Abstract
Social cognitive impairment is a core feature of schizophrenia and plays a critical role in poor community functioning in the disorder. However, our understanding of the relationship between key biological variables and social cognitive impairment in schizophrenia is limited. This study examined the effect of sex on the levels of social cognitive impairment and the relationship between social cognitive impairment and social functioning in schizophrenia. Two hundred forty-eight patients with schizophrenia (61 female) and 87 healthy controls (31 female) completed five objective measures and one subjective measure of social cognition. The objective measures included the Facial Affect Identification, Emotion in Biological Motion, Self-Referential Memory, MSCEIT Branch 4, and Empathic Accuracy tasks. The subjective measure was the Interpersonal Reactivity Index (IRI), which includes four subscales. Patients completed measures of social and non-social functional capacity and community functioning. For objective social cognitive tasks, we found a significant sex difference only on one measure, the MSCEIT Branch 4, which in both patient and control groups, females performed better than males. Regarding the IRI, females endorsed higher empathy-related items on one subscale. The moderating role of sex was found only for the association between objective social cognition and non-social functional capacity. The relationship was stronger in male patients than female patients. In this study, we found minimal evidence of a sex effect on social cognition in schizophrenia across subjective and objective measures. Sex does not appear to moderate the association between social cognition and functioning in schizophrenia.
Subject terms: Schizophrenia, Human behaviour
Introduction
Social cognitive impairment is a core feature of schizophrenia. During the past two decades, a large body of work has shown the pervasive nature of social cognitive impairment and its critical role in poor functioning in schizophrenia1–3. However, surprisingly little is known about whether key biological variables, such as sex, moderate the level of social cognitive impairment and the strength of the association between social cognitive impairment and community functioning in schizophrenia.
While less is known about sex difference in social cognition in schizophrenia, several studies have shown the effect of sex on other core features of schizophrenia. For instance, female patients with schizophrenia tend to have older age of onset4,5, better premorbid functioning4, and better social functioning6,7. Better social functioning of female patients raises an interesting question as to whether any key determinants of functioning, such as non-social cognition and social cognition, may also differ between male and female patients. For non-social cognition, studies on sex differences in schizophrenia have produced mixed findings, such that some found better performance in female than male patients8–10, whereas others found the opposite11,12 or no difference between female and male patients13–15. As these studies focused on different domains of non-social cognition, it is possible that sex differences in cognition in schizophrenia may vary across non-social cognitive domains. The inconsistent findings of these studies raise a possibility that sex differences in social cognition in schizophrenia may differ depending on the type of measures (e.g., subjective versus objective measures), which in turn may affect the relationships between social cognition and functioning.
There is a pervasive impression that compared to males, females are generally better at processing social information, including emotional expressions. Several studies have empirically examined this possibility in healthy populations using both subjective and objective social cognitive measures across multiple domains of social cognition. For subjective social cognitive measures, on which participants self-reported their social cognitive abilities, females reported higher empathy16 and higher emotional intelligence17, compared to males. Studies with objective social cognitive tasks present a more nuanced pattern of female advantage in processing social stimuli. A majority of studies on sex differences examined emotion identification or emotion discrimination using face stimuli. Several studies failed to find sex differences17,18, while some found slightly better performance in females for emotion recognition, especially for negative emotions19–21. Similarly, when discriminating emotional body movement of point-light walkers, females performed slightly better at discriminating emotional body movement of point-light walkers22 or comparably to males23. Females performed slightly better at understanding thoughts of another person24 or the emotional state of another person25 compared to males. Thus, it appears that sex differences in healthy samples are more consistently found using subjective versus objective social cognitive measures.
Several studies examined sex differences in social cognition in schizophrenia using objective social cognitive tasks. Female and male patients showed comparable performance when recognizing facial emotions26–28 or understanding the thoughts of another person (i.e., mental state attribution)26–28. While these findings suggest a lack of sex difference in schizophrenia, most studies employed only objective social cognitive tasks and primarily focused on perception of emotional expressions or mental state attribution. Thus, it remains to be determined whether sex differences in schizophrenia exist for subjective social cognition or whether sex differences are present for other social cognitive domains beyond emotion perception and mental state attribution.
To examine sex differences in social cognition in schizophrenia, this study presents a secondary analysis of data from a two-site case-control study, Social Cognition and Functioning in Schizophrenia (SCAF)29,30. Specifically, by adapting paradigms from social cognitive and affective neuroscience, the SCAF project assessed several social cognitive domains that have not been previously examined in schizophrenia, including self-referential memory and empathic accuracy. The SCAF project also included a subjective social cognitive measure of empathy. Thus, this data set is well suited to examine the following research questions: (1) whether there are sex differences in the levels of social cognitive performance of schizophrenia patients and (2) whether sex moderates the associations between social cognition and functioning in schizophrenia.
