Abstract
Cell behaviors and functions show distinct contrast in different mechanical microenvironment. Numerous materials with varied rigidity have been developed to mimic the interactions between cells and their surroundings. However, the conventional static materials cannot fully capture the dynamic alterations at the bio-interface, especially for the molecular motion and the local mechanical changes in nanoscale. As an alternative, flexible materials have great potential to sense and adapt to mechanical changes in such complex microenvironment. The flexible materials could promote the cellular mechanosensing by dynamically adjusting their local mechanics, topography and ligand presentation to adapt to intracellular force generation. This process enables the cells to exhibit comparable or even higher level of mechanotransduction and the downstream ‘hard’ phenotypes compared to the conventional stiff or rigid ones. Here, we highlight the relevant studies regarding the development of such adaptive materials to mediate cell behaviors across the rigidity limitation on soft substrates. The concept of ‘soft overcomes the hard’ will guide the future development and application of biological materials.
Keywords: Cell, Mechanotransduction, Intracellular force, Biomaterials, Soft materials
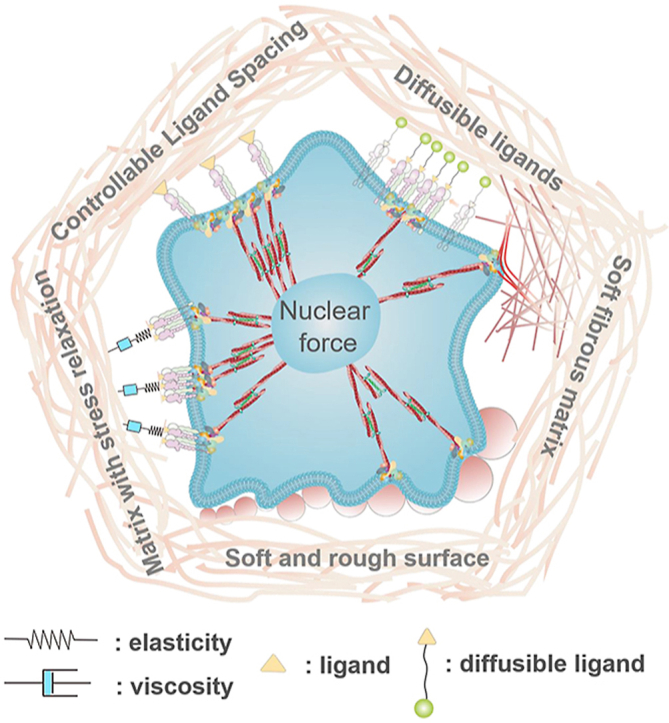
Graphical abstract
Highlights
-
•
Summarize the soft materials promoting intracellular force and the “hard” downstream phenotypes.
-
•
Understand the soft materials dynamically adapting to the mechanical changes at the cell-environment interface.
-
•
Summarize the general mechanosensing mechanism of the cells on/in adaptable soft materials.
1. Introduction
The mechanics of local microenvironments (e.g., matrix stiffness) are known to strongly affect cell behaviors such as migration, proliferation, differentiation and metabolism [[1], [2], [3], [4]]. Meanwhile, cells could exert myosin-generated contractile forces on surroundings to remodel their extracellular matrix (ECM) during adhesion [5,6]. In fact, the intracellular force instead of the environmental mechanics is the direct regulator for gene expression and phenotype decision [7]. Therefore, understanding the force-based communications between cells and their surroundings plays the central role in characterizing the molecular basis of various physiological processes and diseases. However, the mechanisms by which individual cells sense surrounding mechanical signals and transduce them into biochemical signals leading to transcriptional regulation in the nucleus (known as mechanotransduction), are far from sufficiently explored.
Many studies have confirmed that cell response to the elements of ECM is mechanics-dependent, which even overrides the influence of biochemical signals [8]. Specifically, the dose, loading kinetics and spatial distributions of the force regulate the force transmission and transduction across the adhesive receptors (e.g., integrin, cadherin) within the cell membranes [6]. High force loading on cells could facilitate cell mechanotransduction. Stiff environment is usually recognized to promote the intracellular force through cell spreading and cytoskeleton assembly by bearing high traction force [7]. One viewpoint suggests that a loading force over a certain threshold may induce the conformational or organizational changes of the force-bearing proteins (talin, integrins, stretch-sensitive ion channels etc.) [9]. These unfolded proteins recruit adhesive and structural proteins for enhancing the force transmission through focal adhesions. Meanwhile, the newly exposed active sites of these unfolded proteins activate the enzymes to transduce the mechanical cues to biochemical factors. The assembled actomyosin generates appropriate traction force to balance the intra and extra-cellular force through adhesive proteins. The traction force is transmitted to the nucleus along the actin filaments, thereby regulates gene expression and guides cell fate [7]. For example, mesenchymal stem cells on stiff substrates (>30 kPa) tend to generate more stress fibers and focal adhesions and prefer osteogenic differentiation. While on soft substrates (<10 kPa), cell adhesion is highly suppressed, and cells undergo adipogenic differentiation [10]. Importantly, the molecular clutch model reveals the transmission of mechanical signals through the clutch is driven by the force loading kinetics [11,12]. Substrate stiffness usually controls the force loading rate. In the actin–talin–integrin–ligand chain models (molecular clutch), the talin unfolding time and the integrin-ligand lifetime which affect cell adhesion comprehensively. When a constant force acts on a talin molecule, the time of talin unfolding decreases exponentially as the loading force rate increases. Meanwhile, integrin-ligand bonding lifetime increases first and then decreases with the loading force rate increase. When the lifetime is enough (beyond a certain rigidity threshold) for the intracellular force to unfold talin on stiff substrate, the cell adhesion can be switched from slip-bond to catch-bond mode to establish the stable force-dependent adhesion as well as to activate mechanotransduction pathways. The integrin-ligand lifetime is greatly extended in the catch-bond mode. In contrast, the force loading rate on the clutch is slower than the integrin debonding speed on the soft matrix, which cannot unfold talin to form stable adhesion [6,8]. However, the adaptable materials can alter the integrin bonding and debonding kinetics to change the integrin bonding lifetime, which may switch cell mechanosensing on soft matrix. Therefore, the cell mechanosensing is not as simple as the traditional opinion that stiff environment stimulates cellular force and enhances the force-dependent cell behaviors (‘hard’ phenotypes).
