Abstract
Tissue engineering has been extensively investigated and proffered to be a potential platform for novel tissue regeneration. The utilization of mesenchymal stem cells (MSCs) from various sources has been widely explored and compared. In this regard, MSCs derived from bone marrow have been proposed and described as a promising cell resource due to their high yield of isolated cells with colony-forming potential, self-renewal capacity, MSC surface marker expression, and multi-lineage differentiation capacities in vitro. However, there is evidence for bone marrow MSCs (BM-MSCs) both in vitro and in vivo from different species presenting identical and distinct potential stemness characteristics. In this review, the fundamental knowledge of the growth kinetics and stemness properties of BM-MSCs in different animal species and humans are compared and summarized. Finally, to provide a full perspective, this review will procure results of current information studies focusing on the use of BM-MSCs in clinical practice.
Keywords: Tissue engineering, mesenchymal stem cells, bone marrow mesenchymal stem cells, stemness characteristics
INTRODUCTION
Tissue engineering (TE) has been proposed as an advance multidisciplinary approach that incorporates principles from biological, biochemical, medical science [1], engineering, and pharmaceutical [2,3] fields. In the TE paradigm, this combination can develop bioartificial substitutes for tissues or organs, which can be used in regenerative medicine, in pharmaceutical, diagnostic, and basic research into cell functions in vivo, and to identify mechanisms involved in the aging process and disease progression [4].
The three main components used in TE are cells, scaffolds, and signaling molecules [5,6]. Among these components, promising cell resources have been investigated to assess their characteristics, especially stemness capability, to provide fundamental knowledge for developing a mimic tissue or organ. Due to their affordable sample collection feature and non-tumorigenicity, multipotent stem cells from various tissues derived from bone marrow, umbilical cord, amniotic fluid, adipose tissue, muscle, dental tissue [7,8], skin [9], kidney [10], liver [11], heart [12], and brain [13] have been investigated. In this regard, a common source of multipotent stem cells is bone marrow mesenchymal stem cells (BM-MSCs), which have been proposed and described as a promising cell resource from various animal sources, including avian [14], murine [15,16], rat [17], rabbit [18], feline [19], canine [20], ovine [21], bovine [22], porcine [23], equine [24], non-human primate [24], and human [25]. BM-MSCs have crucial stemness abilities, including self-renewal ability, MSCs surface marker expression, and multipotency [26,27]. However, BM-MSCs isolated from different species have been reported to exhibit both similarities and differences in the potency of their stemness characteristics, both in vitro and in vivo [28].
There is no previous literature review comparing the characteristics of BM-MSCs from various species. Previous review articles had only discussed in detail BM-MSCs isolated from one species; for example, murine BM-MSCs [29], feline BM-MSCs [30], canine BM-MSCs [31], or human BM-MSCs [29]. This review presents a comprehensive comparison of the stemness characteristics of BM-MSCs from different species in vitro, linking the information to translational research in humans and animals. Moreover, to present a full perspective, current information studies focusing on the practical use of BM-MSCs in animal models and clinical case reports have been summarized.
HISTORY OF BM-MSCs
Bone marrow-derived MSCs were the first MSCs to be described. In 1996, BM-MSCs were described as fibroblast-like cells with colony-forming ability and differentiation potential [32]. In the 1980s, researchers started to refer to these cells as osteogenic stem cells and bone marrow stromal cells, names that refer back to the source of the cell from bone marrow stroma and its osteogenic differentiation potential [33]. Later, in 1991, Caplan [34] proposed changing the terminology of osteogenic and stromal into mesenchymal. Further, in 2006, due to confusion about the terms mesenchymal stem/stromal cell, the International Society for Cell Therapy (ISCT) recommended adding the tissue origin of the cells to the name. Therefore, currently, bone marrow-derived MSCs are known as BM-MSCs.
COLLECTION SITE OF BM-MSCs
Bone marrow used for MSCs isolation can be obtained from different types of bones in different species. In humans, BM-MSCs may be isolated from sternum [35], vertebral body [36], iliac crest [36,37] and femoral shaft [36]. In animals, BM-MSCs may be sourced from different places according to the species; for example, proximal humerus, femur, and iliac crest are common areas for bone marrow aspiration in canines and felines [38,39]. Whereas, in laboratory animals such as rats [40] and mice [41], bone marrow for BM-MSCs isolation is obtained upon euthanasia from the whole femur or tibia.
STEMNESS CHARACTERISTICS OF BM-MSCs
Stemness, a natural property of MSCs, refers to the capacity of MSCs to maintain self-renewal and an undifferentiated state [42]. Stemness is important for quiescence, proliferation, and regeneration through the interaction between MSCs and their microenvironment [42,43]. In 2006, ISCT proposed several criteria to define human MSCs, including the ability to adhere to a plastic culture surface, expression of MSC surface marker antigens, and potency in multilineage differentiation [44]. However, different species may possess unique stemness characteristics, especially those associated with utilizing MSCs from specific species. Thus, in this review, we have described the MSC morphology, stemness characteristics, including their pluripotency genes, clonogenicity, cell growth kinetic, senescence, surface antigen markers, and multipotency.
