Abstract
Low vitamin D levels have been associated with cognitive decline; however, few randomized trials have been conducted. In a trial, we evaluated vitamin D3 supplementation on cognitive decline. We included participants aged 60+ years (mean[SD] = 70.9[5.8] years) free of cardiovascular disease and cancer in two substudies in the VITAL 2 × 2 randomized trial of vitamin D3 (2000 IU/day of cholecalciferol) and fish oil supplements: 3424 had cognitive assessments by phone (eight neuropsychologic tests; 2.8 years follow-up) and 794 had in-person assessments (nine tests; 2.0 years follow-up). The primary, pre-specified outcome was decline over two assessments in global composite score (average z-scores of all tests); substudy-specific results were meta-analyzed. The pooled mean difference in annual rate of decline (MD) for vitamin D3 versus placebo was 0.01 (95% CI − 0.01, 0.02; p = 0.39). We observed no interaction with baseline 25-hydroxyvitamin-D levels (p-interaction = 0.84) and a significant interaction with self-reported race (p-interaction = 0.01). Among Black participants (19%), those assigned vitamin D3 versus placebo had better cognitive maintenance (MD = 0.04, 95% CI 0.01, 0.08, similar to that observed for Black participants 1.2 years apart in age). Thus, vitamin D3 (2000 IU/day cholecalciferol) supplementation was not associated with cognitive decline over 2–3 years among community-dwelling older participants but may provide modest cognitive benefits in older Black adults, although these results need confirmation.
Trial registration ClinicalTrials.gov; VITAL (NCT01169259), VITAL-DEP (NCT01696435) and VITAL-Cog (NCT01669915); the date the registration for the parent trial (NCT01169259) was submitted to the registry: 7/26/2010 and the date of first patient enrollment in either of the ancillary studies for cognitive function in a subset of eligible VITAL participants: 9/14/2011.
Subject terms: Neurology, Randomized controlled trials
Introduction
Vitamin D is a fat-soluble steroid hormone essential for bone and muscle health. Yet, the discovery of vitamin D’s autocrine pathways in multiple cell types has stimulated interest in its role in brain function1–14. Specifically, the vitamin D receptor is expressed in the cerebral cortex and hippocampus, critical for cognition and memory. In animals, vitamin D deficiency has been linked with deficits in brain development and aging15–17.
In humans, observational studies have implicated low vitamin D in cognitive impairment and dementia18–20, although the literature has been mixed21,22. Observational studies have used varying definitions of low vitamin D and of cognitive impairment/dementia; further, the issue of reverse causation is important, as low vitamin D concentrations may have resulted from lifestyle changes associated with incipient cognitive impairment/dementia. Three major previous randomized clinical trials on cognitive change23–25 have not shown benefits of vitamin D3. However, even a large trial (n = 4143) with 7.8 years of follow-up that observed no effect of vitamin D3 and calcium supplements on cognitive decline compared with placebo24 used a low dose of vitamin D3 (400 IU/day).
Thus, we investigated whether vitamin D3 supplementation of 2000 IU/day may delay cognitive decline over 2–3 years compared to placebo among healthy participants aged 60+ years in VITAL (VITamin D and OmegA-3 TriaL; NCT01169259)26–28, a randomized trial of vitamin D3 and omega-3 fatty acids in the prevention of major chronic diseases. In addition, we conducted pre-specified subgroup analyses by race and baseline blood vitamin D levels, given that supplementation may have stronger effects on subgroups with relatively lower blood vitamin D levels, including Black adults who are also at higher risk of cognitive decline29–31 and in whom we had observed suggestively different effects of vitamin D supplementation in the parent VITAL trial32.
Results
The 3424 participants in VITAL-Cog were aged 60–91 years (mean = 71.9; SD = 5.4) at the first cognitive assessment (Table 1); 58.9% were women; 22.2% were Black participants and 49.8% had some years of post-graduate studies. The mean change over an average of 2.8 years of follow-up was − 0.25 (SD = 0.49) in those assigned to placebo and − 0.24 (SD = 0.48) in those assigned to vitamin D. In CTSC-Cog (Table 1), the 794 participants were aged 60–87 years (mean = 67.1; SD = 5.3) at the first cognitive assessment; 50.4% were women; 5.7% were Black participants and 55.5% had some post-graduate education. The mean change over a mean of 2.0 years of follow-up was 0.09 (SD = 0.39) in those assigned to placebo and 0.08 (SD = 0.40) in those assigned to vitamin D.
Table 1.
Baseline characteristics of participants aged 60+ years in the VITAL cognitive substudy by vitamin D supplement assignment for VITAL-Cog (n = 3424) and CTSC-Cog (n = 794).
| VITAL-Cog (n = 3424) | CTSC-Cog (n = 794) | |||
|---|---|---|---|---|
| Vitamin D Group (n = 1710) | Placebo Group (n = 1714) | Vitamin D3 Group (n = 396) | Placebo Group (n = 398) | |
| Mean (SD) | ||||
| Age at 1st interview, yearsa (n = 2984 in VITAL-Cog; n = 776 in CTSC-Cog) |
71.9 (5.4) (n = 1480) |
71.8 (5.4) (n = 1504) |
66.9 ± 5.2 (n = 385) |
67.3 ± 5.4 (n = 391) |
| Age at 2nd interview, yearsa (n = 2923 in VITAL-Cog; n = 515 in CTSC-Cog) |
73.3 (5.7) (n = 1466) |
73.4 (5.7) (n = 1457) |
69.2 ± 5.1 (n = 254) |
69.8 ± 5.6 (n = 261) |
| Cognitive test scores at 1st interview | ||||
| VITAL-Cog only tests | ||||
| TICS | 33.9 (2.8) | 34.0 (2.8) | – | – |
| OTMT-Part A (s) | 10.7 (3.9) | 10.3 (3.3) | – | – |
| OTMT-Part B (s) | 38.0 (24.2) | 37.9 (24.1) | – | – |
| Digit span backwards | 6.7 (2.3) | 6.8 (2.4) | – | – |
| CTSC-Cog only tests | ||||
| 3MS | – | – | 94.8 ± 4.9 | 94.9 ± 4.4 |
| TMT-Part A (s) | – | – | 29.2 ± 11.5 | 29.8 ± 9.4 |
| TMT-Part B (s) | – | – | 80.0 ± 42.9 | 82.5 ± 44.2 |
| Vegetable naming test | – | – | 15.6 ± 4.6 | 15.4 ± 4.5 |
| Common tests across VITAL-Cog and CTSC-Cog | ||||
| TICS 10-word list recall-immediate | 4.6 (1.7) | 4.7 (1.7) | 4.7 ± 1.3 | 4.7 ± 1.3 |
| TICS 10-word list recall-delayed | 2.7 (1.9) | 2.7 (1.9) | 2.0 ± 1.8 | 1.9 ± 1.7 |
| EBMT-immediate | 9.6 (1.7) | 9.6 (1.8) | 9.7 ± 1.7 | 9.7 ± 1.6 |
| EBMT-delayed | 9.3 (1.8) | 9.3 (1.9) | 9.3 ± 1.7 | 9.3 ± 1.7 |
| Animal naming test | 19.4 (5.5) | 19.7 (5.6) | 21.1 ± 5.9 | 20.3 ± 6.1 |
| Global composite score | − 0.02 (0.57) | 0.02 (0.57) | 0.02 (0.63) | − 0.02 (0.56) |
| Baseline serum 25(OH)D (ng/mL) | 32.2 (9.8) | 32.5 (9.6) | 28.0 (8.3) | 29.1 (9.1) |
| n (%) | ||||
| Omega-3 assignment | ||||
| Active group | 844 (49.4%) | 855 (49.9%) | 198 (50.0%) | 198 (49.8%) |
| Placebo group | 866 (50.6%) | 859 (50.1%) | 198 (50.0%) | 200 (50.3%) |
| Sex | ||||
| Female | 1011 (59.1%) | 1005 (58.6%) | 205 (51.8%) | 195 (49.0%) |
| Male | 699 (40.9%) | 709 (41.4%) | 191 (48.2%) | 203 (51.0%) |
| Self-reported race/ethnicity | ||||
| Non-Hispanic White | 1184 (71.3%) | 1245 (73.8%) | 341 (88.1%) | 345 (89.2%) |
| Black | 387 (23.3%) | 356 (21.1%) | 18 (4.7%) | 26 (6.7%) |
| Other race/ethnicityb | 90 (5.4%) | 85 (5.0%) | 28 (7.2%) | 16 (4.1%) |
| Highest attained education | ||||
| High school or under | 189 (11.1%) | 183 (10.7%) | 29 (7.3%) | 34 (8.5%) |
| College | 678 (39.7%) | 664 (38.9%) | 138 (34.9%) | 152 (38.2%) |
| Post-graduate studies | 842 (49.3%) | 861 (50.4%) | 228 (57.7%) | 212 (53.3%) |
| Depressionc | ||||
| No | 1363 (82.7%) | 1383 (82.9%) | 324 (83.7%) | 309 (79.8%) |
| Yes | 285 (17.3%) | 285 (17.1%) | 63 (16.3%) | 78 (20.2%) |
3MS Modified Mini-Mental Status exam (range = 0–100)33; CTSC Clinical and Translational Science Collaborative Center for VITAL in Boston, MA; EBMT East Boston Memory Test (range = 0–12)34; OTMT Oral Trail Making Test (range = 0–120 s)35,36; SD standard deviation; TICS Telephone Interview for Cognitive Status (range = 0–41)37; TMT Trail Making Test (range = 0–150 s for part A and range = 0–300 s for part B)38,39.
