Abstract
Cardiovascular diseases (CVD) are the leading cause of global mortality and their pathogenesis lies mainly in the atherosclerotic process. There are close connections linking oxidative stress and inflammation to endothelial dysfunction, atherosclerosis and, consequently, to CVD. This review focuses on the role of xanthine oxidoreductase (XOR) and its products on the development of chronic inflammation and oxidative stress, responsible for atheromatous plaque formation. Evidence is reported that an excessive level of XOR products favors inflammatory response and plaque development, thereby promoting major cardiovascular risk factors. Also, the relationship between hyperuricemia and hypertension as well as between XOR activity and CVD is confirmed. In spite of the increasing number of clinical studies investigating the output of cardiovascular patients treated with urate-lowering therapies (including uricosuric drugs, XOR inhibitors and recombinant uricase) the results are still uncertain. The inhibition of XOR activity appears more promising than just the control of uricemia level in preventing cardiovascular events, possibly because it also reduces the intracellular accumulation of urate, as well as the production of reactive oxygen species. However, XOR inhibition also reduces the availability of the multifaced mediator nitric oxide and, at present, can be recommended only in hyperuricemic patients.
Keywords: Atherosclerosis, Cardiovascular diseases, Hypertension, Nitric oxide, Reactive oxygen species, Uric acid, Xanthine oxidoreductase
Graphical abstract
Highlights
-
•
Oxidative stress and inflammation are strictly related to atherosclerosis and CVD.
-
•
XOR products can bring on oxidative stress and inflammation.
-
•
XOR and uric acid in serum are implicated in cardiovascular diseases.
-
•
XOR products favor plaque development, promoting major cardiovascular risk factors.
-
•
The nitrate reductase activity of XOR has protective outcomes.
Abbreviations
- COX2
cyclooxygenase 2
- CVD
cardiovascular diseases
- eNOS
endothelial nitric oxide synthase
- DAMPs
damage-associated molecular patterns
- FAD
flavin adenine dinucleotide
- HDL
high-density lipoprotein
- HIF-1α
inducible factor-1α
- HMGB1
high mobility group box chromosomal protein 1
- hXDH
human gene of XOR
- IL-1β
interleukin-1β
- LDL
low-density lipoproteins
- MAP
mitogen-activated protein
- Moco
molybdopterin cofactor
- mTOR
mammalian target of rapamycin
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NLRP3
NACHT, LRR and PYD domain-containing protein 3
- RAGE
receptor for advanced glycation end products
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TGF-β
transforming growth factor-beta
- UA
uric acid
- VLDL
very low-density lipoprotein
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
Xanthine oxidoreductase
1. Introduction
According to the Global Burden of Disease Study 2019, cardiovascular diseases (CVD) are the leading cause of global mortality, their incidence is increasing all over the world and is almost doubled in the past thirty years, mainly because of population growth and aging. In nearly half of the cases, CVD deaths are due to ischemic heart disease and mortality is greater in men than in women before age 80, whereupon the pattern reverses. Among the risk factors for CVD, hypertension appears to be the most prevalent one, but also one of those with the greatest opportunities for control, through an appropriate dietary intake, increased physical activity and/or specific medications [1].
A recent publication reports the relationship between inflammation markers and blood pressure in the early stages of CVD development. Inflammatory mediators contribute to the development of hypertension by increasing the production of reactive oxygen (ROS) and nitrogen (RNS) species, inducing the release of cytokines, which increase vascular resistance through the production of angiotensinogen and angiotensin II, as well as sodium and volume retention. The study was conducted on a population of healthy black and white young people who were followed for 4.5 years in South Africa. A significant positive association between the level of inflammatory mediators and the increase in blood pressure was observed only in white participants, emphasizing the role of genetic characteristics in the early development of hypertension [2].
An unbalanced overproduction of ROS causes oxidative stress that can be responsible for chronic inflammation, cell and tissue damage, mutagenesis and cancer, alteration of biological molecules and aging. On the other hand, a proper level of ROS is useful for the fine tuning of a number of physiologic functions, such as the enhancement of regulatory T cell differentiation that modulates the adaptative response, as well as the regulation of cell adhesion, proliferation, differentiation and migration, which are essential for embryogenesis, stem cell differentiation and wound repair [3].