Morphology of BM-MSCs
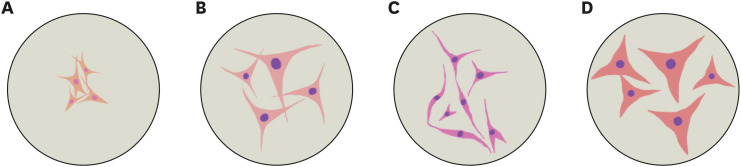
Morphological features are important in defining MSCs as they present as fibroblast-like cells. It has been reported that this feature is possessed by BM-MSCs derived from human [45], canine [46,47], feline [19], rat [48], and murine [49] sources. To the best of our knowledge, murine MSCs are smaller than human, canine, and feline cells. The morphological features y of BM-MSCs from those groups are illustrated in Fig. 1.
Fig. 1. Comparative morphology of BM-MSCs. Morphology (representative figures) of murine (A), canine (B), feline (C), and human (D) BM-MSCs are illustrated.
BM-MSCs, bone marrow mesenchymal stem cells.
Self-renewal and proliferation potential of BM-MSCs
To maintain pluripotency, transcriptional factors (TFs), including Oct4, Sox2, and Nanog have important roles [50]. In addition, Rex1 has been considered a pluripotency marker [51]. It has been reported that pluripotency TFs are expressed in all BM-MSCs derived from human [51,52,53], canine [46,54], rat [55], and murine [56] sources. Moreover, Ki67 is a proliferation marker, based on its reported expression in BM-MSCs derived from rat [55] and canine [46] but not human [57] sources. However, to date, there are no records of Rex1 and Ki67 expressions in feline and murine BM-MSCs, respectively.
The self-renewal capability of MSCs can be assessed by clonogenicity or colony-forming unit assays [58,59]. Our review noted that colonies were formed in human [59], canine [46,60,61], feline [62], rat [63,64], and murine [65,66,67] BM-MSCs.
The kinetics of cell growth show that proliferation is an MSC stemness characteristic. Kinetic studies allow population doubling time (PDT) to be measured and evaluated. Murine BM-MSCs from passage 2 have been reported to have a PDT of more than 80 h at week 4 and 8 [68]. On the other hand, rat BM-MSCs have a PDT of 20–30 h in passages 1 to 3, which rises markedly to 50 h and 130 h in passages 4 and 5, respectively [69]. Contrastingly, another study noted that the PDT of rat BM-MSCs decreased by up to 20 h as the passage number increased [70]. In canines, BM-MSCs derived from several large-sized dog breeds show a PDT increase of up to 100 h after 25 days of culture [60]. Meanwhile, in humans, the PDT was shorter in early-stage cultures (before passage 6) than in late-stage cultures (after passage 6), occurring at less than 48 and 96 h, respectively [57]. Unfortunately, there is no evidence of PDT in cultured feline BM-MSCs, but a different method, MTT assay, showed that feline cells were proliferative up to 120 h after low-density seeding [71]. Another study revealed that feline BM-MSCs showed exponential growth at passage 1 and followed stationary or decreasing growth patterns up to passage 3 [62].
Senescence of cells has been associated with shortened telomeres, causing irreversible cell cycle arrest [72] and leading to proliferation dysfunction in MSCs [73]. Hence, it is important to evaluate senescence by assessing senescence-associated β-galactosidase (SA-β-Gal) expression. One study observed that senescence occurred in late-stage culture (passage 6 and above) of human BM-MSCs [57]. Similarly, SA-β-Gal increased linearly as canine BM-MSCs passage numbers increased [60]. In contrast, there was no indication of SA-β-Gal presence in rat BM-MSCs at PD100 [64]. That result is supported by the previous study showing SA-β-Gal absence in passage 2 of rat BM-MSCs culture but presence in passage 6 [69]. On the other hand, the presence of senescence was relatively low in passages 3 and 4 of murine BM-MSCs [74,75]. However, no feline BM-MSCs senescence study has been reported.
MSCs surface marker expression
According to ISCT, cells must express CD73, CD90, and CD105 as well as negative CD11b, CD14, CD19, CD34, CD45, CD79a, and human leukocyte antigen (HLA)-DR surface markers in order to be considered MSCs. Research reported by Petrenko et al. [76] showed that human BM-MSCs, in addition to meeting the ISCT standard, expressed other MSCs surface markers such as CD10, CD29, CD44, CD133, HLA-ABC, MSCA-1, and SSEA-4. Surface markers in human MSCs differ from those of animal MSCs; for example, in canine and feline MSCs, MHC-1, CD29, and CD105 were expressed [19]. However, canine MSCs also moderately express other markers such as CD90, CD166, and CD73 [20,77,78]. Rat BM-MSCs express CD29, CD44, CD54, CD 90, and CD166 [70]. In contrast, the expressions of MSCs surface markers in mice slightly differ across strains; C57BL, DBA1, and FVBN mice express the SCA-1 surface marker, which is not expressed in BALB/C mice [79]. However, another study reported that BALB/C expressed SCA-1 [80]. Moreover, that study showed that CBA/Ca, ICR and BALB/C mice were positive for CD106 with increasing passage. The absence of endothelial and hematopoietic cell markers CD45, CD11b, and CD34 expressions, per the ISCT standard, were reported in canine, rat, and mouse BM-MSCs [81,82].
