Abstract
Study Objectives:
The first-line treatment of obstructive sleep apnea syndrome in children is adenotonsillectomy, but this may result in perioperative respiratory adverse events (PRAEs). The primary aim of this study is to examine whether the McGill oximetry score (MOS) and other polysomnography parameters can predict major PRAEs following adenotonsillectomy. We secondarily evaluated the MOS interrater reliability and correlation with other polysomnography parameters.
Methods:
This retrospective study included all children aged 0–18 years who underwent preoperative polysomnography between June 2010 and January 2016 prior to adenotonsillectomy at a tertiary pediatric institution. Oximetries from polysomnograms were assigned an MOS. Univariable and multivariable models for prediction of major PRAEs were constructed. MOS was correlated with polysomnography parameters and interrater reliability was evaluated.
Results:
This study included 106 children; 15 had a major PRAE. A multivariable prediction model that combined MOS and age showed evidence for the ability to predict major PRAEs with an area under the receiver operating characteristic curve of 0.68 (95% confidence interval: 0.52, 0.84), whereby increased MOS and younger age were associated with PRAEs, but apnea-hypopnea index was not. MOS had excellent interrater reliability (κ = 0.95) and was highly correlated with oxygen saturation nadir and cumulative time percentage with oxygen saturation less than 90%.
Conclusions:
A prediction model including MOS and age may predict PRAEs following adenotonsillectomy. This suggests that nocturnal oximetry provides the most essential information of polysomnography measures to direct postoperative monitoring following adenotonsillectomy.
Citation:
Xiao L, Barrowman N, Momoli F, et al. Polysomnography parameters as predictors of respiratory adverse events following adenotonsillectomy in children. J Clin Sleep Med. 2021;17(11):2215–2223.
Keywords: obstructive sleep apnea, polysomnography, adenotonsillectomy, perioperative respiratory adverse events, McGill oximetry score
BRIEF SUMMARY
Current Knowledge/Study Rationale: The first-line treatment of obstructive sleep apnea syndrome in children is adenotonsillectomy, but this may result in perioperative respiratory adverse events (PRAEs). The primary aim of this study is to explore polysomnography parameters as predictors for PRAEs following adenotonsillectomy.
Study Impact: Our findings suggest that a model encompassing the McGill oximetry score and age may be able to predict the occurrence of major PRAEs following adenotonsillectomy. McGill oximetry score was the best predictor of major PRAEs as compared with other polysomnography parameters. This suggests that nocturnal oximetry provides the most essential information to direct postoperative monitoring following adenotonsillectomy in children with obstructive sleep apnea syndrome.
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a disorder characterized by partial or complete upper airway obstruction resulting in gas exchange abnormalities and sleep disruption. It is a major cause of morbidity in the pediatric population and occurs at an estimated incidence of 1–6%.1,2 The detection and treatment of OSAS is essential as it appears that the condition may be associated with long-term complications, including neurocognitive and behavioral impairments,3–5 cardiovascular disease,6 and growth difficulties.2,7 The first-line treatment for OSAS with adenotonsillar hypertrophy is adenotonsillectomy (AT). Although relatively safe,8 AT is not without its risks and may result in perioperative respiratory adverse events (PRAEs) that range from major events such as postobstructive pulmonary edema and upper airway obstruction to minor, transient hypoxemia. Although rare, death and anoxic brain injury attributed to a respiratory event have been reported.9–11 Children with medical comorbidities are at higher risk of PRAEs following AT,12–16 with reported major PRAE rates requiring intervention of 15–27%14,16–19 compared to 1.4% in otherwise healthy children.20
Overnight, attended, in-laboratory polysomnography (PSG) results have been used to inform decision making regarding the optimal postoperative monitoring environment following AT,2,21 with increased apnea-hypopnea index (AHI) and low oxygen saturation nadir frequently cited as predictors of PRAEs.12,17,18,22–24 However, there is no consensus regarding the PSG parameter cutoffs that necessitate inpatient monitoring following AT.2,21 Furthermore, AHI and oxygen saturation nadir are not consistently accurate predictors of PRAEs.20,25,26 In light of emerging evidence that additional measures of gas exchange are more strongly associated with PRAEs,24,26 this inconsistency may be related to the fact that each individual parameter is unable to fully capture the magnitude of gas exchange abnormalities. Theoretically, a measure that simultaneously evaluates multiple dimensions of gas exchange would perform better at predicting PRAEs. We were specifically interested in the McGill oximetry score (MOS), which is a validated 4-level severity score based on both the frequency and depth of desaturation events.27 It is considered a measure of accumulated nocturnal hypoxemic burden. Although it is problematic to use in diagnosing OSAS due to its low sensitivity,28 it has shown potential to predict PRAEs as well as to direct disposition planning following AT.27,29,30 To date, no other studies have compared the MOS with other PSG parameters as a predictor of PRAEs in children with OSAS undergoing AT.
We hypothesized that PSG parameters that considered depth, duration, and frequency of desaturation would perform better than traditional metrics in predicting major PRAEs after AT. The primary aim of this study was to examine whether the MOS and other PSG parameters can predict major PRAEs following AT. We secondarily sought to evaluate the MOS by examining its interrater reliability and correlating it with other PSG parameters.
METHODS
Study population
This retrospective cohort study included all children aged 0 to 18 years who received a baseline diagnostic PSG at the Children’s Hospital of Eastern Ontario between June 2010 and January 2016 within 1 year prior to palatine tonsillectomy, adenoidectomy, or AT. All children who received concurrent surgeries at the time of AT were excluded from the study. We also excluded children whose preoperative PSGs were deemed poor quality by the interpreting pediatric sleep medicine physician based on absent or reduced rapid eye movement sleep, poor sleep efficiency, and technical issues due to poor tolerance of PSG setup. In the case that a child received 2 or more baseline PSGs prior to AT, we included the information from the PSG closest to the time of surgery.
