Abstract
Background
HIV-positive people who inject drugs (PWID) experience stigma related to their substance use and HIV, with adverse consequences to their health care utilization and mental health. To help affected individuals cope with their intersectional stigma and reduce its negative impact on health and health care, we adapted a behavioral stigma coping intervention for this HIV key population.
Objective
To conduct a randomized controlled trial (RCT) testing the ‘Stigma Coping to Reduce HIV risks and Improve substance use Prevention and Treatment’ (SCRIPT) intervention, a community-based, adapted form of Acceptance and Commitment Therapy (ACT), for PWID living with HIV in St. Petersburg, Russia.
Methods
We recruited 100 PWID living with HIV from civil society organizations (CSO) delivering harm reduction and HIV prevention services in St. Petersburg, Russia. We randomized participants 2:1 to receive either the intervention (three adapted ACT sessions in a group format over one month and usual CSO care) or usual CSO care alone. ACT aims to help affected individuals cope with stigma by increasing their psychological flexibility to handle stigma-related negative expectations, emotions and experiences. The primary outcomes were satisfaction with the intervention, and changes in HIV and substance use stigma scores.
Conclusions
Stigma coping interventions targeting HIV-positive PWID outside of formal health care settings may help them confront negativities in their lives originating from intersectional stigma and reduce stigma's impact as a health care barrier.
Keywords: Russia, HIV, PWID, Stigma, Behavioral, PLWH
Highlights
-
•
HIV-positive people who inject drugs face stigma due to both substance use and HIV.
-
•
Interventions are scarce to help this population reduce stigma as care barrier.
-
•
We recruited participants outside of the formal health care setting in Russia.
-
•
We partnered with multiple organizations to recruit and retain stigmatized people.
-
•
We evaluated the intervention's effects on stigma, health care, & health outcomes.
Nomenclature
- 1 SCRIPT
Stigma Coping to Reduce HIV risks and Improve substance use Prevention and Treatment
- 2 ACT
Acceptance and Commitment Therapy
- 3 CSO
Civil Society Organization
- 4 ARC
Addiction Rehabilitation Center
- 5 UIPHP
Ukrainian Institute on Public Health Policy
1. Introduction
The HIV epidemic continues to grow in the Russian Federation (Russia), with an increase in incident infections by 10–15% each year [[1], [2], [3]]. Almost half of all new HIV diagnoses in Russia are among people who inject drugs (PWID) [4]. PWID with HIV experience multiple layers of stigma related with their substance use, HIV status, and other identities, such as gender and involvement in criminal activities. The stigma that PWID with HIV experience can be understood as a label, marking and identifying these individuals to be devalued based on socially defined characteristics [5]. Sociologists have historically defined stigma as the social exclusion and dehumanization of marginalized individuals resulting in social status loss [6,7]. As a consequence of the process of stigmatization, social interactions are characterized by devaluation, exclusion, blame, and rejection, derived from actual or anticipated adverse social judgements of an individual or group with a specific health problem [8,9]. Stigma can be mainly classified into 1) external stigma: attitudes from other people and groups such as providers, family, communities, or law enforcement, 2) structural stigma: translation of external stigma and discriminatory attitudes into norms, laws, and policies, and 3) stigma manifestations among affected people: endorsement of the perceived public attitudes by individuals with the identity that is stigmatized, and anticipated or actual experiences of shame and devaluation [10,11]. External stigma, such as beliefs about someone living with HIV, can lead to discrimination towards the marginalized individual, which can then prompt the affected person to endorse others’ attitudes and consequently experience negative feelings about themselves (internalized stigma) [12].
Internalized, stigma-related thoughts and feelings, through fear and shame, can adversely impact all components of the HIV care cascade, including testing, diagnosis, treatment, and adherence [[13], [14], [15]]. Individuals in HIV key populations, such as PWID, almost always experience intersectional stigma, or multiple layers of stigma, by virtue of their substance use and other marginalized identities [8,[16], [17], [18]]. Understanding the interaction between HIV and substance use stigma is vital to understanding the experience of PWID living with HIV, and to recognizing the impact on physical and mental health outcomes [19,20]. Fueled by criminalization of substance use and the legal ban on opioid agonist treatment (i.e., methadone and buprenorphine), structural stigma is pervasive in Russia and has led PWID with HIV to experience increased discrimination, delaying progress towards the UNAIDS 95-95-95 targets [[21], [22], [23], [24], [25]].
While external stigma persists in most settings globally, stigma interventions should also focus on internalized stigma, helping PWID who are living with HIV to cope with intersectional substance use and HIV stigma experiences [26]. Previous quantitative analyses of an existing cohort of PWID living with HIV in addiction care in St. Petersburg, Russia, showed intersectional stigma's adverse association with health care access and utilization [27]. People with less access to a formal health care setting might experience even worse stigma [27]. Due to social resistance and policy within Russia, external stigma interventions on a public scale are not currently implemented. Consequently, within or outside of the formal health care setting in Russia, there are no internalized stigma interventions to support PWID with HIV cope with intersectional internalized stigma.
