Abstract
To date the study of ocean acidification on fish otolith formation has been mainly focused on larval and juvenile stages. In the present pilot study, wild-captured adult Atlantic cod (Gadus morhua) were exposed to two different levels of pCO2, 422µatm (ambient, low pCO2) or 1091µatm (high pCO2), for a period of 30 weeks (from mid-October to early April 2014–2015) in order to study the effects on otolith size, shape and CaCO3 crystallization amongst other biological parameters. We found that otoliths from cod exposed to high pCO2 were slightly smaller (− 3.4% in length; − 3.3% in perimeter), rounder (− 2.9% circularity and + 4% roundness) but heavier (+ 5%) than the low pCO2 group. Interestingly, there were different effects in males and females; for instance, male cods exposed to high pCO2 exhibited significant changes in circularity (− 3%) and roundness (+ 4%) compared to the low pCO2 males, but without significant changes on otolith dimensions, while females exposed to high pCO2 had smaller otoliths as shown for length (− 5.6%), width (− 2%), perimeter (− 3.5%) and area (− 4.8%). Furthermore, while the majority of the otoliths analysed showed normal aragonite deposition, 10% of fish exposed to 1091µatm of pCO2 had an abnormal accretion of calcite, suggesting a shift on calcium carbonate polymorph crystallization in some individuals under high pCO2 conditions. Our preliminary results indicate that high levels of pCO2 in adult Atlantic cod might affect otolith growth in a gender-specific way. Our findings reveal that otoliths from adult cod are affected by ocean acidification, and we believe that the present study will prompt further research into this currently under-explored area.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10695-021-01016-6.
Keywords: Adult cod, Calcite, Gender, Otolith, Ocean acidification
Background
Rising levels of atmospheric CO2 are leading to an increase in the average ocean CO2 partial pressure (pCO2), which translates to a decrease in environmental pH, a phenomenon known as ocean acidification (Caldeira 2005). Elevated pCO2 levels in sea water modify the saturation states of the different calcium carbonate (CaCO3) polymorphs (Jones et al. 2017) and alter the formation/dissolution rates of CaCO3-based structures (Hofmann et al. 2010). It is known that organisms with external calcium carbonate structures, such as mollusc shells, arthropod exoskeletons or coral “skeletons”, will be particularly sensitive to ocean acidification owing to the increase in carbonate dissolution rates that will weaken such structures (Hofmann et al. 2010). Otoliths are CaCO3-based structures located in the inner ear of fish and are responsible for hearing, balance and navigation (Popper et al. 2005). Otoliths are formed extracellularly through the accretion of CaCO3 in the form of aragonitic crystals, which are integrated into a protein-based matrix (Allemand et al. 2007). Otolith formation is a very dynamic process and varies significantly in response to environmental conditions (such as temperature or pH), fish ontology (such as age, gender, size or sexual maturation) and physiological status (nutrition, spawning or stress) (Morales-Nin 2000; Bestgen and Bundy 1998; Radtke and Fey 1996).
Research on the effects of ocean acidification on fish otolith formation has increased in recent years (Heuer and Grosell 2014) with some studies reporting an overall increase of otolith size and/or density for some species (Bignami et al. 2013; Checkley et al. 2009a; Maneja et al. 2013; Pimentel et al. 2014; Réveillac et al. 2015; Coll-Lladó et al. 2018). In addition, studies in gilthead sea bream (Sparus aurata) larvae have shown that exposure to 2000µatm of pCO2 for a period of 15 days also promotes the replacement of aragonite crystals (the common CaCO3 polymorph found in otoliths) by calcite in 21% of the individuals (Coll-Lladó et al. 2018). While calcite is a common CaCO3 polymorph in invertebrates, it is rarely found in fish otoliths, and only some primitive species of fish have calcite, combined with vaterite, as the main CaCO3 polymorph in their otoliths (Pracheil et al. 2017). Vaterite, but not calcite, otoliths are commonly found in many aquaculture-reared fish species (Gauldie et al. 1997; Whitley et al. 1999), and some authors have suggested that vaterite deposition is the result of abnormally high growth rates (Reimer et al. 2017) or high animal density in the farms (Austad et al. 2021). Functionally, transmission of sound waves through otoliths is significantly influenced by the size of the otolith and the presence of non-aragonite CaCO3 polymorphs, indicating that ocean acidification might have a negative impact on fish hearing and navigation if size and/or CaCO3 composition are affected (Bignami et al. 2013; Radford et al. 2021; Reimer et al. 2016). Despite advances in understanding the effects of ocean acidification on otolith formation, the vast majority of studies have been focusing on larval and juvenile stages.
Atlantic cod (Gadus morhua) is a commercially and ecologically important species with a wide distribution throughout the North Atlantic Ocean (Hylen et al. 2008) and considerable intraspecific stock-dependent differences in terms of growth rate, age at maturity, timing of spawning and life expectancy (Brander 2005). The majority of studies on otolith formation simulating ocean acidification conditions use relatively short exposure times to high pCO2 levels on fish embryos, larvae or juveniles (Checkley et al. 2009b; Coll-Lladó et al. 2018; Jarrold and Munday 2018; Réveillac et al. 2015), while experiments on adults are scarce. To understand how long-term exposure to pCO2 levels predicted for the year 2100 (Stocker 2013, appr. global average end of the century, RCP 8.5) would affect otolith growth and CaCO3 crystallization in adult North East Arctic Migratory Cod (Skrej), wild-captured adult fish were exposed to predicted (1091µatm; high pCO2) or ambient (422µatm; low pCO2) pCO2 levels for a period of 30 weeks, and otolith size, shape and main CaCO3 polymorph were thereafter analysed.
Methods
Ethics statement
This study was carried out in strict accordance with the laboratory regulations applicable in Norway. The application was approved by the National Regulatory Committee on the Ethics of Animal Experiments (Permit TRANSCOD project FOTS id 6915 and ACIDCOD project FOTS id 7346). All conditions and samplings were conducted to minimize suffering and stress.
As a result of the size of the fish used in the present work, and in order to ensure animal’s welfare, two adjacent tanks of 25m3 (25,000 L) were used with identical environmental conditions apart from the experimental treatment (low or high carbon dioxide) since replication of treatments were not possible.