aCharacteristics as of randomization unless noted otherwise; for categorical variables, the percentages do not add to 100% due to rounding errors and numbers do not add to the total due to missing values, which were taken out of descriptive statistical analyses. In the VITAL-Cog, 501 completed only the baseline, 440 completed only the 2nd assessment and 2483 completed both assessments. In the CTSC-Cog, 497 completed both assessments, 279 completed only the baseline and 18 completed only the 2nd assessment.
b“Other race/ethnicity” includes “Non-Black/African-American Hispanic”, “Asian”, “Native Hawaiian or other Pacific Islander” or “American Indian/Alaska Native”.
cDepression is defined as a lifetime history of a depression diagnosis or of treatment for depression; current use of antidepressants; experiencing two or more weeks of depression in the past 2 years or scoring 10 points or higher on the Patient Health Questionnaire-8.
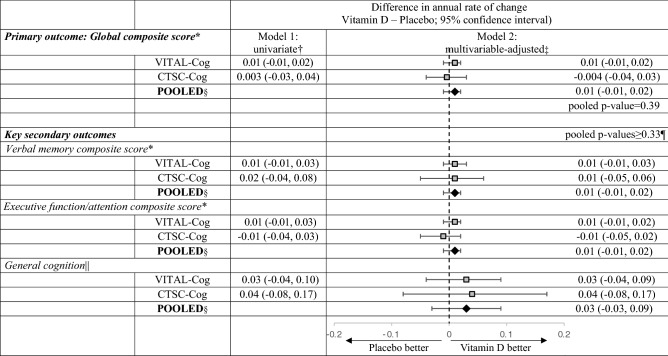
In VITAL-Cog, we did not observe an effect of vitamin D3 supplementation on cognitive function at the end of follow-up (mean = 2.8 years (range = 1.4–4.3 years); Table 2): the least squares mean for the global score was − 0.28 standard units (SE = 0.01) for the vitamin D3 group and − 0.26 (SE = 0.02) for the placebo group (mean difference = − 0.02, 95% CI − 0.06, 0.02). We observed no multivariable-adjusted differences in the global score annual rate of decline by assignment (Table 3; model 2, multivariable-adjusted mean difference = 0.01, 95% CI − 0.01, 0.02). Similarly, multivariable-adjusted differences in annual rates of decline were not significant for the secondary outcomes: 0.01 (95% CI − 0.01, 0.03), verbal memory composite score; 0.01 (95% CI − 0.01, 0.02), executive function/attention score and 0.03 (95% CI − 0.04, 0.09), TICS.
Table 2.
Cognitive function at two assessments by Vitamin D supplement assignment, for VITAL-Cog participants aged 60 + years, (n = 3424) assessed by telephone and for CTSC-Cog participants aged 60 + years, (n = 794) assessed in person.
| VITAL-COG (n = 3424; telephone assessments) | CTSC-COG (n = 794; in-person assessments) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D Group | Placebo Group | Difference in score at each timepoint (Vitamin D -Placebo; 95% CI)c | Vitamin D Group | Placebo Group | Difference in score at each timepoint (Vitamin D-Placebo; 95% CI)c | ||||||
| N | Mean (SE)c | N | Mean (SE)c | N | Mean (SE) | N | Mean (SE) | ||||
| Primary outcome | Primary outcome | ||||||||||
| Global composite scoreb | Difference in scorec | Global composite scoreb | Difference in scorec | ||||||||
| 1st assessment score | 14,800 | − 0.04 (0.01) | 1504 | − 0.01 (0.01) | − 0.03 (− 0.07, 0.01) | 1st assessment scorec | 385 | 0.02 (0.03) | 391 | − 0.03 (0.03) | 0.05 (− 0.04, 0.13) |
| 2nd assessment score | 1466 | − 0.28 (0.01) | 1457 | − 0.26 (0.02) | − 0.02 (− 0.06, 0.02) | 2nd assessment scorec | 254 | 0.11 (0.03) | 261 | 0.06 (0.03) | 0.05 (− 0.04, 0.14) |
| Secondary outcomes | Secondary outcomes | ||||||||||
| Verbal memory composite scoreb | Difference in scorec | Verbal memory composite scoreb | Difference in scorec | ||||||||
| 1st assessment score | 14,800 | − 0.02 (0.02) | 1504 | − 0.01 (0.02) | − 0.01 (− 0.06, 0.04) | 1st assessment scorec | 385 | 0.01 (0.04) | 391 | − 0.02 (0.03) | 0.03 (− 0.07, 0.13) |
| 2nd assessment score | 1466 | − 0.01 (0.02) | 1457 | − 0.02 (0.02) | 0.01 (− 0.04, 0.07) | 2nd assessment scorec | 254 | 0.17 (0.04) | 261 | 0.11 (0.04) | 0.07 (− 0.05, 0.18) |
| Executive function/attention compositeb scorea | Difference in scorec | Executive function/attention compositeb scorea | Difference in scorec | ||||||||
| 1st assessment score | 14,800 | − 0.05 (0.02) | 1504 | 0.01 (0.02) | − 0.05 (− 0.10, − 0.01) | 1st assessment scorec | 385 | 0.03 (0.04) | 391 | − 0.05 (0.03) | 0.08 (− 0.02, 0.18) |
| 2nd assessment score | 1466 | − 0.53 (0.02) | 1457 | − 0.49 (0.02) | − 0.04 (− 0.08, 0.01) | 2nd assessment scorec | 254 | 0.04 (0.04) | 261 | − 0.03 (0.04) | 0.06 (− 0.04, 0.16) |
| TICS | Difference in scorec | 3MS | Difference in scorec | ||||||||
| 1st assessment score | 14,800 | 33.81 (0.07) | 1504 | 33.92 (0.07) | − 0.12 (− 0.31, 0.08) | 1st assessment scorec | 385 | 94.80 (0.25) | 391 | 94.86 (0.22) | − 0.05 (− 0.71, 0.60) |
| 2nd assessment score | 1466 | 33.92 (0.07) | 1457 | 33.97 (0.07) | − 0.05 (− 0.26, 0.16) | 2nd assessment scorec | 254 | 95.69 (0.23) | 261 | 95.52 (0.24) | 0.17 (− 0.48, 0.82) |
3MS Modified Mini-Mental Status exam (range = 0–100)33; CI confidence interval; CTSC Clinical and Translational Science Collaborative center for VITAL in Boston, MA; TICS Telephone Interview of Cognitive Status (range = 0–41)37.
