Abstract
Despite strong preclinical evidence for the ability of corticotropin releasing factor 1 (CRF1) antagonists to regulate alcohol consumption, clinical trials have not yet demonstrated therapeutic effects of these compounds in alcohol use disorder (AUD) patients. Several confounding factors may limit the translation of preclinical CRF1 research to patients, including reliance on experimenter-administered alcohol instead of voluntary consumption, a preponderance of evidence collected in male subjects only, and an inability to assess the effects of alcohol on specific brain circuits. A population of particular interest is the CRF1-containing neurons of the central amygdala (CeA). CRF1 CeA neurons are sensitive to ethanol, but the effects of alcohol drinking on CRF signaling within this population are unknown. In the present study, we assessed the effects of voluntary alcohol drinking on inhibitory control of CRF1+ CeA neurons from male and female CRF1:GFP mice using ex vivo electrophysiology and determined the contributions of CRF1 signaling to inhibitory control and voluntary alcohol drinking. Chronic alcohol drinking produced neuroadaptations in CRF1+ neurons that increased the sensitivity of GABAA receptor-mediated sIPSCs to the acute effects of alcohol, CRF, and the CRF1 antagonist R121919, but these adaptations were more pronounced in male versus female mice. The CRF1 antagonist CP-154,526 reduced voluntary alcohol drinking in both sexes and abolished sex differences in alcohol drinking. The lack of alcohol-induced adaptation in the female CRF1 system may be related to the elevated alcohol intake exhibited by female mice and could contribute to the ineffectiveness of CRF1 antagonists in female AUD patients.
Keywords: Astressin 2B, CP-154, 526, ethanol, Female, GABA, R121919
INTRODUCTION
Alcohol use disorder (AUD) is a debilitating psychiatric illness for which few therapies exist and no widely effective treatment is yet available. Converging evidence from multiple species has pointed to the amygdala, and more specifically the corticotropin-releasing factor (CRF) system of the amygdala, as an important target for the effects of alcohol exposure on brain function (1). However, two recent clinical trials using CRF1 receptor antagonists failed to demonstrate efficacy on alcohol craving, alcohol cue reactivity, and stress-related alcohol cue reactivity (2, 3) despite strong preclinical evidence for a functional role of the CRF1 receptor in alcohol drinking. The CRF1 receptor remains an attractive target for pharmacological intervention in alcohol dependence (4), but more information about the complexity of CRF signaling in the amygdala and preclinical models that better reflect human AUD patients are necessary to allow preclinical research to translate to human patients.
The rodent and human central amygdala (CeA) is a primary region for CRF activity in the brain. The effects of acute and chronic alcohol on the CeA have been assessed in terms of both the CRF peptide and the CRF1/CRF2 receptors. Binge-like alcohol drinking produces increases in CRF immunoreactivity in the mouse CeA (5). The CRF1 receptor has also been shown to mediate the effects of acute alcohol on GABAergic inhibitory transmission within the mouse CeA (6, 7). Chronic alcohol drinking increases CRF mRNA in the rat CeA (8), and chronic alcohol vapor exposure alters sensitivity to the effects of CRF and CRF1 antagonists in rat CeA (9, 10). CRF activity in the CeA has also been shown to functionally regulate alcohol drinking in rodents (11). These findings strongly suggest a role for CRF/CRF1 activity in the effects of alcohol on the CeA, but do not speak to the role of discrete brain circuits within the CeA in the effects of alcohol on the amygdala. The CeA is comprised of many neuronal types, and distinct cell populations have been shown to play very different roles in the context of stress reactivity and fear learning (see (1) for review). Previous work suggests that similar population-specific differences in CRF circuitry may be important for understanding the effects of alcohol on CeA function. The CRF1-containing (CRF1+) population of the CeA is generally insensitive to the effects of acute alcohol on GABAergic transmission, but receives tonic inhibitory input from acute alcohol-sensitive CRF1-lacking (CRF1-) neurons (12). Chronic alcohol vapor exposure blunts this tonic inhibition in CRF1+ neurons while enhancing tonic inhibition in CRF1- neurons, and increases signaling from CRF1+ neurons to downstream targets in the BNST (13). These findings suggest that the effects of alcohol on inhibitory control of CeA circuitry are cell-type specific, and that CRF1+ neurons display selective plasticity following chronic alcohol exposure. However, the effects of alcohol on CRF/CRF1 signaling within the CRF1+ population of the CeA remain unknown.
An additional barrier to the translation of preclinical findings regarding CRF and alcohol is significant differences between preclinical animal models and alcohol consumption in AUD patients. Many rodent models of alcohol exposure rely on experimenter-administered alcohol (via inhalation, injection, or oral gavage) or forced alcohol consumption (via liquid diet or providing alcohol as the only drinking solution during testing). Voluntary alcohol self-administration has been shown to differently enhance brain reward (14) and synaptic plasticity (15) as compared with experimenter-administered alcohol, and represents a more faithful reproduction of human alcohol consumption. Notably, voluntary alcohol drinking in Marchigian Sardinian (msP) rats blunted sensitivity to the CRF1 antagonist R121919 (9) whereas chronic alcohol vapor enhanced sensitivity to R121919 in Sprague-Dawley rats (10), suggesting that voluntary alcohol drinking may produce different effects than vapor exposure on CeA CRF1 activity. There is also evidence to suggest that experimenter-administered alcohol but not voluntary alcohol consumption engages stress signaling in rodents (16) and that pyrazole injections used in alcohol vapor exposure may increase corticosterone signaling in mice (17). Therefore, alcohol exposure procedures that rely on voluntary alcohol drinking may be particularly important in translating the interactions of alcohol and CRF activity to the human condition.
Finally, a third variable that may hinder the translation of CRF pharmacology to the clinic is an almost exclusive reliance on data from male subjects (see [4] for review). As stress circuitry is exquisitely sensitive to the influence of circulating hormones, it is unsurprising that the CRF system is a site of significant sexual dimorphism in the rodent and human brain (19). Within the CeA, Crf mRNA (20) and CRF receptor 1 (CRF1) binding (21) are higher in female versus male rats. However, males have shown greater sensitivity to the effects of early life (22, 23) and adult stressors (24) on CeA CRF signaling and subsequent CeA activation. CRF1+ CeA neurons also exhibit sex differences in sensitivity to the effects of acute alcohol, as well as CRF1 agonists and antagonists (25). Despite these clear sex differences in CeA CRF, to date only a single study has investigated sex differences in the effects of alcohol drinking on the CeA CRF system. Retson and colleagues (26) found that alcohol drinking activated CeA CRF neurons and enhanced the response of these neurons to stress selectively in male but not female rats. The possibility that the CeA CRF system displays alcohol-induced plasticity in males but not females would strongly suggest sex differences in the ability of CRF/CRF1-directed therapies to functionally regulate alcohol intake and dependence. Further investigation of these sex differences is necessary to clarify the contributions of CRF activity to alcohol use in both males and females.
Here, we employed a model of voluntary home-cage alcohol drinking to avoid engaging the CRF circuitry via stress as opposed to alcohol effects alone, and to directly assess the role of voluntary alcohol self-administration on CeA CRF circuitry. We used a transgenic CRF1 reporter line to specifically determine the effects of chronic alcohol drinking on CRF1+ neurons of the CeA, and used both male and female mice to examine the influence of sex on these effects. We report evidence for sex-specific effects of acute alcohol on GABAergic signaling in male and female CeA CRF1+ neurons following alcohol drinking, characterize the effects of alcohol drinking on CRF regulation of inhibitory control in CRF1 neurons, investigate the contribution of the CRF1 and CRF2 receptors to the effects of CRF in male and female mice, and demonstrate sex differences in CRF1 regulation of voluntary alcohol drinking.
MATERIALS AND METHODS
Animals
All experiments were conducted in male and female adult mice (19–30 g) from a transgenic bacterial artificial chromosome (BAC) reporter line expressing green fluorescent protein (GFP) under the control of the CRF1 promotor on a C57BL6/J background (27). Mice were bred at the University of North Carolina at Chapel Hill and maintained with ad libitum access to food, water, and environmental enrichment (nesting materials). All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and as approved by the Institutional Animal Care and Use Committee. Full experimental details are described in the Supplement.
Voluntary Alcohol Drinking
Voluntary intermittent alcohol drinking was established in mice as previously described (28). Male and female CRF1:GFP mice were placed in isolation housing in a clean cage with modified cage lid and nestlet in a vivarium on a reverse light/dark cycle (lights on at 19:00) for one week prior to drinking for habituation. Average age on isolation day was PND 106 for males and PND 114 for females. Beginning at 10:00 (three hours into the dark cycle) the home cage water bottle was replaced with one sipper tube with double-ball bearing containing 20% alcohol and one sipper tube containing tap water (alcohol mice) or two sipper tubes containing water (water mice). Alcohol and water intake were recorded 24 hours after tubes on. After 24 hours all subjects received two sipper tubes containing tap water. This pattern of intermittent two-bottle choice alcohol drinking continued for 28 days (14 total alcohol drinking days) after which subjects were used in electrophysiological recording, immunohistochemistry or behavioral pharmacology experiments. For all experimental procedures, tissue was extracted at 10:00 on Day 29, 24 hrs after the final alcohol drinking day, such that all mice had no alcohol in blood or brain but would anticipate alcohol delivery on the experimental day. This procedure facilitated the examination of the effects of chronic alcohol exposure on brain physiology and function without the confounding variable of acute alcohol on board. Average age on tissue extraction or drug treatment was PND 136 for males and PND 144 for females.
Electrophysiological Recording
Male and female (n =10/sex for alcohol mice and 9/sex for water mice) CRF1:GFP mice consumed alcohol or water in the home cage as described above for four weeks. On the final water day, subjects were rapidly decapitated and brains placed in a beaker containing ice-cold high sucrose solution (in mM): sucrose 206.0; KCl 2.5; CaCl2 0.5; MgCl2 7.0; NaH2PO4 1.2; NaHCO3 26; glucose 5.0; HEPES 5. 300 μm coronal sections were sliced on a vibrating microtome (Leica VT1000S, Leica Microsystems, Buffalo Grove, IL) and incubated in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) (in mM): NaCl 120; KCl 2.5; EGTA 5; CaCl2 2.0 MgCl2 1.0; NaH2PO4 1.2; NaHCO3 26; Glucose 1.75; HEPES 5 for 30 min at 37 °C, followed by 30 min at room temperature (RT, 21–22 °C) for equilibration. Patch pipettes (4–6 MΏ; King Precision Glass Inc., Claremont, CA) were filled with internal solution [in mM: potassium chloride (KCl) 145; EGTA 5; MgCl2 5; HEPES 10; Na-ATP 2; Na-GTP 0.2] to record electrical activity. Data were acquired with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 2–5 kHz, digitized (Digidata 1550B; Molecular Devices), and stored using pClamp 10 software (Molecular Devices). Series resistance was continuously monitored with a hyperpolarizing 10 mV pulse; cells with axis resistance >15 MΩ were excluded from the data set. CRF1 receptor-containing neurons were identified via GFP expression using fluorescent optics and brief (<2 s) episcopic illumination as previously described (29) and were primarily found in the medial CeA. During voltage clamp recording (Vhold= −60mV), electrophysiological properties of cells were determined by pClamp 10 Clampex software using a 10 mV pulse delivered after breaking into the cell. Current clamp recording (Ihold= −70pA) was performed immediately after breaking into the cell in order to determine the firing type of each neuron included in the data sets. Isolation of GABAA sIPSCs was achieved through selective pharmacological blockade with the glutamate receptor blockers 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20μM) and DL-2-amino-5-phosphonovalerate (AP-5, 50 μM) and the GABAB receptor antagonist GCP55845A (1μM). The selective CRF1 antagonist R121919 (R12) was chosen for assessment of CRF1-mediated effects as prior evidence indicates that this drug produces sex differences in inhibitory control of CRF1+ neurons (25). The potent and selective CRF2 receptor antagonist Astressin 2B (A2B) was chosen for assessment of CRF2-mediated effects as it has been well-established in electrophysiological recordings in the CeA (30, 31).
Behavioral Pharmacology
Male and female CRF1-GFP mice (n=10/sex) consumed alcohol and water in the home cage as described above for four weeks. Mice were then habituated to i.p. vehicle injections of 0.5% high-viscosity carboxymethylcellulose (CMC, Thermo Fisher Scientific, Rockford, IL) 30 minutes prior to bottles on until drinking following injection had stabilized (five days). Then, all subjects were pretreated with either CMC or the CRF1 antagonist CP-154,526 (10 mg/kg, i.p.) in a randomized order 30 minutes prior to drinking over two separate drinking days occurring seven days apart in a within-subjects design. One week after the last drinking test day, mice were randomly assigned to CMC or CP pretreatment 30 minutes prior to a two-hour open field locomotor activity test in a between-subjects design. Locomotor activity was measured in Plexiglas activity monitor chambers (27.9 cm2; ENV-510, Med Associates, Georgia, VT) connected to computers for data acquisition. Two sets of 16 pulse-modulated infrared photobeams were located on opposite walls and recorded X–Y ambulatory movements. The mouse’s position in the open field was assessed every 100 ms to quantify distance traveled (in meters). Overhead illumination of the open field enabled the assessment of anxiety-like behavior via time spent and distance traveled in the center versus the surround of the field.
Drugs
Alcohol for mouse drinking solution (20% v/v) was prepared using 95% ethyl alcohol (Pharmco Products Inc., Brookfield, CT) in tap water. DNQX, AP-5, CGP55845A, CRF peptide, and Astressin-2B were purchased from Tocris Bioscience (Ellisville, MO). SR-95531 (gabazine, GBZ; 100 μM) was purchased from Sigma-Aldrich. The CRF1 antagonist R121919 was supplied by Neurocrine Biosciences, Inc. (San Diego, CA). Stock solutions were prepared in ultra-pure water or DMSO (DNQX only), stored at 20°C, and diluted to final experimental concentration in aCSF on the day of testing. Doses for electrophysiology experiments were chosen based on their ex vivo effects in CeA neurons from prior reports (10, 25). CP-154,526 was purchased from Tocris Bioscience and was selected for use in behavioral pharmacology experiments due to a robust literature describing its effects on alcohol drinking in mice (32–34).
