Abstract
Background
Intra-articular blood causes irreversible joint damage, whilst clinical differentiation between haemorrhagic joint effusion and other effusions can be challenging. An accurate non-invasive method for the detection of joint bleeds is lacking. The aims of this phantom study were to investigate whether magnetic resonance imaging (MRI) T1 and T2 mapping allows for differentiation between simple and haemorrhagic joint effusion and to determine the lowest blood concentration that can be detected.
Methods
Solutions of synovial fluid with blood concentrations ranging from 0 to 100% were scanned at 1.5, 3, and 7 T. T1 maps were generated with an inversion recovery technique and T2 maps from multi spin-echo sequences. In both cases, the scan acquisition times were below 5 min. Regions of interest were manually drawn by two observers in the obtained T1 and T2 maps for each sample. The lowest detectable blood concentration was determined for all field strengths.
Results
At all field strengths, T1 and T2 relaxation times decreased with higher blood concentrations. The lowest detectable blood concentrations using T1 mapping were 10% at 1.5 T, 25% at 3 T, and 50% at 7 T. For T2 mapping, the detection limits were 50%, 5%, and 25%, respectively.
Conclusions
T1 and T2 mapping can detect different blood concentrations in synovial fluid in vitro at clinical field strengths. Especially, T2 measurements at 3 T showed to be highly sensitive. Short acquisition times would make these methods suitable for clinical use and therefore might be promising tools for accurate discrimination between simple and haemorrhagic joint effusion in vivo.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41747-021-00251-z.
Keywords: Haemarthrosis, Image interpretation (computer-assisted), Magnetic resonance imaging, Phantoms (imaging), Synovial fluid
Key points
T1 and T2 mapping can differentiate simple and haemorrhagic effusion in vitro.
T1 and T2 relaxation times decreased as the blood concentration increased.
The lowest blood concentrations detected using T1 mapping was 10% at 1.5 T.
The lowest blood concentrations detected using T2 mapping was 5% at 3 T.
Background
Haemarthrosis may be caused by (repeated) trauma or by bleeding disorders like haemophilia and Von Willebrand disease. The intra-articular blood has harmful effects on different joint components [1, 2]. These harmful effects already occur after relatively short exposure to small amounts of intra-articular blood as shown in vitro [3].
Clinical symptoms of joint bleeding, such as pain, swelling, and reduced functionality, are not specific for joint bleeding as these are observed in different joint conditions like arthritis, osteoarthritis, and other arthropathies as well [4–6]. Identification of joint effusion by imaging studies for example is not specific for joint bleeds, because effusion is also observed in healthy joints [7], arthropathy flare-ups, and arthritis [4, 8–10]. Nevertheless, being able to distinguish between a joint bleed and another cause of joint effusion is clinically relevant, especially in patients with bleeding disorders like haemophilia, since the different diagnoses require different treatments [4, 11, 12].
The current reference standard for the analysis of joint effusion is a joint aspiration. However, it is an invasive method that could introduce the potential risk of infection or a haemorrhagic procedure which could result in false-positive outcomes. Therefore, joint aspiration might not be desirable in all cases, e.g. in patients with an increased bleeding risk [11, 13].
Ultrasound is increasingly used in addition to physical examination since it is a non-invasive method that can accurately assess joint effusion and changes in synovial tissue [11, 14–16], it is widely available, and provides real-time information [11, 14–16]. Moreover, previous studies have shown that ultrasonography is sensitive to differentiate between simple and complex joint effusion [17, 18]. However, limitations of ultrasound are the operator dependency and therefore a substantial inter-observer variation of findings [11, 19].
Magnetic resonance imaging (MRI) is a reliable tool for the assessment of inflammatory and degenerative joint changes, due to its excellent contrast in tissues and fluids [10, 14]. Large haemarthroses may show fluid-fluid levels or thrombi on MRI. However, the accuracy of standard MRI protocols for the detection of early or minor joint bleeding has not been established yet [17, 18]. Therefore, an accurate non-invasive reference standard to detect joint bleeding is lacking.
Whilst standard MRI protocols are unable to accurately detect limited amounts of intra-articular blood, dedicated MRI sequences might be able to detect and quantify small amounts of blood within the synovial fluid. Since synovial fluid and iron-containing blood have different relaxation properties, quantification of longitudinal (T1) and transverse (T2) relaxation times with T1 and T2 mapping should in principle be able to assess the presence of blood in the synovial fluid.
In this phantom study, we investigated the potential of quantitative T1 and T2 relaxometry MRI protocols as non-invasive tools to differentiate between simple and haemorrhagic joint effusion at 1.5, 3, and 7 T. Specifically, we aimed to establish the minimal blood concentration in the synovial fluid which can be detected using these quantification methods.
Methods
Sample preparation
All patients or their legal guardians approved the use of their remnant samples for method development, validation, and research purposes, in agreement with the institutional regulations (Art. 8, University Medical Center Utrecht Biobank Regulations; version June 19, 2013). The synovial fluid used consisted of pooled residual synovial fluid from clinical joint aspirations in four patients with rheumatoid arthritis (n = 1), juvenile arthritis (n = 1), and unknown disease (n = 2). The synovial fluid residuals were visually inspected to confirm clear, non-purulent, non-haemorrhagic aspirates, kept frozen at − 80 °C, and were defrosted 24 h before being pooled to one volume of synovial fluid. The blood used was venous whole blood (haemoglobin 9.8 mmol/L), from a healthy male volunteer, collected into lithium-heparin tubes. The freshly withdrawn blood was mixed with the pooled synovial fluid residuals.
