Abstract
Study Objectives:
Our objective was to determine the prevalence of elevated right ventricular pressure (RVP) as a surrogate marker for pulmonary hypertension in children with obstructive sleep apnea syndrome (OSAS) undergoing echocardiography.
Methods:
This was a retrospective chart review of children ages 2–21 years diagnosed with OSAS by an overnight polysomnogram who underwent cardiac echocardiogram to screen for pulmonary hypertension within 6 months of polysomnogram in a tertiary inner-city pediatric hospital. The primary outcome was elevated RVP defined by estimated RVP ≥ 25 mm Hg above right atrial pressure or ventricular septal configuration consistent with elevated RVP.
Results:
A total of 174 children were included. The median (interquartile range) age was 8.9 (5.5–13.1) years with 59.2% male, 41.4% Hispanic, and 25.9% non-Hispanic Black patients. The prevalence of obesity was 72.0% and severe or very severe OSAS was present in 93.1%. The median (interquartile range) apnea-hypopnea index was 28.3 events/h (18.8–52.7 events/h). Seven children (4.0%) had elevated RVP. There was no association between elevated RVP and age, sex, race, body mass index percentile, apnea-hypopnea index, oxygen nadir, or severe OSAS (apnea-hypopnea index ≥ 10 events/h).
Conclusions:
Elevated RVP was rare and was not associated with OSAS severity. The prevalence in this cohort is higher than the prevalence of pulmonary hypertension noted in similar studies (0%–1.8%), which may be related to differences in methodology or unassessed cohort characteristics. Further effort to determine the optimal role for pulmonary hypertension screening in pediatric OSAS is needed.
Citation:
Bitners AC, Arens R, Mahgerefteh J, et al. Prevalence of elevated right ventricular pressure in children with obstructive sleep apnea syndrome undergoing pulmonary hypertension screening. J Clin Sleep Med. 2021;17(11):2225–2232.
Keywords: echocardiogram, obstructive sleep apnea syndrome, pediatrics, pulmonary hypertension
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea syndrome is linked to cardiovascular risk in children, including pulmonary hypertension (PH); however, there are few studies reporting the prevalence of PH in children with obstructive sleep apnea syndrome. Furthermore, the risk factors for PH are unknown, which has hindered the development of evidence-based screening guidelines.
Study Impact: Although obesity and severe obstructive sleep apnea syndrome are often assumed to be risk factors for PH, some children with elevated right ventricular pressure in this study were of normal weight and did not have severe obstructive sleep apnea syndrome at the time of this study. This suggests that screening strategies that rely on these indications alone may not be adequate to identify children with PH.
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is characterized by partial or complete airway obstruction during sleep leading to ventilatory disturbance and sleep fragmentation.1 The prevalence in children is 2%–4%.2 Although the pathophysiology is multifactorial, the most common cause of pediatric OSAS is hypertrophy of the tonsils and adenoids. Accordingly, adenotonsillectomy is the first-line treatment in the majority of cases.3,4
OSAS in children is linked to a variety of adverse effects, including neurocognitive impairment, behavioral problems, metabolic dysregulation, and systemic inflammation.5,6 There is also increased cardiovascular risk mediated by OSAS-related blood pressure dysregulation, hypoxia-induced inflammation, and endothelial dysfunction.6 Pulmonary hypertension (PH) and cor pulmonale due to OSAS have also been reported in children.7,8 Although cardiac catheterization is the gold standard in diagnosis of PH, echocardiography is the preferred method of screening for PH in children, as it can also provide an estimate of the right ventricular pressure (RVP) and ventricular function but has the advantage of being minimally invasive.9 Because OSAS confers increased cardiac risk, recent clinical practice guidelines indicate that some children may require cardiac screening prior to surgery.4,9 However, there is no consensus regarding which children would benefit from screening or which method of screening is most appropriate. Furthermore, risk factors for cardiac morbidity in OSAS are unknown.
Other groups have recently examined the relationship between OSAS and PH. A study by Burns et al10 found a 1.8% prevalence of PH in children with OSAS; however, OSAS was the indication for cardiology referral in only 6.3% of patients, and most children with PH had known congenital heart disease. Other work, by Teplitzky and colleagues,11 found no cases of PH in a cohort of children with severe OSAS undergoing PH screening prior to adenotonsillectomy. Furthermore, neither study found an association between OSAS severity and cardiovascular outcomes. We sought to extend this work by reporting the prevalence of PH in a larger, more ethnically and racially diverse cohort of children who had been referred for PH screening specifically due to a diagnosis of OSAS.