Fish husbandry and experimental conditions
Adult cods (N = 135) were caught in October 2014 in the Barents Sea at 70°15′ N, 19°00′ E and transported to NOFIMA’s Centre for Marine Aquaculture in Tromsø, Norway. The animals were sexed and measured before being equally distributed between the two 25m3 tanks with a continuous supply of water from the adjacent fjord. The CO2 was added to the header tank of the high CO2 experimental condition simulating a future climate scenario (Stocker 2013, global average end of the century, RCP 8.5), while no CO2 was added to the control tanks. From December 2014 to January 2015, a total of 20 animals from the low pCO2 and 13 from the high pCO2 died as a result of infections, 6 animals per group were sampled at the middle of the experimental period for other analyses, while the rest of animals (41 for low pCO2 and 49 for high pCO2) survived until being euthanized and sampled at the end of the experiment. In addition to total alkalinity (AT) and total carbon (CT), in situ pCO2 was estimated weekly (average values for the two treatments presented in Table 1, for more details of the methodology, see SI Stiasny et al. 2016). As a proxy for pCO2, the water pH (pHNBS) was continuously monitored using an IKS Aquastar Controller unit (Germany), which further controlled the CO2 inflow into the treatment tank. A handheld probe was used to measure the daily temperature, salinity and oxygen concentration. Water temperature and salinity were kept at ambient levels, while the light regime was adjusted to local sunrise and sunset on a weekly basis. In order to minimize any possible tank effect, experimental tanks were identical in structure and support systems and were placed 2 m of each other. All environmental conditions (light, temperature, salinity, etc.) except pH were the same during the whole experimental period. The fish were fed ad libitum with frozen capelin (Mallotus villosus) three times per week. Animals were caught with a landing net and euthanized using a MS222 overdose (200 mg/L) (tricaine methanesulfonate TMS, metacaine, finquel). All fish were sexed and weighed, and standard length (SL) measured for each individual. Otoliths were then extracted by cutting through the post parietal bones, removed with forceps and stored in labelled paper bags at room temperature until further analysis.
Table 1.
Average sea water carbonate parameters during the experimental period
| Treatment | T (Co) | CT (µmol/L) | AT (µeq L−1) | pCO2 (µatm) | pHNBS |
|---|---|---|---|---|---|
| Low-pCO2 | 4.6 ± 0.3 | 2154.9 ± 1.9 | 2285.9 ± 3.5 | 422.3 ± 4.3 | 8.02 ± 0.01 |
| High-pCO2 | 4.6 ± 0.4 | 2274.7 ± 29.2 | 2288.2 ± 3.06 | 1091.5 ± 282.8 | 7.64 ± 0.10 |
T temperature, CT total carbon, At total alkalinity, pCO2 CO2 partial pressure. Values are expressed as mean ± SD
Otolith measurements
Right and left otoliths from individual animals (81 otoliths from the low pCO2 group and 98 otoliths for the high pCO2 group) were photographed using a digital camera fixed in a support (RS2 2 XA Copylizer, Kaiser Fototechnik, Germany) against a black background, homogeneous light and a millimetre scale for internal calibration. Otolith length (OL), width (OR), area (OA) and perimeter (OP) were measured twice using ImageJ software (Girish and Vijayalakshmi 2004) to ensure accuracy in the measurements (Supplementary File 1). Otolith total weight (OW) was measured using a digital scale (Sartorius, Germany, 0.01 g precision). For better comparison between otolith measurements from animals of different sizes, an adjusted means (least squares) was used for all measurements (see the statistics section below).
Otolith shape indexes, generally used for discrimination between stocks, were estimated based on previous literature (Pothin et al. 2006; Leguá et al. 2013) as follows:
Raman spectroscopy
Right side otoliths were immersed in a 1% sodium hypochlorite solution for 10 min to remove all organic material on the surface followed by several washes with distilled water. Raman spectra were recorded from the otoliths surface with a Horiba Jobin Yvon LabRam HR instrument using 514 nm excitation wavelength and a 50 × magnification, long working distance objective. Laser intensity was attenuated using neutral density filters to prevent laser-induced transformation of the polymorph.
Statistical analysis
All statistical analyses and graphs were conducted using R-Studio v.1.1.419 (RStudio Team 2015). Data normality and homogeneity of variance were estimated using the Shapiro–Wilk and Levene’s tests, respectively. A Box-Cox transformation approach was used to transform non-normally distributed data, and normality assumptions were estimated again. OL, OR, OP and OA measurements were analysed using linear mixed models (lme4 R-package) (Bates et al. 2015) with treatment as a fixed factor and SL and gender as covariants, while head side (side) was considered as random factor. When data was re-analysed for each gender, mixed models with SL as covariant and side as a random factor were used. Since animal body weight was highly correlated to SL (> 0.80 Pearson’s correlation) (Supplementary File 2), the parameter was not included in the model.
Shape indexes (Cicl, Rect, Rd and Ellip) are not directly affected by the length of the animal (SL was excluded from the statistical model), but they can be affected by the age of the animal. While empirical estimation of cod age by ring counting was not performed in the present pilot study, previous research has found that otolith weight (OW) correlates well with fish age (Bermejo 2014; Campana and Fowler 2012; Pawson 2006). Therefore, OW was used as a proxy for age and was included in the statistical model as covariant. Adjusted means (least squares) and standard errors (SE) for all parameters analysed were extracted from the mixed models using the lsmeans package (Russel 2016).
Unless otherwise indicated, values directly measured (raw data) are shown as mean ± SD, while adjusted values for the different parameters are shown as least squares ± SE. Pearson’s test was used to estimate the correlation between the different parameters. The signification threshold was established as P-value (P) < 0.05.
All graphs were produced using the ggplot2 R-build package (Wickham 2016). R-regression plots include 95% confidence intervals estimated using the geom_smooth (method = "lm") flag.
Results
Fish length and weight
The number of male cod in the present study was slightly higher than females in both low pCO2 (57% males) and high pCO2 (60% males) groups. The standard length (SL) measured and body weight at the end of the experimental period ranged from 68 to 105 cm and 4220 to 11400 g (Table 2). Animals from low pCO2 and high pCO2 groups had similar standard lengths (83.01 ± 5.71 and 86.19 ± 7.93 cm, respectively), while animals from the high pCO2 group were slightly heavier (7009 ± 1687 g) than from the low pCO2 group (6746 ± 1540 g) (P = 0.05). In both groups, female cods were significantly heavier (7656 ± 1790 g) and longer (87.25 ± 8.3 cm) than males (6693 ± 1422 g and 83.56 ± 5.94 cm), indicating sexual dimorphism (Table 3). Furthermore, although not significant, both males and females from the high pCO2 group were heavier (6745 ± 1492 and 7688 ± 1835 g) and longer (84.30 ± 6.6 and 89.24 ± 8.9 cm) compared to males and females from the low pCO2 group (6285 ± 1286 and 7180 ± 1720 g; 82.09 ± 4.67 and 84.23 ± 6.77 cm).
Table 2.