In natural tissue, the ECM is highly dynamic that exhibits rate-dependent behaviors such as non-linear viscoelasticity or thermodynamic instability [1,13,14]. Cells secrete proteins and enzymes as well as exert force to remodel the ECM for adapting the microenvironment to their requirement. Meanwhile, various physical signals of the deformed microenvironment, such as viscoelasticity, topographic features, and ligand re-presentation provide multiple stimulations to regulate cellular behaviors. Thus, the ECM remodeling provides a force-feedback loop for cells [9]. For instance, the stiffness of the cardiac matrix increases after myocardial infarction due to the formation of fibrotic scar [15]. Bone resorption by cell-secreted proteases as in microgravity results in more porous and weaker ECM networks, while bone growth by cell-secreted and reinforced ECM occurs with weight-bearing exercise [16]. Therefore, the mechanical properties of ECM are not always constant but change over time. It provides new challenges for researchers such as how to capture and track these dynamic interactions in such complex microenvironment, how to decouple the chemical cues from mechanical cues, and how to integrate the dynamic mechanics to cellular mechanotransduction etc.
To address these challenges, new material models are required to mimic the complex biochemical and biophysical cues of ECM. Not only do they support regular cell adhesion, but also are sensitive enough to respond to the tiny mechanical changes at the cell-materials interface. One emerging strategy to achieve this goal is to design flexible materials to create self-adaptive microenvironment for cells. Unlike the stiff or soft static materials, these flexible materials could adapt to some typical biological processes across multiple time and space scales. They can locally deform to permit complex cellular functions while maintaining their long-term integrity. These properties on the one hand enable them to be good candidates to study cell-environment interactions, on the other hand make them well-suited for biotechnology and medical applications.
It should be noticed that the flexible materials are usually soft. The soft materials exhibit irreplaceable advantages in biomedical applications. They require lower concentration of backbone polymers or lower degree of cross-linking, which can degrade faster to support tissue growth and limit the immune response [17,18]. Here we focus on the recent studies of regulating cell mechanoresponse through these flexible biomaterials. The soft deformable materials and the soft materials with controllable ligand presentation are the main catalogs of flexible materials that can adapt to cell adhesion to promote the intracellular force generation. As a result, these ‘soft materials’ successfully stimulate the ‘hard’ cell phenotypes, e.g., cell adhesion, stem cell osteogenic differentiation.
2. Deformable materials
2.1. Fibrous matrix
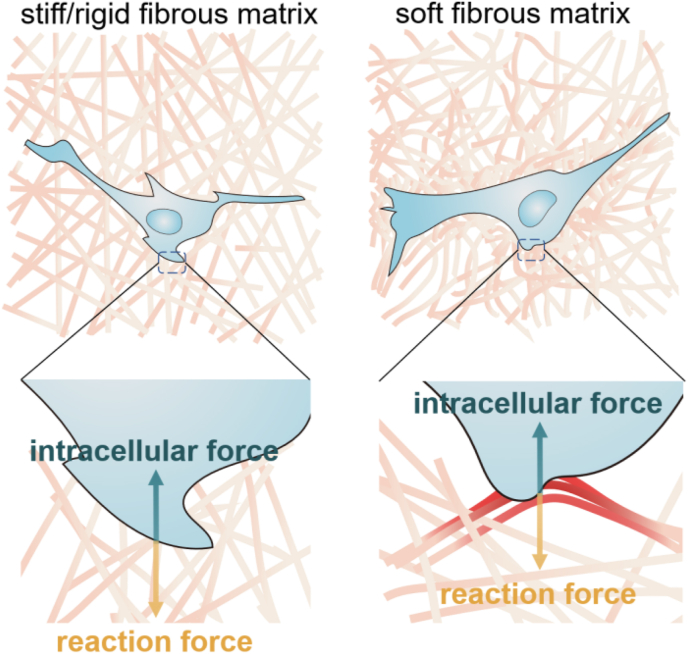
Natural ECM has a complex micro/nano topological structure. Its mechanical properties mainly depend on three components: elastin fiber, fibrillary collagen, and glycosaminoglycans. Elastin and collagen fibers usually provide stiffness, strength, extensibility and resilience to the tissue [19,20]. The fibrous structure of the natural ECM is often mimicked by biomaterials [[21], [22], [23]]. Compared with a highly crosslinked polymer chain in bulk hydrogels with the same components, the stiffness of a single fiber in fibrillar network is lower. It thus can be easily remodeled by cell traction force. Chen et al. prepared RGD decorated dextran nanofiber network via electrospinning to mimic the ECM structure(Fig. 1) [24]. As the stiffness of the fiber network decreases, both cell spread area and proliferation increase, which is in contrast to cell mechanoresponse on conventional flat hydrogels. It has been revealed that cellular traction force deforms the very soft fibers and recruits them to the cell spread region. The deformed network generates extra counterforce to cell adhesive points and offers cells more adhesive area. Therefore, it promotes the focal adhesion (FA) formation and FAK phosphorylation to activate the cell mechanotransduction. The fiber stiffness, fiber-fiber welding, and fiber density synergistically affect the cell mechanosensitive behaviors [24]. The stiff fibrous networks that cannot be deformed by cells fail to stimulate cell spreading. The similar phenomenon has been observed on self-assembled native collagen fibers [25]. It suggests that cells pull the collagen network to alter the local arrangement to increase the local stiffness [21]. Baker et al. have revealed cell-mediated aggregation of the fibrous matrix through actomyosin-generated force [26]. The deformed collagen fibers form bundles, which carry significant tensile forces to guide cell migration [27]. Matrix aggregation no longer occurs by inhibiting cell contractility. Besides fiber stiffness, high fiber density and/or crosslinking degree inhibit matrix aggregation as well. Asgari et al. have revealed that the nanoscale assembles of Col-I and Col-III could regulate the topography and stiffness of heterotypic fibrils in a ratio-dependent manner. The viscoelasticity of the fibrils is directly affected by the external force loading rate. These properties make the collagen fibrils adapt to the physiological conditions and requirements of different tissues at different developmental stages [28]. In general, cells have the impression of active and passive physical factors in the surrounding fiber networks. Cells actively apply force to the surroundings for adhesion and are also passively affected by physical factors such as matrix deformation.
Fig. 1.
Scheme of a cell adhered on stiff/rigid and soft fibrous matrix. The cell-mediated soft fiber recruitment promotes intracellular force to the similar or even higher level comparing with the force on stiff/rigid fibers.
2.2. Rough hydrogel
Besides the fibrous pattern, the topographical features of natural ECM range from nanometers to micrometers. Increasing evidence has suggested that the micro/nano structures play essential roles in regulating cell adhesion and mechanosensing [[29], [30], [31], [32]]. The concept of using ECM-mimicking topographic information to direct cell function has been widely accepted in the design of various biomaterials [33,34]. For instance, surface roughness mediates cell adhesion and the downstream behaviors. As realized on the rigid surfaces, such as titanium [33], semiconductor [35], glass [36], hydroxyapatite [34], and polymers (stiffness ranges from MPa to GPa), the low roughness (nanometer range) promotes cell spreading and intracellular force generation, but the high roughness (sub-micrometer and micro range) inhibits them.