Although the prevalence of PH in children with OSAS is not well described, recognition of PH in children is critical both to avoid acute postoperative complications and to manage the associated long-term sequelae. For example, when children with PH undergo elective surgery, the procedure should be performed in consultation with a cardiologist and an anesthesiologist experienced in the management of PH.9 PH increases the risk of complications, including laryngospasm, oxygen desaturation, pulmonary edema, postoperative upper airway obstruction, and respiratory arrest and is associated with a 20-fold increase in the mortality rate compared to rates for all children undergoing anesthesia or sedation.12,13 Children with PH should also be evaluated with serial echocardiograms and monitored for disease progression.9,14 The potential for morbidity and mortality underscores the importance of recognizing PH in children so that they can be managed appropriately to mitigate perioperative complications and address long-term sequelae.
The purpose of this study was to report the prevalence of PH in children with OSAS at our institution who had been referred to cardiology for echocardiographic evaluation by pediatric otolaryngology and pediatric pulmonology/sleep medicine. Additionally, we aimed to identify clinical or demographic factors associated with PH in these children. We believed this information would aid in the management of children with OSAS by assisting care teams in identifying the children at greatest risk of cardiovascular sequelae who might benefit from PH screening. Adopting a targeted approach to cardiac evaluation in children with OSAS could optimize the screening process and avoid excessive echocardiography screening in this population.
METHODS
Participants
This observational, retrospective cohort study included children (aged 2–21 years) who underwent polysomnography and echocardiography within 6 months between January 1, 2016 and September 23, 2019. Children were eligible for inclusion if they had OSAS, underwent echocardiography to screen for OSAS-related PH, and were not previously followed by pediatric cardiology. Children considered to be at high risk of PH were referred to cardiology by either pediatric otolaryngology or pediatric sleep medicine, but there were no standard criteria for referral in place during the study period. Children aged < 2 years were excluded because that is the cutoff at which our institution’s pediatric otolaryngology group performs adenotonsillectomy as first-line treatment for upper airway obstruction and obstructive sleep apnea. Patients with mixed or primarily central sleep apnea were excluded. Other exclusion criteria included prior tracheotomy, incomplete medical record, and airway surgery (eg, adenotonsillectomy) within 1 year of the echocardiogram or polysomnogram (PSG). This study was approved by the Institutional Review Board at the Children’s Hospital at Montefiore/Albert Einstein College of Medicine.
Measures
The following information was collected from the medical record: age on day of echocardiogram, sex, race/ethnicity, body mass index (BMI) on day of echocardiogram, medical comorbidities (allergies, eczema, allergic rhinitis, asthma, anemia, bronchopulmonary dysplasia, developmental delay, diabetes mellitus, genetic syndromes, gastroesophageal reflux disease, sickle cell disease), PSG parameters, and echocardiogram findings. BMI percentile was categorized as follows: underweight (<5%), normal weight (5%–84%), overweight (85%–94%), mild obesity (95%–119% of 95th percentile), moderate obesity (120%–139% of 95th percentile), and severe obesity (>140% of 95th percentile).15,16
Echocardiography
Echocardiography was performed using standard protocols at the Children’s Hospital at Montefiore in an echocardiography lab accredited by the Intersocietal Accreditation Commission.17,18 The original echocardiogram reports generated by pediatric cardiologists were reviewed to determine whether there was evidence of elevated RVP. Elevated RVP was defined by estimated RVP ≥ 25 mm Hg above right atrial pressure or ventricular septal configuration consistent with elevated RVP according to accepted practice.19,20 Specifically, if the interventricular septum was flattened in systole, the RVP was interpreted to be between one-half of systemic pressure and systemic pressure. If the septum bowed toward the left ventricle in systole, RVP was interpreted to be at or above systemic pressure.
Polysomnography
All children underwent overnight attended polysomnography. PSGs were scored according to standard criteria.21 The following PSG parameters were recorded: total sleep time (TST), sleep efficiency, arousal index, awakening index, apnea-hypopnea index (AHI), central apnea index, obstructive apnea index, arterial oxygen saturation (SpO2) at baseline, SpO2 nadir, end-tidal CO2 (EtCO2) at baseline, maximum EtCO2, and percentage of TST spent with SpO2 < 90%. Polysomnography was performed at 1 of 10 sleep labs, with the majority (89%) being performed in 1 of 3 sleep centers (the pediatric sleep laboratory at our institution and 2 nearby private sleep centers that serve both children and adults). OSAS was defined as AHI ≥ 2 events/h and/or obstructive apnea index ≥ 1 event/h.22 OSAS was classified was mild (1 ≤ AHI < 4.9 events/h), moderate (5 ≤ AHI < 10 events/h), or severe (AHI ≥ 10 events/h).4 An additional classification of very severe (AHI ≥ 30 events/h)23 was used in some analyses.
