Abstract
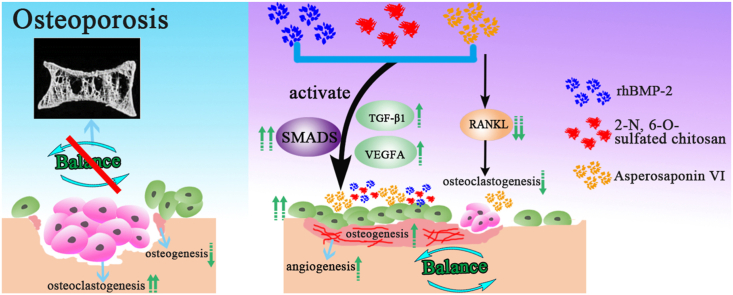
Osteoporosis is a reduction in skeletal mass due to the decrease of osteogenic ability and the activation of the osteoclastic function. Inhibiting bone resorption and accelerating the new bone formation is a promising strategy to repair the bone defect of osteoporosis. In this study, we first systematically investigated the roles of Chinese medicine Asperosaponin VI (ASP VI) on osteogenic mineralization of BMSCs and osteoclastogenesis of BMMs, and then explored the synergistic effect of ASP VI and BS (BMP-2 immobilized in 2-N, 6-O-sulfated chitosan) on bone formation. The result showed that ASP VI with the concentration lower than 10−4 M contributed to the expression of osteogenic gene and inhibited osteoclastic genes RANKL of BMSCs. Simultaneously, ASP VI significantly reduced the differentiation of mononuclear osteoclasts in the process of osteoclast formation induced by M-CSF and RANKL. Furthermore, by stimulating the SMADs, TGF-β1, VEGFA, and OPG/RANKL signaling pathways, ASBS (ASP VI and BS) substantially enhanced osteogenesis, greatly promoted angiogenesis, and suppressed osteoclastogenesis. The findings provide a new perspective on osteoporosis care and prevention.
Keywords: Asperosaponin VI, rhBMP-2, Osteoporosis, Osteogenesis, Osteoclastogenesis
Graphical abstract
Highlights
-
•
Asperosaponin VI down-regulated the osteoclastic genes RANKL expression.
-
•
First combined Asperosaponin VI with rhBMP-2 and 2-N, 6-O-sulfated chitosan.
-
•
Show high osteogenic activity and excellent anti-osteoclastogenesis.
-
•
Explored mechanism on promoting osteogenesis and inhibiting osteoclastogenesis.
1. Introduction
Bone homeostasis is crucial to maintain the normal physiology of the bones. Osteoporosis is a musculoskeletal disorder mainly affecting the elderly female population [1]. It is characterized by reduced bone mineral density and bone quality due to the decrease of osteogenic ability and the enhancement of osteoclastic function [2]. As a result, inhibiting bone resorption while accelerating new bone formation is a promising technique for repairing the osteoporosis-related bone defect.
Some anti-osteoporosis drugs such as Estrogen, Raloxifene, Bisphosphonates, Calcitonin and Parathyroid hormone (PTH) [3,4] are applied in clinic. These drugs are limited due to their high costs and side effects. For example, because of a lack of blood supply at the defect site, drug concentration is often inadequate [[5], [6], [7]]. Hormone replacement therapy is only used for women with an increased risk of osteoporosis [8]. Recently, Chinese herbal medicines with cheap cost and slight side effect have been used to treat the osteoporosis [9]. However, those traditional Chinese medicine played a limited role in anti-osteoporosis merely by regulating the kidney and spleen function, promoting blood circulation and removing blood stasis [[10], [11], [12], [13]].
Dipsacus asper Wall (named Xu Duan in Chinese) belongs to a perennial herb. Dipsacus asper Wall's root is used to treat knee pain, low back pain, rheumatoid arthritis, traumatic hematoma, abortion threats, and bone fractures [14]. Asperosaponin VI (ASP VI) is an active component extracted from Dipsacus asper Wall. Studies revealed that ASP VI could inhibit adipogenic differentiation of preadipocyte 3T3-L1 and mouse bone marrow stromal cells ST-2 [15]. Furthermore, ASP VI was found to be effective in promoting bone marrow stem cell proliferation and osteogenic differentiation [[16], [17], [18]] and inhibiting the osteoclast absorption [19]. Dipsacus extract was also found to prevent the mass loss of OVX-induced bone and deterioration of trabecular microarchitecture [20]. ASP VI has been currently used by oral treatment. Furthermore, Previous studies mainly focused on the processing, isolation of chemical active constituents and pharmacological effects of ASP VI. However, the molecular biological mechanism on anti-osteoporosis and how to regulate the osteogenesis and osteoclastogenesis of ASP VI remains unclear.
Obviously, if a scaffold combined with ASP VI with efficient inhibition bone resorption, the excellent bone-inducing activity, osteoporosis and its bone defects will be effectively prevented and repaired. Bone morphogenetic protein 2 (BMP-2) is such an osteo-inductive factor and approved by FDA in spinal fusion treatment [21]. It can induce osteoblast differentiation, increase alkaline phosphatase activity and promote bone formation. However, BMP-2 is unstable with a short half-life and easily degraded under physiological conditions. Moreover, high dosages to be effective pose cost and safety issues. Hence, it is important to immobilize the BMP-2 for improving the efficiency and mediating the osteogenesis of BMP-2.
Our previous work showed that 2-N, 6-O-sulfated chitosan (26CSC) could successfully bind with BMP-2 and activate the osteogenic capacity of BMP-2. ALP expression, matrix mineralization and osteoinductive activity of BMP-2 were enhanced significantly even in low usage after binding with 26CSC. More specifically, 26CSC increased rhBMP-2 bioactivity and facilitated rhBMP-2-mediated angiogenesis [[22], [23], [24], [25]].
Inspired by biological effects of ASP VI and BS (BMP-2 immobilized in 26CSC), we herein propose to enhance the bone repair in osteoporotic defect by implanting ASP VI and BS. Based on this, we systematically explored the effects of ASP VI on osteogenic mineralization and osteoclastic absorption of BMSCs by characterizing the cell compatibility, extracellular matrix mineralization, calcium deposition and gene expression. And the effect of anti-osteoclastogenesis on BMMs by TRAP staining was also studied in this part. Furthermore, ASP VI and BS were combined and the interaction among ASP VI, BMP-2 and 26CSC were evaluated by cell proliferation and adhesion, extracellular matrix mineralization and calcium deposition, gene and protein expression. It is expected to achieve a synergistic effect in promoting bone formation and inhibiting bone resorption and then to regulate the balance between osteogenesis and osteoclastogenesis.
2. Materials and methods
2.1. BMSCs culture
Sprague-Dawley (SD) rats (male, 80–100g weight) were from Shanghai Jiesijie Experimental Animal Co., Ltd. The BMSCs were isolated from the tibias and femurs of SD rats as described in literatures [26,27]. The washed cells (Passage 0) from the bone marrow were cultured in α-minimum (α-MEM; Gibco, USA) containing 15% fetal bovine serum (FBS; Gibco, USA) for two days. After half of the culture medium was replaced by fresh medium, cells were cultured for another two days with α-MEM (10% FBS). Non-adherent cells were gently withdrawn, and adherent cells were cultured after reaching 90% confluence. In this study, BMSCs from passages 2–3 were used in experiments in vitro.