Experimental Design and Statistical Analysis
All data analyses and visualizations were completed with Prism v. 8.0 (GraphPad, La Jolla, CA). Intermittent alcohol and water drinking were represented as mean intake (g/kg or mL/kg, respectively) ± SEM and mean alcohol preference (%) ± SEM and analyzed via two-way repeated measures (RM) ANOVA (sex X time, repeated by time). Membrane characteristics between water and alcohol drinking mice were represented as mean ± SEM and were compared via two-way ANOVA (sex X drinking solution). Frequency and amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) were analyzed and manually confirmed using a semi-automated threshold-based detection software (Mini Analysis, Synaptosoft Inc.). sIPSC characteristics were determined from baseline and experimental drug application containing a minimum of 65 events each. Event data were represented as mean ± SEM. sIPSCs were analyzed for between-sex differences via three-way RM ANOVA (sex X drinking group X dose, repeated by dose) and for within-sex effects of alcohol drinking via two-way RM ANOVA (drinking group X dose, repeated by dose). Effects of alcohol, CRF, R121919 and Astressin 2B on sIPSCs were also represented as mean ± SEM percent change from baseline. Percent change data were analyzed for independent significance using a one-sample t-test and within-sex drinking groups were compared via unpaired two-tailed t-test for independent samples (with Welch’s correction where appropriate). Behavioral pharmacology data were represented as mean alcohol intake (g/kg) ± SEM. Drug effects were evaluated by two-way RM ANOVA (sex X dose, repeated by dose) and via two-tailed t-test to assess effects within each sex and sex differences under each treatment condition, respectively. Open field locomotor activity was represented as mean distance traveled ± SEM and compared via two-way ANOVA (sex X dose) for both total distance traveled and for time spent in center. Sidak’s multiple comparisons test was used to investigate all significant interactions. α was set at 0.05 for all comparisons.
RESULTS
Voluntary alcohol drinking in male and female CRF1:GFP mice
Voluntary drinking was established in male and female CRF1-GFP mice using an intermittent access two-bottle choice procedure over a period of four weeks (Figure 1A). In the alcohol-drinking group, female mice achieved a higher dose of alcohol intake than male mice [F(1,58) = 33.47, p < 0.0001; Figure 1B]. Alcohol intake also increased over time in male and female mice [F(13,754) = 6.79, p < 0.0001]. Raw alcohol intake was not significantly different between sexes (male mice averaged 3.27 ± 0.05 mL/24hr and female mice averaged 3.44 0.05 mL/24hr; data not shown). In the water-drinking group, female mice consumed more water than male mice [F(1,56) = 6.984, p = 0.0106; Figure 1C]. Water intake did not significantly change over time (p > 0.05). Female mice in the water-drinking group exhibited significantly greater raw water intake than male mice [F(1, 16) = 11.05, p = 0.0043]. Female average water consumption was 4.28 ± 0.09 mL and male average water consumption was 3.56 ± 0.08 mL (data not shown). On the final drinking day before tissue collection (Day 14), female mice achieved a significantly greater dose of alcohol (25.3 ± 6.4 g/kg) than male mice [18.6 ± 4.5 g/kg; t(48) = 4.197, p = 0.0001] and individual intake in both sexes was variable (Figure 1D). In water-drinking mice, Day 14 intake was also greater in female mice [103.9 ± 79.7 mL/kg] than male mice [58.5 ± 55.9 mL/kg; t(56) = 2.511; p = 0.0149] and individual intake in both sexes was variable (Figure 1E). Together, these data indicate that female CRF1:GFP mice consume more alcohol and water than male CRF1:GFP mice, consistent with reports in other mouse strains.
Figure 1. Sex differences in voluntary alcohol drinking in CRF1:GFP mice.
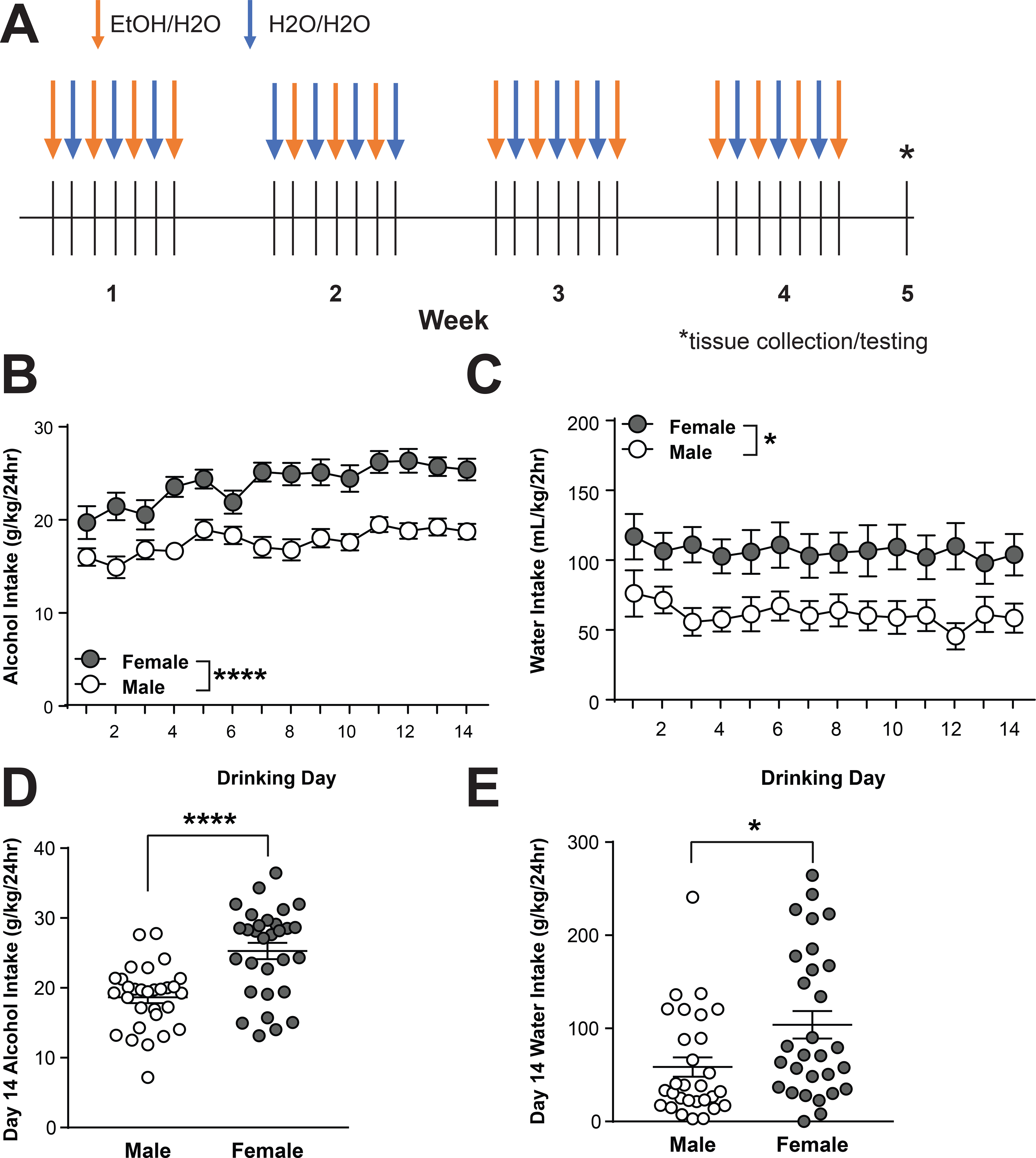
(A) Experimental timeline of intermittent access two-bottle choice drinking procedure. (B) 24-hour alcohol intake in male (n= 30) and female (n=30) CRF1:GFP mice. Female mice consumed significantly more alcohol than male mice (****p < 0.0001 by two-way RM ANOVA.) (C) 24-hour water intake in male (n=29) and female (n=29) CRF1:GFP mice. Female mice consumed significantly more water than male mice (*p < 0.05 by two-way RM ANOVA.) (D) Individual and average alcohol intake on the final drinking day (Day 14). Female mice consumed significantly more alcohol than male mice (****p < 0.0001 by unpaired t-test.) (E) Individual and average water intake on the final drinking day (Day 14). Female mice consumed significantly more water than male mice (*p < 0.05 by unpaired t-test.)
Basal inhibitory control of CRF1 neurons following voluntary alcohol drinking
We next assessed the effects of a four-week history of voluntary alcohol drinking on CRF1+ neurons of the medial CeA. Male and female CRF1+ medial CeA neurons exhibited two firing patterns, consistent with prior reports (12, 25): low-threshold bursting (LTB) and regular spiking (RS; Figure 2A, left), with equivalent proportions of each firing type across neurons from both sexes and drinking solutions (Figure 2A, right). Alcohol drinking did not alter membrane characteristics (Figure 2B), although female CRF1+ neurons had lower resting membrane potential than male CRF1+ neurons irrespective of drinking group [F(1, 227) = 7.77, p = 0.0058; Figure 2B). Baseline GABAA receptor-mediated sIPSC frequency and amplitude were also not altered by alcohol drinking in male (Figure 2C) or female (Figure 2D) CRF1+ neurons. Together, these findings suggest that voluntary alcohol drinking does not produce changes in baseline GABAA receptor-mediated inhibitory transmission in CeA CRF1+ neurons from male or female mice.
Figure 2. Voluntary alcohol drinking does not alter basal activity of CRF1+ neurons.
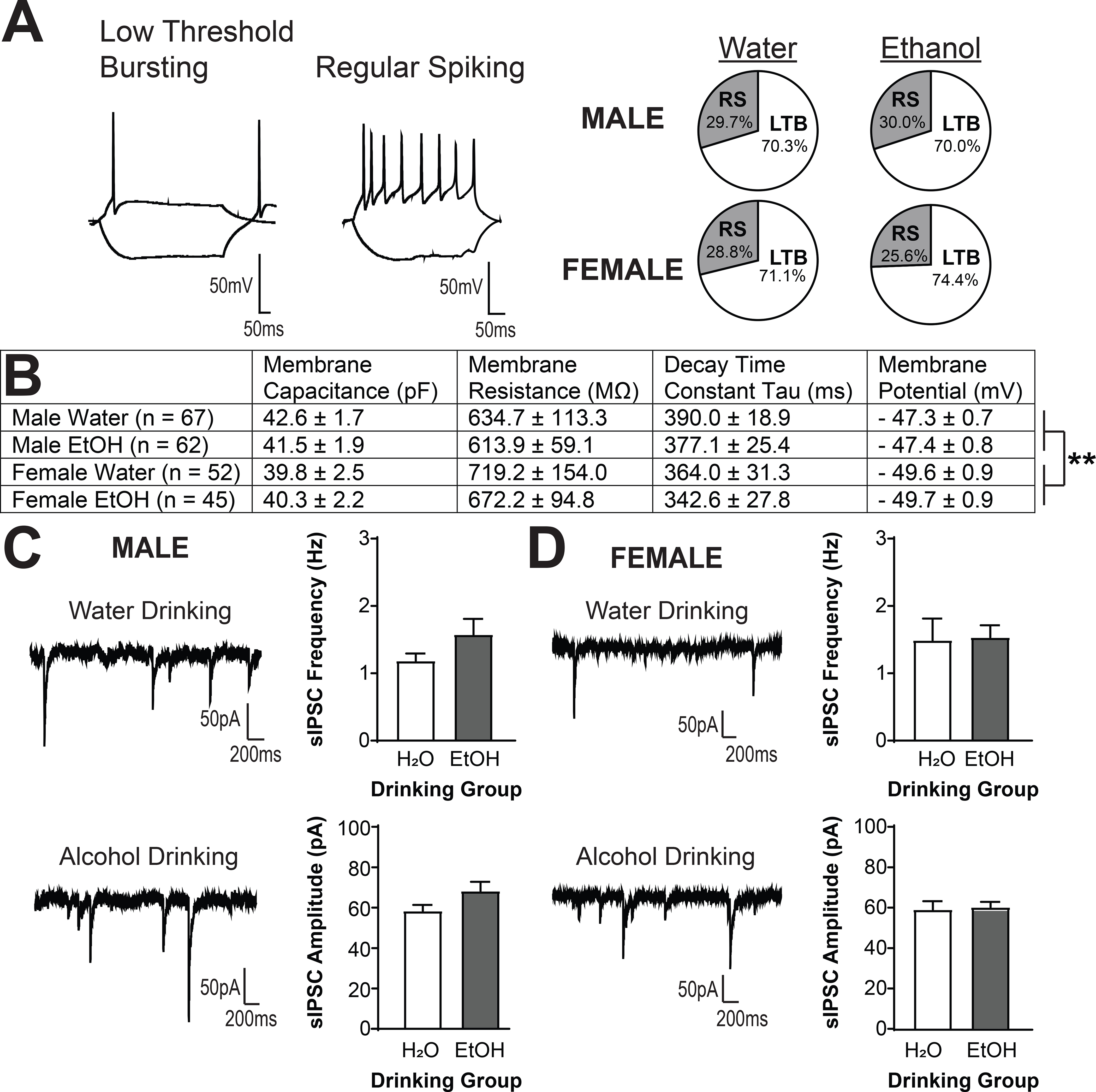
(A) Representative current clamp recordings depicting low-threshold bursting (left) and regular spiking (middle) firing patterns exhibited by male and female CeA GFP+ (hereafter, CRF1+) neurons. No effects of alcohol drinking on firing type was observed in males (top right) or females (bottom right). (B) Summary of membrane characteristics of male and female CeA CRF1+ neurons. No significant effects of alcohol drinking or sex on membrane capacitance, membrane resistance or decay time constant tau emerged. Resting membrane potential was slightly lower in neurons from female mice regardless of drinking solution (**p < 0.01 by two-way ANOVA). (C) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (top left) or alcohol (bottom left). Alcohol drinking did not alter sIPSC frequency (top right) or amplitude (bottom right). (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (top left) or alcohol (bottom left). Alcohol drinking did not alter sIPSC frequency (top right) or amplitude (bottom right).
