Abstract
Background
Naringin and its aglycone naringenin are citrus-derived flavonoids with several pharmacological effects. On the other hand, the mechanism for the anti-diabetic effects of naringenin and naringin are controversial and remain to be clarified further.
Objective
This study examined the relationship between glucose uptake and AMP-activated protein kinase (AMPK) phosphorylation by naringenin and naringin in high glucose-treated HepG2 cells.
Methods
Glucose uptake was measured using the 2-NBDG fluorescent D-glucose analog. The phosphorylation levels of AMPK and GSK3β (Glycogen synthase kinase 3 beta) were observed by Western blotting. Molecular docking analysis was performed to evaluate the binding affinity of naringenin and naringin to the γ-subunit of AMPK.
Results
The treatment with naringenin and naringin stimulated glucose uptake regardless of insulin stimulation in high glucose-treated HepG2 cells. Both flavonoids increased glucose uptake by promoting the phosphorylation of AMPK at Thr172 and increased the phosphorylation of GSK3β. Molecular docking analysis showed that both naringenin and naringin bind to the γ-subunit of AMPK with high binding affinities. In particular, naringin showed higher binding affinity than the true modulator, AMP with all three CBS domains (CBS1, 3, and 4) in the γ-subunit of AMPK. Therefore, both naringenin and naringin could be positive modulators of AMPK activation, which enhance glucose uptake regardless of insulin stimulation in high glucose-treated HepG2 cells.
Conclusions
The increased phosphorylation of AMPK at Thr172 by naringenin and naringin might enhance glucose uptake regardless of insulin stimulation in high glucose treated HepG2 cells.
Keywords: Naringenin, naringin, glucose uptake, AMPK phosphorylation, molecular docking
INTRODUCTION
AMPK activation is involved in non-insulin mediated glucose uptake, suggesting it is a potential strategy to enhance glucose uptake in an insulin-resistant state, such as Type 2 diabetes [1]. AMPK is activated by a low energy status (increased AMP/ADP:ATP) and regulates the metabolic process and energy homeostasis by triggering ATP-generating processes, including glucose uptake and fatty acid oxidation [2]. The activation of AMPK regulates insulin-independent glucose uptake via stimulating GLUT2 translocation and regulating the systemic glucose homeostasis [3]. Once activated, AMPK phosphorylates GSK3, which is responsible for maintaining glucose homeostasis [4,5]. The phosphorylation and inhibition of GSK3 activate glycogen synthase (GS), which is a target for glycogen synthesis [6]. Therefore, AMPK is an important pharmaceutical target for the treatment of diabetes [1].
AMPK is inactive unless phosphorylated on the α-subunit activation loop at Thr172. On the other hand, AMP binding to the γ-subunit enhances Thr172 phosphorylation by upstream kinases, liver kinase B1 (LKB1), and Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) [7]. A previous study reported that flavonoids and other polyphenolic compounds are possible activators of AMPK, which have similar functions to adenosine nucleotides [8,9].
Naringin is a flavanone glycoside found in grapes and citrus fruits. Naringenin is formed by the attachment of two rhamnose units to the 7-carbon of naringin (Fig. 1A) [10]. For naringenin and naringin, diverse biological activities of therapeutic interests have been described, including anti-diabetic effects [11,12]. On the other hand, the mechanisms through which these flavonoids promote glucose uptake in the insulin-resistant state remains to be clarified.
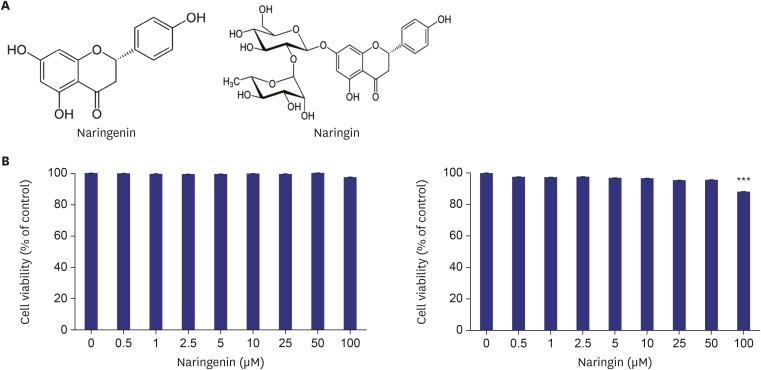
Fig. 1. Effect of naringenin and naringin on the viability of HepG2 cells. Chemical structures of naringenin and naringin (A). Effect of naringenin and naringin on the HepG2 cell viability. HepG2 cells were cultured at a density of 1 × 105 in a 96 well plate. After reaching confluence, the cells were treated with 0–100 µM of flavonoids for 24 h, and the cell viability was measured using a MTT assay (B). The values are the mean ± SE.