Results
Demographic and clinical characteristics
Table 1 shows the demographical and clinical characteristics of the sample separated by sex. For age and parental education, we did not find any significant effect. For personal education and MCCB Neurocognitive Composite Score, we only found significant group effects. Within the schizophrenia group, we found a significant sex effect on SANS total (F(1,244) = 63.49, p < 0.05, η2p = 0.025), but not on age of onset and BPRS total. Female patients with schizophrenia showed lower levels of negative symptoms assessed with SANS compared to male patients with schizophrenia. For functional capacity and community functioning, we found a significant sex effect on MASC total (F(1,236) = 9.42, p < 0.01, η2p = 0.038) and on RFS total (F(1,331) = 8.20, p < 0.01, η2p = 0.024), but not on UPSA total. Female patients with schizophrenia showed higher levels of functional capacity on social domain and better community functioning compared to male patients with schizophrenia.
Table 1.
Demographic and clinical characteristics.
| Patients | Controls | |||
|---|---|---|---|---|
| Female (N = 61) | Male (N = 187) | Female (N = 31) | Male (N = 56) | |
| Age | 42.4 (12.4) | 42.1 (12.4) | 42.3 (9.6) | 42.7 (10.4) |
| Personal Education (yrs)a | 12.7 (1.8) | 12.5 (1.7) | 14.7 (1.9) | 14.7 (1.9) |
| Parental Education (yrs) | 13.8 (2.9) | 13.5 (3.1) | 13.4 (2.6) | 13.3 (2.8) |
| Ethnicity | ||||
| Hispanic | 5 | 16 | 3 | 6 |
| Not Hispanic | 56 | 171 | 28 | 50 |
| Race | ||||
| Asian | 1 | 6 | 1 | 2 |
| Hawaiian/other Pacific Islander | 0 | 1 | 1 | 0 |
| Black | 29 | 73 | 10 | 15 |
| White | 30 | 99 | 18 | 38 |
| More than one race | 1 | 8 | 1 | 1 |
| Age of onset (yrs) | 22.4 (9.9) | 21.1 (5.9) | ||
| SANSb | 7.0 (3.1) | 8.1 (3.2) | ||
| BPRS | 45.9 (13.3) | 45.1 (13.8) | ||
| UPSAb | 0.77 (0.12) | 0.72 (0.13) | ||
| MASC | 3.68 (0.46) | 3.46 (0.49) | ||
| RFSb | 18.1 (5.3) | 17.2 (4.5) | ||
| MCCB neurocognitive compositec | 33.5 (13.0) | 29.8 (12.7) | 47.7 (12.6) | 45.9 (12.1) |
Values are given as mean (standard deviation).
SANS the Scale for the Assessment of Negative Symptoms, BPRS the Brief Psychiatric Rating Scale-24 item, UPSA the University of California at San Diego Performance-based Assessment, MASC the Maryland Assessment of Social Competence, RFS the Role Functioning Scale, MCCB MATRICS Cognitive Consensus Battery.
aA significant effect of group (F(1,331) = 75.11, p < 0.001, η2p = 0.185) indicating that patients had lower levels of personal education than controls.
bSignificant sex difference within the patient group.
cA significant effect of group (F(1,326) = 84.44, p < 0.001, η2p = 0.206) indicating that patients showed poorer performance than controls.
Objective and subjective social cognitive tasks
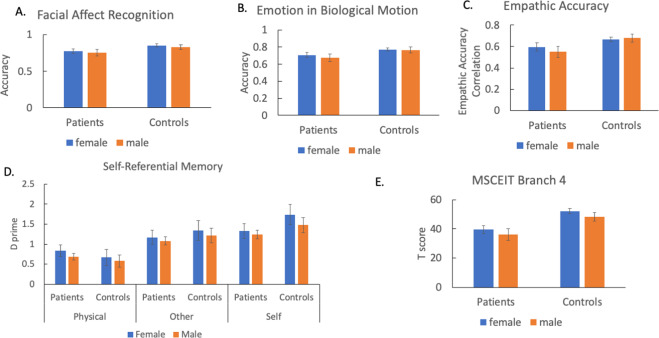
Figures 1 and 2 show performance of patients and controls on objective and subjective cognitive tasks, respectively. Tables 2 and 3 show statistics from two-way ANOVAs and a repeated measures ANOVA. For Facial Affect Recognition task, Emotion in Biological Motion task and Empathic Accuracy task, we did not find any significant effect involving sex. Similarly, no significant main effect of sex or significant interaction involving sex was found for the Self-Referential Memory task. For the MSCEIT Branch 4, we found a significant effect of sex such that female participants performed better than male participants, and this sex effect did not differ between patients and controls as evidenced by a non-significant sex by group interaction.
Fig. 1. Performance of patients and controls on objective social cognitive tasks.
A Facial affect recognition, B Emotion in biological motion, C Empathic accuracy, D Self-referential memory, and E MSCEIT branch 4. Error bars indicate 95% confidence interval. MSCEIT the Mayer–Salovey–Caruso Emotional Intelligence Test 2.0.