ROS are mainly produced by mitochondrial electron transport chain and by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, xanthine oxidoreductase (XOR), aldehyde oxidase and uncoupled endothelial nitric oxide synthase (eNOS). Their overproduction may contribute to the development of heart failure, since oxidative stress can cause endothelial dysfunction, characterized by impaired anti-inflammatory and anticoagulation activities and altered vascular tone, as well as apoptosis and contractile dysfunction of cardiomyocytes and remodeling of the extracellular matrix [4].
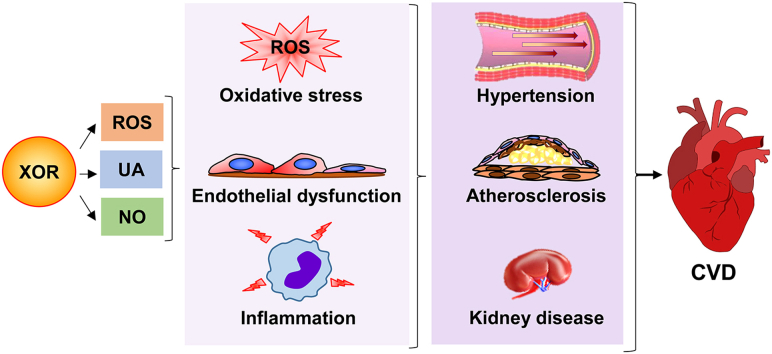
The present review focuses on the role of XOR activities and products in the pathogenesis of oxidative stress and chronic inflammation, leading to atherosclerotic alterations, which represent the ground for the development of CVD.
2. Xanthine oxidoreductase: activities and products
XOR is a molybdo-flavoenzyme that is ubiquitous in living kingdoms from prokaryotic to eukaryotic organisms in a highly conserved form, because all enzymes in this family likely evolved from common ancestral XOR genetic components [5]. The main XOR activity, i.e. the catabolism of hypoxanthine to xanthine and xanthine to uric acid, is carried out by xanthine dehydrogenase (XDH, EC 1.17.1.4) in most living beings, while xanthine oxidase activity (XO, EC 1.17.3.2) is present only in mammals. In uricase-free higher primates, including humans, XOR performs the last two steps of purine catabolism and has a rate-limiting function, which precludes the salvage pathway of purine nucleotides, as its products, xanthine and uric acid, are irreversible [6].
The human gene of XOR (hXDH) is located on chromosome 2 and its expression is subjected to strict regulatory control, possibly because of promoter suppression through a repressor protein [7]. The highest expression of hXDH occurs in epithelial cells of the liver, gut and breast during lactation, although a high level of XOR activity is also associated with endothelial cells, because serum XOR, which mainly results from hepatocyte turnover, readily binds to heparin-binding glycosaminoglycans on the endothelial surface [8]. hXDH expression and XOR activity can be increased by low oxygen tension, as well as inflammatory cytokines, hormones and growth factors through upregulation of XOR transcription and/or post-translational activation. In humans, the serum level of XOR is very low, except during pathological conditions inducing tissue damage, particularly to liver [9].
XOR gene expression generates a NAD+-dependent XDH. In mammals, XO activity results from a post-translational modification of the enzyme that can be reversible, if due to the formation of two disulphide bonds between four specific Cys residues, or irreversible, if caused by a partial proteolysis of the inter-domain linker peptide containing these crucial residues. If only one pair of sulfhydryl groups is oxidized, during the conversion from XDH to XO, an intermediate XOR form can be generated, having both NAD+ and O2 as the electron acceptor [10].
XOR usually has dehydrogenase activity inside the cells and oxidase activity outside, as in biological fluids, with the exception of activated leukocytes, which show XO activity that, through a univalent and divalent electron transfer to O2, generates superoxide ion (O2•−) and hydrogen peroxide (H2O2), respectively. These ROS activate endothelial cells and are functional to the cytocidal phase of phagocytosis. The transition from XDH to XO occurs physiologically when XOR is secreted into milk by lactating breast cells or leak out from dead cells, such as from hepatocytes into serum and from enterocytes into gastrointestinal lumen, where the presence of proteolytic enzymes causes further transformation into irreversible XO. During lactation, XOR induces apical membrane reorganization of mammary cells and contributes to apocrine secretion of milk-fat droplets. The reversible or irreversible XDH transition to XO also occurs in a number of pathological conditions, such as hypoxia and reoxygenation, ischemia and reperfusion, viral infection, toxic tissue injury, radiation damage, organ preservation and transplantation. Under these circumstances, XOR-derived ROS can activate an inflammatory response and amplify tissue damage through their cytotoxic effects [6].