Solutions with blood concentrations of 0, 2.5, 5, 10, 25, 50, 75, and 100% were prepared, and a volume of 3 mL was injected into nuclear magnetic resonance tubes (Wilmad LabGlass, WG-1000-8, Vineland, NJ, USA) (Fig. 1a). To ensure the blood was deoxygenated before starting the scanning sessions, the samples were kept at 37 °C for 24 h. Outside the scanner, the samples were kept in a stove at 37 °C and were manually shaken prior to scanning to maintain homogeneous mixtures.
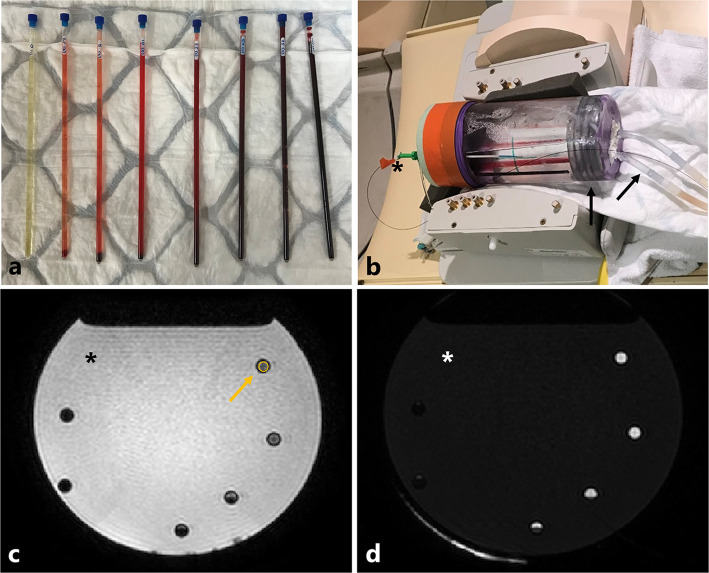
Fig. 1.
a Nuclear magnetic resonance tubes with different ratios of synovial fluid and blood. Blood percentage from left to right: 0%, 2.5%, 5%, 10%, 25%, 50%, 75%, and 100%. b Phantom setup: a cylindrical water-filled holder with six samples, horizontally placed in a 16-channel knee coil using a 3-T MRI system. The phantom was equipped with a fiberoptic thermometer (the asterisk in all images) and a heating system based on heat exchange between water in the heating system (black arrows) and the water or oil volume in the phantom. c Scan image from the T1 mapping sequence. For each tube, a region of interest was manually placed within the tube region (example in yellow). d Scan image from the T2 mapping sequence
Phantom
The samples were horizontally placed in batches of six samples in a cylindrical holder filled with water at 1.5 T and 3 T or mark-oil at 7 T, to reduce magnetic susceptibility effects around the samples. Details can be found in the supplementary materials (Fig. S1). The temperature inside the phantom was monitored using a fiberoptic thermometer (Luxtron Corp., Santa Clara, CA, USA), and was kept stable around 37 °C during the scanning session using a heating system based on heat exchange between water in the heating system and the water or oil volume in the phantom (Fig. 1b).
MRI data acquisition
The experimental data were acquired at 3 field strengths, using 1.5, 3, and 7 T systems (Philips Achieva, Best, The Netherlands). An 8-channel head coil and a 16-channel knee coil (Philips Achieva, Best, The Netherlands) were used at 1.5 and 3 T, respectively. A 32-channel head coil (Nova Medical, Wilmington, MA, USA) was used at 7 T. All acquisitions were performed in a time span of 25 h using the 1.5-T, the 3-T, and the 7-T scanners, in that chronological order.
T1 mapping
Images for the T1 measurements were obtained using a multislice inversion recovery technique with a gradient-echo echo-planar imaging readout (Fig. 1c) [20]. The T1 mapping strategy consists of inverting the longitudinal magnetisation in the imaging volume using an adiabatic inversion pulse and subsequently sampling the recovery curve by changing the order in which the slices are acquired after the inversion. The details of the MRI protocols are reported in Table 1.
Table 1.
