Abstract
Background
Management of patients with sentinel lymph node (SLN) positive melanoma has changed dramatically in the last few years such that completion lymph node dissection (CLND) has become uncommon, and many patients receive adjuvant immunotherapy or targeted therapy. This study seeks to characterize patterns and predictors of early recurrence in this setting.
Methods
All patients with primary cutaneous melanoma undergoing sentinel lymph node biopsy (SLNB) between 3/2016–12/2019 were identified. The subset with a positive SLN who did not undergo CLND were examined for further analysis of outcomes and predictors of recurrence.
Results
Overall, 215 patients with SLN-positive melanoma who did not have CLND were identified. Adjuvant systemic therapy was administered to 102 (47%), with 93% of this subset receiving immunotherapy (n=95). Median follow-up from SLNB was 20 months (IQR 12–28.5 months); 57 patients (27%) recurred during this time. The SLN basin was the most common site of recurrence (n=38, 67% of recurrence), with isolated nodal recurrence being the most common first site of recurrent disease (n=22, 39% of recurrence). On multivariable analysis, lymphovascular invasion (LVI) of the primary tumor, 2 or more involved nodes and >1 mm nodal deposit were independently associated with higher rates of nodal relapse.
Conclusion
Nodal recurrence is a primary driver of early disease relapse for patients with SLN-positive melanoma who do not undergo CLND in the era of effective adjuvant systemic therapy. LVI, ≥2 nodes or >1 mm nodal disease identify patients at particularly high risk of nodal relapse.
INTRODUCTION
In the last four years, there have been dramatic changes in the management of patients with melanoma found to have clinically occult nodal disease at the time of sentinel lymph node biopsy (SLNB). Previously, standard therapy for such patients was completion lymph node dissection (CLND). In the context of high-risk pathological features in the nodal basin, patients could be offered adjuvant nodal radiation therapy (RT) and/or adjuvant systemic therapy with interferon, but most patients received surgery alone. After DeCOG-SLT and MSLT-2 showed a regional control benefit to CLND without improvement in melanoma-specific survival (MSS), CLND became less commonly performed after a positive SLNB1–3.
Subsequently, significant advances were seen in systemic therapy options. KEYNOTE-54 and CheckMate-238 showed a recurrence-free survival benefit from adjuvant anti-PD1 therapy, and COMBI-AD showed a significant relapse-free survival benefit from adjuvant BRAF and MEK directed therapy. All three studies required CLND prior to systemic therapy, excluded patients with prior adjuvant RT and showed adjuvant therapy was generally well tolerated4–6. It is worth noting that the smallest microscopic nodal disease was under-represented across these studies. Specifically, CheckMate-238 excluded AJCC 7 Stage IIIA patients, while KEYNOTE-54 and COMBI-AD included AJCC 7 Stage IIIA patients but required enrollees with this designation to have >1 mm nodal disease.
Patterns of recurrence and predictors of regional control in patients with SLN-positive melanoma who did not undergo CLND in the era of newer adjuvant systemic therapeutics are not well characterized. Data from recent studies of patients undergoing nodal dissection suggest nodal recurrence continues to be a persistent challenge. Specifically, KEYNOTE-54 showed that even after CLND, at a median follow-up of 3 years, 13.2% of patients who received adjuvant pembrolizumab developed isolated locoregional recurrence as the first site of relapse7. In addition, a recent multi-institutional retrospective study reported that 43% of Stage III-IV resectable melanoma patients had confined locoregional disease as the first site of relapse8. While such relapses can potentially be salvaged, nodal disease and its treatment can be associated with significant morbidity. The current study seeks to quantify patterns of recurrence for patients with SLN-positive melanoma in the contemporary therapeutic era and investigate factors that might predict for relapse. In particular, this study seeks to identify which subset of sentinel lymph node positive patients who do not go on to CLND are at highest risk of nodal recurrence and may thereby benefit from additional regional-nodal directed therapy.
PATIENTS & METHODS
Patient cohort
We identified all patients with primary cutaneous melanoma who underwent SLNB at our institution between 3/2016–12/2019. Indications for SLNB have been previously described9. The subset of patients with at least one positive lymph node who did not undergo CLND were identified for further analysis. With institutional review board approval, the electronic medical record of each patient was reviewed to collect information related to patient demographics, primary tumor features, nodal disease characteristics and clinical outcomes.
Clinical follow-up
Our institutional practice is to follow SLNB positive patients who do not have CLND every 3–4 months for 2 years followed by every 6 months for years 3–5. Follow-up includes history, physical, ultrasound of the draining nodal basin and cross-sectional imaging of the chest, abdomen and pelvis, similar to the monitoring performed for MSLT-2. For patients with nodal disease of the head and neck, cross sectional imaging of the neck and involved nodal basin are included. Dedicated CNS imaging is also performed annually for surveillance.