Alcohol drinking sensitizes male but not female CRF1+ neurons to the effects of acute alcohol
To investigate whether voluntary alcohol drinking altered the response of CRF1+ neurons to acute alcohol, we used focal application of alcohol (44 mM) and examined GABAA receptor-mediated sIPSCs in CRF1+ neurons from male water (n = 9 cells from 3 mice) and alcohol drinking mice (n = 13 cells from 5 mice) and female water (n = 8 cells from 5 mice) and alcohol drinking mice (n = 9 cells from 5 mice). Analysis via three-way RM ANOVA revealed a significant Acute Ethanol X Sex interaction [F(1,36) = 4.22, p = 0.0472), indicating that the effects of acute ethanol on sIPSC frequency were different between the two sexes. To understand the interaction and visualize the results, data were also analyzed via two-way RM ANOVA within each sex. In male CRF1+ neurons (Figure 3A), focal application of alcohol produced a drinking solution X dose interaction [F(1,18) = 24.08, p = 0.0516; Figure 3B, left]. Analysis with Sidak’s multiple comparisons test indicated that CRF1+ neurons from water-drinking mice did not exhibit a significant change in sIPSC frequency (p > 0.05) whereas CRF1+ neurons from alcohol-drinking mice exhibited a strong trend towards an increase in sIPSC frequency (p = 0.0545). When data were analyzed as percent change from baseline, opposite responses were observed in neurons from water and alcohol-drinking mice [t(20) = 2.679, p = 0.0144]. Acute alcohol reduced sIPSC frequency in CRF1+ neurons from water drinking males [84.85 ± 16.04%; t(8) = 2.833, p =0.0220], but increased sIPSC frequency in CRF1+ neurons from alcohol drinking males [127.8 ± 12.74%; t(12) = 2.185, p = 0.0495; Figure 3B, right ]. These findings were not accompanied by any changes in sIPSC amplitude (p > 0.05 for all comparisons; Figure 3C), indicating that acute alcohol application altered presynaptic GABA release onto CRF1+ neurons without affecting postsynaptic response. CRF1+ neurons from female mice (Figure 3D) were comparatively insensitive to acute alcohol, with no changes in sIPSC frequency (p > 0.05 for all comparisons, Figure 3E) or amplitude (p > 0.05 for all comparisons, Figure 3F) between water and alcohol groups. Together, these findings suggest that voluntary alcohol drinking alters the effects of acute alcohol on GABA release onto CRF1+ neurons from male, but not female, mice.
Figure 3. Voluntary alcohol drinking reverses the response of CRF1+ neurons to acute alcohol in males but not females.
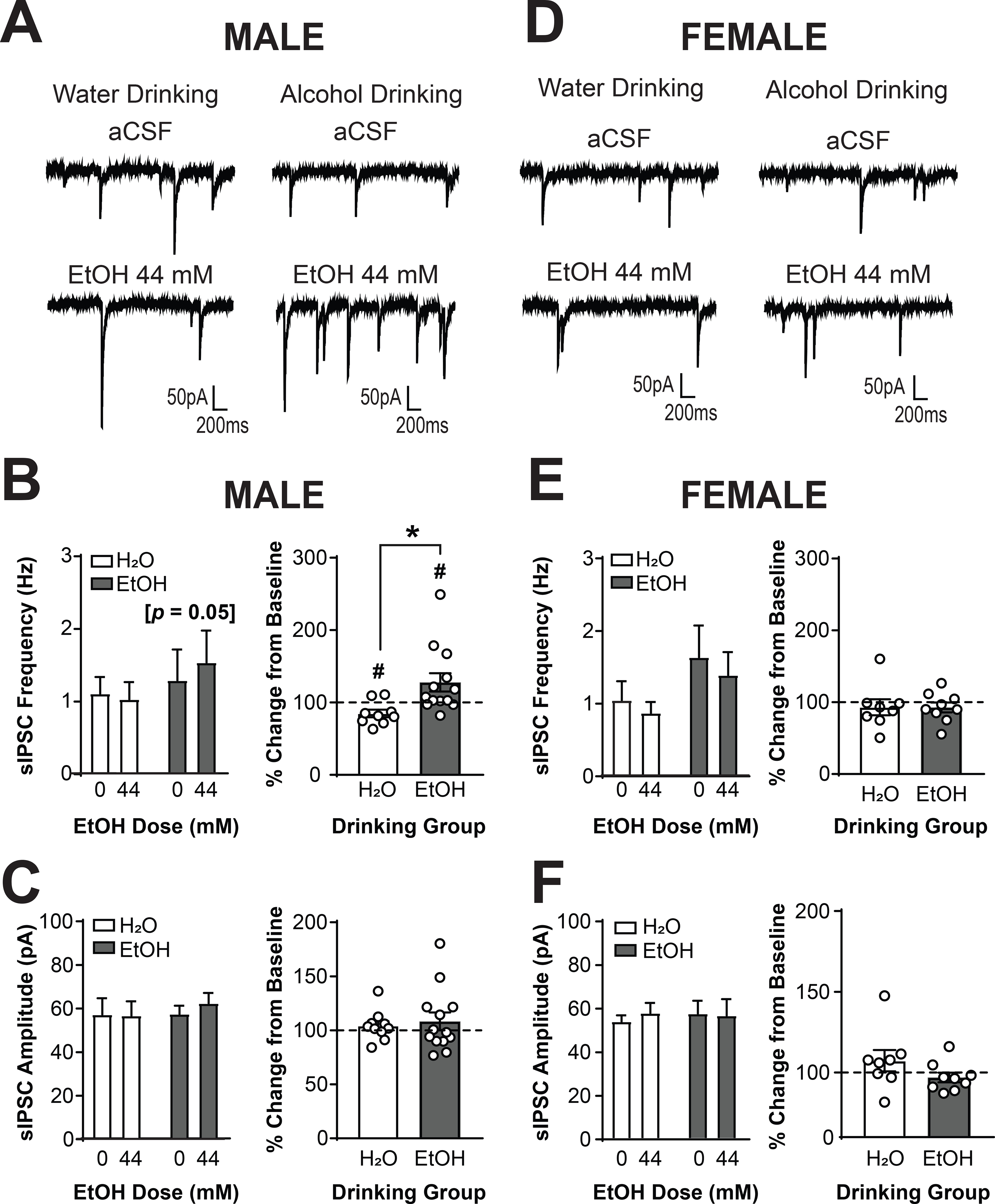
(A) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or alcohol (44 mM; bottom). (B) Summary of effects of acute alcohol on sIPSC frequency (left) and effects of alcohol normalized into percent change from baseline (right). CRF1+ neurons from alcohol drinking mice exhibited a significantly increased sIPSC frequency in response to acute alcohol whereas CRF1+ neurons from water drinking mice did not ([*]p = 0.05 by Sidak’s multiple comparisons test). A significant difference between drinking groups emerged (*p < 0.05 by two-tailed t-test). CRF1+ neurons from water drinking mice exhibited a significant decrease from baseline in response to acute alcohol, whereas CRF1+ neurons from alcohol drinking mice exhibited a significant increase from baseline in response to acute alcohol (#p < 0.05 by one-sample t-test). (C) Summary of the effects of acute alcohol on sIPSC amplitude (left) and effects represented as percent change from baseline (right). sIPSC amplitude was not affected by acute alcohol superfusion in male water or alcohol drinking mice. (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or alcohol (44 mM; bottom). (E) Summary of the effects of acute alcohol on sIPSC frequency (left) and effects represented as percent change from baseline (right). sIPSC frequency was not affected by acute alcohol superfusion in female water or alcohol drinking mice. (F) Summary of the effects of acute alcohol on sIPSC amplitude (left) and effects represented as percent change from baseline (right). sIPSC amplitude was not affected by acute alcohol superfusion in female water or alcohol drinking mice.
Voluntary alcohol drinking enhances CRF signaling in CRF1+ CeA neurons
We next determined the effects of focal CRF (200 nM) application on CRF1+ neurons from male water (n = 10 cells from 6 mice) and alcohol drinking mice (n = 10 cells from 5 mice) and female water (n = 12 cells from 7 mice) and alcohol drinking mice (n = 12 cells from 6 mice). Analysis via Three-Way RM ANOVA revealed main effects of CRF dose [F(1,38) = 28.55, p <0.0001] and drinking group [F(1,38) = 5.38, p = 0.0259] as well as interactions of CRF dose X sex [F(1,38) = 6.71, p = 0.0135] and CRF dose X drinking group [F(1,38) = 6.91, p = 0.0123], indicating that the effects of CRF on sIPSC frequency depended upon both sex and alcohol drinking history. To understand the interactions and visualize the results, data were also analyzed via two-way RM ANOVA within each sex. In males (Figure 4A), CRF produced a dose X drinking group interaction on sIPSC frequency [F(1,16) = 4.201; p = 0.0572; Figure 4B, left]. Analysis with Sidak’s multiple comparisons test indicated that CRF significantly increased sIPSC frequency in CRF neurons from alcohol drinking mice (p = 0.0002), but neurons from water-drinking mice only exhibited a trend for increased sIPSC frequency (p = 0.0776). When data were analyzed as percent change from baseline, no significant between-group differences emerged [t(10.03) = 1.451; p = 0.1773]. CRF increased sIPSC frequency in neurons from water-drinking [161.1 ± 15.585; t(8) = 3.922, p = 0.0044] and alcohol-drinking [228.0 ± 43.38%; t(8) = 2.951, p = 0.0184; Figure 4B, right]. Together, these findings suggest that CRF enhances presynaptic GABA release onto CRF1+ neurons from male mice, and that alcohol drinking enhances this effect. In female mice (Figure 4D), a main effect of CRF emerged [F(1,22) = 4.46; p = 0.0563; Figure 4E, left], indicating that CRF increased sIPSC frequency in neurons from both drinking groups. When data were normalized against baseline, however, a significant difference between drinking groups was evident [t(22) = 2.083, p = 0.0491; Figure 4E, right]. Neurons from alcohol drinking mice exhibited a significant increase from baseline in sIPSC frequency [170.7 ± 19.15%; t(11) = 3.694, p = 0.0035] whereas neurons from water drinking mice did not [118% ± 16.35%; t(11) = 1.118, p = 0.2874]. Therefore, a history of voluntary alcohol drinking enhances the sensitivity of CRF1+ neurons from both male and female mice to the effects of acute CRF application on presynaptic GABA release onto CRF1+ neurons.
Figure 4. Voluntary alcohol drinking sensitizes male and female CRF1+ neurons to the effects of CRF.
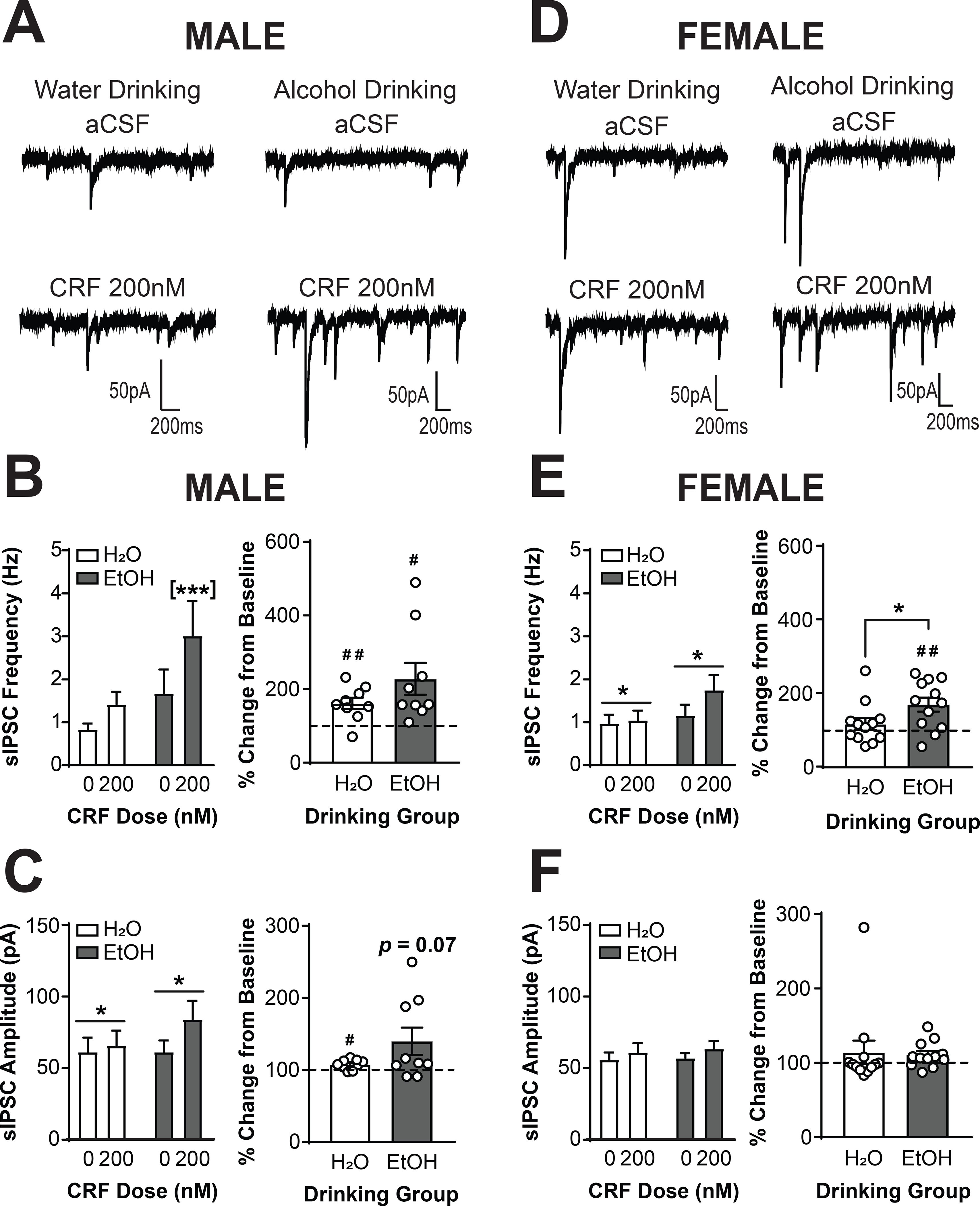
(A) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or CRF (200 nM; bottom). (B) Summary of the effects of CRF on sIPSC frequency (left) and effects of CRF normalized into percent change from baseline (right). CRF1+ neurons from alcohol drinking mice exhibited a significant increase in sIPSC frequency whereas CRF1+ neurons from water drinking mice did not ([***]p < 0.001 by Sidak’s multiple comparisons test). When data were transformed into percent change from baseline, CRF1+ neurons from water and alcohol drinking mice demonstrated an increase in frequency from baseline (#p < 0.05, ##p < 0.01 by one-sample t-test). (C) Summary of the effects of CRF on sIPSC amplitude (left) and effects of CRF normalized into percent change from baseline (right). Focal application of CRF enhanced sIPSC amplitude in male CRF1+ neurons (*p < 0.05 by two-way RM ANOVA). CRF1+ neurons from water drinking mice demonstrated a significant increase from baseline (#p < 0.05 by one-sample t-test). CRF1+ neurons from alcohol drinking mice demonstrated a trend for an increase from baseline (p = 0.07 by one-sample t-test). (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or CRF (200 nM; bottom). (E) Summary of the effects of CRF on sIPSC frequency (left) and effects of CRF normalized into percent change from baseline (right). Focal application of CRF enhanced sIPSC frequency in female CRF1+ neurons in both drinking groups (*p < 0.05 by two-way RM ANOVA). However, when data were transformed into percent change from baseline, a significant difference between drinking groups emerged (*p < 0.05 by two-tailed t-test). CRF1+ neurons from water drinking mice were insensitive to focal application of CRF whereas CRF1+ neurons from alcohol drinking mice exhibited a significant increase in sIPSC frequency from baseline (##p < 0.01 by one-sample t-test). (F) Summary of the effects of CRF on sIPSC amplitude (left) and effects represented as percent change from baseline (right). sIPSC amplitude was not affected by CRF superfusion in female water or alcohol drinking mice.
