinopathy in patients with OSA. Vascular endothelial dysfunction leading to arteriolar changes was proposed as the mechanism. Wang et al19 evaluated 133 patients and reported an increased arteriole-to-venule ratio in patients with severe OSA compared with patients with less severe OSA. The proposed mechanism is hyperactivity of the sympathetic catecholamine system, hypersecretion of catecholamine, and high plasma catecholamine concentrations.
Decreased vessel density
OCT-A is a noninvasive dyeless exam that evaluates retinal layers and vessels using light dispersion. The vascular plexus map can be established from the difference between static (retina tissues) and dynamic (vessels) structures.15 Yu et al20 used OCT-A to evaluate retinal vascular density in 69 patients with OSA; they found that parafoveal and peripapillary vessel density decreased with increasing OSA severity (as assessed by the AHI and Saturation of Peripheral Oxygen [SpO2]), and these changes were most prominent in the peripapillary area due to the vessel caliber and vascular origin.20 The authors concluded that OCT-A is a useful and reliable noninvasive tool for monitoring retinal vessels in patients with OSA. They also proposed that the decrease in retinal vascular density in patients with OSA results from intermittent hypoxemia and fluctuations in blood flow, which leads to oxidative stress, inflammation, endothelial dysfunction, atherosclerosis, and subsequent reduction in vessel diameter.
Retinal vein occlusion
Retinal vein occlusion (RVO) is the second most frequent retinal vascular disorder and a common cause of blindness (Figure 1).21 Systemic diseases such as arterial hypertension, peptic ulcer disease, cerebrovascular disease, chronic pulmonary hypertension, diabetes, hyperlipidemia, smoking, and thyroid diseases have been associated with RVO, and OSA has also been recently associated with RVO.21,22 Agard et al21 reported a high prevalence of OSA in patients with RVO after a prospective evaluation of 114 patients with RVO and controls matched for age, sex, and disease. The authors concluded that OSA is an independent risk factor for RVO.
Wang et al23 evaluated 30 patients with central RVO and 30 controls with no systemic disease. They found that AHI and oxygen desaturation index scores were significantly higher in patients with central RVO, and they speculated that OSA was a risk factor for central RVO. In patients with OSA, the authors speculated that RVO is due to repetitive and prolonged periods of apnea during sleep, leading to transient hypoxia, sympathetic overdrive, and decreased oxygen saturation. The increased hypercoagulable state, elevated lipoprotein levels, increased arterial blood pressure, and increased intracranial pressure, leading to increased vascular resistance and elevated pressure in the optic nerve head.22–25
Macular choroidal thickness
The choroid is the most vascularized system of the body, with different vessel calibers, and is responsible for ocular metabolism control. Systemic oxygen levels control oxygen levels in the choroidal layers, and the subfoveal choroidal thickness may change in inflammatory diseases.26 The influence of systemic oxygen and carbon dioxide levels in the choroidal circulation is not fully understood. The proposed pathophysiology is increased retinal vessel diameter and vascular blood flow induced by hypoxia and hypercapnia.27
After evaluating 74 patients with OSA and 33 controls, Karaca et al27 concluded that the subfoveal choroidal thickness was not significantly different between patients with severe OSA and mild and moderate OSA. Similarly, Yuvacı et al28 found no significant difference in subfoveal choroidal thickness between 54 patients with OSA and 18 controls.
In contrast, Xin et al29 evaluated 53 eyes of patients with OSA and 12 eyes of 12 controls without OSA and found a significantly thinner choroid in patients with severe OSA. The authors proposed that alternating hypoxia and arousal from sleep and daytime sleepiness may stimulate the sympathetic nervous system, leading to downstream effects and structural changes in choroidal blood vessels. Ozge et al30 evaluated 84 eyes of 42 patients with OSA and 112 eyes of 56 controls and found a significantly thicker choroid at 0.5 to 1.5 mm from the fovea in patients with OSA compared with the control group; also, the choroidal thickness was negatively correlated with AHI in patients with OSA. The proposed pathophysiology is that hypoxemia leads to increased intracranial pressure and, in turn, increased choroidal thickness.
Central serous chorioretinopathy
Central serous chorioretinopathy (CSC) is an idiopathic choroidal disease that presents as a serous neurosensory retina and retinal pigmented epithelium detachment (Figure 1). Its proposed pathophysiological mechanism is increased choroidal permeability, increased choroid hydrostatic pressure, and retinal pigmented epithelium breakdown due to increased serum cortisol and catecholamine levels.31,32 According to these authors, the pathophysiology in patients with OSA is similar to that of patients with CSC; both features increased sympathetic activity and increase cortisol levels due to disruption of the hormone regulatory response and activation of the hypothalamic-pituitary-adrenal axis, which may lead to vascular endothelial dysfunction.