***p < 0.0005 vs. control.
The present study hypothesized that naringenin and naringin increase glucose uptake by activating the AMPK pathway in high glucose-treated HepG2 cells. For this, this study examined the effects of naringenin and naringin on the glucose uptake and the phosphorylation of AMPK and GSK3β in high glucose-treated HepG2 cells. In addition, molecular docking analysis was conducted to observe the binding affinity of naringenin and naringin to the γ-subunit of AMPK.
MATERIALS AND METHODS
Cell culture and treatment
HepG2 cells (KCLB 42707) were obtained from the Korean Cell Line Bank (KCLB) (Seoul, Korea) and cultured routinely in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, USA) in a humidified atmosphere containing 5% CO2 at 37°C. After reaching confluence, the cells were then seeded in culture plates for further experiments.
The insulin-resistant HepG2 cell model was established using the reported method with slight modifications [13]. Briefly, after seeding in 96 well plates, the cells were serum-starved for 12 h. and incubated in serum-free DMEM (Gibco, USA) containing either normal concentrations of glucose (5.5 mM D-glucose) or high concentrations of glucose (30 mM D-glucose) with or without the flavonoids (Sigma-Aldrich, USA) (10 and 50µM) and metformin (Sigma-Aldrich, USA) (2 mM) for an additional 24 h. The high glucose-treated cells were used as the insulin-resistant model. The cells were stimulated with or without 100 nM insulin (Sigma-Aldrich, USA) for 30 min before harvesting.
Cell viability assay
The HepG2 cells were seeded in 96-well plates at a cell density of 1 × 105 cells/well and cultured overnight using routine culture media. After reaching confluence, the cells were treated with different concentrations (0–100 µM) of the flavonoids for 24 hrs. The cell culture medium was removed, and fresh medium containing 10% Ez-cytox (DogenBio, Korea) was added to each well, according to the manufacturer's instructions. The plates were incubated for 3 h at 37°C and 5% CO2. The cell viability indicated by formazan production was measured using an ELISA microplate reader (TECAN, Austria) at 450 nm.
2- NBDG glucose uptake
HepG2 cells were cultured in 96-well plates. After reaching confluence, the cells were serum-starved for 12 h and incubated in a serum-free medium containing either normal or high glucose concentrations with or without the samples (flavonoids 10 µM and 50 µM) for an additional 24 h. The cells were incubated with 40 μM 2-NBDG [2-N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino-2-deoxy-d-glucose] (Invitrogen, Carlsbad, USA) with or without 100 nM insulin (Sigma-Aldrich, USA) for 30 min at 37°C. The cells were washed three times with PBS, and the fluorescent images were taken from IncuCyte ZOOM Fluorescent Microscope at 20× magnification (Essen BioScience, Inc. USA). IncuCyte ZOOM Fluorescent Processing Software was used to measure the total fluorescent intensities of each well.
Western blotting
The cells were washed with PBS (Gibco, USA) and lysed with ice-cold RIPA buffer (Tech and Innovation, Korea) containing protease inhibitor mixture. The whole-cell lysates were centrifuged at 12,000 rpm for 10 min. The supernatant was separated, and the amount of protein was assessed using a Bradford assay (Bio-Rad Laboratories, USA). Equal amounts of protein from the cell homogenates were subjected to SDS PAGE and transferred to PVDF membranes. The membranes were probed with the primary antibodies, GSK3β, p-GSK3β, AMPK, and p-AMPK (Cell signaling technology, USA), and detected with peroxidase-conjugated secondary antibodies for 1hr at room temperature. β-actin (Thermofisher, USA) was used as a loading control. A chemiluminescence bioimaging instrument (NeoScience Co., Ltd., Korea) was used to detect the proteins of interest. Densitometry analysis was performed using ImageJ analysis software.