Biometric data and otolith measurements in Atlantic cod
| Low pCO2 (N = 81) | High pCO2 (N = 98) | Diff (%) | |
|---|---|---|---|
| Bw (g) | 6746 ± 1540 | 7009 ± 1687 | |
| SL (cm) | 83.01 ± 5.71 | 86.19 ± 7.93 | |
| OW (mg) | 760 ± 130 (0.78 ± 0.01)* | 800 ± 150 (0.82 ± 0.01) | + 5 |
| OL (mm) | 20.06 ± 1.41 (20.7 ± 0.10)* | 20.12 ± 1.45 (20.0 ± 0.10) | − 3.4 |
| OR (mm) | 9.85 ± 0.64 (9.97 ± 0.06) | 10.07 ± 0.70 (10.18 ± 0.06) | + 2 |
| OP (mm) | 51.76 ± 3.48 (52.7 ± 0.33)* | 51.64 ± 3.32 (51.0 ± 0.37) | − 3.3 |
| OA (mm2) | 152.21 ± 19.30 (154 ± 1.90) | 156.35 ± 19.21 (157 ± 1.70) | + 1 |
| OD | 4.97 ± 0.40 (5.01 ± 0.03) | 5.10 ± 0.45 (5.13 ± 0.03) | + 2.5 |
| Cicl | 17.60 ± 1.40 (17.7 ± 0.15)* | 17.06 ± 1.23 (17.2 ± 0.17) | − 2.9 |
| Rd | 0.48 ± 0.03 (0.47 ± 0.003)* | 0.49 ± 0.03 (0.49 ± 0.003) | + 4 |
| Rect | 0.77 ± 0.03 (0.77 ± 0.003) | 0.77 ± 0.03 (0.77 ± 0.003) | + 0 |
| Ellip | 0.34 ± 0.03 (0.34 ± 0.003) | 0.33 ± 0.03 (0.33 ± 0.004) | − 3 |
Average values and adjusted means (in brackets) for Atlantic cod exposed to 429 (low pCO2) and 1091µatm (high_pCO2) for Bw body weight, SL standard length and OW otolith weight, OL otolith length, OR otolith radius, OP otolith perimeter, OA otolith area, OD = OW/OA otolith density, Cicl circularity, Rd roundness, Rect rectangularity and Ellip ellipticity. Unadjusted data is expressed as Mean ± SD and adjusted means as least squares ± SE. Differences (Diff) between adjusted means are expressed as a percentage. The number of individual otoliths analysed for each group is indicated (N)
Significant differences between groups for the pCO2 treatment are indicated with an asterisk when P < 0.05 (all P-values are in Supplementary File 3)
Table 3.
Biometric data and otolith measurements in male and female Atlantic cod
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Low pCO2 (N = 34) | High pCO2 (N = 38) | Diff (%) | Low pCO2 (N = 47) | High pCO2 (N = 60) | Diff (%) | |
| Bw (g) | 7180 ± 1720 | 7688 ± 1835 | 6285 ± 1286 | 6745 ± 1492 | ||
| SL (cm) | 84.23 ± 6.77 | 89.24 ± 8.97 | 82.09 ± 4.67 | 84.30 ± 6.60 | ||
| OW (mg) | 0.78 ± 0.15 (0.80 ± 0.01)* | 0.83 ± 0.14 (0.85 ± 0.02) | + 6 | 740 ± 113 (0.75 ± 0.01) | 774 ± 148 (0.77 ± 0.01) | + 2.5 |
| OL (mm) | 20.68 ± 1.67 (21.5 ± 0.23)* | 20.53 ± 1.40 (20.3 ± 0.20) | − 5.6 | 19.63 ± 1.01 (19.7 ± 0.18) | 19.87 ± 1.44 (19.8 ± 0.15) | + 0.5 |
| OR (mm) | 10.12 ± 0.56 (10.3 ± 0.09) | 10.18 ± 0.59 (10.1 ± 0.08) | − 2 | 9.65 ± 0.63 (9.73 ± 0.09) | 9.99 ± 0.76 (9.95 ± 0.07) | + 2.3 |
| OP (mm) | 53.19 ± 4.06 (54.3 ± 0.57)* | 52.65 ± 2.77 (52.4 ± 0.51) | − 3.5 | 50.76 ± 2.60 (51.0 ± 0.45) | 51.03 ± 3.53 (50.9 ± 0.37) | + 0 |
| OA (mm2) | 160.69 ± 31.30 (168 ± 2.80)* | 161.27 ± 16.50 (160 ± 2.51) | − 4.8 | 146.42 ± 15.39 (148 ± 2.39) | 153.46 ± 20.46 (152 ± 1.94) | + 4.6 |
| OD | 4.89 ± 0.45 (5.05 ± 0.04)* | 5.20 ± 0.42 (5.26 ± 0.04) | + 4 | 5.02 ± 0.35 (4.99 ± 0.04) | 5.02 ± 0.45 (5.08 ± 0.03) | + 1.8 |
| Cicl | 17.60 ± 1.38 (17.7 ± 0.24) | 17.19 ± 1.32 (17.3 ± 0.22) | − 2.3 | 17.59 ± 1.45 (17.5 ± 0.20)* | 16.97 ± 1.18 (17.0 ± 0.16) | − 3 |
| Rd | 0.48 ± 0.02 (0.47 ± 0.005) | 0.49 ± 0.03 (0.49 ± 0.005) | + 4 | 0.48 ± 0.03 (0.48 ± 0.005)* | 0.49 ± 0.03 (0.50 ± 0.003) | + 4 |
| Rect | 0.76 ± 0.02 (0.77 ± 0.005) | 0.77 ± 0.03 (0.77 ± 0.005) | + 0 | 0.77 ± 0.02 (0.77 ± 0.003) | 0.77 ± 0.02 (0.77 ± 0.003) | + 0 |
| Ellip | 0.34 ± 0.02 (0.34 ± 0.004) | 0.33 ± 0.02 (0.33 ± 0.004) | − 3 | 0.33 ± 0.03 (0.34 ± 0.005) | 0.32 ± 0.03 (0.33 ± 0.004) | − 3 |
Average values and adjusted means (in brackets) of female and male Atlantic cod exposed to 429 and 1091µatm pCO2 for Bw body weight, SL standard length and OW otolith weight, OL otolith length, OR otolith radius, OP otolith perimeter, OA otolith area, OD = OW/OA otolith density, Cicl circularity, Rd roundness, Rect rectangularity and Ellip ellipticity. Unadjusted data is expressed as Mean ± SD and adjusted means as least squares ± SE. Differences (Diff) are expressed as a percentage of the differences between adjusted means. The number of individual otoliths per group is indicated (N)
Significant differences between groups for the pCO2 treatment are indicated with an asterisk when P < 0.05 (actual P-values are in Supplementary File 4)
Otolith morphology
When visually inspected, all otoliths showed the characteristic shape associated to Atlantic cod (McBride et al. 2010), and 90% of them had a smooth and homogeneous white surface (Supplementary File 1). However, we also found 5 animals from the high pCO2 group (10% of the total) showing an abnormal deposition covering up to 10% of the surface for one or both otoliths.