aIn the VITAL-Cog, 2483 completed both assessments, 501 completed only the baseline, 440 completed only the 2nd assessment. In the CTSC-Cog, 497 completed both assessments, 279 completed only the baseline and 17 completed only the 2nd assessment.
bIn the VITAL-Cog: global score is a composite score representing the mean of the z-scores of 8 tests: TICS (range 0–41), immediate and delayed recalls of the East Boston Memory Test, category fluency (animal naming test), delayed recall of the TICS 10-word list, oral trails making test A, oral trails making test B and digit span backwards. Verbal memory score is a composite score representing the mean of the z-scores of 4 tests: the immediate and delayed recalls of both the TICS 10-word list and the East Boston Memory Test. Executive function/attention score is a composite score representing the mean of the z-scores of 4 tests: trails making test A and B, category fluency tests (naming animals), and digit-span backwards. In the CTSC-Cog: the global score is a composite score representing the mean of the z-scores of 9 tests: 3MS, immediate and delayed recalls of the East Boston Memory Test, category fluency tests (naming animals and vegetables), the immediate and delayed recalls of a 10-word list and trail-making tests A and B. Verbal memory score was defined the same way as in VITAL-Cog. Executive function/attention score is a composite score representing the mean of the z-scores of 4 tests: trails making tests A and B, category fluency tests (naming animals and vegetables).
cLeast squares means and standard errors and differences of least squares means and standard errors were derived from univariate models.
Table 3.
Meta-analysis of the mean differences (95% CI) in change over time among VITAL-Cog participants (n = 3424) and CTSC-Cog participants (n = 794), by Vitamin D supplement assignment.
3MS Modified Mini-Mental Status exam (range = 0–100)33, CI confidence interval; CTSC Clinical and Translational Science Collaborative center for VITAL in Boston, MA; TICS Telephone Interview of Cognitive Status (range = 0–41)37.
aFor definitions of the global scores and the key secondary outcomes for the two populations, see footnotes for Table 2.
bFrom linear mixed models of cognitive performance: model 1 includes time since randomization modelled as a continuous variable, vitamin D assignment, and their interaction.
cFrom linear mixed models of cognitive performance: model 2 is model 1 with adjustment for 6 additional variables, omega-3 assignment (yes/no), sex (male/female), age at randomization (years), race/ethnicity (non-Hispanic white, black, other race/ethnicity), education (high school or under, college, graduate school), history of depression (yes/no; see footnote in Table 1 for definition), and the six interaction terms (products with time since randomization).
dPooled using Dersimonian and Laird fixed-effects method for meta-analysis40 except for general cognition where the p for heterogeneity across the two substudies was 0.04 and results were meta-analyzed with random-effects.
eDue to the differences in scale between the TICS (0–41) used in VITAL-Cog and 3MS (range 0–100) used in CTSC-Cog, for pooling purposes, the 3MS scores were multiplied by 0.41 for conversion to the same scale as the TICS scores. As the p for heterogeneity across the two substudies was 0.04, the results were meta-analyzed with Dersimonian and Laird method incorporating random-effects40.
fNone of the effects for the secondary outcomes were significant at Bonferroni-adjusted p-value of 0.0167 (= 0.05/3 secondary outcomes).
In CTSC-Cog (Table 2), we did not observe an effect of vitamin D3 supplementation on cognition at the end of follow-up (mean 2.0 years (range = 1.0–3.1 years)): the least squares mean for the global score was 0.11 (SE = 0.03) for the vitamin D3 group and 0.06 (SE = 0.03) for the placebo group (mean difference = 0.05, 95% CI − 0.04, 0.14). The multivariable-adjusted mean difference in the global score annual rate of decline was − 0.004 (95% CI − 0.04, 0.03; p = 0.83; Table 3). Similarly, multivariable-adjusted mean differences in annual rates of decline for secondary cognitive systems were not significant: 0.01 (95% CI − 0.05, 0.06) for the verbal memory composite score; − 0.01 (95% CI − 0.05, 0.02) for the executive function/attention score and 0.04 (95% CI − 0.08, 0.17) for the 3MS score (re-scaled to have range 0–41 points like the TICS). Results for individual tests are in Table S1.
We observed no heterogeneity in the results by substudy (p for heterogeneity ≥ 0.28); thus, the multivariable-adjusted results were meta-analyzed (Table 3). The pooled effect of vitamin D3 supplementation was a mean difference in the annual rate of decline of 0.01 (95% CI − 0.01, 0.02; p = 0.39) for the global score; 0.01 (95% CI − 0.01, 0.02), verbal composite score; 0.01 (95% CI − 0.01, 0.02), executive function/attention composite score; and 0.03 (95% CI − 0.03, 0.09), for general cognition (TICS/3MS).