2.2. Osteogenesis and osteoclastic analysis of ASP VI
2.2.1. Cell proliferation assay
Cell counting Kit-8 (CCK-8; Beyotime, Shanghai) was used to assess the impact of different concentrations of ASP VI on the behavior of BMSCs (2 × 103 cells/well, 96-well plates). The concentrations of ASP VI were 10−6 M, 10−5 M, 10−4 M and 10−3 M respectively. At 1, 2 and 3 days, CCK-8 were added to each well. The plate was then incubated at 37 °C for 2 h in the dark. The activity of cells was calculated by reading OD values at 450 nm of a microplate reader (Spectra-Max M2). The background value was calculated by the absorbance at 650 nm. All samples were tested with three replicates and expressed as mean ± SD.
2.2.2. Assay of alkaline phosphatase (ALP) activity
BMSCs were seeded at 1 × 104 cells/well in 24-well plates at 37 °C in 5% CO2. After 1 day culture, various sample extracts supplemented with ASP VI mineralization induction medium (50 mM ascorbic acid, 10 mM b-glycerophosphate, 100 nM dexamethasone and ASP VI with different concentrations of 10−7 M, 10−6 M, 10−5 M and 10−4 M respectively) were used to replace the culture medium. The ALP activities of BMSCs were detected after 4, 7 and 14 days of culture. 500 μL NP-40 cell lysate (Beyotime, Shanghai) was added to every well and cultured for 90 min at 37 °C to ensure that all cells were completely lysed. The total protein was measured according to BCA standard curve. 50 μL lysate was taken from each well and added to 96 well plate. After adding 100 μL 1 mg/mL PNPP-Na solution, the plate was incubated at 37 °C for 2 h. The optical density (OD) was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad, Hercules, CA). The terminal ALP value was determined according to the following equation:
2.2.3. ALP staining
BMSCs were seeded at a density of 1 × 104 cells/well in 24-well plates. After 1 day attachment, various sample extracts supplemented with ASP VI mineralization induction medium (50 mM ascorbic acid, 10 mM b-glycerophosphate, 100 nM dexamethasone and ASP VI with different concentrations of 10−7 M, 10−6 M, 10−5 M and 10−4 M respectively) were used to replace the culture medium. After 7 days, the cells were fixed with 300 μL 2.5% glutaraldehyde for 15 min. Subsequently, 250 μL ALP staining solution (BCIP/NBT alkaline phosphatase kit, Beyotime, Shanghai) was added and stained the cells for 30 min at 25 °C away from light. Finally, cells were washed three times in PBS before inverted fluorescence microscope observation.
2.2.4. Alizarin Red S staining
Alizarin Red S (ARS; Cyagen Biosciences Inc., Guangzhou) staining was performed to assess the BMSC mineralization stimulated by ASP VI with different concentrations during the late stage of osteogenesis after 14 days of culture (1 × 104 cells/well). Washed three times with PBS, the fixed BMSCs was stained with 500 μL of ARS dye solution for 120 min at room temperature. Samples were photographed with an inverted fluorescence microscope.
2.2.5. Quantitative realtime-PCR analysis
Gene expression was tested by a quantitative real-time reverse transcription PCR (RT-qPCR) and iTaq Universal SYBR Green supermix on an ABI 7300 PCR system (Bio-Rad, USA). After incubation with different concentrations of ASP VI (0, 10−6, 10−5 and 10−4 M) for 4, 7 and 14 days, total RNAs were extracted from BMSCs. The iScript cDNA Synthesis Kit (Bio-Rad) and 1200 ng of total RNA were used for cDNA synthesis by a quantiscript reverse transcriptase (Applied Biosystem). RT-qPCR was carried out with a hot-start denaturation stage at 95 °C for 30 s, followed by recording fluorescence strength for 40 cycles. Osteogenic-related genes (such as alkaline phosphatase (ALP), runt-related gene 2 (RUNX2), BMP-2, type Ι collagen (Col I), and VEGF as well as osteoclast-related gene RANKL were all expressed. Table S1 included a list of all primers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a template-free negative regulation. Each individual counterpart's relative gene expression was expressed as a fold change with the control group as a reference value (set to 1). All samples were carried out in triplicate.
2.2.6. Preparation and culture of mouse bone marrow-derived monocytes
To examine the effect of ASP VI on osteoclastogenesis in vitro, mouse bone marrow-derived monocytes (BMMs) were isolated as previously described. Briefly, bone marrow cells collected from tibiae and femurs were incubated in a T75 flask in α-MEM containing 10% (v/v) FBS and 30 ng/mL macrophage colony-stimulating factor (M-CSF; PeproTech, Rocky Hill, NJ, USA) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. After incubation for 3 days, the medium was changed to remove non-adherent cells and impurities, and the adherent cells were used as BMMs. The incubation was continued for an additional 4 days. After reaching 90% confluence, the BMMs were harvested through trypsin digestion and seeded into culture plates for further experiments.
2.2.7. Cell viability
The effects of ASP VI concentration on the viability of BMMs were investigated using the CCK-8 assay. BMMs (1 × 104/well) were plated in 96-well plates in triplicate and incubated with medium containing different concentration of ASP VI (10−6 M, 10−5 M and 10−4 M). After 1, 3, or 5 days of culture, cell viability was analyzed by CCK-8 assay according to the manufacturer's instructions. Cells cultured with standard media were used as the control.
2.2.8. Osteoclastogenesis induced with different groups
BMMs (8 × 103/well) were plated and incubated in 96-well plates in triplicate. After 24 h of culture, the medium was replaced with osteoclast differentiation medium (30 ng/mL M-CSF, 100 ng/mL Receptor Activator for Nuclear Factor-κB Ligand (RANKL; PeproTech)) containing different concentration of the ASP VI (10−6 M, 10−5 M and 10−4 M). The culture medium was changed every 48 h. For the control group, cells were cultured in the osteoclast differentiation medium. After 3 or 5 days of culture, cells were immobilized and stained using a TRAP staining kit.
2.3. Synergistic effect of ASP VI and BS
2.3.1. Cell proliferation and adhesion of ASP VI with BS
Cell proliferation: the effect of ASP VI with BS on the activity of BMSCs was tested by CCK-8 (2 × 104 cells/well, 24-well plates). The samples were grouped and listed in Table 1. The optimal concentrations of B and S were 400 ng/mL and 25.6 μg/mL according to our previous work [[28], [29], [30]].
Table 1.
Samples for cell proliferation and ALP expression.
| Samples | BMP-2(ng/mL) | 26CSC(μg/mL) | ASP VI (mol/L) |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| ASBS-0 | 0 | 0 | 10–5 |
| ASBS-1 | 400 | 25.6 | 0 |
| ASBS-2 | 400 | 25.6 | 10–6 |
| ASBS-3 | 400 | 25.6 | 10–5 |
| ASBS-4 | 400 | 25.6 | 10–4 |
At predetermined time points (1, 2 and 3 days), the culture solution was replaced with fresh medium. Each well was added with CCK-8. The plate was then incubated at 37 °C for 120 min (without light). The activity of cells was calculated using a microplate reader to read OD values at 450 nm (Spectra-Max M2). The background value was calculated by the absorbance at 650 nm. All samples with different ASP VI concentrations were tested in triplicate.
