Abstract
Background & Aims:
Eosinophilic gastritis (EG) and eosinophilic duodenitis (EoD), characterized by chronic gastrointestinal (GI) symptoms and increased numbers or activation of eosinophils and mast cells in the GI tract, are likely underdiagnosed. We aimed to determine rates of EG and EoD and number of biopsies required to optimize detection using screening data from a randomized trial of lirentelimab (AK002), an antibody against siglec-8 that depletes eosinophils and inhibits mast cells. We also characterized endoscopic features and symptoms of EG and EoD.
Methods:
Subjects with moderate-to-severe GI symptoms, assessed daily through a validated patient-reported outcome questionnaire, underwent endoscopy with a systematic gastric and duodenal biopsy protocol and histopathologic evaluation. EG diagnosis required presence of ≥30 eosinophils/high-powered field (eos/hpf) in ≥5 hpfs and EoD required ≥30 eos/hpf in ≥3 hpfs. We analyzed diagnostic yields for EG and EoD and histologic, endoscopic, and clinical findings.
Results:
Of 88 subjects meeting symptom criteria, 72 were found to have EG and/or EoD (EG/EoD), including patients with no prior diagnosis of EG/EoD. We found that GI eosinophilia was patchy and that examination of multiple biopsies was required for diagnosis—an average of only 2.6/8 gastric biopsies and 2.2/4 duodenal biopsies per subject met thresholds for EG/EoD. Evaluation of multiple non-overlapping hpfs in each of 8 gastric and 4 duodenal biopsies was required to capture 100% of EG/EoD cases. Neither endoscopic findings nor symptom severity correlated with eosinophil counts.
Conclusions:
In an analysis of patients with moderate-to-severe GI symptoms participating in a clinical trial of lirentelimab for EG/EoD, we found eosinophilia to be patchy in gastric and duodenal biopsies. Counting eosinophils in at least 8 gastric and 4 duodenal biopsies is required to identify patients with EG/EoD, so they can receive appropriate treatment. NCT03496571
Keywords: EGID, histology, eosinophilic enteritis, immune cell
Introduction
Eosinophilic gastrointestinal diseases are chronic inflammatory conditions characterized by persistent gastrointestinal (GI) symptoms and increased numbers and activation of eosinophils in the GI tract.(1–3) These disorders are classified by presence and location of tissue eosinophils, but aberrant activation of mast cells also appears to contribute to pathogenesis.(4–9) Dysregulation of inflammatory signaling pathways leads to type-2 immunity, activation and/or accumulation of eosinophils and mast cells, and alterations in GI physiology.(10–13) Patients with eosinophilic gastritis and/or eosinophilic duodenitis (EG/EoD) have nonspecific symptoms such as abdominal pain, early satiety, and diarrhea.(1–3) They have reduced quality of life, due to the effects of their symptoms on daily functions, lack of adequate treatment options, and frustration from delayed diagnosis.(14, 15)
Studies indicate that EG/EoD is underdiagnosed,(16–18) potentially due to lack of diagnostic guidelines, perceived rarity, and symptoms that are shared with other GI disorders, such as irritable bowel syndrome (IBS) or functional dyspepsia.(16) Without diagnostic guidelines, there is uncertainty about the number and location of biopsies required for optimal diagnostic yield,(1, 19) in contrast to the detailed biopsy protocols for detection of eosinophilic esophagitis (EoE).(20–24) Until recently, histologic criteria for diagnosis of EG/EoD were not well defined; experts and the Food and Drug Administration now accept ≥30 eosinophils per high-powered field (eos/hpf) in ≥5 hpfs in stomach biopsies and/or in ≥3 hpfs in duodenum biopsies as the diagnostic criteria for clinical trials.(1, 25)
Heightened awareness and standardization of diagnostic guidelines have improved detection of EoE(24)—the same is possible for EG/EoD. The first randomized trial for EG/EoD established the therapeutic potential of lirentelimab (AK002), a monoclonal antibody against siglec-8 on eosinophils and mast cells, in patients with EG/EoD.(25) Our primary aim was to analyze screening data from this prospective, multi-center trial to estimate the diagnostic yield of EG/EoD among patients with moderate-to-severe GI symptoms and determine the number of gastric and duodenal biopsies required to optimize detection. Our secondary aim was to analyze endoscopic features and characteristics of these patients.
Materials and Methods
Study design and study subjects
We analyzed screening data from the ENIGMA study (Clinicaltrials.gov identifier: NCT03496571), which enrolled patients 18–80 years old with moderate-to-severe GI symptoms who met histologic criteria for EG/EoD confirmed by esophagogastroduodenoscopy (EGD) with biopsy and histopathologic evaluation. Safety and efficacy endpoints have been published.(25) Diagnoses of functional disorders, which were previously made clinically, were obtained from patient medical histories at the time of study enrollment. We examined the number of biopsies needed to detect EG/EoD and analyzed baseline histologic features, endoscopic appearance, and symptom data collected during screening. Subjects who met symptom criteria but did not meet histologic criteria for EG/EoD were included in the present analysis as a comparison group. All authors had access to the study data and reviewed and approved the final manuscript. EG/EoD refers to histologic disease in the stomach (EG), duodenum (EoD), or both (EG+EoD). Individual participant data will not be shared.
Symptom assessment
Patient symptoms were measured daily using an electronic patient-reported outcome (PRO) questionnaire (see protocol in supplementary material) that assessed severity of abdominal pain, nausea, vomiting, early satiety, loss of appetite, abdominal cramping, bloating, and diarrhea. Individual symptom scores ranged from 0 (no symptoms) to 10 (worst); the maximum daily total symptom score was 80 (higher score indicates greater severity). (25) (26) To be eligible for EGD, subjects were required to have moderate-to-severe GI symptoms: average daily individual symptom score of ≥3 over 7 days for at least 1 of 3 symptoms (abdominal pain, diarrhea, and/or nausea) on the PRO questionnaire for at least 2 weeks.
Endoscopy and biopsy protocol
Patients’ esophagus, stomach, and duodenum were assessed by EGD. Features in the stomach were scored by individual investigators according to the Eosinophilic Gastritis Endoscopic Reference System (EG-REFS), which scores 7 parameters in fundus, corpus, and antrum. Total EG-REFS scores range from 0 to 46 (Table S1). A global endoscopic severity score for the stomach was also calculated for each subject (scores range from 0 to 10, with 10 indicating most severe).
For the standardized biopsy protocol, a minimum of 12 biopsies were collected during EGD (Figure 1): 4 specimens from separate areas of the gastric antrum (2–5 cm proximal to the pylorus), 4 from separate areas of the gastric corpus (2 from the proximal lesser curvature and two from the greater curvature), and 4 of duodenal mucosa from the second and third part of the duodenum. Up to 2 extra specimens each from the stomach and duodenum could be collected from areas of interest identified during EGD, at the discretion of endoscopists(25).
Figure 1. Biopsy and Histopathology Protocol and EG/EoD Diagnostic Criteria.
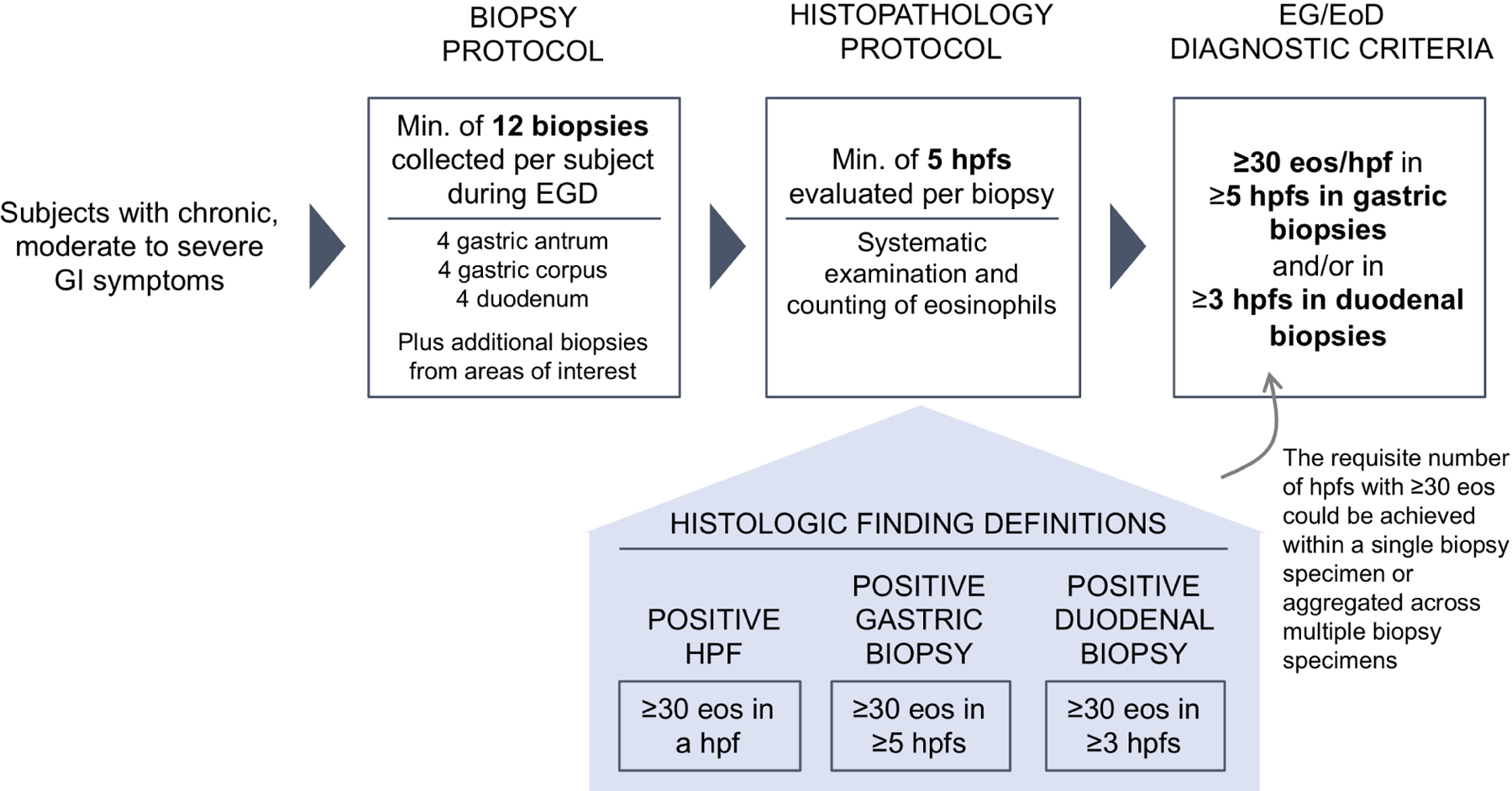
Criteria used for systematic biopsy collection, histopathologic examination, and diagnosis of EG/EoD.