Multipotency of BM-MSCs
The multipotency capacity of BM-MSCs derived from human, canine, feline, rat, and murine sources to differentiate into osteogenic, adipogenic, chondrogenic, and neurogenic lineages are summarized in Table 1.
Table 1. Comparative differentiation ability of multilineage bone marrow mesenchymal stem cells.
BM-MSCs-BASED TE IN PRACTICE
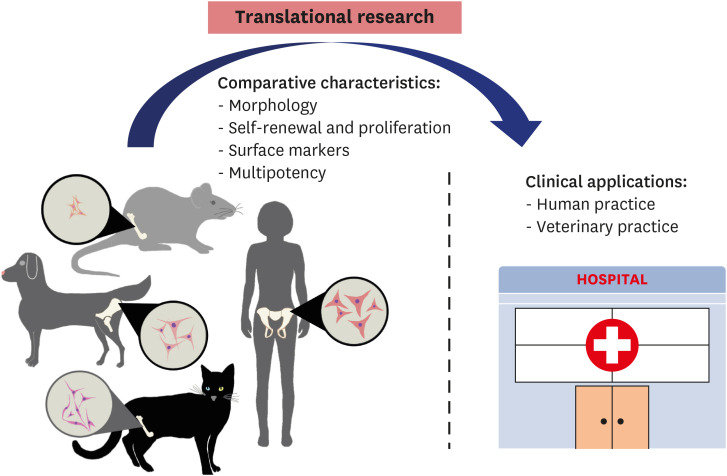
Bone marrow-derived MSCs have been suggested as potential therapeutic agents in improving transplantation-related functional and pathological recovery from several diseases due to their main characteristics: self-renewal, rapid proliferation in vitro, abundant sources for isolation, and high differentiation capability. Indeed, several preclinical studies and clinical trials have been conducted in animal test models, including murine, rat, rabbit, feline, canine, ovine, bovine, and porcine models, to further translational medicine research in humans and determine the effectiveness of BM-MSCs for treatment. A scheme for moving translational research into practice is depicted in Fig. 2.
Fig. 2. Conceptualization of translational research to clinical application. Comparative MSCs characteristics from difference sources of bone marrow-MSCs in vitro used to translate research results into human and animal clinical applications is shown in the diagram.
MSCs, mesenchymal stem cells.
Truong et al. [83] evaluated the treatment effect of murine BM-MSCs on mouse liver cirrhosis induced by carbon tetrachloride. BM-MSCs transplantation reduced inflammation scores and an absence of cirrhosis at 21 days after BM-MSCs injection via a peripheral vein [83].
Other studies demonstrated that BM-MSCs transplantation is a promising treatment strategy for bleomycin-induced pulmonary fibrosis in rats. During the early and late BM-MSCs transplantation period, there was a reduction in pulmonary fibrosis and alveolitis [84]. In addition, BM-MSCs transplantation has been performed in a cerebral artery occlusion rat model [85]. That study noted that BM-MSCs transplantation enhanced endogenous neural progenitor cell migration in rats in a cerebral ischemic condition, which was helpful for restorative cerebral ischemic management.
Autologous and allogeneic feline BM-MSCs intrarenal administration to three cats with chronic kidney disease (CKD) has been investigated. A mild decrease in serum creatinine, a stable body weight, and mild improvement in proteinuria and urine specific gravity were statistically significant after 21 days. In the same study, a transplantation application to treat kidney injury (AKI) in an ischemic kidney model was also investigated. Five cats were administrated for feline BM-MSCs via jugularis catheters; however, the treatment had no effect in cats with AKI, although it was effective in CKD- and AKI-induced rodent models [30].
Another study carried out by Gomes et al. [31] reported on subconjunctival transplantation of BM-MSCs in dogs with experimental corneal ulcer, and BM-MSCs were observed in the injured region. In canine dermatology, wound healing of cutaneous inflammation was accelerated by allogeneic transplantation of BM-MSCs in a Beagle dog [86].
In orthopedics, it has been possible to observe the treatment of osteosarcoma by using canine BM-MSCs and rhBMP-2 [87,88]. Another BM-MSCs application has been shown in a canine orbital wall defect model. After 24 wk, successful bone repair of an orbital wall bone defect was achieved by seeding an autologous canine BM-MSCs onto ß-tricalcium phosphate scaffold for in vivo implantation [89,90]. Success was also achieved in a canine segmental bone defect model [91,92,93]. One of the studies revealed that new bone formation was present at 12 wk post-implant in a Beagle dog [93]. Furthermore, the use of BM-MSCs implantation in fracture repair has been successful in animal models. Autologous and/or exogenous BM-MSCs [94,95] were cultured, loaded onto ceramic cylinders, and implanted into a critically sized segmental bone defect (rat femur); after 8 wk, the implants promoted bone formation for fracture healing.