Assessment of outcome
The date of first recurrence was designated as the date of first biopsy-confirmed relapse. Subsequent recurrences were annotated as the date of an imaging finding consistent with disease progression. Non-nodal locoregional recurrence (NNLRR) was defined as either local recurrence (melanoma within 2 cm of the primary excision scar) or in-transit recurrence (melanoma >2 cm from the primary excision but proximal to the draining nodal basin). Nodal recurrence was defined as nodal disease in the same nodal basin in which a positive SLN was previously found.
Statistical methods
Descriptive statistics were used to evaluate baseline characteristics. Student’s T-test was used to analyze continuous numerical data. Two-tailed Fisher’s exact test and chi-squared analyses were used to analyze categorical data. Time-to-event analyses were calculated from the date of SLNB. Non-nodal locoregional control (NNLRC) was defined as the proportion of patients without evidence of NNLRR. Nodal control (NC) was defined as the proportion of patients without evidence of nodal recurrence. NNLRR and nodal recurrences at any time, regardless of prior relapse events were included, respectively, in these metrics. The distant metastasis-free (DMF) rate was defined as the proportion of patients without evidence of distant metastasis. The Kaplan-Meier method was used to estimate actuarial rates of NNLRC, NC, being DMF, disease-free survival (DFS), and MSS. Log-rank tests were used to identify patient factors, primary tumor and nodal disease factors, and adjuvant therapy modalities that were associated with clinical outcome. Variables were considered to show a statistically significant association if p<0.05. To construct multivariable Cox proportional hazards models, variables with an association with outcome defined as p<0.10 were initially included. Backward stepwise selection was then used to refine the multivariable models. Statistical analysis was performed in JMP Pro version 15.0 (SAS Institute) and Python 3.7 (Python Software Foundation).
RESULTS
Patient and primary tumor characteristics
During the study period, 1,440 patients with cutaneous melanoma at our institution were identified as undergoing SLNB without CLND while having no other evidence of disease. Of this population, 215 patients (14.9%) were found to have clinically occult melanoma involving a SLN. This population served as the basis for further analysis.
Patient and primary tumor characteristics are shown in Table 1. The majority of primary tumors were located in the extremities (n=105; 49%). Sixty percent (n=129) of tumors had at least one high risk primary tumor pathologic feature, defined as >4 mm Breslow thickness, perineural invasion (PNI), lymphovascular invasion (LVI), ≥20 mitosis/mm2 (as per Thompson et al.10) or ulceration). Most patients had mutational analysis performed, which at minimum included BRAF immunohistochemistry and sequencing but often included a more comprehensive DNA-based mutational panel which is often run at our center at the time of recurrence or metastasis (n=166; 77%). BRAF mutations were found in 79 tumors (48% of tumors tested).
Table 1.
Patient, disease and therapy characteristics
| Demographics | Lymph node characteristics | |||||
|---|---|---|---|---|---|---|
| Median age (yrs, IQR) | 65 (51–74) | n | % | |||
| n | % | Nodal basin evaluated | ||||
| Sex | Head & neck | 38 | 18% | |||
| Female | 79 | 37% | Axilla | 108 | 50% | |
| Male | 136 | 63% | Inguinal | 69 | 32% | |
| Race | - | SLNB procedure | ||||
| White | 190 | 88% | Only SLN removed | 189 | 88% | |
| Hispanic | 20 | 9% | Additional LN removed | 26 | 12% | |
| Black | 1 | 0% | Nodal disease location | |||
| Asian | 2 | 1% | Extracapsular extension | 21 | 10% | |
| Other | 2 | 1% | Subcapsular | 190 | 88% | |
|
|
||||||
| Primary tumor characteristics | Intraparenchymal | 126 | 59% | |||
|
|
||||||
| Median Breslow depth (mm, IQR) | 2.4 (1.3–4.4) | Intravascular | 11 | 5% | ||
| n | % | Median number of lymph nodes removed | ||||
| Primary site | - | Total (IQR) | 2 (2–4) | |||
| Head & neck | 35 | 16% | Sentinel (IQR) | 2 (1.5–3) | ||
| Trunk | 75 | 35% | Median number of lymph nodes positive | |||
| Upper extremity | 50 | 23% | Total (IQR) | 1 (1–2) | ||
| Lower extermity | 55 | 26% | Sentinel (IQR) | 1 (1–2) | ||
| Histology | - | Median largest nodal deposit (mm, IQR) | 1.05 (<0.1–3) | |||
|
|
||||||
| Lentigo maligna | 8 | 4% | Adjuvant therapy | |||
|
|
||||||
| Nodular | 47 | 22% | n | % | ||
| Superficial | 125 | 58% | Immunotherapy | 95 | 44% | |
| Acral | 25 | 12% | Nivolumab | 87 | 40% | |
| Not specified | 10 | 5% | Pembrolizumab | 4 | 2% | |
| Pathologic features | - | Ipilimumab | 4 | 2% | ||
| Satellitosis | 29 | 13% | Dabrafenib + Trametinib | 7 | 3% | |
| PNI | 36 | 17% | Radiation therapy | 17 | 8% | |
| LVI | 75 | 35% | To primary tumor | 12 | 6% | |
| Ulceration | 86 | 40% | To draining nodal basin | 9 | 4% | |
|
|
||||||
| >1 mitosis/mm2 | 175 | 81% | ||||
| Mutational Status | 166 | 77% | ||||
| n | % tested | |||||
| BRAF | 79 | 37% | ||||
| NRAS | 29 | 13% | ||||
| cKit | 6 | 3% | ||||
| Other | 35 | 16% | ||||
| None detected | 15 | 7% | ||||
| Not evaluated | 49 | 23% | ||||
Abbreviations: IQR, interquartile range; LN, lymph node; LVI, lymphovascular invasion; PNI, perineural invasion; yrs, years RT, radiation therapy; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; sys tx, systemic therapy.