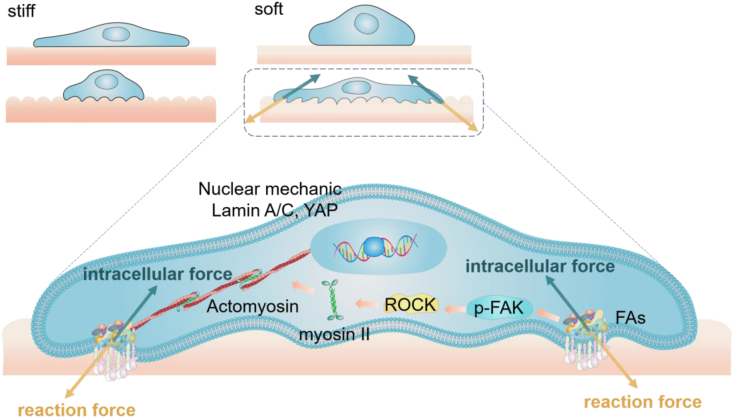
Hydrogels are the most widely used biomaterials for mimicking ECM chemical and physical parameters, especially for ECM elasticity, due to the convenient fabrication and functionalization methods. However, it is difficult to construct ECM-mimicking micro/nano topological structures on the hydrogel surface. We have developed roughness gradient gelatin methacryloyl hydrogels (roughness ranges from 50 nm to 1 μm) with physiological stiffness (about 3–30 kPa) [37]. The rough surfaces are printed from mussel-inspired catecholic coating [[38], [39], [40]] via silicone molds. Cell spread area increases with the increasing roughness on soft hydrogels (Fig. 2), which is distinct from the trend on stiff hydrogels or rigid surfaces as described above. The FA area, FAK phosphorylation level, Lamin A/C assembly level, YAP nuclear localization and osteogenic differentiation of the cells are all in line with the trend of cell spread area, indicating the enhanced intracellular force generation induced by the rough features of the soft hydrogels. Similar to the soft fibrous matrix, the adhered cells exert traction force on the tip structures of the rough hydrogels. The force deforms the rough surface of the soft hydrogels, which offers counterforce and more adhesive sites allowing cells to generate higher intracellular force. Instead, the force fails to deform the stiff or rigid tip structures. As a result, the cell membrane is confined in the micro-size groove structures on the surface, which impedes cell adhesion. Overall, the deformation of the soft and flexible matrix changes the local mechanical properties of the environment and the density of binding sites, which optimizes the cell adhesion in the local area, thereby enhancing intracellular force generation and mechanotransduction.
Fig. 2.
Surface roughness drives cellular mechanoresponse. Cells sense the synergy of roughness and stiffness stimuli. Soft and rough surface adapt to cellular traction force to initiate the mechanotransduction pathways.
2.3. Hydrogel with stress relaxation
Most of the studies only focus on elastic materials, but natural tissues and ECM components are not linearly elastic materials, they are in fact viscoelastic. For example, many of our tissues exhibit stress relaxation or a decreasing elastic modulus over time (from tens to hundreds of seconds) when a constant strain is applied [41]. The viscoelastic matrixes can dissipate energy via stress relaxation and creep, while the elastic matrixes store energy and maintain a constant elastic coefficient under stress. There are various molecular mechanisms producing viscoelasticity in hydrogels. Since viscosity corresponds to energy dissipation, any molecular phenomenon that dissipates energy will cause viscoelasticity [42]. Non-covalently or some reversibly crosslinked hydrogels are usually viscoelastic. These crosslinking points are dynamic. The debonding and rebounding process allows the polymer matrix to flow under applied stress or strain [43]. Notably, the viscoelasticity of the matrix has been revealed to regulate cell mechanosensing and mechanoresponse (Fig. 3). Chaudhuri et al. have found that cells spread better on the soft hydrogels with stress relaxation compared with the elastic hydrogels with the same modulus [41]. Traditionally, cell adhesion and proliferation are considered to be inhibited on the soft matrix. However, the matrix stress relaxation successfully compensates for the effect of reduced stiffness on cell adhesion by enabling ligand accumulation after matrix softening [44,45]. This process activates integrin adhesion, actin assembly, and myosin contractility and thereby promotes cell mechanotransduction. It must be emphasized that high ligand density is required to achieve the integrin clustering threshold [46,47]. The ligand accumulation can also lead to the enhancement of local stiffness [43]. In the energy sight, the energy is dissipated through the yield of the matrix, which leads to a reduction of stored energy, so that the cells generate more work for spreading on the viscoelastic substrate than on the elastic matrix with the same elasticity. Similar results were also found in 3D culture microenvironment. Faster stress relaxation or enhanced creep in hydrogel systems such as alginate, hyaluronic acid and collagen promotes cell spreading, cycle progression, mitosis and differentiation [[48], [49], [50], [51]]. For example, short PEG spacers are covalently coupled with the ionically crosslinked alginate to prepare viscoelastic hydrogels. In the case of the same initial modulus, the hydrogels with lower alginate molecular weight exhibit faster stress relaxation. The hydrogels with stress relaxation exhibit improved cell adhesion and spreading as well as the osteogenic differentiation of stem cells [48]. Notably, unlike cell adhesion on 2D with unlimited space, spacing confinement in 3D hydrogels is also one of the key parameters to regulate cell mechanosensing [52]. Normally, the assembly and force generation of the actomyosin cytoskeleton require enough space in expanding cells [53]. However, the stress relaxation offsets the space limitation of the 3D matrix even independent of cell adhesion. Therefore, the adhesive cells combine adhesion-independent and adhesion-mediated mechanisms to sense matrix viscoelasticity [54]. This provides a new perspective that, besides degradability and pore size, the matrix viscoelasticity also governs confinement in 3D culture. Recently, the Anseth group enhances the hydrogel stress relaxation via reversible non-covalent bonding or dynamic covalent bonding. These hydrogels can better adapt to the cell adhesion and mechanoresponse [55,56].