Study design
This study was approved by the Children’s Hospital of Eastern Ontario Research Ethics Board (18/69X). All data were assembled into a secure Research Electronic Data Capture database (Vanderbilt University, Nashville, TN, USA).
Overnight, attended, in-laboratory baseline PSGs were conducted and scored by sleep technologists according to the American Academy of Sleep Medicine recommendations.31 A full-night PSG was performed with standard PSG montage employed, including 6-lead electroencephalogram, 2-lead electrooculogram, submental and leg electromyogram, electrocardiogram, nasal pressure and/or thermistor monitoring, chest and abdominal movement monitoring by impedance, and end-tidal and/or transcutaneous carbon dioxide monitoring (Natus Xltek, Oakville, Ontario, Canada). Synchronous video monitoring was employed throughout the study. Oxygen saturation was recorded by pulse oximetry with motion resistant technology (Masimo). All PSGs were interpreted by 1 of 2 pediatric sleep medicine physicians according to American Academy of Sleep Medicine criteria.31 PSG parameters examined included total AHI, central AHI, obstructive AHI, oxygen saturation nadir, baseline mean oxygen saturation asleep, 3% oxygen desaturation index (ODI3), peak end-tidal or transcutaneous carbon dioxide level, and cumulative time percentage with oxygen saturation less than 90% (CT90).
All oximetries obtained from PSGs were assigned an MOS by 2 scorers based on the criteria outlined by Nixon and colleagues.27 An MOS of 1 has a stable oxygen saturation baseline with no more than 3 desaturations below 90%. An MOS of 2 to 4 has at least 3 clusters of desaturation and more than 3 desaturation events below 90%. An MOS of 2 has 3 or more desaturation events below 90%, an MOS of 3 has 3 or more desaturation events below 85%, and an MOS of 4 has 3 or more desaturation events below 80%.27 The scorers, a senior pediatric resident physician (L.X.) and a pediatric respiratory medicine physician (F.P.), each received instruction on the rules and application of the MOS prior to scoring oximetries. They undertook practice scoring to ensure that there was concordance prior to initiating the study. The MOS was assigned based on the oxygen saturation trend graphs and oxygen saturation parameters extracted from each PSG. Scorers were blinded to other clinical and PSG information as well as to each other’s answers. Discordant scoring results were resolved by a third score applied by another pediatric respiratory medicine physician with expertise in sleep medicine (S.L.K.).
Paper medical charts were reviewed for demographic characteristics and postoperative course. Induction and maintenance of anesthesia were performed using inhalational and/or intravenous techniques. Both opioids and dexamethasone were routinely administered intraoperatively. Postoperatively, children were either admitted for monitoring or discharged home as a day procedure. The decision to admit children for postoperative monitoring was made on an individual basis rather than based on a protocol. PRAEs requiring intervention were tracked from the time of arrival to the post-anesthetic care unit up to 30 days following AT. We included PRAEs such as hypoxemia, upper airway obstruction, and postobstructive pulmonary edema. The interventions to manage PRAEs were chosen based on the authors’ experiences and included supplemental oxygen, diuretics, inhaled medication administration, jaw thrust, nasal/oral airway placement, bag/mask ventilation, noninvasive positive-pressure ventilation, and intubation. In recognition that many children require a minimal level of respiratory or upper airway support immediately following extubation and general anesthesia, we defined major PRAEs as hypoxemia requiring supplemental oxygen beyond 1 hour postoperatively or upper airway support beyond 30 minutes post-extubation. All other forms of respiratory support that were required prior to hospital discharge were included as a PRAE. It is standard practice at our institution to provide supplemental oxygen for sustained desaturation below 92% and for frequent intermittent desaturations below this threshold. Continuous oxygen saturation monitoring is utilized immediately postoperatively in the post-anesthetic care unit and then individualized to the need of the child based on the risk of decompensation for the remainder of the hospital admission. All emergency department visits and readmissions for respiratory complications at the Children’s Hospital of Eastern Ontario within 30 days of AT were recorded.
Statistical analysis
Demographic and clinical characteristics of study participants were summarized descriptively. Median and interquartile range (IQR) or mean and standard deviation, as appropriate, were used to describe continuous variables. Discrete variables were summarized using frequency and percentage. For each of the prespecified potential predictors of a major PRAE, a univariable analysis was performed to determine the area under the receiver operating characteristic (ROC) curve. Predictors with a large area under the ROC curve were prioritized for inclusion into a multivariable model for PRAEs. Age (under 3 years, 3 to 5 years, and 6 years or older) was selected a priori for inclusion. Given the limited number of major PRAEs, it was determined that only 1 additional single degree of freedom predictor could be included in the model. A multivariable logistic regression model was then fitted. Odds ratios and 95% confidence intervals (CIs) were reported, as well as the area under the ROC curve for the model with a 95% CI. Subgroup analysis based on the presence of major comorbidities was undertaken for the multivariable logistic regression model that had the largest area under the ROC curve. A κ value was calculated to measure the interrater reliability of the MOS. Spearman correlation was used to examine the association between MOS and PSG parameters. Two-sided P values are reported. All analyses were carried out using R statistical software version 4.0.2.32
RESULTS
Baseline demographics
A total of 106 children were included in this study with a median age of 6.5 years (IQR: 4.5, 9.5 years) at the time of surgery (Table 1). There were major comorbidities in 32 of 106 children (30%), including Down syndrome (n = 14), seizures (n = 4), craniofacial malformations (n = 3), Chiari 1 malformation (n = 3), prematurity (n = 3), achondroplasia (n = 2), neuromuscular disease (n = 1), metabolic disease (n = 1), sickle cell anemia (n = 1), and congenital cardiac disease (n = 1). Notably, 1 child had more than 1 major comorbidity. Ninety-four children underwent AT, 4 underwent tonsillectomy, and 8 underwent adenoidectomy. There were 4,153 ATs, tonsillectomies, or adenoidectomies performed in 2010 to 2016 inclusive at our institution. Our study includes 2.6% of all children (106/4153) who underwent AT, tonsillectomy, or adenoidectomy, as well as diagnostic PSG of acceptable quality, in that time frame. There were 90 of 106 children (84.9%) who underwent planned postoperative admission, 3 of 106 (2.8%) who had an unplanned postoperative admission, and 13 of 106 children (12.3%) who were discharged home on the same day of surgery.