Nodal characteristics
Nodal disease factors are also shown in Table 1. Most patients had only sentinel nodes evaluated but 26 patients (n=12%) had additional nodes pathologically evaluated. These nodes were designated as “non-sentinel” by the attending surgeon. It was more likely that “non-sentinel” nodes were evaluated in the head and neck nodal basin (n=13, 34% of patients with SLN involvement at this site).
The distribution of the number of nodes evaluated and nodal deposit size are shown in Supplemental Figure 1. The median number of total lymph nodes evaluated per patient was 2 (IQR 2–4) with 50 patients (23%) having a single SLN examined. The median number of positive SLNs was 1 (IQR 1–2). Two or more involved nodes were found in 27% (n=58). The median size of the largest nodal deposit was 1.05 mm (IQR <0.1–3). Sixty-five percent of patients had a nodal deposit of greater than 0.5 mm (n=139) while 50% had a nodal deposit of greater than 1 mm (n=108). Thirty-five patients (16%) had small nodal deposits that were quantified by the number of cells involved; for purposes of analysis these were annotated as being <0.1 mm. Twenty one (10%) of patients had nodal disease with extracapsular extension.
Adjuvant therapy
Also shown in Table 1 are details of adjuvant therapy. Overall, adjuvant systemic therapy was administered to 102 patients (47%). Immunotherapy was the most common therapeutic class used (n=95; 93% of adjuvant systemic therapy) with nivolumab being the most common agent (n=87; 92% of immunotherapy). Seven patients with BRAF mutant melanoma received adjuvant dabrafenib and trametinib (3%). Variables associated with adjuvant systemic therapy usage are shown in Supplemental Table 1. Younger age, presence of LVI and BRAF mutation were each significantly associated with increased likelihood of adjuvant systemic therapy use, while nodal deposit size, more nodes involved or presence of ECE were not. Of note, patients undergoing SLNB in 2018–2019 were more likely to receive adjuvant systemic therapy than patients undergoing SLNB in 2016–2017 (58% vs. 25%, OR 4.1, 95% CI 2.2–7.9, p<0.0001). Overall, meeting KEYNOTE-54 and COMBI-AD eligibility criteria was significantly associated with adjuvant systemic therapy use across the full study period (Supplemental Table 1 and 2).
Use of adjuvant RT was uncommon. Twelve patients (6%) received adjuvant RT to the primary site. Nine patients received RT to the involved nodal basin. All patients receiving nodal RT had at least 2 involved sentinel nodes with the median size of the largest nodal deposit being 2.25 mm. Two patients receiving nodal RT had ECE. Four of the 9 patients receiving RT to the involved nodal basin (44%) also received adjuvant systemic therapy with all 4 receiving immunotherapy. All patients receiving adjuvant RT received 30 Gy in 5 fractions as per our institutional adjuvant RT standard.
Clinical outcomes
At a median follow-up of 20 months (IQR 12–28.5 months), fifty-seven patients recurred (27%) and 28 died (13%) (Table 2). The cause of death was attributed to melanoma for 14 (50% of deaths). All patients who died of unknown or other causes did not have metastatic disease at last follow-up. Isolated nodal recurrence was the most common first site of relapse (39%; n=22/57). Including patients who developed synchronous non-nodal locoregional recurrence (NNLRR) and nodal recurrence in the absence of distant disease, a total of 46% (n=26/57) had nodal involvement as an initial site of recurrent disease. Thirty percent of patients developed distant metastasis as a site of first recurrence (n=17/57), including 4 with synchronous nodal disease.
Table 2.
Patterns of recurrence
| n | % total | ||
|---|---|---|---|
| Recurrence at any time | 57 | 27% | |
| Alive at last follow-up | 187 | 87 | |
|
| |||
| % recurrence | |||
|
| |||
| First recurrence | |||
| Non-nodal locoregional recurrence | 14 | 25% | |
| Nodal only | 22 | 39% | |
| Distant only | 13 | 23% | |
| Local + nodal | 4 | 7% | |
| Nodal + distant +/− other | 4 | 7% | |
| Any combo, not nodal | 0 | 0% | |
Abbreviations: combo, combination.