Histopathologic evaluation
Biopsy samples were evaluated by a single pathologist, blinded to demographic, clinical, and endoscopic information, who quantified gastric and duodenal eosinophils/hpf and tryptase-positive gastric and duodenal mast cells/hpf using a standardized protocol (see supplement). The histologic criteria for EG were detection of ≥30 eos/hpf in ≥5 hpfs in gastric biopsy specimens and for EoD were detection of ≥30 eos/hpf in ≥3 hpfs in duodenal specimens. The requisite number of hpfs with ≥30 eos could be achieved within a single biopsy specimen or from multiple biopsy specimens (Figure 1, Figure S1). Other morphologic features were graded according to the Updated Sydney System and The Marsh Scale Classification, respectively.(27, 28)
Results
Subject characteristics and diagnostic yield
Baseline characteristics for patients who met the symptom criteria, and the subsets of patients who did vs did not meet the histologic criteria for EG/EoD, are shown in Table 1. Of note, 51 had no history of EG/EoD; of these, 26 met symptom criteria and underwent EGD with biopsy and 15 met histologic criteria for EG/EoD (Figure S2). The rates of discovery of EG/EoD were 58% (15/26) among subjects with moderate-to-severe symptoms, based on the study PRO measure, and 29% (15/51) among all subjects with no history of EG/EoD. Prior diagnoses other than EG/EoD included gastroesophageal reflux, functional dyspepsia, IBS, and other functional GI disorders.
Table 1.
Characteristics of Study Subjects
| Met Symptom Criteria (n=88) | Met Symptom Criteria and Histologic Criteria for EG/EoD (n=71) | Met Symptom Criteria but not Histologic Criteria for EG/EoD (n=16) | |
|---|---|---|---|
| Mean age, years (range) | 43 (18–78) | 42 (18–74) | 47 (19–78) |
| Female sex, n (%) | 54 (61%) | 43 (60%) | 11 (69%) |
| White, n (%) | 80 (91%) | 66 (92%) | 14 (88%) |
| Weight, mean kg (range) | 81 (47–171) | 78 (47–171) | 74 (49–102) |
| Total symptom score at baseline, mean ±SD | 30 ±14 | 31±14 | 26±14 |
| History of asthma, allergic rhinitis, atopic dermatitis, and/or food allergy | 52 (59%) | 48 (67%) | 4 (25%) |
| Absolute eosinophil count | |||
| Mean ±SD | 654±951 | 791±1026 | 107±66 |
| Subjects with ≥250/µl, n (%) | 45 (63%) | 43 (75%) | 0 (0%) |
| Subjects with ≥500/µl, n (%) | 26 (36%) | 26 (46%) | 0 (0%) |
| Prior history, n (%) | |||
| Eosinophilic gastritis and/or eosinophilic duodenitis (EG/EoD) | 62 (70%) | 57 (79%) | 5 (31%) |
| Functional gastrointestinal disorder (IBS, functional abdominal pain, functional diarrhea, or functional constipation) | 26 (30%) | 24 (33%) | 2 (13%) |
| Gastroesophageal reflux, acid reflux, or heartburn | 26 (30%) | 24 (33%) | 2 (13%) |
| Peptic ulcer | 9 (10%) | 9 (13%) | 0 (0%) |
| Chronic gastritis/duodenitis | 6 (7%) | 4 (6%) | 2 (13%) |
| Physician-guided treatment, n (%) | |||
| Proton-pump inhibitor | 40 (45%) | 35 (49%) | 5 (31%) |
| Diet modification | 12 (14%) | 11 (15%) | 1 (6%) |
| Low-dose systemic corticosteroida | 9 (14%) | 7 (10%) | 2 (13%) |
| Swallowed topical corticosteroid | 8 (9%) | 7 (10%) | 1 (6%) |
Prednisone ≤10mg daily or equivalent as a pre-existing regimen and taken throughout the study.
Between groups with vs without a history of EG/EoD, there was a similar distribution of disease type (EG only, EG+EoD, EoD only), overall symptom severity (mean total symptom scores of 31 ±14 and 32 ±13, respectively), and individual symptom scores (Table S2). Other characteristics, such as rate of allergic comorbidities, did not differ significantly among groups; peripheral blood absolute eosinophil counts were significantly lower among subjects without a history of EG/EoD.
Number of biopsies required to maximize diagnostic yield
A minimum of 8 biopsies from the stomach and 4 from the duodenum were required to identify all 72 subjects with EG/EoD. Analysis of the results from the systematic biopsy and histopathologic evaluation protocol (Figure 1) found that each additional gastric and duodenal biopsy collected during EGD incrementally increased the percent of EG/EoD cases captured (Figure 2). Furthermore, the capture rate depended on the required number of positive hpfs (hpfs with ≥30 eos) used to define a case. For example, if a threshold of ≥5 positive hpfs is used to define a case of EG, a single gastric biopsy captured 4% (2/45) and 8 biopsies captured 84% (38/45) of cases. Among subjects with EG, 71% (32/45), 82% (37/45), and 56% (25/45) met this histologic criteria in gastric corpus, antrum, or both, respectively. A similar diagnostic yield was observed for biopsies taken from the corpus vs the antrum; 4 biopsies from the gastric corpus plus 4 from the antrum (8 total biopsies) were required to detect all cases of EG (Figure S3). When we used a threshold of ≥3 positive hpfs to define a case of EoD, a single duodenal biopsy captured 23% (14/62) and 4 biopsies captured 92% (57/62) of cases. Five EoD subjects met the diagnostic threshold based on positive hpfs from multiple duodenal biopsies (4 EoD subjects) and/or from duodenal biopsies collected from areas of special interest during EGD (1 EoD subject). A minimum of 4 biopsies from the duodenum and using a threshold of ≥1 positive hpf was required to detect all cases of EoD.
Figure 2. Number of Biopsies Required to Optimize Detection of EG/EoD.