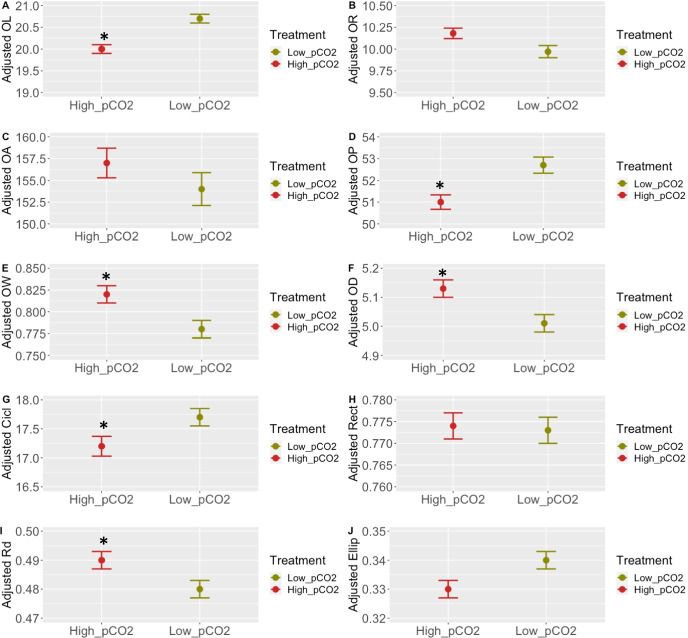
As expected, all otolith measurements were significantly correlated to SL and OW (ρ = 0.73; P < 0.001), thickness (ρ = 0.50; P < 0.001), OL (ρ = 0.56; P < 0.001), OR (ρ = 0.55; P < 0.001), OP (ρ = 0.50; P < 0.001) and OA (ρ = 0.63; P < 0.001) (Supplementary File 2); therefore, SL was included as a covariant in the mixed models to study otolith measurements, and adjusted means were used for comparisons between treatments. Only OL and OP were significantly affected by the pCO2 treatment (P = 0.03 and P = 0.04, respectively). Adjusted means of the measurements showed a 3.4% and 3.3% reduction in OL and OP for individuals of the high pCO2 group (Fig. 1; Table 2; Supplementary File 3). Otolith weight (OW) and density (OD) were a 5% and 2.5% higher in animals of the high pCO2 group (Table 2).
Fig. 1.
Adjusted means for otolith measurements and shape indexes from Atlantic cod exposed to 1091 (high pCO2) and 422µatm (low pCO2) for a period of 30 weeks. Values are expressed as adjusted mean ± SE for 422 (low pCO2; N = 81) (green) and 1091µatm (high pCO2; N = 98) (red) groups. Asterisks indicate significant differences between treatments (*P < 0.05). Otolith length (OL) (A), otolith width (OR) (B), otolith area (OA) (C), otolith perimeter (OP) (D), otolith weight (OW) (E), otolith density (OD) (F), circularity (Cicl) (G), rectangularity (Rect) (H), roundness (Rd) (I) and ellipticity (Ellip) (J)
The different pCO2 treatments also had a significant effect on roundness (Rd) (4% increase in high pCO2 group; P = 0.04) and circularity (Cicl) (− 2.9% reduction in high pCO2 group; P = 0.03) (Fig. 1; Table 2; Supplementary File 3). We also found that OW had a significant effect on Cicl and Rect (P < 0.001 for both cases), but treatment*OW interaction was not significant for any of the shape indexes (Fig. 1; Table 2; Supplementary File 3), suggesting that differences in shape between treatments were not affected by age.
Effect of animal gender on otolith morphometry and shape
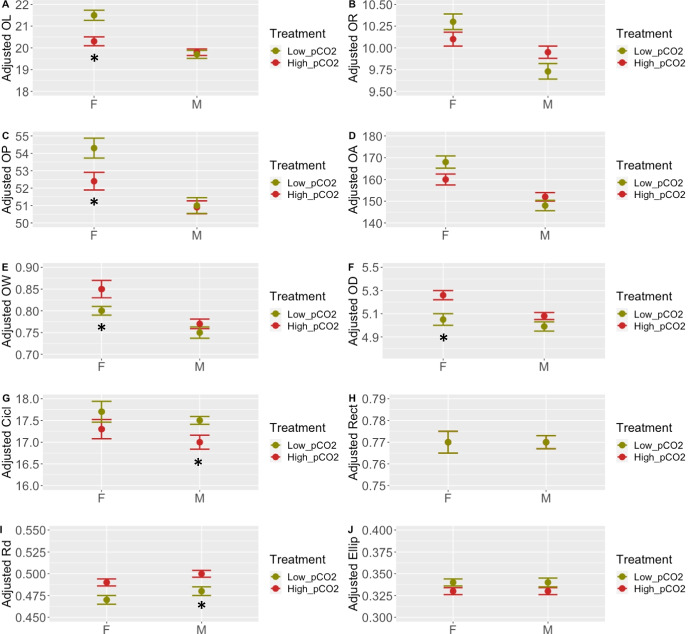
OW, OL, OR, OP, OA, and OD parameters had a significant gender*treatment interaction (Supplementary File 4), indicating a different response to pCO2 treatments between males and females. To determine the existence of any gender-specific susceptibility, the data was re-analysed separately for males and females (Fig. 2; Table 3; Supplementary File 4 and 5).
Fig. 2.
Adjusted means for otolith measurements and shape index from males (M) and females (F) of Atlantic cod exposed to 1091 (high pCO2; male otoliths N = 60, female otoliths N = 38) and 422µatm (low pCO2; male otoliths N = 47, female otoliths N = 34) for a period of 30 weeks. Asterisks indicate significant differences between treatments (*P < 0.05). Otolith length (OL) (A), otolith width (OR) (B), otolith area (OA) (C), otolith perimeter (OP) (D), otolith weight (OW) (E), otolith density (OD) (F), circularity (Cicl) (G), rectangularity (Rect) (H), roundness (Rd) (I) and ellipticity (Ellip) (J)
Again, all otolith measurements strongly correlated to SL for both genders (Supplementary File 5), and SL was included as a co-variant in the models. We found that females from the high pCO2 treatment had smaller (OL, OP and OA were − 5.6, − 3.5 and − 4.8% smaller; P = 0.03, 0.03 and 0.04, respectively) and heavier (OW and OD increased + 6% and + 4%; P = 0.03 and P = 0.02, respectively) otoliths than females of the low pCO2 group (Fig. 2; Table 3, Supplementary File 4).