Analysis via Three-Way RM ANOVA also revealed a main effect of CRF on sIPSC amplitude [F(1, 37) = 9.533, p = 0.0038], indicating a similar effect of CRF on sIPSC amplitude in both sexes and both drinking conditions. When analyzed by two-way RM ANOVA, CRF application increased sIPSC amplitude in cells from male CRF1+ neurons [F(1,15) = 6.925, p = 0.0189; Figure 4C, left]. When normalized against baseline, no between-group differences emerged [t(8.237) = 1.671, p = 0.1321; Figure 4C, right]. The sIPSC amplitude increase in neurons from water drinking mice was significant but modest (107.6 ± 2.314%; t(8) = 3.3, p = 0.0109), whereas the increase in neurons from alcohol drinking mice was greater but only trended towards significance [139.7 ± 19.02%; t(8) = 2.085, p = 0.0705; Figure 4C, right]. This finding indicates an increased postsynaptic response following CRF application in male neurons, which may be driven primarily by large effects in neurons from alcohol-drinking males. CRF application in female mice did not significantly alter sIPSC amplitude in either drinking group (p > 0.05 for all comparisons; Figure 4F), suggesting that the main effect in the Three-Way RM ANOVA was driven primarily by neurons from male mice. Together, these findings suggest that CRF enhances the postsynaptic response to GABA in CRF1+ neurons, although this effect is likely driven by large effects in the male EtOH drinking mice.
Sex differences in activity and alcohol sensitivity of the CRF1 receptor
To establish whether alcohol drinking produced differences in activity at the CRF1 receptor, we assessed the effects of the CRF1 antagonist R121919 (1μM) on sIPSCs in CRF1+ neurons from male water (n= 7 cells from 5 mice) and alcohol drinking mice (n= 7 cells from 3 mice) and female water (n= 11 cells from 8 mice) and alcohol drinking mice (n=10 cells from 7 mice). Analysis via Three-Way RM ANOVA revealed main effects of R121919 dose [F(1, 32) = 7.10, p = 0.0120] and drinking group [F(1, 32) = 4.90, p = 0.0341]. To understand and visualize the effects, data were also analyzed via Two-Way RM ANOVA within each sex. In male mice (Figure 5A), R121919 failed to significantly alter sIPSC frequency (p > 0.05 for all comparisons; Figure 5B). In female mice (Figure 5D), R121919 significantly reduced sIPSC frequency in both drinking groups [main effect of R121919, F(1,19) = 14.19, p = 0.0013; Figure 5E, left]. When data were normalized as percent change from baseline the magnitude of the reduction was similar in both drinking groups (p > 0.05, Figure 5E, right), although CRF1+ neurons from alcohol drinking female mice showed a significant reduction from baseline [82.07 ± 7.50%; t(9) = 2.39, p = 0.0406] whereas the reduction in CRF1+ neurons from water drinking female mice was not significantly different than baseline [85.13 ± 10.93%; p > 0.05]. This suggests that the main effect of R121919 dose seen in the Three-Way RM ANOVA analysis was driven primarily by effects in female CRF1+ neurons. R121919 did not alter sIPSC amplitude in CRF1+ neurons from either drinking group in both sexes (p > 0.05 for all comparisons; Figure 5C and 5F).Together, these findings indicate that the CRF1 receptor regulates basal GABA release onto CRF1+ neurons, an effect which may be driven by large effect sizes in females, and that voluntary alcohol drinking does not alter this regulation.
Figure 5. The CRF1 receptor regulates inhibitory control of female but not male CRF1+ neurons.
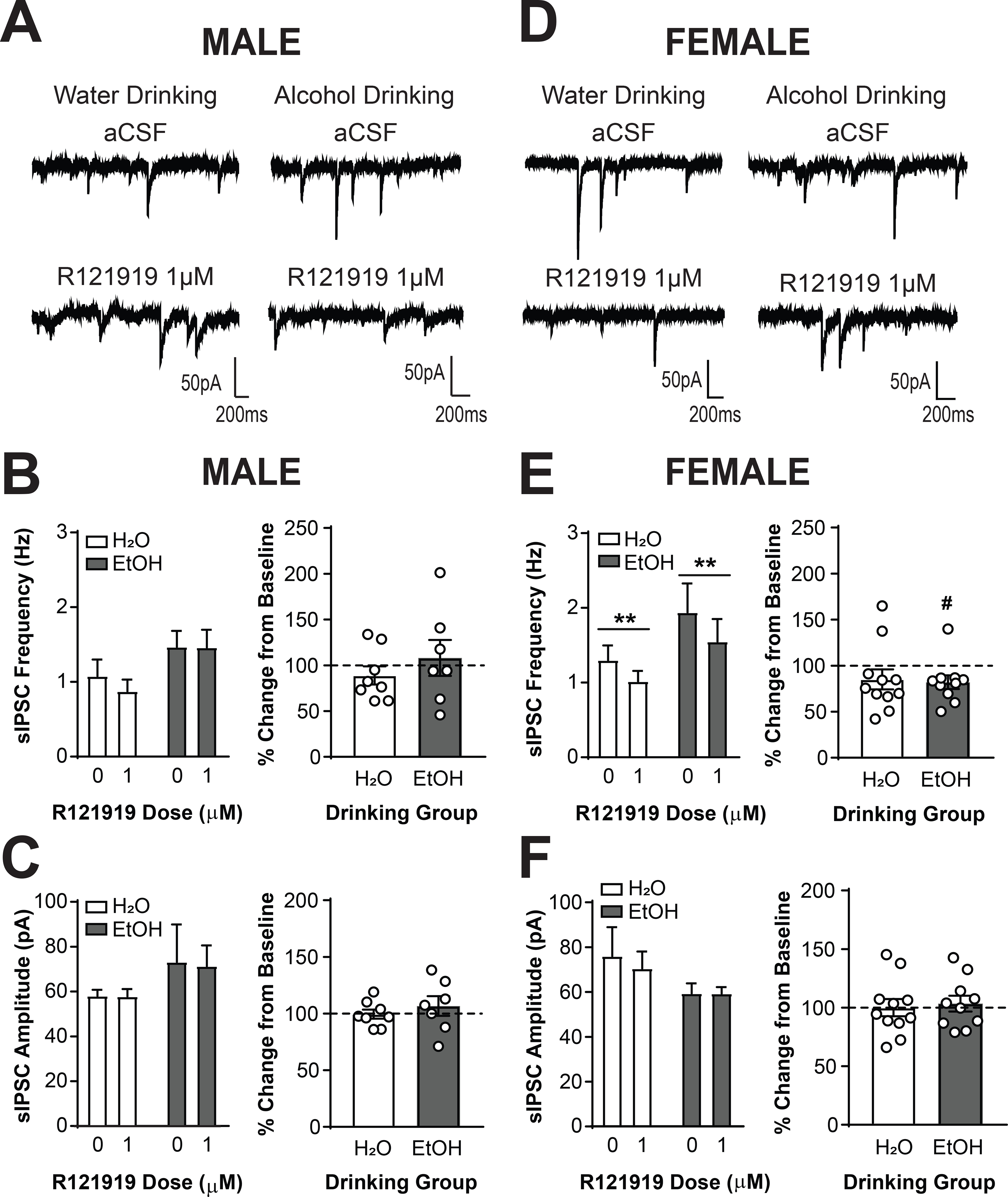
(A) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or R121919 (1 μM, bottom). (B) Summary of the effects of R121919 on sIPSC frequency (left) and effects of R121919 normalized into percent change from baseline (right). Focal application of R121919 did not alter sIPSC frequency in male CRF1+ neurons from both drinking groups. (C) Summary of the effects of R121919 on sIPSC amplitude (left) and effects of R121919 normalized into percent change from baseline (right). Focal application of R121919 did not alter sIPSC amplitude in male CRF1+ neurons from both drinking groups. (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or R121919 (1 μM, bottom). (E) Summary of the effects of R121919 on sIPSC frequency (left) and effects of R121919 normalized into percent change from baseline (right). Focal application of R121919 significantly reduced sIPSC frequency in female CRF1+ neurons from both drinking groups (**p < 0.01 by two-way RM ANOVA). When data were normalized to percent change from baseline, no significant difference in effect size between groups emerged but, CRF1+ neurons from alcohol drinking mice exhibited a significant decrease in sIPSC frequency from baseline whereas CRF1+ neurons from water drinking mice did not (#p > 0.05 by one-sample t-test). (F) Summary of the effects of R121919 on sIPSC amplitude (left) and effects of R121919 normalized into percent change from baseline (right). Focal application of R121919 did not alter sIPSC amplitude in female CRF1+ neurons from both drinking groups.
We next determined the extent to which the effects of focal CRF application involved the CRF1 receptor by pretreating slices with R121919 (1μM) prior to co-application of R121919 and CRF (200 nM). CRF1+ neurons from male mice drinking water (n = 6 cells from 4 mice) or alcohol (n = 6 cells from 3 mice) and female mice drinking water (n = 11 cells from 8 mice) or alcohol (n = 8 cells from 6 mice) were sampled. Analysis via Three-way RM ANOVA revealed a main effect of CRF dose [F(1,27) = 13.12, p = 0.0012], a CRF dose X drinking group interaction [F(1,27) = 7.21, p = 0.0122], and a CRF dose X sex X drinking group interaction [F(1,27) = 5.62, p = 0.0251], indicating that the effects of CRF depended upon both sex and drinking group. To understand the interactions and visualize the data, data were also analyzed via Two-Way RM ANOVA within each sex. In pretreated CRF1+ neurons from male mice (Figure 6A), co-application of CRF + R121919 produced a dose X drinking group interaction [F(1,10) = 8.412, p = 0.0158; Figure 6B, left]. Analysis with Sidak’s multiple comparisons test indicated that CRF still enhanced sIPSC frequency in CRF1+ neurons from water-drinking mice (p = 0.0351). However, CRF did not elicit an increase in sIPSC frequency in CRF1+ neurons from alcohol-drinking mice despite the enhanced effect of CRF we had previously observed in this population (p = 0.4126). When data were analyzed as a percent change from baseline, this differential response between drinking groups was also evident [t(10) = 2.24, p = 0.0490; Figure 6B, right]. CRF significantly increased sIPSC frequency from baseline in CRF1+ neurons from water-drinking males [146.2 ± 17.04%; t(5) = 2.713, p = 0.0421] but not in CRF1+ neurons from alcohol-drinking males [96.06 ± 14.54%; t(5) = 0.2709, p = 0.7973]. Therefore, the alcohol drinking-induced potentiation of the effects of CRF in male CRF1+ neurons requires the CRF1 receptor, and alcohol drinking produced a more prominent role for the CRF1 receptor in the effects of exogenous CRF. In female mice (Figure 6D), CRF1+ neurons from both drinking groups still displayed a CRF-induced increase in sIPSC frequency in the presence of R121919 [main effect of CRF, F(1,17) = 21.08, p = 0.0003; Figure 6E, left]. When data were analyzed as percent change from baseline, the increase in sIPSC frequency induced by CRF + R121919 was of comparable magnitude in both drinking groups [t(11.15) = 1.711, p = 0.1147; Figure 6E, right]. CRF in the presence of R121919 significantly increased sIPSC frequency from baseline in CRF1+ neurons from water [166.8 ± 22.93%; t(10) = 2.914, p = 0.0155] and alcohol-drinking [126.4 ± 5.56%; t(7) = 4.761, p = 0.0021] female mice. Taken together, these findings suggest a sex-specific recruitment of CRF1 signaling in male but not female CRF1+ neurons following voluntary alcohol drinking that functionally regulates CRF-induced changes in presynaptic GABA release. Analysis of sIPSC amplitude by both Three-Way and Two-Way RM ANOVA indicated no effects of CRF in the presence of R121919 in either sex (p > 0.05 for all comparisons; Figure 6C and 6F), suggesting that the effects of CRF on sIPSC amplitude seen in male CRF1+ neurons (Figure 4C) require the CRF1 receptor.
Figure 6. The CRF1 receptor is required for alcohol-induced plasticity of CRF signaling in male but not female CRF1+ neurons.