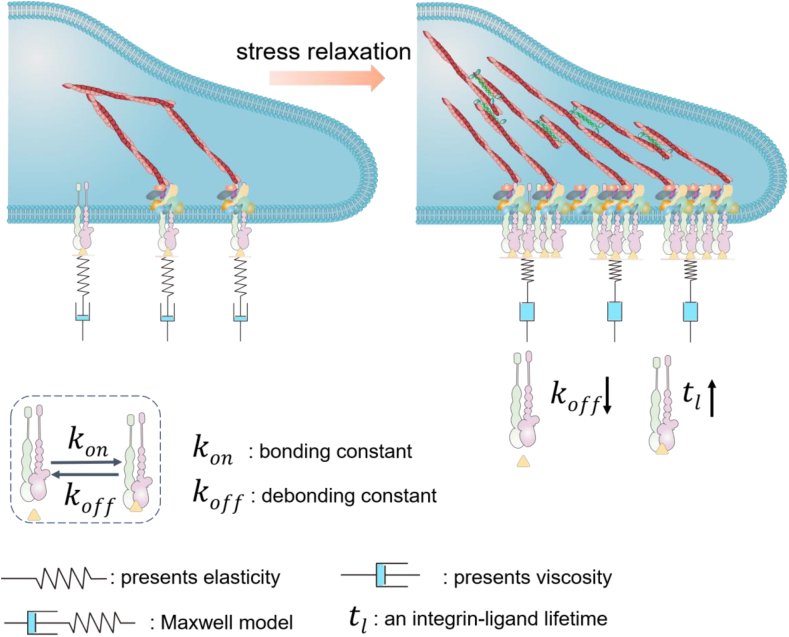
Fig. 3.
Cell adhesion on the matrix with stress relaxation. The optimal stress relaxation rate reduces the retrograde actin flow, maximizes the integrin-ligand lifetime on soft substrate, and effectively improves cell adhesion. The force exerted by the cells increases, when the relaxation time exceeds the time required for the integrins to fully connect to the matrix. The substrate stress relaxation will slow down the integrin-ligand disconnection, which prolongs the lifetime of each signal integrin-ligand bonds. When the lifetime is over the time to unfold structural protein talin, the single integrins can be clustered to form FAs to stabilize the cell adhesion (slip bond to catch bond transition).
A speed-dependent adhesion model has been proposed to describe the effect of stress relaxation on cell adhesion [57]. The force exerted by the cells increases, when the relaxation time exceeds the time required for the integrins to fully connect to the matrix. The substrate stress relaxation will slow down the integrin-ligand disconnection, which prolongs the lifetime of each signal integrin-ligand bond [58]. When the lifetime is over the time to unfold structural protein talin, the integrins can be clustered to form FAs to stabilize the cell adhesion (slip bond to catch bond transition) [59]. When the relaxation time is shorter than the time required for the integrin-ligand interaction, the substrate will directly convert to the final stress state. The effect of viscosity becomes negligible and the cell adhesion will be even weaker than on the elastic hydrogel with the same initial modulus. When the relaxation time is too long and exceeds the lifetime of FAs, the substrate does not relax during the adhesion maturation. Cell adhesion is consistent with on the elastic substrate [57].
2.4. Stress-stiffening hydrogel
Many filamentous biopolymers such as F-actin, microtubules fibrin and vimentin show strain stiffening properties [53]. However, the effects of stress stiffening on cell mechanoresponse are very difficult to be achieved by biomaterials. Rowan et al. proposed a polyisocyanopeptide-based hydrogel (0.2–0.4 kPa) with stress-stiffening effect for cells in three dimensions [60]. Cells can freely expand in the hydrogels in the beginning of spread as the stiffness is still low. Afterwards, the stress acting over the hydrogels surrounding the cells makes the microenvironment being stiffer. The cells which have extended already get increasing mechanical stimuli and have enough space to organize their cytoskeleton. Thus, these cells successfully generate intracellular force in the hydrogels with low initial stiffness, which promotes the osteogenic differentiation of the stem cells. Further studies have confirmed the microtubule-associated protein DCAMKL1, which acts as the inhibitor of RUNX2, participates in the mechanotransduction under stress-stiffening environment. As the stress-stiffening happens, RUNX2 is activated to initiate the osteogenic differentiation process.
2.5. Degradable hydrogel
Natural ECM is biodegradable, which enables the cells to remodel the microenvironment to adapt to their functions. Increasing evidences have demonstrated that ECM degradation regulates cell adhesion and differentiation, especially in the 3D microenvironment. Cells in 3D could degrade the hydrogels to obtain enough space for assembling their actomyosin cytoskeleton, which generates intracellular force and regulates mechanotransduction [61]. The substrate degradation also affects cell mechanosensing on the 2D surface without space limitation. Ding et al. have introduced the biodegradable polyethylene glycol (PEG)-based hydrogels and revealed that rapid degradation promotes the osteogenic differentiation of the stem cells on soft hydrogels. The degradation rate even takes precedence effect over the matrix stiffness [62]. Similar result was also found in a study of neural progenitor cell stemness in a 3D hydrogel [52]. The neural progenitor cell stemness is strongly related to degradability rather than gel stiffness. These results together reveal that the degradability could increase cell-mediated matrix remodeling and then enhance the cell mechanoresponse. Besides offering space for cells in 3D, the degradable matrix can offer a relatively high stiffness for a relatively larger bonding constant (kon) in the initial cell adhesion step. Then, the degradation can make the matrix flexible, which may decrease the debonding constant (koff) to increase the lifetime of integrin-ligand adhesion. It is similar to the effect of stress relaxation. Meanwhile, the degradation will change the ligand presentation, which will be discussed below.
2.6. Diffusible ligands
As described above, the natural ECM is both viscoelastic and degradable. The cells in vivo are located in a highly dynamic environment that directly contact the ECM through receptor-ligands interactions. Cells sense the microenvironment through membrane receptors, which support cell adhesion and initiate signaling pathways. Therefore, the dynamic spatiotemporal distribution of ligands plays a central role in cell-ECM interactions [63].
At the molecular level, ECM dynamics lead to ligand diffusion at the adhesive interface. The lipid bilayer is the most frequently used model to study the ligand diffusion. Salmeron-Sanchez et al. have designed two types of RGD functionalized lipid bilayer with different diffusion rates. Cells can feel higher traction force on the surface with lower diffusion rate (higher viscosity), which promotes the formation of FAs and slows down the actin flow [64].
