Abstract
Introduction and importance
Bone cement implantation syndrome (BCIS) typically occurs during bone cementation and prosthesis insertion. Almost all of the models used to explain BCIS are based on studies related to hip arthroplasty. BCIS induced by bone cement implantation in superficial wounds has not been reported.
Case presentation
We report the case of a 37-year-old man with chronic osteomyelitis of the left tibia (Cierny - Mader type II), who developed BCIS after covering the infected bone surface with antibiotic-loaded bone cement. BCIS presented as acute massive pulmonary exudation and hypoxemia. Early application of awake prone positioning effectively improved oxygenation and lung injury, and prevented the need for a more advanced means of respiratory support. The patient was discharged without any clinical complications on postoperative day 15.
Clinical discussion
We assessed the cause of acute respiratory events in this patient using Naranjo Adverse Drug Reaction Probability Scale, and BCIS was finally considered. Despite the lack of “driving force” (e.g., increased intramedullary pressure), the bone cement monomer may be absorbed into circulation through the wound surface due to its penetrability. Subsequent immune-mediated amplification resulted in pulmonary exudation and hypoxemia. As a pathophysiologically change-oriented strategy, effective drainage after awake prone positioning significantly improved clinical outcomes.
Conclusion
Bone cement monomer absorbed through the wound should be of concern, and pathophysiological change-oriented management should be emphasized in BCIS.
Keywords: Bone cement implantation syndrome, Chronic osteomyelitis, Awake prone positioning, Pulmonary exudation, Hypoxemia
Highlights
-
•
Bone cement monomer absorbed through the wound surface should be of concern.
-
•
Even a small amount of bone cement monomer can trigger severe organ damage due to immune amplification.
-
•
Prone position can be used for the management of bone cement implantation syndrome characterized by pulmonary exudation.
1. Introduction
Bone cement implantation syndrome (BCIS) is considered a unique complication of cemented orthopedic surgeries, and is characterized by varying degrees of respiratory and circulatory dysfunctions [1]. To date, the mechanisms of BCIS are still unclear and cannot be explained by a single model. However, the preventive and therapeutic measures for BCIS are largely based on the theories of pathogenesis [2], [3]. Elucidating the mechanisms from multiple perspectives, as well as the etiology and pathophysiology and prophylaxis and treatment, will be beneficial for the management of BCIS.
We highlight a case of BCIS caused by antibiotic-loaded bone cement implant, in which awake prone positioning (PP) played a pivotal role in improving oxygenation and facilitating recovery from lung injury. This case report has been reported in line with the SCARE Criteria [4].
2. Case-report
2.1. Case presentation
A 37-year-old man with no family, drug, psychosocial or personal histories, was referred to the Sichuan Provincial Orthopedic Hospital for postoperative osteomyelitis following tibial fracture fixation. He had a left tibiofibular fracture due to a fall, and underwent internal fixation of a tibial fracture with screw/plate osteosynthesis in a local hospital about 10 mo prior. More than 6 mo ago, the skin of the left calf appeared red and swollen and an ulcer had formed. He was treated with antibiotics and dressing changes for several months at several medical institutions, but his symptoms did not improve.
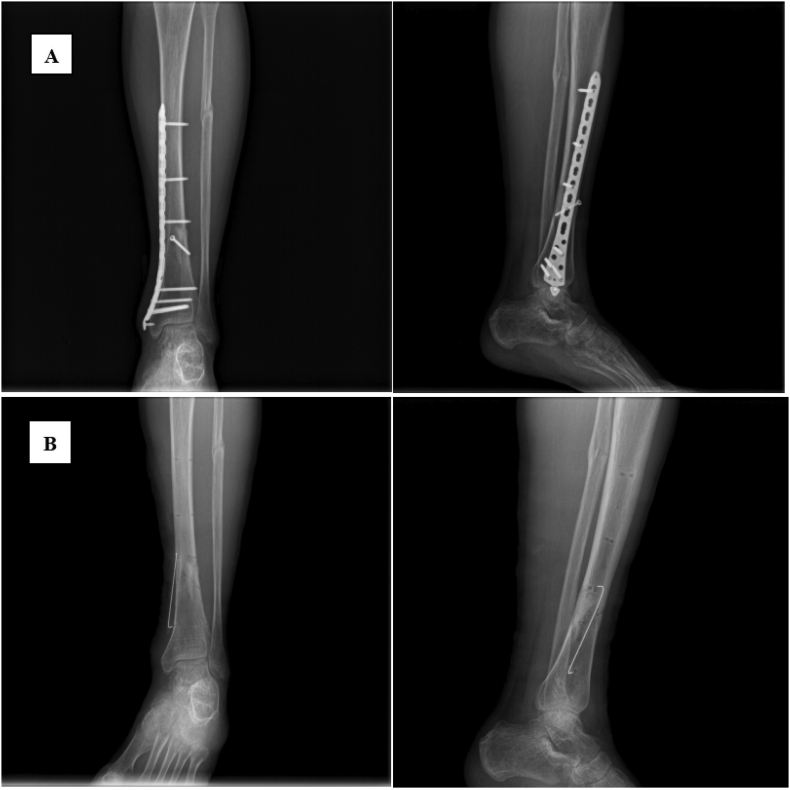
Physical examination revealed a mildly swollen left calf with a 3 mm ulcer on the medial aspect of its lower segment. Laboratory tests on admission revealed higher triglycerides (5.61 mmol/L; normal range: <2.3 mmol/L), total cholesterol (7.49 mmol/L; normal range: <2.55 mmol/L), low-density lipoprotein (4.33 mmol/L; normal range: <3.37 mmol/L), and apolipoprotein B (1.58 g/L; normal range: 0.6–1.1 g/L) compared to reference values. Fatty liver was detected by abdominal ultrasound. Radiological examination showed thinning cortical bone in the lower tibia as well as bone heterogeneity (Fig. 1A). Chronic osteomyelitis of the left tibia (Cierny - Mader type II) was diagnosed.