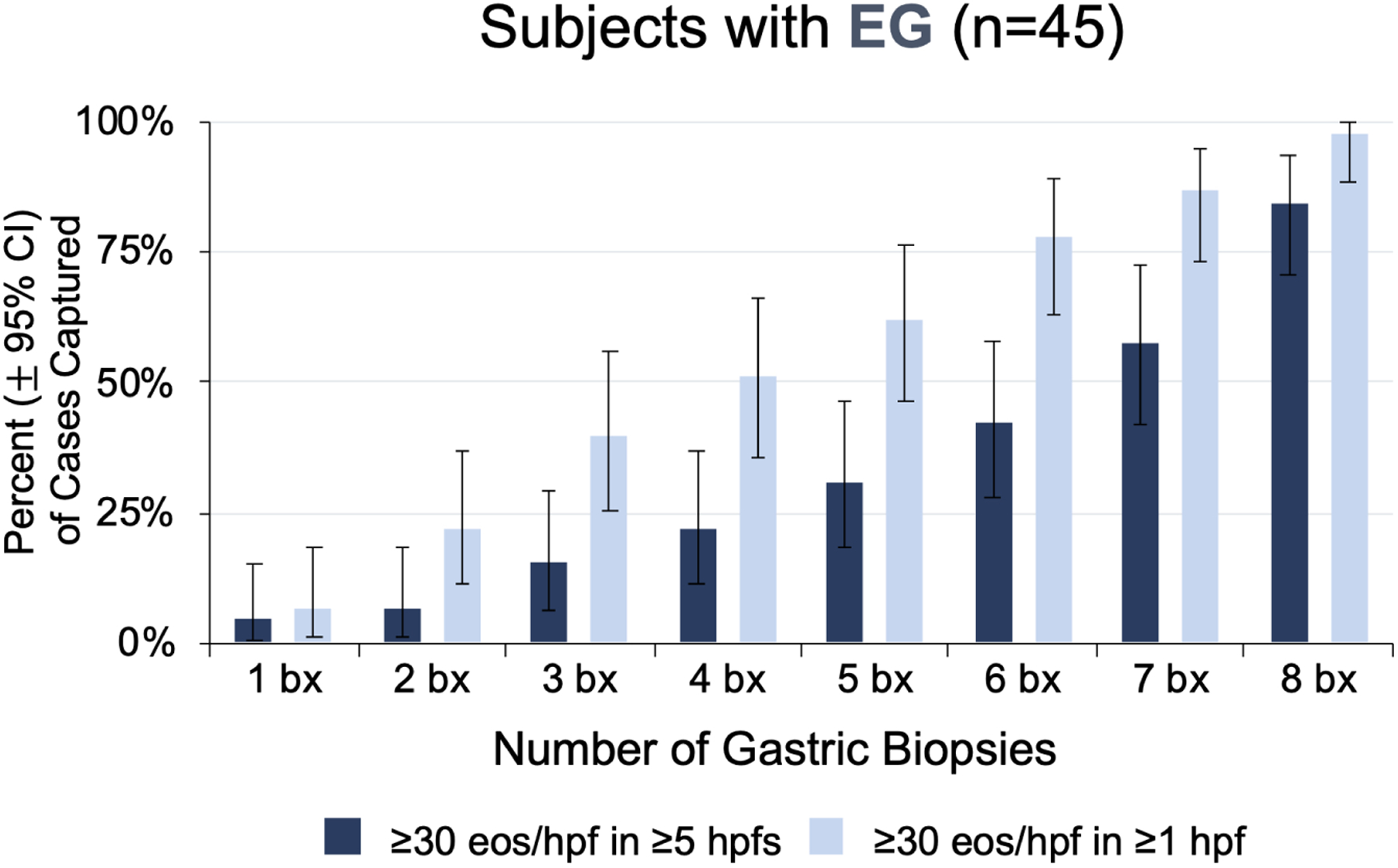
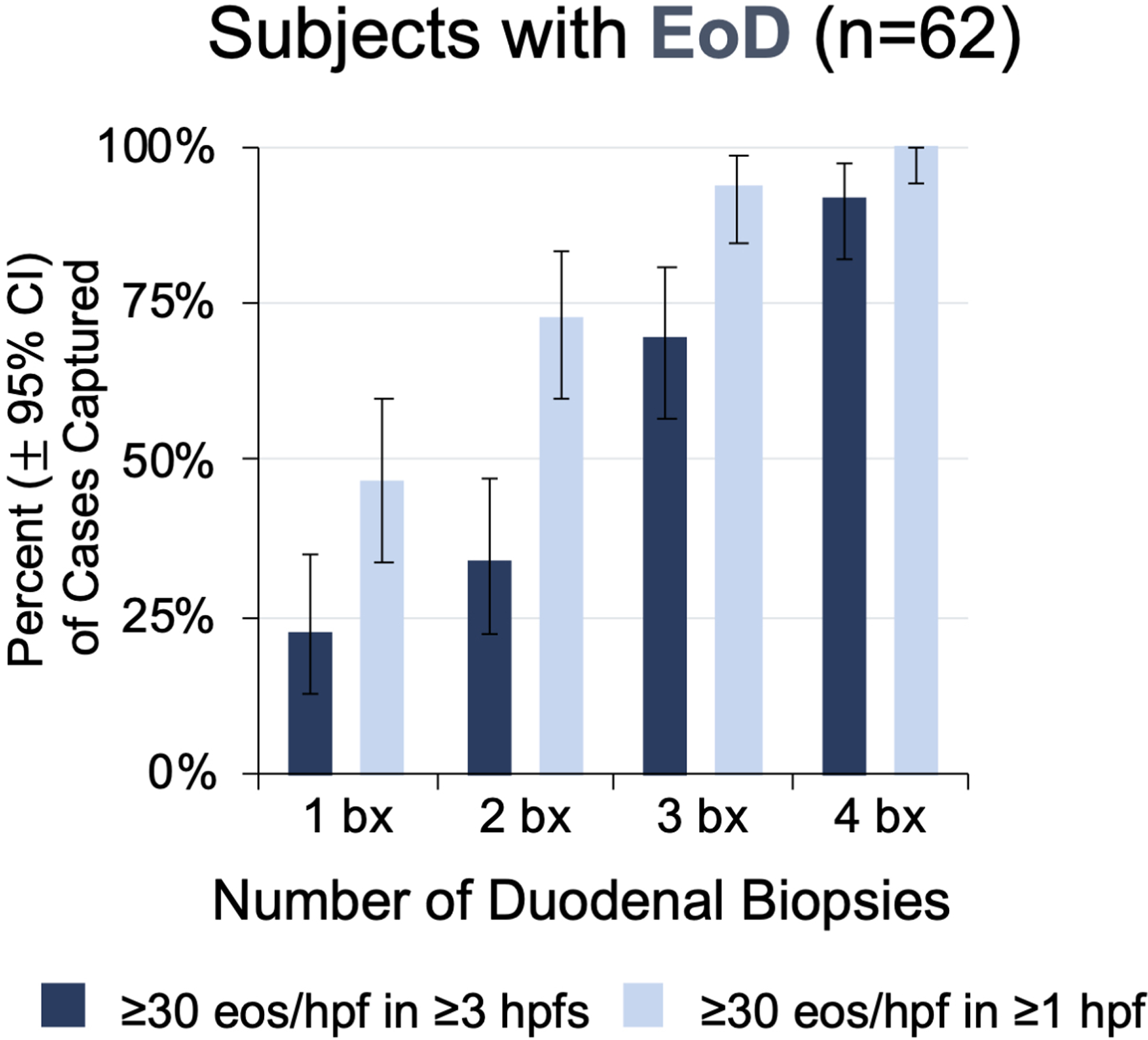
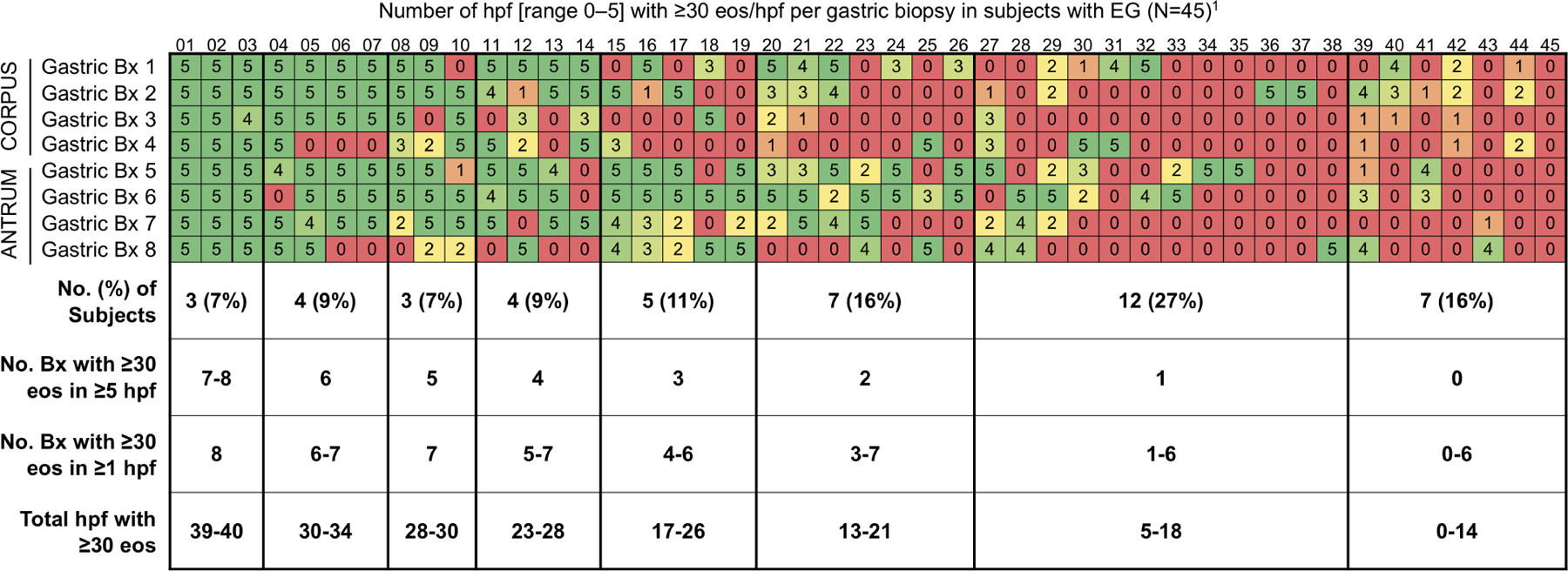
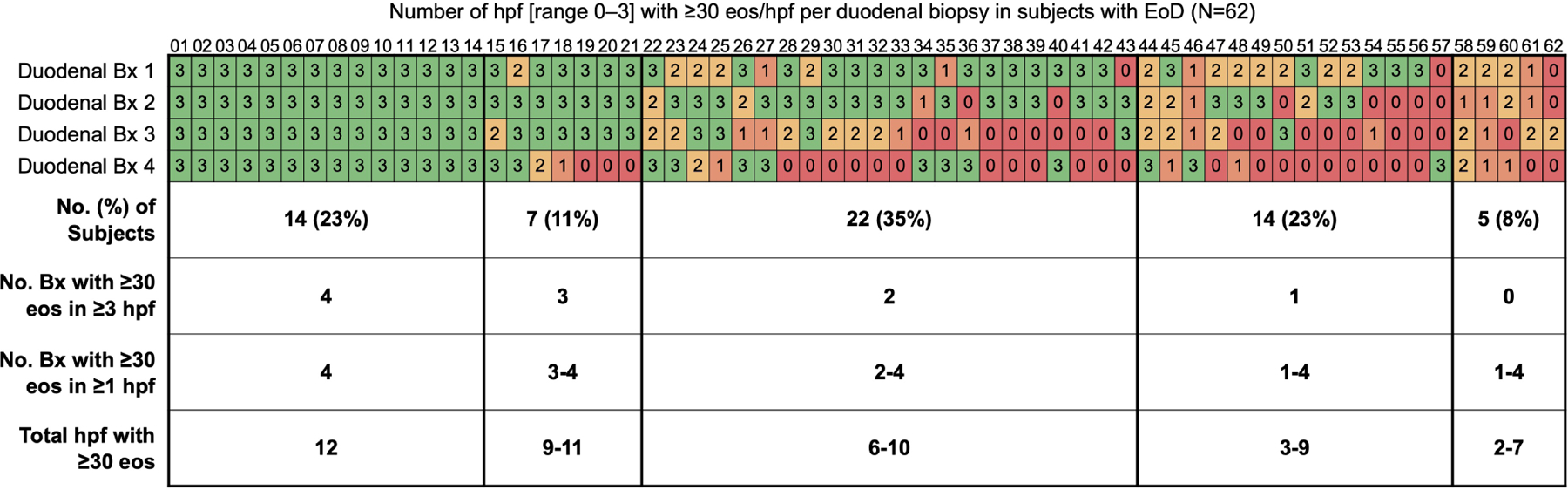
(A, B) Cumulative percent of EG cases (A) and EoD cases(B) that would be captured by minimum number of biopsies out of a total of 8 gastric and 4 duodenal biopsies collected per subject. (C, D) Heatmaps showing the number of positive hpfs per biopsy collected during screening EGD for each EG/EoD subject. Vertical columns contain data for each subject with EG or EoD, horizontal rows contain data for each biopsy, and each cell contains the number of positive hpfs (those with ≥30 eos); cells are color-coded on a scale from green to red, with red indicating fewer positive hpfs. Diagnostic thresholds could be met with any combination of 5 hpfs in the stomach and/or any combination of 3 hpfs in the duodenum. (C) Heatmap of 45 subjects with EG, showing number of positive hpfs in each of 8 gastric biopsies (4 from corpus and 4 from antrum). (D) Heatmap of 62 subjects with EoD, showing number of positive hpfs in each of 4 duodenal biopsies. One patient in each analysis (C, D) did not meet the histologic threshold with the standard 8 gastric and 4 duodenal biopsies but did meet the threshold with collection of additional gastric and duodenal biopsies from areas of interest during screening EGD, as permitted per protocol.
Variation in location and appearance of eosinophilic infiltrate
Tabular heatmaps were created to visualize the patchiness of eosinophilic infiltrate in gastric and duodenal biopsies on a per-patient and per-biopsy basis (Figure 2). For example, Figure 2C (a heatmap of 45 subjects with EG), shows 5 positive hpfs in each of 8 gastric biopsies (4 from corpus and 4 from antrum) from subject 01. Subject 17 had only 3 biopsies with 5 positive hpfs each; 3 other biopsies had 0 positive hpfs and the other 2 biopsies had 2 positive hpfs. Subject 44 had no biopsies with 5 positive hpfs—this patient met the histologic criteria for EG based on total positive hpfs among all biopsies. An average of only 2.6 of 8 total gastric biopsies had ≥30 eos/hpf