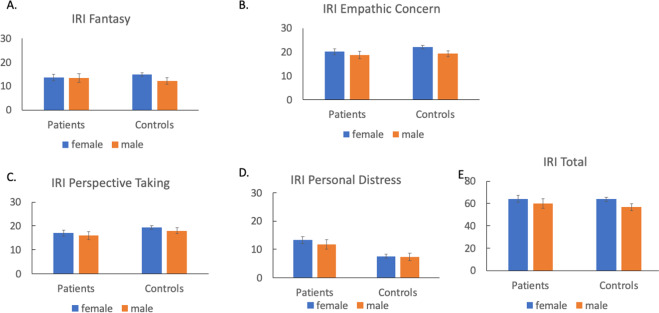
Fig. 2. Performance of patients and controls on the subjective social cognitive measure.
A IRI fantasy, B IRI empathic concern, C IRI perspective taking, D IRI personal distress, and E IRI Total. Error bars indicate 95% confidence interval. IRI the Interpersonal Responsivity Index.
Table 2.
Performance on objective social cognitive measures.
| Inferential statistics | P value | Effect size (η2p) | 95% confidence interval of parameter estimatesa | |
|---|---|---|---|---|
| Facial affect recognitionb | ||||
| Group | F(1,329) = 20.14 | <0.001 | 0.06 | [−0.11, −0.04] |
| Sex | F(1,329) = 1.88 | NS | 0.01 | |
| Group by sex | F(1,329) = 0.001 | NS | 0.00 | |
| Emotion in biological motionb | ||||
| Group | F(1,323) = 21.76 | <0.001 | 0.06 | [−0.121, −0.05] |
| Sex | F(1,323) = 1.19 | NS | 0.00 | |
| Group by sex | F(1,323) = 0.57 | NS | 0.00 | |
| Self-referential memory | ||||
| Group | F(1,325) = 1.98 | NS | 0.01 | |
| Sex | F(1,325) = 2.82 | NS | 0.01 | |
| Group by sex | F(1,325) = 0.10 | NS | 0.00 | |
| Condition | F(2,650) = 227.04 | <0.001 | 0.41 | |
| Condition by group | F(2,650) = 21.08 | <0.001 | 0.06 | |
| Condition by sex | F(2,650) = 0.46 | NS | 0.00 | |
| Condition by sex by group | F(2,650) = 1.41 | NS | 0.00 | |
| Empathic accuracyb | ||||
| Group | F(1,316) = 24.13 | <0.001 | 0.07 | [−0.17, −0.08] |
| Sex | F(1,316) = 0.71 | NS | 0.00 | |
| Group by sex | F(1,316) = 2.15 | NS | 0.01 | |
| MSCEIT branch 4b,c | ||||
| Group | F(1,326) = 69.03 | <0.001 | 0.18 | [−15.72, −8.96] |
| Sex | F(1,326) = 5.83 | <0.05 | 0.02 | [−1.08, 8.82] |
| Group by sex | F(1,326) = 0.02 | NS | 0.00 | |
MSCEIT the Mayer–Salovey–Caruso Emotional Intelligence Test 2.0.
aA 95% confidence interval for the parameter estimate is reported for significant group or sex effects.
bFemales performed better than controls.
cPatients performed worse than controls.
Table 3.
Performance on subjective social cognitive measures.
| Inferential statistics | P value | Effect size (η2p) | 95% confidence interval of parameter estimatesa | |
|---|---|---|---|---|
| IRI fantasy | ||||
| Group | F(1,329) = 0.00 | NS | 0.00 | |
| Sex | F(1,329) = 4.46 | NS | 0.01 | |
| Group by sex | F(1,329) = 3.25 | NS | 0.01 | |
| IRI empathic concernb | ||||
| Group | F(1,329) = 4.18 | NS | 0.01 | |
| Sex | F(1,329) = 11.43 | <0.01 | 0.03 | [0.79, 4.79] |
| Group by sex | F(1,329) = 1.42 | NS | 0.00 | |
| IRI perspective takingc | ||||
| Group | F(1,329) = 11.32 | <0.01 | 0.03 | [−3.45, −0.56] |
| Sex | F(1,329) = 3.32 | NS | 0.01 | |
| Group by sex | F(1,329) =0.73 | NS | 0.00 | |
| IRI personal distressc | ||||
| Group | F(1,329) = 61.78 | <0.001 | 0.16 | [2.97, 5.86] |
| Sex | F(1,329) = 1.98 | NS | 0.01 | |
| Group by sex | F(1,329) = 1.20 | NS | 0.00 | |
| IRI totalb | ||||
| Group | F(1,329) = 1.03 | NS | 0.00 | |
| Sex | F(1,329) = 11.58 | <0.01 | 0.03 | [1.65, 12.42] |
| Group by Sex | F(1,329) = 0.77 | NS | 0.00 | |
IRI the interpersonal reactivity index.
aA 95% confidence interval for the parameter estimate is reported for significant group or sex effects.
bPatients performed worse than controls.
cFemales performed better than controls.
For the IRI, on Empathic Concern and Fantasy subscale, we found a significant sex effect, but no interaction between sex and group. Female participants reported significantly higher scores on Empathic Concern, and this pattern did not differ between patients and controls. On the Fantasy subscale, a sex effect was no longer significant after correcting for multiple comparisons. On the Perspective Taking and Personal Distress subscales, no effect involving sex was significant. Finally, for IRI total score, we found a significant effect of sex after correcting for multiple comparisons, but no group by sex interaction. Across both patient and control groups, females had higher IRI total scores.