Table 1.
Baseline demographics.
| Variable | Values |
|---|---|
| Age at PSG, median (IQR), y | 6.0 (4.1, 9.1) |
| Age at surgery, median (IQR), y | 6.5 (4.5, 9.5) |
| Male, n (%) | 48 (45.3) |
| BMI z-score, mean (SD) | 0.8 (1.4)* |
| Obesity, n (%) | 33 (32.4)* |
| AHI, median (IQR), events/h | 9.7 (4.2, 16.2) |
| Obstructive AHI, median (IQR), events/h | 3.6 (0.1, 10.6) |
| Central AHI, median (IQR), events/h | 3.1 (1.2, 7.2) |
| Baseline mean oxygen saturation asleep, median (IQR), % | 97.0 (96.5, 98.0) |
| Oxygen saturation nadir, median (IQR), % | 84.0 (80.0, 90.0) |
| 3% Oxygen desaturation index, median (IQR), events/h | 6.2 (2.2, 13.3) |
| Peak carbon dioxide, median (IQR),† mm Hg | 51.0 (47.0, 58.0)‡ |
| McGill oximetry score, median (IQR) | 2.0 (1.0, 3.0) |
n = 106. *4 missing values. †Highest of end-tidal or transcutaneous carbon dioxide. ‡Twenty-one missing values. AHI = apnea-hypopnea index, BMI = body mass index, IQR = interquartile range, PSG = polysomnography, SD = standard deviation,.
On preoperative PSG, the median AHI was 9.7 events/h (IQR: 4.2, 16.2 events/h) and the median obstructive AHI was 3.6 events/h (IQR: 0.1, 10.6 events/h). The median oxygen saturation nadir was 84.0% (IQR: 80.0%, 90.0%) and the baseline mean oxygen saturation asleep was 97.0% (IQR: 96.5%, 98.0%). There were 38 of 106 children (36%) with an MOS of 1, 37 of 106 (35%) with an MOS of 2, 16 of 106 (15%) with an MOS of 3, and 15 of 106 (14%) with an MOS of 4.
PRAEs
Fifteen children were deemed to have a major PRAE requiring intervention (Table 2). Hypoxemia was the most common PRAE, occurring in 14 children, with 2 of these children also requiring support for upper airway obstruction. One child had upper airway obstruction but did not require supplemental oxygen. There were no children who required diuretics, bag/mask ventilation, noninvasive positive-pressure ventilation, or reintubation. The majority of children were admitted for postoperative monitoring (93/106, 87.7%) and the median length of stay was 1 day (range: 1–5 days). There were no delayed respiratory complications that resulted in an emergency department visit or readmission to hospital within 30 days of AT.
Table 2.
Perioperative respiratory adverse events.
| Child | Age at Surgery (years) | Medical Comorbidities | MOS | PRAE |
|---|---|---|---|---|
| 1 | 2.7 | Chiari malformation type 1, prematurity | 2 | Hypoxemia requiring supplemental oxygen* |
| 2 | 4.5 | None | 4 | Hypoxemia requiring supplemental oxygen* |
| 3 | 5.5 | None | 2 | Hypoxemia requiring supplemental oxygen* |
| 4 | 7.4 | Chiari malformation type 1 | 1 | Hypoxemia requiring supplemental oxygen* |
| 5 | 8.5 | Prematurity | 4 | Hypoxemia requiring supplemental oxygen* |
| 6 | 6.2 | None | 2 | Hypoxemia requiring supplemental oxygen* |
| 7 | 3.5 | Trisomy 21 | 3 | Hypoxemia requiring supplemental oxygen* |
| 8 | 4.0 | None | 4 | Hypoxemia requiring supplemental oxygen* |
| 9 | 5.7 | None | 1 | Hypoxemia requiring supplemental oxygen* |
| 10 | 1.8 | None | 2 | Upper airway obstruction requiring oral airway† |
| 11 | 9.7 | None | 1 | Upper airway obstruction requiring oral airway† |
| Hypoxemia requiring supplemental oxygen* | ||||
| 12 | 5.9 | Achondroplasia | 3 | Hypoxemia requiring supplemental oxygen* |
| 13 | 10.8 | Congenital muscular dystrophy | 4 | Hypoxemia requiring supplemental oxygen* |
| 14 | 2.3 | Prematurity | 2 | Upper airway obstruction requiring nebulized epinephrine† |
| Hypoxemia requiring supplemental oxygen* | ||||
| 15 | 10.9 | None | 2 | Hypoxemia requiring supplemental oxygen* |
*Beyond 1 hour after arrival in postanesthetic care unit. †Beyond 30 minutes post-extubation. MOS = McGill oximetry score, PRAE = perioperative respiratory adverse event.
Predictors of PRAEs
We were unable to confirm an increased major PRAE occurrence with an increased MOS on univariate analysis. There were 3 of 38 children (7.9%) with an MOS of 1 who had a PRAE, 6 of 37 (16.2%) with an MOS of 2 who had a PRAE, 2 of 16 (12.5%) with an MOS of 3 who had a PRAE, and 4 of 15 (26.7%) with an MOS of 4 who had a PRAE. This did not reach statistical significance (area under the ROC curve: 0.62; 95% confidence interval [CI]: 0.47, 0.77) (Table 3). Similarly, we were unable to confirm an association between younger age and PRAEs (area under the ROC curve: 0.61; 95% CI: 0.45, 0.71). Other PSG parameters including total AHI, obstructive AHI, oxygen saturation nadir, baseline oxygen saturation asleep, CT90, and ODI3 were not predictive of PRAEs following AT on univariate analysis.