MRI protocols for T1 mapping and T2 mapping with turbo spin-echo and sensitivity encoding acceleration, at 1.5, 3, and 7 T
| Field strength | T1 mapping | T2 mapping* | ||||
|---|---|---|---|---|---|---|
| 1.5 T | 3 T | 7 T | 1.5 T | 3 T | 7 T | |
| Number of slices | 11 | 11 | 11 | 5 | 5 | 5 |
| Number of inversions | 11 | 11 | 11 | 32 | 32 | 32 |
| Voxel size (mm) | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Field of view (mm) | 128 × 128 | 128 × 128 | 128 × 128 | 128 × 128 | 128 × 128 | 128 × 128 |
| Slice thickness (mm) | 5 | 5 | 5 | 5 | 5 | 5 |
| Inversion time spacing (ms) | 315 | 315 | 907 | – | – | – |
| Repetition time (ms) | 10,000 | 10,000 | 10,000 | 3000 | 3000 | 7800 |
| Echo time (ms) | 7.8 | 7.8 | 4.33 | 20–950 | 20–950 | 30–960 |
| Flip angle | 15° | 15° | 15° | 90° | 90° | 90° |
| EPI frequency encoding bandwidth (Hz) | 614.6 | 614.6 | 1179 | – | – | – |
| Readout bandwidth (Hz/pixel) | 77.1 | 77.1 | 144.2 | 227 | 227 | 208 |
| EPI factor | 5 | 5 | 5 | – | – | – |
| Parallel reduction factor in-plane | 2 | 2 | 2 | 2 | 2 | 2 |
| Scan duration (s) | 220 | 220 | 220 | 252 | 168 | 632 |
EPI Echo-planar imaging, *with turbo-spin echo and sensitivity encoding acceleration
T2 mapping
T2 mapping was performed using a multi-slice multiple spin-echo sequence, where images are acquired as the signal decays after the initial slice excitation (Fig. 1d). The different echo times (TEs) are separated by the echo spacing. To limit the scan duration, different acceleration techniques were applied: turbo-spin echo (TSE), echo-planar imaging, and a combination of TSE acceleration with sensitivity encoding (SENSE). The details of the sequences are reported in Table 1.
For T2 measurements, a parameter of particular significance in the pulse sequence is the echo spacing [21]. To verify that measured T2 values were independent of the echo spacing, four additional scans with different echo spacing (echo spacing 10, 20, 30, and 40 ms, respectively) were acquired at 1.5 T (information available in the Supplementary material).
Image analysis
T1 and T2 fitting
Image processing was done offline using MatLab 2018a (MathWorks, Natick, MA, USA). T1 and T2 maps were obtained by voxelwise fitting the fit functions to the signal intensity (S) from the data points using a Levenberg-Marquardt non-linear least squares method. To obtain T1, the signal was fitted as S = S0 (1 − 2 e−TI/T1), where S0 is the spin density and TI is the inversion delay. To obtain T2, the signal was fitted as S = S0 (e−TE/T2), where TE is the echo time [22].
Postprocessing
The quantitative evaluation was based on the mean T1 or T2 values from regions of interest (ROIs) within each tube. The T1 and T2 standard deviation was assessed using the spatial standard deviation over the voxels inside the ROIs placed on the maps. Normally distributed data were reported as mean values with standard deviations. The normality of the data was evaluated by the Kolmogorov-Smirnov test. Finally, for each field strength, the mean T1 and T2 across the scan batches were calculated for different blood concentrations. To determine the lowest blood concentration that could be reliably measured by the T1 and T2 mapping, a method based on the guideline EPI17, by Clinical and Laboratory Standards Institute [23], was used. The synovial fluid, i.e. 0% blood concentration sample, was used as a blank sample. The limit of blank (LoB) was determined according to the guideline EPI17. The limit of detection (LoD) definition used by the Clinical and Laboratory Standards Institute was adapted to allow the discrimination of the highest apparent T1 or T2 likely to be reliably distinguished from the LoB among different blood concentrations. The LoD was determined by the following:
where μx % blood is the mean T1 or T2 and σx % blood is the standard deviation of T1 or T2 for a given blood concentration of x%, fulfilling the condition of LoD < LoB. The blood concentration in percent corresponding to the LoD was defined as the detection threshold.
Interrater reliability and agreement
To estimate the reproducibility of the measurements on the obtained T1 and T2 maps, all ROIs were independently determined by two raters, both relatively inexperienced in clinical radiology (FL, 4 months clinical radiology; BL, 4 years investigative radiology). Interrater reliability was assessed by intraclass correlation coefficient (ICC) for T1 and T2 mapping at the clinically available field strengths (1.5 and 3 T). ICC values range from 0 to 1, where values < 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and > 0.90 indicate poor, moderate, good, and excellent reliability, respectively. ICC estimates and their 95% confidence intervals (CI) were calculated using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 26.0.0.1, Armonk, NY, USA) based on a single rater, absolute agreement, 2-way random effects model. Interrater agreement was assessed by the interrater limit of agreement (LoA) and graphically displayed in the Bland-Altman method with plots [24] for the previously mentioned sequences and field strengths.
Results
Imaging was successful at all field strengths. Scan images for T1 mapping presented Gibbs ringing artefacts at 7 T, which hampered the analysis of the 75% and in part of the 100% blood concentration samples. Moreover, it was not possible to measure T2 values for blood concentration above 25% at 7 T because of the complete signal loss due to the short T2 of blood. A temperature between 35 and 38 °C was maintained in the phantom during the scanning sessions. Blood clot formation and sedimentation were observed to some extent in the samples during scanning.
T1 measurements
The mean T1 values as a function of blood concentration for each field strength are shown in Fig. 2. Overall, the estimated T1 values showed an inverse dependence on the blood concentration for all field strengths. The blood detection thresholds for T1 were ≥ 10% at 1.5 T, ≥ 25% at 3 T, and ≥ 50% at 7 T. The T1 estimates for blood and synovial fluid, the LoB (ms), the LoD (ms), and the blood detection threshold (blood percentage) are reported in Table 2.
