Abstract
Evidence suggests that transient receptor potential (TRP) ion channels dysfunction significantly contributes to the physiopathology of metabolic and neurological disorders. Dysregulation in functions and expression in genes encoding the TRP channels cause several inherited diseases in humans (the so-called ‘TRP channelopathies’), which affect the cardiovascular, renal, skeletal, and nervous systems. This study aimed to evaluate the expression of ion channels in the forebrain of rats with diet-induced obesity (DIO). DIO rats were studied after 17 weeks under a hypercaloric diet (high-fat diet, HFD) and were compared to the control rats with a standard diet (CHOW). To determine the systemic effects of HFD exposure, we examined food intake, fat mass content, fasting glycemia, insulin levels, cholesterol, and triglycerides. qRT-PCR, Western blot, and immunochemistry analysis were performed in the frontal cortex (FC) and hippocampus (HIP). After 17 weeks of HFD, DIO rats increased their body weight significantly compared to the CHOW rats. In DIO rats, TRPC1 and TRPC6 were upregulated in the HIP, while they were downregulated in the FC. In the case of TRPM2 expression, instead was increased both in the HIP and in the FC. These could be related to the increase of proteins and nucleic acid oxidation. TRPV1 and TRPV2 gene expression showed no differences both in the FC and HIP. In general, qRT-PCR analysis were confirmed by western blot analysis. Immunohistochemical procedures highlighted the expression of the channels in the cell body of neurons and axons, particularly for the TRPC1 and TRPC6. The alterations of TRP channel expression could be related to the activation of glial cells or the neurodegenerative process presented in the brain of the DIO rat highlighted with post synaptic protein (PSD 95) alterations. The availability of suitable animal models may be useful for studying possible pharmacological treatments to counter obesity-induced brain injury. The identified changes in DIO rats may represent the first insight to characterize the neuronal alterations occurring in obesity. Further investigations are necessary to characterize the role of TRP channels in the regulation of synaptic plasticity and obesity-related cognitive decline.
Key words: TRP ion channel, obesity, brain, high-fat diet, synaptic proteins
Introduction
Obesity can harm the brain and compromise performance on intelligence tests.1 High-fat diet (HFD)-induced obesity revealed modifications in hippocampal structure, learning and memory deficits, and impaired executive function. HFD also leads to a reduction in markers of neurogenesis, synaptic plasticity, and neuronal growth.2 Early-stage obesity produces a decrease in dendritic spine density on pyramidal neurons, as well as a decrease in synaptic protein levels in the prefrontal cortex (PFC) and perirhinal cortex (PRC), but not the hippocampus (HIP).3
Ion channels are involved in obesity and neurodegenerative diseases and regulate various physiological processes, such as electrical conduction in the neuronal cells and the release of neurotransmitters. 4 Ion channels are known to govern three prominent roles in regulating membrane physiology that is setting up the membrane potential of cells, where the movement of ions across the membrane constitutes a potential gradient that determines resting potentials of the membrane and generates action potentials. These ion channels are involved with neurotransmitter release, hormonal secretion, muscular contraction, and altered gene expression by a modulation of the constitute electric signal flow, which triggers intracellular signaling cascades.5
Transient receptor potential channels (TRPs) act as intracellular ion channels, mainly as Ca2+ release channels, regulating the function of cellular organelles such as endosomes and lysosomes.6 They are highly expressed in the HIP and can modulate brain development, neuronal excitability, hippocampal persistent activity, synaptic plasticity, including the pre- and post-synaptic processes or neurogenesis. In addition, they are involved in the basic hippocampal function, such as different memory and learning processes. 7 TRP ions channels family is involved in sensory (pheromone signaling, taste transduction, nociception, and temperature sensation) and homeostatic functions (such as Ca2+ and Mg2+ reabsorption and osmoregulation), as well as muscle contraction and vasomotor control. Their dysfunction contributes to the etiology of several diseases.6,8
The mammalian TRP channel family core consists of subfamilies of classical TRP channels, which are cation receptor-related TRP channels (TRPC1-TRPC7), vanilloid receptor-related TRP channels (TRPV1-TRPV6), melastatin-related TRP channels (TRPM1-TRPM8), polycystin-related TRP channels (TRPP1- TRPP2) and mucolipin subfamily TRP cation channel (TRPML1- TRPML3).9
They regulate diverse neuronal and glial functions including developmental and homeostatic functions of the brain. Recent studies show that dysregulation of the TRP channel functions is involved in various pathological events of neurological and psychiatric disorders.10
TRPV1 was first found to be expressed in the pain-sensitive neurons of the dorsal root ganglion and trigeminal ganglion neurons. TRPV1 was also identified in the multiple non-neuronal cell types as well as in the terminals of spinal and peripheral nerves.11 TRPV1 activation promotes microglial migration and cell death by causing mitochondrial damage mediated by Ca2+ influx.12
TRPV2 is said to be expressed in cultured hippocampus neurons and colocalizes with TRPV1 in the rat cortex, suggesting that this receptor family has more functional variety.13 Moreover TRPV2 may have a role in regulating neuronal activity in response to changes in lipid metabolism.14 The TRPC subfamily contains seven members: TRPC1-TRPC7. All TRPC channels form Ca2+- permeable nonselective cation channels and are highly expressed in diverse regions of the brain. They generally work as receptoroperated cation channels and have been implicated in various cellular functions including neuronal firing, synapse transmission, gene expression, migration, neurite elongation, and growth cone guidance.15
TRPC1 is an important way for Ca2+ entry influx in platelets, smooth muscle cells, and B-lymphocytes.16-19 Furthermore, TRPC1 regulates the slow excitatory postsynaptic potential, which is triggered by the neuronal metabotropic glutamate receptor mGluR1.20 TRPC1 is involved in brain-derived neurotrophic factor (BDNF)- and netrin-1-induced axon guidance.21 It is activated by leptins and mediates the depolarizing effects of leptins in hypothalamic proopiomelanocortin neurons.22 Furthermore, knockdown of TRPC1 reduced the degree of neuronal progenitor cell proliferation by cell cycle arrest.23 The expression of TRPC6 is highest in the lung and brain.24 Furthermore, TRPC6 is important for neuronal survival25 and synaptogenesis in neural development.26 In particular TRPC6 is required for BDNF-induced axon guidance and neuron survival by activating Ca2+ signaling.27 Overexpression of TRPC6 slowed neuritogenesis and increases the number of spines in hippocampus neurons, resulting in improved spatial memory and learning. TRPC6 thus contributes to behavioral and synaptic plasticity.28
TRPM2 is a Ca2+ permeable cationic channel expressed in the brain, in particular in microglial cells and different peripheral cell types.29 It is an oxidative stress-sensitive channel.30 In brain oxidative stress may activate and upregulate TRPM2 to cause a neuroinflammatory response.31
TRP channels have been shown to play an important role in obesity and neurodegenerative diseases. In fact, they are regulated by different metabolic processes such as adipogenesis, inflammation, lipid composition, food intake, body weight, insulin secretion, and blood pressure, which are implicated in the development of obesity.32
Based on the evidence that TRP channels regulate hormone release, energy expenditure, and neurotransmitter release in control and obese and/or diabetic conditions,33 we aimed to evaluate the expression of ion channels in the forebrain of diet-induced obese (DIO) rats. DIO rats provide a useful animal model that shares several features with human obesity. An HFD represents the etiology of obesity in modern societies. Dietary supplementation of natural ligands of TRP channels has been shown to have potential beneficial effects in obese and diabetic conditions.33 Therefore, DIO models are convenient for increasing our understanding of the effects of diet and the development of subsequent obesity on nervous system alterations.