The pCO2 levels had a significant effect on high pCO2 males’ otolith Cicl and Rd (− 3% and + 4% changes, respectively; P = 0.03 and 0.04) compared to males of the low pCO2 group (Table 3; Fig. 2; Supplementary File 4). No significant changes were found in males’ otolith dimensions.
OW (as a proxy for age) significantly influenced shape indexes (Cicl, Rect and Round on males and Rect and Round in females) (Supplementary File 4), suggesting that age had an overall effect on otolith shape, as we would expect from a mixture of animals of different ages. However, the treatment*OW interaction was not significant for any of the indexes (Supplementary File 4), suggesting that age does not explain the differences found between treatments.
CaCO3 polymorph identification
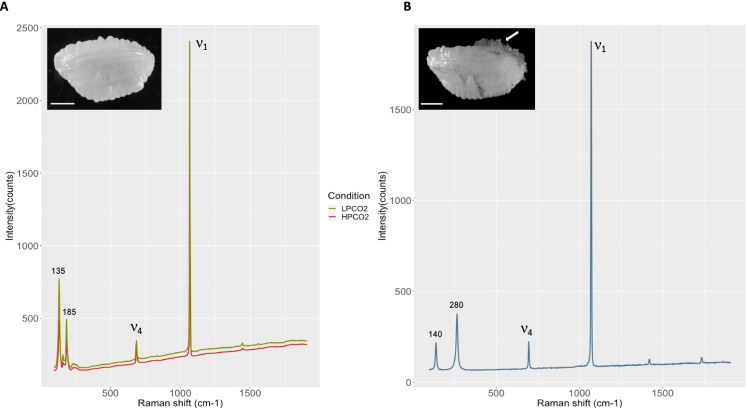
Raman readings were obtained from the surface of 5 otoliths from each condition. All readings showed the characteristic Raman shifts corresponding to the ν1 and ν4 vibrational modes of the CaCO3 lattice (1070 cm−1, 680–690 cm−1 and 135 cm−1) together with a peak at 190 cm−1 specific to aragonite (Fig. 3). Raman measurements were also taken from the abnormal depositions identified in 10% of the high pCO2 individuals showing a characteristic calcite profile with the common peaks of the CaCO3 lattice and a calcite-specific peak at 280 cm−1 (Fig. 3).
Fig. 3.
Raman spectrometry on cod otoliths exposed to 1091 and 422µatm pCO2 for a period of 30 weeks. Aragonite Raman reading obtained from high pCO2 (red) and low pCO2 (green) normal otoliths (A). Raman reading obtained from an abnormal deposition found in 10% of the high pCO2 individuals (B) showing a typical calcite profile. Calcium carbonate lattice ν1 and ν4 peaks are indicated, and the shift positions of relevant peaks are indicated. Scale bar indicates 5 mm
Discussion
In the present study, we found indications that exposure of adult Atlantic cod to 1091µatm pCO2 for 30 weeks had a significant, but gender-specific, effect on otolith morphology. Otoliths from females exposed to high pCO2 treatment (High pCO2) were relatively smaller compared to low pCO2 females (a reduction of 2–6% for OL, OR, OP and OA in females from the high pCO2), while otoliths from males exposed to the high pCO2 treatment appeared slightly rounder (− 3% in Cicl and + 4% in Rd) when compared to those from the low pCO2 group (Supplementary File 6). Our observations contrast with previous studies on fish larvae where either an increase or no change in otolith size was observed in response to high pCO2 such as for Atlantic cod (Maneja et al. 2013), cobia (Bignami et al. 2013), Baltic cod (Frommel et al. 2013) or gilthead sea bream (Coll-Lladó et al. 2018). However, previous studies have not normally distinguished between males and females in those species where it is possible.
There is no conclusive explanation for the different gender susceptibility to high pCO2 observed in the present study. There is the possibility that it might be linked to energy budget constraints between growth, development and acid–base regulation. Otolith growth, like somatic growth, is limited by the energy availability (Fablet et al. 2011), and increased energy expenditure to counteract ocean acidification effects may hamper otolith formation. It is known that cod females have higher energy demands for gonad development than males (Gjedrem 2000; Karlsen et al. 1995). Changes in otolith structure (opacity pattern) are observed in some species in response to spawning (Katayama 2018) likely as a result of the energy budget constraints during that period (Irgens 2018). Similarly, differences in otolith growth are observed in fish species that change gender during their lifecycle, linked to changes in growth rate (Walker and McCormick 2009). Animals used in the present study (caught in October) spawned during March of the following year, and their weight was significantly reduced during that period, but this reduction was more prominent in females from the high pCO2 group (Supplementary File 7). Since females had a higher energy demand for gonadal development and egg production, the energy budget of high pCO2 females would have been more constrained trying to maintain the acid–base balance, reducing somatic growth capacity and, consequently, otolith growth when compared to low pCO2 females. In addition to changes in size, we found a significant increase in average otolith weight (+ 6%; P = 0.04) and density (+ 4%; P = 0.02) of otoliths (using OW/OA as a proxy) in females and also, but not significantly, in males exposed to high pCO2 levels (Fig. 2; Table 3). These results are in agreement with previous studies in which an increase in otolith density was observed in response to higher pCO2 (Bignami 2013). It has also been suggested that endolymph pCO2 and HCO3 increase when exposed to ocean acidification conditions in parallel to plasma levels, leading to enhanced carbonate deposition in the otoliths of some species (Grosell 2019). The increase in otolith density seems to indicate that there is an increase in CaCO3 accretion that is not translated into larger otoliths. One possible hypothesis is that while the CaCO3 fraction of the otolith was increasing, the protein fraction in adult cod decreased as a result of changes in acid/base physiology, although this possibility needs to be further investigated. The present results should be considered as a pilot study since important information from a sampling before the experimental period was not performed. Furthermore, the animals were not treated with calcein or any similar dye to determine how much CaCO3 was deposited during the experimental period between the two experimental groups.