Fig. 1.
Radiographic images of the left tibiofibula. A: Preoperative; B: Postoperative.
2.2. Treatment
Surgical treatment was determined by both the patient and the surgeons. After blocking the left femoral and sciatic nerve with 0.2% ropivacaine, anesthesia was induced by administration of propofol, sufentanil, and rocuronium, and a No. 4 laryngeal mask was placed. Sevoflurane inhalation and continuous injection of propofol were applied for the maintenance of anesthesia. The original internal fixation device was removed and the chronically-infected membrane between the plate and bone was scraped. The wound surface was cleaned with normal saline, and vancomycin-loaded bone cement was used to cover the bone wound (approximately 2 cm × 5 cm). After the procedure, the tourniquet was deflated. The surgical duration was 95 min, the blood loss was 50 mL, intravenous infusion volume was 1200 mL, and urine volume was 200 mL. The laryngeal mask was removed when the patient awakened from anesthesia. He complained of mild dyspnea. The heart rate was 110 beats/min, blood pressure was 130/82 mmHg, respiratory rate was 28 breaths/min, and pulse oxygen saturation (SpO2) was 90%–91% (O2 was administered via face mask at 10 L/min). Considering the unstable oxygenation, the patient was transferred to an intensive care unit (ICU) under O2 therapy and monitoring.
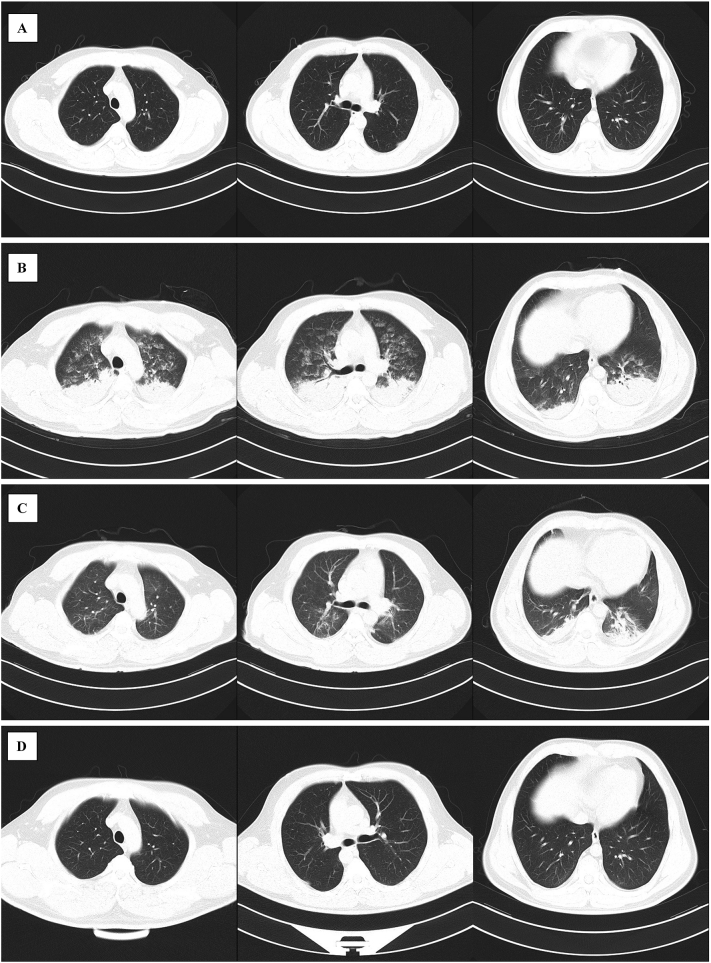
Arterial blood gas analysis showed hypoxemia (Table 1). Ultrasound showed no abnormalities in the size, chamber, valve, and systolic and diastolic function of the heart. However, diffuse B-lines were found in the lungs. Hydrocortisone and furosemide were administered, but the patient's oxygenation status did not improve. A chest CT examination was implemented under monitoring and showed multiple patchy shadows and ground-glass opacities scattered around the texture, with extensive consolidation in the lower lobes of the lung (Fig. 2B). Based on the radiographic findings, awake PP was considered. As soon as the patient was transferred to the prone position, a choking cough occurred. Subsequently, a large amount of pink foamy sputum was expectorated, followed by a significant increase in SpO2. After 2 h of awake PP, SpO2 was maintained at 97% to 98% with 5 L/min O2 administered with nasal cannula. The first awake PP lasted 8 h. During the next 48 h, PP was intermittently performed for a total of 12 h, according to the patient's tolerance.