Table 3.
Univariate analysis of PRAE predictors.
| Covariate | Degrees of Freedom | Area Under the Curve (95% CI) |
|---|---|---|
| McGill oximetry score | 1 | 0.62 (0.47, 0.77) |
| Age category* | 2 | 0.61 (0.45, 0.76) |
| Oxygen saturation nadir | 1 | 0.60 (0.42, 0.78) |
| Obstructive AHI | 1 | 0.59 (0.41, 0.76) |
| Baseline asleep oxygen saturation | 1 | 0.58 (0.41, 0.74) |
| Total AHI | 1 | 0.54 (0.38, 0.71) |
| CT90 | 1 | 0.52 (0.34, 0.69) |
| Oxygen desaturation index | 1 | 0.52 (0.35, 0.68) |
*Categorized into < 3 years, 3–5 years, ≥ 6 years. CI = confidence interval, AHI = apnea-hypopnea index, CT90 = cumulative time percentage with oxygen saturation less than 90%, PRAE = perioperative respiratory adverse event.
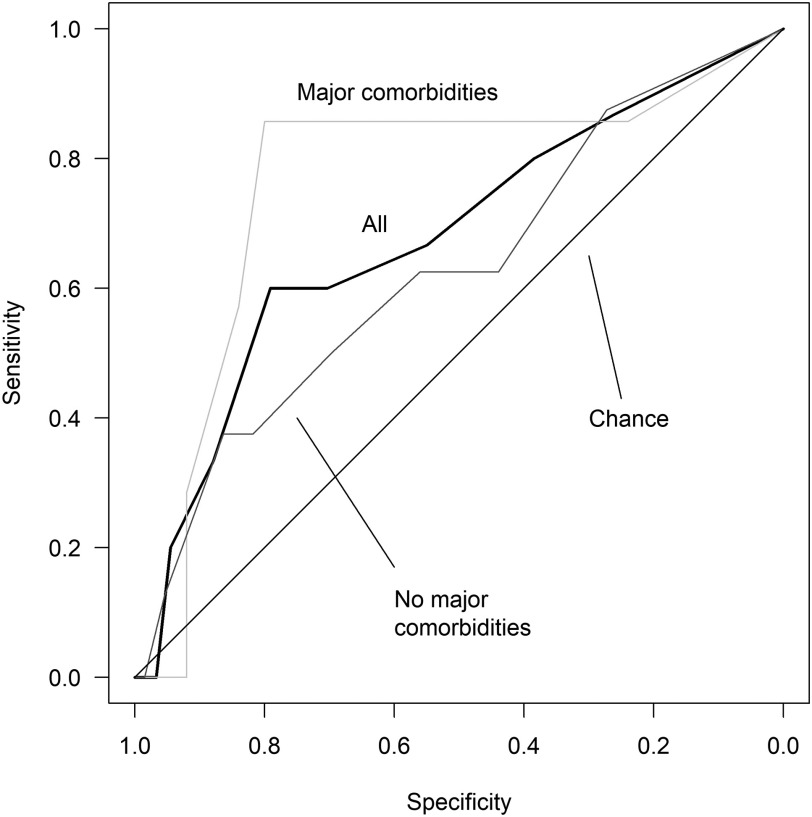
A multivariable prediction model that combined MOS and age showed evidence for the ability to predict PRAEs with an area under the ROC curve of 0.68 (95% CI: 0.52, 0.84) (Table 4). We found evidence that the same model may predict PRAEs following AT in children with major comorbidities (area under the ROC curve: 0.77; 95% CI: 0.53, 1.00). We were unable to confirm whether this model could predict PRAEs in children without major comorbidities (area under the ROC curve: 0.63; 95% CI: 0.40, 0.85). There was no significant difference for the predictive ability of the model based on the presence of major comorbidities (P = .41). Figure 1 shows the ROC curve for the total population as well as subgroups of children with and without major comorbidities. We also tested a multivariable prediction model with age and oxygen saturation nadir, because it was the term that performed second best to the MOS as a univariate predictor. This model performed nearly as well, with an area under the ROC curve of 0.66 (95% CI: 0.49, 0.82) (Table 5).
Table 4.
Multivariable model with McGill oximetry score and age.
| PRAE n/N (%) | Univariable Odds Ratio (95% CI) | P | Multivariable Odds Ratio (95% CI) | P | |
|---|---|---|---|---|---|
| McGill oximetry score | 1.49 (0.90, 2.50) | .12 | 1.43 (0.83, 2.46) | .19 | |
| Age category (years) | .19 | .26 | |||
| ≥ 6 | 6/57 (10.5) | 1.00 (ref) | 1.00 (ref) | ||
| 3 to 5 | 6/41 (14.6) | 1.46 (0.42, 5.02) | 1.29 (0.36, 4.52) | ||
| < 3 | 3/8 (37.5) | 5.10 (0.88, 27.02) | 4.30 (0.72, 23.23) |
The area under the receiver operating characteristic curve is 0.68 (95% CI: 0.52, 0.84). CI = confidence interval, PRAE = perioperative respiratory adverse event, ref = reference.
Figure 1. Receiver operating characteristic curve for the multivariable prediction model combining McGill oximetry score and age.
A multivariable model combining McGill oximetry score and age predicted major perioperative respiratory adverse events in children following adenotonsillectomy with an area under the receiver operating characteristic curve of 0.68 (95% CI: 0.52, 0.84). The area under the receiver operating characteristic curve was 0.77 (95% CI: 0.53, 1.00) for the subgroup of children with major comorbidities and 0.63 (95% CI: 0.40, 0.85) for the subgroup of children without major comorbidities. CI = confidence interval.
Table 5.