Fig. 2.
Mean T1 and T2 value estimates for the different blood concentrations, at 3 field strengths. For 7 T, some of the estimates were not available, because artefacts or relaxation times were too short to be measured
Table 2.
Experimentally estimated T1 and T2 values for synovial fluid, blood, LoB, LoD, and blood detection threshold at 1.5, 3, and 7 T
| Field strength | Relaxation time synovial fluid (ms) | Relaxation time blood (ms) | LoB (ms) | LoD (ms) | Blood detection threshold | |
|---|---|---|---|---|---|---|
| T1 | 1.5 T | 2641 ± 60 | 1258 ± 33 | 2541 | 2526 | ≥ 10% |
| 3 T | 2719 ± 72 | 1310 ± 33 | 2600 | 2560 | ≥ 25% | |
| 7 T | 2355 ± 145 | 1272 ± 160 | 2117 | 2040 | ≥ 50% | |
| T2 | 1.5 T | 845 ± 56 | 93 ± 11 | 753 | 298 | ≥ 50% |
| 3 T | 592 ± 13 | 28 ± 20 | 579 | 546 | ≥ 5% | |
| 7 T | 281 ± 5 | Not measurable | 272 | 218 | = 25% |
Results are reported as mean values, and the standard deviations as data were normally distributed (Kolmogorov-Smirnov test, p > 0.05)
Blood blood concentration 100%, Blood detection threshold blood concentration corresponding to the LoD, LoB limit of blank, LoD limit of detection, synovial fluid blood concentration 0%
T2 measurements
At 1.5 T, different protocols for T2 mapping were employed (Fig. 3). With all the protocols, the T2 exhibited an inverse dependence on the blood concentration. The three accelerated T2 mapping methods showed good agreement with the reference scan (echo spacing 30 ms) in almost all cases. A large T2 standard deviation was identifiable for the 5% blood concentration with the EPI acceleration technique.
Fig. 3.
Effect of different acceleration techniques on T2 estimates at 1.5 T. T2 exhibited an inverse dependence on the blood concentration with all acceleration techniques. The three accelerated T2 mapping methods showed good agreement with the reference scan (echo spacing 30 ms), except from the 5% blood concentration measurement using the EPI acceleration technique. EPI, echo-planar imaging; TSE, turbo spin-echo; TSE+SENSE, turbo spin-echo with sensitivity encoding
The mean T2 values for different blood concentrations using multi-slice TSE/SENSE-accelerated sequences at each field strength are shown in Fig. 2. The blood detection thresholds for T2 were ≥ 50% at 1.5 T, ≥ 5% at 3 T, and equal to 25% at 7 T. The T2 estimates for the blood and synovial fluid, the LoB (ms), the LoD (ms), and the blood detection threshold (blood percentage) are reported in Table 2.
Interrater reliability and agreement
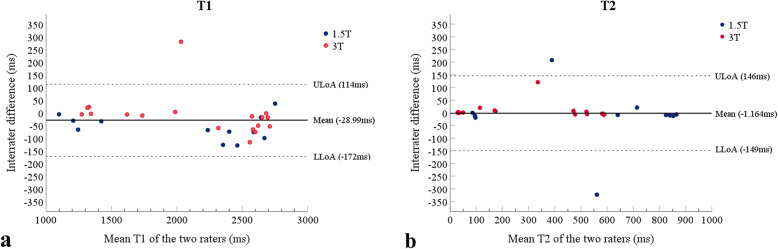
Interrater reliability as assessed by the ICC was excellent for both T1 (0.991, 95% CI 0.980–0.996) and T2 (0.969, 95% CI 0.937–0.985) measurements at 1.5 and 3 T. The interrater LoA, which reflects the deviation of the measurements performed by the two observers compared to the mean value, was 143.15 ms for T1 and 147.51 ms for T2 (Fig. 4).
Fig. 4.
Bland-Altman plots for interobserver agreement regarding T1 and T2 measurements at 1.5 and 3 T. Horizontal dashed lines indicate the upper and lower limit of agreement from the mean by the two observers (ULoA/LLoA). For T1 measurements, the mean was − 28.99 ms, with a limit of agreement (LoA) equal to 143 ms. For T2 measurements, the mean was − 1.164 ms with LoA equal to 148 ms
Discussion
Our study presented T1 and T2 mapping MRI protocols aimed at detecting low blood concentrations in joints. We showed that different concentrations of deoxygenated blood and synovial fluid can be identified in vitro using quantification of the relaxation times at 1.5, 3, and 7 T. At all three field strengths, T1 and T2 relaxation times decreased as the blood concentration increased. Moreover, we were able to determine the detection thresholds of the T1 and T2 mapping approaches, i.e. the lowest blood concentration likely to be reliably distinguished from synovial fluid. For T1, the detection threshold was 10% at 1.5 T, 25% at 3 T, and 50% at 7 T. For T2, the detection threshold was 50% for 1.5 T, at 5% for 3 T, and is at least equal to 25% for 7 T.