Thus, we sought to evaluate a stigma intervention targeting internalized substance use and HIV stigma among PWID who are living with HIV, who are not currently on ART [28].
To target internalized substance use and HIV stigma, we adapted an Acceptance and Commitment Therapy (ACT) intervention to the context in St. Petersburg, Russia [29]. We chose ACT because a literature review of interventions for internalized stigma toward HIV and substance use revealed its preliminary effectiveness for people with addictions [29,30]. ACT is a behavioral intervention that guides people to cope with stigma by detaching from judgmental and self-critical thinking; confronting shame, fear, and other emotions; and focusing on taking effective action connected to their values, for example through seeking HIV treatment or engaging in addiction recovery or harm reduction services [31]. The SCRIPT study was a randomized controlled trial (RCT) comparing usual civil society organization (CSO) care and ACT to usual CSO care alone. The trial examined 1) intervention feasibility and 2) the intervention's effect on substance use and HIV internalized stigma scores, furthering an understanding of possible coping techniques for PWID living with HIV who experience internalized stigma.
2. Materials and methods
Stigma Coping to Reduce HIV risks and Improve substance use Prevention and Treatment (SCRIPT) was an RCT enrolling 100 HIV-positive PWID to implement and evaluate the feasibility of the adapted ACT intervention. We also tested the effects of the ACT intervention plus usual CSO care compared to solely usual CSO care on the reduction of internalized HIV and substance use stigma scores, and on HIV care entry, substance use care engagement, and substance use frequency. Eligible participants were randomly assigned at a ratio of 2:1 to either the ACT intervention or the control group (civil society organization's standard of care as described). The intervention comprised of an adapted form of ACT in three group sessions.
2.1. Study setting
We conducted this study at two sites: Humanitarian Action, a CSO, and the outpatient Addiction Rehabilitation Center (ARC) of City Addiction Hospital, both located in St. Petersburg, Russia. Usual care provided by Humanitarian Action includes free harm reduction and HIV prevention services in mobile units (outreach busses) through medical, social, and psychological resources to individuals who use substances. From October 2019 to September 2020, the Humanitarian Action staff recruited participants at their service sites [32].
Following pre-screening at Humanitarian Action, we referred eligible participants to the outpatient ARC of City Addiction Hospital to conduct the intervention and all study procedures in this facility. This government-funded medical facility in St. Petersburg delivers outpatient addiction rehabilitation services to residents [33]. Participants did not receive any intervention or treatment services at the ARC beyond the study procedures and ACT sessions as described below.
2.2. Participants and recruitment
Table 1 lists study eligibility criteria [Table 1]. Screening occurred in two steps: 1) a phone screening initiated on the bus by interaction with Humanitarian Action staff; and 2) an in-person screening for those participants who were eligible after the phone screening at the ARC. Research Assessors (RAs) who were ARC staff (psychologists and social workers), conducted phone screening and in-person screening. When a client entered a Humanitarian Action bus for standard services, the outreach worker provided information about the SCRIPT study through the study pamphlet and flyer. If the client expressed interest in participating and being screened, the outreach worker immediately connected the client with the ARC study team through the study phone number, which was answered by an RA at the ARC. During the phone call, the RA provided a detailed description of the study and conducted the phone screening to evaluate whether the client met study inclusion criteria. After confirming study eligibility, the ARC RA scheduled the client for an in-person screening at the rehabilitation center and provided navigation directions. After the phone call, the Humanitarian Action staff additionally provided written directions to the ARC from the bus location. The client was scheduled for the visit at the ARC, where the RA met with the client in a private location to explain the study in more detail and conducted in-person screening to confirm the client's eligibility for the study. Once the participant was confirmed as eligible, the RA administered informed consent procedures and enrolled the client into the study. The participant then provided contact information for themselves and at least two alternative contacts. The baseline assessment took place the same day.
Table 1.
SCRIPT eligibility criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age 18 years or older | Not fluent in Russian |
| HIV-positive (self-report) | Cognitive impairment precluding informed consent |
| Current injection drug use (past 30 days) | Participants in the main study did not participate in the practice portion of the study. |
| Not currently on ART | Acute severe psychiatric illness (i.e., answered yes to any of the following: past three month active hallucinations; mental health symptoms prompting a visit to the emergency room or hospital; mental health medication changes due to worsening symptoms; presence of suicidal plans) and research clinical observation (i.e. clinical observation or prior knowledge of sever personality disorder; past three months active mania; past three months active psychosis) |
| Provision of two contacts to assist with follow-up | |
| Address within St. Petersburg or districts within 100 km of St. Petersburg | |
| Not enrolled in any other research studies | |
| Possession of a home or mobile phone | |
| Ability and willingness to comply with all study protocols and procedures over 6 months | |
| Available at the specific days of the week and times that the group sessions will be occurring for the subsequent 3–4 weeks (to ensure that participants randomized into the intervention arm will be able to receive the intervention) |
Inclusion and exclusion criteria for the Stigma Coping to Reduce HIV risks and Improve substance use Prevention and Treatment (SCRIPT) study.