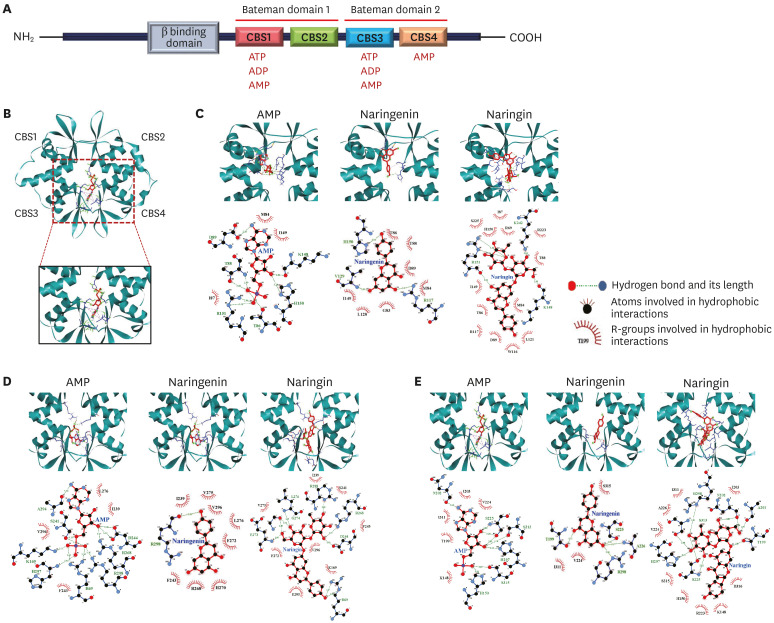
Molecular docking
The crystal structure of AMPK in complexes with AMP (PDB ID; 2V8Q) was obtained from Protein Data Bank (PDB). The sitemap tool (Schrodinger Software, Germany) was used to identify the four CBS domains (CBS1, CBS2, CBS3, and CBS4) in the γ-subunit of AMPK. All the AMP molecules in the γ-subunit of AMPK were removed for docking of the flavonoids. The 3D structures of AMP and flavonoids (naringenin and naringin) were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and minimized energetically using PyRx software (Python Prescription 0.8, The Scripps Research Institute). The grid box used for focused docking was set to 26 × 44 × 46 Å to ensure the structure of the γ-subunit of AMPK. The docking experiments were carried out using the AutoDock Vina module (Molecular Graphics Lab, The Scripps Research Institute, USA). The best-docked pose was selected based on binding energy, and 3D images were generated using the PyMOL (The PyMOL Molecular Graphics System, Ver.2.5.0, Schrodinger, LLC, USA). The docked complex of AMPK was optimized further, validated, and explored using the Discovery Studio visualizer (Ver.21.1.0.20298). The hydrogen bond and hydrophobic interactions between the receptor and ligand were analyzed using the Ligplot program [14].
Statistical analysis
The values are presented as the mean ± SE for each group. Statistical analysis was performed using IBM SPSS Statistics (Ver.17.0; USA) and one-way analysis of variance with Tukey's post hoc tests for multi-group comparisons. The p values < 0.05 were considered significant.
RESULTS
Cell viability
The MTT assay was performed to assess the cellular toxicity of naringenin and naringin on HepG2 cells. No significant cellular toxicity was observed in HepG2 cells at flavonoid concentrations up to 50 μM (Fig. 1B). Accordingly, the experiments were conducted using the non-toxic concentrations of naringenin and naringin (10 and 50 µM).
Glucose uptake
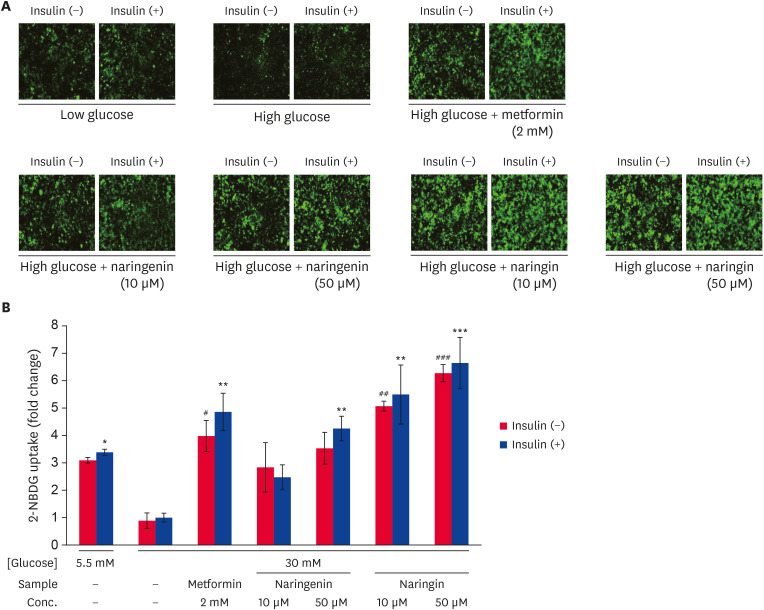
The effects of naringenin and naringin on the glucose uptake in high glucose-treated HepG2 cells were determined using a 2-NBDG uptake assay (Fig. 2). The glucose uptake was reduced in the high glucose-treated HepG2 cells compared to low glucose-treated cells. In contrast, the treatment of metformin (positive control) resulted in a significant increase (p < 0.005) the 2-NBDG uptake compared to the high glucose-treated HepG2 cells (Fig. 2A and B). In addition, the naringenin and naringin treatment increased the 2-NBDG glucose uptake in high glucose treated cells, regardless of insulin stimulation (Fig. 2A and B). In particular, the naringin treatment showed higher glucose uptake than the naringenin treatment in high glucose treated cells. The results suggest that naringenin and naringin enhanced the glucose uptake in high glucose-treated HepG2 cells regardless of insulin stimulation.