Materials and Methods
Animal handling and tissue processing
The brains used for this study were the same of the male Wistar rats sacrificed as described in the previous paper by Micioni Di Bonaventura et al.34 The animals of 5 weeks of age (Charles River Inc., Wilmington, MA, USA; n=20; 250-275 g body weight) were divided into two groups: DIO rats (n=12) and control rats (CHOW) (n=8).34
DIO rats were fed with high fat (45%) diet ad libitum (D12451, Research Diets, Inc., New Brunswick, NJ, USA; 4.73 kcal/g) and after 5 weeks (12 weeks of age), the obese phenotype starts to be developed. DIO rats were fed for further 12 weeks (24 weeks of age). The CHOW rats were fed with the standard diet (4RF18, Mucedola, Settimo Milanese, MI, Italy; 2.6 kcal/g). All procedures were carried out following the Institutional Guidelines and complied with the Italian Ministry of Health (Prot. n. 1610/2013) and associated guidelines from the European Communities Council Directive. The protocol was approved by the Ethics Committee of the University of Camerino (n. 7/2012, June 6th, 2012). After 17 weeks of HFD, rats were anesthetized with carbon dioxide and sacrificed. The brains were taken based on the anatomical depots. A portion of the brain was frozen in liquid nitrogen and stored at −80°C for gene expression and biochemical analysis. For paraffin embedding, the right cerebral hemisphere was fixed in 4% paraformaldehyde solution 0.1 M PBS, pH 7.4 for 48 h. After, the samples were gradually dehydrated in ethanol and then embedded in paraffin wax. Brain sections (10 μm thick) were cut using a microtome, collected on slides, and processed for morphological staining and immunohistochemistry (IHC).
Gene expression
Total RNA was extracted from the frontal cortex (FC) and HIP with the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), using the QiaCube (Qiagen), and reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). cDNA products were used as a template for polymerase chain reaction Quantitative Real-Time PCR (qRT-PCR) performed using the IQ5 Multicolor Real-time PCR detection system (Bio-Rad), the RT² SYBR Green qPCR Mastermix (Qiagen). QuantiTect Primer Assays were specified in Table 1. Specific control primer: Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All samples were assayed in triplicates on the same plate. The qRT-PCR parameters were 10 min at 95°C followed by 45 cycles of 95°C for 15 s and 60°C for 40 s. Measurement of GAPDH levels was used to normalize mRNA contents, and target gene levels were calculated by the 2-ΔΔCt method.
Western blot analysis
Samples (0.1±0.02 g) were homogenized in a Mixer Mill MM300 with lysis buffer containing protease inhibitor cocktail (Sigma Aldrich, Milan, Italy). After two centrifugations at 13,000 rpm (10 min at 4°C), aliquots of the supernatant were used for protein assay against a standard of bovine serum albumin (BSA) using a Bradford protein assay according to the manufacturer’s protocol. An equal amount of proteins (40 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. Transblotted membranes were incubated with polyclonal antibodies, as detailed in Table 2. The specificity of the immune reaction was assessed using antibodies pre-adsorbed with peptides used for generating them. Blots were then washed in PBS + 0.5% of TritonX-100 (PBS-T) and incubated with the horseradish-peroxidase-linked secondary antibody IGg (Bethyl Lbs., Montgomery, TX, USA) a dilution of 1:5000 for 120 min at room temperature. Positive bands were visualized by an enhanced chemiluminescence system (LiteAblot® Turbo, Cat. N. EMP012001; Euroclone, Pero, MI, Italy). To normalize protein loadings, membranes were stripped and incubated with a mouse monoclonal antibeta- actin antibody IGg2b (Monoclonal, Mouse Cat. N A2228, Sigma Aldrich) at a dilution of 1:3000 in PBS-T overnight at 4°C. Band intensities were measured by densitometric analysis with Image Lab software of ChemiDoc XRS (Bio-Rad Labs, Segrate, MI, Italy).
Immunohistochemistry
A microtome was used to make slices of the left hemisphere of the brain from each rat. Optimal antibody concentration was established in a series of preliminary experiments. Slides were incubated overnight at 4°C with primary antibodies (Table 2). Non-specific binding of IgGs was prevented by incubating them with BSA 3% in PBS-T for 1 h. The product of the immune reaction was then revealed by incubating slides for 30 min at 25°C with the specific biotinylated secondary IgGs (IgG h+l biotinylated; Bethyl) of antigoat, anti-mouse, and anti-rabbit diluted 1:200 in PBS-T. The immune reaction was then revealed with diaminobenzidine (0.05% 3-3'-diaminobenzidine dissolved in 0.1% H2O2) as a substrate. Slides were then washed, counterstained with hematoxylin, mounted on coverslips, and viewed under a light microscope. The intensity of immunostaining was assessed microdensitometrically by NIS Element Software as previously described,34 selecting, by a specific option of the program, the area of immunoreactive neurons, and considering an arbitrary value of “0” as the intensity of staining in the negative control and a value of “256”, as maximum value of density of immunoreaction.