We were unable to identify quantitative studies differentiating T1 and T2 for blood and synovial fluid at 1.5, 3, and 7 T. However, previous studies have reported on the relaxation times of blood and/or synovial fluid at various magnetic field strengths in in vitro and in vivo settings (Table 3). Our estimates of synovial fluid were in line with the relaxation times reported by previous works [25, 28]. Variations can be attributed to the intersubject variability of relaxation times in the synovial fluid [32, 33] or to the differences in the pulse sequence parameters, e.g. type of sequence, acquisition techniques, T1 and T2 estimation method, and echo times [25, 26, 28]. As for blood relaxation times, previous studies reported higher values than ours at 1.5, 3, and 7 T [26, 27, 30, 31]. This discrepancy probably results from a higher blood oxygen level [29, 31]: up to 95% oxygenated blood for reported values in the other studies [26], compared to fully deoxygenated blood in this study. At 7 T, estimates in the blood appeared to be different. However, the study at 7 T should only be considered as a proof of concept.
Table 3.
T1 and T2 relaxation times of blood and synovial fluid at different field strengths: a comparison of the experimental values with literature values
| Field strength | Synovial fluid | Blood | |||||
|---|---|---|---|---|---|---|---|
| Experimental values | Literature values | Author | Experimental values | Literature values | Author | ||
| T1 (ms) | 1.5 T | 2641 ± 60 | 2850 ± 280 | Gold [25] | 1258 ± 33 | 1440 ± 120 | Stanisz [26] |
| 1480 ± 61 | Zhang [27] | ||||||
| 3 T | 2719 ± 72 | 3620 ± 320 | Gold [25] | 1310 ± 33 | 1932 ± 85 | Stanisz [26] | |
| 2564 ± 270 | Jordan [28] | 1649 ± 68 | Zhang [27] | ||||
| 1584 ± 5 | Lu [29] | ||||||
| 7 T | 2355 ± 145 | 4813 ± 700 | Jordan [28] | 1272 ± 160 | 2212 ± 53 | Dobre [30] | |
| 2087 ± 131 | Zhang [27] | ||||||
| T2 (ms) | 1.5 T | 845 ± 56 | 1210 ± 140 | Gold [25] | 93 ± 11 | 290 ± 30 | Stanisz [26] |
| 3 T | 592 ± 13 | 767 ± 50 | Gold [25] | 28 ± 20 | 275 ± 50 | Stanisz [26] | |
| 652 ± 113 | Jordan [28] | 60 ± 6 | Krishnamurty [31] | ||||
| 7 T | 281 ± 5 | 324 ± 60 | Jordan [28] | Not measurable* | 21 ± 4 | Krishnamurty [31] | |
Experimental values Values measured in this study in region of interests within the synovial fluid (0% blood) and 100% blood tubes reported as means and standard deviations, Literature values Mean values found in other studies with corresponding standard deviations, *T2 times too short to be measured
Furthermore, we observed a lower blood detection threshold with T1 mapping at 1.5 T than at 3 T and the opposite for T2 mapping. This behaviour can be related to the compartmentalisation of paramagnetic haemoglobin in the blood cells, which is reminiscent of the way superparamagnetic iron oxide particles are distributed. The relaxation effects of these iron oxide contrast agents are strongly dependent on the physical characteristics of the individual nanoparticles and are influenced by their local concentration as well as the applied field strength [34]. In our case, a higher detection threshold is caused by a higher mean value and a bigger standard deviation. T1 measurements at 1.5 T were more precise than at 3 T, and the T1 relaxivity (the inverse of the relaxation time, i.e. 1/T1) of clustered paramagnetic compounds is higher at 1.5 T than at 3 T [35, 36]. Thus, due to a combination of stronger T1 effect and smaller T1 standard deviation at 1.5 T compared to 3 T, the LoD for T1 is lower at 1.5 T. The opposite can be observed for T2, where the effect of paramagnetic compartments, such as blood cells or iron oxide, increases with field strength [34, 36].
The current study reports on the performance of the developed MRI protocols in a phantom. Therefore, these results cannot be readily translated into patient settings. However, the observed trends and values give useful indications of T1 and T2 quantification in patients since the phantom mimics the conditions in a human joint. Firstly, the small volume of the scanned samples resembles the volume of synovial fluid in joints without large effusion. Secondly, the phantom was scanned at a constant temperature of 37 °C to simulate the intra-articular temperature. Thirdly, deoxygenated blood was used for the preparation of biological samples to ensure a stable oxygenation level throughout the scan session. Deoxygenated intra-articular blood is also expected in clinical and research settings after the expected patient delay between bleed onset and presentation at the clinic.
Nevertheless, the nature of the biological fluids employed in this study introduces some challenges on the experimental side. The blood used showed clot formation and sedimentation in the samples during scanning, despite heparinisation of the blood and the manual mixing before scanning. As a result of an inhomogeneous distribution of blood, observed T1 and T2 relaxation times might not fully correspond to values of homogeneous mixtures. However, clot formation and sedimentation can be expected to some extent in vivo as well. Therefore, imprecision of measured values in vivo can be expected too and cannot be prevented either. Furthermore, the reported T1 and T2 relaxation times were measured for blood with a haemoglobin level of 9.8 mmol/L, and relaxation times could vary with differences in haemoglobin levels.