Otolith shape indexes are not commonly assessed in response to ocean acidification, and just a few xMunday et al. 2011; Réveillac et al. 2015) or no effects (Checkley et al. 2009b) have been reported. The otolith shape, proportions and lobe number in Atlantic cod have a genetic component but, together with the otolith weight, they are also affected by age and environmental factors, such as temperature or diet (e.g. Mille et al. 2016; Vignon and Morat 2010). Otolith parameters in cod, such as otolith weight and number of lobes, can rapidly change during the first years of life (0 to 3 years) but at a much slower rate in the following years (Campana and Fowler 2012; Irgens 2018). In the present study we used the otolith weight (OW) as a proxy for the animal’s age, and their values ranged from a minimum of 0.5 to a maximum of 1.02 g (average 0.75–0.80 g), indicating age diversity as it could be expected for wild populations. Statistical analysis showed that OW had a significant effect on the majority of shape indexes analysed, suggesting they were influenced by age. However, OW*treatment interaction was not significant in any case, and, therefore, differences observed between groups were independent of age. This is possibly due to the similar OW/age distribution between the groups (Supplementary File 2 and 5). Studies based on mathematical models already suggested that larger otoliths would generate sound wave displacement with a possible detrimental effect on hearing (Bignami et al. 2013). A recent study by Radford et al. (2021) has confirmed some of the predictions from these models, showing that otolith size and fluctuating asymmetry under conditions of ocean acidification cause a decline in low frequency hearing in fish (Radford et al. 2021). It is possible that if important changes in the shape are not followed by modifications in the inner ear morphology, this might lead to physical constraints between the two compartments and have consequences on sound propagation and hearing sensitivities. Changes observed in the present study were minor but raise the possibility that chronic exposure to high pCO2 would produce larger differences. However, more detailed studies about the relationship between otolith shape and inner ear morphology in response to ocean acidification and in vivo analysis of hearing capabilities are needed.
Otolith formation is strongly dependent on the endolymph composition. The growth of the otolith reflects both the aragonite saturation state (Ω) of the endolymph (indicating the concentration of and Ca+2 available to produce the mineral phase) and its organic composition (Payan et al. 2004). Dissolved inorganic carbon occurs as H2CO3, and , with the formation of favoured at high pH. Proton secretion from the endolymph serves to increase endolymph pH and likely promotes aragonite formation (Payan et al. 1998). In the vast majority of otoliths examined, aragonite was the predominant CaCO3 polymorph; however, approximately 10% of the high pCO2 fish group showed calcite deposition on the otolith surface (Fig. 3). Although very rare, aragonite replacement by calcite can occur spontaneously in wild populations (Oliveira et al. 1996), but the reasons are still unknown. Some authors have suggested that aragonite can be replaced by other polymorphs such as vaterite when growth rates are increased (Reimer et al. 2017) or animal density is high (Austad et al. 2021). While possible changes in growth rate might be the reason for calcite deposition, the fact that only 10% of the animals were affected makes that assumption unlikely. Under normal circumstances, the endolymph is more alkaline (pH = 8.0) than the plasma (pH = 7.2–7.6), partly determined by the concentration of bicarbonate and the relatively high levels of CO2 in the endolymph (30 mmol−1) compared to the plasma (8–12 mmol−1) (Campana 1999; Payan et al. 2004). Studies in vitro have shown that CaCO3 precipitates as calcite when the pH of the media increases (Ren et al. 2013). It is possible that the increase of and pCO2 in the high pCO2 animals could increase the alkalinity of the saccular endolymph beyond a certain threshold that would facilitate the replacement of aragonite by calcite. Another possibility might be potential changes in the protein matrix composition driving this polymorph replacement. Previous studies have shown that the otolith’s organic matrix plays a crucial role in carbonate crystallization (Falini et al. 2005; Ren et al. 2013), so if the protein matrix composition is altered by more acidic pH, that might lead to calcite deposition. Hughes et al. (2004) reported that abnormal calcite otoliths are formed in zebrafish when otopetrin-1 was inhibited in knockout fish, demonstrating the importance of the protein matrix for otolith formation (Hughes et al. 2004). However, since only 10% of the individuals in our study appeared to be affected, it might be an indication of genetic susceptibility of some individuals to deposit calcite in response to high pCO2. Calcite deposition has also been observed in 21% of gilthead sea bream larval otoliths when exposed to 2000µatm of pCO2 (Coll-Lladó et al. 2018), and this was attributed to a significant degree of heritability (h2 = 0.44–0.55%), suggesting genetic susceptibility to calcite deposition. Although it is possible that Atlantic cod showing calcite deposition were genetically more susceptible to crystallize calcite in response to high pCO2, we cannot rule out other factors, and, therefore, further research is needed to confirm this hypothesis.
While the current study has some limitations, the results obtained give us some perspective of how ocean acidification might impact adult fish. Some of the limitations include the difficulty to replicate treatments due to welfare considerations, so despite keeping equal environmental parameters (such as light, temperature and diet) between tanks, we cannot rule out the tank effect; although most of the studies in which a tank effect has been observed, this was attributable to environmental or treatment differences (Speare et al. 1995). Also, to overcome the intrinsic variability present on wild populations, we did not perform a pre-experimental sampling in order to have enough statistical power; therefore, the information about the otoliths before starting the experimental trial is lacking. Finally, wild populations have a mixture of animals of different ages, but empirical estimation of cod’s age by counting the annual growth increments was not performed and otolith weight (OW) was used as a proxy for age since both parameters correlate well (Bermejo 2014; Campana and Fowler 2012; Pawson 2006). In the present study, growth rates were similar between groups, with a significant weight reduction during the spawning occurred during ~ 24 weeks after capture (Supplementary File 5). Since no significant differences in weight, standard length or OW (as estimation of age) were found between the two experimental groups, we believe the differences between otolith parameters between the two groups were likely determined by the effect of pCO2.
Conclusions
Exposure of adult cod to 1091µatm of pCO2 for a period of 30 weeks had a relatively small but significant effect on otolith size and shape in a gender-dependent way. Males exposed to 1091µatm had rounder otoliths whereas female otoliths were smaller. We also found calcite aggregations in 10% of the animals exposed to 1091µatm pCO2, suggesting individual susceptibility to deposit calcite. Despite the experimental limitations of the present pilot study, our results suggest that even when otoliths are well formed and during periods of slow growth (such as in adults), exposure to high pCO2 still has a significant effect on their formation.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
CC, CRB, MS and FM planned the fish experimental setup. FHM, MS, CRB, GG and CC were responsible of fish maintenance and sample collection. NA, CCLL and DGS processed the samples. PBW did the Raman measurements. CCLL and DGS took otoliths measurements, calculated shape indexes and performed statistical analyses. CCLL and DGS wrote the manuscript with inputs from all the authors.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Funding was provided by the Bonus Baltic Sea research and development programme (Art 185) BIO-C3 project, funded jointly by the EU and the BMBF (Grant No. 03F0682A), BIOACID project (Biological Impacts of Ocean Acidification: Grant No. 03F0655K) funded by the German Ministry for Education and Research (BMBF), and the EU AQUAEXCEL transnational access grant for aquaculture infrastructures. Part of the work was supported by the FINEAQUA-project (Grant Number 12212001) funded by the Program for the Future Economy (financed by the European Regional Development Fund, the Federal Republic of Germany and the state of Schleswig–Holstein), the Centre for Marine Aquaculture, Tromsø, and the Marine Alliance for Science and Technology for Scotland (MASTS) Small Grant initiative (SG330 and SG407), and their support especially by the staff of the centre for Marine Aquaculture is gratefully acknowledged. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. PBW would like to thank the Royal Society for the award of an Industry Fellowship. DGS is a Serra Húnter Tenure-Track lecturer. This study was carried out in strict accordance with the laboratory regulations applicable in Norway. The application was approved by the National Regulatory Committee on the Ethics of Animal Experiments (Permit TRANSCOD project FOTS id 6915 and ACIDCOD project FOTS id 7346).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/27/2022
The original version of this paper was updated to add the missing compact agreement Open Access funding note.