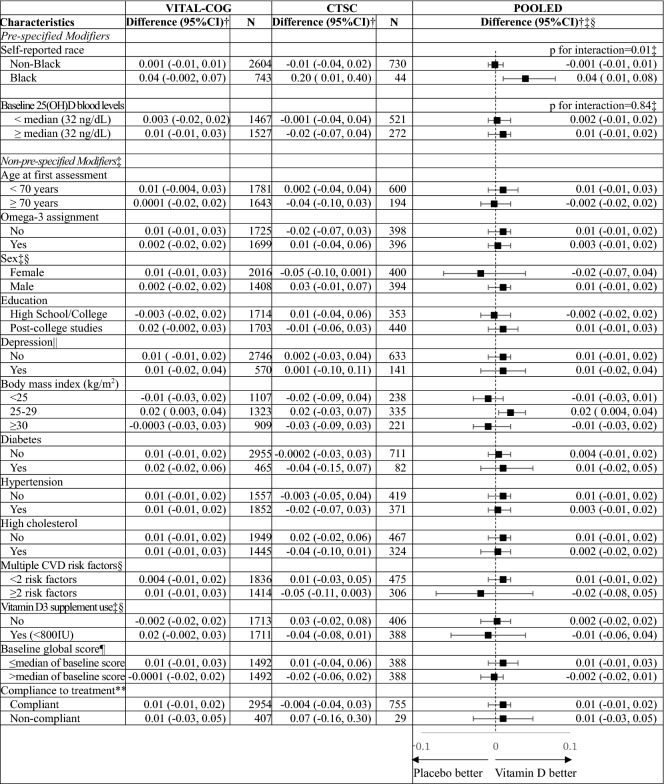
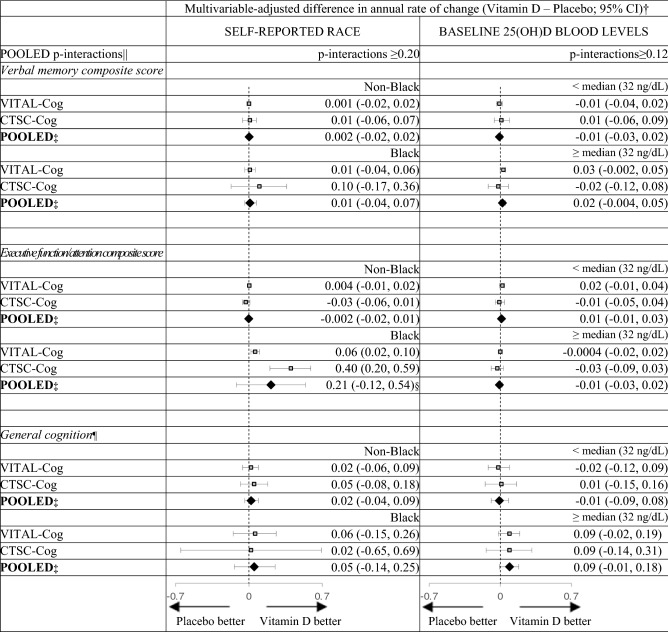
For pre-specified interaction analysis (Table 4) by race for the global composite score, we observed a significant interaction (pooled p-interaction = 0.01), where among Black participants, the vitamin D3 group showed a significantly slower rate of decline than placebo (pooled multivariable-adjusted mean difference in annual rate of decline = 0.04, 95% CI 0.01, 0.08), but not in other races (pooled multivariable-adjusted mean difference = − 0.001, 95% CI − 0.01, 0.01). To help interpret these results, among Black participants, at the 2nd assessment in VITAL-Cog, those on vitamin D had a 0.03 standard units higher global score performance than those on placebo; this difference was equivalent to that observed with Black participants who were 1.2 years apart in age, indicating an overall modest effect. The beneficial vitamin D3 effect among Black participants was stronger for the executive function/attention score, where the effect was equivalent to the difference observed between Black participants who were 3.8 years apart in age (when the more conservative fixed effects summary was used for estimation (Table 5 footnote)). We observed no significant interaction with the other pre-specified modifier of baseline blood 25(OH)D concentrations for the global score (pooled p-interaction = 0.84; Table 4) or the secondary outcomes (pooled p-interaction ≥ 0.12; Table 5). Finally, we observed no significant interactions for the other 13 effect modifiers evaluated for the global score (pooled p-interactions ≥ 0.18; Table 4; for secondary outcomes, see Table 6).
Table 4.
Mean difference (95% CI) in rate of change in global score between vitamin D and placebo group: effect modification by risk factors for cognitive decline.
CI confidence interval; CTSC-Cog, subset that received in-person interviews at the Harvard Clinical and Translational Science Collaborative center for VITAL in Boston, MA; CVD cardiovascular disease; VITAL-Cog subset that received telephone cognitive interviews in VITAL.
For definitions of the global scores for the two populations, see footnote for Table 2.
aMean difference in annual rate of decline of vitamin D—placebo groups from multivariable-adjusted linear mixed models: see footnotes for Table 3. The stratified analyses were done among those with non-missing data on the effect modifier.
bInteraction terms across the two substudies were pooled using Dersimonian and Laird fixed-effects method for meta-analysis. There was significant heterogeneity (p < 0.05) for two pooled p-interactions, and for these, random effects were incorporated into the meta-analysis; the pooled p-for interaction was 0.48 for sex and 0.66 for Vitamin D supplement use (< 800 IU) outside of the trial. None of the interaction terms for the non-pre-specified modifiers were significant at the Bonferroni-adjusted p-value of 0.0038 (= 0.05/13 subgroup analyses): pooled p for interaction ≥ 0.18.
cStratum-specific estimates were pooled using Dersimonian and Laird fixed-effects method for meta-analysis. There was significant heterogeneity (p-het < 0.05) for three strata, and for these, random effects were incorporated into the meta-analysis and presented in the Table. For reference, the fixed effects meta-analyzed pooled estimates were: 0.004 (95% CI − 0.01, 0.02) for females (p-het = 0.03), 0.003 (95% CI − 0.02, 0.02) for those with multiple CVD risk factors (p-het = 0.04) and 0.01 (95% CI − 0.01, 0.02) for those using Vitamin D supplements (< 800 IU) outside of the trial (p-het = 0.03).
dSee footnote in Table 1 for definition of depression.
eMedian for the global score was 0.05 in both the VITAL-Cog and the CTSC-Cog.
fCompliance is defined as taking ≥ 2/3rd of pills on all of the follow-up questionnaires between the first and the second cognitive assessment and not initiating out-of-study fish oil supplementation.
Table 5.
Pooled results across VITAL-Cog and CTSC-Cog for mean difference in annual rate for the secondary outcomes for vitamin D-Placebo: effect modification by race and blood 25(OH)D levels for cognitive decline.
3MS Modified Mini-Mental Status exam (range = 0–100)33; CI confidence interval; CTSC Clinical and Translational Science Collaborative center for VITAL in Boston, MA; TICS Telephone Interview of Cognitive Status (range = 0–41)37.
For definitions of the secondary outcomes for the two populations, see footnotes for Table 2.
aFrom multivariable-adjusted linear mixed models of cognitive performance (model 2) as described in footnote in Table 3.
bPooled using Dersimonian and Laird fixed-effects method for meta-analysis40 unless otherwise noted.
cPooled using Dersimonian and Laird random-effects method for meta-analysis40 as the p for heterogeneity was 0.001; if fixed effects methods are used, the pooled estimate was 0.07 (95% CI 0.03, 0.12).
dNot significant at Bonferroni-corrected p-value of 0.0167 (= 0.05/3 outcomes).
eDue to the differences in scale between the TICS (0–41) used in VITAL-Cog and 3MS (range 0–100) used in CTSC-Cog, for pooling purposes, the 3MS scores were multiplied by 0.41 for conversion to the same scale as the TICS scores.
Table 6.
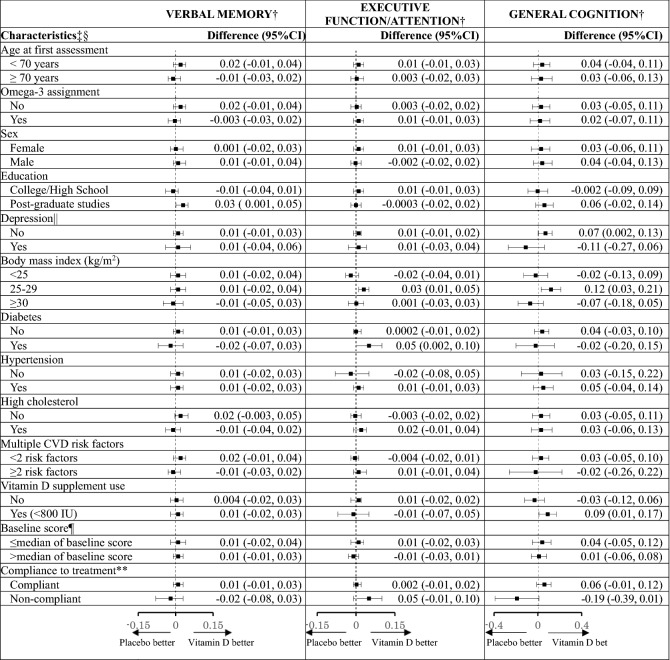
Pooled results across VITAL-Cog and CTSC-Cog for mean difference in annual rate for the secondary outcomes for vitamin D-Placebo: effect modification by risk factors for cognitive decline.