Known risk factors for CSC include male sex, smoking, hyperopia, pregnancy, systemic arterial hypertension, corticosteroid use, and type A personality. OSA is also considered a risk factor for CSC.32,33 Brodie et al31 evaluated 48 patients with OSA and found no significant difference in CSC risk compared with controls. In contrast, a meta-analysis by Wu et al32 concluded that patients with CSC are more likely to have OSA based on 7,238 patients from 6 studies; however, OSA severities were not distinguished.
Macular edema
Increased macular thickness may occur in diabetes, age-related macular disease, and other neovascular retinal pathologies.34 OSA has been studied as a factor influencing the response to anti–vascular endothelial growth factor (-VEGF) treatment in patients with macular edema. Intermittent hypoxia and hypercapnia lead to increased VEGF.35 These authors evaluated 103 patients with age-related macular disease and 100 controls using the Berlin OSA risk questionnaire and found that poor responders to intravitreal anti-VEGF had a significantly higher risk for OSA. Schaal et al36 prospectively evaluated 38 patients with exudative age-related macular disease. They compared the bevacizumab response in groups with and without continuous positive air pressure (CPAP) treatment, the gold-standard treatment for OSA.36 The authors concluded that untreated OSA might underlie nonresponsiveness to anti-VEGF therapy. CPAP treatment may decrease the macular volume and amount of subretinal fluid, reducing the required number of intraocular injections. The proposed mechanism is dysregulation of fluid balance, leading to an increase in intravascular hydrostatic pressure due to the renin-angiotensin-aldosterone system’s alteration.
Diabetic retinopathy
Diabetic retinopathy (DR) is a microvascular complication of diabetes mellitus and the leading cause of blindness in adults worldwide. OSA is considered a risk factor for DR (Figure 1).37 OSA may worsen diabetic maculopathy by increasing inflammatory cytokine production due to repeated nocturnal desaturation episodes. OSA also increases oxidative stress and advanced glycation product levels.38 Based on an evaluation of 99 patients, these authors found that the mean AHI was significantly higher in patients with diabetes and macular edema than in patients with diabetes without macular edema. Chang et al37 reviewed 6 studies that included a total of 317 patients stratified according to DR severity and OSA severity and found associations between severe OSA and the presence and severity of DR.37 They reported a 2- to 3-fold increased risk of DR, proliferative DR, and macular edema in patients with mild–moderate OSA. West et al38 performed a randomized controlled trial of 131 patients. They found that CPAP treatment should be administered to the OSA population but should not be used as an alternative treatment for diabetic macular edema and did not identify improvement in visual acuity and retinal photographs. Smith et al39 retrospectively compared DR prevalence in patients treated with CPAP and concluded that DR was significantly less prevalent in the CPAP-compliant group than in the noncompliant group. Nishimura et al40 evaluated 131 patients and found that AHI during rapid eye movement sleep was independently and significantly associated with DR.
Limitations
Although OSA may lead to many vascular changes, most studies only evaluated patients cross-sectionally. Few prospective studies and clinical trials have assessed retinal changes and improvement after clinical treatment.
DISCUSSION
OSA is an underdiagnosed and underestimated syndrome with a high frequency in the adult population.2 OSA may have multiple systemic repercussions—significantly, vascular changes.6–9 OSA may also increase inflammatory status with numerous systemic vascular changes, such as increased carotid artery intima-media thickness, increased systemic arterial pressure, and endothelial damage.41
The retina is a terminal microvascular system with well-established anatomical alterations in systemic vascular diseases such as diabetes and systemic arterial hypertension. New noninvasive retinal exams like OCT and OCT-A better to help understand retinal and choroidal apnea modifications, including changes in retinal anatomy or vasculature. As a microvascular system, damage to the retina may correlate with OSA severity. A better understanding of the pathophysiology and ophthalmic consequences of OSA could enable better treatments and prevention of microvascular and endothelial damage.
Vascular changes in patients with OSA reported in the literature include vascular tortuosity, decreased vascular density, and venular occlusion risk. Choroidal thickness variation remains controversial; some groups report no change in patients with OSA, while others report thinning or even increased thickness. Other unidentified variables appear to underlie choroidal involvement and more studies are warranted to elucidate this concern.