Statistical analysis
Statistical analysis was performed using Prism software version 8.4.2 (GraphPad Software, San Diego, CA). Descriptive statistics were obtained for the study variables. In comparing the characteristics of children with normal vs elevated RVP, categorical variables were summarized using counts and percentages, while continuous variables were expressed as means and standard deviations for normally distributed variables and as medians and interquartile ranges for variables that were not normally distributed. Categorical data was analyzed using the chi-square or Fisher’s exact test as appropriate. Continuous variables were analyzed using the Mann-Whitney U test. Statistical significance was defined as α < 0.05.
Because one of the children with elevated RVP had sickle cell disease and there is an increased prevalence of PH in children with this disease,24 a separate posthoc analysis of the data was performed that excluded all children with sickle cell disease (n = 5).
RESULTS
Participants
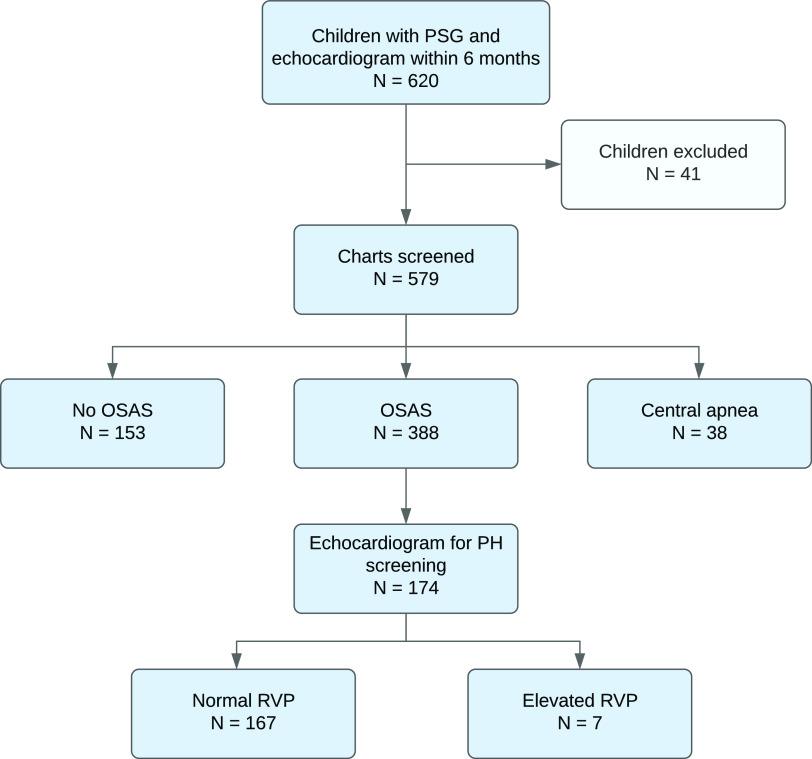
A total of 620 children aged 2–21 who underwent echocardiogram and PSG were identified (Figure 1). Forty-one children were excluded for the following reasons: incomplete medical record (n = 5), prior tracheotomy (n = 10), and airway surgery within 1 year of the echocardiogram or PSG (adenoidectomy [n = 1], adenotonsillectomy [n = 21], tonsillectomy [n = 2], epiglottectomy [n = 1], supraglottoplasty with adenoidectomy, turbinate reduction, tonsillectomy, and lingual reduction [n = 1]). Of the remaining 579 children, 388 had OSAS. Their medical records were manually reviewed to determine the indication for echocardiography. One hundred seventy-four children were referred to cardiology for OSAS-related PH screening and thus were included in the study.
Figure 1. Flow chart of study participants.
OSAS = obstructive sleep apnea syndrome, PH = pulmonary hypertension, PSG = polysomnogram, RVP = right ventricular pressure.
Demographic information and comorbidities
Demographic and clinical characteristics of children included in the study are presented in Table 1. The median (interquartile range [IQR]) age was 8.9 (5.5–13.1) years and 59.2% were male. The most common racial/ethnic groups were Hispanic (41.4%) and non-Hispanic Black (25.9%). The median BMI percentile was 98.9 (IQR 90.2–99.7). Of the 168 children with BMI data, 121 (72.0%) were obese, defined as BMI at or above the 95th percentile. A prior history of tonsillectomy with or without adenoidectomy was noted for 29 (16.7%) children. Demographic characteristics between those with normal RVP and elevated RVP were similar. The participants’ medical comorbidities are displayed in Table 2.
Table 1.