2.3. Randomization
The randomization sequence, with an allocation ratio of 2:1, was created using random block size in R, version 3.5.2 and uploaded to the REDCap platform, an electronic data collection tool, and used to reduce selection bias and make the sequence less predictable [34,35]. The unequal randomization ratio allowed to gather more data on the ACT intervention, given that this innovative intervention was developed for and implemented in this population for the first time.
2.4. Intervention
The SCRIPT study utilized two interventions: ACT and usual CSO care (intervention arm) compared to usual CSO care alone (control arm).
2.4.1. Intervention group
The ACT intervention developed for the SCRIPT study was a culturally adapted version of Acceptance and Commitment Therapy (ACT), designed to help people cope with stigma and related thoughts and emotions; as well as take action to counter stigma's adverse consequences on health behaviors [31].
2.4.1.1. Acceptance and Commitment Therapy content
Sessions occurred in groups of approximately five participants and followed a manual based on prior work by Luoma et al. (2008, 2012), that was culturally adapted to the Russian context by the study team, with the assistance of a Russian ACT trainer [29,30]. The goal of the group sessions was to help participants overcome negative thoughts and emotions related to stigma-related shame and negative judgements of self and others. We modified standard ACT exercises to focus on how participants respond to shame and stigma so as to encourage substance use disorder recovery, harm reduction, and health care utilization. Interventionists trained participants to utilize techniques focused on mindfully observing and accepting indications of internalized, anticipated, or experienced stigma manifestations (e.g., shame or fear), in order to diminish their conditioned association with potentially ineffective or harmful behavior (i.e. health care avoidance, or drug use and sex risk behaviors). The intervention helped participants identify and commit to meaningful values and life goals that could guide more effective and healthier behavior.
We audio recorded all sessions for supervision purposes and to monitor intervention fidelity.
2.4.1.2. Intervention logistics
We scheduled the three ACT intervention group sessions with five planned participants in each group, for 2 h each, in weekly succession. The sessions occurred at the ARC and were led by two interventionists (clinical psychologists) trained in ACT by peer-reviewed ACT trainers. If a participant missed the first session, the RA scheduled the participant for the subsequent group. If a participant missed the second session, the RA invited the participant for an individual make-up session, prior to the scheduled third session. If a participant missed the third session, this was a missed session, but the RA invited the participant to complete subsequent 1-month and 6-month assessments.
2.4.2. Control group
Both intervention and control groups received the usual care from the CSO, which included counseling and referral to addiction treatment clinics (for detoxification and rehabilitation care, as opioid agonist treatment is not available in Russia) [36] and to HIV treatment clinics (for ART and HIV care) in St. Petersburg. Both groups also were given printed flyers with drug harm reduction, HIV care, and safer sex information.
2.5. Compensation
RAs compensated participants for their travel and time in Russian rubles equivalent to approximately 30 USD at the time of the study (2019–2020) for each of the three intervention sessions and all in-person assessment visits. For assessments that took place over the phone instead of in-person, participants received partial compensation in equivalent of approximately 8 USD. This procedure was modified during the pandemic, with participants receiving full compensation for their follow-up visits even if they were completed over the phone. In order to dis-incentivize coming late to group, participants who arrived 1 h or later to a session received partial compensation in equivalent of approximately 15 USD. If a participant arrived to a session with less than 30 min remaining, they received no compensation for that session.
2.6. Outcomes
We evaluated implementation of the intervention with the following outcomes: 1) proportion of participants satisfied with the intervention at 1 month from an overall satisfaction score (primary implementation outcome, assessed among intervention arm participants); 2) rate of participation in three intervention sessions; and 3) fidelity to the intervention. We evaluated the effectiveness of the intervention on the following outcomes: 1) change in substance use and HIV internalized stigma at 1 month (primary effectiveness outcomes); 2) initiation of HIV care (ART) at 6 months; 3) engagement in substance use care at 6 months; 4) change in total number of injections in the past 30 days at 6 months.
The proportion of participants satisfied post-intervention at 1-month was defined as the proportion of participants with a mean score of 3 or greater on a Likert scale (1 = low, 5 = high) of three items that signified overall satisfaction ("1. How much did you enjoy attending the ACT trainings?", "2. Did the ACT trainings meet or exceed your expectations?", and "3. Do you think the ACT trainings would be useful in helping others with HIV and substance use?"). Data for other domains of satisfaction were collected for exploratory analyses.
This study was registered with ClinicalTrials.gov (NCT03695393).