Table 1.
Postoperative laboratory findings and oxygen therapy.
| Parameter | 1 h | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|---|
| Arterial pH | 7.34 | 7.34 | 7.36 | 7.41 | 7.47 |
| PaO2 (mmHg) | 51 | 79 | 79 | 83 | 194 |
| PaCO2 (mmHg) | 44 | 43 | 48 | 44 | 37 |
| SaO2 (%) | 83 | 95 | 95 | 96 | 100 |
| SB (mmol/L) | 22.8 | 22.8 | 25.7 | 27.9 | 27.5 |
| Lactate (mmol/L) | 3.2 | 3.4 | 2.6 | 3.7 | 2.1 |
| WBC (×109 per L) | – | 15.8 | 10.8 | 11.2 | – |
| Hemoglobin (g/L) | – | 149 | 130 | 140 | – |
| Platelets (×109 per L) | – | 291 | 214 | 237 | – |
| hs-TnT (ng/mL) | – | 0.005 | – | 0.007 | – |
| pro-BNP (pg/mL) | – | 84.16 | – | 667.8 | – |
| PCT (ng/mL) | – | 0.936 | – | 0.301 | – |
| Oxygen therapy | FM | NC | NC | NC | NC |
| Flow (L/min) | 10 | 5 | 3 | 3 | 3 |
Abbreviations: FM, Face mask; hs-TnT, High-sensitivity cardiac troponin T; NC, Nasal catheter; PaCO2, Partial pressure of carbon dioxide; PaO2, Arterial partial pressure of oxygen; PCT, Procalcitonin; pro-BNP, pro-brain natriuretic peptide; SaO2, Blood oxygen saturation; SB, Standard bicarbonate; WBC, White blood cell.
Fig. 2.
Chest computed tomography scan obtained during hospitalization. A: Before surgery; B: 3 h after surgery; C: 3 d after surgery; D: 12 d after surgery. Before and after surgery, significant changes occurred as well as the gradual dissipation of pulmonary lesions over time.
2.3. Outcome and follow-up
On the third postoperative day, the patient was transferred to the general ward because his oxygenation status was markedly improved and most of the lung lesions had dissipated (Table 1, Fig. 2C). Chest CT showed almost no abnormalities on postoperative day 12 (Fig. 2D). The patient was discharged on postoperative day 15 without any clinical complications. His outpatient follow-up at 4 wk. after surgery showed no clinical symptoms or chest abnormalities upon physical examination. Despite the unexpected perioperative complication, the patient was satisfied with our treatment because his lower limb lesions had improved markedly.
3. Discussion and conclusion
3.1. Discussion
The patient developed serious pulmonary edema after debridement and vancomycin-loaded bone cement implantation, and cardiogenic and airway obstruction-related factors were excluded. These symptoms were puzzling. Referring to a previous study [3], Naranjo Adverse Drug Reaction Probability Scale was used to assess the association between intraoperative drug use (including vancomycin) and subsequent symptoms. This scoring system clearly defines the causal relationship between drugs and adverse events at four levels: definite, probable, possible, and doubtful [5]. The bone cement was given a score of 6 in this scoring system, corresponding to probable. Therefore, BCIS was diagnosed.
Current perspectives on the mechanisms of BCIS mainly include embolic and immunological models. Embolization is commonly used to explain BCIS occurring in hip arthroplasty. This theory is based on the fact that some amount of emboli, which may be composed of bone cement microparticle, bone, fat, marrow, or air, has been detected by ultrasonography or autopsy of the right heart and pulmonary vessels [3], [6]. The driving force for emboli to enter the circulation is related to the increased intramedullary pressure during the procedure. In our case, as the surgeon only used a “flaky” vancomycin-loaded bone cement to cover the bone wound, “pressure” contributing to the embolization appeared to be lacking. It is worth noting that the bone cement monomer may be absorbed through the wound due to its penetrating properties [7].