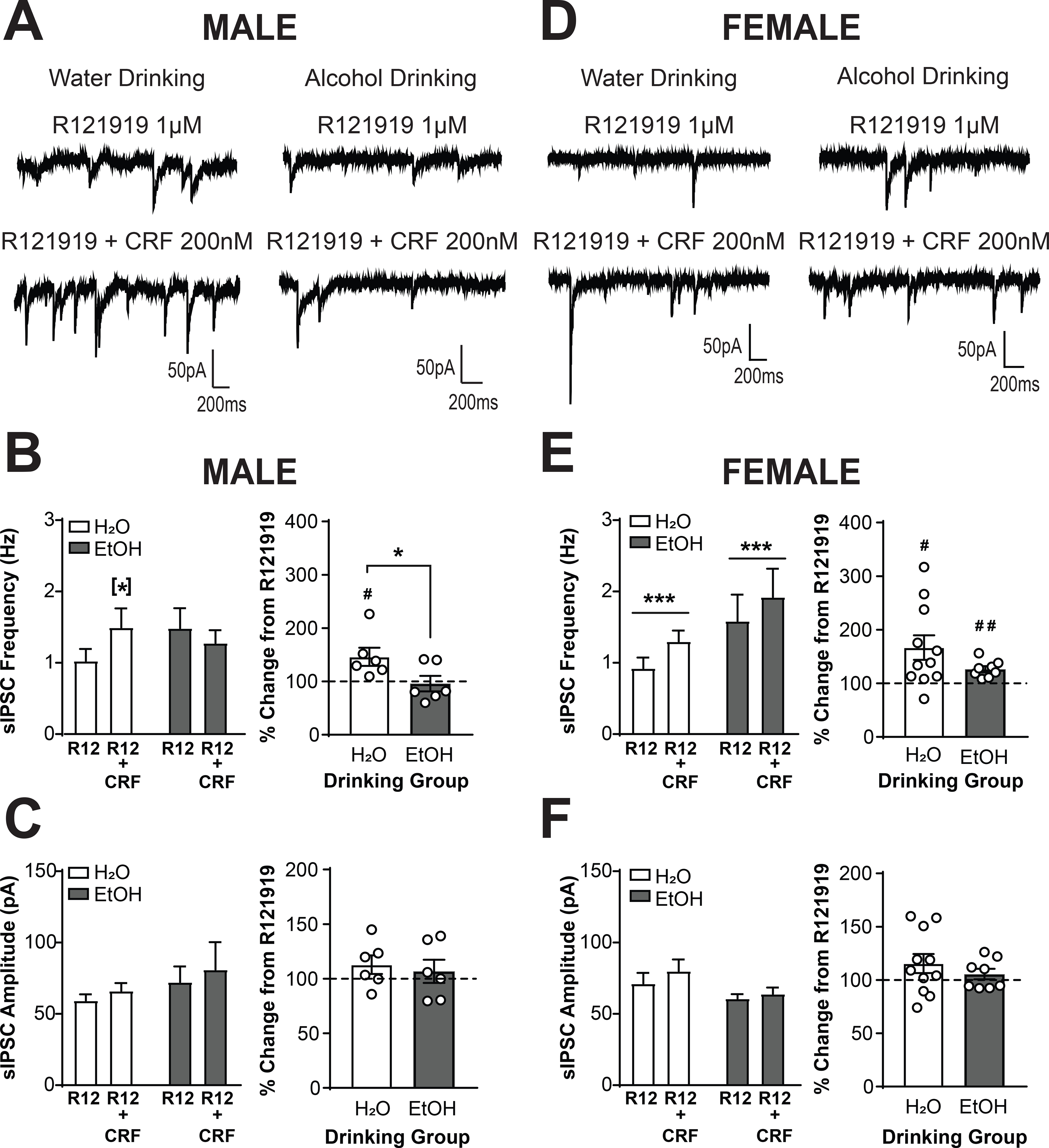
(A) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (left) or alcohol (right) during superfusion of R121919 (1 μM, top) or R121919 + CRF (200 nM; bottom). The traces shown are from the same neurons as depicted in Figure 5A. (B) Summary of the effects of R121919+CRF on sIPSC frequency (left) and effects of R121919+CRF normalized into percent change from baseline (right). CRF1+ neurons from water drinking mice exhibited a significant increase in sIPSC frequency whereas CRF1+ neurons from alcohol drinking mice did not ([*]p < 0.05 by Sidak’s multiple comparisons test). When data were normalized to percent change from R121919 alone, a significant difference between drinking groups emerged (*p < 0.05 by two-tailed t-test). In CRF1+ neurons from water drinking mice, CRF still induced a significant increase from baseline in the presence of R121919 whereas in CRF1+ neurons from alcohol drinking mice CRF failed to stimulate an increase in sIPSC frequency from baseline (#p < 0.05 by one-sample t-test). (C) Summary of the effects of R121919+CRF on sIPSC amplitude (left) and effects of R121919+CRF normalized into percent change from baseline (right).CRF failed to increase sIPSC amplitude in male CRF1+ neurons from both drinking groups following R121919 pretreatment. (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (left) or alcohol (right) during superfusion of R121919 (1 μM, top) or R121919 + CRF (200 nM; bottom). The traces shown are from the same neurons depicted in Figure 5D. (E) Summary of the effects of R121919+CRF on sIPSC frequency (left) and effects of R121919+CRF normalized into percent change from baseline (right). Co-application of CRF + R121919 following R121919 pretreatment significantly increased sIPSC frequency in female CRF1+ neurons from water and alcohol drinking mice (***p < 0.001 by two-way RM ANOVA). When data were normalized to percent change from baseline, no difference between drinking groups was evident. CRF still induced a significant increase in sIPSC frequency from baseline in the presence of R121919 in CRF1+ neurons from water drinking and alcohol drinking mice (#p < 0.05, ##p > 0.01 by one-sample t-test). (F) Summary of the effects of R121919+CRF on sIPSC amplitude (left) and effects of R121919+CRF normalized into percent change from baseline (right).CRF failed to increase sIPSC amplitude in female CRF1+ neurons from both drinking groups following R121919 pretreatment.
Sex differences in activity, but not alcohol sensitivity, of the CRF2 receptor
To establish whether alcohol drinking altered activity at the CRF2 receptor, we assessed the effects of the CRF2 antagonist Astressin 2B (200 nM) on sIPSCs in CRF1+ neurons from male mice drinking water (n = 9 cells from 4 mice) or alcohol (n = 11 cells from 5 mice) and female mice drinking water (n= 9 cells from 4 mice) or alcohol (n = 10 cells from 6 mice). Analysis via Three-Way RM ANOVA revealed a main effect of Astressin 2B dose [F(1,40) = 6.48, p = 0.0149]. To understand and visualize the effects, data were also analyzed via Two-Way RM ANOVA within each sex. In male mice (Figure 7A), Astressin 2B reduced sIPSC frequency in both drinking groups [main effect of Astressin 2B, F(1,23) = 5.368, p =0.0298; Figure 7B, left]. However, when data were normalized as percent change from baseline, a differential response by drinking group was evident [t(23) = 2.349, p = 0.0278; Figure 7B, right]. CRF1+ neurons from water-drinking mice did not exhibit a significant change from baseline in sIPSC frequency [104.0 ± 10.25%; t(10) = 0.3917, p = 0.7035], whereas CRF1+ neurons from alcohol-drinking mice exhibited a significant reduction from baseline in sIPSC frequency [78.13 ± 5.571%; t(13) = 3.926, p = 0.0017]. These findings indicate that the CRF2 receptor regulates baseline presynaptic GABA release onto male CRF1+ neurons, and this CRF2 regulation may be enhanced by alcohol drinking. Conversely, in female mice (Figure 7D) Astressin 2B (200nM) did not alter sIPSC frequency (p > 0.05 for all comparisons, Figure 7E) in either drinking group. These findings indicate that the main effect of Astressin 2B seen in the Three-Way RM ANOVA was predominantly driven by effects in male CRF1+ neurons. In both male and female neurons, CRF2 antagonism did not produce any significant changes in sIPSC amplitude in either drinking group (p > 0.05 for all comparisons; Figure 7C and 7F), although there was a trend for a small between drinking group difference in percent change from baseline in male CRF1+ neurons [t(14.46) = 1.981, p = 0.0669; Figure 7C, right].Together, these findings indicate that the CRF2 receptor regulates baseline GABA release onto CRF1+ neurons, and that this regulation is more pronounced in male versus female neurons.
Figure 7. The CRF2 receptor regulates inhibitory control in male but not female CRF1+ neurons.
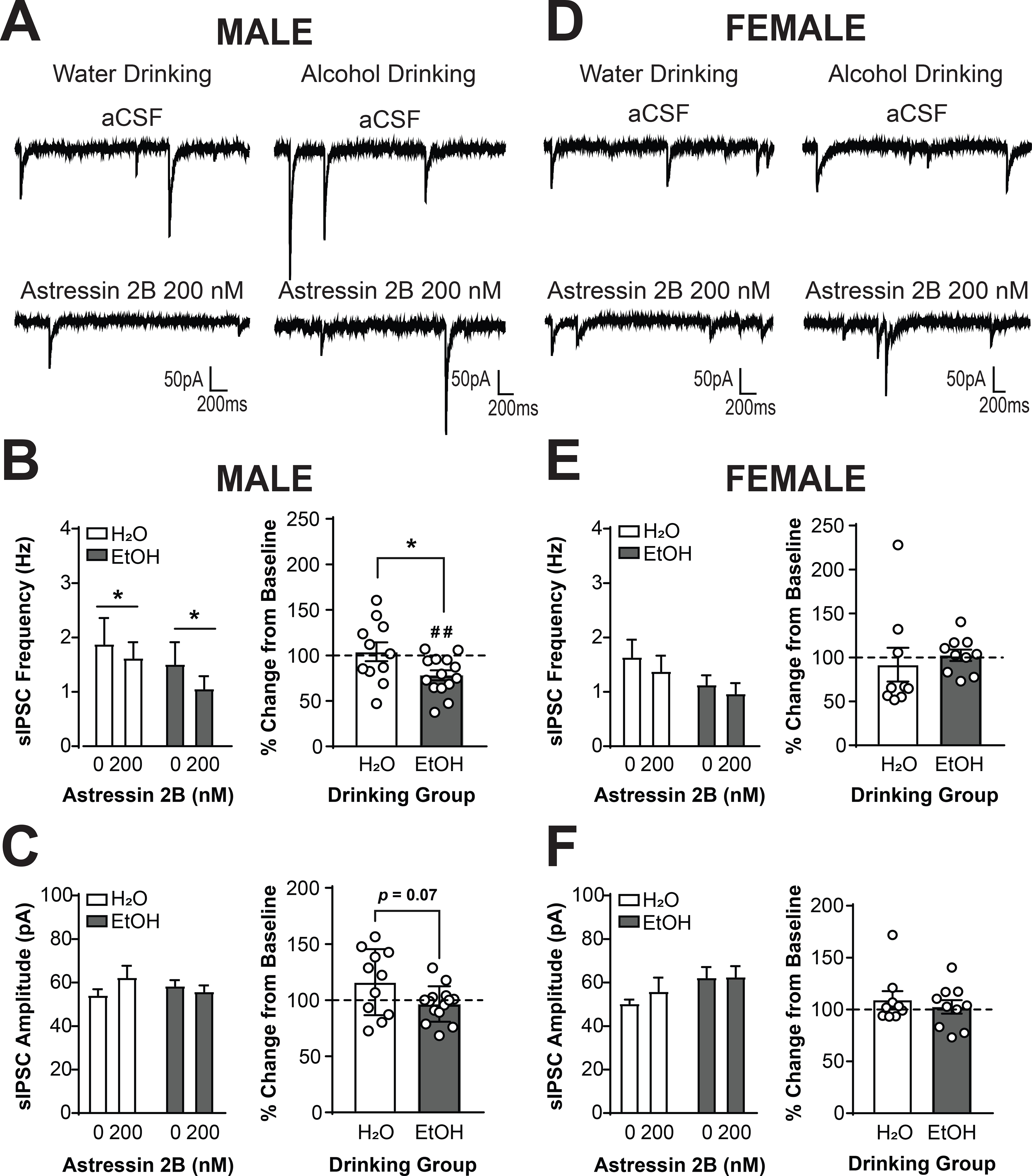
(A) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or Astressin 2B (200nM, bottom). (B) Summary of the effects of Astressin 2B on sIPSC frequency (left) and effects of Astressin 2B normalized into percent change from baseline (right). Focal application of Astressin 2B significantly reduced sIPSC frequency in male CRF1+ neurons (*p < 0.05 by two-way RM ANOVA). When data were normalized to percent change from baseline, a significant difference between drinking groups emerged (*p < 0.05 by two-tailed t-test). In CRF1+ neurons from water drinking mice, Astressin 2B did not produce a change from baseline in sIPSC frequency whereas in CRF1+ neurons from alcohol drinking mice Astressin 2B produced a significant decrease in sIPSC frequency from baseline (##p < 0.01 by one-sample t-test). (C) Summary of the effects of Astressin 2B on sIPSC amplitude (left) and effects of Astressin 2B normalized into percent change from baseline (right). Astressin 2B (200 nM) did not alter sIPSC amplitude in male CRF1+ neurons from either drinking groups. A trend towards a difference in percent change in amplitude was observed between drinking groups (p = 0.07), but neither group exhibited a substantial change from 100%. (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (left) or alcohol (right) during superfusion of aCSF (top) or Astressin 2B (200 nM, bottom). (E) Summary of the effects of Astressin 2B on sIPSC frequency (left) and effects of Astressin 2B normalized into percent change from baseline (right).Focal application of Astressin 2B did not alter sIPSC frequency in female CRF1+ neurons from either drinking group. (F) Summary of the effects of Astressin 2B on sIPSC amplitude (left) and effects of Astressin 2B normalized into percent change from baseline (right). Astressin 2B also did not alter sIPSC amplitude in female CRF1+ neurons from either drinking group.