Cell adhesion: In 48 well plates, BMSCs were seeded on the sterilized scaffolds surface (4 × 104 cells/well). After 1 day, original medium was changed into the medium showed in Table 1. After 24 h of culture, the cells were fixed by 2.5% glutaraldehyde for 20 min and successively dehydrated by 30%, 50%, 60%, 70%, 80%, 90% and 100% gradient ethanol. The samples were then treated with isopentyl acetate and vacuum dried for 24 h. Scanning electron microscope (SEM; Hitachi s-4800, Japan) was performed to observe the cells morphology attached on the scaffolds.
2.3.2. Alkaline phosphatase (ALP) activity assay
The ALP activity of ASBS for BMSCs was assayed as described in 2.3.1.
2.3.3. ALP staining and ARS assay
ALP Staining and ARS of the samples in Table 1, investigated the osteogenic mineralization effect of ASBS on BMSCs. The details of staining and assay are same as described in 2.2.3 and 2.2.4.
2.3.4. Osteogenesis and osteoclastogenesis
The genes expression of samples in Table 2 was assessed by RT-qPCR. BMSCs were cultured for 4 days. RT-qPCR was used to measure the expression of osteogenic-related genes [BMP-2, Type 1 collagen (Col I), Osteocalcin (OCN), TGF-β1, VEGF, Alkaline phosphatase (ALP), Osterix (OSX), Runt-related gene 2 (Runx2), and Smad1/4/5/8] as well as an osteoclast-related gene (RANKL) and OPG. Table S2 lists all of the RT-qPCR primer sequences.
Table 2.
Samples for RT-qPCR and Western blot.
| Samples | BMP-2(ng/mL) | 26CSC(μg/mL) | ASP VI(M) |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| ASBS-P0 | 400 | 0 | 0 |
| ASBS-P1 | 0 | 25.6 | 0 |
| ASBS-P2 | 400 | 25.6 | 0 |
| ASBS-P3 | 0 | 0 | 10–5 |
| ASBS-P4 | 400 | 0 | 10–5 |
| ASBS-P5 | 0 | 25.6 | 10–5 |
| ASBS-P6 | 400 | 25.6 | 10–5 |
The expression of Smad 5 and Col I protein in BMSCs induced by different media (Control, ASBS-P0, ASBS-P2, ASBS-P3, ASBS-P4 and ASBS-P6) was detected by Western blot. (1.5 × 105 cells/well, 6-well plate).
After 4 days, proteins were extracted from cells by RIPA buffer (Beyotime, Shanghai) containing 1 mM PMSF. The protein concentration was calculated according to BCA standard curve. The protein's concentrations of each group were equaled with PBS and SDS-PAGE sample loading buffer (5X, Beyotime, Shanghai), the equaled proteins were boiled at 100 °C for 5 min and then stored at −80 °C. SDS-PAGE was used to isolate the equal amount of proteins, which were then moved to a PVDF membrane (Millipore, USA). BeyoGel™ Plus Precast PAGE Gel (Hepes, 10%, 10 pores) and BeyoGel™ Plus SDS-PAGE Hepes Electrophoresis Buffer (20X) and Western Transfer Buffer (Beyotime, Shanghai) were used in this procedure. After blocking for 15 min at room temperature with QuickBlock™ blocking buffer (Beyotime, Shanghai), the membrane was incubated overnight at 4 °C with rabbit polyclonal antibodies against Smad5, Col I and β-actin (1:1000 dilution, Cell Signaling Technology). The membranes were washed with TBST and probed for 2 h at room temperature with anti-rabbit lgG HRP-linked antibody (1:1000, Cell Signaling Technology). Tanon™ High-sig ECL western blotting substrate was used to detect the protein signals.
2.4. Statistical analysis
The mean ± SD of three independent experiments was used to measure all results. One-way analysis of variance (ANOVA) was used in the statistical analysis, and a Student-Newman-Keuls (SNK) post hoc test was used to assess statistical significance. P < 0.05 was used to determine statistical significance.
3. Results and discussion
3.1. BMSCs cytocompatibility with different concentrations of ASP VI
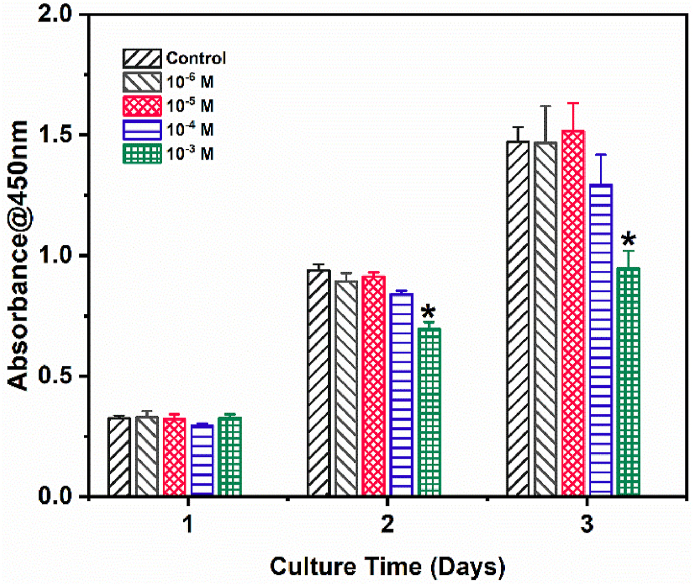
To explore the effect of ASP VI on BMSCs cytocompatibility, CCK-8 assay was performed by culturing the cells with different concentrations of ASP VI. The cells number in all groups increased with the culture time (Fig. 1). The result indicated that cells have a benign proliferation with ASP VI. After incubation for 3 d, the OD values of the Control, 10−6 M, 10−5 M and 10−4 M groups were not substantially different, while the OD value of the 10−3 M group was significantly lower than Control (P<0.05). It indicated that ASP VI had good cytocompatibility with BMSCs when the concentration of ASP VI was lower than 10−4 M. However, the BMSCs proliferation had no significant difference among different concentrations of ASP VI.
Fig. 1.
BMSCs cytocompatibility with different concentration of ASP VI by CCK-8 assay (*, p < 0.05).