in ≥5 hpfs; 42% of subjects (19/45) had 0 or 1 gastric biopsies that met this criterion. A similar distribution was observed in biopsies from the 62 subjects with EoD (with or without EG): an average of only 2.2 of 4 total duodenal biopsies had ≥30 eos/hpf in ≥3 hpfs; 31% of subjects (19/62) had 0 or 1 duodenal biopsies that met this criterion (Figure 2D).
In addition to the high variability in eosinophil count among biopsies, there was substantial variability among distinct hpfs within a single biopsy (Figure 3A). Of all biopsies with ≥30 eos in ≥1 hpf, 20% of gastric biopsies (39/200) and 30% of duodenal biopsies (59/194) had only 1 or 2 positive hpfs of the ≥5 non-overlapping hpfs that were evaluated per biopsy (Figure 2C, D).
Figure 3. Histologic Features.
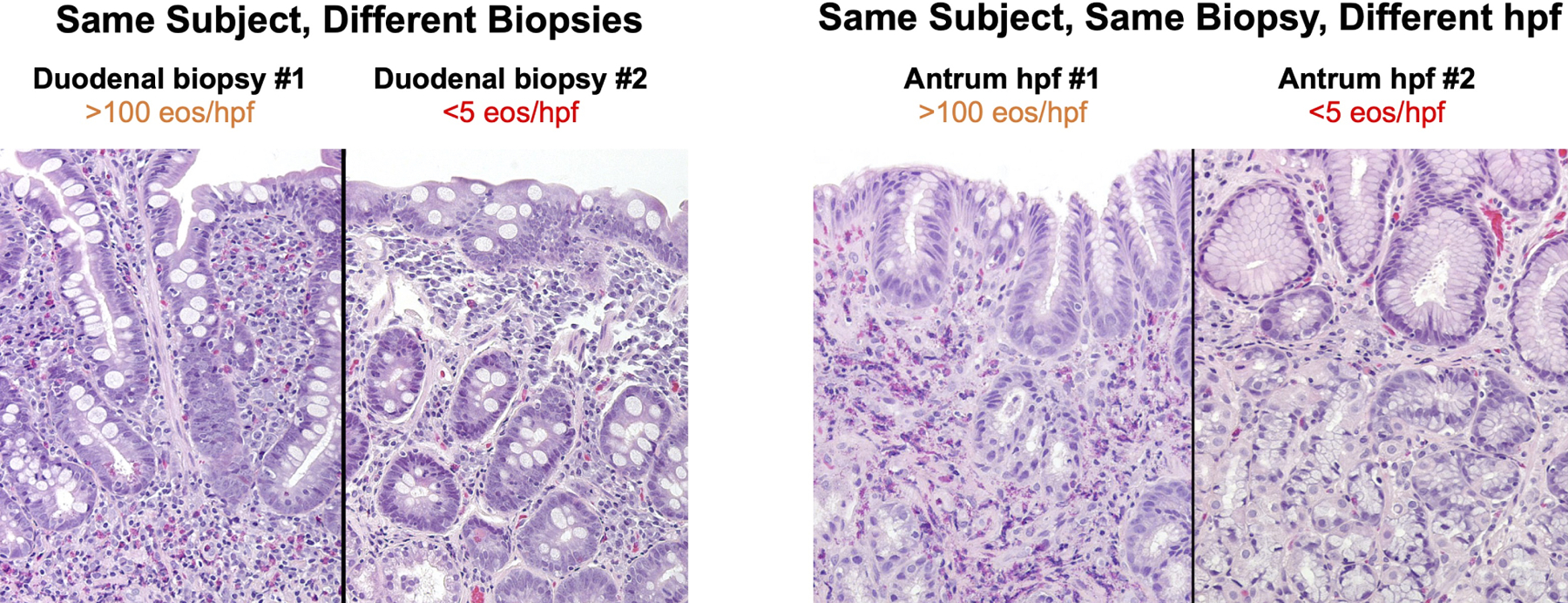
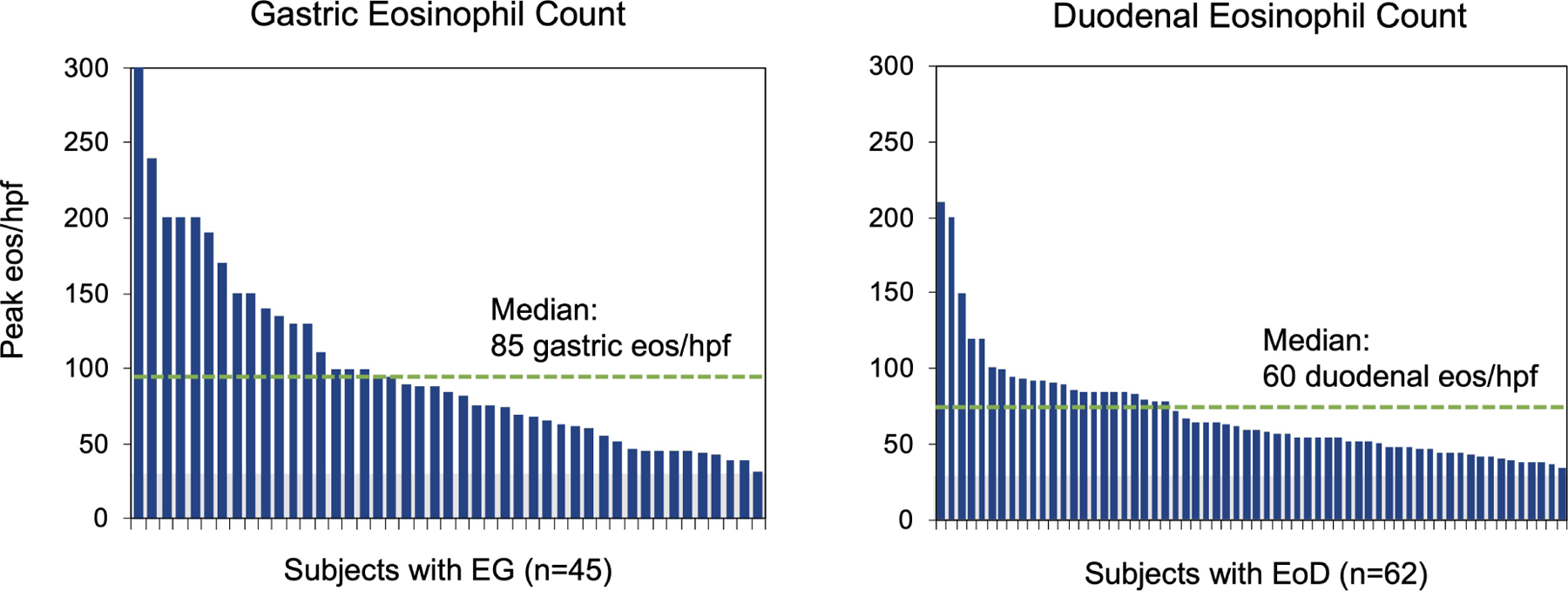
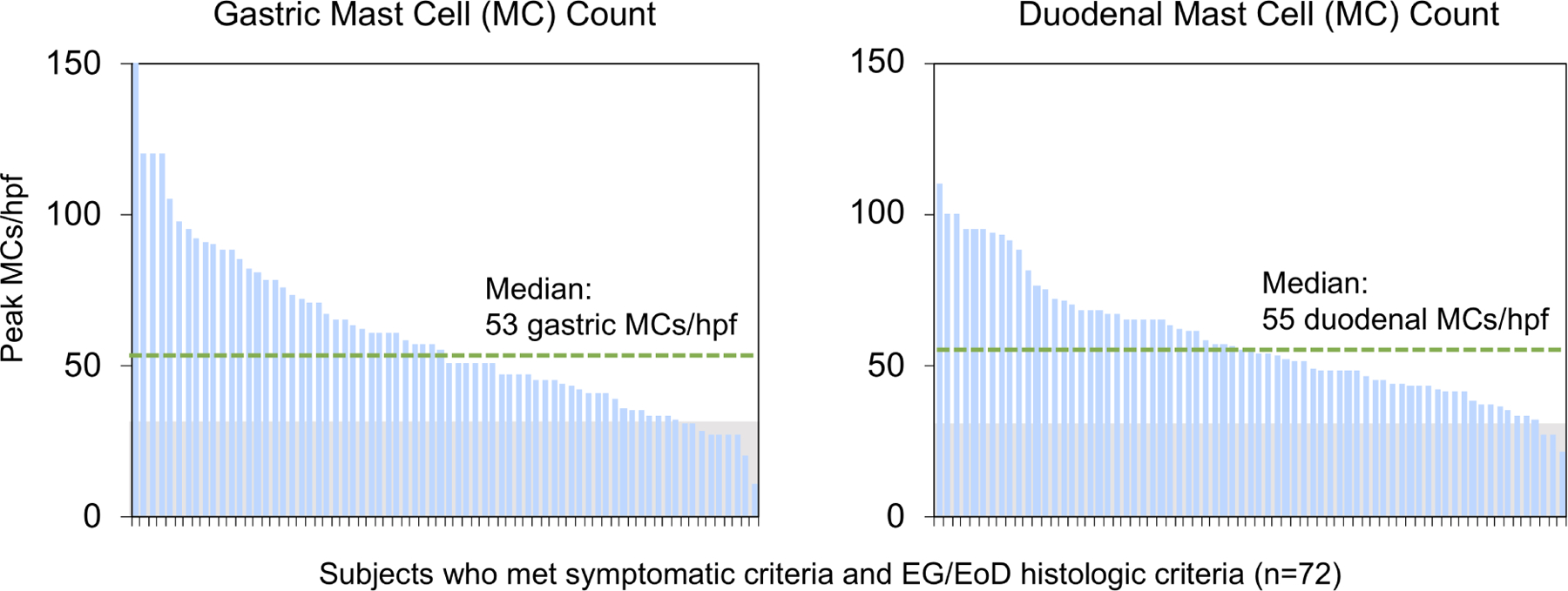
(A) High-power (20X) images of EG/EoD specimens showing patchiness of eosinophils in different biopsies from the same subject (left) and among different hpfs from the same biopsy (right). (B) Peak number of gastric (left) and duodenal (right) eos/hpf in subjects with EG and EoD. Green dotted line indicates median peak eos/hpf. (C) Peak gastric (left) and duodenal (right) mast cell (MC) counts in subjects who met histologic criteria for EG/EoD. The histologic criteria for elevated MCs is ≥30 MCs/hpf (above gray area)(29, 31–34); green dotted line indicates median peak MCs/hpf in patients with EG/EoD.
Subjects with no history of EG/EoD had fewer positive gastric biopsies than subjects with a history (P<.001), but there was no association with number of positive duodenal biopsies (Table S3). Subjects with EG+EoD had a similar number of positive biopsies compared to subjects with only EG or EoD (Table S4). The number of positive biopsies was not associated with use of systemic and/or swallowed steroids (Table S5). Symptom severity (total symptom score) did not differ significantly among EG/EoD subjects with few (0 or 1), compared to those with multiple (≥2), positive biopsies (Figure S4).