The moderating role of sex in relationships between social cognition and functioning
Table 4 presents findings from linear regression analyses that examined the moderating role of sex in relationships between social cognition and functioning. As the findings did not change when negative symptoms assessed with SANS, neurocognition assessed with MCCB, age, or site were included in the model, we present findings without these covariates below. Bivariate correlations among variables in male and female patients are presented in the Supplement.
Table 4.
Linear multiple regression analyses to examine the moderating role of sex in associations between social cognition and functioning.
| Step 1 | Step 2a | Step 3b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | AIC | R2 | ΔR2 | AIC | R2 | ΔR2 | AIC | Unstandardized coefficientsc | |||
| Female | Male | ||||||||||
| Social cognitive composite | UPSA | 0.343** | −1075 | 0.345** | 0.002 | −1074 | 0.363** | 0.018* | −1079 | 0.05** | 0.093** |
| MASC | 0.071** | −379 | 0.097** | 0.026* | −384 | 0.098** | 0.001 | −382 | |||
| RFS | 0.079** | 1058 | 0.080** | 0.001 | 1057 | 0.081** | 0.001 | 1059 | |||
| IRI total | UPSA | 0.000 | −971 | 0.013 | 0.013 | −972 | 0.013 | 0.000 | −970 | ||
| MASC | 0.023** | −362 | 0.055** | 0.032** | −368 | 0.055** | 0.000 | −366 | |||
| RFS | 0.002 | 1125 | 0.026 | 0.024** | 1118 | 0.026 | 0.000 | 1120 | |||
AIC Akaike Information Criterion, UPSA the University of California at San Diego Performance-based Assessment, MASC the Maryland Assessment of Social Competence, RFS the Role Functioning Scale, IRI the Interpersonal Reactivity Index.
*p < 0.05; **p < 0.01.
aStep 2 included sex as a dummy variable.
bStep 3 included interaction between sex and predictors.
cFor significant interactions, unstandardized coefficients are presented. The significance of unstandardized coefficients was examined using t-tests.
With a social cognitive composite score as a predictor and UPSA as a predicted variable, we found a significant interaction between sex and social cognition such that the relationship between a social cognitive composite and UPSA was stronger in male than in female patients. For the MASC, we observed a significant effect of social cognition and a significant effect of sex, but no significant interaction. Sex did not moderate the association between objective social cognition and MASC. For RFS, we observed only a significant effect of social cognition.
Regarding the relationships between subjective social cognition and functioning, we found a significant effect of the IRI total and a significant effect of sex, but no interaction between IRI total and sex when predicting MASC. The strength of association between the IRI total and MASC did not differ between female and male patients. For UPSA and RFS, we did not find any significant effect.
Discussion
This study examined the effect of sex on the levels of social cognitive impairment and the relationship between social cognition and functioning (functional capacity and functional outcome). Overall, the findings of this study do not strongly support a female advantage for social cognitive ability. For objective social cognitive tasks, we found a significant sex effect, but no sex by group interaction on the MSCEIT Branch 4, a measure of emotional regulation. Females performed better than males, and this effect was similar across patients and controls. A sex effect was not found on other objective social cognitive measures. Regarding subjective social cognition in both patient and control groups, females reported greater empathic concern than males. We did not find any sex differences on other subscales of the IRI. Finally, we found that sex moderated the association between objective social cognition and non-social functional capacity. This relationship between objective social cognition and functional capacity was stronger in male than female patients. However, sex did not moderate the relationships between objective social cognition and other measures of functioning. Nor did sex moderate the relationship between subjective social cognition and functioning in schizophrenia.
In this study, female patients showed less severe negative symptoms, better functional capacity in the social domain, and better community functioning than male patients. These findings add to the existing literature on sex differences in schizophrenia6,7, suggesting that the course of illness differs between female and male schizophrenia patients. In this context, it is notable that we did not find strong evidence on sex difference in social cognition, a key determinant of poor functioning in schizophrenia. The lack of sex effect is consistent with recent studies showing comparable performance between female and male patients on social cognitive tasks15,27. Further, our regression analyses showed that sex moderated the relationship between objective social cognition and UPSA, but not other measures of functioning. Overall, the role of social cognition in community functioning in schizophrenia does not seem to differ much between female and male patients, suggesting that any intervention for improving social cognition is likely to have similar effects in both female and male patients.
For objective social cognitive measures across both patients and controls, females performed better than males on a measure of emotion regulation, consistent with a previous study25. However, across both groups, females and males performed similarly on the measures of emotion identification, emotional biological motion and empathic accuracy. This is consistent with previous studies in healthy individuals that showed the lack of sex differences in emotion identification17,18 and emotional biological motion perception23. Thus, it appears that females and males recognize or infer emotional social cues in a similar way but diverge when asked to regulate emotional responses in a social situation. As this study did not include any measures at a neural level, the question remains as to whether this pattern of sex differences across emotional domains exists at the neural level. Whereas other objective measures on emotional processing that this study employed primarily relied on visual stimuli or video clips, the MSCEIT Branch 4 used vignettes of social situations that required participants to rely on a language processing ability. It remains to be determined whether females and males perform differently on social cognitive tasks with greater demand on language processing. Beyond emotional processing, this study also found that females and males performed in a comparable way on the measure of self-referential memory. This is consistent with a recent neuroimaging study31 in which females and males showed a similar pattern of neural activations related to self-referential processing.