3.2. Mineralization activities of ASP VI
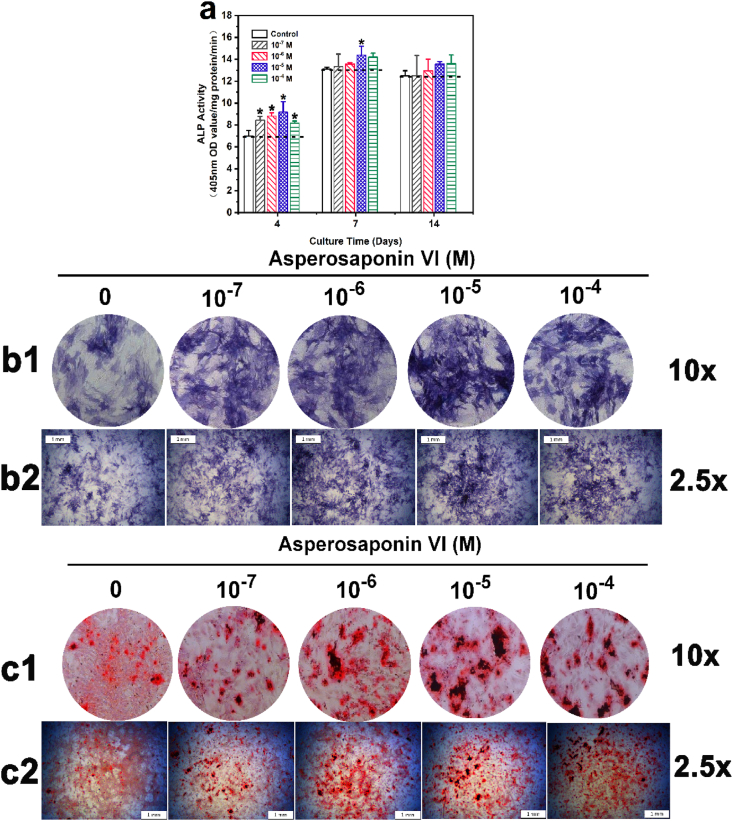
To screen out whether ASP VI affects osteogenic differentiation of BMSCs, the ALP activity assay, ALP staining and ARS were carried out. In Fig. 2a, the ALP value ascended with the prolongation of culture time. After 4-day culture, there was significant difference among Control, 10−7 M, 10−6 M, 10−5 M and 10−4 M groups. In addition, after culturing for 7 and 14 days, this difference was lessened. However, the groups with ASP VI, especially the 10−5 M group, remained slightly higher than the Control.
Fig. 2.
ASP VI enhanced osteogenic differentiation of BMSCs. a. ALP activity of BMSCs after culture for 4, 7 and 14 d (*, p < 0.05); b. ALP staining of BMSCs after culture for 7 d (b1 with magnification of 10 × ; b2 with magnification of 2.5 × ); c. ARS assay of BMSCs after culture for 14 d (c1 with magnification of 10 × ; c2 with magnification of 2.5 × ).
ALP Staining and ARS were used to describe the dose effect of ASP VI on osteogenic differentiation after 7 and 14 d induction. Fig. 2(b1 and b2)b showed that the colors (blue) of ALP staining in all experimental groups were much darker than the Control, which indicated that ASP VI could promote the early osteogenesis. Alizarin red can chelate with calcium ions to form a dark-red precipitate complex calcium nodule, which occurs in the mineralization stage and is one of the markers of late osteogenesis. Fig. 2(c1 and c2) showed that compared to the Control, there was a substantial increase on the number of mineralized dark-red nodules in ASP VI groups. Both ALP and AR Staining demonstrated that the 10−5 M group had the most significant ability of osteogenic mineralization, which was consistent with the ALP activity result. Therefore, the above results showed that ASP VI enhanced ALP activity and calcium deposition of BMSCs. The concentration of 10−5 M was used in subsequent qPCR assay for the gene and protein expression.
3.3. Effect of ASP VI on osteogenic genes expression of BMSCs
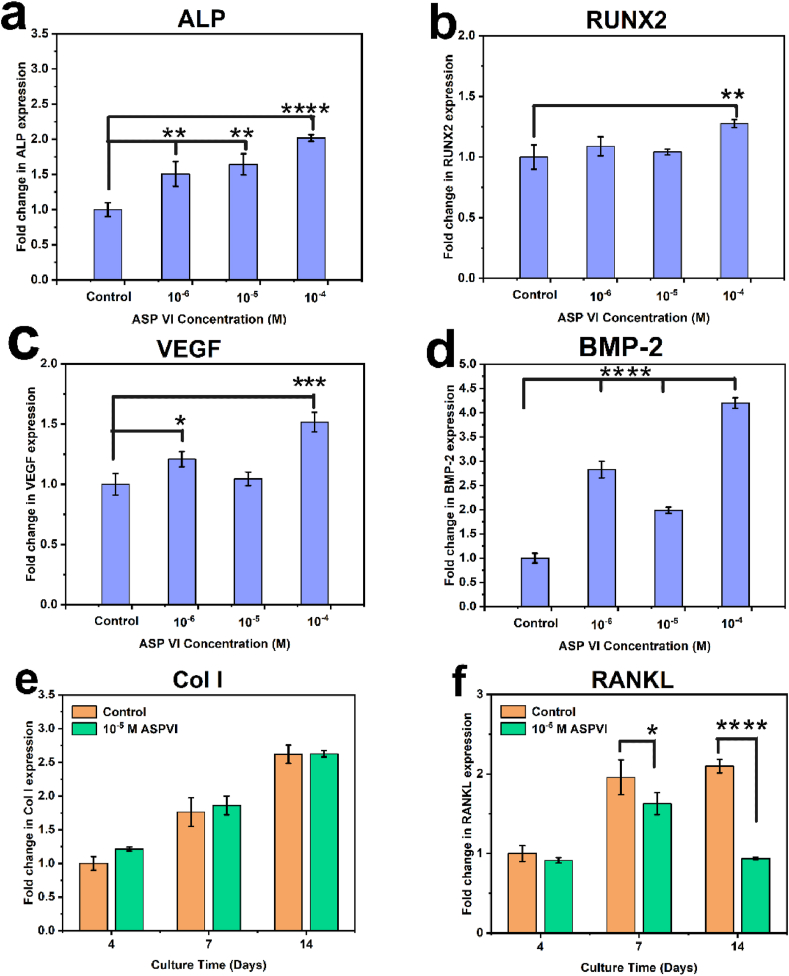
To further examine the effect of ASP VI with different concentrations on osteogenic genes’ expression of BMSCs, we cultured BMSCs with Control, 10−6 M, 10−5 M and 10−4 M groups, respectively. BMP-2 can be transcribed into BMP-2 protein to promote osteogenesis. RUNX2 is a key link in BMP-2. ALP is one of the most important markers of osteogenic differentiation. Type I collagen (Col I) can be transcribed into protein to promote collagen mineralization and osteogenic differentiation. Interestingly, Fig. 3 displayed that the five osteogenic genes of ALP, RUNX2, VEGF, BMP-2 and Col I were all upregulated with the addition of ASP VI. With the role of ASP VI, VEGF was also up-regulated. These results indicated that BMSCs differentiation and osteogenesis were enhanced with the effect of ASP VI by activating BMP-2 signaling pathway.
Fig. 3.
Genes expression of BMSCs stimulated by ASP VI. (a–d) ALP, RUNX2, VEGF, BMP-2 expression of BMSCs after 4 d incubation; (e–f) Col I, RANKL expression of BMSCs after 4 d, 7 d and 14 d incubation. (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
RANKL is an osteoclast differentiation gene. Fig. 3 showed that the osteoclast gene RANKL was downregulated with the addition of ASP VI. The decrease of RANKL indicated that ASP VI inhibited the expression of osteoclast genes, which further inhibited the osteoclast differentiation. Combining with osteogenic and osteoclastic factors, it was obvious that ASP VI increased the expression of osteogenic genes while inhibiting the expression of osteoclastic genes, facilitating the formation of new bone.