We next determined the extent to which the effects of focal CRF application involved the CRF2 receptor by pretreating slices with Astressin 2B (200 nM) prior to co-application of Astressin 2B and CRF (200 nM). CRF1+ neurons from male mice drinking water (n = 9 cells from 3 mice) or alcohol (n = 11 cells from 4 mice) and female mice drinking water (n = 8 cells from 4 mice) or (n = 7 cells from 4 mice) were sampled. Analysis via Three-Way RM ANOVA revealed a main effect of CRF dose [F(1,31) = 22.00, p < 0.0001]. To understand and visualize the effects, data were also analyzed within each sex via Two-Way RM ANOVA. In pretreated CRF1+ neurons from water drinking male mice (Figure 8A), CRF + Astressin 2B increased sIPSC frequency [main effect of CRF, F(1,18) = 23.64, p = 0.0001; Figure 8B, left]. When data were normalized to percent change from Astressin 2B alone, a different response between drinking groups was evident [t(13.11) = 2.21, p = 0.0455; Figure 8B, right]. CRF significantly increased sIPSC frequency in CRF1+ neurons from water-drinking mice [135.7 ± 11.66%; t(8) = 3.06, p = 0.0156], but the increase in alcohol-drinking mice was larger [204.3 ± 28.79%; t(10) = 3.624, p = 0.0047]. These findings indicate that the effects of CRF on sIPSC frequency, and the enhanced effect of CRF in CRF1+ neurons from alcohol drinking males, do not require the CRF2 receptor. Similarly, Astressin 2B (200nM) pretreatment was insufficient to block the CRF-induced increase in sIPSC frequency in female CRF1+ neurons (Figure 8D) from both drinking groups [main effect of CRF, F(1,13) = 5.905, p = 0.0303; Figure 8E, left]. The increase in sIPSC frequency induced by CRF + Astressin 2B was of comparable magnitude in both drinking groups (p > 0.05; Figure 8E, right), consistent with the effects of CRF alone. The increase in water drinking females was large but not significantly different from baseline [172.1 ± 49.06%; t(7) = 1.469, p = 0.1852] whereas the increase in alcohol drinking females was visually greater and significantly increased from baseline [250.7 ± 58.06%; t(6) = 2.596, p = 0.0409]. This result suggests that unlike the CRF1 receptor, the CRF2 receptor is not required for the alcohol drinking-induced enhancement of the effects of CRF on sIPSC frequency.
Figure 8. Alcohol-induced plasticity in the effects of CRF does not require the CRF2 receptor in male and female CRF1+ neurons.
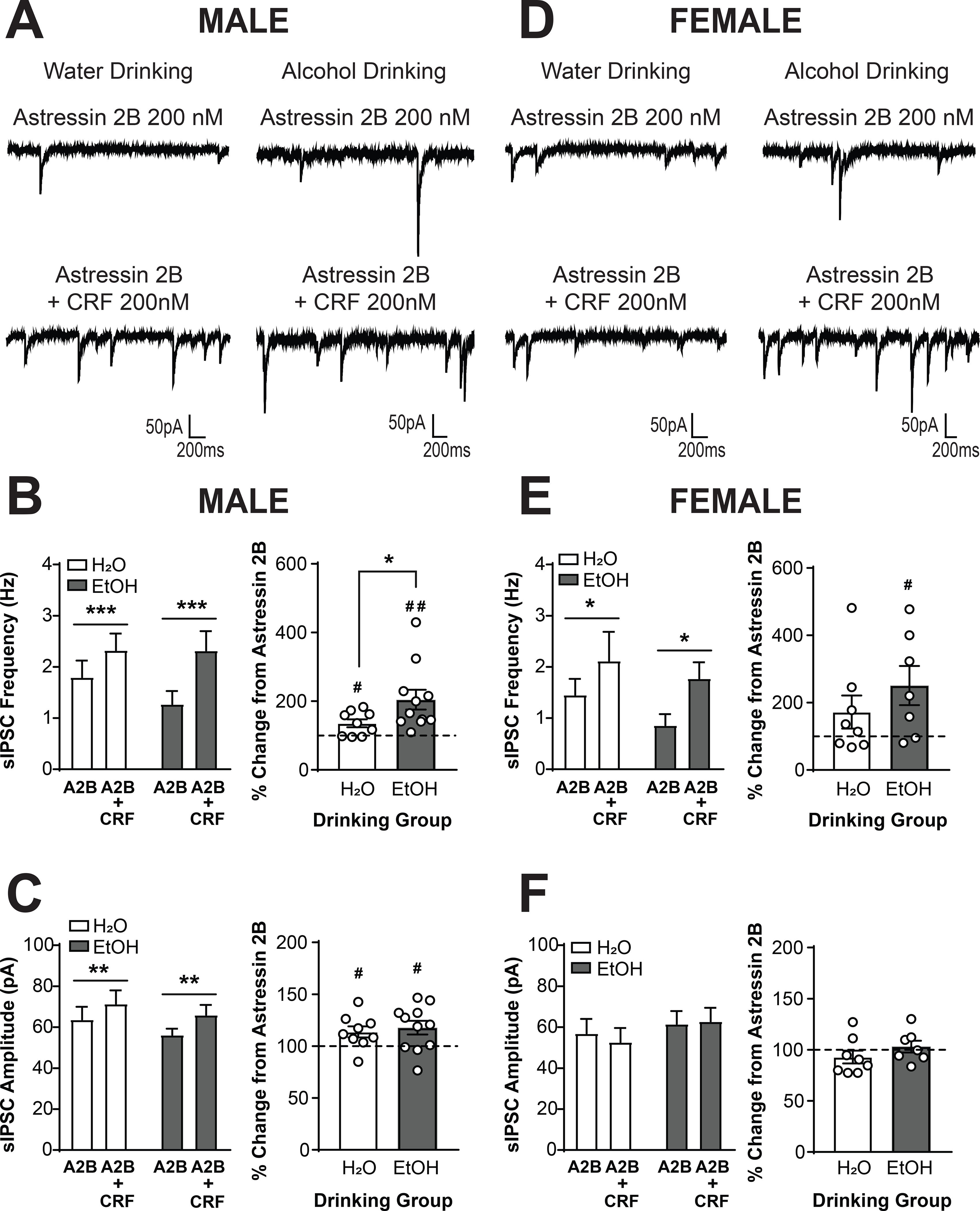
(A) Representative traces of sIPSCs in CRF1+ neurons from male mice drinking water (left) or alcohol (right) during superfusion of Astressin 2B (200 nM, top) or Astressin 2B + CRF (200 nM;bottom). The traces shown are from the same neurons as depicted in Figure 7A. (B) Summary of the effects of Astressin 2B + CRF on sIPSC frequency (left) and effects of Astressin 2B + CRF normalized into percent change from baseline (right). Co-application of CRF + Astressin 2B following Astressin 2B pretreatment significantly increased sIPSC frequency in male CRF1+ neurons from water and alcohol drinking mice (***p < 0.001 by two-way RM ANOVA). When data were normalized to percent change from Astressin 2B alone, a significant difference between drinking groups was evident (*p < 0.05 by two-tailed t-test). CRF induced a significant increase in sIPSC frequency from baseline in the presence of Astressin 2B in CRF1+ neurons from water and alcohol drinking mice, but the increase was significantly greater in neurons from alcohol drinking mice (#p < 0.05 ##p < 0.01 by one-sample t-test). (C) Summary of the effects of Astressin 2B + CRF on sIPSC amplitude (left) and effects of Astressin 2B + CRF normalized into percent change from baseline (right). CRF + Astressin 2B significantly increased sIPSC amplitude in male CRF1+ neurons from both drinking groups (**p < 0.01 by two-way RM ANOVA). When data were normalized to percent change from baseline, CRF still stimulated an increase in sIPSC amplitude in the presence of Astressin 2B in CRF1+ neurons from both water drinking and alcohol drinking mice (#p < 0.05 by one-sample t-test). (D) Representative traces of sIPSCs in CRF1+ neurons from female mice drinking water (left) or alcohol (right) during superfusion of Astressin 2B (200 nM, top) or Astressin 2B + CRF (200 nM; bottom). The traces shown are from the same neurons as depicted in Figure 7D. (E) Summary of the effects of Astressin 2B + CRF on sIPSC frequency (left) and effects of Astressin 2B + CRF normalized into percent change from baseline (right). Co-application of CRF + Astressin 2B following Astressin 2B pretreatment significantly increased sIPSC frequency in female CRF1+ neurons from water and alcohol drinking mice (*p < 0.05 by two-way RM ANOVA). When data were normalized to percent change from baseline, CRF still induced a significant increase in sIPSC frequency from baseline in the presence of R121919 in CRF1+ neurons from alcohol drinking mice ( #p < 0.05 by one-sample t-test). The percent increase from baseline in water drinking mice was not statistically significant, but significant difference in effect size emerged between the two drinking groups. (F) Summary of the effects of Astressin 2B + CRF on sIPSC amplitude (left) and effects of Astressin 2B + CRF normalized into percent change from baseline (right). CRF + Astressin 2B did not alter sIPSC amplitude in female CRF1+ neurons from both drinking groups.
Analysis of sIPSC amplitude data via Three-Way RM ANOVA indicated a CRF dose X sex interaction, F(1,31) = 8.04, p = 0.0080. To understand the interaction and visualize the data, Two-Way RM ANOVA was performed in each sex. In males, CRF still produced an increase in sIPSC amplitude in the presence of Astressin 2B in CRF1+ neurons [main effect of CRF, F(1,18) = 12.78, p = 0.0022; Figure 8C, left]. When data were analyzed as percent change from baseline, this increase was similar in neurons from both drinking groups (p > 0.05; Figure 8C, right]; neurons from water-drinking [113.7 ± 5.375%; t(8) = 2.548, p = 0.0343] and alcohol-drinking [117.7 ± 6.617%; t(10) = 2.68, p = 0.0231] mice both evinced a significant increase from baseline. In female CRF1+ neurons there were no effects of CRF in the presence of Astressin 2B on sIPSC amplitude, consistent with the effects of CRF alone (p > 0.05 for all comparisons; Figure 8E). Taken together, these findings suggest that the CRF2 receptor is not required for the effects of acute CRF on GABA release or postsynaptic response to GABA, nor the enhanced effect of CRF seen in male CRF1+ neurons from alcohol drinking mice.
CRF1 regulation of alcohol drinking in male and female CRF1:GFP mice
CRF1 antagonists have previously been shown to functionally regulate alcohol drinking in male mice (32), but to date their effects in females have not been explored and no study has compared effects in males and females in parallel. We used a systemic injection of the CRF1 antagonist CP-154,526 in male and female CRF1:GFP mice to evaluate the functional contributions of the CRF1 receptor to voluntary alcohol drinking in both sexes. Following baseline voluntary alcohol drinking, male and female CRF1:GFP mice were habituated to i.p. injections and pretreated with the CRF1 antagonist CP-154,526 (10mg/kg) 30 minutes prior to drinking. Consistent with baseline 24-hour intake, female CRF1:GFP mice consumed more alcohol than male mice at the 2-hour interval [F(1,18) = 7.604, p = 0.0130; Figure 9A]. CP-154,526 also reduced alcohol drinking in both sexes [F(1,18) = 29.28, p = 0.0001; Figure 9A]. Post-hoc t-tests evaluating the effects of CP-154,526 in each sex indicated that the reduction was not significant in male mice [t(9) = 1.227, p = 0.2508; Figure 9B, top left] but was significant in female mice [t(9) = 5.881, p = 0.0002; Figure 9B, top right] mice. Post-hoc t-tests evaluating sex differences under vehicle and CP-154, 526 conditions indicated that the sex differences evident after vehicle treatment [t(18) = 2.552, p = 0.0200; Figure 9B, bottom left] were abolished by CP-154,526 treatment [t(18) = 1.491, p = 0.1532; Figure 9B, bottom right]. Treatment with CP-154,526 was not associated with changes in water intake (p > 0.05 for all comparisons; Figure 9C), locomotor activity (p > 0.05 for all comparisons; Figure 9D), or anxiety-like behavior (p > 0.05 for all comparisons; Figure 9E). Together, these findings indicate that the CRF1 receptor functionally regulates alcohol drinking in both male and female mice and may contribute to sex differences in alcohol intake.
Figure 9. The CRF1 antagonist CP-154,526 abolishes sex differences in voluntary alcohol drinking.