However, a causal relationship between bone cement monomer and lung injury in this patient based solely on the above explanation seems far-fetched, as asymptomatic embolic events are not uncommon [8], [9]. Immunological models may be a bridge connecting the wound-absorbed bone cement monomer to acute pulmonary exudation. As the possibility of hydrostatic pulmonary edema was ruled out in this case, massive exudation of the lungs were attributed to hyperpermeability of the pulmonary capillaries. The direct effects of bone cement monomer, hypersensitivity, complement-mediated cytotoxicity, and waterfall release of cytokines may play important roles in this amplification effect, further illustrating that cement emboli may act as a mediator rather than a single mechanical force to promote BCIS development.
PP, first proposed in the 1970s, facilitates the drainage of secretions, increases ventilation for pulmonary atelectasis, and optimizes ventilation-perfusion matching. The physiological improvements and clinical benefits based on PP have been a subject of debate [10]. In 2013, the PROSEVA trial demonstrated a significant benefit of PP in reducing 28-d and 90-d mortality in patients with severe acute respiratory distress syndrome (ARDS), and PP has mainly been applied to cases of severe ARDS with PaO2/FiO2 < 100 mmHg in subsequent clinical practice [11], [12]. With the COVID-19 pandemic, awake PP has been tentatively used in hypoxemic non-intubated COVID-19 patients, and significant improvement in oxygenation has been found. However, more clinical evidence is needed to determine whether non-sedated, non-intubated patients with COVID-19 really benefit from this therapy [13].
BCIS in the reported patient presented as acute massive pulmonary exudation and hypoxemia without significant circulatory disturbance. Implementation of awake PP is, on the one hand, a pathophysiology-oriented management protocol, and on the other hand, a concern about the adverse effects of inadequate pulmonary drainage on long-term lung function. The rapid improvements in oxygenation and chest imaging of this patient were largely attributed to effective drainage after awake PP.
3.2. Conclusion
Bone cement monomer absorbed through the wound surface should be of concern, which may also cause serious organ damage due to immune amplification. Pathophysiological change-oriented management should be emphasized in BCIS.
Funding
No financial support was received for the completion of this study.
Ethical approval
This study is exempt from ethnical approval in our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
None.
Guarantor
Zhijun Qin takes full responsibility of the work.
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
Zhijun Qin, Yang Deng and Man Li managed the patient and wrote the first draft.
Xia Li reviewed the manuscript.
All authors read and approved the final manuscript.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript.
References
- 1.Hamal P.K., Poudel P.R., Singh J. Grade III bone cement implantation syndrome in malignant lung cancer patient: a case report. BMC Anesthesiol. 2018;18:28. doi: 10.1186/s12871-018-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dradjat R.S., Pradana A.S., Putra D.P., Hexa Pandiangan R.A., Cendikiawan F., Mustamsir E. Successful management of severe manifestation bone cemented implantation syndrome during hemiarthroplasty surgery in patient with multiple comorbidities: a case report. Int. J. Surg. Case Rep. 2021;78:331–335. doi: 10.1186/s12871-018-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra A., Sharma A., Palaniswamy C., El-Oshar S., Desai P., Yazbeck M., et al. Diagnosis and management of bone cement implantation syndrome: case report and brief review. Am. J. Ther. 2013;20:121–125. doi: 10.1097/MJT.0b013e31820b3de3. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Liang R., Borgundvaag B., McIntyre M., Thwaites C., Ragan K., Wyllie A. Evaluation of the reproducibility of the Naranjo adverse drug reaction probability scale score in published case reports. Pharmacotherapy. 2014;34:1159–1166. doi: 10.1002/phar.1496. [DOI] [PubMed] [Google Scholar]
- 6.Razuin R., Effat O., Shahidan M.N., Shama D.V., Miswan M.F. Bone cement implantation syndrome. Malays. J. Pathol. 2013;35:87–90. [PubMed] [Google Scholar]
- 7.Hines C.B. Understanding bone cement implantation syndrome. AANA J. 2018;86:433–441. [PubMed] [Google Scholar]
- 8.Sultan P., Seligman K., Carvalho B. Amniotic fluid embolism: update and review. Curr. Opin. Anaesthesiol. 2016;29:288–296. doi: 10.1097/ACO.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 9.Rothberg D.L., Makarewich C.A. Fat embolism and fat embolism syndrome. J. Am. Acad. Orthop. Surg. 2019;27:e346–e355. doi: 10.5435/JAAOS-D-17-00571. [DOI] [PubMed] [Google Scholar]
- 10.Scholten E.L., Beitler J.R., Prisk G.K., Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151:215–224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 12.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit. Care. 2020;24:28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhazzani W., Evans L., Alshamsi F., Møller M.H., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU: first update. Crit. Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]