Numerous reports have shown that BM-MSCs have potency as treatments for congenital, degenerative, vascular, traumatic, and iatrogenic conditions. In veterinary medicine, BM-MSCs have assisted in the reproduction of endangered species and have been used for generating transgenic animals, producing biomedical models, and for pathological condition treatment through transplantation [31]. Some clinical trials have been carried out with BM-MSCs in companion animals, such as osteoarthritis, tendon ligament injury, and intervertebral disk degeneration trials in dogs and horses [96,97] and CKD in cats [98].
In humans, potential applications of BM-MSCs in bone TE have been studied in various bone defects. In a segmental long bone defect of the distal tibia fracture of a 58-year-old woman, bone was actively formed 6 wk after a xenogeneic transplant [99]. Another successful application was in a long-bone defect hip trauma, with bone formation present at 3–6 wk after implantation of microspheres co-immobilized with alginate [100]. Moreover, systemic infusion of allogeneic BM-MSCs produced new bone formation in children with severe osteogenesis imperfecta three months after osteoblast engraftment [101]. Overall, the efficacy of transplantation of BM-MSCs appears to be more effective in acute rather than chronic stage diseases. The advantages and disadvantages of BM-MSCs are summarized in Table 2.
Table 2. Advantages and disadvantages of bone marrow mesenchymal stem cells.
| Advantages | Disadvantages |
|---|---|
| Multiple site collection [107] | Invasive harvesting procedure [108] |
| Capacity of self-renewal [109] | In vitro cell expansion difficulty [108] |
| Multilineage differentiation potential [109] | Limited replication lifespan [110] |
| Easy and high yield of isolation cells [108] | Require younger donors [111] |
| Supporting evidence, both in vitro and in vivo [107] | |
| High osteogenic differentiation performance [110] | |
| Potential for the treatment of several diseases [110] | |
| Autologous, allogeneic, and xenologous are possible [110] | |
| Free from ethical issues [110] | |
| The primary source of stem cells [110] | |
| Higher migration capacity [112] | |
| Lower risk immune rejection [112] | |
| Less susceptible to mutations [113] |
CONCLUSION
To be referred to as stem cells, the cells must show stemness characteristics: pluripotency, gene expression, proliferation, kinetic cell growth, and senescence. The expression of pluripotency genes, such as Oct4, Sox2, and Nanog discriminates stem cells from other cells through the capability to differentiate into other types of cells. Moreover, the growth kinetic, proliferation, and senescence features of stem cells are important in determining their self-renewal property. Understanding the differences and uniqueness of BM-MSCs stemness characteristics across species is critical when maximizing their potential in clinical practice and translational research. Therefore, elucidation of stemness characteristics is crucial in the utilization of BM-MSCs in TE and clinical applications in both human and animal practice.
Footnotes
Funding: Sawangmake C was supported by research supporting grant of the Faculty of Veterinary Science; Chulalongkorn Academic Advancement into Its 2nd Century Project; Veterinary Stem Cell and Bioengineering Research Unit, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University; and Government Research Fund. This work was supported by The First International Conference of Advanced Veterinary Science and Technologies for Sustainable Development (ICAVESS) on April 28–29 2021 in Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Conflict of Interest: The authors declare no conflict of interest.
- Conceptualization: Purwaningrum M, Nantavisai S.
- Data curation: Purwaningrum M, Jamilah NS, Purbantoro SD, Nantavisai S.
- Supervision: Nantavisai S, Sawangmake C.
- Writing - original draft: Purwaningrum M, Jamilah NS, Purbantoro SD.
- Writing - review and editing: Purwaningrum M, Nantavisai S.