Similar to objective social cognitive measures, we found sex differences on the IRI Empathic Concern subscale, but not on other subscales. The Empathic Concern subscale involves one’s emotional responses to others (e.g., feeling compassion). The Personal Distress subscale concerns one’s own feelings of anxiety or distress in social situations, and the Perspective Taking subscale asks one’s tendency to take another’s perspective in social situations. Taken together, our findings from the subjective social cognitive measure suggest that females may endorse greater emotional responses, such as sympathy or compassion toward others, but these greater emotional responses to others do not result in greater distress or anxiety. It is possible that the greater emotional regulation of females we observed with the objective social cognitive task may play a role in modulating one’s own emotional feeling in the presence of greater emotional reactivity to others.
The findings of this study also raise a question as to what factors other than social cognition may be related to better community functioning in female patients. For example, higher cognitive reserve has been implicated in better social functioning in schizophrenia32. It is possible that female patients may have higher cognitive reserve. Schizophrenia patients tend to overestimate their ability to accurately perform on social cognitive tasks33, which was related to poorer community functioning in schizophrenia34. It will be important to carefully examine whether these variables may differentially affect community functioning in female compared to male patients.
Our study had several limitations. The study included chronic patients, so it remains to be determined whether a similar pattern of sex differences is observed in patients with recent-onset psychosis or in individuals at risk for developing psychosis. Similarly, as this study employed behavioral measures, it needs to be examined whether a similar pattern of sex differences in social cognition in schizophrenia is present at a neural level. This study only included one measure of subjective social cognition, so it will be important to examine whether sex differences can be observed on other domains of social cognition assessed with subjective measures. Finally, as the study sample was not balanced on sex, it will be important to replicate these findings using a more balanced sample that also better represents the general population of patients.
In summary, this two-site case-control study used a large battery of measures across multiple social cognitive domains to examine the effect of sex on the levels of social cognitive impairment and the relationship between social cognitive impairment and functioning in schizophrenia. Our findings suggest that the sex difference in social cognition in schizophrenia is not strong and may vary from domain to domain. Sex moderated the relationship between objective social cognition and non-social functional capacity, but not other measures of functioning. Our finding of sex not moderating the relationship between social cognition and community functioning in schizophrenia also suggests that social cognition is less likely to explain better community functioning of female versus male patients with schizophrenia.
Methods
Participants
This study included 248 patients with schizophrenia and 87 healthy controls from two sites: (1) University of California, Los Angeles (UCLA)—outpatient treatment facilities in the Los Angeles area and mental health clinics at the VA Greater Los Angeles Healthcare System (VAGLAHS) and (2) University of North Carolina (UNC)—Chapel Hill Schizophrenia Treatment and Evaluation Program and community mental health clinics in the Chapel Hill area. Healthy controls were recruited through internet advertisements. All participants provided written informed consents after procedures were fully explained, as approved by the Institutional Review Boards at University of California Los Angeles, VAGLAHS, and UNC.
Selection criteria have been described elsewhere29. Briefly, for patients they included: (1) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of schizophrenia based on a Structured Clinical Interview for DSM-IV (SCID)35, (2) age between 18 and 60 years, (3) sufficient competence in English language to understand testing procedures, (4) no clinically significant neurological disease as determined by medical history, (5) no history of serious head injury, (6) no evidence of substance or alcohol abuse in the month previous to testing, (7) no sedatives or benzodiazepines within 12 h of testing, (8) no history of intellectual disability or developmental disability, and (9) clinical stability. Selection criteria for community controls were: (1) age between 18 and 60 years, (2) sufficient competence in English language to understand testing procedures, (3) no clinically significant neurological disease as determined by medical history, (4) no psychotic disorder, bipolar disorder, or recurrent major depressive disorder according to SCID-I, (5) no schizotypal, avoidance, schizoid or paranoid personality disorder according to SCID-II, (6) no family history of psychotic disorders among first-degree relatives, (7) no history of substance or alcohol dependence and no substance or alcohol abuse in the month previous to testing, and (8) no sedatives or benzodiazepines within 12 h of testing.
Clinical symptoms of patients were assessed with the Scale for the Assessment of Negative Symptoms (SANS)36 and the Brief Psychiatric Rating Scale (BPRS)37. Diagnostic interviews, BPRS, and SANS were administered by trained diagnosticians. To characterize neurocognitive ability of participants, we administered the MATRICS Consensus Cognitive Battery (MCCB)38,39. The MCCB includes six different non-social cognitive domains (speed of processing, attention and vigilance, working memory, verbal learning, visual learning, and reasoning/problem solving).