Beside its role in purine catabolism, XOR can metabolize a number of endogenous and exogenous compounds [8], including different drugs [11]. In addition, XOR possesses a nitrite reductase activity, generating nitric oxide (NO), and a NADH oxidase activity, producing ROS especially in hypoxic, acidic and inflammatory conditions. In turn, the interaction between O2•− and NO determines the formation of the RNS peroxynitrite (Fig. 1A) [12].
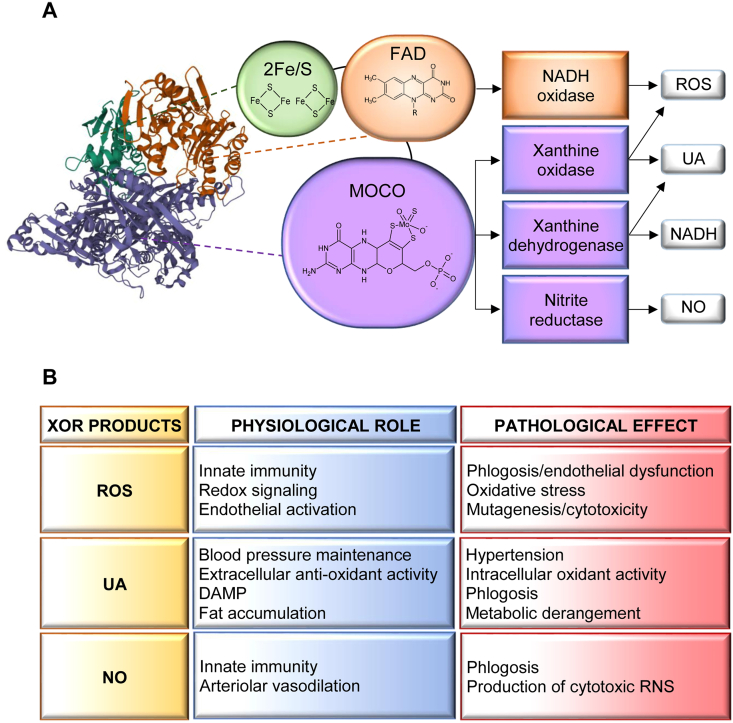
Fig. 1.
A) Xanthine oxidoreductase (XOR) monomer structure, enzymatic activity and products. XOR (PDB number 3B9J) is a homodimer of about 300 kDa. Each subunit is composed of three domains connected by unstructured regions: the 20-kDa N-terminal domain (green) containing two non-identical iron-sulfur clusters (2Fe/S), the 40-kDa intermediate domain (orange) with a flavin adenine dinucleotide (FAD) cofactor and the 85-kDa C-terminal domain (violet) characterized by a molybdopterin cofactor containing a molybdenum atom (Moco). Xanthine oxidase and dehydrogenase activities produce uric acid (UA) and reduced nicotinamide adenine dinucleotide (NADH) or superoxide ion and hydrogen peroxide (ROS). The nitrate and nitrite reductase activities generate nitric oxide (NO) and the NADH oxidase activity produces ROS [13]. B) Physio-pathological roles of XOR products. XOR products have physiological roles at low levels, but they exert pathological effects at high levels. ROS play a redox signaling role that is implicated in many cellular functions, such as activation, proliferation and migration, that are essential for innate immunity. A high level of ROS can cause oxidative stress or endothelial dysfunction, can induce mutagenesis or cytotoxicity. UA supports blood pressure by activating the renin-angiotensin system, but hyperuricemia promotes hypertension; it is a free radical scavenger in biological fluids, but its intracellular accumulation induces oxidative stress; it increases cyclooxygenase 2 expression and, when released from dead cells, stimulates macrophages by acting as a damage-associated molecular pattern (DAMP), thus having a pro-phlogistic effect; it promotes physiological fat accumulation; however, hyperuricemia induces lipidic and glycidic metabolism derangements that can favor the development of metabolic syndrome [19]. NO regulates vascular tone by dilating blood vessels and contributes to innate immunity both by activating endothelium and by generating cytotoxic reactive nitrogen species (RNS) [17,23]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The XOR protein consists of two identical subunits of approximately 150 kDa, each composed of three domains linked by hinge regions. The domains are characterized by the presence of different cofactors: two non-identical iron-sulfur clusters (2Fe/S) in the 20-kDa N-terminal domain, a flavin adenine dinucleotide (FAD) cofactor in the 40-kDa intermediate domain and a molybdenum atom-containing molybdopterin cofactor (Moco) in the 85-kDa C-terminal domain. The two iron-sulfur redox centers allow electron flow to move between the Moco site, where purine oxidation and nitrite reduction occur, and the FAD site, where NAD + or O2 are reduced or NADH is oxidized. The Moco site may be inhibited by allopurinol or febuxostat, which have a pharmacological use to lower uricemia in gout, but do not affect NADH oxidase activity. Main products of XOR activities are uric acid, produced by XDH and XO, ROS, produced by XO and NADH oxidase and NO, produced by nitrite reductase (Fig. 1A) [13].