References
- Allemand D, Mayer‐Gostan N, De Pontual H, Boeuf G, Payan P (2007) Fish otolith calcification in relation to endolymph chemistry. Handbook of Biomineralization: Biological aspects and structure formation 291–308
- Austad B, Vøllestad LA, Foldvik A. Frequency of vateritic otoliths and potential consequences for marine survival in hatchery-reared Atlantic salmon. J Fish Biol. 2021;98:1401–1409. doi: 10.1111/jfb.14683. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software Artic. 2015;67:1–48. [Google Scholar]
- Bermejo S. The benefits of using otolith weight in statistical fish age classification: a case study of Atlantic cod species. Comput Electron Agric. 2014;107:1–7. doi: 10.1016/j.compag.2014.06.001. [DOI] [Google Scholar]
- Bestgen K, Bundy J. Environmental factors affect daily increment deposition and otolith growth in young Colorado squawfish. Trans Am Fish Soc. 1998;127:105–117. doi: 10.1577/1548-8659(1998)127<0105:EFADID>2.0.CO;2. [DOI] [Google Scholar]
- Bignami S, Enochs IC, Manzello DP, Sponaugle S, Cowen RK. Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc Natl Acad Sci. 2013;110:7366–7370. doi: 10.1073/pnas.1301365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander KM. Cod recruitment is strongly affected by climate when stock biomass is low. ICES J Mar Sci. 2005;62:339–343. doi: 10.1016/j.icesjms.2004.07.029. [DOI] [Google Scholar]
- Caldeira K. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005;110:C09S04. [Google Scholar]
- Campana S. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser. 1999;188:263–297. doi: 10.3354/meps188263. [DOI] [Google Scholar]
- Campana S, Fowler G (2012) Age determination without tears: statistical estimation of silver hake (Merluccius bilinearis) age composition on the basis of otolith weight and fish length
- Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. Elevated CO2 enhances otolith growth in young fish. Science. 2009;324:1683–1683. doi: 10.1126/science.1169806. [DOI] [PubMed] [Google Scholar]
- Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. Elevated CO2 enhances otolith growth in young fish. Science. 2009;324:1683. doi: 10.1126/science.1169806. [DOI] [PubMed] [Google Scholar]
- Coll-lladó C, Giebichenstein J, Webb PB, Bridges CR (2018) Ocean acidification promotes otolith growth and calcite deposition in gilthead sea bream (Sparus aurata) larvae. Sci Rep:1–28 [DOI] [PMC free article] [PubMed]
- Fablet R, Pecquerie L, de Pontual H, Høie H, Millner R, Mosegaard H, Kooijman SALM. Shedding light on fish otolith biomineralization using a bioenergetic approach. PLoS One. 2011;6:1–7. doi: 10.1371/journal.pone.0027055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini G, Fermani S, Vanzo S, Miletic M, Zaffino G. Influence on the formation of aragonite or vaterite by otolith macromolecules. Eur J Inorg Chem. 2005;1:162–167. doi: 10.1002/ejic.200400419. [DOI] [Google Scholar]
- Frommel AY, Schubert A, Piatkowski U, Clemmesen C (2013) Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar Biol 160:1825–1834
- Gauldie RW, Sharma SK, Volk E (1997) Micro-Raman spectral study of vaterite and aragonite otoliths of the coho salmon, Oncorhynchus kisutch. Comp Biochem Physiol A Physiol 118:753–757
- Girish V, Vijayalakshmi A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 2004;41:47. [PubMed] [Google Scholar]
- Gjedrem T. Genetic improvement of cold-water fish species. Aquac Res. 2000;31:25–33. doi: 10.1046/j.1365-2109.2000.00389.x. [DOI] [Google Scholar]
- Grosell M. 4 - CO2 and calcification processes in fish. In: Grosell M, Munday PL, Farrell AP, Brauner CJBT-FP, editors. Carbon Dioxide. Cambridge: Academic Press; 2019. pp. 133–159. [Google Scholar]
- Heuer RM, Grosell M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. AJP Regul Integr Comp Physiol. 2014;307:R1061–R1084. doi: 10.1152/ajpregu.00064.2014. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA. The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu Rev Ecol Evol Syst. 2010;41:127–147. doi: 10.1146/annurev.ecolsys.110308.120227. [DOI] [Google Scholar]
- Hughes I, Blasiole B, Huss D, Warchol ME, Rath NP, Hurle B, Ignatova E, Dickman JD, Thalmann R, Levenson R et al (2004) Otopetrin 1 is required for otolith formation in the zebrafish Danio rerio. Dev Biol 276:391–402 [DOI] [PMC free article] [PubMed]
- Hylen A, Nakken O, Nedreaas K. Northeast Arctic cod: fisheries, life history, stock fluctuations and management Nor spring-spawning herring Northeast Arct. cod. Trondheim: Tapir Acad. Press; 2008. pp. 83–118. [Google Scholar]
- Irgens C (2018) Otolith structure as indicator of key life history events in Atlantic cod (Gadus morhua)
- Jarrold M, Munday P (2018) Diel CO2 cycles do not modify juvenile growth, survival and otolith development in two coral reef fish under ocean acidification. Mar Biol 165
- Jones EM, Fenton M, Meredith MP, Clargo NM, Ossebaar S, Ducklow HW, Venables HJ, de Baar HJW. Ocean acidification and calcium carbonate saturation states in the coastal zone of the West Antarctic Peninsula. Deep Sea Res II Top Stud Oceanogr. 2017;139:181–194. doi: 10.1016/j.dsr2.2017.01.007. [DOI] [Google Scholar]
- Karlsen Ø, Holm JC, Kjesbu OS (1995) Effects of periodic starvation on reproductive investment in first-time spawning Atlantic cod (Gadus morhua L.). Aquaculture 133:159–170
- Katayama S. A description of four types of otolith opaque zone. Fish Sci. 2018;84:735–745. doi: 10.1007/s12562-018-1228-z. [DOI] [Google Scholar]