Oxidative stress evaluation
Oxidative stress markers were evaluated in brain areas homogenates. The proteins oxidation status was investigated using the OxyBlot Protein Oxidation Detection Kit (Merck Millipore, Burlington, MA, USA; Cat. N. S7150).35,36 Briefly, 20 μg of proteins were separated in 10% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. Transblotted membranes were incubated with specific primary and secondary antibodies diluted in 1% BSA in PBS-T. Positive bands were visualized by an enhanced chemiluminescence system (LiteAblot® Turbo, Cat. N. EMP012001; Euroclone) and the intensity of bands revealed by densitometry analysis with Image Lab software of ChemiDoc XRS (BioRad, Italy). In sections of brains monoclonal antibody specific for 8-oxo-2′- deoxyguanosine (8-oxo-dG), was used according to the manufacturer’s protocol (Table 2). After the incubation with the goat antimouse secondary antibody (Alexa Fluor 488), the sections were viewed using an Olympus BX51 Fluorescence Microscope Olympus Corporation, Europa SE & CO. KG, Germany). Percentage of area of immunoreaction was measured with the Nikon NIS Element software.
Table 1.
Probe and primer used for qRT-PCR.
| cDNA probe/prime | Assay ID | Company |
|---|---|---|
| TRPV1 | QT00180782 | Qiagen |
| TRPV2 | QT00187698 | Qiagen |
| TRPC1 | QT00190715 | Qiagen |
| TRPC6 | QT00195804 | Qiagen |
| TRPM2 | QT00444311 | Qiagen |
| GAPDH | QT00199633 | Qiagen |
Table 2.
Primary antibodies using for Western blotting (WB) and immunohistochemistry (IHC).
| Primary antibody | Clone | Host animal | Company | WB dilution | IHC dilution |
|---|---|---|---|---|---|
| TRPV 1 | Polyclonal | Goat | Santa Cruz Biotek, USA Cat. N. SC-12498 | 1:500 | 1:25 |
| TRPC 1 | Polyclonal | Rabbit | Alomone Labs, Israel Cat. N. ACC-010 | 1:200 | 1:100 |
| TRPC 6 | Monoclonal | Rabbit | Alomone Labs, Israel Cat. N. ACC-017 | 1:200 | 1:100 |
| TRPM 2 | Monoclonal | Rabbit | Alomone Labs, Israel Cat. N. ACC-043 | 1:200 | 1:50 |
| SYN | Monoclonal | Mouse | Chemicon Millipore,USA Clone SY38 Cat. N. MAB5258. | 1:500 | 1:100 |
| PSD 95 | Monoclonal | Mouse | Sigma Aldrich, Italy Clone 7E3-IB8, Cat. N. P-246 | 1:200 | |
| 8-oxo-dG | Monoclonal | Mouse | Trevigen, Gaithersburg, MD, USA Cat. N. 4354-MC-050 | 1:200 |
Data analysis
The averages of different parameters investigated were calculated from single animal data, and group means ± SEM were then derived from mean single animal values. The significance of the differences between the averages was analyzed by Student’s t-test using Prism software. The significance level was set for p<0.05 to evaluate the difference between the studied groups.
Results
General parameters
As showed in the previously published study,34 body weight was increased in DIO rats in comparison to CHOW rats. After 17 weeks, the bodyweight of DIO rats (682.8±17.1 g) was significantly higher in comparison to CHOW rats (557±10.7 g; p<0.01 vs DIO rats). The obese phenotype, as showed in previous study,34 induced alterations of different physiological and blood parameters. Systolic blood pressure was higher in DIO rats (140.3±8.1 mmHg) after 17 weeks of HFD compared to age-matched CHOW rats (110.9±6.1 mmHg p<0.05 vs DIO). Both glycemia (126.8±6.1 mg/dl) and insulin levels (1.06±0.05 μg/L) in the DIO rats were statistically higher compared to CHOW rats (respectively 91.6±5.1 mg/dl and 0.73±0.05 μg/L p<0.05 vs DIO rats). The HFD did not significantly affect total cholesterol and triglycerides levels.34
Gene expression changes of TRP channels
qRT-PCR revealed gene expression levels of considered TRP channels that are known from the literature to be involved in cognitive decline. Results of the qRT-PCR analysis for the gene expression of the TRPV1, TRPV2, TRPC1, TRPC6, and TRPM2 in the FC and HIP were summarized in Figure 1A-E. TRP channels expression levels of DIO rats were altered in comparison to that of CHOW rats used as controls. Results showed that the gene expression of TRPV1 was slightly upregulated in both the FC and HIP of DIO rats compared to CHOW rats (Figure 1A), and on the other hand, TRPV2 gene expression slightly downregulated in both brain areas of DIO rats compared to CHOW (Figure 1B). Compared to CHOW rats gene expression, in the FC of DIO rats, TRPC1 and TRPC6 were downregulated while TRPM2 was upregulated. As regards the HIP of DIO rats, TRPC1, TRPC6 and TRPM2 were upregulated compared to the CHOW (Figure 1 C-E).
Figure 1.
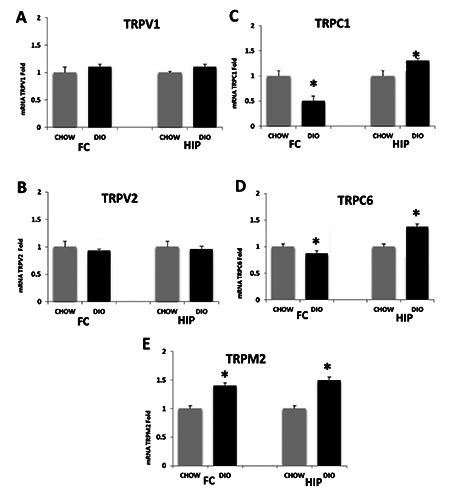
Gene expression analysis by qRT-PCR in the frontal cortex (FC) and hippocampus (HIP). A) TRPV1; B) TRPV2; C) TRPC1; D) TRPC6; E) TRPM2. CHOW, rats fed with standard diet; DIO, rats fed with high-fat diet. Data are expressed as folds comparing DIO with CHOW rats used as control. Data are the mean ± SEM. *p<0.05 vs CHOW rats.