References
- 1.de Isla N, Huseltein C, Jessel N, Pinzano A, Decot V, Magdalou J, et al. Introduction to tissue engineering and application for cartilage engineering. Biomed Mater Eng. 2010;20(3):127–133. doi: 10.3233/BME-2010-0624. [DOI] [PubMed] [Google Scholar]
- 2.Gholami A, Dadkhah K, Anijdan SHM. Nanofiber and stem cell to bone, cartilage and muscle tissue engineering. Scholars Acad J Biosci. 2015;3(7):624–626. [Google Scholar]
- 3.Yamzon JL, Kokorowski P, Koh CJ. Stem cells and tissue engineering applications of the genitourinary tract. Pediatr Res. 2008;63(5):472–477. doi: 10.1203/PDR.0b013e31816a704a. [DOI] [PubMed] [Google Scholar]
- 4.Caddeo S, Boffito M, Sartori S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front Bioeng Biotechnol. 2017;5:40. doi: 10.3389/fbioe.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2(1):403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S, Langer R. Advances in tissue engineering. Curr Top Dev Biol. 2004;61:113–134. doi: 10.1016/S0070-2153(04)61005-2. [DOI] [PubMed] [Google Scholar]
- 7.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--Part II: Clinical applications. J Prosthodont Res. 2012;56(4):229–248. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Yuan C, Liu Z, Geng T, Li X, Wei L, et al. Characteristic comparison between canine and human dental mesenchymal stem cells for periodontal regeneration research in preclinical animal studies. Tissue Cell. 2020;67:101405. doi: 10.1016/j.tice.2020.101405. [DOI] [PubMed] [Google Scholar]
- 9.Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, et al. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364(9429):172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- 10.Leuning DG, Reinders ME, Li J, Peired AJ, Lievers E, de Boer HC, et al. Clinical-grade isolated human kidney perivascular stromal cells as an organotypic cell source for kidney regenerative medicine. Stem Cells Transl Med. 2017;6(2):405–418. doi: 10.5966/sctm.2016-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Kehdy H, Pourcher G, Zhang W, Hamidouche Z, Goulinet-Mainot S, Sokal E, et al. Hepatocytic differentiation potential of human fetal liver mesenchymal stem cells: in vitro and in vivo evaluation. Stem Cells Int. 2016;2016:6323486. doi: 10.1155/2016/6323486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Paula DR, Capuano V, Filho DM, Carneiro AC, de Oliveira Crema V, de Oliveira LF, et al. Biological properties of cardiac mesenchymal stem cells in rats with diabetic cardiomyopathy. Life Sci. 2017;188:45–52. doi: 10.1016/j.lfs.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Khatri M, O’Brien TD, Sharma JM. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev. 2009;18(10):1485–1492. doi: 10.1089/scd.2008.0223. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30(8):896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 16.Tropel P, Noël D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295(2):395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Kumar K, Das K, Madhusoodan AP, Kumar A, Singh P, Mondal T, et al. Rat bone marrow derived mesenchymal stem cells differentiate to germ cell like cells. bioRxiv. 2018 Epub ahead of print. doi: 10.1101/418962. [Google Scholar]
- 18.Tan SL, Ahmad TS, Selvaratnam L, Kamarul T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J Anat. 2013;222(4):437–450. doi: 10.1111/joa.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30(8):879–886. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- 20.Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;8(1):150. doi: 10.1186/1746-6148-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Q, Wang Z, Liu W, Shi Y, Cui L, Cao Y. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg. 2001;12(6):586–593. doi: 10.1097/00001665-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2005;319(2):243–253. doi: 10.1007/s00441-004-1012-5. [DOI] [PubMed] [Google Scholar]
- 23.McDaniel JS, Antebi B, Pilia M, Hurtgen BJ, Belenkiy S, Necsoiu C, et al. Quantitative assessment of optimal bone marrow site for the isolation of porcine mesenchymal stem cells. Stem Cells Int. 2017;2017:1836960. doi: 10.1155/2017/1836960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012;21(2):273–283. doi: 10.1089/scd.2010.0589. [DOI] [PubMed] [Google Scholar]
- 25.Sharma AK, Bury MI, Marks AJ, Fuller NJ, Meisner JW, Tapaskar N, et al. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells. 2011;29(2):241–250. doi: 10.1002/stem.568. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH. The advantages and limitations of mesenchymal stem cells in clinical application for treating human diseases. Osteoporos Sarcopenia. 2018;4:150. doi: 10.1016/j.afos.2018.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suto EG, Mabuchi Y, Suzuki N, Suzuki K, Ogata Y, Taguchi M, et al. Prospectively isolated mesenchymal stem/stromal cells are enriched in the CD73+ population and exhibit efficacy after transplantation. Sci Rep. 2017;7(1):4838. doi: 10.1038/s41598-017-05099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28(5):905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quimby JM, Borjesson DL. Mesenchymal stem cell therapy in cats: current knowledge and future potential. J Feline Med Surg. 2018;20(3):208–216. doi: 10.1177/1098612X18758590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes IS, de Oliveira VC, Pinheiro AO, Roballo KCS, de Araujo GSM, Veronezi JC, et al. Bone marrow stem cell applied in the canine veterinary clinics. Pesqui Vet Bras. 2017;37:1139–1145. [Google Scholar]