Measures
Five objective measures and one subjective measure of social cognition were administered. The objective measures include Facial Affect Identification29, Emotion in Biological Motion29, Self-Referential Memory29,40, Empathic accuracy29,41 and the Mayer–Salovey–Caruso Emotional Intelligence Test 2.0 (MSCEIT) Branch 442, and the subjective measure was the Interpersonal Reactivity Index (IRI)43. As details of each measure are provided elsewhere29,43, we briefly describe each measure below.
In the Facial Affect Identification task, participants were asked to decide which emotional expressions a face conveyed on each trial. The primary dependent measure was percent accuracy.
For the Emotion in Biological Motion, participants were asked to decide which emotion (fear, anger, happiness, sadness or neutral) was described by the movement of a point-light walker stimulus. The primary dependent measure was percent accuracy.
For Self-Referential Memory task, participants first completed an encoding phase in which they decided whether a trait word described themselves (“self-referential” condition), whether the word indicated a desirable trait (“other” condition), and whether it was upper case. After a delay period, participants were presented one word at a time and asked to decide if the word was presented during the encoding phase. The primary dependent measure was an index of sensitivity (d’) for recognition of words.
We used two versions of an Empathic Accuracy task, and approximately half of the sample took the older version, and the other half took the newer version29. The key difference between the two versions was the diversity of individuals featured in the videos (i.e., targets), as the newer version was developed to include a broader range of age, racial and ethnic diversity. The dependent measure was the mean correlation across clips between the ratings of the targets on their own emotion and the participant’s ratings of the targets’ emotion. We did not find any performance difference between participants who received the older version and participants who received the newer version (see Supplemental material for details).
The MSCEIT Branch 4, Managing Emotion, assessed emotion regulation in oneself and one’s relationship with others using vignettes. Specifically, participants are presented with vignettes of various social situations along with the solution to cope with the emotions depicted in these vignettes. Participants are asked to indicate how effective each solution is using a scale ranging from 1 (very ineffective) to 5 (very effective).
Finally, the IRI was used as a measure of subjective social cognitive ability. The IRI, as a measure of empathy, consists of four subtests, each assessing a different aspect of empathy. The Fantasy subscale measures a tendency to transpose oneself into the feelings of a character in a movie or book. The Perspective Taking scale measures how a person will spontaneously adopt someone else’s point of view. The Empathic Concern Scale assesses feelings of sympathy or concern towards the other. The Personal Distress Scale measures feelings of personal distress in unpleasant interpersonal situations. We analyzed both the total IRI index and the four subscales separately.
In addition to measures of social cognition, we assessed functional capacity and community functioning of patients. Functional capacity was assessed using the University of California at San Diego Performance-Based Skills Assessment UPSA44; and the Maryland Assessment of Social Competence MASC45;. The UPSA consists of role-play simulation tasks that measure a participant’s ability to negotiate real-world tasks. As a measure of social skills (i.e., functional capacity on social domain), the MASC employs a role-play approach in which participants are responsible for taking the conversation forward in a series of common interpersonal problems. Four role play scenarios were videotaped and coded by trained raters who achieved a median interclass coefficient of 0.85 on a set of 10 videos that were derived from a separate sample. Community Functioning was assessed with the Role Functioning Scale RFS46;.
Statistical analysis
First, to examine whether female and male patients with schizophrenia differ on demographic and clinical characteristics, we conducted a series of two-way ANOVA with group and sex as between-subject factors for age, personal education, and parental education, and one-way ANOVA for clinical characteristics. Second, to examine whether female and male schizophrenia patients show different levels of performance on objective and subjective social cognitive tasks, we conducted a series of two-way ANOVAs with sex and group as between-subject factors for all tasks except the Self-Referential Memory task. For the Self-Referential Memory task, we conducted a repeated measures ANOVA with condition as within-subject factor and group and sex as between-subject factors. Significance thresholds for the objective social cognitive tasks were set at p = 0.05 because each cognitive task is considered a separate task that assesses a distinct social cognitive domain. The subjective social cognitive measure, the IRI, includes four subscales and a total score; thus, significance thresholds for the subjective social cognitive task were set at p = 0.01 (0.05/5). All p values represent two-tailed tests. For these analyses, we also report effect size (i.e., partial eta square) along with statistics. The general rule of thumb regarding the magnitude of effect size for partial eta square is: 0.01 = small effect, 0.06 = medium effect, and 0.14 for large effect47.
Third, to examine whether sex moderates the associations between social cognition and functioning (i.e., functional capacity and community functioning) within the schizophrenia group, we conducted linear multiple regression analyses for objective social cognition and subjective social cognition separately. For objective social cognition, a social cognitive composite score was created by calculating the mean of the standardized objective social cognition variables using the mean and standard deviation of the control group. In the first block, the social cognitive composite score was entered, which allowed to compare the findings of this study to previous work on the relationship between social cognition in schizophrenia. Sex (dummy coded) was entered in the second block. In the third block, the interaction between sex and the social cognitive composite was entered. A significant interaction would indicate that sex moderated the relationship between social cognition and functioning in schizophrenia. A similar regression analysis was conducted for subjective social cognition using IRI total score, such that IRI total score was entered in the first block, followed by sex in the second block, and a sex by IRI total interaction in the third block. Significance thresholds represent two-tailed tests and were set at a p = 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work is supported by National Institute of Mental Health (MH087618 to M.F.G. and MH113856 to J.L.).