Multivariable model with oxygen saturation nadir and age.
| PRAE n/N (%) | Univariable Odds Ratio (95% CI) | P | Multivariable Odds Ratio (95% CI) | P | |
|---|---|---|---|---|---|
| Oxygen saturation nadir | 0.97 (0.93, 1.01) | .11 | 0.97 (0.93, 1.01) | .16 | |
| Age category (years) | .19 | .25 | |||
| ≥ 6 | 6/57 (10.5) | 1.00 (ref) | 1.00 (ref) | ||
| 3 to 5 | 6/41 (14.6) | 1.46 (0.42, 5.02) | 1.35 (0.38, 4.72) | ||
| < 3 | 3/8 (37.5) | 5.10 (0.88, 27.02) | 4.44 (0.74, 24.04) |
The area under the receiver operating characteristic curve is 0.66 (95% CI: 0.49, 0.82). CI = confidence interval, PRAE = perioperative respiratory adverse event, ref = reference.
MOS
The MOS had excellent interrater reliability with a κ value of 0.95. There were discordant results for 4 of 106 children (3.7%) based on the scores assigned by the initial 2 scorers. Scoring discordance only occurred for oximetries that received a MOS of 1 to 3, and the discrepancy was no greater than 1 in each instance. All discrepancies were resolved by a third scorer. There was perfect agreement for all oximetries that received an MOS of 4.
We found that the MOS was highly correlated with oxygen saturation nadir and CT90, with Spearman coefficients of −0.73 (95% CI: −0.80, −0.62; P < .001) and 0.85 (95% CI: 0.79, 0.90; P < .001), respectively. MOS was moderately correlated with ODI3 with a Spearman coefficient of 0.61 (95% CI: 0.47, 0.71; P < .001) (Table 6).33 Total AHI, obstructive AHI, and central AHI were not strongly correlated with the MOS, although total AHI was higher with increasing MOS. Children with an MOS of 1 had a median AHI of 4.7 events/h (IQR: 2.0, 10.1 events/h), with MOS of 2 had a median AHI of 9.8 events/h (IQR: 5.1, 15.7 events/h), with an MOS of 3 had a median AHI of 12.7 events/h (IQR: 9.7, 18.3 events/h), and with an MOS of 4 had a median AHI of 16.3 events/h (IQR: 9.4, 31.7 events/h).
Table 6.
Spearman correlation of polysomnography parameters with McGill oximetry score.
| Polysomnography Parameter | Median (IQR) | Correlation with MOS Spearman Coefficient (95% CI) | P |
|---|---|---|---|
| Apnea-hypopnea index, events/h | 9.7 (4.2, 16.2) | 0.43 (0.26, 0.57) | < .001 |
| Obstructive apnea-hypopnea index, events/h | 3.6 (0.1, 10.6) | 0.30 (0.12, 0.46) | .002 |
| Central apnea-hypopnea index, events/h | 3.1 (1.2, 7.2) | 0.19 (–0.01, 0.36) | .06 |
| Oxygen saturation nadir, % | 84.0 (80.0, 90.0) | –0.73 (–0.80, –0.62) | < .001 |
| Baseline mean oxygen saturation asleep, % | 97.0 (96.5, 98.0) | –0.20 (–0.38, –0.01) | .04 |
| CT90, % | 0.1 (0.0, 0.4) | 0.85 (0.79, 0.90) | < .001 |
| Oxygen desaturation index, events/h | 6.2 (2.2, 13.3) | 0.61 (0.47, 0.71) | < .001 |
CI = confidence interval, CT90 = cumulative time percentage with oxygen saturation less than 90%, IQR = interquartile range, MOS = McGill oximetry score.
DISCUSSION
This study confirmed our hypothesis that the MOS performed better than traditional PSG parameters such as the AHI in predicting major PRAEs following AT. The combination of the MOS and age was predictive of major PRAEs in a heterogeneous population of children undergoing AT. The MOS correlated well with other measures of nocturnal hypoxemia including the oxygen saturation nadir and CT90, but better represents the accumulated hypoxemic burden as it assesses both depth of desaturation and time spent desaturated. Furthermore, the MOS is easy to perform and apply reliably.
PRAEs
We report a major PRAE rate of 14% (15/106) within a heterogenous population of children with varying medical complexity who were cared for at an academic tertiary care center. This is a substantial complication rate but is lower than in previously reported studies of children with OSAS and varying medical complexity who have major PRAE rates of 15–27%.14,16,17 Our population had milder OSAS than previous study populations and we hypothesize that this may have contributed to the lower PRAE rate. Notably, our population had a median oxygen saturation nadir of 84.0% (IQR: 80.0%, 90.0%) and median obstructive AHI of 3.6 events/h (IQR: 0.1, 10.6 events/h), which would not meet criteria for admission post-AT according to the American Academy of Otolaryngology–Head and Neck Surgery Guidelines.21 Additionally, we employed stringent criteria to define major PRAEs, whereby only those requiring intervention and occurring outside of the immediate postoperative period were included. We believe that this definition of major PRAEs is a strength of our study as it reflects clinically significant events that may alter decision making regarding the ideal postoperative monitoring environment and duration of monitoring.