Western blot and immunohistochemistry
Exposure of membranes of FC and HIP to anti-TRPV1 antibody caused the development of approximately 80 kDa (Figure 2A). Comparative densitometric analysis of different bands in brain portions of experimental groups, normalized to the respective reference protein intensity of immune reaction (Figure 2 A,B), revealed an increased expression in DIO animals of TRPV1 in the HIP compared to age-matched controls (Figure 2 A,B), these data were in accordance with IHC (Figure 2 C,D). Exposure of membranes to anti-TRPC1 and TRPC6 antibodies developed a 100 kDa band and 95 kDa band, respectively (Figures 3A and 4A). In this case, the presence of the proteins in the FC appears lower than in the HIP. A decrease in the expression and immunoreaction of TRPC1 was evident in the DIO rats compared to the CHOW control in the FC, but not in HIP (Figure 3 A-D). On the contrary, the expression as well as immunoreaction intensity of TRPC6 did not show significant modification in the FC in DIO rats compared to CHOW rats (Figure 4 A-D). As shown in figure 3, TRPC1 was present on the membrane of pyramidal neurons of the 5th (V) layer of the FC (Figure 3C) and pyramidal neurons of the CA1 subfield of the HIP (Figure 3C); while TRPC6 was present on the membrane of pyramidal neurons of the 6th (VI) layer of the FC (Figure 4C) and pyramidal neurons of the CA3 subfield of the HIP (Figure 4C).
For TRPM2, a band of 110kDa was revealed in the FC and HIP of the different experimental groups (Figure 5A). The intensities of the band, normalized for the corresponding references, increased in both FC and HIP of DIO rats compared to CHOW rats (Figure 5B). IHC analysis demonstrated that TRPM2 was expressed on the membrane of pyramidal neurons of the FC and pyramidal neurons in CA1 (Figure 5C) and CA3 subfields of HIP (data not showed). Particularly, densitometric analysis confirmed that TRPM2 expression in brain areas of DIO rats was higher than the controls (Figure 5D). To evaluate the possible effects of obesity and the involvement of ion channels on the synaptic components, we studied the expression of synaptic cleft proteins. Exposure of membranes of FC and HIP to anti-Synaptophysin (SYN, pre-synaptic components) antibody caused the development of a bands at 38 and 36 kDa (Figure 6A). Comparative densitometric analysis of different bands with the same molecular weight in different brain portions of CHOW and DIO rats of 17 weeks of high-caloric diet, normalized to the respective references protein intensity of immune reaction revealed an increased expression of SYN in DIO groups compared to the CHOW in HIP (Figure 6A), also in accordance with IHC (Figure 6C). The exposure of membranes to anti-PSD 95 (post-synaptic component) antibody caused the development of a band at 95kDa (Figure 6B). Comparative densitometric analysis of different bands revealed a decreased expression of the protein in the DIO group compared to the CHOW, both in the FC and HIP (Figure 6B).
Figure 2.
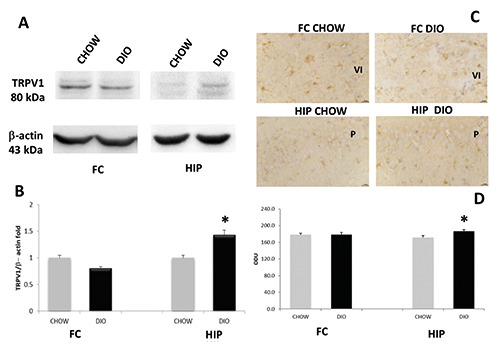
Protein expression of TRPV1. Immunochemical analysis (A) in the frontal cortex (FC) and in the hippocampus (HIP) of rats fed with standard diet (CHOW) and high-fat diet (DIO). The densitometric analysis of bands (B) is expressed as a ratio between the optical density of protein and reference protein (β-actin) where the value of the CHOW is set at 1. Immunohistochemical analysis (C) in the 6th layer of frontal cortex (VI) and in the CA1 subfield of hippocampus. P, pyramidal neurons. Calibration bar: 25 μm. The densitometric analysis of positive area (D) is expressed as an arbitrary optical density unit (ODU). Data are the mean ± SEM. *p<0.05 vs CHOW rats.
Oxidative stress analysis
The results of OxyBlot analysis showed an increase of oxidized proteins concentration in the DIO animals compared to the control one, both in FC and HIP (Figure 7 A,B). The 8-oxo-dG was used as a biomarker of oxidative damage in the nucleic acids. The pictures show representative images of 8-oxo-dG in the neurons of 5th layer of FC and in the pyramidal neurons of CA1 subfield of HIP (Figure 7C), both in the nuclei and cytoplasm. The immunofluorescence showed that 8-oxo-dG-positive area was increased in the DIO compared to CHOW both in the FC and HIP (Figure 7D).
Discussion
Accumulating evidence indicates that obesity adversely affects the central nervous system (CNS) and in particular cognitive function. 1,37 Our data support the hypothesis that DIO represents an interesting animal model for investigating brain damage due to obesity. Diet-induced obesity in rats provides a useful animal model sharing several common features with human obesity,38 including a polygenic mode in inheritance. Many studies have shown in different strains of rodents that, after exposure to a HFD, some animals become obese (DIO) while others remain lean (DR).38-40 Before the onset of obesity, outbred DIO-prone rats manifest several abnormalities of nervous system function, which might predispose them to develop obesity when offered a 31% HFD.34 Previously, we reported blood-brain barrier (BBB) alterations, neuronal loss, astrogliosis, and microglial cells activation in the FC and the HIP of DIO rats compared to the CHOW.34 Here, we assessed the HFD modulation of the expression of TRP channels both in the FC and in the HIP. Studies have indicated that TRP channels, ubiquitously expressed throughout the brain,10 play a key role in the regulation of physiological functions, as well as in pathological ones, including cardiovascular, neurological, metabolic, or neoplastic disorders.6-8,41,42 Moreover, mutations in genes encoding TRP channels are the cause of several inherited diseases in humans (the so-called ‘TRP channelopathies’) that affect the cardiovascular, renal, skeletal, and nervous systems.43 Since TRP channels can be opened and activated in response to various stimuli, their hyperactivation can induce neuronal loss and excitotoxicity, which are closely associated with neurodegenerative diseases. On the contrary, Ca2+ homeostasis is crucial to the normal physiological functions of neurons, such as growth, differentiation, and survival.42
Figure 3.