Demographic information and clinical characteristics.
| Variable | All Patients (n = 174) | Normal RVP (n = 167) | Elevated RVP (n = 7) | P |
|---|---|---|---|---|
| Age, y | 8.9 (5.5–13.1) | 8.9 (5.5–13.2) | 10.5 (5.9–12.2) | .55 |
| Male | 103 (59.2) | 100 (59.9) | 3 (42.9) | .45 |
| Race/ethnicity | .70 | |||
| Hispanic | 72 (41.4) | 70 (41.9) | 2 (28.6) | |
| Non-Hispanic Black | 45 (25.9) | 42 (25.1) | 3 (42.9) | |
| Non-Hispanic White | 9 (5.2) | 8 (4.8) | 1 (14.3) | |
| Asian | 1 (0.6) | 1 (0.6) | 0 | |
| Other | 15 (8.6) | 15 (9.0) | 0 | |
| Unknown | 32 (18.4) | 31 (18.6) | 1 (14.3) | |
| BMI percentile* | 98.9 (90.2–99.7) | 98.9 (89.3–99.6) | 99.8 (93.0–99.9) | .25 |
| Underweight | 9 (5.2) | 9 (5.4) | 0 | |
| Normal weight | 30 (17.2) | 29 (17.4) | 1 (14.3) | |
| Overweight | 8 (4.6) | 7 (4.2) | 1 (14.3) | |
| Mild obesity | 32 (18.4) | 32 (19.2) | 0 | |
| Moderate obesity | 36 (20.7) | 34 (20.4) | 2 (28.6) | |
| Severe obesity | 53 (30.5) | 50 (29.9) | 3 (42.9) | |
| Obesity | 121 (72.0) | 116 (72.0) | 5 (71.4) | >.99 |
| Tonsils and/or adenoids removed | 29 (16.7) | 27 (16.2) | 2 (28.6) | .33 |
| OSAS severity | .08† | |||
| Mild (1 ≤ AHI < 4.9 events/h) | 4 (2.3) | 4 (2.4) | 0 | |
| Moderate (5 ≤ AHI < 10 events/h) | 8 (4.6) | 6 (3.6) | 2 (28.6) | |
| Severe (10 ≤ AHI < 30 events/h) | 78 (44.8) | 76 (45.5) | 2 (28.6) | |
| Very severe (AHI ≥ 30 events/h) | 84 (48.3) | 81 (48.5) | 3 (42.9) |
Categorical variables are presented as counts and percentages. Continuous variables are presented as median (interquartile range). P values were calculated using Mann-Whitney U, Fisher’s exact, or chi-square test between groups as appropriate. *BMI percentile (and therefore obesity status) was unavailable or unspecified (due to age 20+) for 6 patients, all of whom had normal RVP. BMI percentile was categorized as follows: underweight (< 5%), normal weight (5%–84%), overweight (85%–94%), mild obesity (95%–119% of 95th percentile), moderate obesity (120%–139% of 95th percentile), and severe obesity (> 140% of 95th percentile). †P value was calculated for severe OSAS (AHI ≥ 10 events/h) vs nonsevere OSAS (AHI < 10 events/h). AHI = apnea-hypopnea index, BMI = body mass index, OSAS = obstructive sleep apnea syndrome, RVP = right ventricular pressure.
Table 2.
Medical comorbidities stratified by RVP.
| Comorbid Condition | Normal RVP (n = 167) | Elevated RVP (n = 7) |
|---|---|---|
| Allergies/eczema/allergic rhinitis | 50 (29.9%) | 1 (14.3%) |
| Anemia | 11 (6.6%) | 1 (14.3%) |
| Asthma | 61 (36.5%) | 2 (28.6%) |
| BPD | 1 (0.6%) | 0 |
| Developmental delay | 10 (6.0%) | 1 (14.3%) |
| Diabetes mellitus | 6 (3.6%) | 0 |
| Genetic syndrome | 12 (7.2%) | 0 |
| GERD | 6 (3.6%) | 0 |
| SCD | 4 (2.4%) | 1 (14.3%) |
Data are presented as counts with percentages. BPD = bronchopulmonary dysplasia, GERD = gastroesophageal reflux disease, RVP = right ventricular pressure, SCD = sickle cell disease.
Echocardiography
Seven children (4.0%) had elevated RVP. Their demographic and clinical characteristics are displayed in Table 3. There was no association between elevated RVP and age, sex, race, BMI percentile, or severe OSAS.
Table 3.
Characteristics of patients with elevated RVP.