The synovial fluid used came in part from patients with known inflammatory diseases. This might have increased the count of inflammatory cells in the synovial fluid mixture compared to the synovial fluid of patients without ongoing inflammatory joint processes. However, the concordance of our results to the values in the literature suggests that the effect of this increase in inflammatory cells on the relaxation time measurements can be considered minimal [25, 28].
From a technical perspective, the methods presented in this phantom study could be suitable for clinical settings, since a scan duration below 5 min of one T1 or T2 mapping sequence allows the use in clinical research and practice as part of the MRI protocol.
Additionally, the MRI sequences were shown to be sufficiently robust to be translated to different field strengths. The MRI protocols were initially developed at 1.5 T. Translation to 3 T was straightforward and effective. However, this choice led to compromises at 7 T. The echo times and spacing chosen for T2 measurements turned out to be suboptimal for estimating the short T2 at high blood concentrations at 7 T. Therefore, this study only shows a proof of concept of T2 measurements at 7 T.
At the clinically relevant magnetic field strengths, 1.5 and 3 T, the high ICC coefficient showed a high level of interrater reliability for the measurements. As supported by interrater agreement analysis, the T1 and T2 values estimated for each sample were independent from the rater as well.
What might be the potential use of these techniques in future patient studies or clinical practice? After in vivo validation, T1 and T2 mapping might be used for non-invasive differentiation between minor joint bleeding and no joint bleeding. The developed MRI sequences might be used to accurately detect low concentrations of blood in case of joint bleeding, whereas simple effusions suggest a non-bleeding aetiology. Therefore, the results of this study might eventually be used for research purposes or even in clinical cases as an objective reference standard to verify or rule out recent joint bleeding.
However, factors of variability should be investigated further, and some precautions need to be adopted for establishing the optimal usage of these sequences in clinics. Since the relaxation times of synovial fluid and blood depend on different conditions [25, 26, 28, 37–39], the effect of changes in variables as field strength and MRI pulse sequences, inter-patient differences in synovial fluid consistency and haemoglobin level, and time between bleed onset and scanning—and thereby differences in the blood oxygenation level—should be taken into account when interpreting the results.
To summarise, this study is providing different tools for the differentiation between simple and haemorrhagic joint effusion in vitro. In vivo validation of the MRI protocols is needed to establish the use in patients. A study in persons without joint bleeding may determine reference values for T1 and T2 of the synovial fluid in vivo that will enable interpretation of T1 and T2 measurements in patients with suspected joint bleeding. Evaluation of a joint with (suspected) bleeding and the contralateral joint without bleeding as a reference may be useful for comparison. Furthermore, in daily practice, the MRI protocol should be adapted according to the MRI field strength and sequence availability. If a 3-T scanner is available, our results indicate that quantitative T2 mapping allows detection of the lowest blood concentration (equal to 5%). In case a 3-T scanner is not available, T1 mapping at 1.5 T allows for the detection of blood concentrations as low as 10%.
This study shows that MRI T1 and T2 mapping techniques may differentiate between simple and haemorrhagic effusion in vitro. The relaxometry protocols were able to detect low blood concentrations in the synovial fluid at clinically available field strengths (1.5 and 3 T). Especially T2 measurements at 3 T MRI appeared to be highly sensitive with the lowest detectable blood concentration of 5%. With limited acquisition times below 5 min, these T1 and T2 mapping techniques might be promising for accurate non-invasive discrimination between simple and haemorrhagic joint effusion in vivo, for example, in patients with bleeding disorders.
Supplementary Information
Additional file 1: Figure S1. Detailed description of the position of the samples in the phatom in the different batches scanned. Percentages correspond to the blood percentage in the sample. a) First batch scanned at all field strenghts. b) Second batch scanned at all field strengths. c) Third batch scanned at 3T. Figure S2. (A) Signal intensity in the ROI vs IR delays for the 0% blood tube at 3T: the experimental data are indicated with blue ● and the fitting with a red line. (B) Analysis of the residuals: each ● represents the residual of each experimental data point from the one predicted with the fitting. The mean of the residual is also reported as dotted line. Figure S3. T2 mapping at 1.5T with different echo spacing on dependence on the blood concentration. Figure S4. T2 mapping at 1.5T (A) and 3 T (B) with a multi-slice accelerated scans and single slice non accelerated scans.
Acknowledgements
We would like to thank Clemens Bos for being the scientific guarantor of this paper, Arno Concepcion for the help in the preparation of the biological samples, and Lieke van den Wildenberg for the technical support at the 7-T MRI scanner.
Abbreviations
- CI
Confidence intervals
- EPI
Echo planar imaging
- ICC
Intraclass correlation coefficient
- LoA
Limit of agreement
- LoB
Limit of blank
- LoD
Limits of detection
- MRI
Magnetic resonance imaging
- ROI
Region of interest
- SENSE
Sensitivity encoding
- TE
Echo time
- TSE
Turbo spin-echo
Authors’ contributions
CB, WF, FN, and LV contributed to the concepualisation of the study. CB, WF, FL, BL, LV, and JZ contributed to the design of the work. CB, WF, FL, and BL acquired, analysed, and interpreted the data. CB and BL created the new software used in the work. FL and BL drafted the article. LB, CB, KF, WF, PJ, LV, and JZ substantially revised the article. All authors read and approved the final manuscript.