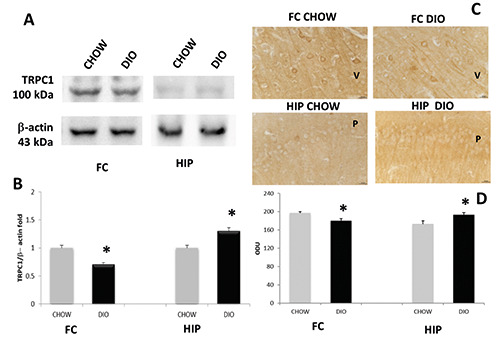
Protein expression of TRPC1. Immunochemical analysis (A) in the frontal cortex (FC) and in the hippocampus (HIP) of rats fed with standard diet (CHOW) and high-fat diet (DIO). The densitometric analysis of bands (B) is expressed as a ratio between the optical density of protein and reference protein (β-actin) where the value of the CHOW is set at 1. Immunohistochemical analysis (C) in the 5th layer of frontal cortex (V) and in the CA1 subfield of hippocampus. P, pyramidal neurons. Calibration bar: 25 μm. The densitometric analysis of positive area (D) is expressed as an arbitrary optical density unit (ODU). Data are the mean ±SEM. *p<0.05 vs CHOW rats.
A modulation in the TRPV expression was found in the hippocampus of obese rats compared to CHOW. TRPV channel activation, especially TRPV1, plays a role in the regulation of neuronal activity and synaptic plasticity as triggering long-term depression at excitatory synapses on hippocampal interneurons, influencing excitatory transmission and protecting spatial learning from the effects of acute stress.10 In this regard, TRPV1 agonists reduce the effects of stress on synaptic plasticity and spatial learning. In astrocytes, several isoforms of TRPV channels are expressed preferentially at the thick cellular processes of astrocytes rather than fine cellular processes and cell bodies. In particular TRPV1 is involved in signaling pathways responsible for the detection of blood-borne molecules in the sensory circumventricular organs.44 Its activation in astrocytes and microglia regulates migration, cytoskeleton remodeling, cytokine production, and chemotactic activity.45 There is little information on the expression and the function of TRPV2 in the rodent brain; it is potentially involved in thermal nociception and hyperalgesia.46
Interestingly, our results showed an upregulation of the TRPC1 and TRPC6 gene in DIO rats in the HIP, while it was downregulated in FC. The same phenomena were reported by Erac et al.47 in aged rat aorta where TRPC6 over expression was related to TRPC1 downregulation. Thus, we speculate the presence of a feedback loop mechanism between them. In addition, hippocampal dysregulation of TRPC6 expression is linked to different neural disorders such as Alzheimer's disease (AD),48 stroke,49 and epilepsy.50 Thus, maintaining an adequate level of TRPC6 expression is important for neuronal functions. Overexpression of TRPC6 in cultured hippocampal neurons increased the density of the dendritic spine, while downregulation of TRPC6 with siRNA reduced the spine density.51,52 TRPC6 transgenic mice exhibited improved spatial learning and memory, suggesting a crucial role of TRPC6 in learning and memory tasks through regulation of synaptic plasticity.52 However, it remains unclear how TRPC6 expression in the neurons is controlled.53 No data are available regarding the effect of TRPC1 in FC and HIP. However, this type of channel in the striatum repressed neuronal cell death by reducing TRPC5 functionality in Huntington’s disease,54 while in Parkinson’s disease (PD), in the substantia nigra, decreased neurotoxicity and increased survival of dopaminergic neurons.55,56 Expression of the different proteins, which are involved in neurotransmission, at the synapses are con-sidered as the markers of neural plasticity. SYN is a presynaptic membrane protein essential for neurotransmission in hippocampal neurons.57 Even if, it was reported that obesity diminishes synaptic markers3 and induces a loss of synaptic protein expression after three months of HFD,58 here we found in DIO animals an impairment only of postsynaptic PSD 95 expression, but not of the presynaptic SYN.
Figure 4.
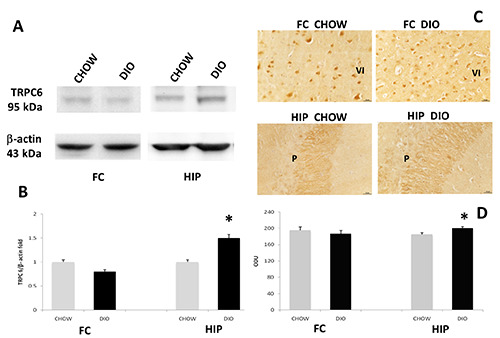
Protein expression of TRPC6. Immunochemical analysis (A) in the frontal cortex (FC) and in the hippocampus (HIP) of rats fed with standard diet (CHOW) and high-fat diet (DIO). The densitometric analysis of bands (B) is expressed as a ratio between the optical density of protein and reference protein (β-actin) where the value of the CHOW is set at 1. Immunohistochemical analysis (C) for TRPC6 in the 6th layer of frontal cortex (VI) and CA3 subfield of hippocampus. P, pyramidal neurons. Calibration bar: 25 μm. The densitometric analysis of positive area (D) is expressed as an arbitrary optical density unit (ODU). Data are the mean ± SEM, *p<0.05 vs CHOW rats.
Concerning TRPM2, it was highly expressed in HIP and FC of HFD-fed rats as showed by immunohistochemical and western blot analysis. TRPM2 is highly expressed in the brain, and even if its physiologic role is not well known, it is involved in insulin secretion. TRPM2 functions as a cellular redox (oxidative stress) sensor and has been implicated in the pathogenesis of bipolar disorder, diabetes, as well as cardiovascular and neurodegenerative disorders such as amyotrophic lateral sclerosis.59 Genetic studies found that single nucleotide polymorphisms (SNPs) in TRPM2 and TRPM7 genes were associated with two related neurodegenerative disorders, Guam amyotrophic lateral sclerosis (ALS-G) and parkinsonism-dementia, respectively.60 TRPM2 channel responds to oxidative stress,61 pro-inflammatory mediators such as tumor necrosis factors alpha (TNFα) and increased amyloid beta-peptide (Aβ)-mediated in the HIP, thus it has been implicated in AD.62-64 The important role of TRPM2 isoforms in cell proliferation and oxidant-induced cell death has been well established, using divergent cell systems and techniques including overexpression, channel depletion or inhibition, and calcium chelation.65 In our results, the modulation of TRPM2 and other TRP channels was correlated to the increase of the oxidative stress in brain areas. In fact, both proteins oxidation and oxidative damage of nucleic acids were elevated in FC and HIP of DIO rats, compared to CHOW. Moreover, this evidence confirms the association of obesity with the increase of oxidative stress and inflammation in the brain, as shown in an animal model of genetic obesity.66
Evidence supports a key role for the TRPM2-mediated Ca2+ signaling in mediating microglial cell activation, generation of proinflammatory mediators, and neuroinflammation, which are of relevance to CNS diseases.63 This is following the significant increase of ionized calcium-binding adaptor molecule 1 (Iba1), which we previously reported both in FC and in the HIP of DIO rats.34 It has been demonstrated that members of TRP canonical, TRP melastatin, and TRP vanilloid subfamilies of TRP channels are highly expressed and regulate diverse neuronal and glial functions including developmental, and homeostatic functions.15,67 In addition, recent studies show that dysregulation of the TRP channel expression and functions contributes to pathological events of neurological and psychiatric disorders.10 The nervous system cellular environment is modulated by the activity of microglia that can induce neuroprotective and neurotoxic effects. Especially in response to pathological conditions, microglia produce free radicals and pro-inflammatory cytokines molecules that can contribute to axon demyelination and neuron death. For this reason, the activation of microglia functions promotes not only defense/repair mechanisms but also improves brain injuries as in neurodegenerative disorders.68 Since the TRP channels contribute to cell osmotic regulation, cytokine production, proliferation, activation, cell death, and oxidative stress responses, it is now well accepted that they play an important role in the regulation of microglia activities.69
Figure 5.