Author contributions
M.F., M.F.G., J.L. conceptualized the study. M.F. obtained the funding of the project. M.F., W.P.H., D.L.P., R.S.K., and J.L. were involved with data collection. M.F. and J.L. analyzed data and wrote the first draft of the manuscript. All the authors reviewed the initial draft of the manuscript and provided comments and edits.
Data availability
The dataset analyzed during the current study can be available upon request.
Code availability
The code used for data analysis can be available upon request.
Competing interests
M.F.G. has been a paid consultant for Biogen, Click Therapeutics, Otsuka, and was a member of the Scientific Board of Cadent. M.F.G. is also an officer in a non-profit organization, MATRICS Assessment, Inc., but receives no financial compensation. W.P.H. is a full-time employee of VeraSci. The remaining authors have no disclosures.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-021-00188-7.
References
- 1.Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18:146–161. doi: 10.1002/wps.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol. Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 3.Halverson TF, et al. Pathways to functional outcomes in schizophrenia spectrum disorders: meta-analysis of social cognitive and neurocognitive predictors. Neurosci. Biobehav. Rev. 2019;105:212–219. doi: 10.1016/j.neubiorev.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Segarra R, et al. Similarities in early course among men and women with a first episode of schizophrenia and schizophreniform disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262:95–105. doi: 10.1007/s00406-011-0218-2. [DOI] [PubMed] [Google Scholar]
- 5.Eranti SV, MacCabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol. Med. 2013;43:155–167. doi: 10.1017/S003329171200089X. [DOI] [PubMed] [Google Scholar]
- 6.Thorup A, et al. Gender differences in first-episode psychosis at 5-year follow-up—two different courses of disease? Results from the OPUS study at 5-year follow-up. Eur. Psychiatry. 2014;29:44–51. doi: 10.1016/j.eurpsy.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Lappin JM, et al. Early sustained recovery following first episode psychosis: Evidence from the AESOP10 follow-up study. Schizophr. Res. 2018;199:341–345. doi: 10.1016/j.schres.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JM, et al. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am. J. Psychiatry. 1998;155:1358–1364. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, et al. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci. Rep. 2017;7:11821. doi: 10.1038/s41598-017-12027-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longenecker J, Dickinson D, Weinberger DR, Elvevag B. Cognitive differences between men and women: a comparison of patients with schizophrenia and healthy volunteers. Schizophr. Res. 2010;120:234–235. doi: 10.1016/j.schres.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlick D, Mattis S, Stastny P, Teresi J. Gender differences in cognition in schizophrenia. Schizophr. Res. 1992;8:69–73. doi: 10.1016/0920-9964(92)90062-a. [DOI] [PubMed] [Google Scholar]
- 12.Lewine RR, Walker EF, Shurett R, Caudle J, Haden C. Sex differences in neuropsychological functioning among schizophrenic patients. Am. J. Psychiatry. 1996;153:1178–1184. doi: 10.1176/ajp.153.9.1178. [DOI] [PubMed] [Google Scholar]
- 13.Zanelli J, et al. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am. J. Psychiatry. 2010;167:78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- 14.Hoff AL, et al. Sex differences in neuropsychological functioning of first-episode and chronically ill schizophrenic patients. Am. J. Psychiatry. 1998;155:1437–1439. doi: 10.1176/ajp.155.10.1437. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, et al. The effects of age and sex on cognitive impairment in schizophrenia: Findings from the Consortium on the Genetics of Schizophrenia (COGS) study. PLoS One. 2020;15:e0232855. doi: 10.1371/journal.pone.0232855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg N. Empathy and related vicarious emotional responses. New Dir. Child Dev. 1989;44:1–7. [PubMed] [Google Scholar]
- 17.Fischer AH, Kret ME, Broekens J. Gender differences in emotion perception and self-reported emotional intelligence: a test of the emotion sensitivity hypothesis. PLoS ONE. 2018;13:e0190712. doi: 10.1371/journal.pone.0190712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimshaw GM, Bulman-Fleming MB, Ngo C. A signal-detection analysis of sex differences in the perception of emotional faces. Brain Cogn. 2004;54:248–250. doi: 10.1016/j.bandc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Connolly HL, Lefevre CE, Young AW, Lewis GJ. Sex differences in emotion recognition: evidence for a small overall female superiority on facial disgust. Emotion. 2019;19:455–464. doi: 10.1037/emo0000446. [DOI] [PubMed] [Google Scholar]
- 20.Thompson AE, Voyer D. Sex differences in the ability to recognise non-verbal displays of emotion: a meta-analysis. Cogn. Emotion. 2014;28:1164–1195. doi: 10.1080/02699931.2013.875889. [DOI] [PubMed] [Google Scholar]