3.4. Effect of ASP VI on suppressed osteoclastogenesis
To examine the effect of ASP VI on osteoclastogenesis in vitro, the experiments of ASP VI directly affect the proliferation of monocytes (Fig. 4) and TRAP staining (Fig. 5) were carried out.
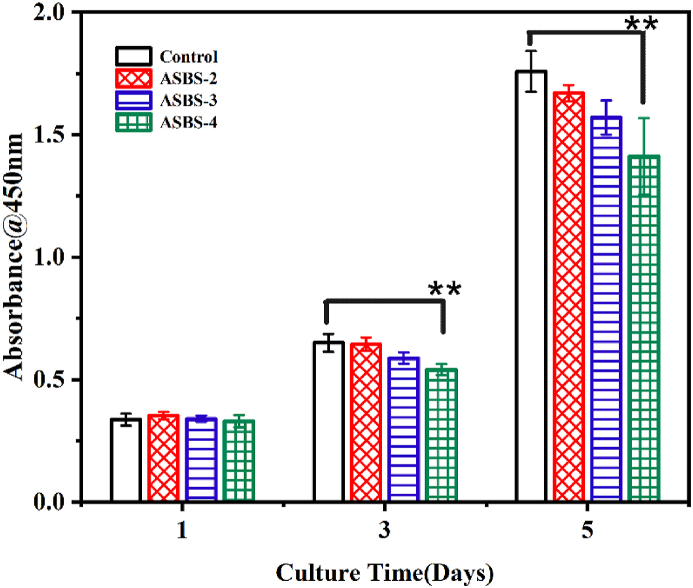
Fig. 4.
BMMs cytocompatibility with ASP VI by CCK-8 assay(**, p < 0.01).
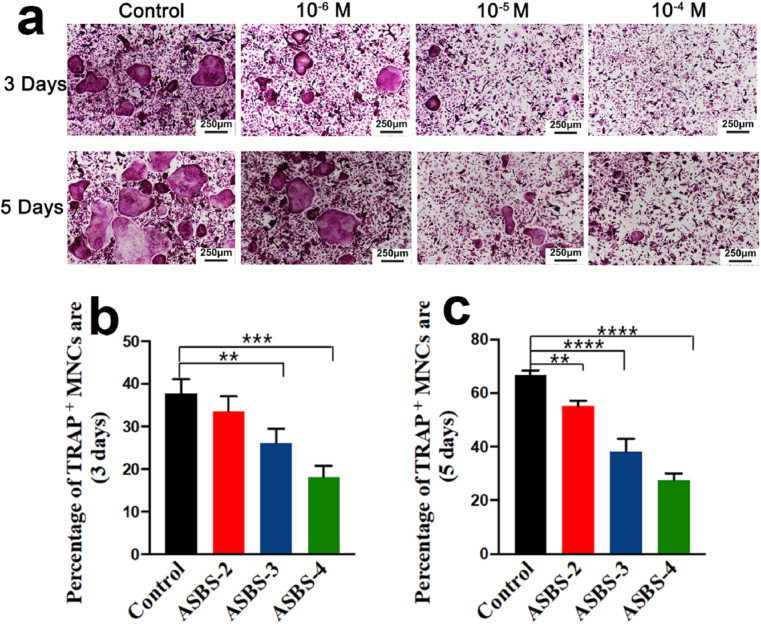
Fig. 5.
ASP VI suppressed RANKL and M-CSF-induced osteoclast differentiation by TRAP staining assay. (a) TRAP staining assay after 3 and 5 days; (b–c) Percentage of TRAP-positive multinucleated cells (MNCs) area. (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
In Fig. 4, BMMs number in all groups increased with the culture time (1, 3 and 5 days). The result indicated that ASP VI had good cytocompatibility with BMMs. In addition, the proliferation of BMMs was gradually inhibited with the increase of ASP VI concentration and the culture time.
In Fig. 5a, after 3 and 5 days of induction, typical morphological changes of osteoclasts were observed in the control group, which indicated that BMMs could differentiate into osteoclasts under the induction of RANKL and M-CSF. Simultaneously, the statistical analysis of osteoclast differentiation in Fig. 5b and c also showed that after the introduction of ASP VI, the number and volume of osteoclasts decreased significantly, indicating that ASP VI significantly reduced the differentiation of mononuclear osteoclasts in a dose-dependent manner. Therefore, ASP VI can inhibit the osteoclast formation in the process of osteoclast formation induced by M-CSF and RANKL.
3.5. Cell proliferation and cell adhesion of ASBS
Although ASP VI promoted osteogenic gene expression while inhibited osteoclast gene expression, its impact on cell proliferation and extracellular matrix mineralization is still limited. To achieve rapid bone regeneration in osteoporotic defect, BMP-2 immobilized in 26CSC (BS) was introduced to stimulate osteogenic differentiation and blood vessel growth to enhance the role of ASP VI [23,[31], [32], [33]].
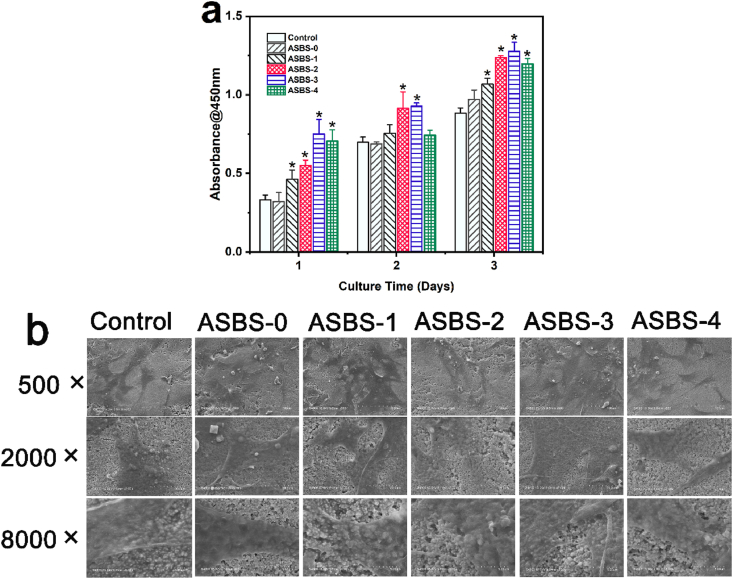
The cell proliferation of ASBS with different concentrations of ASP VI and the Control were shown in Fig. 6a. After 1, 2 and 3 d incubation, the cell numbers increased with the increase of incubation time. No obvious differences were found between the Control and ASBS-0 group only with ASP VI. However, there were obvious improvements in ASBS-1∼ASBS-4 compared with the Control. Furthermore, the OD value of ASBS-2, ASBS-3 and ASBS-4 was much higher than that of ASBS-1 only with BS. The result indicated that ASBS-2, ASBS-3 and ASBS-4 possessed the excellent proliferation of BMSCs both with ASP VI and BS, especially for ASBS-3 with 10−5 M ASP VI. The result revealed that ASP VI together with BS significantly enhanced BMSCs cell proliferation.
Fig. 6.
a. BMSCs proliferation with ASBS by CCK-8 assay. b. SEM observations for BMSCs morphology with ASBS (*, p < 0.05).