| Sex | Age (y) | Race | BMI Percentile (% of 95th percentile) | AHI (events/h) | SpO2 Nadir | Peak EtCO2 | RVP Estimate | Comorbid Conditions |
|---|---|---|---|---|---|---|---|---|
| F | 11 | Black | 99.8 (170) | 156.4 | 21% | 57 | > 1/2 systemic-systemic | Developmental delay, prematurity, asthma |
| F | 10 | Black | 99.9 (236) | 87.5 | 61% | * | 35–40 mm Hg above RAP | Prediabetes, iron deficiency anemia |
| M | 15 | Unknown | 7.8 | 23.4 | 85% | 47 | 30–35 mm Hg above RAP | Allergic rhinitis, SCD |
| F | 5 | Black | 99.9 (120) | 6.3 | 66% | 53 | 25–30 mm Hg above RAP | — |
| M | 12 | Black | 93.0 | 7.5 | 89% | 44 | 25 mm Hg above RAP | Elevated blood pressure, asthma |
| M | 10 | Other | 98.9 (126) | 20.2 | 40% | 59 | > 1/2 systemic– systemic | — |
| F | 4 | White | 99.9 (155) | 38.8 | 70% | * | 25–30 mm Hg above RAP | — |
Data not available. AHI = apnea-hypopnea index, BMI = body mass index, EtCO2 = end-tidal CO2, RAP = right atrial pressure, RVP = right ventricular pressure, SCD = sickle cell disease, SpO2 = arterial oxygen saturation.
Polysomnography
PSG parameters are displayed in Table 4. The median AHI was 28.3 events/h (IQR 18.9–52.6) for children with normal RVP and 23.4 events/h (IQR 7.5–87.5) for children with elevated RVP (P = .75). The median SpO2 nadir was 77.0% (IQR 69.0–85.0) for children with normal RVP and 66.0% (IQR 40.0–85.0) for children with elevated RVP (P = .14). The median peak EtCO2 was 51.0 mm Hg (IQR 47.0–54.0) in children with normal RVP and 53.0 mm Hg (IQR 45.5–58.0) in children with elevated RVP (P = .65). There was no association between elevated RVP and AHI, SpO2 nadir, peak EtCO2, or with any of the other PSG parameters (TST, sleep efficiency, central apnea index, baseline SpO2, %TST with SpO2 < 90%, and baseline EtCO2). The severity of OSAS was mild, moderate, and severe in 2.3%, 4.6%, and 44.8% of children, respectively. Very severe OSAS was present in 48.3% of cases.
Table 4.
Polysomnographic data.
| Variable | Normal RVP (n = 167) | Elevated RVP (n = 7) | P |
|---|---|---|---|
| TST (min) | 360 (323–399) | 329 (214–369) | .08 |
| Sleep efficiency (%) | 84.1 (74.0–90.1) | 83.1 (50.6–89.9) | .41 |
| Arousal index (events/h)¶ | 24.7 (16.4–39.4) | 22.0 (17.5–53.1) | .95 |
| Awakening index (events/h)# | 4.1 (2.6–5.5) | 3.7 (3.2–56.4) | .54 |
| AHI (events/h) | 28.3 (18.9–52.6) | 23.4 (7.5–87.5) | .75 |
| CAI (events/h) | 0.3 (0.0–1.6) | 0.25 (0.0–1.5) | .90 |
| Baseline SpO2 (%)‡ | 98 (97–100) | 99 (94–100) | .96 |
| SpO2 nadir (%) | 77.0 (69.0–85.0) | 66.0 (40.0–85.0) | .14 |
| % TST with SpO2 < 90% | 2.3 (0.28–8.1) | 6.1 (0.20–20.8) | .44 |
| Baseline EtCO2 (mm Hg)§ | 40.0 (36.3–42.8) | 39.0 (38.3–41.3) | .92 |
| Peak EtCO2 (mm Hg)* | 51.0 (47.0–54.0) | 53.0 (45.5–58.0) | .65 |
Medians (interquartile range) are provided. P values were calculated using the Mann-Whitney U test. ¶Data available for 107 patients with normal RVP, 5 patients with elevated RVP. #Data available for 89 patients with normal RVP, 5 patients with elevated RVP. ‡Data available for 157 patients with normal RVP, 7 patients with elevated RVP. §Data available for 80 patients with normal RVP, 4 patients with elevated RVP. *Data available for 97 patients with normal RVP, 5 patients with elevated RVP. AHI = apnea-hypopnea index, CAI = central apnea index, EtCO2 = end-tidal CO2, RVP = right ventricular pressure, SpO2 = arterial oxygen saturation, TST = total sleep time.
Posthoc analysis excluding patients with sickle cell disease
Five children in the study population had sickle cell disease, 1 with elevated RVP and 4 with normal RVP. The subgroup analysis excluding these children revealed that severe or very severe OSAS was more common in the group with normal RVP (95.1%) than with elevated RVP (66.7%), and this difference reached significance (P = .04). In an analysis comparing PSG parameters between the 2 groups, a significantly lower SpO2 nadir was observed in the group with elevated RVP (63.5% vs 77.0%, P = .0496).