OSA is a risk factor for CSC. It is more common among patients with central serous choroidopathy; however, previous studies have not distinguished OSA severity. More studies are needed to elucidate the exact correlation between OSA and CSC.
OSA is more prevalent among poor responders to intravitreal anti-VEGF and is considered a risk factor for macular edema and diabetic retinopathy. The importance of OSA should not be overlooked in patients undergoing treatment for DR or age-related macular disease.
Ciccone et al41 reported improved endothelial vascular function after CPAP treatment based on assessments using flow-mediated vasodilation. Nevertheless, few studies have evaluated retinal parameters after CPAP treatment, and studies with a greater number of patients of differing OSA severity are needed.
CPAP treatment decreases serum lactate through improving nocturnal hypoxia and subsequent increase in pH and mean SpO2%.42
Big-data and artificial intelligence are changing how medical care is driven. In ophthalmology, multimodal exams such as retinal images are applied mainly in diagnosis and follow-up of retinal diseases.43 There are available public datasets of retinal exams such as EYEPACS with 88,702 images44 and Messidor 1 and 245 with 1784 images, but the main focus of datasets is DR and classifications of normal or abnormal images. So far, there isn’t a publicly available ophthalmological dataset of patients with OSA.
In summary, OSA is a prevalent syndrome with many systemic vascular changes. The retina and choroid are the most affected ocular structures, with primarily vascular changes. New noninvasive technologies such as OCT and OCT-A could better help understand retinal structures and might help clarify the ophthalmological repercussions of OSA.
DISCLOSURE STATEMENT
All authors contributed to article writing and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI,
apnea-hypopnea index
- CPAP,
continuous positive airway pressure
- CSC,
central serous choroidopathy
- DR,
diabetic retinopathy
- OCT,
optical coherence tomography
- OCT-A,
optical coherence tomography angiography
- OSA,
obstructive sleep apnea
- RVO,
retinal vein occlusion
- VEGF,
vascular endothelial growth factor
REFERENCES
- 1. Kohler M , Stradling JR . Mechanisms of vascular damage in obstructive sleep apnea . Nat Rev Cardiol. 2010. ; 7 ( 12 ): 677 – 685 . [DOI] [PubMed] [Google Scholar]
- 2. Tufik S , Santos-Silva R , Taddei JA , Bittencourt LRA . Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study . Sleep Med. 2010. ; 11 ( 5 ): 441 – 446 . [DOI] [PubMed] [Google Scholar]
- 3. Anitua E , Duran-Cantolla J , Almeida GZ , Alkhraisat MH . Predicting the night-to-night variability in the severity of obstructive sleep apnea: the case of the standard error of measurement . Sleep Sci. 2019. ; 12 ( 2 ): 72 – 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bittencourt LR , Suchecki D , Tufik S , et al . The variability of the apnoea-hypopnoea index . J Sleep Res. 2001. ; 10 ( 3 ): 245 – 251 . [DOI] [PubMed] [Google Scholar]
- 5. Eckert DJ , White DP , Jordan AS , Malhotra A , Wellman A . Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets . Am J Respir Crit Care Med. 2013. ; 188 ( 8 ): 996 – 1004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold J , Sunilkumar M , Krishna V , Yoganand SP , Kumar MS , Shanmugapriyan D . Obstructive sleep apnea . J Pharm Bioallied Sci. 2017. ; 9 ( 5, Suppl 1 ): S26 – S28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quan SF , Gillin JC , Littner MR . Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force . Sleep. 1999. ; 22 ( 5 ): 667 – 689 . [PubMed] [Google Scholar]
- 8. Bittencourt LRA and Caixeta EC . Critérios diagnósticos e tratamento dos distúrbios respiratórios do sono: SAOS . J Bras Pneumol. 2010. ; 36 ( suppl 2 ): 23 – 27 . [DOI] [PubMed] [Google Scholar]
- 9. Slowik JM , Collen JF . Obstructive Sleep Apnea. In: StatPearls. Treasure Island, FL: StatPearls Publishing; ; 2021. . [PubMed] [Google Scholar]