2.7. Assessments
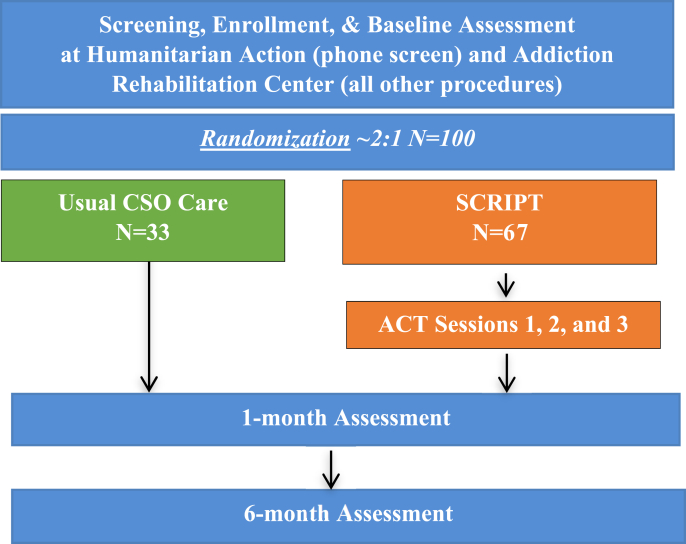
RAs assessed all participants at baseline, one, and six months post-enrollment [Fig. 1]. All follow-up study visits took place at the ARC. Table 2 lists baseline and follow-up study assessments [Table 2]. Trained RAs administered all sections of the assessments using REDCap, an electronic database.
Fig. 1.
Study design and procedures for the Stigma Coping to Reduce HIV risks and Improve substance use Prevention and Treatment (SCRIPT) study.
Table 2.
Assessment table of participants in SCRIPT study (n = 100).
| Administered Assessment | Baseline | 1-Month | 6-Month |
|---|---|---|---|
| Demographics [[40], [41], [42]] | X | ||
| ART Use and Adherence [43] | X | X | X |
| HIV Sex Risk Behaviors [44,45] | X | X | X |
| HIV Risk Categories [46,47] | X | ||
| HIV Disclosure [48,49] | X | X | |
| HIV Stigma [50,51] | X | X | X |
| Substance Use Stigma (SASSS) [52] | X | X | X |
| Stigma-Related Rejection Scale (SRS) [53] | X | ||
| Acceptance and Action (AAQ-II – SA) [54] | X | X | X |
| Patient Health Questionnaire (PHQ-9) [55] | X | X | X |
| Anxiety (GAD-7) [56] | X | X | X |
| Partner Violence and Sexual Assault [57] | X | ||
| Alcohol Use: AUDIT-C [58] | X | ||
| Perceived Alcohol Stigma [59] | X | ||
| Drug Use [60,61] | X | X | X |
| Overdose | X | X | |
| Social Support Scale [62] | X | X | |
| VR-12 Health Survey [63,64] | X | X | |
| Health Care Utilization [65] | X | X | |
| Abbreviated PTSD Checklist [66] | X | ||
| Involvement with Police | X | X | |
| ±Satisfaction with the Intervention | X | ||
| *HIV Testing History [67] | X |
±Intervention group participants only.
*If this information is not collected at baseline, RAs will collect this information at a later study visit.
Administered assessment measures at the baseline, 1-month, and 6-month study visits for participants in the Stigma Coping to Reduce HIV risks and Improve substance use Prevention and Treatment (SCRIPT) study.
2.8. Serious adverse events/safety monitoring
RAs and interventionists determined a participant's ability to continue to participate in the study from clinical observation. They appropriately documented and reported any event meeting the criteria for an adverse event, serious adverse event, or unanticipated problem. As this was a behavioral intervention, this was a minimal-risk study, however in the event that a study staff member believed a participant was at risk of hurting themselves and/or others, appropriate action was taken with a defined suicide and homicide safety protocol.
2.9. Data management
Specialists from the Ukrainian Institute on Public Health Policy (UIPHP) designed, developed, and maintained the electronic data collection and database systems which compiled participant data, intervention adherence information, scheduling, and participant tracking. UIPHP implemented procedures for external data quality control, including pre-programmed skip patterns, real-time range checks, and internal logic to minimize missing data. For data collection questionnaires, where data were directly entered into the study record, the RA, local study coordinator, and the overall study coordinator reviewed records on a regular schedule to ensure data quality.
2.10. Analytic methods
The main purpose of this study was to assess implementation and effects of the intervention on stigma scores and health outcomes to inform future, larger effectiveness trials. In this pilot project, the sample size was justified by an 80% power to detect an absolute difference of 40% (i.e., 60% vs. 20% in the intervention and control arms, respectively) in the proportions of care initiation, with an estimated 90 evaluable subjects, assuming 10% loss to follow-up of the initial sample of n = 100. We expected 14 participants to be enrolled each month. Given the lengths of follow-up of 6 months, this allowed for enrollment of 100 participants.
This study will use an intent-to-treat analysis that includes all participants according to their randomized assignment. To assess whether there appears to be any differences across treatment arms, all variables at baseline and follow-up visit will undergo descriptive analyses. We will use Chi-square test to compare differences in proportions and T-test or Mood's median to explore differences in means and medians, respectively.
Primary outcomes will be proportion of participants satisfied with the intervention from an overall satisfaction score and change in total substance use and in HIV stigma scores between baseline and 1 month after randomization. Initiation of ART at 6 months, engagement in substance use care at 6 months, change in the number of injections in the past 30 days at 6 months, as well as participation in all three intervention sessions and fidelity to the intervention will be assessed as secondary outcomes.