Of the 26 patients whose first recurrence included nodal disease in the absence of distant metastasis, 21 were planned for therapeutic nodal dissection, 3 patients refused surgery or were thought surgically unfit, 1 patient received nodal TVEC and 1 patient did not have subsequent follow-up after recurrence. Of those planned for nodal dissection, 11 were planned for neoadjuvant immunotherapy and 3 were planned for neoadjvuant BRAF/MEK targeted therapy. Seven patients were found to have metastatic disease while on neoadjvuant therapy and did not ultimately go to surgery. With a median follow-up of 9 months from nodal recurrence, 11 patients (42%) had stable disease on active therapy, 10 patients (38%) had either died or had disease that was not controlled at last follow-up, 3 patients (12%) were without evidence of disease for >6 months off melanoma-directed therapy, and 2 patients had inadequate follow-up to assess disease control. Overall, for the full patient population (n=215), 2-year actuarial event rates were as follows: NNLRC-88%, nodal control- 82%, distant disease control-86%, DFS-74%, melanoma-specific survival (MSS)-95%, and overall survival-88% (Supplemental Figure 2).
Further analysis was also performed on the subset of 102 patients who received adjuvant systemic therapy. In this cohort, the median follow-up was 17 months (IQR 12–24 months). During this time, 26 patients developed disease recurrence (25%). The most common first site of relapse included nodal disease in the absence of distant metastasis for 50% of patients (n=13) with 42% having isolated nodal recurrence (n=11) and 8% having concurrent NNLRR and nodal recurrence (n=2). Isolated NNLRR was experienced by 38% (n=10) and 12% had distant metastasis (n=3). For this subset, 18-month actuarial event rates were similar to the overall dataset: NNLRC-87%, nodal control-80%, distant disease control-94%, DFS-76%, MSS-94%, and overall survival-89%.
Predictors of outcome
Univariate predictors of outcome across the full study population are shown in Table 3. Breslow thickness < 4 mm and LVI predicted for higher local, nodal and distant relapse. Microsatellitosis predicted for higher rates of locoregional disease recurrence while ulceration, greater nodal disease burden and ECE predicted for higher rates of nodal and distant disease recurrence. The use of adjuvant systemic therapy was not associated with any outcome. As might be expected, patients who developed nodal recurrence as a first site of relapse had significantly likelihood of subsequent distant metastasis (2-year distant disease control 92% without first nodal relapse vs. 50% after first nodal relapse, p<0.005).
Table 3.
Univariate predictors of outcome
| 2yr NNLRC | NNLRC p-value | 2yr NC | NC p-value | 2yr DMF | DMF p-value | 2yr DFS | DFS p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Female | 92% | 0.09 | 85% | 0.51 | 85% | 0.75 | 75% | 0.66 | |
| Male | 85% | 80% | 86% | 72% | |||||
| Age, yrs | |||||||||
| <65 | 89% | 0.26 | 88% | 0.08 | 94% | 0.02 | 83% | <0.005 | |
| ≥65 | 87% | 76% | 79% | 64% | |||||
| Primary site | |||||||||
| Head & neck | 86% | 0.26 | 79% | 0.73 | 76% | 0.21 | 67% | 0.54 | |
| Trunk/extremity | 88% | 82% | 88% | 75% | |||||
| Breslow thickness | |||||||||
| <4 mm | 91% | 0.02 | 85% | 0.019 | 88% | 0.022 | 78% | <0.005 | |
| >4 mm | 80% | 76% | 78% | 60% | |||||
| Microsatellites | |||||||||
| Absent | 92% | <0.005 | 85% | <0.005 | 86% | 0.34 | 78% | <0.005 | |
| Present | 60% | 92% | 85% | 39% | |||||
| Ulceration | |||||||||
| Absent | 90% | 0.19 | 85% | 0.012 | 88% | 0.02 | 79% | <0.005 | |
| Present | 84% | 77% | 82% | 64% | |||||
| PNI | |||||||||
| Absent | 89% | 0.28 | 81% | 0.93 | 85% | 0.11 | 74% | 0.019 | |
| Present | 83% | 84% | 86% | 67% | |||||
| LVI | |||||||||
| Absent | 93% | <0.005 | 91% | <0.005 | 89% | 0.01 | 83% | <0.005 | |
| Present | 77% | 60% | 79% | 53% | |||||
| ≥20 mitoses/mm2 | |||||||||
| No | 90% | <0.005 | 83% | 0.069 | 86% | 0.49 | 75% | 0.04 | |
| Yes | 69% | 69% | 79% | 57% | |||||
| >1 mm nodal deposit | |||||||||
| No | 89% | 0.61 | 91% | <0.005 | 94% | <0.005 | 84% | <0.005 | |
| Yes | 87% | 72% | 77% | 62% | |||||
| ≥2 lymph nodes positive | |||||||||
| No | 88% | 0.99 | 85% | 0.008 | 91% | <0.005 | 79% | <0.005 | |
| Yes | 89% | 71% | 70% | 57% | |||||
| Extracapsular extension | |||||||||
| No | 88% | 0.93 | 84% | 0.007 | 86% | <0.005 | 74% | <0.005 | |
| Yes | 89% | 61% | 78% | 61% | |||||
| AJCC 8e Stage | |||||||||
| IIIA | 94% | 0.1 | 94% | <0.005 | 96% | <0.005 | 91% | <0.005 | |
| IIIB-D | 85% | 75% | 80% | 63% | |||||
| Adjuvant systemic therapy | |||||||||
| No | 91% | 0.51 | 84% | 0.49 | 82% | 0.25 | 74% | 0.81 | |
| Yes | 85% | 80% | 89% | 72% | |||||
| Adjuvant immunotherapy | |||||||||
| No | 91% | 0.35 | 83% | 0.71 | 82% | 0.2 | 74% | 0.91 | |
| Yes | 84% | 81% | 90% | 73% | |||||
Abbreviations: DFS, disease-free survival, DMF, distant metastasis-free, LC, local control; LVI, lymphovascular invasion; NC, nodal control; NNLRC, non-nodal locoregional control (local and in-transit disease control); PNI, perineural invasion; yrs, years.