Predictors of major PRAEs
Overall, major PRAEs are quite difficult to predict, particularly in populations of children with predominantly mild to moderate OSAS, such as ours. It remains elusive how age and comorbidities modulate the severity of OSAS and the frequency and severity of PRAEs in patients presenting for AT.34 The effect of comorbidities on a multivariable prediction model including MOS and age to predict PRAEs in a group of children following AT could not be completely elucidated given the limitations of the sample size. Although we did not demonstrate that PSG parameters are significant predictors of PRAEs on univariate modeling, our results suggest that the MOS may be more predictive of PRAEs than traditionally used metrics such as AHI. Indeed, we found that a prediction model with MOS and age performed slightly better at predicting PRAEs in comparison to a model with oxygen saturation nadir and age. Nixon and colleagues27 also reported that a higher MOS is associated with PRAEs following AT, but they did not compare the predictive ability of the MOS with other PSG parameters. Jamieson and colleagues30 found that major PRAEs were more common among children with a preoperative MOS of 2 to 4. These children were also more likely to have an abnormal postoperative oximetry on the first postoperative night compared with children with a preoperative MOS of 1. PSG gas exchange parameters, such as the MOS, may perform better at predicting major PRAEs because they are a direct measure of accumulated nocturnal hypoxemic burden as compared with the AHI, which is confounded by the arousal response. We hypothesize that a blunted arousal response in children with OSAS predisposes to postoperative gas exchange abnormalities. Generally, arousals from sleep occur during respiratory events and result in sleep fragmentation but are protective of gas exchange abnormalities by immediately terminating major obstructive events. Children with OSAS may have higher arousal thresholds to the most prominent respiratory stimuli, hypercapnia and respiratory effort,35,36 resulting in a delayed arousal to upper airway obstruction and subsequently more frequent or severe desaturations. The AHI includes respiratory events with decreased respiratory flow that may be associated with desaturation or arousal; it is unable to discern children with protective arousal responses from those who do not have protective arousal responses. In contrast, gas exchange parameters such as the MOS and ODI3 directly measure respiratory events that result in desaturation with absent or delayed arousal responses, and may therefore be more predictive of PRAEs following AT.
Our finding that the MOS has the potential to predict PRAEs is quite important, as no other PSG parameters have been found to predict PRAEs in populations with mostly mild OSAS. Konstantinopoulou and colleagues20 similarly evaluated the association between PSG parameters and complications following AT in children with predominantly mild OSAS. They did not report any significant associations of PRAEs with AHI, oxygen saturation nadir, CT90, peak end-tidal carbon dioxide, or percent time with end-tidal carbon dioxide greater than 50 mm Hg. Dalesio and colleagues25 also found that PSG parameters were not significantly associated with postoperative oxygen desaturation in multivariable regression analysis. Unlike other PSG measures, the MOS has the ability to better quantify accumulated nocturnal hypoxemic burden, which likely enhances its ability to predict PRAEs accurately. The MOS can be derived from nocturnal oximetry by a trained assessor and the oximetry could potentially be completed in the home setting. Furthermore, it is a convenient rating system that requires a median time of 9 seconds per study to apply (IQR: 2, 45 seconds).27 The degree of accumulated nocturnal hypoxemic burden appears to be responsible for a chronic low-grade systemic inflammatory response, the modulation of which may influence anesthetic and drug-related adverse respiratory related events through a γ-aminobutyric acid (GABA) receptor–mediated mechanism.37–39 The role of steroids and nonsteroidal anti-inflammatory agents including celecoxib to modulate the perioperative inflammatory response and influence the incidence of PRAEs after AT is unclear.40,41
MOS
Similarly to Nixon and colleagues,27 we found excellent interrater reliability among individuals with varying degrees of prior experience using the MOS. However, a prominent learning curve could be observed whereby clarifications of technical scoring criteria were initially required in the scoring process.
As expected, the MOS was strongly correlated with measures of oxygen saturation including oxygen saturation nadir, CT90, and ODI3. However, unlike Nixon and colleagues27 who demonstrated that the MOS was associated with AHI, we did not find that the MOS was strongly correlated with total, central, or obstructive AHI. The 4% oxygen desaturation index and AHI have previously been reported to correlate,42 so it would be expected that the MOS, which measures the frequency and depth of desaturation, may also be expected to correlate with AHI. However, the scoring criteria for AHI do not require desaturation,31 and this would allow for the discordance between AHI and measures of saturation including the MOS. Furthermore, it has been suggested that oxygen desaturation and AHI correlation decreases with milder OSAS42,43 and this likely contributed to our results as our study population had milder OSAS as reflected by an obstructive AHI median of 3.6 events/h.
Generalizability and limitations
Our institution serves as a large referral center with a wide catchment area. As such, our study population includes a heterogenous sample of children with varying medical comorbidities as well as children without additional comorbidity. The main limitation to the generalizability of our study is that only a subset of children with suspected OSAS, who are deemed at highest risk, will undergo PSG prior to AT at our institution. Our study only included 2.6% of all children who underwent AT, tonsillectomy, or adenoidectomy from 2010 to 2016 inclusive due to the scarcity of access to PSGs in our region.44 The decision to complete a PSG prior to AT is made on an individual basis as opposed to a protocol; therefore, it is difficult to determine the precise reasons why PSG is completed preoperatively in a subset of children who undergo AT. Despite this, our study population had milder OSAS compared with previously studied populations of children. Another limitation is our small sample size, which restricted the number of variables that could be included in our multivariable regression analysis. It also influenced our ability to detect statistically significant predictors; indeed, CIs for the area under the ROC curve crossed 0.5 for each studied predictor in univariate and multivariate analysis. Additionally, we were unable to examine carbon dioxide parameters as predictors of PRAEs due to technically unreliable measurements in many children. Future study of carbon dioxide parameters could provide further evidence that gas exchange parameters are the most important predictors for PRAEs. Finally, we were unable to account for the varying skill levels of care providers or variation in benzodiazepine premedication or opioid dose, which may have influenced our PRAE rate.45,46
CONCLUSIONS
We found evidence that a model including MOS and age may have the ability to predict major PRAEs following AT in a population of children with predominantly mild OSAS. This is a significant finding as it has historically been quite difficult to predict PRAEs within this population. Our findings suggest that nocturnal oximetry provides the most essential information to direct postoperative monitoring following AT. The use of MOS from nocturnal oximetry in place of PSGs to predict PRAEs could be advantageous due to its accessibility, better tolerance, and potential cost savings. Although the absence of desaturations does not exclude OSAS, the risk for a major PRAE is low. Future studies should further validate the use of MOS in a larger and more diverse population of children with OSAS undergoing AT and consider other multidimensional measures of gas exchange that capture the accumulated nocturnal hypoxemic burden.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Children’s Hospital of Eastern Ontario, Ottawa, Canada. Dr. Katz has received a speaker honorarium from Biogen, unrelated to this work. The other authors report no conflicts of interest.