Because the ACT intervention is delivered in the form of group sessions, individuals within the same group will be correlated. We will use linear probability model for binary outcomes and linear regression for continuous outcomes with robust standard errors to account for within cluster/correlation due to the group sessions in the ACT intervention. For continuous outcomes, we will use quartile regression models with robust standard errors in case of major departures from normality. Models for primary outcomes (changes in stigma scores) will be adjusted for baseline stigma score, injecting frequency, history of ART, and depressive symptoms to account for residual confounding. This set of covariates was defined by the study team based on the literature and considering the sample size. Models for secondary outcomes will be unadjusted except for continuous outcomes calculated as change; they will be adjusted for the baseline scores. We will calculate effect sizes using Cohen’s d for numerical outcomes and Cohen’s h for categorical outcomes to understand the clinical significance of observed differences. We will conduct analyses in R, version 4.0.5 (Copyright (C) 2021 The R Foundation for Statistical Computing).
2.11. Protection of study participants and their data
The Institutional Review Boards of Boston University Medical Campus and First St. Petersburg Pavlov State Medical University approved the SCRIPT study. All study participants completed written informed consent.
All data collection occurred on a secure, web-based system, located on a secure server within the UIPHP domain [37]. The system used various methods to protect against malicious users who may attempt to identify and exploit any security vulnerabilities in the system, such as secure logins, user privilege settings, automatic log outs after inactivity, and audit trail logs of all user activity and pages viewed by every user. Participant contact information was kept in separate programs from research data.
3. Discussion
The SCRIPT study assessed an intervention designed to help PWID living with HIV in Russia to cope with internalized substance use and HIV stigma and provides effect sizes on stigma scores, health care utilization, and other outcomes. This community-based study aimed to engage those who are already connected to harm reduction CSOs, to the formal HIV and addiction health care systems. The SCRIPT study provided effect size data to appropriately power subsequent effectiveness and implementation trials for this stigma intervention.
Beyond assessing health care utilization and ART initiation, future studies should also measure retention in HIV care, adherence to ART, and viral load suppression. Other intervention design enhancements could include bundling the ACT intervention with other support, including mental health counseling, social support networks, or case management. Studying intervention delivery by peers or patient navigators could improve the reach of this approach, and potentially allow for scale-up in resource-constrained settings. Future studies should investigate intervention adoption and variations of exposure (number and time length of sessions) and timing (temporal spacing of sessions). Other variants could include individual versus group delivery of the intervention, as well as utilizing telemedicine and embodied conversational agents, a computer-generated interface that utilizes human verbal and non-verbal characteristics [38]. We implemented this study with CSOs, as they are in close proximity to people who are out of care, who are the most vulnerable and most stigmatized. Most importantly, other researchers should investigate how to utilize the ACT intervention in different settings.
A particular strength of this study is that it examines a stigma coping intervention for PWID living with HIV outside of the formal health care system in Russia. Engaging PWID living with HIV in medical care is a high priority in Russia, as only 10% of this population in St. Petersburg are accessing HIV treatment [39]. This trial was conducted in Russia, where public stigma towards PWID and people living with HIV remains high, and for that reason, the results from this study may be applied to other settings with pervasive intersectional public stigma. As both public and internalized stigma remain a high barrier to health care utilization, this research is integral towards reaching Russia's and global HIV targets and reaching the goal of zero discrimination.
Clinical trial registration details
This study was registered with ClinicalTrials.gov through the National Institutes of Health - Stigma, Risk Behaviors and Health Care Among HIV-infected Russian People Who Inject Drugs (SCRIPT): NCT03695393.
Ethics approval and consent to participate
The SCRIPT study was approved by the Institutional Review Boards of Boston University Medical Campus and Pavlov University.
Funding
This work was supported by the National Institute on Drug Abuse (NIDA) under Grant R00DA041245 and Grant K99DA041245; and by the Providence/Boston Center for AIDS Research under Grant P30AI042853. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. The authors report no conflict of interest.
Author contributions
Sarah L. Rossi: Writing – original draft; Visualization; Project administration. Yuliia Sereda: Data curation; Formal analysis; Methodology; Software; Writing – review & editing. Jason B. Luoma: Conceptualization; Methodology; Resources; Supervision; Writing – review & editing. Nikolai Pavlov: Conceptualization; Resources; Supervision. Olga Toussova: Investigation; Writing – review & editing. Janna Vasileva: Investigation. Kristina Abramova: Investigation. Sally Bendiks: Project administration; Writing – review & editing. Tetiana Kiriazova: Writing – review & editing. Marina Vetrova: Writing – review & editing. Elena Blokhina – Conceptualization; Project administration; Supervision; Writing – review & editing. Evgeny Krupitsky – Conceptualization. Dmitry Lioznov – Conceptualization; Resources; Supervision. Sara Lodi – Conceptualization; Formal analysis; Methodology. Karsten Lunze – Conceptualization; Funding acquisition; Methodology; Supervision; Writing – original draft.
Acknowledgements
We thank Aga Bereznicka for her assistance in preparing this manuscript.
Contributor Information
Sarah L. Rossi, Email: sarah.rossi@bmc.org.