- 21.Wingenbach TSH, Ashwin C, Brosnan M. Sex differences in facial emotion recognition across varying expression intensity levels from videos. PLoS ONE. 2018;13:e0190634. doi: 10.1371/journal.pone.0190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alaerts K, Nackaerts E, Meyns P, Swinnen SP, Wenderoth N. Action and emotion recognition from point light displays: an investigation of gender differences. PLoS ONE. 2011;6:e20989. doi: 10.1371/journal.pone.0020989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isernia S, Sokolov AN, Fallgatter AJ, Pavlova MA. Untangling the ties between social cognition and body motion: gender impact. Front. Psychol. 2020;11:128. doi: 10.3389/fpsyg.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkland RA, Peterson E, Baker CA, Miller S, Pulos S. Meta-analysis reveals adult female superiority in “Reading the Mind in the Eyes” test. North Am. J. Psychol. 2013;15:121–146. [Google Scholar]
- 25.Cabello R, Sorrel MA, Fernandez-Pinto I, Extremera N, Fernandez-Berrocal P. Age and gender differences in ability emotional intelligence in adults: a cross-sectional study. Dev. Psychol. 2016;52:1486–1492. doi: 10.1037/dev0000191. [DOI] [PubMed] [Google Scholar]
- 26.Danaher H, Allott K, Killackey E, Hester R, Cotton S. An examination of sex differences in neurocognition and social cognition in first-episode psychosis. Psychiatry Res. 2018;259:36–43. doi: 10.1016/j.psychres.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 27.Pinkham AE, Kelsven S, Kouros C, Harvey PD, Penn DL. The effect of age, race, and sex on social cognitive performance in individuals with schizophrenia. J. Nerv. Ment. Dis. 2017;205:346–352. doi: 10.1097/NMD.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarra-Ventura G, et al. Gender differences in social cognition: a cross-sectional pilot study of recently diagnosed patients with schizophrenia and healthy subjects. Can. J. Psychiatry. 2018;63:538–546. doi: 10.1177/0706743717746661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern RS, et al. Adapting social neuroscience measures for schizophrenia clinical trials, Part 2: trolling the depths of psychometric properties. Schizophr. Bull. 2013;39:1201–1210. doi: 10.1093/schbul/sbt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green MF, Lee J, Ochsner KN. Adapting social neuroscience measures for schizophrenia clinical trials, Part 1: ferrying paradigms across perilous waters. Schizophr. Bull. 2013;39:1192–1200. doi: 10.1093/schbul/sbt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung J, et al. Exploring sex differences in the neural correlates of self-and other-referential gender stereotyping. Front. Behav. Neurosci. 2019;13:31. doi: 10.3389/fnbeh.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amoretti S, et al. Cognitive reserve as an outcome predictor: first-episode affective versus non-affective psychosis. Acta Psychiatr. Scand. 2018;138:441–455. doi: 10.1111/acps.12949. [DOI] [PubMed] [Google Scholar]
- 33.Jones MT, et al. Confidence, performance, and accuracy of self-assessment of social cognition: a comparison of schizophrenia patients and healthy controls. Schizophr. Res Cogn. 2020;19:002–002. doi: 10.1016/j.scog.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silberstein JM, Pinkham AE, Penn DL, Harvey PD. Self-assessment of social cognitive ability in schizophrenia: association with social cognitive test performance, informant assessments of social cognitive ability, and everyday outcomes. Schizophr. Res. 2018;199:75–82. doi: 10.1016/j.schres.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. W. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (Biometrics Research Department, 1997).
- 36.Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS) (The University of IOWA, 1984).
- 37.Ventura J, et al. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int. J. Methods Psychiatr. Res. 1993;3:227–243. [Google Scholar]
- 38.Nuechterlein KH, et al. The MATRICS Consensus Cognitive Battery: Part 1. test selection, reliability, and validity. Am. J. Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 39.Nuechterlein, K. H. & Green, M. F. MATRICS Consensus Cognitive Battery (MATRICS Assessment, Inc., 2006).
- 40.Harvey PO, Lee J, Horan WP, Ochsner K, Green MF. Do patients with schizophrenia benefit from a self-referential memory bias? Schizophr. Res. 2011;127:171–177. doi: 10.1016/j.schres.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol. Med. 2011;41:2297–2304. doi: 10.1017/S0033291711000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer, J. D., Salovey, P. & Caruso, D. R. Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) User’s Manual (MHS Publishers, 2002).
- 43.Horan WP, et al. Structure and correlates of self-reported empathy in schizophrenia. J. Psychiatr. Res. 2015;66-67:60–66. doi: 10.1016/j.jpsychires.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr. Bull. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- 45.Bellack AS, Brown CH, Thomas-Lohrman S. Psychometric characteristics of role-play assessments of social skill in schizophrenia. Behav. Ther. 2006;37:339–352. doi: 10.1016/j.beth.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.McPheeters HL. Statewide mental health outcome evaluation: a perspective of two southern states. Community Ment. Health J. 1984;20:44–55. doi: 10.1007/BF00754103. [DOI] [PubMed] [Google Scholar]
- 47.Cohen, J., Milles, J. & Shevlin, M. Applying Regression and Correlations: A Guide For Students and Researchers (Sage, 2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during the current study can be available upon request.
The code used for data analysis can be available upon request.