Predictors of outcome for the subset of patients receiving adjuvant systemic therapy (n=102) are shown in Supplementary Table 4. Specifically, in this subset, LVI and greater nodal disease burden predicted for increased risk of nodal relapse. DFS was predicted by similar variables in addition to >4 mm Breslow thickness, microsatellitosis and ECE.
An additional univariate analysis for predictors of outcome was also conducted for the subset of the overall patient population who had nodal disease >1 mm (n=108). Similar factors were found to predict for risk of nodal relapse and DFS (Supplemental Table 3). In this subset of patients with >1 mm nodal disease, adjuvant systemic therapy was also not found to predict for DFS in the context of small numbers (Supplemental Figure 3).
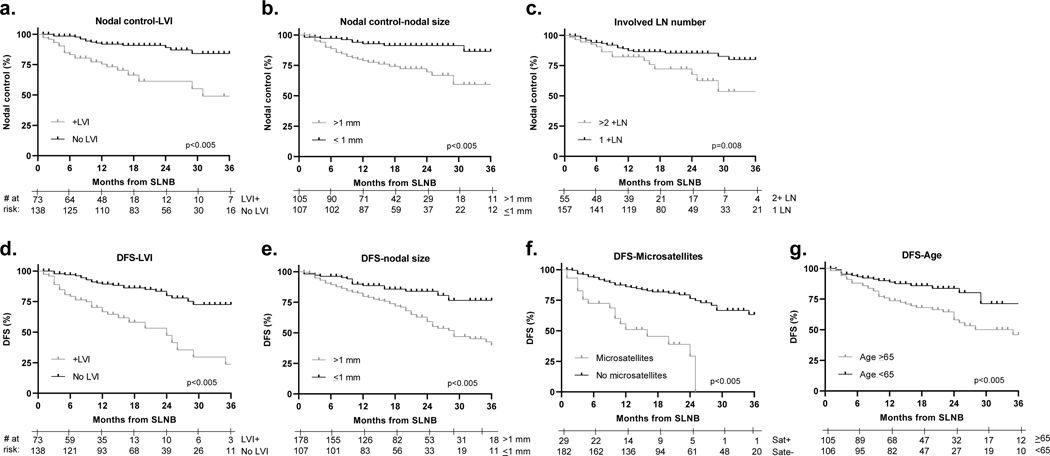
Multivariable models of predictors of clinical outcomes for the full study population are shown in Table 4. More advanced AJCC 8th edition staging was excluded from this analysis given that stage group represents a combination of other primary and nodal factors. Worse NNLRC was independently predicted by the presence of microsatellites or LVI. Worse nodal disease control was independently associated with LVI, ≥2 involved nodes and >1 mm nodal disease (Figure 1). Risk of distant metastasis was associated with the same three parameters. The multivariable model for DFS showed that LVI, >1 mm nodal disease, microsatellites and age ≥65 years were associated with worse outcome.
Table 4.
Multivariate models of outcome
| HR (95% CI) | p-value | ||
|---|---|---|---|
| Non-nodal locoregional control | |||
| Microsatellites | 3.4 (1.4–8.2) | 0.009 | |
| LVI | 2.7 (1.1–6.4) | 0.023 | |
| Nodal disease control | |||
| LVI | 3.84 (1.90–7.76) | <0.005 | |
| ≥2 nodes | 2.14 (1.07–4.26) | 0.03 | |
| >1 mm node | 2.21 (1.00–4.92) | 0.04 | |
| Distant disease control | |||
| LVI | 2.24 (1.04–4.82) | 0.039 | |
| ≥2 nodes | 2.51 (1.15–5.48) | 0.023 | |
| >1 mm node | 2.51 (1.00–6.60) | 0.048 | |
| Disease-free survival | |||
| LVI | 2.36 (1.32–4.23) | 0.0037 | |
| >1 mm node | 2.29 (1.23–4.22) | 0.0057 | |
| Microsatellites | 2.73 (1.48–5.02) | 0.0023 | |
| Age ≥65 | 1.87 (1.06–3.30) | 0.026 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion.