- 10. Siwasaranond N , Nimitphong H , Manodpitipong A , et al . The relationship between diabetes-related complications and obstructive sleep apnea in type 2 diabetes . J Diabetes Res. 2018. ; 2018 : 9269170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolk R , Shamsuzzaman ASM , Somers VK . Obesity, sleep apnea, and hypertension . Hypertension. 2003. ; 42 ( 6 ): 1067 – 1074 . [DOI] [PubMed] [Google Scholar]
- 12. Eckert DJ , Jordan AS , Merchia P , Malhotra A . Central sleep apnea: pathophysiology and treatment . Chest. 2007. ; 131 ( 2 ): 595 – 607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu C , Chen M-S , Yu H . The relationship between obstructive sleep apnea and obesity hypoventilation syndrome: a systematic review and meta-analysis . Oncotarget. 2017. ; 8 ( 54 ): 93168 – 93178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karger RA , White WA , Park W-C , et al. Prevalence of floppy eyelid syndrome in obstructive sleep apnea-hypopnea syndrome . Ophthalmology. 2006. ; 113 ( 9 ): 1669 – 1674 . [DOI] [PubMed] [Google Scholar]
- 15. Kashani AH , Chen C-L , Gahm JK , et al . Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications . Prog Retin Eye Res. 2017. ; 60 : 66 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maram J , Srinivas S , Sadda SR . Evaluating ocular blood flow . Indian J Ophthalmol. 2017. ; 65 ( 5 ): 337 – 346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohsenin A , Mohsenin V , Adelman RA . Retinal vascular tortuosity in obstructive sleep apnea . Clin Ophthalmol. 2013. ; 7 : 787 – 792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fraser CL , Bliwise DL , Newman NJ , et al . A prospective photographic study of the ocular fundus in obstructive sleep apnea . J Neuroophthalmol. 2013. ; 33 ( 3 ): 241 – 246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X-Y , Wang S , Liu X , Ding X , Li M , Han D-M . Retinal vascular morphological changes in patients with extremely severe obstructive sleep apnea syndrome . Chin Med J (Engl). 2017. ; 130 ( 7 ): 805 – 810 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu J , Xiao K , Huang J , Sun X , Jiang C . Reduced retinal vessel density in obstructive sleep apnea syndrome patients: an optical coherence tomography angiography study . Invest Ophthalmol Vis Sci. 2017. ; 58 ( 9 ): 3506 – 3512 . [DOI] [PubMed] [Google Scholar]
- 21. Agard E , El Chehab H , Vie A-L , Voirin N , Coste O , Dot C . Retinal vein occlusion and obstructive sleep apnea: a series of 114 patients . Acta Ophthalmol. 2018. ; 96 ( 8 ): e919 – e925 . [DOI] [PubMed] [Google Scholar]
- 22. Kwon HJ , Kang EC , Lee J , Han J , Song WK . Obstructive sleep apnea in patients with branch retinal vein occlusion: a preliminary study . Korean J Ophthalmol. 2016. ; 30 ( 2 ): 121 – 126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y-H , Zhang P , Chen L , et al . Correlation between obstructive sleep apnea and central retinal vein occlusion . Int J Ophthalmol. 2019. ; 12 ( 10 ): 1634 – 1636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glacet-Bernard A , Leroux les Jardins G , Lasry S , et al . Obstructive sleep apnea among patients with retinal vein occlusion . Arch Ophthalmol. 2010. ; 128 ( 12 ): 1533 – 1538 . [DOI] [PubMed] [Google Scholar]
- 25. Chou K-T , Huang C-C , Tsai D-C , et al . Sleep apnea and risk of retinal vein occlusion: a nationwide population-based study of Taiwanese . Am J Ophthalmol. 2012. ; 154 ( 1 ): 200 – 205.e1 . [DOI] [PubMed] [Google Scholar]
- 26. Karahan E , Zengin MO , Aydin R , et al . Correlation of choroidal thickness with serum cortisol level . Clin Exp Optom. 2015. ; 98 ( 4 ): 362 – 365 . [DOI] [PubMed] [Google Scholar]
- 27. Karaca EE , Ekici F , Yalçın NG , Çiftçi TU , Özdek Ş . Macular choroidal thickness measurements in patients with obstructive sleep apnea syndrome . Sleep Breath. 2015. ; 19 ( 1 ): 335 – 341 . [DOI] [PubMed] [Google Scholar]
- 28. Yuvacı İ , Pangal E , Bayram N , et al . Evaluation of posterior ocular changes using enhanced depth imaging-optical coherence tomography in patients with obstructive sleep apnea syndrome . Arq Bras Oftalmol. 2016. ; 79 ( 4 ): 247 – 252 . [DOI] [PubMed] [Google Scholar]