The effect of ASBS on cell adhesion was observed by SEM in Fig. 6b. Cells spread on the surface of β-TCP/PLGA composite scaffolds in all groups. It was clear that synapses spread more obviously and the pseudopodia was more transparent with the combined effects of ASP VI and BS. Especially, ASBS-3 group possessed the best cell adhesion with fully covered synapses and more transparent pseudopods. In a word, the observations on cell proliferation and cell adhesion demonstrated that ASBS contributed to the significant promotion for BMSCs proliferation with favorable synaptic spread and pseudopodia extension.
3.6. ASBS enhanced the ALP activity and calcium deposition
To further investigate the extracellular matrix mineralization and calcium deposition effect of ASBS, the assay of ALP activity, ALP and ARS Staining were carried out. As an anionic dye, Alizarin Red S can chelate with calcium ion to form complex and produce a red-black precipitate which was called calcium nodules. Fig. 7a showed that the addition of BS significantly improved the osteogenic differentiation of BMSCs with ASBS-2, ASBS-3 and ASBS-4 after culturing for 4, 7 and 14 days (P<0.05). Furthermore, the ALP values of ASBS-2, ASBS-3 and ASBS-4 were not only higher than ASBS-1 only with BS but also higher than ASBS-0 only with ASP VI at 7 and 14 d. Among these groups, ASBS-3 displayed the highest ALP value. The result further indicated that the synergistic effect between ASP VI and BS significantly promoted the expression of ALP activity.
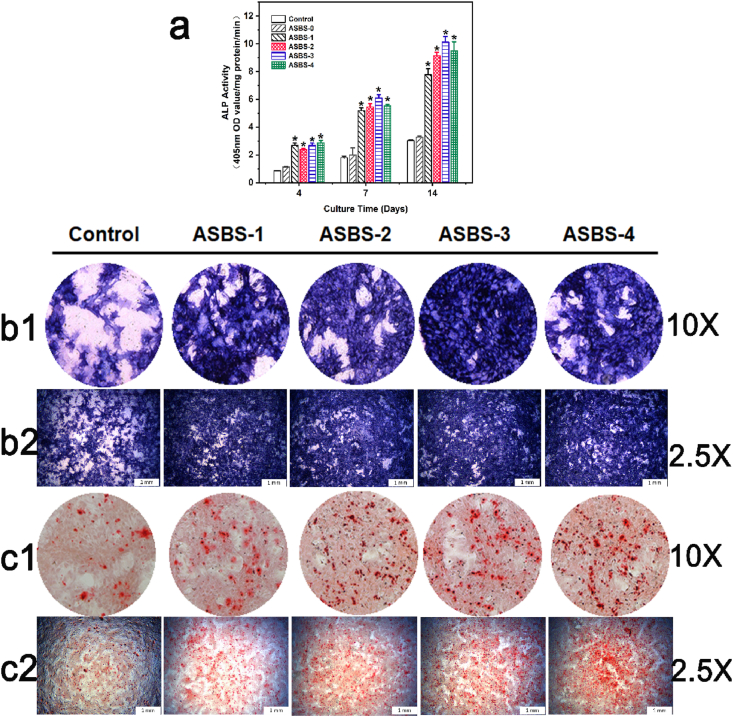
Fig. 7.
ASBS enhanced the ALP activity and calcium deposition of BMSCs a. ALP activity of BMSCs after 4, 7 and 14 d (*, p < 0.05); b. ALP staining of BMSCs after culture for 7 d (b1 with magnification of 10 × ; b2 with magnification of 2.5 × ); c. ARS assay of BMSCs after culture for 14 d (c1 with magnification of 10 × ; c2 with magnification of 2.5 × ).
The ALP Staining (7 d) results are plotted in Fig. 7b1 and Fig. 7b2. Under the synergy effect of ASP VI and BS, BMSCs showed a darker color after staining, especially ASBS-3 group induced 10−5 M ASP VI. ARS staining in Fig. 7c1 and Fig. 7c2 showed that the number of mineralized nodules marked in dark-red in ASBS-2, ASBS-3 and ASBS-4 groups were significantly increased after culturing for 14d. The results demonstrated that the calcium deposition of BMSCs were promoted with the synergy of BS and ASP VI.
The above ALP and ARS staining results showed that extracellular matrix mineralization was significantly promoted with the interaction of ASP VI and BS, which was consistent with the ALP activity assay result. In addition, ASBS with the concentration of 10−5 M of ASP VI was used in subsequent gene and protein expression assay.
3.7. Gene and protein expression of ASBS
Osteoporosis is a complex physiological process involved with osteoblasts, osteoclasts, angiogenesis and their elaborate interactions by multiple signaling pathways. To further explore the synergistic effect mechanism on osteogenesis between ASP VI and BS, the gene and protein expression assay were carried out and the results were shown in Fig. 8.
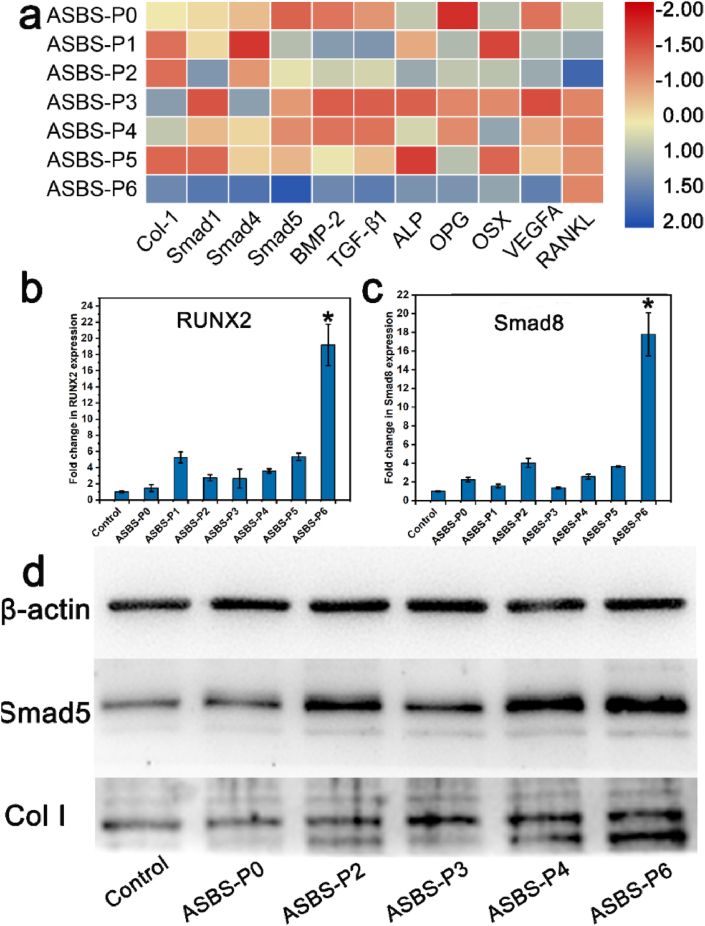
Fig. 8.
Gene and protein expression of ASBS. (a–c) PCR assay of osteogenic-related genes (BMP-2, Col I, OSX, Smad1/4/5/8, ALP, Runx2, TGF-β1, VEGFA) and osteoclastic-related genes (OPG, RANKL). (d) Western Blot assay of osteogenic-related proteins (Smad5 and Col I) (*, p < 0.05).