3MS Modified Mini-Mental Status exam; CI confidence interval; CTSC-Cog subset that received in-person interviews at the Harvard Clinical and Translational Science Collaborative center for VITAL in Boston, MA; TICS Telephone Interview of Cognitive Status; VITAL-Cog subset that received telephone interviews in VITAL.
From multivariable-adjusted linear mixed models of cognitive performance (model 2) as described in footnote in Table 3.
aFor definitions of the verbal memory and executive function scores for the two populations, see footnotes for Table 2. For general cognition, due to the differences in scale between the TICS (0–41) and 3MS (range 0–100), for pooling purposes, the 3MS scores were multiplied by 0.41 for conversion to the same scale as the TICS scores.
bInteraction terms across the two substudies were pooled using Dersimonian and Laird fixed-effects method for meta-analysis. For a few interactions where there was significant heterogeneity (p < 0.05) for the estimate across the two substudies, random effects were incorporated into the meta-analysis. Among these non-pre-specified modifiers for secondary outcomes, none of the pooled p-interactions were significant at Bonferroni-adjusted p-value of 0.0038 (= 0.05/13 subgroup analyses), except for three nominally significant pooled p-interactions for education for verbal memory (p = 0.04), diabetes for executive function/attention (p = 0.04); compliance for general cognition (p = 0.01).
cStratum-specific estimates were pooled using Dersimonian and Laird fixed-effects method for meta-analysis. For a few strata where there was significant heterogeneity (p < 0.05) for the estimate across the two substudies, random effects were incorporated into the meta-analysis. For reference, the fixed effects pooled estimate for executive function/attention is 0.002 (95% CI − 0.02, 0.02) for those without hypertension; 0.01 (95% CI − 0.01, 0.03) for those taking Vitamin D supplements (< 800 IU) outside of the trial; and the pooled estimate for general cognition is − 0.001 (95% CI − 0.08, 0.08) for those without hypertension and 0.03 (95% CI − 0.06, 0.13) for those with multiple CVD risk factors.
dFor the definition of depression, see footnote in Table 1.
eFor the verbal memory score, the median was -0.02 standard units in VITAL-Cog and 0.02 in the CTSC-Cog; for the executive memory/attention score, the median was 0.04 in VITAL-Cog and 0.02 in the CTSC-Cog; for TICS, the median was 34 in VITAL-Cog and for the 3MS in CTSC-Cog, the median was 96 (equivalent to 39 on the transformed variable to have the same range as the TICS).
fCompliance is defined as taking ≥ 2/3rd of pills on all of the follow-up questionnaires between the first and the second cognitive assessment and not initiating out-of-study fish oil supplementation.
In sensitivity analyses where we restricted the analyses in both substudies to those who reported no hearing impairment (68% in VITAL-Cog; 86% in CTSC-Cog; pooled mean difference in the annual rate of decline in the global score was 0.01 (95% CI − 0.01, 0.02; p = 0.37)) or restricted the analyses to those enrolled from the 1st assessment in VITAL-Cog (pooled mean difference was 0.01 (95% CI − 0.01, 0.02; p = 0.39)) or restricted the analyses in CTSC-Cog to those who did not have neuropsychiatric disorders or possible dementia at baseline (72%; pooled mean difference was 0.01 (95% CI − 0.01, 0.02; p = 0.40)) or restricted the analyses in both substudies to those who were in the top 90% of performance in each outcome (to avoid floor effects and to remove those with possible dementia, especially in VITAL-Cog; pooled mean difference was − 0.001 (95% CI − 0.01, 0.01; p = 0.93)), results were similar to the main results. Results also did not differ when we additionally adjusted for practice effects (pooled mean difference was 0.01 (95% CI − 0.01, 0.02; p = 0.31)) or when we additionally adjusted for season of cognitive assessment (pooled mean difference was 0.01 (95% CI − 0.01, 0.02; p = 0.38)).
Discussion
In this randomized trial among generally healthy community-dwelling older participants followed for 2–3 years, supplementation with 2000 IU/day of vitamin D3 was not associated with cognitive decline. No effects were observed for the primary outcome as well as secondary outcomes of verbal memory, executive function/attention and global cognition and for both substudies where cognition was assessed by phone or in person. However, a pre-specified subgroup analysis showed cognitive benefits over time for vitamin D3 supplementation versus placebo among Black participants, but not by levels of 25(OH)D. Because the subgroup analyses by race may be due to chance, the results should be interpreted with caution and confirmed in future studies.
Randomized trials of vitamin D and cognitive decline21,22,41 in relatively healthy populations have shown conflicting results, with most reporting no benefit from supplementation, despite vitamin D’s potential neuroprotective anti-inflammatory and antioxidant effects7–11,42. In the largest (n = 4143) and longest trial (7.8 years duration) where women aged 65+ years received vitamin D3 (400 IU/day) and calcium (1000 mg/day) or placebo24, no association was observed between vitamin D3/calcium treatment and incident cognitive impairment or dementia, although the vitamin D3 dose was low, and was one fifth of our dose. Thus, our findings are important in suggesting that even much higher doses of vitamin D3 do not provide meaningful cognitive benefits overall. Similarly, in two European randomized trials of 2000 IU/day of vitamin D3 compared to placebo43 (n = 2157) or 800 IU/day44 (n = 273) of vitamin D3 assessed over 343 and 244 years among community-dwelling older persons observed no differences in change in cognitive function by intervention. Four small studies (n < 400)23,45–47 have evaluated even higher doses of vitamin D3 but were short-term (≤ 1 year of treatment) and also have not observed significant overall differences in cognitive change. Among Black adults, in a 3-year study among 260 older women (aged 65–73 years) where higher doses of vitamin D3 (individualized doses of ≥ 2400 IU/day needed to maintain serum 25(OH)D ≥ 30 ng/mL) and calcium (1200 mg/day) was compared to placebo and calcium (1200 mg/day), Owusu et al.48 observed no difference in change in MMSE performance, similar to our null finding for general cognition for Black participants; change in executive function was not assessed in this study. Thus, our study adds to the literature in that it was a long-term, large study (n > 4200) testing a relatively high dose of vitamin D3 for long durations and had a relatively large representation of Black participants.
In subgroup analyses, we observed that in Black participants, vitamin D3 supplementation was significantly associated with better cognitive maintenance in the global score and executive function/attention score. This is consistent with studies that have observed that vitamin D3 deficiency is associated most prominently with deficits in executive function41,45,49. While the interaction by race and baseline blood 25(OH)D levels were pre-specified, these subgroup findings were not adjusted for multiple comparisons and thus, should be interpreted with caution. We had hypothesized a priori that vitamin D3 might have particular benefits in Black participants who had lower 25(OH)D concentrations at baseline; yet, given the lack of a significant effect modification by baseline blood 25(OH)D levels in this substudy and the main trial28, the reasons for the specific benefits in Black participants remain unclear. A future evaluation using novel biomarkers of vitamin D (e.g., vitamin D binding protein (VDBP) or free 25(OH)D)29,30,50,51 and genetic variants for VDBP52–54 that show difference in distribution across race/ethnicity groups may be insightful. Also, it is notable that Black participants had a higher prevalence of diabetes and other cardiovascular risk factors, and lower baseline blood 25(OH)D levels, education and baseline cognitive scores, which were characteristics of subgroups for which vitamin D had a suggestively stronger beneficial effect, particularly for executive function; thus, the concentration of a multitude of risk factors for cognitive decline may have led to stronger benefits of vitamin D in Black participants.