DISCUSSION
Our findings help characterize the prevalence of PH in a largely non-White, urban patient population with a high prevalence of obesity. The main finding of this study is a 4.0% prevalence of elevated RVP in children with OSAS screened for PH. Furthermore, there was no association with elevated RVP and any clinical or demographic variables, including severity of OSAS. The prevalence of elevated RVP in this cohort of children is higher than the observed prevalence of PH in similar studies. Teplitzky et al11 studied 47 children with severe OSAS (AHI > 30 events/h) screened for PH prior to adenotonsillectomy and found no functional or structural echocardiogram abnormalities. The higher prevalence in our study (4% vs 0%) may be attributable to methodological differences or unmeasured cohort characteristics. Of note, the studies used the same method of determining increased RVP as recommended by standard guidelines.17 In addition to screening for PH, Teplitzky and colleagues measured 16 echocardiographic parameters to evaluate the left ventricle, right ventricle, and pulmonary hemodynamics. In their analysis, PSG parameters were poorly predictive of echocardiographic outcome variables. Specifically, AHI was not predictive of any of the 16 echocardiographic variables they studied. This finding is consistent with our observation that RVP was not associated with severity of OSAS. In fact, of the 7 children we identified with elevated RVP, 4 had an AHI < 30 events/h, and AHIs as low as 6.3 and 7.5 events/h were observed. Of the 2 children with moderate OSAS, 1 had a history of obesity and persistent severe obstructive symptoms despite prior tonsillectomy 26 months before the PSG, while the other had no prior surgery but did have a diagnosis of hypertension.
In another cohort of 163 children with OSAS from a military health system database who were referred to cardiology, Burns et al10 reported a 1.8% prevalence of PH using criteria similar to our study. Of the 3 children with PH, 2 had mild OSAS (AHI 1.1, 1.5 events/h) and 1 had moderate disease (AHI 8.2 events/h). Two children had a known history of congenital heart disease and 1 was referred to cardiology for an electrocardiogram abnormality and was found to have a subaortic membrane. Although our study is similar to the Burns et al study in that they both employed PSG and echocardiography, the study methodologies differ significantly in the referral pattern for PH screening. In the Burns et al study, the most frequent indication for cardiology referral was congenital heart disease, and only 10 patients (6.3%) were referred for their OSAS. This differs from our study, in which all children were referred to cardiology for OSAS. Inclusion of children with congenital heart disease likely increased the prevalence of PH in the study by Burns et al, where 2 out of 3 children with PH had a known history of cardiac disease that likely contributed to the development of PH. In light of these differences in study design, the 4.0% prevalence of PH noted in this study—more than double that in the Burns et al study—is noteworthy. The higher prevalence in our population may also be related to differences in the baseline population of the 2 studies. The Burns et al study included children in a military health system in Texas, whereas our institution serves an inner-city population in Bronx, NY. Patients of White race composed almost half of the population in the Burns et al cohort but represented only 5% of our population. For unclear reasons, it has been observed that Black children tend to have more severe OSAS than children from other racial groups.22 Patients in our cohort also tended to be older (median age 8.9 vs 7.6 years).
In addition to determining the prevalence of PH in this cohort, a second study objective was to identify risk factors for elevated RVP. Our findings were consistent with prior studies10,11 that have not detected an association between elevated RVP and either OSAS severity or BMI score. Analysis of the polysomnographic parameters also did not reveal any significant differences (Table 4). Given the modest sample size, the lack of significant findings may be attributable to inadequate statistical power, and larger studies are recommended to further explore possible clinical or demographic risk factors for PH in this population. Although a variety of strategies have been proposed, at present there is no consensus regarding specific indications for PH screening in OSAS across pediatric specialties. Recent tonsillectomy guidelines published by the American Academy of Otolaryngology–Head and Neck Surgery state that preoperative cardiac evaluation may be required in children with severe OSAS4; however, there are no specific recommendations regarding which children specifically warrant evaluation. Other authors suggest that preoperative echocardiogram is warranted if the overnight oxygen nadir on PSG reaches 70%25 or if systemic hypertension or signs of right ventricular dysfunction are present.26 Guidelines for pediatric PH published by the American Heart Association and American Thoracic Society recommend echocardiography for children with severe OSAS.9 While these guidelines rely on OSAS severity to guide screening recommendations, our findings show that children with nonsevere OSAS may have elevated RVP and suggest that other clinical or demographic characteristics play a role in the development of PH. Identifying these other risk factors is a crucial component of developing evidence-based screening guidelines.