Among subjects with EG and EoD, the median peak eosinophil counts were 85 and 60 eos/hpf in gastric and duodenal biopsies, respectively (Figure 3). In most cases, eosinophils did not form sheets that were readily visible under low-power magnification; they could be found in isolation or in clusters, aggregates, or microabscesses. Eosinophils were mostly located in the lamina propria—in the spaces between glands or foveola—and degranulation ranged from minimal to diffuse. Histologic abnormalities in gastric morphology were present in 22/45 subjects with EG (49%), with reactive gastropathy in 12/45 (27%) and chronic inflammation in 9/45 (20%) (Figure S5). Histologic abnormalities in duodenal morphology, such as lymphocytosis or villus changes, were found in only 3/62 subjects with EoD (5%). Median peak gastric and duodenal mast cell counts were 53 and 55/hpf, respectively, in subjects with EG and EoD (Figure 3C). In subjects with EG/EoD, gastric and duodenal mast cell counts correlated with gastric and duodenal eosinophil counts (Figure S6).
Correlations with endoscopic and histologic findings
Based on EG-REFS (Table S1), gastric abnormalities (granularity, raised lesion/nodularity, erythema, friability, folds, erosion/ulceration, pyloric stenosis) were either not present (score=0) or mild (score=1) in most subjects with EG (Figure 4). Most abnormalities were in the antrum: mild erythema in 24/44 subjects (55%) and mild granularity in 15/44 (34%). Among EG subjects, 40/44 (91%) had a total score ≤15. Total EG-REFS did not correlate with total symptom score, peak gastric eos/hpf, or peak gastric mast cells/hpf in subjects with EG (Figure S7). There were no significant differences in rates of each abnormality in subjects with EG vs without EG (Figure 4C). Global endoscopic scores were a median 4.5 (IQR, 2.0–6.0) for subjects with EG and 3.0 (IQR, 1.8–5.3) for subjects with moderate-to-severe GI symptoms but without EG (possible score of 10) (Figure S8). Total, corpus-specific, and antrum-specific EG-REFS did not differ significantly subjects who met histologic criteria for EG vs those who did not (Figure 4D).
Figure 4. Endoscopic Appearance.