Limitations of our study warrant consideration. First, in VITAL-Cog, cognitive assessments were conducted over the phone; however, our validation of the telephone cognitive assessment with in-person assessment showed reasonable validity, and the main results were similar in CTSC-Cog, with in-person cognitive assessments. While telephone interviews increased participation, it is possible that there was more misclassification in outcome assessment and that subtle changes were missed compared to in-person assessments. Our trial included mostly healthy, well-educated individuals (> 50% had post-graduate studies); this likely led to modest observed cognitive decline and few participants being vitamin D deficient. Both factors may have limited our ability to detect modest effects of vitamin D3 supplements on cognition. Also, while a dose of 2000 IU/day was used in the study, it is possible that the optimal dose for brain health might be higher, although the literature has been inconsistent43,48,55. Finally, the follow-up period of 2–3 years, with only two assessments, may have been too short to detect effects of vitamin D3 supplementation, particularly in a healthy population at relatively lower risk for cognitive decline. Although in VITAL-Cog, we did observe cognitive decline in the placebo group over 2.8 years follow-up, additional studies with longer durations of follow-up and more cognitive assessments among those at highest risk of vitamin D deficiency and cognitive decline would be important.
Our study had several strengths. This was a randomized trial including > 4200 participants, with high rates of follow-up and adherence to the assigned treatment group. In particular, there was a relatively high proportion of Black participants (19%), who are at high risk for vitamin D insufficiency30,56–58. Also, we were able to investigate the effect of vitamin D3 supplements on multiple cognitive domains.
In conclusion, among generally well-educated healthy adults aged 60+ years, supplementation of vitamin D3 (2000 IU/day) did not slow cognitive decline over 2–3 years, although there were modest benefits observed specifically in Black older adults that should be confirmed in future studies.
Methods
Study design, randomization and masking, and procedures
VITAL trial
VITAL26–28 is a completed large randomized, double-blind, placebo-controlled, 2 × 2 factorial clinical trial of vitamin D3 (vitamin D3[cholecalciferol], 2000 IU/day) and marine omega-3 fatty acid (Omacor® fish oil, eicosapentaenoic acid + docosahexaenoic acid, 1 g/day) oral supplements in the primary prevention of cancer and cardiovascular disease. Participants were free of cancer (except non-melanoma skin cancer) and cardiovascular disease. Participants (n = 25,871 US men aged ≥ 50 and women aged ≥ 55 years) were randomized from 2011 to 2014 and were required to limit using out-of-study supplemental vitamin D3 to ≤ 800 IU/day, supplemental calcium to ≤ 1200 mg/day, and to avoid using omega-3 fatty acid supplements. Supplementation with 2000 IU/day vitamin D3 for one year in VITAL led to a 40% mean increase in 25-hydroxyvitamin D (25(OH)D) levels (from 29.8 to 41.8 ng/mL)28. The VITAL trial main phase has been completed, and its trial design (including details on randomization and masking)26 and main findings have been published27,28. The marine n-3 arm results for the cognitive substudies have been analyzed separately59.
Participants
We used data from two distinct subsets of VITAL participants. Although cognitive function was not the main planned outcome to be evaluated in the parent VITAL trial, assessing cognitive function was planned before the start of the trial and baseline cognitive function assessments were planned to occur before randomization as much as possible. One subset (VITAL-Cog; NCT01669915); n = 3424) completed cognitive assessments by phone with randomization and again 2.8 years later. Another subset (CTSC-Cog; n = 794 in an ancillary study of depression (VITAL-DEP; NCT01696435)) completed in-person cognitive assessments with randomization and again 2.0 years later.
In VITAL-Cog, the baseline cognitive interview was conducted from September 2011 through April 2014 (mean = 1 month before randomization; range of 1.2 years before to 0.5 years after randomization (1.31% done > 1 month after randomization); Fig. 1a). Of 3658 eligible people as of April 2014 and we attempted to contact, 241 (7%) were unreachable, and of 3417 contacted, 3271 (96%) participated. We further excluded 262 participants who were also in the CTSC-Cog, leaving 3009 participants (2984, including 317 Black participants, with complete scores on all tests and 25 with scores missing on some tests). For the 2nd cognitive assessment (February 2013 to June 2016), of the 3009 who participated in the 1st assessment, 58 died (2%) and 322 were unreachable (11%). Of the 2629 contacted, 100 (4%) refused, and 2529 (96%) participated (2501 with complete scores on all tests and 28 with scores missing on some tests).
Figure 1.
(a) Flow of Participants in the VITAL-Cog Ancillary Study to the VITAL Trial. (b) Flow of Participants in the subset of CTSC-Cog participants in the VITAL-DEP Ancillary Study to the VITAL Trial.
To allow for enough time for follow-up assessments within the trial period and because we had reached the target of 3000 participants, we stopped administering baseline cognitive assessments in April 2014, even though there were additional eligible participants. However, to increase the number of Black participants, at the initiation of 2nd assessments, we invited 618 additional eligible Black participants (Fig. 1a: aged 60+ years at randomization and willing to be part of VITAL-Cog). Of 618 Black participants, 141 (23%) could not be contacted. Of 477 contacted, 48 refused (10%) and 429 (90%) participated (November 2014 to June 2016; 422 with complete scores on all tests; and 7 with scores missing on some tests). Thus, the total number of unique individuals in VITAL-Cog was 3424: 2984 with complete baseline assessments and 2923 (= 2501 + 422 new Black participants) with complete follow-up assessments.
In CTSC-Cog, the baseline assessment occurred from January 2012 to March 2014 (mean = 0.5 month before randomization; range of 3.0 months before to within 1 month after randomization). For CTSC-Cog (Fig. 1b), we excluded 229 participants aged < 60 years and four people who refused participation, leaving 821 participants (776 with complete scores on all tests and 45 with scores missing on some tests). A 2-year follow-up in-person interview was conducted from January 2014 through April 2016. Of 821 who participated in the baseline assessment, 3 died (0.4%), 217 (26%) were ineligible for VITAL-DEP due to their baseline assessments showing neuropsychiatric disorders, 6 (1%) showed neuropsychiatric disorders and possible dementia, 55 (7%) refused; and 540 participated (515 with complete scores on all tests and 25 with scores missing on some tests). The total number of unique individuals in CTSC-Cog was 794 (including 44 Black participants): 776 with complete baseline assessments and 515 with complete follow-up assessments.
Standard protocol approvals, registrations, and patient consents
The research followed the Declaration of Helsinki, and this substudy protocol was approved by the institutional review board of the Brigham and Women’s Hospital. Written informed consent was obtained directly from VITAL participants and CTSC-Cog participants or from their legally authorized representatives/next of kin26; for VITAL-Cog, completion of cognitive tests was considered as implied consent.
Participants and outcomes: VITAL-Cog study population and telephone cognitive function assessments
In VITAL-Cog substudy, the eligibility criteria were age 60+ years and in the screening questionnaire, being willing to participate in cognitive function assessments. Cognition was assessed by telephone by trained interviewers, with eight neuropsychological tests assessing general cognition (Telephone Interview of Cognitive status (TICS; range = 0–41 points)), verbal memory, and executive function/attention (see details in the “Supplementary Methods”). We derived a global composite score by averaging the z-scores of the eight individual tests (based on the baseline test distributions). When generating composite scores for the 2nd assessment, the baseline means and SDs of scores from VITAL-Cog were used. Our primary, pre-specified outcome was the annual rate of change of the global composite score, and for secondary outcomes, we also evaluated the TICS and composite scores for verbal memory and executive function/attention (“Supplementary Methods”).