Funding
This research is supported by the Young Researchers Grant from the European Society of Musculoskeletal Radiology (ESSR).
Declarations
Ethics approval and consent to participate
All patients or their legal guardians approved the use of their remnant samples for method development, validation, and research purposes, in agreement with Article 8. “Processing, storing, release and use of human biological material in accordance with ‘no objection’ procedure” of the University Medical Center Utrecht Biobank Regulations (version June 19, 2013).
Consent for publication
Not applicable
Competing interests
The authors of this manuscript declare relationships with the following companies: WF has received research grants from NovoNordisk and Pfizer which were paid to the institution. LV received a research grant from CSL Behring which was paid to the institution and reports to be in the advisory boards of Swedish Orphan Biovitrum BV (sobi), Tremeau Pharmaceuticals and CSL Behring. KF has received speaker's fees from Bayer, Baxter/Shire, Biotest, CSL Behring, Octapharma, Pfizer, NovoNordisk; performed consultancy for Baxter/Shire, Biogen, CSL-Behring, Freeline, NovoNordisk, Pfizer, Roche and SOBI; and has received research support from Bayer, Pfizer, Baxter/Shire, and Novo Nordisk. PJ reported to perform consultancy for Sanifit and Inozyme. The Radiology department of the University Medical Center Utrecht received research support from Philips.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Flora H. P. van Leeuwen and Beatrice Lena are joint first authors.
References
- 1.Pulles AE, Mastbergen SC, Schutgens REG, et al. Pathophysiology of hemophilic arthropathy and potential targets for therapy. Pharmacol Res. 2017;115:192–199. doi: 10.1016/j.phrs.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Jansen NWD, Roosendaal G, Lafeber FPJG. Understanding haemophilic arthropathy: an exploration of current open issues. Br J Haematol. 2008;143:632–640. doi: 10.1111/j.1365-2141.2008.07386.x. [DOI] [PubMed] [Google Scholar]
- 3.Jansen NWD, Roosendaal G, Bijlsma JWJ, et al. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: an in vitro study. Arthritis Rheum. 2007;56:199–207. doi: 10.1002/art.22304. [DOI] [PubMed] [Google Scholar]
- 4.Timmer MA, Pisters MF, de Kleijn P, et al. Differentiating between signs of intra-articular joint bleeding and chronic arthropathy in haemophilia: a narrative review of the literature. Haemophilia. 2015;21:289–296. doi: 10.1111/hae.12667. [DOI] [PubMed] [Google Scholar]
- 5.Ceponis A, Wong-Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19:790–798. doi: 10.1111/hae.12175. [DOI] [PubMed] [Google Scholar]
- 6.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 7.Foppen W, van der Schaaf IC, Witkamp TD, Fischer K. Is joint effusion on MRI specific for haemophilia? Haemophilia. 2014;20:582–586. doi: 10.1111/hae.12338. [DOI] [PubMed] [Google Scholar]
- 8.Eerdekens M, Peerlinck K, Staes F, et al. Clinical gait features are associated with MRI findings in patients with haemophilic ankle arthropathy. Haemophilia. 2020;26:333–339. doi: 10.1111/hae.13925. [DOI] [PubMed] [Google Scholar]
- 9.Lundin B, Ljung R, Pettersson H. MRI scores of ankle joints in children with haemophilia - comparison with clinical data. Haemophilia. 2005;11:116–122. doi: 10.1111/j.1365-2516.2005.01061.x. [DOI] [PubMed] [Google Scholar]
- 10.Burke CJ, Alizai H, Beltran LS, Regatte RR. MRI of synovitis and joint fluid. J Magn Reson Imaging. 2019;49:1512–1527. doi: 10.1002/jmri.26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26:1–158. doi: 10.1111/hae.14046. [DOI] [PubMed] [Google Scholar]
- 12.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheum. 2020;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 14.Doria AS, Keshava SN, Mohanta A, et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am J Roentgenol. 2015;204:W336–W347. doi: 10.2214/AJR.14.12501. [DOI] [PubMed] [Google Scholar]
- 15.Foppen W, van der Schaaf IC, Beek FJA, et al. Diagnostic accuracy of point-of-care ultrasound for evaluation of early blood-induced joint changes: comparison with MRI. Haemophilia. 2018;24:971–979. doi: 10.1111/hae.13524. [DOI] [PubMed] [Google Scholar]
- 16.Mandl P, Ciechomska A, Terslev L, et al. Implementation and role of modern musculoskeletal imaging in rheumatological practice in member countries of EULAR. RMD Open. 2019;5:e000950. doi: 10.1136/rmdopen-2019-000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen S, Lu X, Ma Y, et al. Musculoskeletal ultrasound for intra-articular bleed detection: a highly sensitive imaging modality compared with conventional magnetic resonance imaging. J Thromb Haemost. 2018;16:490–499. doi: 10.1111/jth.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regi SS, Livingstone RS, Kandagaddala M, et al. Ultrasound and magnetic resonance imaging for the detection of blood: an ex-vivo study. Haemophilia. 2021;27:488–493. doi: 10.1111/hae.14303. [DOI] [PubMed] [Google Scholar]