- 29. Xin C , Wang J , Zhang W , Wang L , Peng X . Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS) . Eye (Lond). 2014. ; 28 ( 4 ): 415 – 421 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ozge G , Dogan D , Koylu MT , et al . Retina nerve fiber layer and choroidal thickness changes in obstructive sleep apnea syndrome . Postgrad Med. 2016. ; 128 ( 3 ): 317 – 322 . [DOI] [PubMed] [Google Scholar]
- 31. Brodie FL , Charlson ES , Aleman TS , et al . Obstructive sleep apnea and central serous chorioretinopathy . Retina. 2015. ; 35 ( 2 ): 238 – 243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu CY , Riangwiwat T , Rattanawong P , Nesmith BLW , Deobhakta A . Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: a systematic review and meta-analysis . Retina. 2018. ; 38 ( 9 ): 1642 – 1651 . [DOI] [PubMed] [Google Scholar]
- 33. Chatziralli I , Kabanarou SA , Parikakis E , Chatzirallis A , Xirou T , Mitropoulos P . Risk factors for central serous chorioretinopathy: multivariate approach in a case-control study . Curr Eye Res. 2017. ; 42 ( 7 ): 1069 – 1073 . [DOI] [PubMed] [Google Scholar]
- 34. Mason RH , Kiire CA , Groves DC , et al . Visual improvement following continuous positive airway pressure therapy in diabetic subjects with clinically significant macular oedema and obstructive sleep apnoea: proof of principle study . Respiration. 2012. ; 84 ( 4 ): 275 – 282 . [DOI] [PubMed] [Google Scholar]
- 35. Nesmith BLW , Ihnen M , Schaal S . Poor responders to bevacizumab pharmacotherapy in age-related macular degeneration and in diabetic macular edema demonstrate increased risk for obstructive sleep apnea . Retina. 2014. ; 34 ( 12 ): 2423 – 2430 . [DOI] [PubMed] [Google Scholar]
- 36. Schaal S , Sherman MP , Nesmith B , Barak Y . Untreated obstructive sleep apnea hinders response to bevacizumab in age-related macular degeneration . Retina. 2016. ; 36 ( 4 ): 791 – 797 . [DOI] [PubMed] [Google Scholar]
- 37. Chang AC , Fox TP , Wang S , Wu AY . Relationship between obstructive sleep apnea and the presence and severity of diabetic retinopathy . Retina. 2018. ; 38 ( 11 ): 2197 – 2206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. West SD , Prudon B , Hughes J , et al. ; ROSA Trial Investigators . Continuous positive airway pressure effect on visual acuity in patients with type 2 diabetes and obstructive sleep apnoea: a multicentre randomised controlled trial . Eur Respir J. 2018. ; 52 ( 4 ): 1801177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith JP , Cyr LG , Dowd LK , Duchin KS , Lenihan PA , Sprague J . The Veterans Affairs Continuous Positive Airway Pressure Use and Diabetic Retinopathy Study . Optom Vis Sci. 2019. ; 96 ( 11 ): 874 – 878 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishimura A , Kasai T , Kikuno S , et al . Apnea hypopnea index during rapid eye movement sleep with diabetic retinopathy in patients with type 2 diabetes . J Clin Endocrinol Metab. 2019. ; 104 ( 6 ): 2075 – 2082 . [DOI] [PubMed] [Google Scholar]
- 41. Ciccone MM , Favale S , Scicchitano P , et al . Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients . Int J Cardiol. 2012. ; 158 ( 3 ): 383 – 386 . [DOI] [PubMed] [Google Scholar]
- 42. Lin T , Huang J-F , Lin Q-C , et al . The effect of CPAP treatment on venous lactate and arterial blood gas among obstructive sleep apnea syndrome patients . Sleep Breath. 2017. ; 21 ( 2 ): 303 – 309 . [DOI] [PubMed] [Google Scholar]
- 43. Kras A , Celi LA , Miller JB . Accelerating ophthalmic artificial intelligence research: the role of an open access data repository . Curr Opin Ophthalmol. 2020. ; 31 ( 5 ): 337 – 350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhaskaranand M , Ramachandra C , Bhat S , et al. Automated Diabetic Retinopathy Screening and Monitoring Using Retinal Fundus Image Analysis. J Diabetes Sci Technol. 2016;10(2):254–261. [DOI] [PMC free article] [PubMed]
- 45. Decencière E , Cazuguel G , Zhang X , et al . TeleOphta: machine learning and image processing methods for teleophthalmology . IRBM. 2013. ; 34 ( 2 ): 196 – 203 . [Google Scholar]