- Leguá J, Plaza G, Pérez D, et al., (2013) Otolith shape analysis as a tool for stock identification of the southern blue whiting, Micromesistius australis. Latin Amercian Journal of Aquatic Research 41(3):479–489. 10.3856/vol41-issue3-fulltext-11
- Maneja RH, Frommel AY, Geffen AJ, Folkvord A, Piatkowski U, Chang MY, Clemmesen C (2013) Effects of ocean acidification on the calcification of otoliths of larval Atlantic cod Gadus morhua. Mar Ecol Prog Ser 477:251–258
- Mille T, Mahé K, Cachera M, Villanueva MC, De Pontual H, Ernande B. Diet is correlated with otolith shape in marine fish. Mar Ecol Prog Ser. 2016;555:167–184. doi: 10.3354/meps11784. [DOI] [Google Scholar]
- Morales-Nin B (2000) Review of the growth regulation processes of otolith daily increment formation. Fisheries Research 46:53–67. 10.1016/s0165-7836(00)00133-8
- Munday PL, Gagliano M, Donelson JM, Dixon DL. Ocean acidification does not affect the early life history development of a tropical marine fish. Mar Ecol Prog Ser. 2011;423:211–221. doi: 10.3354/meps08990. [DOI] [Google Scholar]
- McBride RS, Hauser JW, Sutherland SJ (2010) Brodeur's Guide to Otoliths of Some Northwest Atlantic Fishes. Northeast Fisheries Science Center 10–04:35
- Oliveira AM, Farina M, Ludka IP, Kachar B. Vaterite, calcite, and aragonite in the otoliths of three species of piranha. Naturwissenschaften. 1996;83:133–135. doi: 10.1007/BF01142180. [DOI] [Google Scholar]
- Pawson M. Using otolith weight to age fish. J Fish Biol. 2006;36:521–531. doi: 10.1111/j.1095-8649.1990.tb03554.x. [DOI] [Google Scholar]
- Payan P, Borelli G, Boeuf G, Mayer-Gostan N (1998) Relationship between otolith and somatic growth: consequence of starvation on acid-base balance in plasma and endolymph in the rainbow trout Oncorhynchus mykiss. Fish Physiol Biochem 19:35–41
- Payan P, De Pontual H, Bœuf G, Mayer-Gostan N. Endolymph chemistry and otolith growth in fish. Comptes Rendus - Palevol. 2004;3:535–547. doi: 10.1016/j.crpv.2004.07.013. [DOI] [Google Scholar]
- Pimentel MS, Faleiro F, Dionisio G, Repolho T, Pousao-Ferreira P, Machado J, Rosa R. Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J Exp Biol. 2014;217:2062–2070. doi: 10.1242/jeb.092635. [DOI] [PubMed] [Google Scholar]
- Popper AN, Ramcharitar J, Campana SE. Why otoliths? Insights from inner ear physiology and fisheries biology. Mar Freshw Res. 2005;56:497–504. doi: 10.1071/MF04267. [DOI] [Google Scholar]
- Pothin K, Gonzalez-Salas P, Chabanet P, et al., (2006) Distinction between Mulloidichthys flavolineatus juveniles from Reunion Island and Mauritius Island (south-west Indian Ocean) based on otolith morphometrics. Journal of Fish Biology 69(1):38–5. 10.1111/j.1095-8649.2006.01047.x
- Pracheil BM, Chakoumakos BC, Feygenson M, Whitledge GW, Koenigs RP, Bruch RM. Sturgeon and paddlefish (Acipenseridae) sagittal otoliths are composed of the calcium carbonate polymorphs vaterite and calcite. J Fish Biol. 2017;90:549–558. doi: 10.1111/jfb.13085. [DOI] [PubMed] [Google Scholar]
- Radford CA, Collins SP, Munday PL, Parsons D. Ocean acidification effects on fish hearing. Proc R Soc B Biol Sci. 2021;288:20202754. doi: 10.1098/rspb.2020.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer T, Dempster T, Warren-Myers F, Jensen AJ, Swearer SE. High prevalence of vaterite in sagittal otoliths causes hearing impairment in farmed fish. Sci Rep. 2016;6:1–8. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer T, Dempster T, Wargelius A, Fjelldal PG, Hansen T, Glover KA, Solberg MF, Swearer SE. Rapid growth causes abnormal vaterite formation in farmed fish otoliths. J Exp Biol. 2017;220:2965–2969. doi: 10.1242/jeb.148056. [DOI] [PubMed] [Google Scholar]
- Ren D, Feng Q, Bourrat X. The co-effect of organic matrix from carp otolith and microenvironment on calcium carbonate mineralization. Mater Sci Eng C. 2013;33:3440–3449. doi: 10.1016/j.msec.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Réveillac E, Lacoue-labarthe T, Oberhänsli F, Teyssié J, Jeffree R, Gattuso J, Martin S. Journal of experimental marine biology and ecology ocean acidification reshapes the otolith-body allometry of growth in juvenile sea bream. J Exp Mar Bio Ecol. 2015;463:87–94. doi: 10.1016/j.jembe.2014.11.007. [DOI] [Google Scholar]
- RStudio Team (2015) RStudio: Integrated Development Environment for R
- Russel VL (2016) Least-Squares Means: The R package lsmeans Journal of Statistical Software 69(1):1–33. 10.18637/jss.v069.i01
- Speare DJ, MacNair N, Hammell KL (1995) Demonstration of tank effect on growth indices of juvenile rainbow trout (Oncorhynchus mykiss) during an ad libitum feeding trial. American Journal of Veterinary Reserach 568(10)1372–9 [PubMed]
- Stiasny MH, Mittermayer FH, Sswat M, Voss R, Jutfelt F, Chierici M, Puvanendran V, Mortensen A, Reusch TBH, Clemmesen C. Ocean acidification effects on Atlantic cod larval survival and recruitment to the fished population. PLoS One. 2016;11:e0155448. doi: 10.1371/journal.pone.0155448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker TF, et al., (2013) Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. The Physical Science Basis
- Vignon M, Morat F. Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Mar Ecol Progr Ser. 2010;411:231–241. doi: 10.3354/meps08651. [DOI] [Google Scholar]
- Walker SPW, McCormick MI. Fish ears are sensitive to sex change. Biol Lett. 2009;5:73–76. doi: 10.1098/rsbl.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999;29:748–751. doi: 10.1016/S0741-5214(99)70329-0. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.