Fig. 2. Effect of naringenin and naringin on glucose uptake. The glucose uptake assay was done using the fluorescent D-glucose analog 2-NBDG. The HepG2 cells were serum-starved for 12 h and incubated in serum-free medium containing either normal (5.5 mM) or high glucose (30 mM) concentrations for an additional 24 h in the presence and absence of samples. The cells were then stimulated with or without insulin (100 nM) and 2-NBDG (50 uM) for 30 min. 2-NBDG uptake by cells was detected by IncuZyte Zoom at 20× magnification. Representative images for each sample (A), Quantitative 2NBDG uptake from each sample (B). The values are mean ± SE.
*p < 0.05, **p < 0.005, and ***p < 0.0005 vs. insulin-stimulated high glucose control and #p < 0.05, ##p < 0.005, and ###p < 0.0005 vs. without insulin-stimulated high glucose control.
Phosphorylation of AMPK
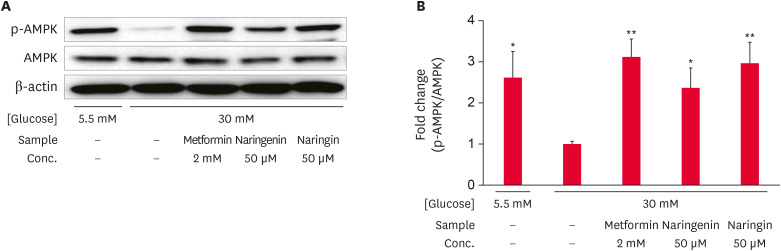
Western blot analysis was conducted to observe the effects of naringenin and naringin on AMPK phosphorylation (Fig. 3). The levels of AMPK phosphorylation (Thr172) in high glucose-treated HepG2 cells were decreased significantly (p < 0.05) compared to low glucose-treated cells. In contrast, the level of AMPK phosphorylation (Thr172) was increased significantly by the metformin, naringenin, or naringin treatments in high glucose-treated HepG2 cells (Fig. 3A and B). The effects of naringin on the levels of AMPK phosphorylation (Thr172) were comparatively higher than the effect of naringenin. The results suggest that both naringenin and naringin increased the level of AMPK phosphorylation in high glucose-treated HepG2 cells.
Fig. 3. Effect of naringenin and naringin on the phosphorylation of AMPK. HepG2 cells were starved in serum-free medium for 12 h and incubated in serum-free medium containing either normal (5.5 mM) or high (30 mM) glucose concentrations with or without different samples for an additional 24 h. The total cell extract was subjected to Western blot to observe the phosphorylation of AMPK. Immunoblot bands of AMPK (A), and the result of quantitative analysis of the ratio of p-AMPK to AMPK (B). Values are the mean ± SE.
AMPK, AMP-activated protein kinase.
*p < 0.05 and **p < 0.05 compared to high glucose control.
Phosphorylation of GSK3β
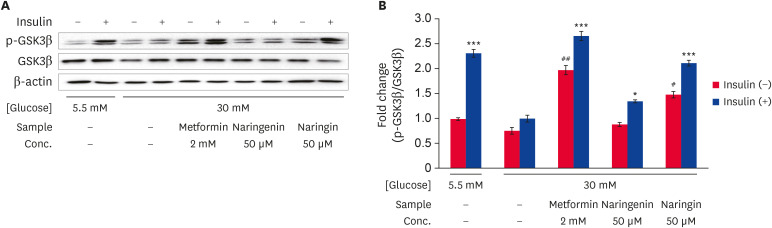
Western blotting was performed to determine the effects of naringenin and naringin on the level of GSK3β phosphorylation (Ser9) (Fig. 4). The phosphorylation levels of GSK3β (Ser9) in high glucose treated HepG2 cells were decreased significantly (p < 0.0005) compared to the low glucose-treated cells. In contrast, the levels of GSK3β phosphorylation were increased by the metformin, naringenin, or naringin treatment compared to high glucose-treated cells in either the presence or absence of insulin. In particular, the treatment of naringin showed significantly higher (p < 0.0005) levels of GSK3β phosphorylation compared to naringenin treatment (Fig. 4A and B). These results suggest that both naringenin and naringin increased the levels of GSK3β phosphorylation (Ser9) in high glucose-treated HepG2 cells regardless of insulin stimulation.