Figure 1.
Predictors of nodal control and disease-free survival. (a) Lymphovascular invasion, (b) ≥0.5 mm nodal disease and (c) ≥2 involved nodes, predict for nodal control. (d) Lymphovascular invasion, (e) ≥0.5 mm nodal disease, (f) ≥2 involved nodes, and (g) microsatellites predict for disease-free survival.
DISCUSSION
The current study represents an analysis of a large cohort of patients with SLN positive melanoma without CLND in the contemporary era. Approximately half of patients received adjuvant immunotherapy or BRAF/MEK targeted therapy. Of the 27% of patients who have thus far recurred, nodal recurrence was the most common site of first relapse. This data illustrate that nodal relapse is a primary driver of early disease recurrence for patients with SLN positive melanoma in the modern era. While MSLT-2, DeCOG-SLT and others have shown that improving nodal disease control does not translate to changes in DFS or overall survival, nodal relapse can be morbid and anxiety provoking for patients1,3. In that context, improving nodal control in the upfront setting remains an important goal.
It is worth noting that the current study population represents a somewhat higher risk cohort than the prior randomized studies that led to the current treatment paradigm of forgoing CLND. Specifically, in the current study, 32% of patients had nodal disease >2 mm, in contrast to DeCOG-SLT where only 7.4% of patients with annotated nodal size met this criteria1. Similarly, 33.2% of patients in MSLT-2 had nodal disease >1 mm in contrast to the current study where 50.2% of patients met this criteria3. In this context, the current study may be particularly well-suited to demonstrated patterns of recurrence in a “real-world” sentinel lymph node positive population. Though it is also worth noting that the 3-year nodal control rate in the observation arm of MSLT-2 was 77% which is generally consistent with the 2-year nodal control rate in the current study of 82%3.
Several recent studies put the current data into context. Work by Bartlett et al. examined outcomes of 370 patients who underwent SLNB in the absence of CLND between 1995–201811. Given the era of study, only 4 patients received adjuvant checkpoint inhibition or targeted therapy. With a median follow-up of 33 months, 42.7% developed recurrence with 39% of first recurrences including nodal disease in the absence of distant metastasis, which is similar though slightly less than the 46% seen in the current study where approximately half of patients received adjuvant checkpoint inhibition or targeted therapy. Work by Farrow et al. examined outcomes of 32 patients who underwent SLNB in the absence of CLND between 2016–201912. In this study 68.8% of patients (n=22) received adjuvant checkpoint inhibition or targeted therapy. With a median follow-up of 10.7 months, 21.9% of patients (n=7) had recurred with 5 recurring in the nodal basin alone, also suggesting in this modern therapeutic context, nodal relapse may be a primary driver of early disease recurrence.
A larger burden of nodal disease, including both number of nodes and size of nodal disease was associated with increased risk of nodal recurrence. Prior studies of melanoma patients with clinically evident nodal disease have shown similar associations13–17. In the context of >1 mm nodal disease being a previously established threshold for defining high risk patients, this was the primary cut-off used to define high risk in the current study. Further multivariate modeling showed a 0.5 mm nodal disease threshold (which included 67% of patients) was even more strongly associated with risk of nodal relapse and DFS (Supplemental Table 5). Prior studies have shown LVI is associated with increased risk of nodal involvement at diagnosis18–21. However, this study is the first, to our knowledge, to demonstrate that LVI in the setting of any magnitude of SLN involvement is associated with a significantly higher rate of early nodal relapse.
It was unexpected that adjuvant systemic therapy was not associated with outcome given that multiple randomized prospective studies have shown a significant advantage of adjuvant immunotherapy and adjuvant targeted therapy for patients with node-positive melanoma4–6. It is notable, however, that the burden of nodal disease in the prospective studies was typically greater than what is seen in the current study. Specifically, patients with sub-mm disease were under-represented in the prior prospective evaluations of adjuvant systemic therapy. In the current dataset, only 132 patients (61%) met KEYNOTE-54 and COMBI-AD eligibility criteria and only 58 patients (27%) met CheckMate-238 eligibility criteria. Of those who met KEYNOTE-54 and COMBI-AD criteria, only 71 (54%) received adjuvant systemic therapy. In addition, given that the current study spans a 4-year era that includes time before the presentation or publication of the adjuvant therapy studies, this finding may be an artifact of unequal application of adjuvant systemic therapy across time as well as selection bias in terms of which patients received this treatment and were available for long term follow-up. While we were unable to find a clear association between adjuvant systemic therapy use and variables such as nodal deposit size, ECE or anatomic nodal basin, in the context of this being a retrospective study there are likely significant unaccounted confounders. In addition, given the time period of analysis, few patients received adjuvant BRAF/MEK directed therapy, such that the primary driver of the adjuvant systemic therapy effect was immunotherapy, specifically nivolumab. We looked for an association between adjuvant systemic therapy and outcome in the latter 2018–2019 period (after the publication of the anti-PD1 studies) and did not find a significant association. However, even in this latter period only 58% of patients received adjuvant systemic therapy, such that it is challenging to draw meaningful conclusions.