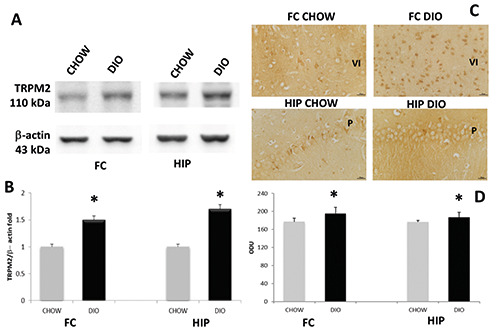
Protein expression of TRPM2. Immunochemical analysis (A) in the frontal cortex (FC) and in the hippocampus (HIP) of rats fed with standard diet (CHOW) and high-fat diet (DIO). The densitometric analysis of bands (B) is expressed as a ratio between the optical density of protein and reference protein (β-actin) where the value of the CHOW is set at 1. Immunohistochemical analysis (C) in the 6th layer of frontal cortex (VI) and in the CA1 subfield of hippocampus. P, pyramidal neurons. Calibration bar: 25 μm. The densitometric analysis of positive area (D) is expressed as an arbitrary optical density unit (ODU). Data are the mean ± SEM. *p<0.05 vs CHOW rats.
The TRPC members, expressed in neurons and glial cells, such as astrocytes, microglia, and oligodendrocytes,70 are involved, via neuronal receptor stimulation by neurotrophic factors or neuropeptides in neuronal firing, synapse transmission, gene expression, migration, neurite elongation, and growth cone guidance. Thus, they play a pivotal role in promoting neuronal survival. Overall, TRPC is involved in synaptic and behavioral plasticity, as spatial learning and memory functions, by influencing the number of spines in hippocampal neurons.51 It has been also demonstrated that they are involved in motor control and coordination.71 It has been demonstrated that TRPC channels are essential in the prevention of neuronal injury after cerebral ischemia so that the reduction in their expression is associated with stroke and cerebral vessel occlusion. In addition, they can be activated by the peptidic hormone leptin causing depolarizing effects in hypothalamic proopiomelanocortin neurons and promoting spine formation in hippocampal neurons.72 TRPM receptors are redox-sensitive Ca2+ permeable channels involved in the protection of neurons from cell death after oxidative stress.44 They contribute to hippocampal synaptic plasticity73 and neuronal development, as evidenced by the fact that TRPM2 inhibition increased while TRPM2 overexpression decreased axonal growth. Moreover, oxidative stress increases the expression of the TRPM2 channel, enhancing the Ca2+ influx leading to a neuroinflammatory response in human astrocytes74 and stimulating reactive oxidative species (ROS)- induced neuronal death. The activation of TRPM channels, mainly TRPM2, has been also associated with oxidative stress-induced ischemic brain damage.75 In cultured microglia, recent findings showed that TRPM members promote nitric oxide production and migration of microglia in an inflammatory state.
Figure 6.
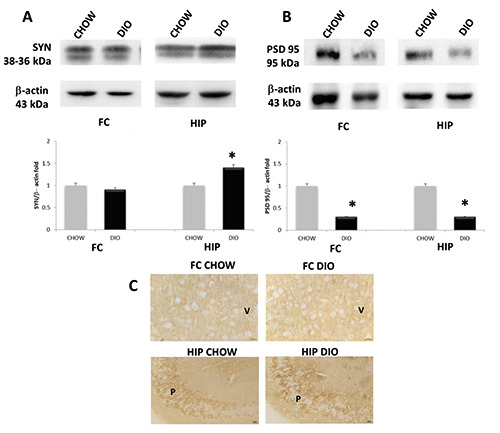
Protein expression of synaptic proteins. Immunochemical analysis of pre-synaptic protein synaptophysin (A) and post-synaptic protein PSD 95 (B) in the frontal cortex (FC) and in the hippocampus (HIP) of rats fed with standard diet (CHOW) and high-fat diet (DIO). The densitometric analysis of bands is expressed as a ratio between the optical density of protein and reference protein (β- actin) where the value of the CHOW is set at 1. Immunohistochemical analysis (C) for the synaptophysin in the 5th layer of frontal cortex (V) and in the CA1 subfield of hippocampus; P, pyramidal neurons. Calibration bar: 25 μm. Data are the mean ± SEM. *p<0.05 vs CHOW rats.
Figure 7.
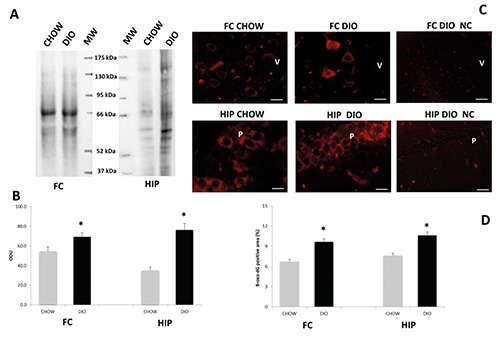
Oxidative stress in brain areas. Lysates of frontal cortex (FC) and hippocampus (HIP) from rats fed with standard diet (CHOW) and high-fat diet (DIO) were immunoblotted using the OxyBlot Protein Oxidation Detection kit. Bars graph (B) reports the values of optical density measured in optical density unit (ODU). Data are mean ± SEM; *p<0.05 vs CHOW rats. Sections of the brain of CHOW and DIO rats were processed for the immunohistochemistry of 8-oxo-dG (C) and immunoreaction expressed as a percentage of positive area (D). The immunoreaction was present more in the cytoplasm than in nuclei of neurons of the 5th layer (V) of FC and in the pyramidal neurons (P) of CA1 subfield of HIP; NC, negative control. Calibration bar: 10 μm.