Fig. 8a–c showed the gene expression of BMSCs. In Fig. 8a, the color in ASBS-P6 group was darker (more blue tendency) than in the other groups for osteogenic related genes (BMP-2, Col I, OSX, Smad1/4/5, ALP, TGF-β1, VEGFA) and OPG. As for RANKL, the color in ASBS-P3∼ASBS-P6 groups with ASP VI was lighter (more red tendency) than in ASBS-P0∼ASBS-P2 groups without ASP VI. The ASBS-P6 group had significantly higher Runx2 and Smad 8 gene expression than the other groups (Fig. 8b and c). Compared with the groups induced only with ASP VI or BS, the expression of genes related to osteogenesis and OPG were significantly up-regulated with the synergy of ASP VI and BS. The Western blot assay further confirmed this result (Fig. 8d). The expression of Smad 5 and Col I protein was enhanced with the both induction of ASP VI and BS. Interestingly, the ASP VI greatly decreased the expression of RANKL signal. That is to say, ASP VI could not only enhance the bone formation of BS but also prevent and inhibit the bone absorption. Therefore, the ASBS played an effective role in osteoporosis by dramatically promoting osteogenic differentiation and angiogenesis and inhibiting osteoclast absorption.
When BMSCs were cultured in the medium with ASBS, ASBS could up-regulate the expression of TGF-β1 and VEGFA genes and thus promote angiogenesis. Simultaneously, ASP VI and BMP-2 stimulated their respective receptors BMP-2R on BMSCs membrane, and then activated SMADs signaling pathway which made the signals translated into the nucleus [34]. Furthermore, Runx2 regulates BMSCs differentiation which can be activated by SMADs [35]. As a downstream effector of Runx2 [36], OSX was active and directly stimulated transcription of Col I [37] and ALP [38]. Finally, the synergy between ASP VI and BS significantly promoted osteogenesis mainly by promoting cell proliferation and differentiation. More importantly, ASP VI played a key role in down-regulating the RANKL expression. The BMSCs stimulated by ASBS-P6 promoted the OPG expression, which plays an important role in bone resorption prevention [39]. The upregulation of OPG as well as the downregulation of RANKL revealed that the ASP VI and BS could inhibit the expression of osteoclast genes, directly leading to the inhibition of osteoclast absorption.
In conclusion, SMADs, TGF-β1, VEGFA, and OPG/RANKL signaling pathways were involved in promoting angiogenesis and suppressing osteoclastogenesis, and played an important role in controlling the balance of osteogenesis and osteoclastogenesis.
4. Conclusions
We first designed the combination of ASP VI and BMP-2 immobilized in 26CSC (ASBS) and investigated its promoting osteogenesis and decreasing osteoclastogenesis-inducing-related gene expression on BMSCs. The result showed that ASP VI enhanced ALP activity and extracellular matrix mineralization with the concentration lower than 10−4 M. ASP VI contributed to the expression of osteogenic gene and inhibited osteoclastic genes RANKL of BMSCs. Simultaneously, ASP VI significantly reduced the differentiation of mononuclear osteoclasts in the process of osteoclast formation induced by M-CSF and RANKL. Furthermore, ASBS, with synergistic effect of ASP VI and BS, significantly improved osteogenesis by promoting the BMSCs proliferation and differentiation. Meanwhile, by stimulating the SMADs, TGF-β1, VEGFA, and OPG/RANKL signaling pathways, ASBS (ASP VI and BS) substantially enhanced osteogenesis, greatly promoted angiogenesis, and suppressed osteoclastogenesis. Based on its high osteogenic activity and excellent inhibition osteoclastic formation, ASBS might be therapeutic candidate for repairing osteoclast-related bone defects.
CRediT authorship contribution statement
Fangping Chen: Conceptualization, Resources, Data curation, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Qing Liang: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft. Lijie Mao: Writing – review & editing. Yanrong Yin: Writing – review & editing. Lixin Zhang: Writing – review & editing. Cuidi Li: Formal analysis, Investigation, Writing – review & editing. Changsheng Liu: Conceptualization, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This investigation was funded by National Key Research and Development Program of China (No.2016YFC1102900), National Natural Science Foundation of China (No. 51772100 and No. 32171342), Shanghai Science and Technology Agriculture Project (No. 202002080002F01474), Shanghai Pujiang Program (16PJD015) and Joint Fund for equipment pre-research of the ministry of education (6141A02022618).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.09.001.
Contributor Information
Fangping Chen, Email: fpchen@ecust.edu.cn.
Cuidi Li, Email: cuidili@sjtu.edu.cn.
Changsheng Liu, Email: csliu@ecust.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Riggs B.L., Melton L., Iii The worldwide problem of osteoporosis: insights afforded by epidemiology. J. Bone. 1995;17:S505–S511. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 2.Manolagas S.C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. J. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 3.Miller P.D. Anti-resorptives in the management of osteoporosis. J. Best Pract Res Cl En. 2008;22:849–868. doi: 10.1016/j.beem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Agirrezabal I., Cabasés J.M., Di Tanna G.L., Sánchez-Iriso E. Inequalities in prescription rates of anti-osteoporosis drugs in primary care in England: a practice-level prescribing data analysis in 2013-2018. J. Bone. 2020;130:115125–115131. doi: 10.1016/j.bone.2019.115125. [DOI] [PubMed] [Google Scholar]
- 5.Tan W., Xi W. Commonly used anti-osteoporosis drugs in clinic. J. Shanghai Medical & Pharmaceutical Journal. 2013;34:6–9. [Google Scholar]
- 6.Cui Y., Feng Z.P. Research progress of anti-osteoporosis drugs. J. Chin J Osteoporos. 2015;21:367–371. [Google Scholar]
- 7.Spangenberg A., Maghsoodi N., Dulnoan D., Moore A.E., Edwards S., Frost M.L., Hampson G. Bone mineral density and body composition are associated with circulating angiogenic factors in post-menopausal women. J. Calcif Tissue Int. 2016;99:608–615. doi: 10.1007/s00223-016-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson J.C. Justification for the use of HRT in the long-term prevention of osteoporosis. J. Maturitas. 2005;51:113–126. doi: 10.1016/j.maturitas.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Wang H.T. TCM medicine laws and advantages for osteoporosis. J. Clinical Journal of Chinese Medicine. 2015;7:111–113. [Google Scholar]
- 10.Tang Z.Y., Li H., Xu Z.J. A study of medication rule of Traditional Chinese Medicine for treatment of osteoporosis. J. J Trad Chin Orthop Trauma. 2019;31:20–22. [Google Scholar]
- 11.Huang H., Pan J., Liu J., Hong K., Xie H., Luo B. Study on rule of drug use in TCM treatment of osteoporosis. J. Acta Chinese Medicine. 2017;32:124–126. [Google Scholar]