It was also unexpected that ECE was not associated with outcomes on our multivariate models. This may be the result of ECE being an uncommon finding in our study population (n=21, 10%), such that drawing significant associations with this variable are challenging. It is also worth noting that the data regarding the association of ECE with increased risk of recurrence has most often been seen in patients with larger volume nodal burden. With macroscopic disease, ECE likely indicates an underlying biologic predisposition for invasion beyond the node15,16,22. While others have found ECE in the context of microscopic nodal disease can be associated with increased risk of non-SLN involvement and worse outcomes overall, whether ECE in the context of small microscopic nodal deposits may be related to chance anatomic location rather than inherent biology is not clear23,24.
We acknowledge the limitations inherent in a retrospective cohort study. In addition to the aforementioned likely selection bias in those who received adjuvant systemic therapy, our median follow-up time was relatively short. It is also worth noting that a relatively smaller proportion of patients with head and neck nodal disease are presented here because many with SLN involvement have historically undergone CLND at our institution.
Overall, this study identifies regional nodal recurrence as a primary driver of early relapse in patients with SLN positive melanoma in the contemporary era. This raises the question of whether there is an intervention with low toxicity that could mitigate this risk. MSLT-2 and DeCOG-SLT showed that CLND significantly decreases nodal recurrence risk but in the absence of an effect on survival endpoints, CLND has fallen out of routine practice. This trend has been the result of physicians generally agreeing that the potential morbidities of CLND outweigh a regional disease control benefit that does not translate to a DFS benefit. However, nodal RT has an excellent short- and long-term toxicity profile and could serve as an alternative option to maximize regional nodal disease control in the absence of CLND. In the current study, adjuvant nodal RT was not associated with an improvement in nodal control but only 4% of patients (n=9) received this treatment and these patients typically had the most aggressive nodal disease. In this context, the lack of benefit in this subset is likely the product of significant selection bias. TROG 02.01 showed that regional nodal RT after CLND for patients with clinically evident, macroscopic high risk nodal involvement significantly decreases nodal recurrence risk with good tolerability25,26. In this context we are currently enrolling patients on a prospective, randomized trial, “MelPORT,” to evaluate the role of adjuvant nodal RT for patients with high risk SLN positive melanoma who are planned for adjuvant immunotherapy (NCT04594187). Based on the current study, eligibility criteria for MelPORT consists of patients with a positive SLN planned for adjuvant immunotherapy with larger nodal burden (defined as ≥2 involved nodes or >0.5 mm nodal disease) or primary tumor LVI. The primary goal of this study is to determine if adjuvant nodal RT can mitigate the risk of early nodal recurrence with an acceptable side effect and quality of life profile.
Supplementary Material
SYNOPSIS.
In the contemporary era of positive sentinel lymph node biopsy being rarely followed by completion lymph node dissection, nodal recurrence is a primary driver of early relapse despite the availability of newer adjuvant systemic therapies.
Acknowledgments
Funding Statement: This work was supported by Cancer Center Support (Core) Grant CA016672 to (PI-Pisters) The University of Texas MD Anderson Cancer Center.
Footnotes
Conflict of Interest Disclosures: Dr. Gershenwald reported serving as a consultant and/or on the advisory board for Merck, Syndax, Castle Biosciences, Novartis, and Bristol Myers Squibb. Dr. Davies reported serving as a consultant to Roche/Genentech, Array, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex and Apexigen, and he has been the PI of research grants to MD Anderson by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, and Oncothyreon. No other disclosures were reported.