Fig. 4. Effect of naringenin and naringin on the phosphorylation of GSK3β. HepG2 cells were starved in serum-free medium for 12 h and incubated in serum-free medium containing either normal (5.5 mM) or high (30 mM) glucose concentrations with or without different samples for an additional 24 h. Before harvesting, the cells were stimulated with or without 100 nM insulin for 30 min. The total cell extract was subjected to Western blot to observe the phosphorylation of GSK3β. Immunoblot band pattern of GSK3β (A), and the results of quantitative analysis of the ratio of p-GSK3β to GSK3β (B). The values are the mean ± SE.
*p < 0.05, ***p < 0.0005 compared with high glucose control and #p < 0.05 and ##p < 0.005 vs. without insulin-stimulated high glucose control.
Molecular docking analysis
Molecular docking analysis was performed to simulate the binding affinity between the flavonoids and γ-subunit of AMPK (Fig. 5). As shown in Fig. 5A, the γ-subunit of AMPK contains four cystathionine β-synthase (CBS) domains. Among the four CBS domains, however, only three CBS (CBS1, CBS3, and CBS4) domains can bind to their modulator, adenosine nucleotides (ATP, ADP, or AMP). Both naringenin and naringin bind to each of the binding sites (CBS1, 3, and 4) in the γ-subunit of AMPK with high binding affinities, which were close to the affinity of AMP (Table 1). With all CBS domains (CBS1, 3, and 4), naringin showed higher binding affinities than the true modulator, AMP (Table 1). In particular, naringin (−9.1 kcal/mol) showed higher binding affinity than naringenin (−7.6 kcal/mol) or AMP (-8.1 kcal/mol) at the CBS4 domain. With all CBS domains (CBS1, 3, and 4), naringin forms more hydrogen bonds or hydrophobic interactions than AMP or naringenin (Table 1). The results suggest that both naringenin and naringin could be potent positive modulators for AMPK activation.
Fig. 5. Molecular docking analysis of naringenin and naringin binding to AMPK. Schematic representation of the γ-subunit of AMPK (A). The view of AMPK γ-subunit structure illustrating nucleotide-binding sites named according to their CBS sequences (B). Docked complexes of AMP, naringenin, and naringin with AMPK at CBS1 (C), CBS 3 (D), and CBS 4 (E). The red arches indicate the residues participating in hydrophobic interactions, and the dotted lines indicate hydrogen bonds. Full red circles denote the residues present in the binding sites of naringenin, naringin, and AMP.
AMPK, AMP-activated protein kinase.
Table 1. Binding energy of AMP and flavonoids at three sites that were identified in the γ-subunit after removing all the co-crystallized ligands.
| Molecule | Description | Binding site of γ-subunit of AMPK | ||
|---|---|---|---|---|
| CBS1 | CBS3 | CBS4 | ||
| AMP | Binding energy (kcal/mol) | −7.8 | −7.4 | −8.1 |
| Hydrogen bonds | D89, R151, T88, T86, K148, H150 | A294, R69, R298, R268, D244, H297, K169, S241 | N202, S225, S313, S315, H150, H297 | |
| Hydrophobic interactions | M84, I149, I87 | L276, I239, F243, V296 | I203, V224, I311, T199, K148 | |
| Naringenin | Binding energy (kcal/mol) | −7.7 | −8.0 | −7.6 |
| Hydrogen bonds | R117, H150, V129 | R298 | A226, R298, T199, S225 | |
| Hydrophobic interactions | D89, I149, L128, G83, T86, T88, M84 | R268, I239, H270, F243, F272, L276, V275, V296 | S315, V224, I311 | |
| Naringin | Binding energy (kcal/mol) | −8.5 | −8.3 | −9.1 |
| Hydrogen bonds | R151, K242, K148 | R298, R268, R69, D244, G273, G247, L276 | R298, N202, A201, H297, S313, S225, T99 | |
| Hydrophobic interactions | R223, R117, D89, R69, S225, T88, T86, W116, M84, H150, I87, I149, L121 | I239, S241, F243, V296, K169, G295, F272, V275 | A226, R223, D316, I311, I203, H150, K148, S315, V224 | |
DISCUSSION
This study examined the effects of naringenin and naringin on glucose uptake and AMPK phosphorylation in high glucose-treated HepG2 cells. The naringenin and naringin treatment showed increased glucose uptake with increased AMPK phosphorylation (Thr172) and GSK3β. Molecular docking simulation showed that both naringenin and naringin bind to the cavity of the γ-subunit of AMPK with high binding affinities, suggesting that the flavonoids could be positive modulators for AMPK activation.