- 32.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 33.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 35.Dias LD, Casali KR, Ghem C, da Silva MK, Sausen G, Palma PB, et al. Mesenchymal stem cells from sternum: the type of heart disease, ischemic or valvular, does not influence the cell culture establishment and growth kinetics. J Transl Med. 2017;15(1):161. doi: 10.1186/s12967-017-1262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drela K, Stanaszek L, Snioch K, Kuczynska Z, Wrobel M, Sarzynska S, et al. Bone marrow-derived from the human femoral shaft as a new source of mesenchymal stem/stromal cells: an alternative cell material for banking and clinical transplantation. Stem Cell Res Ther. 2020;11(1):262. doi: 10.1186/s13287-020-01697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fragkakis EM, El-Jawhari JJ, Dunsmuir RA, Millner PA, Rao AS, Henshaw KT, et al. Vertebral body versus iliac crest bone marrow as a source of multipotential stromal cells: Comparison of processing techniques, tri-lineage differentiation and application on a scaffold for spine fusion. PLoS One. 2018;13(5):e0197969. doi: 10.1371/journal.pone.0197969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YP, Hong HP, Lee YH, Liu IH. The canine epiphyseal-derived mesenchymal stem cells are comparable to bone marrow derived-mesenchymal stem cells. J Vet Med Sci. 2015;77(3):273–280. doi: 10.1292/jvms.14-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townsend FI., 3rd Bone marrow aspiration in dogs and cats. Lab Anim (NY) 2008;37(11):497–498. doi: 10.1038/laban1108-497. [DOI] [PubMed] [Google Scholar]
- 40.Smajilagić A, Aljičević M, Redžić A, Filipović S, Lagumdžija A. Rat bone marrow stem cells isolation and culture as a bone formative experimental system. Bosn J Basic Med Sci. 2013;13(1):27–30. doi: 10.17305/bjbms.2013.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4(1):102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 42.Mushtaq M, Kovalevska L, Darekar S, Abramsson A, Zetterberg H, Kashuba V, et al. Cell stemness is maintained upon concurrent expression of RB and the mitochondrial ribosomal protein S18-2. Proc Natl Acad Sci U S A. 2020;117(27):15673–15683. doi: 10.1073/pnas.1922535117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aponte PM, Caicedo A. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017;2017:5619472. doi: 10.1155/2017/5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Chang W, Wei H, Zhang K. Comparison of the biological characteristics of mesenchymal stem cells derived from bone marrow and skin. Stem Cells Int. 2016;2016:3658798. doi: 10.1155/2016/3658798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nantavisai S, Pisitkun T, Osathanon T, Pavasant P, Kalpravidh C, Dhitavat S, et al. Systems biology analysis of osteogenic differentiation behavior by canine mesenchymal stem cells derived from bone marrow and dental pulp. Sci Rep. 2020;10(1):20703. doi: 10.1038/s41598-020-77656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humenik F, Cizkova D, Cikos S, Luptakova L, Madari A, Mudronova D, et al. Canine bone marrow-derived mesenchymal stem cells: genomics, proteomics and functional analyses of paracrine factors. Mol Cell Proteomics. 2019;18(9):1824–1835. doi: 10.1074/mcp.RA119.001507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Li C, Gong H. Morphological and functional changes in bone marrow mesenchymal stem cells in rats with heart failure. Exp Ther Med. 2017;13(6):2888–2892. doi: 10.3892/etm.2017.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, et al. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc. 2008;40(8):2649–2654. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136(14):2311–2322. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhandari DR, Seo KW, Roh KH, Jung JW, Kang SK, Kang KS. REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells. PLoS One. 2010;5(5):e10493. doi: 10.1371/journal.pone.0010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matic I, Antunovic M, Brkic S, Josipovic P, Mihalic KC, Karlak I, et al. Expression of OCT-4 and SOX-2 in bone marrow-derived human mesenchymal stem cells during osteogenic differentiation. Open Access Maced J Med Sci. 2016;4(1):9–16. doi: 10.3889/oamjms.2016.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, et al. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20(5):915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- 54.Shahsavari A, Weeratunga P, Ovchinnikov DA, Whitworth DJ. Pluripotency and immunomodulatory signatures of canine induced pluripotent stem cell-derived mesenchymal stromal cells are similar to harvested mesenchymal stromal cells. Sci Rep. 2021;11(1):3486. doi: 10.1038/s41598-021-82856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fafián-Labora J, Fernández-Pernas P, Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, et al. Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci Rep. 2015;5(1):16765. doi: 10.1038/srep16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudhary JK, Rath P. A simple method for isolation, propagation, characterization, and differentiation of adult mouse bone marrow-derived multipotent mesenchymal stem cells. J Cell Sci Ther. 2017;8:261. [Google Scholar]
- 57.Bellotti C, Capanni C, Lattanzi G, Donati D, Lucarelli E, Duchi S. Detection of mesenchymal stem cells senescence by prelamin A accumulation at the nuclear level. Springerplus. 2016;5(1):1427. doi: 10.1186/s40064-016-3091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro . Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 59.Kazemi S, Parivar K, Roudbari NH, Yaghmaei P, Sadeghi B. Growth kinetic comparison of human mesenchymal stem cells from bone marrow, adipose tissue and decidua. Med Sci. 2020;24:223–234. [Google Scholar]