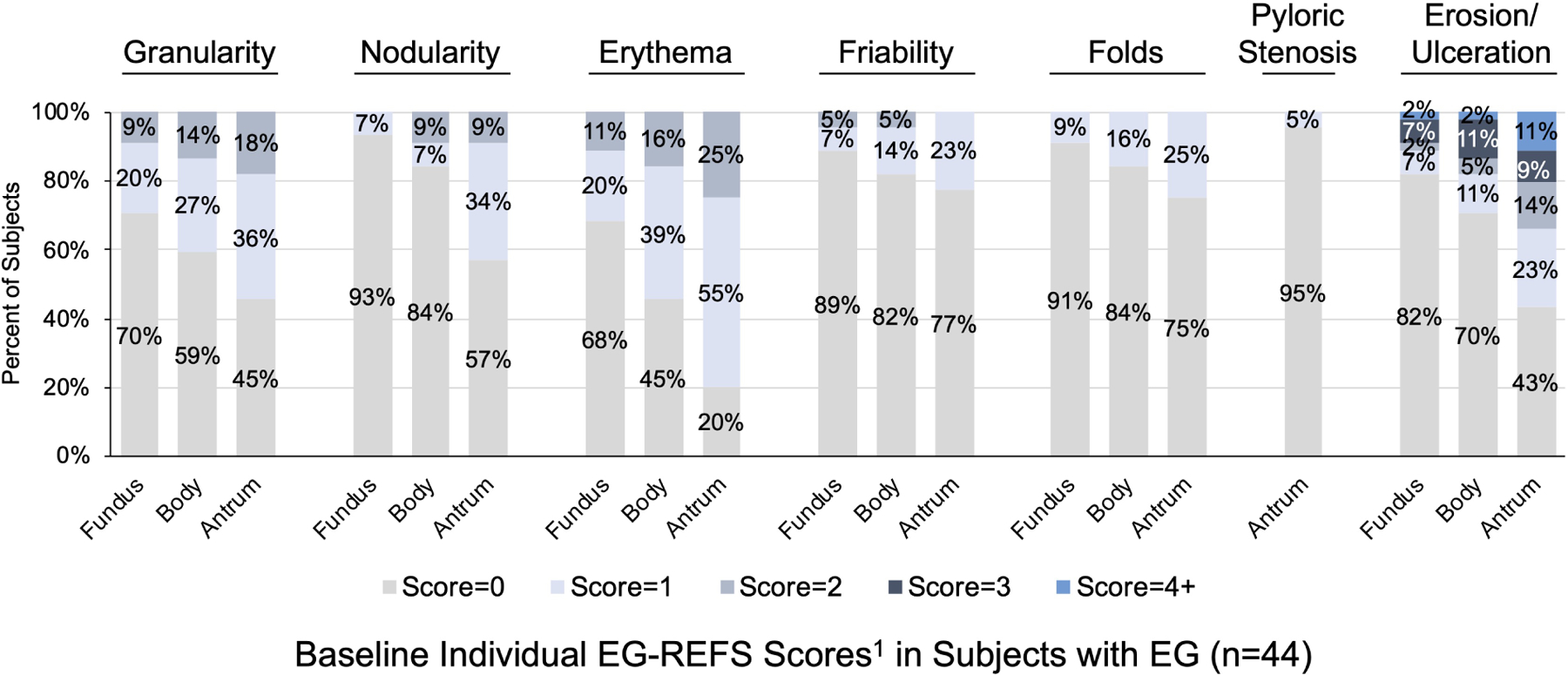
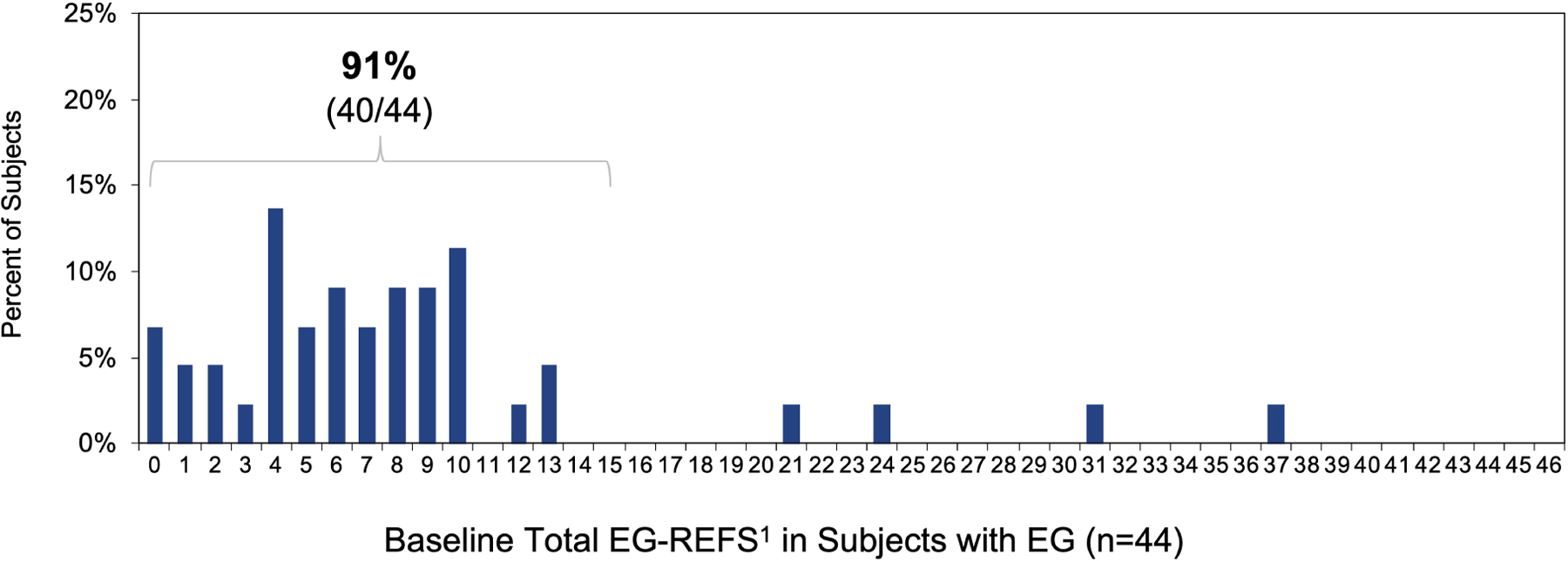
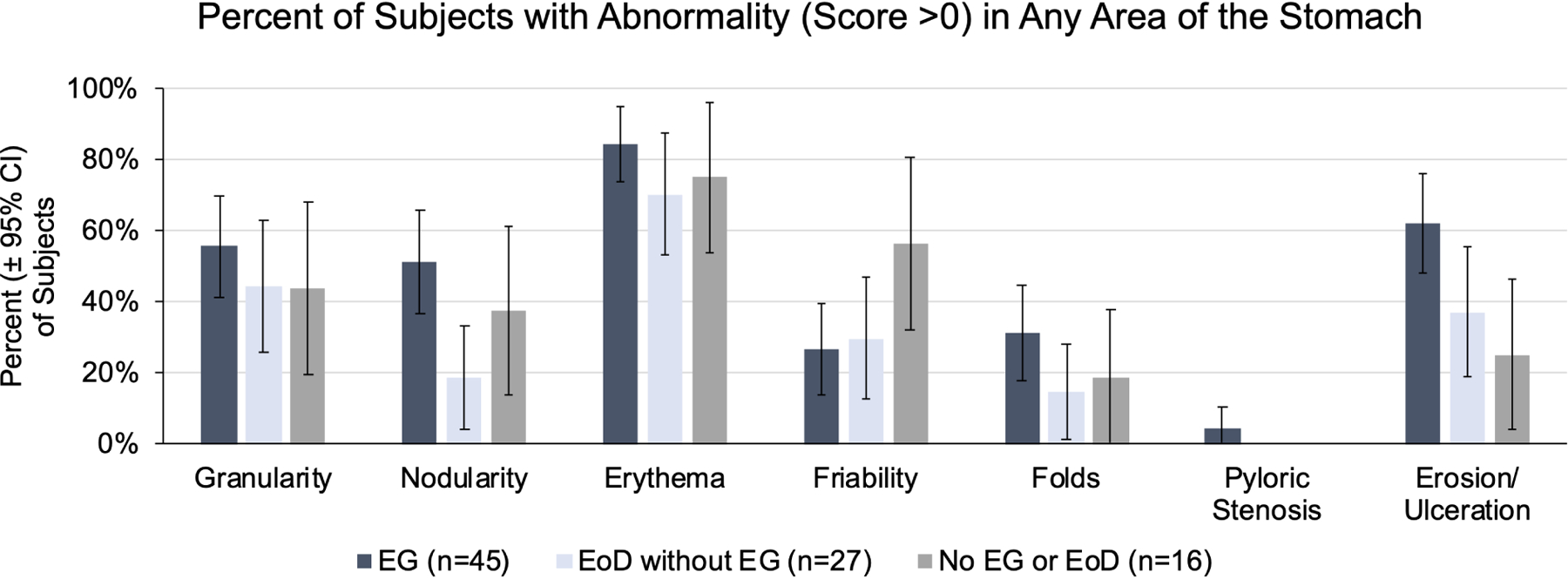
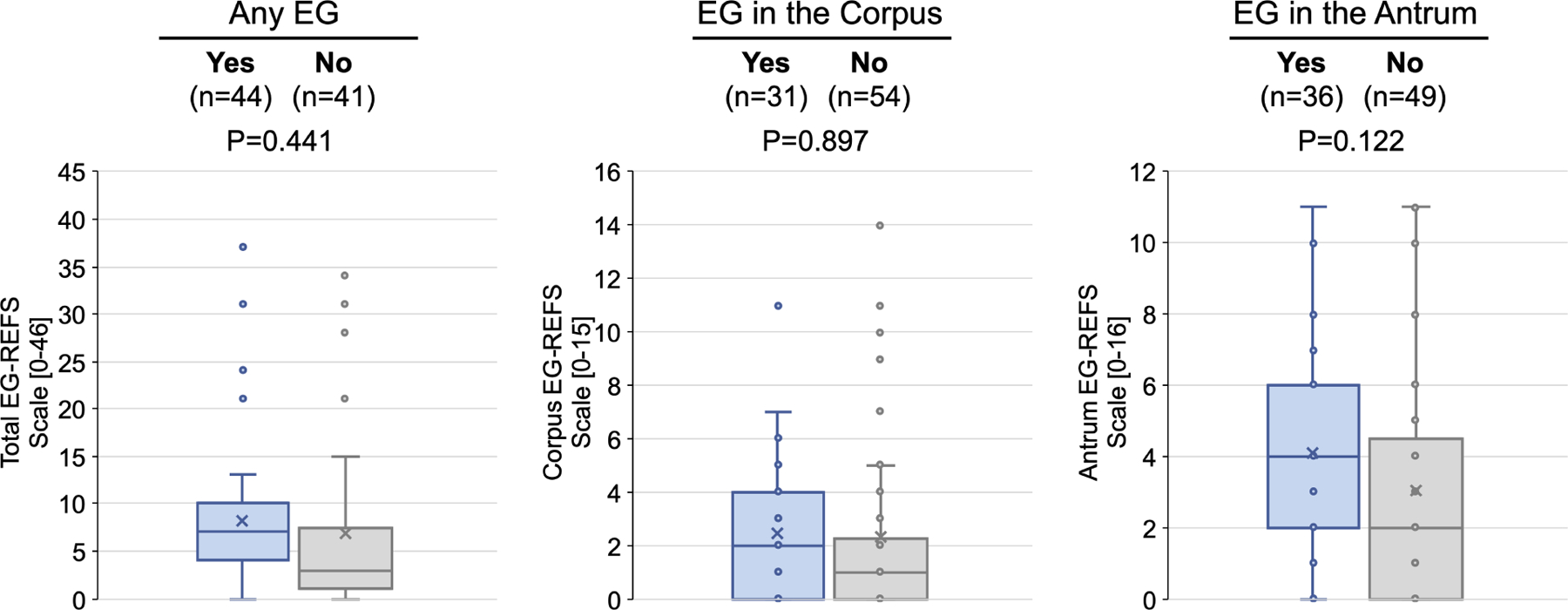
(A) Percent of EG subjects with each EG-REFS score (0= normal and 1=mild). (B) Percent of subjects with each EG-REFS. (C) Percent of subjects with endoscopic abnormalities (EG-REFS >0) in any area of stomach. (D) Box and whisker plot (Tukey method) of subjects with (blue) and without (gray) histologic EG. No EG indicates subjects who did not meet histologic criteria for EG or EoD (n=15) or who met histologic criteria for only EoD (n=26). One subject from the EG group and 2 from the No EG group were not assessed by EG-REFS at screening and are not included in analysis. P value calculated by 2-sample t test.