Naringenin and naringin enhanced the glucose uptake significantly regardless of the insulin stimulation in high glucose treated HepG2 cells, as observed previously [15]. Hepatic glucose uptake is largely an insulin-independent process, which contributes to whole-body glucose homeostasis [16]. For example, hepatic glucose uptake can be enhanced by the entry of glucose into the portal vein, which stimulates a portal glucose signal [17]. When hyperglycemia occurs, plasma glucose enters the cytoplasm of the hepatocytes through GLUT2, which is an insulin-independent system [18]. In this process, AMPK suppresses gluconeogenesis in the liver and promotes glucose uptake in peripheral tissues [19].
In the liver, AMPK enhances glucose uptake by up-regulating the expression of GLUT2 [18]. In addition, the activation of AMPK suppresses hepatic gluconeogenesis and promotes glycogen synthesis by the direct phosphorylation of its substrates, including GSK3β, which will ultimately increase the glucose uptake by the liver [20,21]. The AMPK-induced phosphorylation of GSK3β inhibited the transcriptional activity of the cAMP response element-binding protein (CREB), a key transcription factor, which regulates the phosphorylation of gluconeogenic enzymes [20].
AMPK is a heterotrimer consisting of catalytic α subunits and regulatory β and γ subunits [22]. The phosphorylation of Thr172 within the activation loop of the α-subunit can cause AMPK activation [7]. AMP is a natural activator of AMPK and binds to the CBS domains of the γ-subunit and indirectly promotes the activity of the catalytic domain in α-subunit [23]. The binding of AMP to the γ-subunit of AMPK stimulates the phosphorylation of Thr172 in the α-subunit by upstream kinases LKB1 and CaMKKβ, and prevents the dephosphorylation of Thr172 in α-subunit [24,25]. In addition, a recent study suggested that transforming growth factor β-activated kinase (TAK1) phosphorylates AMPK on Thr172 through AMP binding [26]. Therefore, AMP binding to CBS causes allosteric activation subsequent to the phosphorylation of Thr172 in the activation loop of the α-subunit, which results in AMPK activation [25].
The molecular docking results suggest that both naringenin and naringin bind to the cavity of γ-subunit of AMPK with high binding affinities. AMP and flavonoids interacted with the R-groups of amino acids in CBS domains 1, 3, and 4 of the γ-subunit. In particular, naringin showed higher binding affinities than AMP in the CBS domains 1, 3, and 4 of the γ-subunit, where naringin forms a larger number of hydrophobic interactions or hydrogen bonds than true modulator, AMP. The results suggest that naringin could be a potent positive modulator for activating AMPK. In addition, both naringenin and naringin enhanced AMPK phosphorylation at Thr172, which might enhance glucose uptake by stimulating GLUT2 translocation in HepG2 cells. These results suggest that both naringenin and naringin could positively modulate AMPK activation, enhancing glucose uptake.
As reported previously, flavonoid glucuronic acids had a beneficial effect on glucose homeostasis with high binding affinities to AMPK [27]. A recent study reported that Lippia citriodora-derived polyphenols act as direct agonists of AMPK by binding to the AMP binding sites of the γ-subunit and the interaction zones between the γ and β-subunits [28]. An in silico study on Chinese medicinal compounds eugenyl β-D-glucopyranoside and 6-O-cinnamoyl-D-glucopyranose revealed similar functions as AMP for AMPK activation [8]. Overall, the molecular docking analysis of the present study confirmed that both naringenin and naringin could be positive modulators of AMPK activation.