The lipid bilayers match cell membrane fluidity, but the diffusion rate is too fast to match the ligand movement in the dynamic ECM. The assembly of the amphiphilic block copolymers offers another model to mimic the dynamic behaviors of natural ECM. The ligand diffusion can be controlled by adjusting the interactions between the assembly blocks. The size and chemical properties of the polymer chains can independently adjust the mechanical properties and the polymer/ligand diffusion on the assembled films [65]. Kourouklis et al. designed the amphiphilic block copolymer 1,2-polybutadiene-b-polyethylene oxide (PB-b-PEO) to create a self-assembled film with adjustable lateral mobility. The RGD polypeptide is immobilized at the terminal of the hydrophilic blocks with a constant density, while the fluidity of the film is controlled by adding the hydrophobic homopolymer poly(isobutylene). The effect of ligand diffusion on cell adhesion is non-linear. The low ligand diffusion rate enhances cell spreading. Meanwhile, the integrin-ligand binding occurs faster and more effectively with higher ligand diffusion rate, resulting in a greater number of FAs but less clustered integrin numbers in a single FA [66]. Further studies reveal that α5β1 and αvβ3 integrins contribute to the cell adhesion on the ligands with high and low diffusion rate, respectively [67].
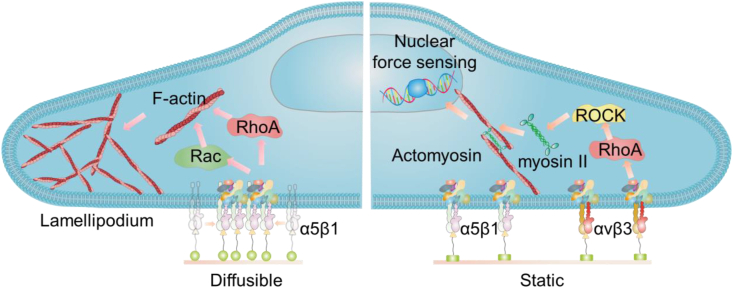
We have revealed the mechanosensing and mechanotransduction pathways of cell adhesion on the ligands with different diffusion rates based on a self-assembly monolayer of amphiphilic block copolymer (Fig. 4) [68]. The highly mobile ligands recruit α5β1 integrins, which further activate the RhoA and Rac pathways, and lead to a force-independent lamellipodium-like cell spreading. In contrast, the formation of actomyosin stress fiber is limited due to the lack of ROCK activation. In comparison, the constantly immobilized ligands activate the intracellular-force-based canonical mechanotransduction pathways to enhance cell adhesion and osteogenic differentiation of stem cells.
Fig. 4.
Ligand diffusion selectively activates α5β1 integrin and initiates Rac and RhoA signaling to enable actomyosin force-independent cell adhesion through lamellipodium. Static ligand stimulates cell adhesion through the cooperation of α5β1 and α5β3 integrin to initiate the canonical actomyosin force-dependent mechanotransduction pathways.
Based on the mechanism study, we have developed self-dynamic interfacial materials to regulate cell behaviors and functions. The RGD functionalized amphiphilic polyglycerol (PG) brushes are immobilized on the substrates via hydrophobic interaction of spiropyran/merocyanine (SP/MC) anchors [69]. In the more hydrophilic MC form, the anchor force of the polymer brushes is about 26 pN, resulting in a higher diffusion rate. Meanwhile, the force increases to about 240 pN in the more hydrophobic SP form, resulting in the almost static state of the polymer brushes. The MC form spontaneously isomerize to SP form, which lasts about 24 h in dark conditions. Therefore, the polymer with MC anchors can activate the α5β1 integrin and Rac signaling in the first a few hours of cell spreading. The following MC-SP isomerization strengthens the cell adhesion to activate αvβ3 integrin and RhoA/ROCK signaling in a stimuli-free manner. These two subsequently activated signaling pathways enhance the intracellular force and promote mechanotransduction, which further improve the osteogenic differentiation of stem cells [69].
2.7. Soft hydrogel with controllable ligand spacing
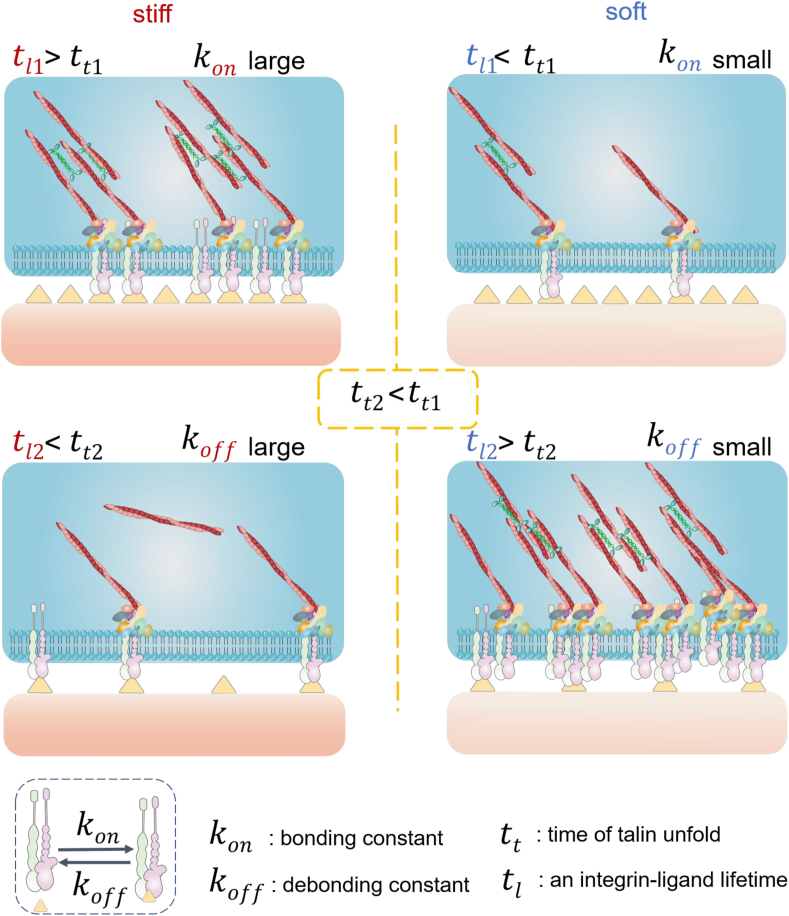
Besides flexible materials, well-ordered ligand spacing can also adapt to intracellular force generation to promote mechanotransduction on soft substrates. The large ligand spacing (>70 nm) causes the unstable cell adhesion and decreased intracellular force on stiff or rigid substrates [45,70]. Conversely, the unexpected phenomenon has been observed that large ligand spacing supports cell adhesion and the assembly of actomyosin cytoskeleton on very soft substrates (lower than a few kilopascal). Roca-Cusachs et al. revealed the mechanism based on the molecular clutch theory [6]. With the decreasing ligand density (i.e., increasing ligand distance), the number of integrin-actomyosin molecular clutch decreases since the activated myosin II molecules distribute along the filamentous actin fibers, each clutch obtains more myosin II molecules and generates larger intracellular traction force. The increasing traction force accelerates the force loading rate, which can be faster than the integrin debonding speed on soft substrates but not on the stiff/rigid substrates. Thus, only the increasing force on soft substrates can unfold talin to switch the slip bonds to catch bonds, resulting in the stable cell adhesion and activated mechanotransduction (Fig. 5). [71].