An adequate level of serum uric acid has several positive effects, while both hypouricemia and hyperuricemia are associated with a number of pathological conditions, including a significant increase in rates of CVD, following a J-shaped curve [14]. In biological fluids uric acid has antioxidant activity, behaving as a free radical scavenger. In this manner uric acid contributes to the non-enzymatic defense system against oxidative stress, preventing cancer, cardiovascular and neurodegenerative diseases.
Mice with haploinsufficiency of urate oxidase showed reduced brain damage and better outcome after brain ischemia. But, only female mice exhibited also a significantly lengthened lifespan [15].
Uric acid supports blood pressure through a number of mechanisms: it increases the expression of cyclooxygenase-2 which upregulates the renin-angiotensin system; it activates NADH oxidase, which decreases the availability of NO, thus neutralizing the NO-induced vasodilation; it switches on the endothelium towards a pro-inflammatory state that contributes to vasoconstriction [16,17]. Furthermore, the urate released by dead cells and the eventual formation of urate crystals represent damage-associated molecular patterns (DAMPs) that are capable of stimulating macrophages and causing inflammation, as occurs in gout. Finally, uric acid influences the hepatic metabolism of lipids and glycides by promoting lipogenesis and fat storage, blocking fat oxidation and inducing gluconeogenesis (Fig. 1B) [9,18]. Hyperuricemia induces lipidic and glycidic metabolism derangements that seem to favor the development of metabolic syndrome [19], even if some experimental evidence contradicts this assumption [20].
XOR-generated ROS and NO play an essential role in innate immunity. They increase the permeability of endothelial cells, which express adhesion molecules for leukocytes. The cytokines released by the activated leukocytes upregulate macrophage XOR expression, which contributes to the antibacterial defense during phagocytosis with its products. In maternal milk, ROS and RNS produced by XOR have bactericidal activity in the digestive apparatus of suckling newborn while sparing commensal flora. A similar protective action against opportunistic bacteria is performed by the XOR released by the enterocytes in the intestinal lumen where the XOR products contribute to the regulation of the intestinal microbiome [21]. XOR-derived ROS and NO also help to modulate the local vascular tone and consequently contribute to blood pressure regulation, in accordance with the endothelial activities of NADPH oxidase and NO synthase (Fig. 1B) [22].
3. Atherosclerosis and reactive oxygen species
Atherosclerosis is a specific form of arteriosclerosis characterized by formation of atheromatous plaques in the arterial wall as a consequence of a chronic inflammatory stimulus, for example mechanical stress, which activates the endothelial lining and promotes the infiltration of monocytes. Oxidative stress has a pro-inflammatory action and contributes to low-density lipoproteins (LDL) oxidation and advanced glycation end-product (AGE) formation, which concur to the atherogenic process. A growing body of evidence supports the hypothesis that elevated XOR activity and hyperuricemia contribute to the development of atherosclerosis that plays an essential role in the pathogenesis of CVD [24]. There is a strict connection linking oxidative stress and inflammation to endothelial dysfunction, atherosclerosis and CVD. An excessive number of oxidants stimulates the pro-inflammatory phenotype of endothelial cells, which modify their shape to allow permeabilization and produce adhesion molecules and pro-coagulating and vasoconstricting mediators, while decreasing the bioavailability of NO and impairing vascular relaxation. Chronic inflammation promotes vascular modifications, through the migration and proliferation of vascular smooth muscle cells, along with the extracellular matrix remodeling that lead to vascular stiffness and hypertension or aneurysmal complications. In vitro, animal and human studies with antioxidants somehow encourage further investigations, although the results so far obtained on hypertension and atherosclerosis are still disappointing [25].