- 19.Costantino F, Carmona L, Boers M, et al. EULAR recommendations for the reporting of ultrasound studies in rheumatic and musculoskeletal diseases (RMDs) Ann Rheum Dis. 2021;80:840 LP–840847. doi: 10.1136/annrheumdis-2020-219816. [DOI] [PubMed] [Google Scholar]
- 20.Clare S, Jezzard P. RapidT1 mapping using multislice echo planar imaging. Magn Reson Med. 2001;45:630–634. doi: 10.1002/mrm.1085. [DOI] [PubMed] [Google Scholar]
- 21.Baldock C, Lepage M, Bäck SÅJ, et al. Dose resolution in radiotherapy polymer gel dosimetry: effect of echo spacing in MRI pulse sequence. Phys Med Biol. 2001;46:449–460. doi: 10.1088/0031-9155/46/2/312. [DOI] [PubMed] [Google Scholar]
- 22.Brown RW, Cheng Y-CN, Haacke EM, et al. Magnetic resonance imaging. 2. Hoboken: Wiley & Sons, Inc.; 2014. Introductory signal acquisition methods; pp. 113–139. [Google Scholar]
- 23.Armbruster DA, Pry T (2008) Limit of blank, limit of detection and limit of quantification. Clin Biochem Rev 29(Suppl 1):S49–52. [PMC free article] [PubMed]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 25.Gold GE, Han E, Stainsby J, et al. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. Am J Roentgenol. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 26.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3 T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Petersen ET, Ghariq E, et al. In vivo blood T 1 measurements at 1.5 T, 3 T, and 7 T. Magn Reson Med. 2013;70:1082–1086. doi: 10.1002/mrm.24550. [DOI] [PubMed] [Google Scholar]
- 28.Jordan CD, Saranathan M, Bangerter NK, et al. Musculoskeletal MRI at 3.0 T and 7.0 T: a comparison of relaxation times and image contrast. Eur J Radiol. 2013;82:734–739. doi: 10.1016/j.ejrad.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H, Clingman C, Golay X, van Zijl PCM. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52:679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 30.Dobre MC, Uğurbil K, Marjanska M. Determination of blood longitudinal relaxation time (T1) at high magnetic field strengths. Magn Reson Imaging. 2007;25:733–735. doi: 10.1016/j.mri.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthy LC, Liu P, Xu F, et al. Dependence of blood T 2 on oxygenation at 7 T: in vitro calibration and in vivo application. Magn Reson Med. 2014;71:2035–2042. doi: 10.1002/mrm.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ. Anisotropy of NMR properties of tissues. Magn Reson Med. 1994;32:592–601. doi: 10.1002/mrm.1910320508. [DOI] [PubMed] [Google Scholar]
- 33.Yao L, Sinha S, Seeger LL. MR imaging of joints: analytic optimization of GRE techniques at 1.5 T. Am J Roentgenol. 1992;158:339–345. doi: 10.2214/ajr.158.2.1370362. [DOI] [PubMed] [Google Scholar]
- 34.Bulte JWM, Vymazal J, Brooks RA, et al. Frequency dependence of MR relaxation times II. Iron oxides. J Magn Reson Imaging. 1993;3:641–648. doi: 10.1002/jmri.1880030414. [DOI] [PubMed] [Google Scholar]
- 35.Kellar KE, Fujii DK, Gunther WHH, et al. Important considerations in the design of iron oxide nanoparticles as contrast agents for Tl-weighted MRI and MRA. Acad Radiol. 2002;9:S34–S37. doi: 10.1016/S1076-6332(03)80391-4. [DOI] [PubMed] [Google Scholar]
- 36.Simon GH, Bauer J, Saborovski O, et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5 T and 3 T clinical MR scanning. Eur Radiol. 2006;16:738–745. doi: 10.1007/s00330-005-0031-2. [DOI] [PubMed] [Google Scholar]
- 37.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta, Gen Subj. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 38.Clark RA, Watanabe AT, Bradley WG, Roberts JD. Acute hematomas: effects of deoxygenation, hematocrit, and fibrin-clot formation and retraction on T2 shortening. Radiology. 1990;175:201–206. doi: 10.1148/radiology.175.1.2315481. [DOI] [PubMed] [Google Scholar]
- 39.van Zijl PCM, Eleff SM, Ulatowski JA, et al. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4:159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Detailed description of the position of the samples in the phatom in the different batches scanned. Percentages correspond to the blood percentage in the sample. a) First batch scanned at all field strenghts. b) Second batch scanned at all field strengths. c) Third batch scanned at 3T. Figure S2. (A) Signal intensity in the ROI vs IR delays for the 0% blood tube at 3T: the experimental data are indicated with blue ● and the fitting with a red line. (B) Analysis of the residuals: each ● represents the residual of each experimental data point from the one predicted with the fitting. The mean of the residual is also reported as dotted line. Figure S3. T2 mapping at 1.5T with different echo spacing on dependence on the blood concentration. Figure S4. T2 mapping at 1.5T (A) and 3 T (B) with a multi-slice accelerated scans and single slice non accelerated scans.