Fig. 5.
The ligand spacing regulates the lifetime of a single integrin-ligand connection. The large ligand spacing on soft matrix adapts to the intracellular force-induced talin unfolding, which switches cell-matrix interaction from slip-bond to catch-bond mode. With the decreasing ligand density (i.e., increasing ligand distance), the number of integrin-actomyosin molecular clutch decreases. Since the activated myosin II molecules distribute along the filamentous actin fibers, each clutch obtains more myosin II molecules and generates a larger intracellular traction force. The increasing traction force accelerates the force loading rate, which can be faster than the integrin debonding speed on soft substrates but not on the stiff/rigid substrates. Thus, only the increasing force on soft substrates can unfold talin to switch the slip bonds catch bonds, resulting in the stable cell adhesion and activated mechanotransduction.
This new phenomenon enables us to develop soft hydrogels adapting to intracellular force generation, which allows stem cells to differentiate to osteoblasts. The soft hydrogels (about 3 kPa) are functionalized with quasi-hexagonally distributed gold nanodots (diameter c.a. 6 nm, distance c.a. 230 nm). The gold nanodots are further decorated by RGD peptide. Since the size of a single gold nanodot is smaller than the size of integrin, each single gold nanodot can only bind to one integrin in principle [72]. This well-designed ligand spacing initiates cell mechanotransduction and leads to the osteogenic differentiation of mesenchymal stem cell [73].
3. Conclusion
Here, we highlight the flexible materials for promoting cell mechanosensing and summarize the design principles and their unique advantages in mechanobiology (Table 1). Compared to the conventional stiff materials, these soft materials adapt to cell mechanosensing and capture the cell-environment dynamics to match the cellular requirements. The adaptation enables better cell adhesion and enhances mechanotransduction, which further activates the ‘hard’ cell phenotypes.
Table 1.
The flexible materials promote cell mechanotransduction.
| Flexible materials | Mechanism for promoting intracellular force | Ref.a |
|---|---|---|
| Soft fiber | Reaction force increase | [21,[24], [25], [26], [27]] |
| Soft rough hydrogel | [37] | |
| Diffusible ligands | Integrin clustering and Rac signaling activation | [[66], [67], [68], [69]] |
| Stress relaxation hydrogel | Integrin binding lifetime increase | [[41], [42], [43]] |
| Ligand spacing hydrogel | [71,73] | |
| 2D degradable hydrogel | [62] | |
| 3D degradable hydrogel | Balancing space and stiffness | [52] |
| Stress-stiffening hydrogel | [60] |
Representative reference.
Although it shows great promise, the development of the adaptive materials remains many challenges. Cells are always changing over time. How to match the time-scale and force-scale of cell actions, how to monitor and quantify the tiny changes within cells and their surroundings, and how to adapt to cell signaling pathways, are the remaining questions. All of these require a deep understanding of cellular mechanosensing and mechanotransduction. Solving these problems and developing adaptive materials will significantly contribute to the tissue engineering and regenerative medicine.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51973129 and No. 32000951), the Sichuan Science and Technology Program (2020YFH0034), the State Key Laboratory of Polymer Materials Engineering, Sichuan University (sklpme2020-2-08), the HKSAR Research Grants Council (RGC) General Research Fund (GRF, no. 14306117), Early Career Scheme (ECS, No. 27202919), the HKU Start-Up Grant and the Seed Fund (No. 202011159019).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Zhiqin Chu, Email: zqchu@eee.hku.hk.
Qiang Wei, Email: wei@scu.edu.cn.
References
- 1.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang G., Li F., Zhao X., Ma Y., Li Y., Lin M., Jin G., Lu T.J., Genin G.M., Xu F. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017;117(20):12764–12850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X., Peng R., Ding J. Cell–material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013;25(37):5257–5286. doi: 10.1002/adma.201301762. [DOI] [PubMed] [Google Scholar]
- 4.Ye K., Wang X., Cao L., Li S., Li Z., Yu L., Ding J. Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. Nano Lett. 2015;15(7):4720–4729. doi: 10.1021/acs.nanolett.5b01619. [DOI] [PubMed] [Google Scholar]
- 5.Wolfenson H., Yang B., Sheetz M.P. Steps in mechanotransduction pathways that control cell morphology. Annu. Rev. Physiol. 2019;81(1):585–605. doi: 10.1146/annurev-physiol-021317-121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elosegui-Artola A., Trepat X., Roca-Cusachs P. Control of mechanotransduction by molecular clutch dynamics. Trends Cell Biol. 2018;28(5):356–367. doi: 10.1016/j.tcb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Sun Q., Wei Q., Zhao C.S. How do the cells sense and respond to the microenvironment mechanics? Chin. Sci. Bull. 2021;66(18):2303–2311. doi: 10.1360/TB-2020-1069. [DOI] [Google Scholar]
- 8.Swaminathan V., Waterman C.M. The molecular clutch model for mechanotransduction evolves. Nat. Cell Biol. 2016;18(5):459–461. doi: 10.1038/ncb3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paluch E.K., Nelson C.M., Biais N., Fabry B., Moeller J., Pruitt B.L., Wollnik C., Kudryasheva G., Rehfeldt F., Federle W. Mechanotransduction: use the force(s) BMC Biol. 2015;13(1):47. doi: 10.1186/s12915-015-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells R.G. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47(4):1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 11.Elosegui-Artola A., Oria R., Chen Y., Kosmalska A., Pérez-González C., Castro N., Zhu C., Trepat X., Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016;18(5):540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 12.Elosegui-Artola A., Bazellières E., Allen M.D., Andreu I., Oria R., Sunyer R., Gomm J.J., Marshall J.F., Jones J.L., Trepat X., Roca-Cusachs P. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 2014;13(6):631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker B.M., Chen C.S. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012;125(13):3015. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., Yan Z., Ren J., Qu X. Manipulating cell fate: dynamic control of cell behaviors on functional platforms. Chem. Soc. Rev. 2018;47(23):8639–8684. doi: 10.1039/c8cs00053k. [DOI] [PubMed] [Google Scholar]