- 60.Bertolo A, Steffen F, Malonzo-Marty C, Stoyanov J. Canine mesenchymal stem cell potential and the importance of dog breed: implication for cell-based therapies. Cell Transplant. 2015;24(10):1969–1980. doi: 10.3727/096368914X685294. [DOI] [PubMed] [Google Scholar]
- 61.Kamishina H, Farese JP, Storm JA, Cheeseman JA, Clemmons RM. The frequency, growth kinetics, and osteogenic/adipogenic differentiation properties of canine bone marrow stromal cells. In Vitro Cell Dev Biol Anim. 2008;44(10):472–479. doi: 10.1007/s11626-008-9137-6. [DOI] [PubMed] [Google Scholar]
- 62.Maciel BB, Rebelatto CLK, Brofman PRS, Brito HFV, Patricio LFL, Cruz MA, et al. Morphology and morphometry of feline bone marrow-derived mesenchymal stem cells in culture. Pesq Vet Bras. 2014;34(11):1127–1134. [Google Scholar]
- 63.Sangeetha P, Maiti S, Divya M, Shivaraju S, Raguvaran R, Rafee MA, et al. Mesenchymal stem cells derived from rat boné marrow (rBM MSC): techniques for isolation, expansion and differentiation. J Stem Cell Res Ther. 2017;3(3):272–277. [Google Scholar]
- 64.Yusop N, Battersby P, Alraies A, Sloan AJ, Moseley R, Waddington RJ. Isolation and characterisation of mesenchymal stem cells from rat bone marrow and the endosteal niche: a comparative study. Stem Cells Int. 2018;2018:6869128. doi: 10.1155/2018/6869128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caroti CM, Ahn H, Salazar HF, Joseph G, Sankar SB, Willett NJ, et al. A novel technique for accelerated culture of murine mesenchymal stem cells that allows for sustained multipotency. Sci Rep. 2017;7(1):13334. doi: 10.1038/s41598-017-13477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y, Lou B, Wu X, Wu R, Wang H, Gao L, et al. Comparative study on in vitro culture of mouse bone marrow mesenchymal stem cells. Stem Cells Int. 2018;2018:6704583. doi: 10.1155/2018/6704583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 68.Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13(6):675–685. doi: 10.3109/14653249.2010.549122. [DOI] [PubMed] [Google Scholar]
- 69.Ridzuan N, Al Abbar A, Yip WK, Maqbool M, Ramasamy R. Characterization and expression of senescence marker in prolonged passages of rat bone marrow-derived mesenchymal stem cells. Stem Cells Int. 2016;2016:8487264. doi: 10.1155/2016/8487264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karaoz E, Aksoy A, Ayhan S, Sariboyaci AE, Kaymaz F, Kasap M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol. 2009;132(5):533–546. doi: 10.1007/s00418-009-0629-6. [DOI] [PubMed] [Google Scholar]
- 71.Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg. 2012;14(2):165–168. doi: 10.1177/1098612X11429224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schultz MB, Sinclair DA. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143(1):3–14. doi: 10.1242/dev.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3(10):640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 74.Wu J, Niu J, Li X, Wang X, Guo Z, Zhang F. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14(1):21. doi: 10.1186/1471-213X-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang K, Huang L, Sun H, Zhu Y, Xiao Y, Huang M, et al. Role of Notch expression in premature senescence of murine bone marrow stromal cells. Prog Nat Sci. 2009;19(5):557–562. [Google Scholar]
- 76.Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep. 2020;10(1):4290. doi: 10.1038/s41598-020-61167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bearden RN, Huggins SS, Cummings KJ, Smith R, Gregory CA, Saunders WB. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study. Stem Cell Res Ther. 2017;8(1):218. doi: 10.1186/s13287-017-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russell KA, Chow NH, Dukoff D, Gibson TW, LaMarre J, Betts DH, et al. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS One. 2016;11(12):e0167442. doi: 10.1371/journal.pone.0167442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 80.Hu Y, Lou B, Wu X, Wu R, Wang H, Gao L, et al. Immunophenotype and differentiation capacity of bone marrow-derived mesenchymal stem cells from CBA/Ca, ICR and Balb/c mice. World J Stem Cells. 2013;5(1):34–42. doi: 10.4252/wjsc.v5.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51(8):723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 82.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89(6):1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 83.Truong NH, Nguyen NH, Le TV, Vu NB, Huynh N, Nguyen TV, et al. Comparison of the treatment efficiency of bone marrow-derived mesenchymal stem cell transplantation via tail and portal veins in CCl4-induced mouse liver fibrosis. Stem Cells Int. 2016;2016:5720413. doi: 10.1155/2016/5720413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang E, Yang Y, Zhang J, Ding G, Chen S, Peng C, et al. Efficacy of bone marrow mesenchymal stem cell transplantation in animal models of pulmonary fibrosis after exposure to bleomycin: A meta-analysis. Exp Ther Med. 2019;17(3):2247–2255. doi: 10.3892/etm.2019.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shiota Y, Nagai A, Sheikh AM, Mitaki S, Mishima S, Yano S, et al. Transplantation of a bone marrow mesenchymal stem cell line increases neuronal progenitor cell migration in a cerebral ischemia animal model. Sci Rep. 2018;8(1):14951. doi: 10.1038/s41598-018-33030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JW, Lee JH, Lyoo YS, Jung DI, Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013;24(2):242–e53. doi: 10.1111/vde.