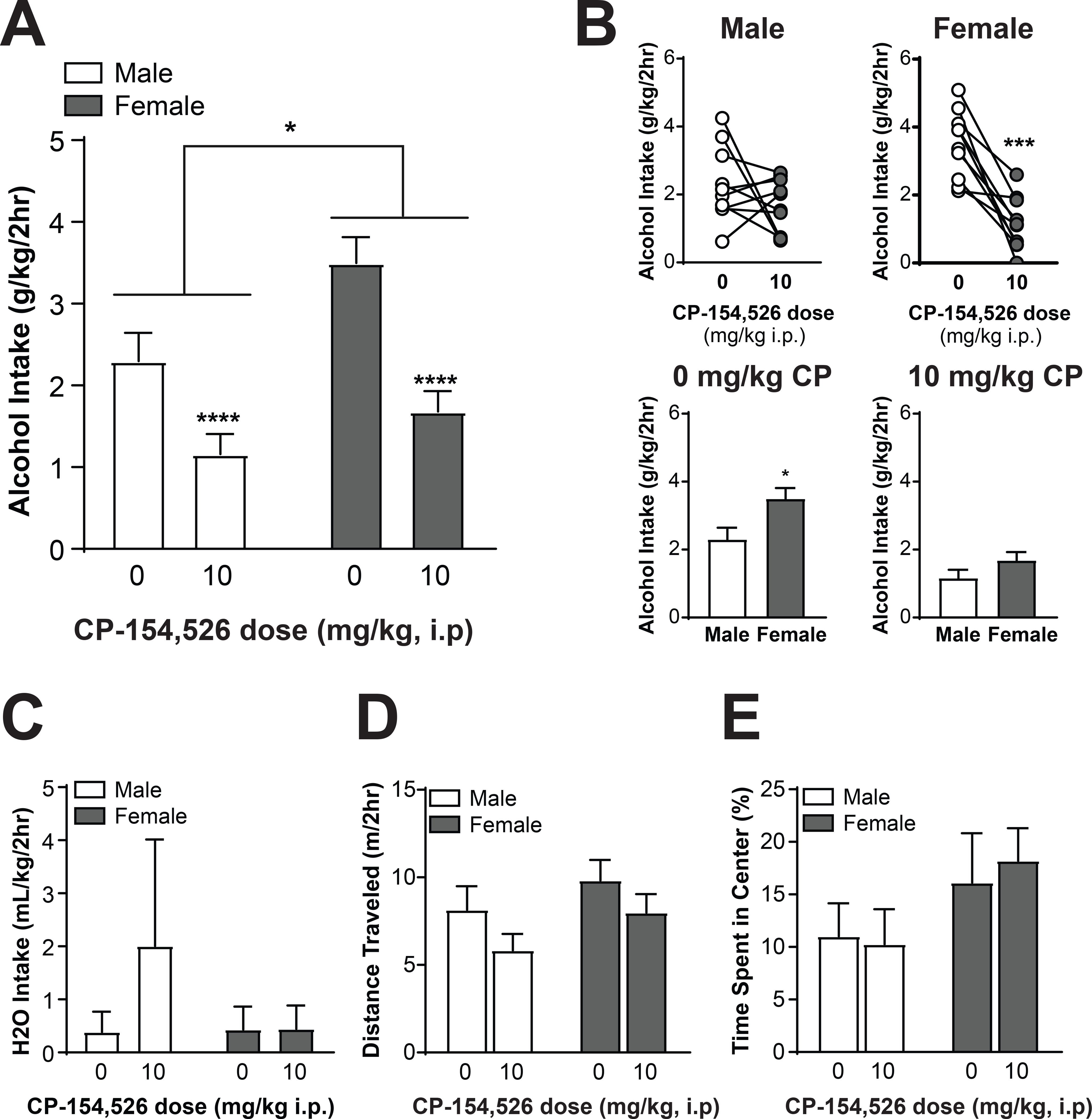
(A) Summary of the effects of CP-154,526 on 2-hour alcohol intake in male and female CRF1:GFP mice. Female alcohol intake was greater than male alcohol intake, and pretreatment with the CRF1 antagonist CP-154,526 (10 mg/kg) reduced alcohol intake in male and female CRF1:GFP mice (*p = 0.05,****p < 0.0001 by two-way RM ANOVA). (B) Summary of the effects of CP-154,526 on alcohol intake within sex (top) and within dose (bottom). When the same intake data were assessed within male subjects (top left), no significant effect of CP-154,526 pretreatment emerged, with 5 out of 10 subjects exhibiting a decrease and 5 out of 10 subjects exhibiting an increase or no change. When intake data were assessed within female subjects (top right), a significant effect for CP-154,526 to decrease alcohol intake was observed, with 9 out of 10 mice exhibiting a decrease and 1 out of 10 mice exhibiting no change (***p < 0.001 by two-tailed t-test). Under vehicle conditions (bottom left), females consumed more alcohol than males (*p < 0.05 by two-tailed t-test). Following CP-154,526 pretreatment (bottom right), this sex difference was abolished. (C) Summary of the effects of CP-154,526 on water intake in male and female CRF1:GFP mice. No significant effect of CP-154,526 pretreatment emerged in either sex. (D) Open-field locomotor activity following pretreatment with vehicle or CP-154,526 (10 mg/kg). No significant effect of CRF1 antagonism emerged in either sex. (E) Time spent in center of open field following pretreatment with vehicle or CP-154,526 (10 mg/kg). No significant effect of CRF1 antagonism emerged in either sex.
DISCUSSION
These experiments are the first investigation of alcohol-induced functional plasticity of the CeA CRF/CRF1 signaling systems specifically in the CRF1+ population of the CeA. In addition, this is the first report comparing differences in alcohol-induced CeA CRF/CRF1 plasticity in female rodents, and the first report of significant sex differences in the responses of the medial CeA CRF1+ neuronal population to alcohol. Female CRF1:GFP mice consumed significantly more alcohol than male mice when intake was adjusted for body weight, and male but not female CRF1+ neurons exhibited altered sensitivity to the actions of acute alcohol following voluntary alcohol drinking. CRF increased sIPSC frequency in CRF1+ CeA neurons from both sexes, but enhanced sIPSC amplitude in a CRF1-dependent manner in male but not female CRF1+ CeA neurons. Baseline inhibitory control of male CRF1+ neurons is partially regulated by the CRF2 receptor but not the CRF1 receptor, whereas basal inhibitory control of female CRF1+ neurons is mediated by the CRF1 but not the CRF2 receptor. Although both male and female CRF1+ neurons exhibit greater CRF-mediated enhancement of sIPSC frequency following voluntary alcohol drinking, in male neurons this increase involves the CRF1 but not the CRF2 receptor, whereas in female neurons these effects persist independent of CRF1 and CRF2 receptor availability. These sex differences in the effects of alcohol on the CeA CRF system may be related to the sex differences we observed in the ability of systemic CRF1 antagonism to regulate alcohol drinking. Importantly, these sex differences do not appear to be a function of alcohol dose, since female mice achieved a higher dose of alcohol during voluntary drinking but were less sensitive than males to alcohol-induced plasticity in CRF1 activity.
Passive, involuntary chronic intermittent ethanol (CIE) vapor exposure has previously been shown to dysregulate basal GABAergic signaling in the CeA of male rodents (10, 13). In the present study using voluntary alcohol drinking, female mice achieved a higher dose of alcohol than male mice. Prior reports in male and female C57BL/6 mice have shown that blood alcohol concentrations in this strain generally correlate with alcohol intake, with female blood alcohol correspondingly higher than male blood alcohol when sex differences are present (35–38). Thus, the increased alcohol dose consumed by female mice likely corresponds to a higher blood and brain concentration of alcohol in female versus male mice. Despite these sex differences in alcohol dose, there were no changes in baseline GABAA-mediated inhibitory control of CRF1+ CeA neurons from either sex. There are also several important differences between the models, including voluntary versus involuntary exposure, engagement of stress circuitry, and the presence of pyrazole in alcohol vapor chambers. Additionally, in the present experiments mice were singly-housed in order to allow individual fluid intake to be recorded. Social isolation is a stressor in rodents (39), and the CRF circuitry can be engaged by isolation stress (18). All subjects in the present experiments were singly housed to allow comparisons without the confounding variable of housing status, but isolation is an important difference between the present findings and prior reports using CIE. In addition, the CIE procedure involves much higher levels of ethanol exposure, including greater levels of intoxication and more regular exposure (repeated days/week over a period of 4–5 weeks). Despite these differences, we do report alterations in the effects of acute alcohol (males only), an increased sensitivity to the effects of CRF (both sexes) and a recruitment of CRF1 signaling (males only) following voluntary alcohol drinking, which are consistent with prior reports using alcohol vapor exposure in males (10, 13). In CRF1+ neurons from alcohol-naïve male mice, acute alcohol application tended to non-significantly reduce sIPSC frequency, consistent with prior reports (12, 13). However, in neurons from alcohol drinking males, acute alcohol enhanced sIPSC frequency. This alcohol drinking-induced reversal of the actions of acute alcohol in the CRF1+ population is consistent with earlier reports that chronic alcohol vapor exposure produces a functional switch in the tonic conductance of male CeA neurons, with CRF1+ neurons specifically undergoing a loss of tonic inhibition following chronic alcohol (13). Importantly, the alcohol-induced adaptation we observed in male mice was absent in CRF1+ neurons from female mice; neither neurons from water nor alcohol drinking female mice exhibited acute alcohol sensitivity. Functional alcohol-induced plasticity of the CeA CRF system is in part what has made this circuitry an attractive target for therapeutic intervention in AUD; an absence of these neuroadaptations in females may be a significant obstacle in the ability of such interventions to translate to human patients, particularly as one of the two clinical trials using CRF1 antagonists was conducted exclusively in women (2).
We also identified sex differences in pre- versus postsynaptic regulation of inhibitory control by CRF. Focal application of CRF (200nM) enhanced sIPSC frequency in male and female CRF1+ neurons, indicating an increase in presynaptic GABA release onto CRF1+ neurons. The source of this CRF-sensitive GABA release into CeA is not clear, and several brain regions may be likely candidates. The bed nucleus of the stria terminalis (BNST) sends inhibitory projections to the CeA (40), and expression of CRF1 in the BNST is robust (41, 42). If BNST-CeA projection fibers express CRF1 they could be responsible for the CRF1-mediated changes in presynaptic GABA release described in the present study, but whether CRF1 is expressed in projection fibers from BNST remains unknown. The CRF1 receptor is also expressed in the locus coeruleus (LC) (43), and the LC-CeA projection has been shown to be important in the expression of aversion learning (44, 45). Whether LC-CeA projection neurons express CRF1, and whether stimulation of LC projection neurons can enhance GABA release in CeA, remains to be explored. An additional possible source for this presynaptic regulation of CeA GABA release could be connectivity from CRF1+ neurons from other amygdala nuclei, such as the basolateral amygdala or intercalated population of amygdala neurons (1). In male but not female neurons, acute CRF also enhanced sIPSC amplitude, indicating a greater postsynaptic response to GABA release. The mechanism of this enhanced response could be postsynaptic alterations in receptor number or availability, or greater stimulation of postsynaptic receptors by the enhanced presynaptic release of GABA, either of which may differ between the sexes. Importantly, in male CRF1+ neurons basal sIPSC frequency was reduced by blockade of the CRF2 but not CRF1 receptor, whereas in female CRF1+ neurons basal sIPSC frequency was reduced by blockage of the CRF1 but not CRF2 receptor. These effects suggest the presence of a CRF tone in the male and female CeA, mediated primarily by CRF2 receptors in the male CeA and CRF1 receptors in the female CeA. The effects of CRF on sIPSC amplitude in male CRF1+ neurons were completely blocked by the CRF1 antagonist R121919 but not the CRF2 antagonist Astressin 2B, suggesting that the CRF effects on amplitude observed in the present study require the CRF1 but not CRF2 receptor.
We also discovered sex differences in alcohol-induced plasticity within the CeA CRF1 system. Importantly, the effects of CRF were enhanced in neurons from alcohol drinking mice of both sexes, but in males this enhancement (and the ability of CRF to enhance GABA release onto CRF1+ neurons at all) was blocked by the CRF1 antagonist R121919. This suggests that in males, alcohol drinking may recruit CRF1 activity to mediate the increased effects of CRF. Although there appears to be a CRF2-mediated CRF tone in the male CeA, blockade of the CRF2 receptor did not prevent the effects of CRF in male CRF1+ neurons. This suggests that CRF1 may outcompete CRF2 when CRF is exogenously applied. In females, CRF1 antagonism did not prevent CRF-induced increases in presynaptic GABA release; in fact, the effects of CRF on neurons from water drinking female mice were more pronounced in the presence of R121919. It therefore appears that alcohol-induced recruitment of the CRF1 receptor is a sex-specific neuroadaptation. The ability of R121919 to potentially enhance the presynaptic effects of CRF in female neurons may imply a more complex synaptic architecture in the female CeA, with multiple CRF1+ neurons forming a microcircuit leading to disinhibition of downstream CRF1+ neurons in the presence of a CRF1 antagonist (46). The mechanisms of alcohol-induced enhancement of CRF effects in females remain unclear, as neither blockade of the CRF1 nor CRF2 receptor prevented these effects. Significant biases in the GPCR signaling of the CRF1 receptor have been reported, with male CRF1 receptors biasing towards β-arrestin and female CRF1 receptors biasing towards Gq (47). This signaling bias may predispose female CRF1 receptors to states of persistent activation after a stimulus initially triggers a response. Our findings may indicate that alcohol-induced CRF1 activation results in similar persistent activation of CRF1 second messenger effects, which may continue despite the presence of antagonists.
Notably, the CRF1 antagonist R121919 did not prevent the effects of acute CRF in water-drinking males and females from either drinking group. Prior reports have demonstrated the ability of CRF1 antagonists to prevent the effects of CRF in CeA neurons from male mice (7). However, these experiments were performed in undifferentiated CeA neurons which likely represents data from a mixed population with a low prevalence of CRF1+ neurons. Additionally, these experiments were performed on action potential-independent mIPSCs whereas the present recordings were made with full circuit activity intact. We have previously demonstrated that CRF1 antagonism is insufficient to prevent the effects of CRF on GABA release specifically in male and female CRF1+ neurons (25), consistent with the present findings, collectively suggesting the potential involvement of other signaling systems and/or subcellular mechanisms.
Alcohol exposure has been shown to differently impact the CRF systems of male and female rodents (18), but to date few studies have probed the ability of alcohol to induce functional changes in female CRF circuitry. We report a number of important sex differences in CRF signaling in CRF1+ CeA neurons, some of which may be particularly vulnerable to alcohol-induced plasticity. First, the CRF1 antagonist R121919 reduced baseline GABA release onto female but not male CRF1+ neurons. This finding suggests greater basal activity of the CRF1 receptor in female versus male CRF1+ CeA neurons, which may underlie the observed greater sensitivity of females to reductions in alcohol drinking induced by systemic CRF1 antagonism. This finding is consistent with our prior report of enhanced sensitivity to the effects of R121919 in female CRF1:GFP mice (25). However, although the drinking model in the present study produced robust alcohol intake, with only 14 drinking days this procedure would not be expected to produce alcohol dependence or pronounced alcohol withdrawal. Previously, CRF1 antagonism has been shown to selectively regulate alcohol drinking in dependent male rodents (10, 48) but not non-dependent drinking procedures (35–37; but see also 32). Our findings suggest that this initial lack of sensitivity may be related to the lack of CRF1-mediated regulation of inhibitory control of male CeA CRF1 neurons. The recruitment of the CRF1 receptor after chronic alcohol exposure may begin to confer behavioral sensitivity to CRF1 regulation of alcohol drinking in males. Because this recruitment does not occur in females, CRF1 antagonism may be insufficient to reduce alcohol intake in dependent female rodents, unlike our non-dependent alcohol intake model. This would produce opposing sensitivities between the sexes, with females sensitive to CRF1 antagonist-induced reductions in non-dependent drinking and males sensitive to reductions in dependent drinking. Our systemic pharmacology approach was chosen to better translate to the administration of drugs to human patients, but has the significant drawback of engaging CRF systems throughout the body and brain and not specifically within the amygdala. CRF activity in the LC and paraventricular nucleus has been shown to be sensitive to the effects of alcohol in a sex-dependent manner (18), and CRF1 expression is also robust in the BNST and within other amygdalar nuclei (1). These regions and others could also contribute to the effects of systemic CRF1 blockade reported here. Additionally, CP-154,526 is a peptide antagonist of the CRF1 receptor whereas R121919 is a small-molecular inhibitor of the CRF1 receptor. Although both compounds have robust antagonist activity at the CRF1 receptor and high selectivity for CRF1 over CRF2, the binding location of these antagonists and pharmacological features of their activity at CRF1 may differ (52).It will be important for future studies to assess the effects of CRF1 antagonists in dependence models using female subjects, to administer CRF1 antagonists directly into the female CeA, and examine the effects of small-molecule CRF1 antagonists in addition to peptide antagonists.