Due to the increased prevalence of PH in children with sickle cell disease, a separate posthoc analysis excluding these patients was performed. In that subgroup, children with elevated RVP had a significantly lower SpO2 nadir on polysomnography (63.5% vs 77.0%). This result is in alignment with several studies of adults with OSAS in observing that hypoxemia is more closely associated with the presence of PH than the severity and frequency of nocturnal respiratory events.27,28 Correlates to adult studies must be interpreted with caution, however, due to the distinct pathophysiology of adult OSAS and the higher prevalence of comorbid cardiopulmonary disease in that population. Nonetheless, our results suggest that markers of hypoxemia such as SpO2 may confer increased PH risk in children, a hypothesis which should be further evaluated in future studies. Although there was no difference in AHI between the 2 groups, children with PH were less likely to have severe OSAS. This agrees with the findings by Burns et al10 and Teplitzky et al11 and further suggests that OSAS severity may not be an indication for PH screening. It is also important to note, however, that this finding may be influenced by the retrospective nature of this study, as the children with nonsevere OSAS may have been referred for echocardiogram due to medical comorbidities or other perceived risk factors. As an example, 1 child with nonsevere OSAS who was referred for echocardiogram had a history of hypertension.
Limitations inherent in the retrospective design include potential selection bias due to practice variability regarding cardiology referral: Patients were most commonly referred by either otolaryngology or pulmonology if severe OSAS was demonstrated on polysomnography, without specific criteria regarding other potential risk factors or predefined thresholds for BMI or AHI that triggered automatic screening. Because participants in this study were referred to cardiology for PH screening, the prevalence detected here likely exceeds the prevalence in the general population of children with OSAS. There was a high prevalence of obesity and severe OSAS in this cohort, which reflects the increased frequency with which these children are referred to cardiology. Variability in referral practices is unavoidable in this retrospective study and highlights the need for prospective studies to better define the role of OSAS in the development of PH. Additionally, the number of children with elevated RVP in this study was small, limiting our ability to identify risk factors. In this study, elevated RVP was used as a surrogate marker for PH. Although cardiac catheterization is the gold-standard diagnostic test, echocardiography is widely accepted as a surrogate screening method and has the additional benefit of being safe and noninvasive. We relied on the original echocardiogram reports to determine the RVP rather than blinded re-reading of the original imaging; however, this reflects the typical clinical setting and practice. The temporal difference in acquisition of PSG and echocardiogram data (up to 6 months) is another limitation of the study; however, the median time between the 2 was 61.5 days, and 48% of children had them performed within 60 days. The time delay to echocardiogram for some patients reflects challenges including missed appointments and difficulty reaching patients despite efforts by our clinical teams to expedite and facilitate cardiology appointment scheduling. Of note, no children had surgical treatment of their OSAS between the PSG and echocardiogram.
In conclusion, this study demonstrates a 4% prevalence of elevated RVP in children with OSAS. This is higher than rates reported in similar studies. Importantly, of 7 children identified with elevated RVP, 2 (29%) had nonsevere OSAS and 2 (29%) were not obese. Although severe disease and obesity are often presumed to be risk factors for the development of PH in children with OSAS, our findings demonstrate that elevated RVP can develop in their absence and suggest that screening strategies that rely heavily on these 2 indications for cardiac evaluation may not identify all children with OSAS who have PH. Recognizing the diagnosis of PH in children is crucial in developing an appropriate management plan that avoids both perioperative complications and long-term sequelae related to the disease. While some societies and authors recommend cardiac or PH screening for certain patients with OSAS,4,9,25,26 there are no universally accepted guidelines. Further effort is needed to identify risk factors for OSAS-related PH in children and to develop optimal screening strategies that balance the importance of identifying PH with the need to utilize health care resources reasonably and justly.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Albert Einstein College of Medicine, Children’s Hospital at Montefiore. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Maximilian C. Stahl and Evan Kominsky for their assistance with data collection.
ABBREVIATIONS
- AHI,
apnea-hypopnea index
- BMI,
body mass index
- EtCO2,
end-tidal CO2
- IQR,
interquartile range
- OSAS,
obstructive sleep apnea syndrome
- PH,
pulmonary hypertension
- PSG,
polysomnogram
- RVP,
right ventricular pressure
- SpO2,
arterial oxygen saturation
- TST,
total sleep time
REFERENCES