Symptom burden
The mean total symptom score of subjects with EG/EoD was 31±14 (Table 1) (33±14 for subjects with EG and 30±13 for subjects with EoD). Ninety percent of subjects had 7 or 8 of the 8 symptoms assessed (Figure S9; early satiety, bloating, abdominal pain, abdominal cramping, loss of appetite, nausea, diarrhea, vomiting)—most occurred on 5 or 6 days out of 7 days, except for diarrhea and vomiting. Across all analyses, symptoms were similar in subjects with EG vs EoD, and between EG subjects with involvement of the gastric corpus vs antrum (Figures S9, S10). There was no correlation between total symptom scores and tissue eosinophil or mast cell counts (Figure S11). Subjects receiving systemic steroids and/or swallowed topical steroid enteric-release capsules at screening (18%, 13/72) did not have a significant differences in peak eosinophil count or total symptom score compared with subjects not receiving steroids (Figure S12). Symptoms were similar between patients who did vs did not meet the histologic criteria for EG/EoD (Figure S13).
Discussion
This study was the first prospective analysis of diagnostic yield and biopsy and histopathology criteria for diagnosis of EG/EoD. We demonstrated that a minimum of 12 biopsies—4 from the gastric corpus, 4 from the gastric antrum, and 4 from the duodenum—were required to detect all cases of EG/EoD. This standardized biopsy protocol, followed by histopathologic analysis including quantification of eosinophils, identified a high rate of EG/EoD among individuals with moderate-to-severe GI symptoms, indicating that EG/EoD is underdiagnosed. Because we observed patchiness of gastric and duodenal eosinophilia, insufficient biopsy sampling in clinical practice might produce false-negative results and missed diagnoses. Implementing a more extensive biopsy protocol and achieving an accurate diagnosis of EG/EoD will enable the initiation of targeted therapy.
The threshold of ≥30 eos/hpf for EG/EoD diagnosis is supported by a recent study indicating that mean eos/hpf in gastric and duodenal biopsies from subjects without known GI disease were 3.8 and 14.6, respectively(29). Although the diagnostic threshold was ≥30 eos/hpf in ≥5 gastric hpfs and/or in ≥3 duodenal hpfs, a fewer number of hpfs might be acceptable for detection of EG/EoD (29). In a sub-analysis, we found that adjusting the requirement to ≥30 eos/hpf in a single gastric and/or duodenal hpf maintained predictive values of 87% for EG and 97% for EoD (Table S6). Simplifying the requirement to a single hpf aligns with the diagnostic criterion for EoE (≥15 eos/hpf in 1 hpf) and may reduce pathologist burden. If ≥30 eos/hpf in 1 hpf were to be used as the diagnostic threshold, it would still be important to examine and systematically count eosinophils in ≥5 non-overlapping hpfs in at least 12 biopsies, to avoid missing areas of eosinophilic infiltrate. Our findings suggest that such areas might be easily missed in practice, because more than half of EG/EoD subjects had no histologic morphology abnormalities other than increased eosinophils. Furthermore, tissue eosinophilia was highly patchy, and even where eosinophils were increased, the sheets of dense eosinophil infiltrates reported in the literature were rarely present (30). Activation of mast cells is also likely to be involved in EG/EoD, but current diagnostic criteria include only quantification of eosinophils (4, 9, 13).
This study also provides the first prospective characterization of endoscopic and clinical features of adults with EG/EoD. Notably, our findings of the nonspecific endoscopic and clinical features of EG/EoD support the concept that many patients remain undiagnosed in current practice. Most subjects in this study had a normal or only mildly abnormal endoscopic appearance; use of medications or diet therapy might have reduced the appearance of endoscopic abnormalities in some subjects, but endoscopic findings did not correlate with tissue eosinophilia. Therefore, endoscopic appearance alone cannot reliably increase clinical suspicion for, or rule out, a diagnosis of EG/EoD. Analysis of data collected daily with a validated PRO questionnaire revealed the high symptom burden of patients with EG/EoD. Many of these symptoms occur in patients with other GI disorders, such as IBS or functional dyspepsia, so patients (particularly if they have normal endoscopic findings) receive misdiagnoses of functional GI disorders. Moreover, peripheral blood absolute eosinophil counts were rarely elevated in subjects with no history of EG/EoD in this study, and should not rule out a diagnosis of EG/EoD. The nonspecific endoscopic and clinical presentation of EG/EoD may initially obscure an accurate diagnosis, but thorough evaluation of GI symptoms followed by EGD with systematic gastric and duodenal biopsy sampling, regardless of endoscopic appearance or absolute eosinophil count, is likely to improve detection.
The main limitation of this study was the modest sample size—studies are needed in larger populations. Studies are needed to characterize the prevalence of EG/EoD in symptomatic subjects using thorough and systematic biopsy and histopathology protocols—a large, prospective, non-interventional study is underway. In the meantime, collection of a minimum of 12 biopsies (4 from the gastric corpus, 4 from the gastric antrum, and 4 from the duodenum) from patients with moderate-to-severe GI symptoms, and systematic counting of tissue eosinophils, will likely identify more patients with EG/EoD. Implementation of these protocols will help to provide EG/EoD patients with definitive diagnoses and enable initiation of targeted treatment to improve their quality of life.
Supplementary Material
What You Need to Know:
BACKGROUND:
Eosinophilic gastritis (EG) and eosinophilic duodenitis (EoD) are underdiagnosed. We analyzed biopsies from subjects with gastrointestinal symptoms in a randomized trial to determine rates of EG and EoD and the number of biopsies required to optimize detection.
FINDINGS:
More than half of subjects (58%) with moderate–severe GI symptoms were found to have EG/EoD. Counting of eosinophils in at least 8 gastric and 4 duodenal biopsies was required to identify patients with EG/EoD.
IMPLICATIONS FOR PATIENT CARE:
EG and EoD are underdiagnosed—it is important to count eosinophils in multiple gastric and duodenal biopsies from patients with moderate-to-severe gastrointestinal symptoms to identify those with EG/EoD, so they can receive appropriate treatment.
Acknowledgments
The authors thank Lori Brashears, R.N., for her role in the management of biopsy sample acquisition and histopathological data collection (funded by Allakos); Cory Mekelburg, B.S., an employee of Allakos Inc, for data analysis assistance; and Jocelyn Hybiske, Ph.D., for writing and editing assistance (funded by Allakos).
Grant/Funding Support: This study was sponsored and funded by Allakos, Inc. The Eosinophilic Gastritis Endoscopic Reference System (EG-REFS) was developed in part by the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR), which is funded by NIH U54 AI117804.