Naringin has a higher anti-diabetic potential than naringenin. In general, glycosides have higher biological activities than their aglycones because the bound sugar modifies the hydrophilicity of glyscosides, enhancing the bioavailability of the compounds [29,30]. On the other hand, the glycoside linkages are unstable under the acidic conditions in the stomach. In addition, the cleavage of sugar moieties of naringin by glycosidase from intestinal bacteria will produce the aglycone, naringenin [31]. Therefore, the intestinal absorption of naringenin should be clarified [32]. In the present study, however, naringin, being a glycoside, had a higher anti-diabetic effect than its aglycone naringenin on HepG2 cells.
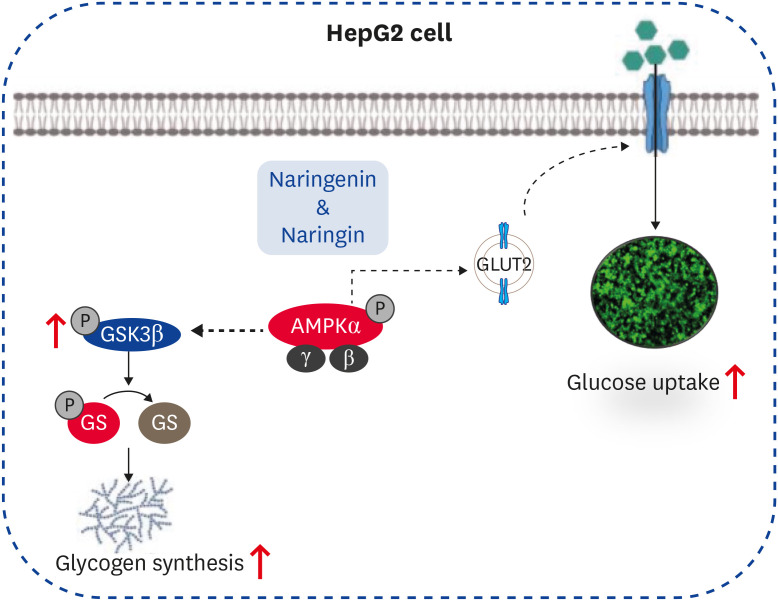
Molecular docking results showed that both naringenin and naringin are potential AMPK activators with high binding affinities to the γ-subunit of AMPK. These findings suggest that the glucose uptake by naringenin and naringin depends on the phosphorylation of AMPK at Thr172, which will enhance glucose uptake by stimulating GLUT2 translocation in HepG2 cells (Fig. 6). Overall, the results suggest that both naringenin and naringin could be positive modulators of AMPK activation, enhancing glucose uptake regardless of insulin stimulation in high glucose-treated HepG2 cells. Nevertheless, further efforts will be needed to define the precise role of naringenin and naringin on the glucose uptake underlying the AMPK pathway in HepG2 cells.
Fig. 6. Proposed model for the enhanced glucose uptake effect of naringenin and naringin on HepG2 cells.
AMPK, AMP-activated protein kinase.
Footnotes
Funding: This work was supported by Korean Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Innovational Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant No.: 11901303).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Han CH, Dayarathne LA.
- Data curation: Ranaweera SS.
- Funding acquisition: Han CH.
- Investigation: Dayarathne LA, Han CH.
- Resources: Han CH.
- Software: Premkumar N, Rajan P.
- Supervision: Han CH.
- Visualization: Dayarathne LA, Han CH.
- Writing - original draft: Dayarathne LA.
- Writing - review & editing: Premkumar N, Rajan P.