Participants and outcomes: CTSC-Cog study population and in-person cognitive function assessments
A subgroup of 1054 VITAL participants received in-person health assessments, including cognitive assessments as part of VITAL-DEP60, by trained interviewers at the CTSC in Boston with randomization (CTSC-Cog). The in-person cognitive battery included nine cognitive tests assessing general cognition (Modified Mini-Mental State (3MS; range = 0–100)33), verbal memory and executive function/attention. The CTSC global composite score, the primary outcome, was calculated as the average of the z-scores for the nine assessments, using the CTSC-Cog baseline means and SDs, for both baseline and follow-up; secondary outcomes included the 3MS and verbal memory and executive function/attention composite scores (“Supplementary Methods”).
Validation study of the VITAL-Cog telephone cognitive assessment
Cognitive assessment by phone has been extensively validated61,62. In VITAL-Cog, we validated our telephone cognitive assessment against in-person assessments among a subset of 181 of the 262 CTSC participants with both assessments who had the two within 1 month of each other. We compared the global composite score derived from scores on the eight tests administered by telephone versus a similar score derived from the nine tests administered in-person. The intraclass correlation between the two modes was 0.64, supporting the validity of our telephone cognitive interview (“Supplementary Methods”).
Statistical analyses
We compared characteristics at randomization by treatment group using Wilcoxon’s rank-sum tests for continuous variables and chi-square tests for proportions. Primary analyses were conducted using the intention to treat principle. For each substudy, linear mixed-effects models with random intercepts were used to estimate the mean change in participants’ scores as a function of time (years between randomization and each assessment), treatment assignment, and their interaction63. We fitted models by maximum likelihood, incorporating the longitudinal correlation within participants (using unstructured covariance structure); for statistical testing, we used Wald tests. We calculated multivariable-adjusted mean differences in annual rate of decline and 95% confidence intervals (CIs); information on covariates at pre-randomization were collected by questionnaires. We used two models: model 1 included just the treatment group, while model 2 was additionally adjusted for age at randomization (years), sex, highest attained education, race, omega-3 treatment arm assignment, and depression history.
In secondary analyses, we evaluated potential effect modification by race and baseline blood vitamin D levels, which were pre-specified given that supplementation may have stronger effects on subgroups with relatively lower blood vitamin D levels such as Black participants29,30. We also evaluated effect modification by testing the 3-way interaction terms in multivariable-adjusted linear mixed models for 13 possible risk factors of cognitive decline (based on self-report on pre-randomization questionnaires): age, sex, omega-3 fatty acid assignment, education, depression, body mass index, diabetes, hypertension, high cholesterol, multiple CVD risk factors, out-of-study vitamin D3 supplement use, baseline score and compliance (over the entire follow-up period).
For the primary outcome of global score and for the two pre-specified subgroup analyses, the significance tests were 2-sided, and the significance level was p-value < 0.05. For the secondary outcomes and subgroup analyses, multiple comparisons were adjusted using Bonferroni corrections.
We first evaluated associations separately by substudy and then pooled the substudy-specific results using the Dersimonian and Laird meta-analytic approach incorporating fixed-effects40. Because the TICS and 3MS had different scales, for pooling, we multiplied the 3MS scores by 0.41 to generate the same scale as the TICS.
In sensitivity analyses, we restricted the analyses in both substudies to those who reported no hearing impairment (68% in VITAL-Cog; 86% in CTSC-Cog), restricted the analyses to those enrolled from the 1st assessment in VITAL-Cog (to ascertain whether missingness in the data can be assumed to be missing at random), restricted the analyses in CTSC-Cog to those who did not have neuropsychiatric disorders or possible dementia at baseline (72%), and restricted the analyses in both substudies to those who were in the top 90% of performance in each outcome. In additional analyses, we additionally adjusted for practice effects by adjusting for the number of prior assessments and in alternate models, we additionally adjusted for the season of cognitive assessment as vitamin D levels may depend on season of the year.
For statistical analyses, we used SAS (SAS release 9.4; SAS Institute Inc, Cary, NC). For the cognitive ancillary substudies, there was no data monitoring committee. This study is registered with ClinicalTrials.gov VITAL-Cog (NCT01669915), VITAL-DEP (NCT01696435) and VITAL (NCT01169259).
Supplementary Information
Acknowledgements
VITAL (NCT01169259), VITAL-DEP (NCT01696435) and VITAL-Cog (NCT01669915) are registered with ClinicalTrials.gov. VITAL-Cog was supported by R01 AG036755; VITAL-DEP was supported by R01 MH091448; and VITAL was supported by Grants U01 CA138962, R01 CA138962, and UL1TR001102 including support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Centre for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple Institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others. The funding sources had no role in the study design; the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Author contributions
J.H.K., O.I.O., F.G., J.E.M. conceptualized the study design, investigation; J.H.K., O.I.O., D.D., F.G., J.E.M. curated the data and provided project administration; J.H.K., N.R.C. and C.M.V. did the formal analysis; J.H.K., O.I.O., F.G., J.E.M. acquired the funding; J.H.K., O.I.O., C.M.V., S.O., J.E.M. wrote the original draft; J.H.K. and C.V.M. accessed and verified the data, and all authors (J.H.K., C.M.V., O.I.O., S.O., M.A., I.L., D.D., J.E.B., N.R.C., F.G., J.E.M.) provided input on the interpretation of the data, reviewed and edited the final submitted draft of the manuscript.
Data availability
The corresponding author can be contacted for de-identified data requests. Analysis proposal requests will require review and approval by the VITAL Publications & Presentations Committee and appropriate IRB approval. Once approved and a data access agreement has been executed, deidentified data generated from this research will be made available to affiliated investigators through secure databases for the prespecified analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02485-8.