- 16.Chen J.C., Castillo A.B., Jacobs C.R. In: Osteoporosis. fourth ed. Marcus R., Feldman D., Dempster D.W., Luckey M., Cauley J.A., editors. Academic Press; San Diego: 2013. Chapter 20 - cellular and molecular mechanotransduction in bone; pp. 453–475. [DOI] [Google Scholar]
- 17.Kong D., Megone W., Nguyen K.D.Q., Di Cio S., Ramstedt M., Gautrot J.E. Protein nanosheet mechanics controls cell adhesion and expansion on low-viscosity liquids. Nano Lett. 2018;18(3):1946–1951. doi: 10.1021/acs.nanolett.7b05339. [DOI] [PubMed] [Google Scholar]
- 18.Wei W., Dai H. Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioactive Materials. 2021;6(12):4830–4855. doi: 10.1016/j.bioactmat.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arribas S.M., Hinek A., González M.C. Elastic fibres and vascular structure in hypertension. Pharmacol. Ther. 2006;111(3):771–791. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Ferruzzi J., Collins M.J., Yeh A.T., Humphrey J.D. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for Marfan syndrome. Cardiovasc. Res. 2011;92(2):287–295. doi: 10.1093/cvr/cvr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall M.S., Alisafaei F., Ban E., Feng X., Hui C.-Y., Shenoy V.B., Wu M. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113(49):14043. doi: 10.1073/pnas.1613058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen J.H., Vincent L.G., Fuhrmann A., Choi Y.S., Hribar K.C., Taylor-Weiner H., Chen S., Engler A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014;13(10):979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Q., Zheng Y., Wang X., Xie R., Ding Y., Wang B., Yu X., Lu Y., Liu L., Li Y., Li M., Zhao Y., Jiao Y., Ye F. Dynamically Re-organized collagen fiber bundles transmit mechanical signals and induce strongly correlated cell migration and self-organization. Angew. Chem. Int. Ed. 2021;60(21):11858–11867. doi: 10.1002/anie.202016084. [DOI] [PubMed] [Google Scholar]
- 24.Baker B.M., Trappmann B., Wang W.Y., Sakar M.S., Kim I.L., Shenoy V.B., Burdick J.A., Chen C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015;14(12):1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie J., Bao M., Bruekers S.M.C., Huck W.T.S. Collagen gels with different fibrillar microarchitectures elicit different cellular responses. ACS Appl. Mater. Interfaces. 2017;9(23):19630–19637. doi: 10.1021/acsami.7b03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson C.D., Wang W.Y., Zaimi I., Jayco D.K.P., Baker B.M. Cell force-mediated matrix reorganization underlies multicellular network assembly. Sci. Rep. 2019;9(1):12. doi: 10.1038/s41598-018-37044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Q., Zheng Y., Wang X., Xie R., Ding Y., Wang B., Yu X., Lu Y., Liu L., Li Y., Li M., Zhao Y., Jiao Y., Ye F. Dynamically Re-organized collagen fiber bundles transmit mechanical signals and induce strongly correlated cell migration and self-organization. Angew. Chem. Int. Ed. 2021;60(21):11858–11867. doi: 10.1002/anie.202016084. [DOI] [PubMed] [Google Scholar]
- 28.Asgari M., Latifi N., Heris H.K., Vali H., Mongeau L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017;7(1):1392. doi: 10.1038/s41598-017-01476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalby M.J., Gadegaard N., Oreffo R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014;13(6):558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 30.Ermis M., Antmen E., Hasirci V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: a review from the tissue engineering perspective. Bioactive Materials. 2018;3(3):355–369. doi: 10.1016/j.bioactmat.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Li S., Yan C., Liu P., Ding J. Fabrication of RGD micro/nanopattern and corresponding study of stem cell differentiation. Nano Lett. 2015;15(3):1457–1467. doi: 10.1021/nl5049862. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Zheng S., Ye K., He J., Shen Y., Cui S., Huang J., Gu Y., Ding J. Cell migration regulated by RGD nanospacing and enhanced under moderate cell adhesion on biomaterials. Biomaterials. 2020;263 doi: 10.1016/j.biomaterials.2020.120327. [DOI] [PubMed] [Google Scholar]
- 33.Boyan B.D., Hummert T.W., Dean D.D., Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 34.Deligianni D.D., Katsala N.D., Koutsoukos P.G., Missirlis Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2000;22(1):87–96. doi: 10.1016/S0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- 35.Bain L.E., Collazo R., Hsu S.-h., Latham N.P., Manfra M.J., Ivanisevic A. Surface topography and chemistry shape cellular behavior on wide band-gap semiconductors. Acta Biomater. 2014;10(6):2455–2462. doi: 10.1016/j.actbio.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Qian W., Gong L., Cui X., Zhang Z., Bajpai A., Liu C., Castillo A.B., Teo J.C.M., Chen W. Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl. Mater. Interfaces. 2017;9(48):41794–41806. doi: 10.1021/acsami.7b16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou Y., Yu L., Xie W., Camacho L.C., Zhang M., Chu Z., Wei Q., Haag R. Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano Lett. 2020;20(1):748–757. doi: 10.1021/acs.nanolett.9b04761. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q., Achazi K., Liebe H., Schulz A., Noeske P.-L.M., Grunwald I., Haag R. Mussel-inspired dendritic polymers as universal multifunctional coatings. Angew. Chem. Int. Ed. 2014;53(43):11650–11655. doi: 10.1002/anie.201407113. [DOI] [PubMed] [Google Scholar]
- 39.Chen X., Gao Y., Wang Y., Pan G. Mussel-inspired peptide mimicking: an emerging strategy for surface bioengineering of medical implants. Smart Materials in Medicine. 2021;2:26–37. doi: 10.1016/j.smaim.2020.10.005. [DOI] [Google Scholar]
- 40.Hou Y., Xie W.Y., Yu L.X., Camacho L.C., Nie C.X., Zhang M., Haag R., Wei Q. Surface roughness gradients reveal topography-specific mechanosensitive responses in human mesenchymal stem cells. Small. 2020;16(10):10. doi: 10.1002/smll.201905422. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhuri O., Gu L., Darnell M., Klumpers D., Bencherif S.A., Weaver J.C., Huebsch N., Mooney D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015;6(1):6365. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhuri O., Cooper-White J., Janmey P.A., Mooney D.J., Shenoy V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584(7822):535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhuri O. Viscoelastic hydrogels for 3D cell culture. Biomaterials Science. 2017;5(8):1480–1490. doi: 10.1039/C7BM00261K. [DOI] [PubMed] [Google Scholar]