Yuliia Sereda, Email: yulia.v.sereda@gmail.com.
Jason B. Luoma, Email: jbluoma@portlandpsychotherapy.com.
Nikolai Pavlov, Email: nickpavlov@icloud.com.
Olga Toussova, Email: otoussova@gmail.com.
Janna Vasileva, Email: jvas333@gmail.com.
Kristina Abramova, Email: kristinaabramova17@mail.ru.
Sally Bendiks, Email: sally.bendiks@bmc.org.
Tetiana Kiriazova, Email: kiriazova@uiphp.org.ua.
Marina Vetrova, Email: mvetrova111@gmail.com.
Elena Blokhina, Email: blokhinaelena@gmail.com.
Evgeny Krupitsky, Email: kruenator@gmail.com.
Dmitry Lioznov, Email: dlioznov@yandex.ru.
Sara Lodi, Email: slodi@bu.edu.
Karsten Lunze, Email: lunze@bu.edu.
Data availability
No data was used for the research described in the article.
References
- 1.UNAIDS “Data Book,”. UNAIDS; 2018. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf [Google Scholar]
- 2.Approving the State Strategy to Combat the Spread of HIV in Russia through 2020 and beyond. Ministry of Healthcare of the Russian Federation; 2016. http://government.ru/en/docs/24983/ [Google Scholar]
- 3.HIV and AIDS in Russia. Avert; 2019. https://www.avert.org/professionals/hiv-around-world/eastern-europe-central-asia/russia [Google Scholar]
- 4.Beyrer C., Wirtz A.L., O'Hara G., Léon N., Kazatchkine M. The expanding epidemic of HIV-1 in the Russian Federation. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link B.G., Phelan J.C. In: Handb. Sociol. Ment. Health. Aneshensel C.S., Phelan J.C., Bierman A., editors. Springer Netherlands; Dordrecht: 2013. Labeling and stigma; pp. 525–541. [DOI] [Google Scholar]
- 6.Link B.G., Phelan J.C. Conceptualizing stigma. Annu. Rev. Sociol. 2001;27:363–385. doi: 10.1146/annurev.soc.27.1.363. [DOI] [Google Scholar]
- 7.Goffman E. Simon & Schuster; New York: 1963. Notes on the Management of a Spoiled Identity. [Google Scholar]
- 8.Turan J.M., Elafros M.A., Logie C.H., Banik S., Turan B., Crockett K.B., Pescosolido B., Murray S.M. Challenges and opportunities in examining and addressing intersectional stigma and health. BMC Med. 2019;17 doi: 10.1186/s12916-018-1246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss M.G., Ramakrishna J. Stigma interventions and research for international health. Lancet Lond. Engl. 2006;367:536–538. doi: 10.1016/S0140-6736(06)68189-0. [DOI] [PubMed] [Google Scholar]
- 10.Gray A.J. Stigma in psychiatry. J. R. Soc. Med. 2002;95:72–76. doi: 10.1258/jrsm.95.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai A.C., Kiang M.V., Barnett M.L., Beletsky L., Keyes K.M., McGinty E.E., Smith L.R., Strathdee S.A., Wakeman S.E., Venkataramani A.S. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay E.S., Rice W.S., Crockett K.B., Atkins G.C., Batey D.S., Turan B. Experienced HIV-related stigma in health care and community settings: mediated associations with psychosocial and health outcomes. J. Acquir. Immune Defic. Syndr. 1999. 2018;77:257–263. doi: 10.1097/QAI.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whetten K., Reif S., Whetten R., Murphy-McMillan L.K. Trauma, mental health, distrust, and stigma among HIV-positive persons: implications for effective care. Psychosom. Med. 2008;70:531–538. doi: 10.1097/PSY.0b013e31817749dc. [DOI] [PubMed] [Google Scholar]
- 14.Facts about HIV stigma | HIV basics | HIV/AIDS | CDC. 2019. https://www.cdc.gov/hiv/basics/hiv-stigma/index.html
- 15.HIV Stigma and Discrimination. Avert; 2015. https://www.avert.org/professionals/hiv-social-issues/stigma-discrimination [Google Scholar]
- 16.Jackson-Best F., Edwards N. Stigma and intersectionality: a systematic review of systematic reviews across HIV/AIDS, mental illness, and physical disability. BMC Publ. Health. 2018;18:919. doi: 10.1186/s12889-018-5861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crenshaw K. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev. 1991;43:1241–1299. doi: 10.2307/1229039. [DOI] [Google Scholar]
- 18.Crenshaw K. Univ. Chic. Leg. Forum. 1989; 2015. Demarginalizing the Intersection of Race and Sex: a Black Deminist Critique of Antidiscrimination Doctrine, Feminist Theory and Antiracist Politics.https://chicagounbound.uchicago.edu/uclf/vol1989/iss1/8 [Google Scholar]
- 19.Earnshaw V.A., Smith L.R., Cunningham C.O., Copenhaver M.M. Intersectionality of internalized HIV stigma and internalized substance use stigma: implications for depressive symptoms. J. Health Psychol. 2015;20:1083–1089. doi: 10.1177/1359105313507964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logie C.H., James Ll, Tharao W., Loutfy M.R. HIV, gender, race, sexual orientation, and sex work: a wualitative study of intersectional stigma experienced by HIV-positive women in Ontario, Canada. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke S.E., Calabrese S.K., Dovidio J.F., Levina O.S., Uusküla A., Niccolai L.M., Abel-Ollo K., Heimer R. A tale of two cities: stigma and health outcomes among people with HIV who inject drugs in St. Petersburg, Russia and Kohtla-Järve. Estonia, Soc. Sci. Med. 1982. 2015;130:154–161. doi: 10.1016/j.socscimed.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunze K., Lunze F.I., Raj A., Samet J.H. Stigma and human rights abuses against people who inject drugs in Russia—a qualitative investigation to inform policy and public health strategies. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0136030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese S.K., Burke S.E., Dovidio J.F., Levina O.S., Uusküla A., Niccolai L.M., Heimer R. Internalized HIV and drug stigmas: interacting forces threatening health status and health service utilization among people with HIV who inject drugs in St. Petersburg, Russia. AIDS Behav. 2016;20:85–97. doi: 10.1007/s10461-015-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russian federation | UNAIDS. https://www.unaids.org/en/regionscountries/countries/russianfederation (n.d.)