The identification of TRP changes and the alterations of synaptic proteins in DIO rats may represent the first insight to characterize and explain the neuronal and glial changes occurring in obesity. The availability of suitable animal models may be useful for investigating pharmacological treatments countering obesity-induced brain injury. Further investigations are necessary to identify the role of TRP channels in the regulation of synaptic plasticity in cognitive decline related to obesity.
Funding Statement
Funding: This research was supported by University of Camerino, Fondo d'Ateneo di Ricerca (FAR 2019).
References
- 1.Belsky DW, Caspi A, Goldman-Mellor S, Meier MH, Ramrakha S, Poulton R, et al. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Am J Epidemiol 2013;178:1461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet 2005;366:1197-209. [DOI] [PubMed] [Google Scholar]
- 3.Bocarsly ME, Fasolino M, Kane GA, La Marca EA, Kirschen GW, Karatsoreos IN, et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci USA 2015;112:15731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodish H, Berk A, Zipursky SL, Freeman WH. Neurotransmitters, synapses, and impulse transmission. In: Lodish H, Berk A, Zipursky SL, Freeman WH, editors. Molecular Cell Biology. New York: W.H. Freeman; 2000. Section 21.4. [Google Scholar]
- 5.Gouaux E, MacKinnon R. Principles of selective ion transport in channels and pumps. Science 2005;310:1461-5. [DOI] [PubMed] [Google Scholar]
- 6.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2010;2:a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gualdani R, Gailly P. How TRPC channels modulate hippocampal function. Int J Mol Sci 2020;21:3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciardo MG, Ferrer-Montiel A. Lipids as central modulators of sensory TRP channels. Biochim Biophys Acta Biomembr 2017;1859:1615-8. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch 2005;451:35-42. [DOI] [PubMed] [Google Scholar]
- 10.Sawamura S, Shirakawa H, Nakagawa T, Mori Y, Kaneko S. TRP channels in the brain: What are they there for? In: Emir TLR, editor. Neurobiology of TRP channels. Boca Raton: CRC Press/Taylor & Francis; 2017. Chapter 16. [PubMed] [Google Scholar]
- 11.Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, et al. Cloning and functional expression of a human orthologue of rat vanilloid receptor 1. Pain 2000;88:205-15. [DOI] [PubMed] [Google Scholar]
- 12.Kim SR, Kim SU, Oh U, Jin BK. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol 2006;177:4322-9. [DOI] [PubMed] [Google Scholar]
- 13.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci 2005;22:825-34. [DOI] [PubMed] [Google Scholar]
- 14.Shibasaki K, Ishizaki Y, Mandadi S. Astrocytes express functional TRPV2 ion channels. Biochem Biophys Res Commun 2013;441:327-32. [DOI] [PubMed] [Google Scholar]
- 15.Vennekens R, Menigoz A, Nilius B. TRPs in the brain. Rev Physiol Biochem Pharmacol 2012;163:27-64. [DOI] [PubMed] [Google Scholar]
- 16.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 2006;13:693-708. [DOI] [PubMed] [Google Scholar]
- 17.Authi KS. TRP channels in platelet function. In: Flockerzi V, Nilius B, editors. Transient receptor potential (TRP) channels. Handbook of experimental pharmacology, vol 179. Springer; 2007. p. 425-43. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 2006;112:744-60. [DOI] [PubMed] [Google Scholar]
- 19.Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, et al. Transient receptor potential 1 regulates capacitative Ca2+ entry and Ca2+ release from endoplasmic reticulum in B lymphocytes. J Exp Med 2002;195:673-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 2003;426:285-91. [DOI] [PubMed] [Google Scholar]
- 21.Shim S, Yuan JP, Kim JY, Zeng W, Huang G, Milshteyn A, et al. Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron 2009;64:471-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu J, Fam Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 2010;30:1560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Chen C, Xhou Z, Xu S, Yu Z. TRPC1 mediated the increase in store-operated Ca2+entry is required for the proliferation of adult hippocampal neuronal progenitor cells. Cell Calcium 2012;51:486-96. [DOI] [PubMed] [Google Scholar]
- 24.Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, et al. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res 2002;109:95-104. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci 2007;10:559-67. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, et al. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 2008;11:741-3. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 2005;434:894-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, et al. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 2008;11:741-3. [DOI] [PubMed] [Google Scholar]
- 29.Kraft R, Harteneck C. The mammalian melastatin related transient receptor potential cation channels: an overview. Pflugers Arch 2005;451:204-11. [DOI] [PubMed] [Google Scholar]
- 30.Malko P, Syed Mortadza SA, McWilliam J, Jiang LH. TRPM2 channel in microglia as a new player in neuroinflammation associated with a spectrum of central nervous system pathologies. Front Pharmacol 2019;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bond CE, Greenfield SA. Multiple cascade effects of oxidative stress on astroglia. Glia 2007;55:1348-61. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Huang W, Wu D, Priestley J V. TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci 2006;24:1-6. [DOI] [PubMed] [Google Scholar]
- 33.Zsombok A, Derbenev AV. TRP Channels as therapeutic targets in diabetes and obesity. Pharmaceuticals (Basel) 2016;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micioni Di Bonaventura MV, Martinelli I, Moruzzi M, Micioni Di Bonaventura E, Giusepponi ME, Polidori C, et al. Brain alterations in high fat diet induced obesity: effects of tart cherry seeds and juice. Nutrients 2020;12:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinelli I, Tomassoni D, Moruzzi M, Roy P, Cifani C, Amenta F, et al. Cardiovascular changes related to metabolic syndrome: Evidence in obese Zucker rats. Int J Mol Sci 2020;21:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinelli I, Tomassoni D, Roy P, Di Cesare Mannelli L, Amenta F, Tayebati SK. Antioxidant properties of alpha-lipoic (thioctic) acid treatment on renal and heart parenchyma in a rat model of hypertension. Antioxidants (Basel) 2021;10:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol 2017;16:465-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 2000;278:R231-7. [DOI] [PubMed] [Google Scholar]