ABBREVIATIONS
- AHI,
apnea-hypopnea index
- AT,
adenotonsillectomy
- CT90,
cumulative time percentage with oxygen saturation less than 90%
- MOS,
McGill oximetry score
- ODI3,
3% oxygen desaturation index
- OSAS,
obstructive sleep apnea syndrome
- PRAE,
perioperative respiratory adverse events
- PSG,
polysomnography
- ROC,
receiver operating characteristic
REFERENCES
- 1. Lumeng JC, Chervin RD . Epidemiology of pediatric obstructive sleep apnea . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 242 – 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584. [DOI] [PubMed] [Google Scholar]
- 3. Kohler MJ, Lushington K, Kennedy JD . Neurocognitive performance and behavior before and after treatment for sleep-disordered breathing in children . Nat Sci Sleep. 2010. ; 2 : 159 – 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biggs SN, Vlahandonis A, Anderson V, et al . Long-term changes in neurocognition and behavior following treatment of sleep disordered breathing in school-aged children . Sleep. 2014. ; 37 ( 1 ): 77 – 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biggs SN, Walter LM, Jackman AR, et al . Long-term cognitive and behavioral outcomes following resolution of sleep disordered breathing in preschool children . PLoS One. 2015. ; 10 ( 9 ): e0139142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith DF, Amin RS . OSA and cardiovascular risk in pediatrics . Chest. 2019. ; 156 ( 2 ): 402 – 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esteller E, Villatoro JC, Agüero A, et al . Obstructive sleep apnea syndrome and growth failure . Int J Pediatr Otorhinolaryngol. 2018. ; 108 : 214 – 218. [DOI] [PubMed] [Google Scholar]
- 8. Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al . Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management . Eur Respir J. 2016. ; 47 ( 1 ): 69 – 94. [DOI] [PubMed] [Google Scholar]
- 9. Goldman JL, Baugh RF, Davies L, et al . Mortality and major morbidity after tonsillectomy: etiologic factors and strategies for prevention . Laryngoscope. 2013. ; 123 ( 10 ): 2544 – 2553. [DOI] [PubMed] [Google Scholar]
- 10. Subramanyam R, Chidambaran V, Ding L, Myer CMIII , Sadhasivam S. Anesthesia- and opioids-related malpractice claims following tonsillectomy in USA: LexisNexis claims database 1984–2012 . Paediatr Anaesth. 2014. ; 24 ( 4 ): 412 – 420. [DOI] [PubMed] [Google Scholar]
- 11. Coté CJ, Posner KL, Domino KB . Death or neurologic injury after tonsillectomy in children with a focus on obstructive sleep apnea: Houston, we have a problem ! Anesth Analg. 2014. ; 118 ( 6 ): 1276 – 1283. [DOI] [PubMed] [Google Scholar]
- 12. Katz SL, Monsour A, Barrowman N, et al . Predictors of postoperative respiratory complications in children undergoing adenotonsillectomy . J Clin Sleep Med. 2020. ; 16 ( 1 ): 41 – 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown KA, Morin I, Hickey C, Manoukian JJ, Nixon GM, Brouillette RT . Urgent adenotonsillectomy: an analysis of risk factors associated with postoperative respiratory morbidity . Anesthesiology. 2003. ; 99 ( 3 ): 586 – 595. [DOI] [PubMed] [Google Scholar]
- 14. McColley SA, April MM, Carroll JL, Naclerio RM, Loughlin GM . Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea . Arch Otolaryngol Head Neck Surg. 1992. ; 118 ( 9 ): 940 – 943. [DOI] [PubMed] [Google Scholar]
- 15. Biavati MJ, Manning SC, Phillips DL . Predictive factors for respiratory complications after tonsillectomy and adenoidectomy in children . Arch Otolaryngol Head Neck Surg. 1997. ; 123 ( 5 ): 517 – 521. [DOI] [PubMed] [Google Scholar]
- 16. Rosen GM, Muckle RP, Mahowald MW, Goding GS, Ullevig C . Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994. ; 93 ( 5 ): 784 – 788 . [PubMed] [Google Scholar]
- 17. Jaryszak EM, Shah RK, Vanison CC, Lander L, Choi SS . Polysomnographic variables predictive of adverse respiratory events after pediatric adenotonsillectomy . Arch Otolaryngol Head Neck Surg. 2011. ; 137 ( 1 ): 15 – 18. [DOI] [PubMed] [Google Scholar]
- 18. Wilson K, Lakheeram I, Morielli A, Brouillette R, Brown K . Can assessment for obstructive sleep apnea help predict postadenotonsillectomy respiratory complications? Anesthesiology. 2002. ; 96 ( 2 ): 313 – 322. [DOI] [PubMed] [Google Scholar]
- 19. Hill CA, Litvak A, Canapari C, et al . A pilot study to identify pre- and peri-operative risk factors for airway complications following adenotonsillectomy for treatment of severe pediatric OSA . Int J Pediatr Otorhinolaryngol. 2011. ; 75 ( 11 ): 1385 – 1390. [DOI] [PubMed] [Google Scholar]
- 20. Konstantinopoulou S, Gallagher P, Elden L, et al . Complications of adenotonsillectomy for obstructive sleep apnea in school-aged children . Int J Pediatr Otorhinolaryngol. 2015. ; 79 ( 2 ): 240 – 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitchell RB, Archer SM, Ishman SL, et al . Clinical practice guideline: tonsillectomy in children (update) . Otolaryngol Head Neck Surg. 2019. 160, 1 _suppl, 1S: S1 – S42. [DOI] [PubMed] [Google Scholar]
- 22. Keamy DG, Chhabra KR, Hartnick CJ . Predictors of complications following adenotonsillectomy in children with severe obstructive sleep apnea . Int J Pediatr Otorhinolaryngol. 2015. ; 79 ( 11 ): 1838 – 1841. [DOI] [PubMed] [Google Scholar]