Abbreviations:
- EG
eosinophilic gastritis
- EoD
eosinophilic duodenitis
- EGD
esophagogastroduodenoscopy
- eos
eosinophils
- eos/hpf
eosinophils per high-powered field
- GI
gastrointestinal
- hpf
high-powered field
- MC
mast cells
- EG-REFS
Eosinophilic Gastritis Endoscopic Reference System
- PRO
patient-reported outcome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Evan S Dellon received research support from Adare, Allakos Inc, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire/Takeda; consulting fees from Abbott, Adare, Aimmune, Allakos, Amgen, Arena, AstraZeneca, Biorasi, Calypso, Celgene/Receptos, Celldex, Eli Lilly, EsoCap, GSK, Gossamer Bio, Parexel, Regeneron, Robarts, Salix, Sanofi, Shire/Takeda; and education grants from Allakos Inc, Banner, and Holoclara. Mirna Chehade received research support from the National Institutes of Health (NIH, R01-AI140133, U54-AI117804), APFED/AAAAI, Shire, Regeneron, Allakos Inc, AstraZeneca, and Danone; consulting fees from Shire, Regeneron, Allakos Inc, Adare, Astra Zeneca and Nutricia; and lecture honoraria from Nutricia, Medscape, and Vindico. Kathryn A Peterson received research support from the NIH, Takeda, Regeneron, Astra Zeneca, GlaxoSmith Kline, Adare, Chobani, and Allakos Inc; consulting fees from Takeda, Regeneron, Allakos, Astra Zeneca, and Eli Lilly; lecture honoraria from Regeneron; and is shareholder in Nexeos Dx. Marc E Rothenberg is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celgene, Astra Zeneca, Arena Pharmaceuticals, GlaxoSmith Kline, Guidepoint, and Suvretta Capital Management (has an equity interest in the first 5 listed); receives royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate; and is an inventor of patents owned by Cincinnati Children’s Hospital. Ikuo Hirano has received research support from Adare, Allakos, AstraZeneca, Meritage, Celgene/Receptos, Regeneron, and Shire/Takeda; as well as consulting fees from Adare, Allakos Inc, Arena, AstraZeneca, Celgene/Receptos, EsoCap, Gossamer Bio, Parexel, Regeneron, Sanofi, and Shire/Takeda. Robert M Genta (as Gastropath LCC) has received a fees for histopathology services from Allakos Inc and consulting fees from Allakos Inc, Ellodi Pharmaceuticals, and RedHill Pharmaceuticals. Nirmala Gonsalves is an advisory board member and received consulting fees from Allakos Inc, royalties from UpToDate, NIH funding, and speaker reimbursements from Medscape. Gary W Falk is a consultant for Allakos Inc, Sanofi, Shire/Takeda, Lucid, and Adare; he received grant support from Allakos Inc, Regerneron, Adare, Shire/Takeda, Lucid, and Sanofi. Joseph A. Murray has received grant support from the NIH, Immunogenix, Teva, Kanyos, Theravance, Johnson and Johnson, Takeda, Allakos Inc, and DBV Technologies (paid to The Mayo Clinic); has support from the Oberkotter Foundation and Board Medical Research Program at CCFA; has served on the advisory board of Amgen, Celimmune, GlaxoSmithKline, Chugai, and Glenmark Pharmaceuticals Ltd; has served as consultant to Senda Bioscences, Inova diagnostics, Ukko Inc, and Intrexon; has a patent with Evelo; and owns equity options in Torax. Lauren T Gehman and Alan T Chang are full-time employees of Allakos Inc and own stocks and stock options for Allakos Inc. Bhupinder Singh and Henrik S Rasmussen are full-time employees of Allakos, Inc, own stocks and stock options for Allakos Inc, and are named on patents from Allakos Inc.
Writing Assistance: Writing and editorial support was provided by Jocelyn Hybiske, PhD, an independent medical writer funded by Allakos, Inc.
Data Transparency Statement: The trial protocol and study materials are available at DOI: 10.1056/NEJMoa2012047. Individual participant data will not be shared.
References
- 1.Gonsalves N. Eosinophilic Gastrointestinal Disorders. Clin Rev Allergy Immunol 2019;57(2):272–85. [DOI] [PubMed] [Google Scholar]
- 2.Egan M, Furuta GT. Eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis. Ann Allergy Asthma Immunol 2018;121(2):162–7. [DOI] [PubMed] [Google Scholar]
- 3.Pesek RD, Rothenberg ME. Eosinophilic gastrointestinal disease below the belt. J Allergy Clin Immunol 2020;145(1):87–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youngblood BA, Brock EC, Leung J, Falahati R, Bochner BS, Rasmussen HS, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 2019;4(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu Blatman KS, Gonsalves N, Hirano I, Bryce PJ. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J Allergy Clin Immunol 2011;127(5):1307–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol 2010;126(1):140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton SM, Kagalwalla AF, Arva NC, Wang MY, Amsden K, Melin-Aldana H, et al. Mast Cell Infiltration Is Associated With Persistent Symptoms and Endoscopic Abnormalities Despite Resolution of Eosinophilia in Pediatric Eosinophilic Esophagitis. Am J Gastroenterol 2020;115(2):224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2013;304(12):G1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell JM, Collins MH, Stucke EM, Putnam PE, Franciosi JP, Kushner JP, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol 2014;134(5):1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunkara T, Rawla P, Yarlagadda KS, Gaduputi V. Eosinophilic gastroenteritis: diagnosis and clinical perspectives. Clin Exp Gastroenterol 2019;12:239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert-Bayo M, Paracuellos I, Gonzalez-Castro AM, Rodriguez-Urrutia A, Rodriguez-Lagunas MJ, Alonso-Cotoner C, et al. Intestinal Mucosal Mast Cells: Key Modulators of Barrier Function and Homeostasis. Cells 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsay DB, Stephen S, Borum M, Voltaggio L, Doman DB. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y) 2010;6(12):772–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Shoda T, Wen T, Caldwell JM, Collins MH, Besse JA, Osswald GA, et al. Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: Multi-site study. J Allergy Clin Immunol 2020;145(1):255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedell A, Taft T, Craven MR, Guadagnoli L, Hirano I, Gonsalves N. Impact on Health-Related Quality of Life in Adults with Eosinophilic Gastritis and Gastroenteritis: A Qualitative Assessment. Dig Dis Sci 2018;63(5):1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandinetti T, Biedermann L, Bussmann C, Straumann A, Hruz P. Eosinophilic Gastroenteritis: Clinical Manifestation, Natural Course, and Evaluation of Treatment with Corticosteroids and Vedolizumab. Dig Dis Sci 2019;64(8):2231–41. [DOI] [PubMed] [Google Scholar]
- 16.Chehade M, Gehman L, Kamboj A, Atkins D. A longitudinal, population-based study of the difficult journey to diagnosis endured by patients with eosinophilic gastritis and eosinophilic gastroenteritis. J Allergy Clin Immunol 2020;145(2):AB40. [Google Scholar]
- 17.Licari A, Votto M, Scudeller L, De Silvestri A, Rebuffi C, Cianferoni A, et al. Epidemiology of Nonesophageal Eosinophilic Gastrointestinal Diseases in Symptomatic Patients: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract 2020;8(6):1994–2003 e2. [DOI] [PubMed] [Google Scholar]
- 18.Alhmoud T, Hanson JA, Parasher G. Eosinophilic Gastroenteritis: An Underdiagnosed Condition. Dig Dis Sci 2016;61(9):2585–92. [DOI] [PubMed] [Google Scholar]
- 19.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis 2015;47(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006;64(3):313–9. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol 2009;104(3):716–21. [DOI] [PubMed] [Google Scholar]
- 22.Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF 3rd. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J Allergy Clin Immunol 2012;130(3):798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Shaheen NJ, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol 2015;28(3):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018;155(4):1022–33 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med 2020;383(17):1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano I, Dellon E, Murray J, Peterson K, Banderas B, Wolley J, et al. Development of a Patient Reported Outcome (PRO) Questionnaire to Assess the Symptoms of Eosinophilic Gastritis and/or Eosinophilic Gastroenteritis (EG/EGE-SQ©). Am J Gastroenterol 2019;44 (Suppl):S694–5. [Google Scholar]
- 27.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20(10):1161–81. [DOI] [PubMed] [Google Scholar]
- 28.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11(10):1185–94. [DOI] [PubMed] [Google Scholar]
- 29.Reed CC, Genta RM, Youngblood BA, Wechsler JB, Dellon ES. Mast Cell and Eosinophil Counts in Gastric and Duodenal Biopsies From Patients With and Without Eosinophilic Gastroenteritis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins MH, Capocelli K, Yang GY. Eosinophilic Gastrointestinal Disorders Pathology. Front Med (Lausanne) 2017;4:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn HP, Hornick JL. Immunoreactivity for CD25 in gastrointestinal mucosal mast cells is specific for systemic mastocytosis. Am J Surg Pathol 2007;31(11):1669–76. [DOI] [PubMed] [Google Scholar]
- 32.Jakate S, Demeo M, John R, Tobin M, Keshavarzian A. Mastocytic enterocolitis: increased mucosal mast cells in chronic intractable diarrhea. Arch Pathol Lab Med 2006;130(3):362–7. [DOI] [PubMed] [Google Scholar]
- 33.Walker MM, Talley NJ, Prabhakar M, Pennaneac’h CJ, Aro P, Ronkainen J, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther 2009;29(7):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima S, Krishnan B, Ota H, Segura AM, Hattori T, Graham DY, et al. Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology 1997;113(3):746–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