In contrast, in males the CRF2 antagonist Astressin 2B reduced baseline GABA release onto CRF1+ neurons. Notably, this effect was most pronounced in male CRF1+ neurons from mice with a history of chronic alcohol drinking. Recruitment of the CRF2 receptor may be a compensatory response to the role of the CRF1 receptor in mediating the enhanced response to CRF exhibited by male neurons following alcohol drinking. The CRF2 receptor is expressed both pre and postsynaptically in the CeA (53, 54) and potential compensation by CRF2 has been proposed as a hypothesis for the failure of CRF1 antagonists to reduce alcohol craving in clinical trials (4). Our findings generally support this suggestion, and raise the possibility that a therapeutic strategy that targets both CRF1 and CRF2 in male subjects may be necessary to achieve a clinical benefit.
The sex-specific effects of alcohol drinking on inhibitory control of CRF1+ neurons are depicted in Figure 10. Overall, alcohol drinking appears to produce adaptations which result in enhanced GABA release onto male and female CRF1+ neurons. However, these effects are CRF1-dependent in male mice but not female mice, and are accompanied by plasticity in the effects of acute alcohol that are absent in female mice. Together, our findings in males and females identify important differences in CRF1/CRF2 signaling in the CeA and shed light on potential sex-specific mechanisms for the failure of CRF1 antagonists in clinical trials. Nevertheless, the CRF1 receptor remains an active target in AUD and anxiety disorders, and interventions that target CRF signaling are still attractive for therapeutic interventions in these disorders. Due to the complexities in CRF1 signaling reported here and elsewhere, further research- particularly in females- will be needed to guide the development of new therapeutics.
Figure 10. Proposed schematic of sex differences in alcohol-drinking induced changes in CRF-mediated inhibitory signaling in CRF1+ CeA neurons.
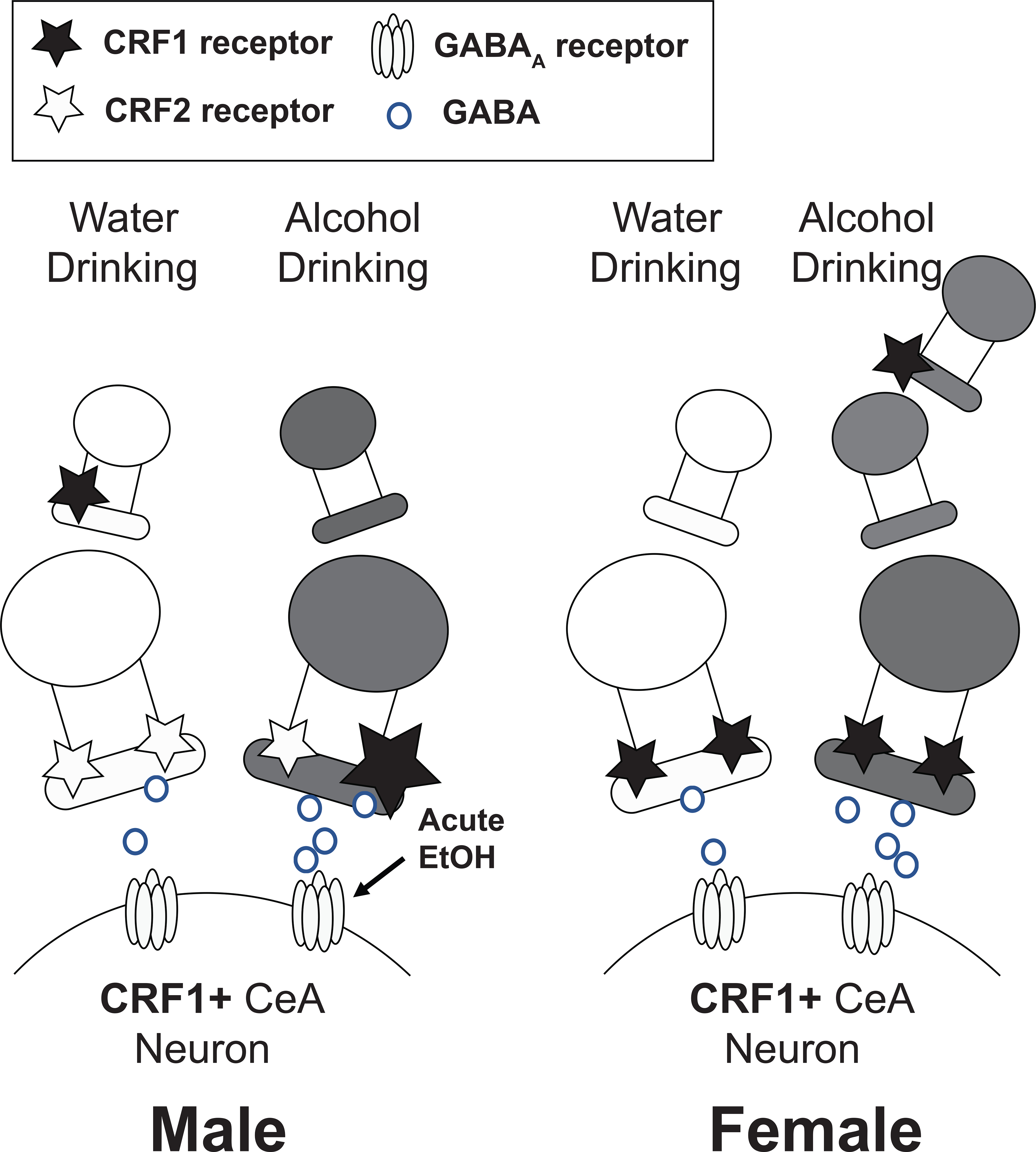
In male mice (left), baseline GABA release onto CRF1+ neurons is regulated by the CRF2 receptor and enhanced by exogenous CRF. A history of alcohol drinking enhances sensitivity to the effects of CRF in a CRF1-dependent manner and appears to produce a greater role for the CRF1 receptor in the actions of CRF than was observed in CRF1+ neurons from water-drinking male mice. A history of alcohol drinking also confers the ability of acute alcohol to enhance GABA release onto CRF1+ neurons, an effect of acute alcohol that was not observed in CRF1+ from water-drinking male mice. In female mice (right), baseline GABA release onto CRF1+ neurons is regulated by the CRF1 receptor and enhanced by exogenous CRF. A history of alcohol drinking enhances sensitivity to the effects of CRF in a CRF1-independent manner, which may suggest a more complex circuitry involving CRF1+ neurons upstream of the CRF1+ recording neuron in female mice. A history of alcohol drinking does not alter the responsivity of female CRF1+ neurons to the effects of acute alcohol.
ACKNOWLEDGEMENTS
This work was supported by the Bowles Center for Alcohol Studies and NIH grants T32AA007573 (AEA), T32NS007431 (MZ), R37 AA014983 (CWH), AA020430 (MH), AA026858 (MH), and AA011605 (CWH and MH).
REFERENCES
- 1.Agoglia AE, Herman MA (2018): The center of the emotional universe: Alcohol, stress, and CRF1 amygdala circuitry. Alcohol. 72:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. (2016): The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology. 41:2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. (2015): The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 40:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spierling SR, Zorrilla EP (2017): Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl). 234:1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, et al. (2012): Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 32:3405–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR (2004): Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 303:1512–1514. [DOI] [PubMed] [Google Scholar]
- 7.Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR (2009): Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal. 9:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lack AK, Floyd DW, McCool BA (2005): Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 36:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, et al. (2013): Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 67:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. (2010): Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 67:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk CK, O’Dell LE, Crawford EF, Koob GF (2006): Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 26:11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman MA, Contet C, Justice NJ, Vale W, Roberto M (2013): Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 33:3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman MA, Contet C, Roberto M (2016): A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J Neurosci. 36:10729–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moolten M, Kornetsky C (1990): Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol. 7:221–225. [DOI] [PubMed] [Google Scholar]
- 15.Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, et al. (2016): Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 79:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koranyi L, Endroczi E, Tal E, Levay G (1987): The effect of acute and chronic ethanol administration on serum corticosterone concentration in rats. Acta Physiol Hung. 69:123–128. [PubMed] [Google Scholar]
- 17.Keith LD, Crabbe JC (1992): Specific and nonspecific effects of ethanol vapor on plasma corticosterone in mice. Alcohol. 9:529–533. [DOI] [PubMed] [Google Scholar]
- 18.Agoglia AE, Crofton EJ, Herman MA (2020): Biological intersection of sex, age, and environment in the corticotropin releasing factor (CRF) system and alcohol. Neuropharmacology. 170:108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panagiotakopoulos L, Neigh GN (2014): Development of the HPA axis: where and when do sex differences manifest? Front Neuroendocrinol. 35:285–302. [DOI] [PubMed] [Google Scholar]
- 20.Janitzky K, Peine A, Krober A, Yanagawa Y, Schwegler H, Roskoden T (2014): Increased CRF mRNA expression in the sexually dimorphic BNST of male but not female GAD67 mice and TMT predator odor stress effects upon spatial memory retrieval. Behav Brain Res. 272:141–149. [DOI] [PubMed] [Google Scholar]
- 21.Weathington JM, Hamki A, Cooke BM (2014): Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Comp Neurol. 522:1284–1298. [DOI] [PubMed] [Google Scholar]
- 22.Prusator DK, Greenwood-Van Meerveld B (2017): Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain. 158:296–305. [DOI] [PubMed] [Google Scholar]
- 23.Brunton PJ, Donadio MV, Russell JA (2011): Sex differences in prenatally programmed anxiety behaviour in rats: differential corticotropin-releasing hormone receptor mRNA expression in the amygdaloid complex. Stress. 14:634–643. [DOI] [PubMed] [Google Scholar]
- 24.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, et al. (2011): Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 6:e28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agoglia AE, Tella J, Herman MA (2020): Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Retson TA, Hoek JB, Sterling RC, Van Bockstaele EJ (2015): Amygdalar neuronal plasticity and the interactions of alcohol, sex, and stress. Brain Struct Funct. 220:3211–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008): Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 511:479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011): Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 35:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agoglia AE, Zhu M, Ying R, Sidhu H, Natividad LA, Wolfe SA, et al. (2020): Corticotropin-Releasing Factor Receptor-1 Neurons in the Lateral Amygdala Display Selective Sensitivity to Acute and Chronic Ethanol Exposure. eNeuro. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varodayan FP, Correia D, Kirson D, Khom S, Oleata CS, Luu G, et al. (2017): CRF modulates glutamate transmission in the central amygdala of naive and ethanol-dependent rats. Neuropharmacology. 125:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, Neugebauer V (2008): Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 28:3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE (2010): CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 35:1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE (2008): The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 32:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, et al. (2008): Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A. 105:9070–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn DA, Hashimoto JG, Cozzoli DK, Helms ML, Nipper MA, Kaufman MN, et al. (2018): Binge Ethanol Drinking Produces Sexually Divergent and Distinct Changes in Nucleus Accumbens Signaling Cascades and Pathways in Adult C57BL/6J Mice. Front Genet. 9:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwok C, Lei K, Pedrozo V, Anderson L, Ghotra S, Walsh M, et al. (2021): Differential importance of nucleus accumbens Ox1Rs and AMPARs for female and male mouse binge alcohol drinking. Sci Rep. 11:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005): Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 84:53–63. [DOI] [PubMed] [Google Scholar]
- 38.Middaugh LD, Kelley BM, Bandy AL, McGroarty KK (1999): Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 17:175–183. [DOI] [PubMed] [Google Scholar]
- 39.Mumtaz F, Khan MI, Zubair M, Dehpour AR (2018): Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 105:1205–1222. [DOI] [PubMed] [Google Scholar]
- 40.Gungor NZ, Yamamoto R, Pare D (2015): Optogenetic study of the projections from the bed nucleus of the stria terminalis to the central amygdala. J Neurophysiol. 114:2903–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983): Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 36:165–186. [DOI] [PubMed] [Google Scholar]
- 42.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. (2000): Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 428:191–212. [DOI] [PubMed] [Google Scholar]
- 43.Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ (2006): Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 23:2991–2998. [DOI] [PubMed] [Google Scholar]
- 44.Gu Y, Piper WT, Branigan LA, Vazey EM, Aston-Jones G, Lin L, et al. (2020): A brainstem-central amygdala circuit underlies defensive responses to learned threats. Mol Psychiatry. 25:640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uematsu A, Tan BZ, Johansen JP (2015): Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learn Mem. 22:444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agoglia AE, Tella J, Herman MA (2020): Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology. 180:108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valentino RJ, Van Bockstaele E, Bangasser D (2013): Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci. 34:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ (2007): Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 86:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilpin NW, Richardson HN, Koob GF (2008): Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 32:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, et al. (2006): Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl). 189:175–186. [DOI] [PubMed] [Google Scholar]
- 51.Sabino V, Kwak J, Rice KC, Cottone P (2013): Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking. Alcohol Clin Exp Res. 37:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C (2006): Recent advances in small molecule antagonists of the corticotropin-releasing factor type-1 receptor-focus on pharmacology and pharmacokinetics. Curr Med Chem. 13:1261–1282. [DOI] [PubMed] [Google Scholar]
- 53.Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R (1998): Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 286:459–468. [PubMed] [Google Scholar]
- 54.Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A (2002): The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol. 136:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]