- 25.Hatzenbuehler M.L. Structural stigma and health inequalities: research evidence and implications for psychological science. Am. Psychol. 2016;71:742–751. doi: 10.1037/amp0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunze K. Determinants of HIV and substance use stigma among people with a history of substance use who are living with HIV in Russia. 2018. http://programme.aids2018.org/Abstract/Abstract/6103
- 27.Vetrova M., Lunze K., Cheng D.M., Lloyd-Travaglini C., Blokhina E. Double stigma among HIV-positive people who inject drugs in Russia and health care outcomes. 2018. https://www.drugabuse.gov/international/abstracts/double-stigma-among-hiv-positive-people-who-inject-drugs-in-russia-health-care-outcomes
- 28.Biancarelli D.L., Biello K.B., Childs E., Drainoni M., Salhaney P., Edeza A., Mimiaga M.J., Saitz R., Bazzi A.R. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend. 2019;198:80–86. doi: 10.1016/j.drugalcdep.2019.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luoma J.B., Kohlenberg B.S., Hayes S.C., Bunting K., Rye A.K. Reducing self-stigma in substance abuse through acceptance and commitment therapy: model, manual development, and pilot outcomes. Addiction Res. Theor. 2008;16:149–165. doi: 10.1080/16066350701850295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luoma J.B., Kohlenberg B.S., Hayes S.C., Fletcher L. Slow and steady wins the race: a randomized clinical trial of Acceptance and Commitment Therapy targeting shame in substance use disorders. J. Consult. Clin. Psychol. 2012;80:43–53. doi: 10.1037/a0026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.S. Hayes, Acceptance & Commitment Therapy, Assoc. Context. Behav. Sci. (n.d.). https://contextualscience.org/act.
- 32.Saint petersburg fund for medical and social programs “humanitarian action,”. https://haf-spb.org/ (n.d.)
- 33.Addiction rehabilitation center No. 1. https://www.spbmed.info/clinics/614/ (n.d.)
- 34.R Core Team R. R Found. Stat. Comput.; 2019. A Language and Envrionment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 35.Efird J. Blocked randomization with randomly selected block sizes. Int. J. Environ. Res. Publ. Health. 2011;8:15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimer R. The policy-driven HIV epidemic among opioid users in the Russian Federation. Curr. HIV AIDS Rep. 2018;15:259–265. doi: 10.1007/s11904-018-0395-y. [DOI] [PubMed] [Google Scholar]
- 37.REDCap general security overview. https://www.iths.org/wp-content/uploads/About-REDCap-Vanderbilt.pdf n.d.
- 38.Provoost S., Lau H.M., Ruwaard J., Riper H. Embodied conversational agents in clinical psychology: a scoping review. J. Med. Internet Res. 2017;19 doi: 10.2196/jmir.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heimer R., Usacheva N., Barbour R., Niccolai L.M., Uusküla A., Levina O.S. Engagement in HIV care and its correlates among people who inject drugs in St Petersburg, Russian Federation and Kohtla-Järve, Estonia, Addict. Abingdon Engl. 2017;112:1421–1431. doi: 10.1111/add.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.T. McLellan, J. Cacciola, D. Carise, T.H. Coyne, H. Categories, Addiction Severity Index Lite - CF, (n.d.) vol. 12.