- 39.Surwit RS, Feinglos MN, McCaskill CC, Clay SL, Babyak MA, Brownlow BS, Plaisted CS, Lin PH. Metabolic and behavioral effects of a high-sucrose diet during weight loss. Am J Clin Nutr 1997;65:908-15. [DOI] [PubMed] [Google Scholar]
- 40.Levin BE, Routh VH. Role of the brain in energy balance and obesity. Am J Physiol 1996;271: R491-500. [DOI] [PubMed] [Google Scholar]
- 41.Smani T, Shapovalov G, Skryma R, Prevarskaya N, Rosado JA. Functional and physiopathological implications of TRP channels. Biochim Biophys Acta 2015;1853:1772-82. [DOI] [PubMed] [Google Scholar]
- 42.Hong C, Jeong B, Park HJ, Chung JY, Lee JE, Kim J, et al. TRP Channels as emerging therapeutic targets for neurodegenerative diseases. Front Physiol 2020;11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 2014;171:2474-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang R, Tu S, Zhang J, Shao A. Roles of TRP channels in neurological diseases. Oxid Med Cell Longev 2020;2020: 7289194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang XL, Wang X, Shao L, Jang GT, Min JW, Mei XY, et al. TRPV1 mediates astrocyte activation and interleukin-1β release induced by hypoxic ischemia (HI). J Neuroinflammation 2019;16:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedungadi T P, Dutta M, Bathina SC, Caterina MJ, Cunningham JT. Expression and Distribution of TRPV2 in rat brain. Exp Neurol 2012;237:223-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erac Y, Selli C, Kosova B, Akcali KC, Tosun M. Expression levels of TRPC1 and TRPC6 ion channels are reciprocally altered in aging rat aorta: implications for age-related vasospastic disorders. Age (Dordr) 2010;32:223-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Lu R, Yang J, Li H, He Z, Jing N, et al. TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production. Nat Commun 2015;6:8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Y, Chen F, Zhang J, Wang T, Wei X, Wu J, et al. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J Mol Neurosci 2013;50:504-13. [DOI] [PubMed] [Google Scholar]
- 50.Zeng C, Zhou P, Jiang T, Yuan C, Ma Y, Feng L, et al. Upregulation and diverse roles of TRPC3 and TRPC6 in synaptic reorganization of the mossy fiber pathway in temporal lobe epilepsy. Mol Neurobiol 2015;52:562-72. [DOI] [PubMed] [Google Scholar]
- 51.Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, et al. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci 2008;121:2301-7. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, et al. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 2008;11:741-3. [DOI] [PubMed] [Google Scholar]
- 53.Qu C, Ding M, Zhu Y, Lu Y, Du J, Miller M, et al. Pyrazolopyrimidines as potent stimulators for transient receptor potential canonical 3/6/7 channels. J Med Chem 2017;60: 4680-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong C, Seo H, Kwak M, Jeon J, Jang J, Jeong EM, et al. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington's disease. Brain 2015;138:3030-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bollimuntha S, Ebadi M, Singh BB. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res 2006;1099:141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, et al. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest 2012;122:1354-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weimer RM, Jorgensen EM. Controversies in synaptic vesicle exocytosis. J Cell Sci 2003;116:3661-6. [DOI] [PubMed] [Google Scholar]
- 58.Shuai Hao, Aditi Dey, Xiaolin Yu, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 2016;51:230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang L-H, Yang W, Zou J, Beech DJ. TRPM2 channel properties, functions and therapeutic potentials. Expert Opin Ther Targets 2010;14:973-88. [DOI] [PubMed] [Google Scholar]
- 60.Plato CC, Galasko D, Garruto RM, Plato M, Gamst A, Craig UK, et al. ALS and PDC of Guam: forty-year follow-up. Neurology 2002;58:765-73. [DOI] [PubMed] [Google Scholar]
- 61.Ovey İS, Naziroğlu M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience 2015;284:225-33. [DOI] [PubMed] [Google Scholar]
- 62.Ostapchenko VG, Chen M, Guzman MS, Xie YF, Lavine N, Fan J, et al. The transient receptor potential melastatin 2 (TRPM2) channel contributes to β-amyloid oligomer-related neurotoxicity and memory impairment. J Neurosci 2015;35: 15157-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malko P, Syed Mortadza SA, McWilliam J, Jiang LH. TRPM2 channel in microglia as a new player in neuroinflammation associated with a spectrum of central nervous system pathologies. Front Pharmacol 2019;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Wei LY, Ding R, Feng Y, Li D, Li C, et al. Predisposition to Alzheimer's and age-related brain pathologies by PM2.5 exposure: Perspective on the roles of oxidative stress and TRPM2 channel. Front Physiol 2020;11:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol 2011;704:531-44. [DOI] [PubMed] [Google Scholar]
- 66.Tomassoni D, Martinelli I, Moruzzi M, Micioni Di Bonaventura MV, Cifani C, Amenta F, et al. Obesity and agerelated changes in the brain of the Zucker Leprfa/fa rats. Nutrients. 2020;12:1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 2014;66:676-814. [DOI] [PubMed] [Google Scholar]
- 68.Skaper SD. Ion channels on microglia: therapeutic targets for neuroprotection. CNS Neurol Disord Drug Targets 2011; 10:44-56. [DOI] [PubMed] [Google Scholar]
- 69.Echeverry S, Rodriguez MJ, Torres YP. Transient receptor potential channels in microglia: Roles in physiology and disease. Neurotox Res 2016;30:467-78. [DOI] [PubMed] [Google Scholar]
- 70.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol 2005;564:737-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartman RE, Kamper JE, Goyal R, Stewart JM, Longo LD. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol Behav 2012;105:1092-7. [DOI] [PubMed] [Google Scholar]
- 72.Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 2010;30:1560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie YF, Belrose JC, Lei G, Tymianski M, Mori Y, Macdonald JF, Jackson MF. Dependence of NMDA/GSK-3β mediated metaplasticity on TRPM2 channels at hippocampal CA3-CA1 synapses. Mol Brain 2011;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee GR, Shin MK, Yoon DJ, Kim AR, Yu R, Park NH, Han IS. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity (Silver Spring) 2013;21:115-22. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu T, MacEy TA, Quillinan N, Klawitter J, Oerraud ALL, Traystman RJ, Herson PS. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab 2013;33:1549-55. [DOI] [PMC free article] [PubMed] [Google Scholar]