REFERENCES
- 1.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8 [DOI] [PubMed] [Google Scholar]
- 2.Leiter U, Stadler R, Mauch C, et al. Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients With Melanoma With Positive Sentinel Node. J Clin Oncol. 2019;37(32):3000–3008. doi: 10.1200/JCO.18.02306 [DOI] [PubMed] [Google Scholar]
- 3.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM., Blank CU, Mandalà M, et al. Pembrolizumab versus placebo after complete resection of high-risk stage III melanoma: New recurrence-free survival results from the EORTC 1325-MG/Keynote 054 double-blinded phase III trial at three-year median follow-up. Journal of Clinical Oncology. 2020;38:(suppl; abstr 10000). doi: 10.1200/JCO.2020.38.15_suppl.1000033052757 [DOI] [Google Scholar]
- 8.Owen C, Larkin J, Shoushtari AN, et al. A multicenter analysis of melanoma recurrence following adjuvant anti-PD1 therapy. JCO. 2019;37. doi: 10.1200/JCO.2019.37.15_suppl.9502 [DOI] [Google Scholar]
- 9.Wong SL, Faries MB, Kennedy EB, et al. Sentinel Lymph Node Biopsy and Management of Regional Lymph Nodes in Melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update. Ann Surg Oncol. 2018;25(2):356–377. doi: 10.1245/s10434-017-6267-7 [DOI] [PubMed] [Google Scholar]
- 10.Thompson JF, Soong S-J, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199–2205. doi: 10.1200/JCO.2010.31.5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett EK, Lee AY, Spanheimer PM, et al. Nodal and systemic recurrence following observation of a positive sentinel lymph node in melanoma. Br J Surg. 2020;107(11):1480–1488. doi: 10.1002/bjs.11621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow NE, Raman V, Williams TP, Nguyen KY, Tyler DS, Beasley GM. Adjuvant Therapy is Effective for Melanoma Patients with a Positive Sentinel Lymph Node Biopsy Who Forego Completion Lymphadenectomy. Ann Surg Oncol. 2020;27(13):5121–5125. doi: 10.1245/s10434-020-08478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershenwald JE, Andtbacka RHI, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26(26):4296–4303. doi: 10.1200/JCO.2007.15.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uppal A, Stern S, Thompson JF, et al. Regional Node Basin Recurrence in Melanoma Patients: More Common After Node Dissection for Macroscopic Rather than Clinically Occult Nodal Disease. Ann Surg Oncol. 2020;27(6):1970–1977. doi: 10.1245/s10434-019-08086-0 [DOI] [PubMed] [Google Scholar]
- 15.Lee RJ, Gibbs JF, Proulx GM, Kollmorgen DR, Jia C, Kraybill WG. Nodal basin recurrence following lymph node dissection for melanoma: implications for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46(2):467–474. doi: 10.1016/s0360-3016(99)00431-9 [DOI] [PubMed] [Google Scholar]
- 16.Pidhorecky I, Lee RJ, Proulx G, et al. Risk factors for nodal recurrence after lymphadenectomy for melanoma. Ann Surg Oncol. 2001;8(2):109–115. doi: 10.1007/s10434-001-0109-2 [DOI] [PubMed] [Google Scholar]
- 17.Kretschmer L, Bertsch HP, Zapf A, et al. Nodal Basin Recurrence After Sentinel Lymph Node Biopsy for Melanoma: A Retrospective Multicenter Study in 2653 Patients. Medicine (Baltimore). 2015;94(36):e1433. doi: 10.1097/MD.0000000000001433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt CR, Panageas KS, Coit DG, Patel A, Brady MS. An increased number of sentinel lymph nodes is associated with advanced Breslow depth and lymphovascular invasion in patients with primary melanoma. Ann Surg Oncol. 2009;16(4):948–952. doi: 10.1245/s10434-009-0331-x [DOI] [PubMed] [Google Scholar]
- 19.White RL, Ayers GD, Stell VH, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Ann Surg Oncol. 2011;18(13):3593–3600. doi: 10.1245/s10434-011-1826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tas F, Erturk K. Histological lymphovascular invasion is associated with nodal involvement, recurrence, and survival in patients with cutaneous malignant melanoma. Int J Dermatol. 2017;56(2):166–170. doi: 10.1111/ijd.13405 [DOI] [PubMed] [Google Scholar]
- 21.Namikawa K, Aung PP, Gershenwald JE, Milton DR, Prieto VG. Clinical impact of ulceration width, lymphovascular invasion, microscopic satellitosis, perineural invasion, and mitotic rate in patients undergoing sentinel lymph node biopsy for cutaneous melanoma: a retrospective observational study at a comprehensive cancer center. Cancer Med. 2018;7(3):583–593. doi: 10.1002/cam4.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosrotehrani K, van der Ploeg APT, Siskind V, et al. Nomograms to predict recurrence and survival in stage IIIB and IIIC melanoma after therapeutic lymphadenectomy. Eur J Cancer. 2014;50(7):1301–1309. doi: 10.1016/j.ejca.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 23.Frankel TL, Griffith KA, Lowe L, et al. Do micromorphometric features of metastatic deposits within sentinel nodes predict nonsentinel lymph node involvement in melanoma? Ann Surg Oncol. 2008;15(9):2403–2411. doi: 10.1245/s10434-008-0024-x [DOI] [PubMed] [Google Scholar]
- 24.Lobo AZC, Tanabe KK, Luo S, et al. The distribution of microscopic melanoma metastases in sentinel lymph nodes: implications for pathology protocols. Am J Surg Pathol. 2012;36(12):1841–1848. doi: 10.1097/PAS.0b013e31826d25f9 [DOI] [PubMed] [Google Scholar]
- 25.Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–597. doi: 10.1016/S1470-2045(12)70138-9 [DOI] [PubMed] [Google Scholar]
- 26.Henderson MA, Burmeister BH, Ainslie J, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–1060. doi: 10.1016/S1470-2045(15)00187-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.