- 41.McLellan A.T., Luborsky L., Cacciola J., Griffith J., Evans F., Barr H.L., O'Brien C.P. New data from the Addiction Severity Index. Reliability and validity in three centers. J. Nerv. Ment. Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 42.United States Department of Health and Human Services . Version 2. National Health Interview Survey; 1997. 2000. (National Center for Health Statistics). [DOI] [Google Scholar]
- 43.Chesney M., Ickovics J.R., Chambers D.B., Gifford A., Neidig J., Zwickl B., Wu A. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 44.Wechsnerg W. 1998. Revised Risk Behavior Assessment, Part I and Part II. [Google Scholar]
- 45.Wechsberg W.M., Browne F.A., Carney T., Myers B., Minnis A., MacDonald R., Ndirangu J.W., Turner L.B., Howard B.N., Rodman N. The Young Women's Health CoOp in Cape Town, South Africa: study protocol for a cluster-randomised trial for adolescent women at risk for HIV. BMC Publ. Health. 2018;18 doi: 10.1186/s12889-018-5665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navaline H.A., Snider E.C., Petro C.J., Tobin D., Metzger D., Alterman A.I., Woody G.E. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res. Hum. Retrovir. 1994;10(Suppl 2):S281–S283. [PubMed] [Google Scholar]
- 47.Questions Adapted from American Red Cross to Ascertain HIV Transmission Route, ((n.d.)).
- 48.Stein M.D., Freedberg K.A., Sullivan L.M., Savetsky J., Levenson S.M., Hingson R., Samet J.H. Sexual ethics. Disclosure of HIV-positive status to partners. Arch. Intern. Med. 1998;158:253–257. doi: 10.1001/archinte.158.3.253. [DOI] [PubMed] [Google Scholar]
- 49.Raj A., Cheng D.M., Levison R., Meli S. J.H. Samet, Sex trade, sexual risk, and nondisclosure of HIV serostatus: findings from HIV-infected persons with a history of alcohol problems. AIDS Behav. 2006;10:149–157. doi: 10.1007/s10461-005-9050-x. [DOI] [PubMed] [Google Scholar]
- 50.Berger B.E., Ferrans C.E., Lashley F.R. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res. Nurs. Health. 2001;24:518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 51.Simbayi L.C., Strebel A., Cloete A., Henda N., Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc. Sci. Med. 2007;64:1823–1831. doi: 10.1016/j.socscimed.2007.01.006. 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luoma J.B., Nobles R.H., Drake C.E., Hayes S.C., O'Hair A., Fletcher L., Kohlenberg B.S. Self-stigma in substance abuse: development of a new measure. J. Psychopathol. Behav. Assess. 2013;35:223–234. doi: 10.1007/s10862-012-9323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luoma J.B., Twohig M.P., Waltz T., Hayes S.C., Roget N., Padilla M., Fisher G. An investigation of stigma in individuals receiving treatment for substance abuse, Addict. Beyond Behav. 2007;32:1331–1346. doi: 10.1016/j.addbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Luoma J., Drake C., Kohlenberg B., Hayes S. Substance abuse and psychological flexibility: the development of a new measure, Addict. Res. Theory. 2011;19:3–13. doi: 10.3109/16066359.2010.524956. [DOI] [Google Scholar]
- 55.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 57.Adapted from the TAJ assessment of the TAJ study. https://www.bumc.bu.edu/care/research-studies/past-research-studies/taj-study/ n.d.
- 58.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 59.Luoma J.B., O'Hair A.K., Kohlenberg B.S., Hayes S.C., Fletcher L. The development and psychometric properties of a new measure of perceived stigma toward substance users. Subst. Use Misuse. 2010;45:47–57. doi: 10.3109/10826080902864712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weatherby N.L., Needle R., Cesari H., Booth R., McCoy C.B., Watters J.K., Williams M., Chitwood D.D. Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street outreach. Eval. Progr. Plann. 1994;17:347–355. doi: 10.1016/0149-7189(94)90035-3. [DOI] [Google Scholar]
- 61.Needle R., Fisher D., Weatherby N. Reliability of self-reported HIV risk behaviors of drug users. Psychol. Addict. Behav. 1995;9:242–250. doi: 10.1037/0893-164X.9.4.242. [DOI] [Google Scholar]
- 62.Antelman G., Smith Fawzi M.C., Kaaya S., Mbwambo J., Msamanga G.I., Hunter D.J., Fawzi W.W. Predictors of HIV-1 serostatus disclosure: a prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania, AIDS Lond. Englera. 2001;15:1865–1874. doi: 10.1097/00002030-200109280-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kazis L.E., Miller D.R., Clark J.A., Skinner K.M., Lee A., Ren X.S., Spiro A., Rogers W.H., Ware J.E. Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. J. Ambul. Care Manag. 2004;27:263–280. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Wu A.W., Revicki D.A., Jacobson D., Malitz F.E. Evidence for reliability, validity and usefulness of the medical outcomes study HIV health survey (MOS-HIV) Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1997;6:481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 65.Miller W.R. Form 90: a structured assessment interview for drinking and related behaviors: test manual: (563242012-001) 1996. [DOI]
- 66.Lang A.J., Stein M.B. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behav. Res. Ther. 2005;43:585–594. doi: 10.1016/j.brat.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 67.HIV/HCV/STI testing status and organizational testing practices questionnaire. 2011. https://www.drugabuse.gov/researchers/research-resources/data-harmonization-projects/seek-test-treat-retain/addressing-hiv-among-vulnerable-populations
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.