- 23. Martins RO, Castello-Branco N, Barros JL, Weber SAT . Risk factors for respiratory complications after adenotonsillectomy in children with obstructive sleep apnea . J Bras Pneumol. 2015. ; 41 ( 3 ): 238 – 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thongyam A, Marcus CL, Lockman JL, et al . Predictors of perioperative complications in higher risk children after adenotonsillectomy for obstructive sleep apnea: a prospective study . Otolaryngol Head Neck Surg. 2014. ; 151 ( 6 ): 1046 – 1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dalesio NM, McMichael DH, Benke JR, et al . Are nocturnal hypoxemia and hypercapnia associated with desaturation immediately after adenotonsillectomy? Paediatr Anaesth. 2015. ; 25 ( 8 ): 778 – 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molero-Ramirez H, Tamae Kakazu M, Baroody F, Bhattacharjee R . Polysomnography parameters assessing gas exchange best predict postoperative respiratory complications following adenotonsillectomy in children with severe OSA . J Clin Sleep Med. 2019. ; 15 ( 9 ): 1251 – 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nixon GM, Kermack AS, Davis GM, Manoukian JJ, Brown KA, Brouillette RT . Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry . Pediatrics. 2004. ; 113 ( 1 Pt 1 ): e19 – e25. [DOI] [PubMed] [Google Scholar]
- 28. Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM . Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea . Pediatrics. 2000. ; 105 ( 2 ): 405 – 412. [DOI] [PubMed] [Google Scholar]
- 29. Horwood L, Brouillette RT, McGregor CD, Manoukian JJ, Constantin E . Testing for pediatric obstructive sleep apnea when health care resources are rationed . JAMA Otolaryngol Head Neck Surg. 2014. ; 140 ( 7 ): 616 – 623. [DOI] [PubMed] [Google Scholar]
- 30. Jamieson K, Soh HJ, Davey MJ, Rimmer J, Horne RSC, Nixon GM . Continuous oximetry recordings on the first post-operative night after pediatric adenotonsillectomy-a case-control study . Int J Pediatr Otorhinolaryngol. 2020. ; 138 : 110313. [DOI] [PubMed] [Google Scholar]
- 31. Berry RB, Brooks R, Gamaldo CE, et al. ; American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications , Version 2.2. Darien, IL: : American Academy of Sleep Medicine; ; 2015. [Google Scholar]
- 32. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. Accessed March 9, 2020.
- 33. Overholser BR, Sowinski KM . Biostatistics primer: part 2 . Nutr Clin Pract. 2008. ; 23 ( 1 ): 76 – 84. [DOI] [PubMed] [Google Scholar]
- 34. Zalan J, Vaccani J-P, Murto KT . Paediatric adenotonsillectomy, part 2: considerations for anaesthesia . BJA Educ. 2020. ; 20 ( 6 ): 193 – 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcus CL, Moreira GA, Bamford O, Lutz J . Response to inspiratory resistive loading during sleep in normal children and children with obstructive apnea . J Appl Physiol 1985. ; 87 ( 4 ): 1448 – 1454. [DOI] [PubMed] [Google Scholar]
- 36. Marcus CL, Lutz J, Carroll JL, Bamford O . Arousal and ventilatory responses during sleep in children with obstructive sleep apnea . J Appl Physiol 1985. ; 84 ( 6 ): 1926 – 1936. [DOI] [PubMed] [Google Scholar]
- 37. Avramescu S, Wang D-S, Lecker I, et al . Inflammation increases neuronal sensitivity to general anesthetics . Anesthesiology. 2016. ; 124 ( 2 ): 417 – 427. [DOI] [PubMed] [Google Scholar]
- 38. Tan H-L, Gozal D, Kheirandish-Gozal L . Obstructive sleep apnea in children: a critical update . Nat Sci Sleep. 2013. ; 5 : 109 – 123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ehsan Z, Mahmoud M, Shott SR, Amin RS, Ishman SL . The effects of anesthesia and opioids on the upper airway: a systematic review . Laryngoscope. 2016. ; 126 ( 1 ): 270 – 284. [DOI] [PubMed] [Google Scholar]
- 40. Redmann AJ, Maksimoski M, Brumbaugh C, Ishman SL . The effect of postoperative steroids on post-tonsillectomy pain and need for postoperative physician contact . Laryngoscope. 2018. ; 128 ( 9 ): 2187 – 2192. [DOI] [PubMed] [Google Scholar]
- 41. Caulfield HM, Cunningham A, Naik R . The use of medical treatment to optimise respiratory function prior to adenotonsillectomy for sleep disordered breathing in the under 3 age group: our experience in one hundred and forty two children . Clin Otolaryngol. 2012. ; 37 ( 6 ): 488 – 491. [DOI] [PubMed] [Google Scholar]
- 42. Tsai C-M, Kang C-H, Su M-C, et al . Usefulness of desaturation index for the assessment of obstructive sleep apnea syndrome in children . Int J Pediatr Otorhinolaryngol. 2013. ; 77 ( 8 ): 1286 – 1290. [DOI] [PubMed] [Google Scholar]
- 43. Dos Santos C, Samuels M, Laverty A, Raywood E . Comparison of oxygen desaturation index and apnoea-hypopnoea index for categorising OSA in children . Eur Respir J. 2018. ; 52 : PA549. [Google Scholar]
- 44. Katz SL, Witmans M, Barrowman N, et al . Paediatric sleep resources in Canada: the scope of the problem . Paediatr Child Health. 2014. ; 19 ( 7 ): 367 – 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taenzer AH, Spence BC . The afferent limb of rapid response systems: continuous monitoring on general care units . Crit Care Clin. 2018. ; 34 ( 2 ): 189 – 198. [DOI] [PubMed] [Google Scholar]
- 46. Habre W, Disma N, Virag K, et al. ; APRICOT Group of the European Society of Anaesthesiology Clinical Trial Network. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe . Lancet Respir Med. 2017. ; 5 ( 5 ): 412 – 425. [DOI] [PubMed] [Google Scholar]