Hypercholesterolemia, hypertension, diabetes mellitus and smoking are established cardiovascular risk factors because they cause the formation of atherosclerotic plaques by enhancing ROS production and decreasing the availability of eNOS-derived NO, which has an anti-atherosclerotic role. Atherogenesis is promoted by oxidative stress that induces oxidative modification of circulating molecules as well as activation of the endothelial cell and monocyte/macrophage activation and smooth muscle cell proliferation (Fig. 2) [26]. Oscillatory shear stress is a pro-atherosclerotic stimulus that enhances the expression and activity of endothelial XOR and markedly increases ROS production by activating NADPH oxidase, which promotes conversion from XDH to XO. In turn, ROS, generated by both NADPH oxidase and XO, oxidize and inactivate tetrahydrobiopterin causing the uncoupling of eNOS due to its cofactor deficiency, thus further increasing oxidative stress. XOR inhibition restores eNOS-dependent vasodilation in aorta rings from hypercholesterolemic rabbits as well as reverses endothelial dysfunction in heavy smokers [23].
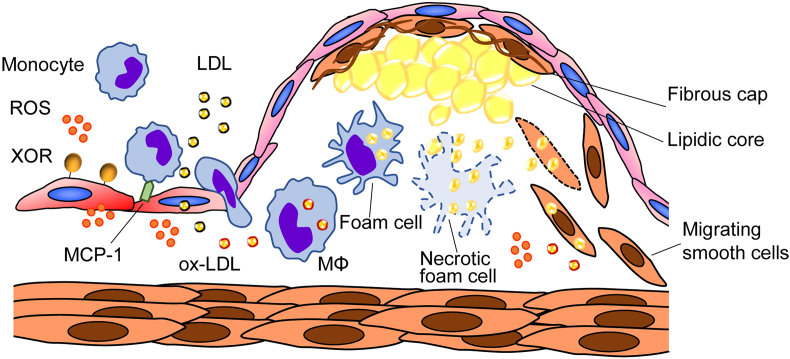
Fig. 2.
Xanthine oxidoreductase (XOR)-promoted plaque formation. Monocyte-derived macrophages turn into foam cells in the artery wall by incorporating reactive oxygen species (ROS)-oxidized low-density lipoproteins (ox-LDL) and contribute to unstable plaques formation by producing inflammatory cytokines, such as monocyte chemoattractant protein-1 (MCP-1), and matrix-degrading metalloproteases, as well as through the generation of a lipid rich necrotic core by finally undergoing death. XOR expression is upregulated in macrophages (MΦ) that infiltrate experimental atherosclerotic plaques [28]. XOR inhibition strongly reduces ROS production, and the in vitro transformation of macrophages into foam cells, and suppresses the overexpression of inflammatory cytokines, adhesion molecules and metalloproteases [27].
In murine J774.1 macrophagic cells, XOR overexpression induced an upregulation of scavenger and VLDL receptors, while decreased the expression of molecules that regulate cellular cholesterol efflux, which in turn was increased by XOR knockdown. Allopurinol strongly reduced the in vitro transformation of murine or human macrophages into foam cells after stimulation with modified LDL or VLDL. In J774.1 cells transformed into foam cells, allopurinol suppressed the overexpression of inflammatory cytokines, adhesion molecules and metalloproteases. All together the above results suggest that ROS produced by macrophage XOR contribute to the development of atherosclerotic diseases by enhancing inflammation and plaque formation [27].