- 44.Maheshwari G., Brown G., Lauffenburger D.A., Wells A., Griffith L.G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000;113(10):1677. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 45.Arnold M., Cavalcanti-Adam E.A., Glass R., Blümmel J., Eck W., Kantlehner M., Kessler H., Spatz J.P. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem. 2004;5(3):383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 46.Huang J., Gräter S.V., Corbellini F., Rinck S., Bock E., Kemkemer R., Kessler H., Ding J., Spatz J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9(3):1111–1116. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S., Wang X., Cao B., Ye K., Li Z., Ding J. Effects of nanoscale spatial arrangement of arginine–Glycine–aspartate peptides on dedifferentiation of chondrocytes. Nano Lett. 2015;15(11):7755–7765. doi: 10.1021/acs.nanolett.5b04043. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., Huebsch N., Lee H.-p., Lippens E., Duda G.N., Mooney D.J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nam S., Chaudhuri O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nat. Phys. 2018;14(6):621–628. doi: 10.1038/s41567-018-0092-1. [DOI] [Google Scholar]
- 50.Lou J., Stowers R., Nam S., Xia Y., Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Nam S., Gupta V.K., Lee H.-p., Lee J.Y., Wisdom K.M., Varma S., Flaum E.M., Davis C., West R.B., Chaudhuri O. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27(Kip1) signaling axis. Science Advances. 2019;5(8) doi: 10.1126/sciadv.aaw6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao M., Xie J., Huck W.T.S. Recent advances in engineering the stem cell microniche in 3D. Adv. Sci. 2018;5(8) doi: 10.1002/advs.201800448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H.-p., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16(12):1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang S., Ma H., Tu H.-C., Wang H.-R., Lin P.-C., Anseth K.S. Adaptable fast relaxing boronate-based hydrogels for probing cell–matrix interactions. Adv. Sci. 2018;5(9):1800638. doi: 10.1002/advs.201800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson B.M., Wilcox D.G., Randolph M.A., Anseth K.S. Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomater. 2019;83:71–82. doi: 10.1016/j.actbio.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong Z., Szczesny S.E., Caliari S.R., Charrier E.E., Chaudhuri O., Cao X., Lin Y., Mauck R.L., Janmey P.A., Burdick J.A., Shenoy V.B. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(12):E2686. doi: 10.1073/pnas.1716620115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng B., Wan W., Huang G., Li Y., Genin G.M., Mofrad M.R.K., Lu T.J., Xu F., Lin M. Nanoscale integrin cluster dynamics controls cellular mechanosensing via FAKY397 phosphorylation. Science Advances. 2020;6(10) doi: 10.1126/sciadv.aax1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfenson H., Yang B., Sheetz M.P. In: Nelson M.T., Walsh K., editors. Vol. 81. Annual Reviews; Palo Alto: 2019. Steps in mechanotransduction pathways that control cell morphology; pp. 585–605. (Annual Review of Physiology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das R.K., Gocheva V., Hammink R., Zouani O.F., Rowan A.E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2016;15(3):318. doi: 10.1038/nmat4483. [DOI] [PubMed] [Google Scholar]
- 61.Wei Q., Young J., Holle A., Li J., Bieback K., Inman G., Spatz J.P., Cavalcanti-Adam E.A. Soft hydrogels for balancing cell proliferation and differentiation. ACS Biomater. Sci. Eng. 2020;6(8):4687–4701. doi: 10.1021/acsbiomaterials.0c00854. [DOI] [PubMed] [Google Scholar]
- 62.Peng Y., Liu Q.-J., He T., Ye K., Yao X., Ding J. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials. 2018;178:467–480. doi: 10.1016/j.biomaterials.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Morgan M.R., Humphries M.J., Bass M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007;8(12):957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennett M., Cantini M., Reboud J., Cooper J.M., Roca-Cusachs P., Salmeron-Sanchez M. Molecular clutch drives cell response to surface viscosity. Proc. Natl. Acad. Sci. U. S. A. 2018;115(6):1192–1197. doi: 10.1073/pnas.1710653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Discher B.M., Won Y.-Y., Ege D.S., Lee J.C.M., Bates F.S., Discher D.E., Hammer D.A. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284(5417):1143. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 66.Kourouklis A.P., Lerum R.V., Bermudez H. Cell adhesion mechanisms on laterally mobile polymer films. Biomaterials. 2014;35(17):4827–4834. doi: 10.1016/j.biomaterials.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 67.Kourouklis A.P., Bermudez H. Integrins direct cell adhesion in a substrate-dependent manner. Cell. Mol. Bioeng. 2015;8(3):488–495. doi: 10.1007/s12195-015-0394-7. [DOI] [Google Scholar]
- 68.Yu L.X., Hou Y., Xie W.Y., Camacho J.L.C., Cheng C., Holle A., Young J., Trappmann B., Zhao W.F., Melzig M.F., Cavalcanti-Adam E.A., Zhao C.S., Spatz J.P., Wei Q., Haag R. Ligand diffusion enables force-independent cell adhesion via activating alpha 5 beta 1 integrin and initiating rac and RhoA signaling. Adv. Mater. 2020;32(29):12. doi: 10.1002/adma.202002566. [DOI] [PubMed] [Google Scholar]
- 69.Yu L., Hou Y., Xie W., Cuellar-Camacho J.L., Wei Q., Haag R. Self-strengthening adhesive force promotes cell mechanotransduction. Adv. Mater. 2020;32(52):2006986. doi: 10.1002/adma.202006986. [DOI] [PubMed] [Google Scholar]
- 70.Deng J., Zhao C.S., Spatz J.P., Wei Q. Nanopatterned adhesive, stretchable hydrogel to control ligand spacing and regulate cell spreading and migration. ACS Nano. 2017;11(8):8282–8291. doi: 10.1021/acsnano.7b03449. [DOI] [PubMed] [Google Scholar]
- 71.Oria R., Wiegand T., Escribano J., Elosegui-Artola A., Uriarte J.J., Moreno-Pulido C., Platzman I., Delcanale P., Albertazzi L., Navajas D., Trepat X., García-Aznar J.M., Cavalcanti-Adam E.A., Roca-Cusachs P. Force loading explains spatial sensing of ligands by cells. Nature. 2017;552(7684):219–224. doi: 10.1038/nature24662. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M., Li C., Yang S., Hirte J., Zhao W., Wei Q., Diao Z., Spatz J.P., Zhao C. Ultra-transparent slippery surface. Smart Materials in Medicine. 2021;2:38–45. doi: 10.1016/j.smaim.2020.10.001. [DOI] [Google Scholar]
- 73.Zhang M., Sun Q., Liu Y., Chu Z., Yu L., Hou Y., Kang H., Wei Q., Zhao W., Spatz J.P., Zhao C., Cavalcanti-Adam E.A. Controllable ligand spacing stimulates cellular mechanotransduction and promotes stem cell osteogenic differentiation on soft hydrogels. Biomaterials. 2021;268:120543. doi: 10.1016/j.biomaterials.2020.120543. [DOI] [PubMed] [Google Scholar]