- 1. Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584. [DOI] [PubMed] [Google Scholar]
- 2. Lumeng JC, Chervin RD . Epidemiology of pediatric obstructive sleep apnea . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 242 – 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D . Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children . Prog Cardiovasc Dis. 2009. ; 51 ( 5 ): 416 – 433. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell RB, Archer SM, Ishman SL, et al . Clinical practice guideline: tonsillectomy in children (update) . Otolaryngol Head Neck Surg. 2019. ; 160 ( 1_suppl ): S1 – S42. [DOI] [PubMed] [Google Scholar]
- 5. Tan H-L, Gozal D, Kheirandish-Gozal L . Obstructive sleep apnea in children: a critical update . Nat Sci Sleep. 2013. ; 5 : 109 – 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith DF, Amin RS . OSA and cardiovascular risk in pediatrics . Chest. 2019. ; 156 ( 2 ): 402 – 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunt CE, Brouillette RT . Abnormalities of breathing control and airway maintenance in infants and children as a cause of cor pulmonale . Pediatr Cardiol. 1982. ; 3 ( 3 ): 249 – 256. [DOI] [PubMed] [Google Scholar]
- 8. Miman MC, Kirazli T, Ozyurek R . Doppler echocardiography in adenotonsillar hypertrophy . Int J Pediatr Otorhinolaryngol. 2000. ; 54 ( 1 ): 21 – 26. [DOI] [PubMed] [Google Scholar]
- 9. Abman SH, Hansmann G, Archer SL, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society . Circulation. 2015. ; 132 ( 21 ): 2037 – 2099. [DOI] [PubMed] [Google Scholar]
- 10. Burns AT, Hansen SL, Turner ZS, Aden JK, Black AB, Hsu DP . Prevalence of pulmonary hypertension in pediatric patients with obstructive sleep apnea and a cardiology evaluation: a retrospective analysis . J Clin Sleep Med. 2019. ; 15 ( 8 ): 1081 – 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teplitzky TB, Pereira KD, Isaiah A . Echocardiographic screening in children with very severe obstructive sleep apnea . Int J Pediatr Otorhinolaryngol. 2019. ; 126 : 109626. [DOI] [PubMed] [Google Scholar]
- 12. Blum RH, McGowan FX Jr . Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implications . Paediatr Anaesth. 2004. ; 14 ( 1 ): 75 – 83. [DOI] [PubMed] [Google Scholar]
- 13. Chau DF, Gangadharan M, Hartke LP, Twite MD . The post-anesthetic care of pediatric patients with pulmonary hypertension . Semin Cardiothorac Vasc Anesth. 2016. ; 20 ( 1 ): 63 – 73. [DOI] [PubMed] [Google Scholar]
- 14. Galiè N, Humbert M, Vachiery JL, et al . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) . Eur Heart J. 2016. ; 37 ( 1 ): 67 – 119. [DOI] [PubMed] [Google Scholar]
- 15. Skinner AC, Skelton JA . Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012 . JAMA Pediatr. 2014. ; 168 ( 6 ): 561 – 566. [DOI] [PubMed] [Google Scholar]
- 16. Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of childhood and adult obesity in the United States, 2011-2012 . JAMA. 2014. ; 311 ( 8 ): 806 – 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai WW, Geva T, Shirali GS, et al. ; Pediatric Council of the American Society of Echocardiography . Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography . J Am Soc Echocardiogr. 2006. ; 19 ( 12 ): 1413 – 1430. [DOI] [PubMed] [Google Scholar]
- 18. Lopez L, Colan SD, Frommelt PC, et al . Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council . J Am Soc Echocardiogr. 2010. ; 23 ( 5 ): 465 – 495. [DOI] [PubMed] [Google Scholar]
- 19. Rudski LG, Lai WW, Afilalo J, et al . Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography . J Am Soc Echocardiogr. 2010. ; 23 ( 7 ): 685 – 713. [DOI] [PubMed] [Google Scholar]
- 20. Jone P-N, Ivy DD . Echocardiography in pediatric pulmonary hypertension . Front Pediatr. 2014. ; 2 : 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berry RB, Albertario CL, Harding SM, et al. ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications , Version 2.5. Darien, IL: : American Academy of Sleep Medicine; ; 2018. . [Google Scholar]
- 22. Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea . N Engl J Med. 2013. ; 368 ( 25 ): 2366 – 2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isaiah A, Hamdan H, Johnson RF, Naqvi K, Mitchell RB . Very severe obstructive sleep apnea in children: outcomes of adenotonsillectomy and risk factors for persistence . Otolaryngol Head Neck Surg. 2017. ; 157 ( 1 ): 128 – 134. [DOI] [PubMed] [Google Scholar]
- 24. Kato GJ, Onyekwere OC, Gladwin MT . Pulmonary hypertension in sickle cell disease: relevance to children . Pediatr Hematol Oncol. 2007. ; 24 ( 3 ): 159 – 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patino M, Sadhasivam S, Mahmoud M . Obstructive sleep apnoea in children: perioperative considerations . Br J Anaesth. 2013. ; 111 ( Suppl 1 ): i83 – i95. [DOI] [PubMed] [Google Scholar]
- 26. Schwengel DA, Sterni LM, Tunkel DE, Heitmiller ES . Perioperative management of children with obstructive sleep apnea . Anesth Analg. 2009. ; 109 ( 1 ): 60 – 75. [DOI] [PubMed] [Google Scholar]
- 27. Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R . Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients . Chest. 1996. ; 109 ( 2 ): 380 – 386. [DOI] [PubMed] [Google Scholar]
- 28. Minai OA, Ricaurte B, Kaw R, et al . Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome . Am J Cardiol. 2009. ; 104 ( 9 ): 1300 – 1306. [DOI] [PubMed] [Google Scholar]