References
- 1.Annweiler C, et al. Vitamin D and ageing: Neurological issues. Neuropsychobiology. 2010;62:139–150. doi: 10.1159/000318570. [DOI] [PubMed] [Google Scholar]
- 2.Llewellyn DJ, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch. Intern. Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing "D"ecline? Mol. Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slusarczyk J, et al. Nanocapsules with polyelectrolyte shell as a platform for 1,25-dihydroxyvitamin d3 neuroprotection: Study in organotypic hippocampal slices. Neurotox. Res. 2016;30:581–592. doi: 10.1007/s12640-016-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 6.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J. Inflamm. (Lond.) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bivona G, et al. Non-skeletal activities of vitamin D: From physiology to brain pathology. Medicina (Kaunas) 2019 doi: 10.3390/medicina55070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bivona G, et al. Standardized measurement of circulating vitamin D [25(OH)D] and its putative role as a serum biomarker in Alzheimer's disease and Parkinson's disease. Clin. Chim. Acta. 2019;497:82–87. doi: 10.1016/j.cca.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Bivona G, et al. The role of vitamin D as a biomarker in Alzheimer's disease. Brain Sci. 2021 doi: 10.3390/brainsci11030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gambino CM, Sasso BL, Bivona G, Agnello L, Ciaccio M. Aging and neuroinflammatory disorders: New biomarkers and therapeutic targets. Curr. Pharm. Des. 2019;25:4168–4174. doi: 10.2174/1381612825666191112093034. [DOI] [PubMed] [Google Scholar]
- 11.Bivona G, Gambino CM, Iacolino G, Ciaccio M. Vitamin D and the nervous system. Neurol. Res. 2019;41:827–835. doi: 10.1080/01616412.2019.1622872. [DOI] [PubMed] [Google Scholar]
- 12.Annweiler C, et al. Dietary intake of vitamin D and cognition in older women: A large population-based study. Neurology. 2010;75:1810–1816. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 13.Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly US population. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarcin O, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J. Clin. Endocrinol. Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 15.Keisala T, et al. Premature aging in vitamin D receptor mutant mice. J. Steroid. Biochem. Mol. Biol. 2009;115:91–97. doi: 10.1016/j.jsbmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, et al. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol. Neurootol. 2008;13:219–230. doi: 10.1159/000115431. [DOI] [PubMed] [Google Scholar]
- 17.Brewer LD, Porter NM, Kerr DS, Landfield PW, Thibault O. Chronic 1alpha,25-(OH)2 vitamin D3 treatment reduces Ca2+-mediated hippocampal biomarkers of aging. Cell Calcium. 2006;40:277–286. doi: 10.1016/j.ceca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 19.Shah I, et al. Low 25OH vitamin D2 levels found in untreated Alzheimer's patients, compared to acetylcholinesterase-inhibitor treated and controls. Curr. Alzheimer Res. 2012;9:1069–1076. doi: 10.2174/156720512803568975. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, et al. Circulating vitamin D levels and Alzheimer's disease: A mendelian randomization study in the IGAP and UK Biobank. J. Alzheimers. Dis. 2020;73:609–618. doi: 10.3233/JAD-190713. [DOI] [PubMed] [Google Scholar]
- 21.Sommer I, et al. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017;17:16. doi: 10.1186/s12877-016-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghajafari F, Pond D, Catzikiris N, Cameron I. Quality assessment of systematic reviews of vitamin D, cognition and dementia. BJPsych. Open. 2018;4:238–249. doi: 10.1192/bjo.2018.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean AJ, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults—A randomised controlled trial. PLoS ONE. 2011;6:e25966. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossom RC, et al. Calcium and vitamin D supplementation and cognitive impairment in the women's health initiative. J. Am. Geriatr. Soc. 2012;60:2197–2205. doi: 10.1111/jgs.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer's disease. J. Alzheimers Dis. 2011;26:477–484. doi: 10.3233/JAD-2011-110149. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manson JE, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson JE, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos. Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powe CE, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canevelli M, et al. Race reporting and disparities in clinical trials on Alzheimer's disease: A systematic review. Neurosci. Biobehav. Rev. 2019;101:122–128. doi: 10.1016/j.neubiorev.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Manson JE, et al. Vitamin D, marine n-3 fatty acids, and primary prevention of cardiovascular disease current evidence. Circ. Res. 2020;126:112–128. doi: 10.1161/CIRCRESAHA.119.314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 34.Albert M, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int. J. Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 35.Abraham E, Axelrod BN, Ricker JH. Application of the oral trail making test to a mixed clinical sample. Arch. Clin. Neuropsychol. 1996;11:697–701. doi: 10.1093/arclin/11.8.697. [DOI] [Google Scholar]
- 36.Bastug G, et al. Oral trail making task as a discriminative tool for different levels of cognitive impairment and normal aging. Arch. Clin. Neuropsychol. 2013;28:411–417. doi: 10.1093/arclin/act035. [DOI] [PubMed] [Google Scholar]
- 37.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 38.Army Individual Test Battery. Manual of Directions and Scoring. (War Department, Adjutant General’s Office, 1944).
- 39.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsycholgical Test Battery: Therapy and Clinical Interpretation. Neuropsychological Press; 1985. [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 41.Annweiler C, et al. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J. Alzheimers Dis. 2013;37:147–171. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- 42.Annweiler C. Vitamin D in dementia prevention. Ann. NY Acad. Sci. 2016;1367:57–63. doi: 10.1111/nyas.13058. [DOI] [PubMed] [Google Scholar]
- 43.Bischoff-Ferrari HA, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: The DO-HEALTH randomized clinical trial. JAMA. 2020;324:1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schietzel S, et al. Effect of 2000 IU compared with 800 IU vitamin D on cognitive performance among adults age 60 years and older: A randomized controlled trial. Am. J. Clin. Nutr. 2019;110:246–253. doi: 10.1093/ajcn/nqz081. [DOI] [PubMed] [Google Scholar]
- 45.Pettersen JA. Does high dose vitamin D supplementation enhance cognition? A randomized trial in healthy adults. Exp. Gerontol. 2017;90:90–97. doi: 10.1016/j.exger.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Castle M, et al. Three doses of vitamin D and cognitive outcomes in older women: A double-blind randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:835–842. doi: 10.1093/gerona/glz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jorde R, et al. Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. J. Neurol. Sci. 2019;396:165–171. doi: 10.1016/j.jns.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Owusu JE, et al. Cognition and vitamin D in older African–American women-physical performance and osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J. Am. Geriatr. Soc. 2019;67:81–86. doi: 10.1111/jgs.15607. [DOI] [PubMed] [Google Scholar]
- 49.Lipowski M, et al. Improvement of attention, executive functions, and processing speed in elderly women as a result of involvement in the nordic walking training program and vitamin D supplementation. Nutrients. 2019 doi: 10.3390/nu11061311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bikle DD, et al. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 51.Brown LL, et al. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health—Expert panel meeting report. BMC Proc. 2018;12:6. doi: 10.1186/s12919-018-0102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun A, Bichlmaier R, Cleve H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): Allelic differences of the common genetic GC types. Hum. Genet. 1992;89:401–406. doi: 10.1007/BF00194311. [DOI] [PubMed] [Google Scholar]
- 53.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): A review of isoelectric focusing studies in human populations. Hum. Genet. 1986;72:281–293. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 54.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr. Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 55.Byrn MA, Adams W, Penckofer S, Emanuele MA. Vitamin D supplementation and cognition in people with type 2 diabetes: A randomized control trial. J. Diabetes Res. 2019;2019:5696391. doi: 10.1155/2019/5696391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J. Nutr. 2005;135:2478–2485. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 57.Holick MF. Bioavailability of vitamin D and its metabolites in black and white adults. N. Engl. J. Med. 2013;369:2047–2048. doi: 10.1056/NEJMe1312291. [DOI] [PubMed] [Google Scholar]
- 58.Harris SS. Vitamin D and African Americans. J. Nutr. 2006;136:1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 59.Kang, J. H. et al. Marine n-3 fatty acids and cognitive change among older adults in the VITAL randomized trial. Accepted abstract #55393 at the 2021 Alzheimer’s Association International Conference. [DOI] [PMC free article] [PubMed]
- 60.Okereke OI, et al. Effect of long-term vitamin d3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471–480. doi: 10.1001/jama.2020.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans DA, Grodstein F, Loewenstein D, Kaye J, Weintraub S. Reducing case ascertainment costs in US population studies of Alzheimer's disease, dementia, and cognitive impairment-Part 2. Alzheimers Dement. 2011;7:110–123. doi: 10.1016/j.jalz.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rapp SR, et al. Validation of a cognitive assessment battery administered over the telephone. J. Am. Geriatr. Soc. 2012;60:1616–1623. doi: 10.1111/j.1532-5415.2012.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; 2004. pp. 103–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author can be contacted for de-identified data requests. Analysis proposal requests will require review and approval by the VITAL Publications & Presentations Committee and appropriate IRB approval. Once approved and a data access agreement has been executed, deidentified data generated from this research will be made available to affiliated investigators through secure databases for the prespecified analysis.