- 12.Zheng S.W., Li Q.C., Xu H., Jiang Z.C. Research progress of the treatment for osteoporosis by TCM. J. Asia-Pacific Traditional Medcine. 2016;12:88–90. [Google Scholar]
- 13.Feng X., Ge J.R. Research progress of TCM in the treatment of primary osteoporosis from the spleen. J. Chin J Osteoporos. 2014;20:968–972. [Google Scholar]
- 14.Hung T.M., Jin W.Y., Thuong P.T., Song K.S., Seong Y.H., Bae K. Cytotoxic saponins from the root ofDipsacus asper wall. J. Arch Pharm Res. 2005;28:1053–1061. doi: 10.1007/BF02977401. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Cui Z., Wang B., Bian Y., Zheng F. Effect of Asperosaponin Ⅵ on adipocyte differentiation in ST-2 cells and its underlying mechanisms. J. Tianjin Medical Journal. 2015;43:1345–1348. [Google Scholar]
- 16.Mi-Shan W.U., Zhao S.Z., Ren L.Z., Wang R., Bai X., Han H.W., Bin L.I. Experimental study of akebia saponin D on the differentiation of rat bone marrow derived mesenchymal stem cells to osteoblasts in vitro via induction. J. Chinese Pharmacological Bulletin. 2012;28:222–226. [Google Scholar]
- 17.Zhang Y.H., Liu C.C., Zhu A.Z., Chen X.Y., Liu G.X., He D.M., Tan G.X. Akebia saponin D promotes differentiation of bone marrow mesenchymal stem cells into osteoblasts through MAPK signaling pathways. J. Chinese Journal of Pathophysiology. 2012;28:1455–1460. [Google Scholar]
- 18.Ke K., Li Q., Yang X., Xie Z., Wang Y., Shi J., Chi L., Xu W., Hu L., Shi H. Asperosaponin VI promotes bone marrow stromal cell osteogenic differentiation through the PI3K/AKT signaling pathway in an osteoporosis model. J. Sci Rep. 2016;6:35233–35239. doi: 10.1038/srep35233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu Y., Li Y., Kong X., Zhang R., Sun Y., Li Q., Li C., Liu L., Wang J., Mei Q. The beneficial effect of Radix Dipsaci total saponins on bone metabolism in vitro and in vivo and the possible mechanisms of action. J. Osteoporos Int. 2012;23:2649–2660. doi: 10.1007/s00198-012-1932-y. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z.G., Zhang R., Li C., Ma X., Liu L., Wang J.P., Mei Q.B. The osteoprotective effect of Radix Dipsaci extract in ovariectomized rats. J. J Ethnopharmacol. 2009;123:74–81. doi: 10.1016/j.jep.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Krishnakumar G.S., Roffi A., Reale D., Kon E., Filardo G. Bone Morphogenic Protein augmentation for long bone healing" response to "Clinical need for bone morphogenetic protein. J. Int Orthop. 2017;41:2417–2419. doi: 10.1007/s00264-017-3595-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H., Qian J., Wang J., Yao W., Liu C., Chen J., Cao X. Enhanced bioactivity of bone morphogenetic protein-2 with low dose of 2-N, 6-O-sulfated chitosan in vitro and in vivo. J. Biomaterials. 2009;30:1715–1724. doi: 10.1016/j.biomaterials.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Cao L., Yu Y., Wang J., Werkmeister J.A., McLean K.M., Liu C. 2-N, 6-O-sulfated chitosan-assisted BMP-2 immobilization of PCL scaffolds for enhanced osteoinduction. J. Mater. Sci. Eng. C. 2017;74:298–306. doi: 10.1016/j.msec.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Cao L., Wang J., Hou J., Xing W., Liu C. Vascularization and bone regeneration in a critical sized defect using 2-N, 6-O-sulfated chitosan nanoparticles incorporating BMP-2. J. Biomaterials. 2014;35:684–698. doi: 10.1016/j.biomaterials.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y., Chen R., Yuan Y., Wang J., Liu C. Affinity-selected polysaccharide for rhBMP-2-induced osteogenesis via BMP receptor activation. J. Appl Mater Today. 2020;20:100681–100689. [Google Scholar]
- 26.Li J., Zhao Z., Liu J., Huang N., Long D., Wang J., Li X., Liu Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF‐β1/Smads pathway. J. Cell Prolif. 2010;43:333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Wang J., Zou Y., Zhang Y., Long D., Lei L., Tan L., Ye R., Wang X., Zhao Z. The influence of delayed compressive stress on TGF-β1-induced chondrogenic differentiation of rat BMSCs through Smad-dependent and Smad-independent pathways. J. Biomaterials. 2012;33:8395–8405. doi: 10.1016/j.biomaterials.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y.Y., Wang J., Liu C.S. Research of quaternized chitosan based hybrid scaffold as a delivery system for rhBMP-2,J. Journal of Functional Materials. J. J Funct Mater. 2014;14:14001–14007. [Google Scholar]
- 29.Li C.D., Chen F.P., Wang J.W., Dai K.R., Liu C.S. Biological property of rhBMP-2 loaded mesoporous calcium silicate/calcium phosphate cement scaffolds fabricated with 3D bioplotting technology. J. International Journal of Orthopaedics,J. Int J Orthop. 2015;36:187–193. [Google Scholar]
- 30.Peng G., Wang J., Liu C. Preparation of dextran/chitosan hydrogel and study as the carriers of growth factor. J. Materials China. 2013;32:599–604. [Google Scholar]
- 31.Tang W., Yu Y., Wang J., Liu H., Pan H., Wang G., Liu C. Enhancement and orchestration of osteogenesis and angiogenesis by a dual-modular design of growth factors delivery scaffolds and 26SCS decoration. J. Biomaterials. 2020;232:119645. doi: 10.1016/j.biomaterials.2019.119645. [DOI] [PubMed] [Google Scholar]
- 32.Kong X., Wang J., Cao L., Yu Y., Liu C. Enhanced osteogenesis of bone morphology protein-2 in 2-N,6-O-sulfated chitosan immobilized PLGA scaffolds. J. Colloids Surf B. 2014;122:359–367. doi: 10.1016/j.colsurfb.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y., Chen R., Sun Y., Pan Y., Tang W., Zhang S., Cao L., Yuan Y., Wang J., Liu C. Manipulation of VEGF-induced angiogenesis by 2-N, 6-O-sulfated chitosan. J. Acta Biomater. 2018;71:510–521. doi: 10.1016/j.actbio.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y.E. Mechanistic insight into contextual TGF-β signaling. J. Curr Opin Cell Biol. 2018;51:1–7. doi: 10.1016/j.ceb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu R., Angel P., Karin M. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-b and bone morphogenetic protein. J. Oncogene. 2002;21:7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., Crombrugghe B.d. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. J. Cell. 2002;108 doi: 10.1016/s0092-8674(01)00622-5. 0-29. [DOI] [PubMed] [Google Scholar]
- 37.Byers B.A., García A.J. Exogenous Runx2 expression enhances in vitro osteoblastic differentiation and mineralization in primary bone marrow stromal cells. J. Tissue Eng. Part A. 2004;10:1623–1632. doi: 10.1089/ten.2004.10.1623. [DOI] [PubMed] [Google Scholar]
- 38.Jang W.G., Kim E.J., Bae I.H., Lee K.N., Kim Y.D., Kim D.K., Kim S.H., Lee C.H., Franceschi R.T., Choi H.S. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. J.Bone. 2011;48:885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. J. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.