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rici RE, Alcântara D, Fratini P, Wenceslau CV, Ambrósio CE, Miglino MA, et al. Mesenchymal stem cells with rhBMP-2 inhibits the growth of canine osteosarcoma cells. BMC Vet Res. 2012;8(1):17. doi: 10.1186/1746-6148-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang W, Lin D, Yu Y, Niu H, Guo H, Yuan Y, et al. Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomater. 2016;32:309–323. doi: 10.1016/j.actbio.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Bi X, Zhou H, Deng Y, Sun J, Xiao C, et al. Repair of orbital bone defects in canines using grafts of enriched autologous bone marrow stromal cells. J Transl Med. 2014;12(1):123. doi: 10.1186/1479-5876-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng Y, Zhou H, Yan C, Wang Y, Xiao C, Gu P, et al. In vitro osteogenic induction of bone marrow stromal cells with encapsulated gene-modified bone marrow stromal cells and in vivo implantation for orbital bone repair. Tissue Eng Part A. 2014;20(13-14):2019–2029. doi: 10.1089/ten.TEA.2013.0604. [DOI] [PubMed] [Google Scholar]
- 91.Zhu Y, Zhang K, Zhao R, Ye X, Chen X, Xiao Z, et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials. 2017;147:133–144. doi: 10.1016/j.biomaterials.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 92.Yuan J, Cui L, Zhang WJ, Liu W, Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous β-tricalcium phosphate. Biomaterials. 2007;28(6):1005–1013. doi: 10.1016/j.biomaterials.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 93.Yuan J, Zhang WJ, Liu G, Wei M, Qi ZL, Liu W, et al. Repair of canine mandibular bone defects with bone marrow stromal cells and coral. Tissue Eng Part A. 2010;16(4):1385–1394. doi: 10.1089/ten.TEA.2009.0472. [DOI] [PubMed] [Google Scholar]
- 94.Kadiyala S, Jaiswal N, Bruder SP. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997;3(2):173–185. [Google Scholar]
- 95.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16(2):155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 96.Mocchi M, Dotti S, Bue MD, Villa R, Bari E, Perteghella S, et al. Veterinary regenerative medicine for musculoskeletal disorders: can mesenchymal stem/stromal cells and their secretome be the new frontier? Cells. 2020;9(6):1453. doi: 10.3390/cells9061453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulus M, Kulus J, Jankowski M, Borowiec B, Jeseta M, Bukowska D, et al. The use of mesenchymal stem cells in veterinary medicine. Med J Cell Biol. 2018;6(3):101–107. [Google Scholar]
- 98.Quimby JM, Webb TL, Gibbons DS, Dow SW. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg. 2011;13(6):418–426. doi: 10.1016/j.jfms.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hesse E, Kluge G, Atfi A, Correa D, Haasper C, Berding G, et al. Repair of a segmental long bone defect in human by implantation of a novel multiple disc graft. Bone. 2010;46(5):1457–1463. doi: 10.1016/j.bone.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 100.Grellier M, Granja PL, Fricain JC, Bidarra SJ, Renard M, Bareille R, et al. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials. 2009;30(19):3271–3278. doi: 10.1016/j.biomaterials.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 101.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 102.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 103.Tharasanit T, Phutikanit N, Wangdee C, Soontornvipart K, Tantrajak S, Kaewamatawong T, et al. Differentiation potentials of canine bone marrow mesenchymal stem cells. Wetchasan Sattawaphaet. 2011;41:79. [Google Scholar]
- 104.Rodprasert W, Nantavisai S, Pathanachai K, Pavasant P, Osathanon T, Sawangmake C. Tailored generation of insulin producing cells from canine mesenchymal stem cells derived from bone marrow and adipose tissue. Sci Rep. 2021;11(1):12409. doi: 10.1038/s41598-021-91774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghasemzadeh-Hasankolaei M, Batavani R, Eslaminejad MB, Sayahpour F. Transplantation of autologous bone marrow mesenchymal stem cells into the testes of infertile male rats and new germ cell formation. Int J Stem Cells. 2016;9(2):250–263. doi: 10.15283/ijsc16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lotfy A, El-Sherbiny YM, Cuthbert R, Jones E, Badawy A. Comparative study of biological characteristics of mesenchymal stem cells isolated from mouse bone marrow and peripheral blood. Biomed Rep. 2019;11(4):165–170. doi: 10.3892/br.2019.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translat. 2015;3:95–104. doi: 10.1016/j.jot.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nantavisai S, Egusa H, Osathanon T, Sawangmake C. Mesenchymal stem cell-based bone tissue engineering for veterinary practice. Heliyon (Lond) 2019;5(11):e02808. doi: 10.1016/j.heliyon.2019.e02808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sawangmake C, Nantavisai S, Osathanon T, Pavasant P. Osteogenic differentiation potential of canine bone marrow-derived mesenchymal stem cells under different β-glycerophosphate concentrations in vitro . Wetchasan Sattawaphaet. 2016;46:617–625. [Google Scholar]
- 110.Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21:1–10. doi: 10.12717/DR.2017.21.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arnhold S, Elashry MI, Klymiuk MC, Wenisch S. Biological macromolecules and mesenchymal stem cells: Basic research for regenerative therapies in veterinary medicine. Int J Biol Macromol. 2019;123:889–899. doi: 10.1016/j.ijbiomac.2018.11.158. [DOI] [PubMed] [Google Scholar]
- 112.Voga M, Adamic N, Vengust M, Majdic G. Stem Cells in Veterinary Medicine-Current State and Treatment Options. Front Vet Sci. 2020;7:278. doi: 10.3389/fvets.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1(1):113–119. doi: 10.1016/j.gendis.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]