References
- 1.Krook A, Wallberg-Henriksson H, Zierath JR. Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc. 2004;36(7):1212–1217. doi: 10.1249/01.mss.0000132387.25853.3b. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J. 2013;37(1):1–21. doi: 10.4093/dmj.2013.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7(5):1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Bridges D, Nakada D, Skiniotis G, Morrison SJ, Lin JD, et al. Inhibition of AMPK catabolic action by GSK3. Mol Cell. 2013;50(3):407–419. doi: 10.1016/j.molcel.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan HD, Kim DY, Quan HY, Kim SJ, Jung MS, Chung SH. Ginsenoside Rg2 induces orphan nuclear receptor SHP gene expression and inactivates GSK3β via AMP-activated protein kinase to inhibit hepatic glucose production in HepG2 cells. Chem Biol Interact. 2012;195(1):35–42. doi: 10.1016/j.cbi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156(6):885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574(Pt 1):7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang HC, Chen CY. In silico design for adenosine monophosphate-activated protein kinase agonist from traditional chinese medicine for treatment of metabolic syndromes. Evid Based Complement Alternat Med. 2014;2014:928589. doi: 10.1155/2014/928589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong Y, Shin SY, Jung Y, Jung H, Ahn S, Chong Y, et al. Flavonoids activating adenosine monophosphate-activated protein kinase. J Korean Soc Appl Biol Chem. 2015;58(1):13–19. [Google Scholar]
- 10.Ishii K, Furuta T, Kasuya Y. Determination of naringin and naringenin in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996;683(2):225–229. doi: 10.1016/0378-4347(96)00114-4. [DOI] [PubMed] [Google Scholar]
- 11.Dhanya R, Arun KB, Nisha VM, Syama HP, Nisha P, Santhosh Kumar TR, et al. Preconditioning L6 muscle cells with naringin ameliorates oxidative stress and increases glucose uptake. PLoS One. 2015;10(7):e0132429. doi: 10.1371/journal.pone.0132429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dayarathne LA, Ranaweera SS, Natraj P, Rajan P, Lee YJ, Han CH. Restoration of the adipogenic gene expression by naringenin and naringin in 3T3-L1 adipocytes. J Vet Sci. 2021;22(4):e55. doi: 10.4142/jvs.2021.22.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005;64(3):207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Kramer B, Rarey M, Lengauer T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins. 1999;37(2):228–241. doi: 10.1002/(sici)1097-0134(19991101)37:2<228::aid-prot8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed OM, Hassan MA, Abdel-Twab SM, Abdel Azeem MN. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed Pharmacother. 2017;94:197–205. doi: 10.1016/j.biopha.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 16.Jung HA, Paudel P, Seong SH, Min BS, Choi JS. Structure-related protein tyrosine phosphatase 1B inhibition by naringenin derivatives. Bioorg Med Chem Lett. 2017;27(11):2274–2280. doi: 10.1016/j.bmcl.2017.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab. 2014;16(Suppl 1):33–40. doi: 10.1111/dom.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo . Adv Nutr. 2012;3(3):286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- 20.Kang OH, Shon MY, Kong R, Seo YS, Zhou T, Kim DY, et al. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement Altern Med. 2017;17(1):341. doi: 10.1186/s12906-017-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan HD, Piao GC. An active part of Artemisia sacrorum Ledeb. suppresses gluconeogenesis through AMPK mediated GSK3β and CREB phosphorylation in human HepG2 cells. Biosci Biotechnol Biochem. 2011;75(6):1079–1084. doi: 10.1271/bbb.100881. [DOI] [PubMed] [Google Scholar]
- 22.Ren Z, Xie Z, Cao D, Gong M, Yang L, Zhou Z, et al. C-Phycocyanin inhibits hepatic gluconeogenesis and increases glycogen synthesis via activating Akt and AMPK in insulin resistance hepatocytes. Food Funct. 2018;9(5):2829–2839. doi: 10.1039/c8fo00257f. [DOI] [PubMed] [Google Scholar]
- 23.Bung N, Surepalli S, Seshadri S, Patel S, Peddasomayajula S, Kummari LK, et al. 2-[2-(4-(trifluoromethyl)phenylamino)thiazol-4-yl]acetic acid (Activator-3) is a potent activator of AMPK. Sci Rep. 2018;8(1):9599. doi: 10.1038/s41598-018-27974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18(4):556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro . J Biol Chem. 2006;281(35):25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 27.Yang LL, Xiao N, Liu J, Liu K, Liu B, Li P, et al. Differential regulation of baicalin and scutellarin on AMPK and Akt in promoting adipose cell glucose disposal. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):598–606. doi: 10.1016/j.bbadis.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Olivares-Vicente M, Sánchez-Marzo N, Encinar JA, de la Luz Cádiz-Gurrea M, Lozano-Sánchez J, Segura-Carretero A, et al. The potential synergistic modulation of AMPK by Lippia citriodora compounds as a target in metabolic disorders. Nutrients. 2019;11(12):2961. doi: 10.3390/nu11122961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morand C, Manach C, Crespy V, Remesy C. Respective bioavailability of quercetin aglycone and its glycosides in a rat model. Biofactors. 2000;12(1-4):169–174. doi: 10.1002/biof.5520120127. [DOI] [PubMed] [Google Scholar]
- 30.Křen V. Glycoside vs. Aglycon: the role of glycosidic residue in biological activity. Glycoscience. Berlin/Heidelberg: Springer-Verlag Berlin Heidelberg; 2008. pp. 2589–2644. [Google Scholar]
- 31.Ribeiro IA, Ribeiro MH. Naringin and naringenin determination and control in grapefruit juice by a validated HPLC method. Food Control. 2008;19(4):432–438. [Google Scholar]
- 32.Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin Pharmacol Ther. 1996;60(1):34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]