In ApoE knockout mice, an established model of atherosclerosis, XOR expression was upregulated in macrophages that infiltrate atherosclerotic plaques and aortic endothelial cells, while febuxostat reduced the level of ROS in the aortic walls and mitigated the development of atherosclerotic lesions. In cultured macrophages, febuxostat suppressed the increase in XOR activity and ROS production, as well as the secretion of inflammatory cytokines, induced by cholesterol crystals, indicating both the atherogenic role of ROS and the pro-inflammatory action of XOR [28].
In vitro experiments showed that uric acid can enter endothelial cells via urate transporters and cause endothelial dysfunction by activating NADPH oxidase, which generates ROS and decreases the NO bioavailability. NO counteracts the development and progression of atherosclerosis, inducing vasodilation and inhibiting leukocyte adhesion, platelet aggregation and proliferation of vascular smooth muscle cells. Uric acid transporter inhibitors, such as probenecid, suppress endothelial dysfunction resulting from oxidative stress and inflammation. Serum uric acid level is closely associated with hypertension, chronic kidney disease and metabolic syndrome. In the clinical setting, impairment of flow-mediated vasodilation, an index of endothelial function, was greater in hyperuricemic patients. Flow-mediated vasodilation resulted also improved by treatment with XOR inhibitors. This improvement is not dependent on the uric acid-lowering effect, as also demonstrated by clinical trials with urate transporter inhibitors that were not able to improve endothelial function, although effective in lowering uricemia [29].
Uricase transgenic mice, with a low level of uric acid either in serum or intracellularly, showed an attenuated inflammatory response to cholesterol crystals injected into the peritoneal cavity. The size of atherosclerotic plaques was significantly smaller in the aortic roots of double ApoE-deficient and uricase transgenic mice fed high-fat diet than in control mice [30]. In vitro, physiological concentration of soluble uric acid may promote the secretion of interleukin-1β (IL-1β) dependent on NACHT, LRR and PYD domain-containing protein 3 (NLRP3) inflammasome from human peripheral blood mononuclear cells via activation of hypoxia inducible factor-1α (HIF-1α) and mitochondrial ROS. This leukocyte inflammatory response is suppressed by the urate-lowering therapy with benzopromarone in vivo in healthy humans [30].
In calcifying bovine aortic interstitial valve cells, endotoxin induced a significant overexpression of osteoblast-like markers, such as alkaline phosphatase, together with an increased expression of enzymes involved in the redox homeostasis, including XOR. l-Arginine, the main precursor of NO, reverts the upregulation of XOR and suppress the inflammatory activation of cardiovascular cells, thus preventing their pro-calcific differentiation. Gene expression analysis reveals that treatment with allopurinol reduced the LPS-induced overexpression of alkaline phosphatase, suggesting a potential involvement of XOR-derived ROS during valve calcification [31].
A high risk for CVD was reported in patients with chronic kidney disease, which showed increased ROS production and decreased NO availability. At least in part, these alterations were mediated by the upregulation of the renin-angiotensin system, which activates inflammation and promotes the progression of atherosclerosis [32].
Obesity and related diseases, such as diabetes and metabolic syndrome, are strongly influenced by the serum level of uric acid, which promotes the inflammasome response and the dysfunction of the adipocytes, in turn responsible for insulin resistance. Furthermore, hyperuricemia leads to vascular and renal damage, thus inducing hypertension [33]. A population-based study including 14,130 US adults founded a strong association between hyperuricemia and dyslipidemia, particularly with the serum level of total cholesterol, LDL cholesterol, triglycerides and apolipoprotein B, as well as with the ratio of triglycerides to HDL cholesterol and between apolipoprotein B and apolipoprotein A-I, while HDL cholesterol is inversely related to serum uric acid level [34]. In addition, several clinical studies showed a significant positive association between serum levels of uric acid and oxidized LDL, as well as inflammation markers, such as C reactive protein (CRP), interleukin 6 (IL‐6) and tumor necrosis factor α (TNF‐α) [35].
A comparison of subjects with or without ischemic brain events showed significantly higher XOR expression in macrophage cells of carotid atherosclerotic plaques along with significantly higher circulating uric acid levels in symptomatic than in asymptomatic patients. Furthermore, the overexpression of XOR in macrophages was associated with a low level of HDL, concurring to the formation of cholesterol crystals in the atheroma and the macrophage inflammatory response. ROS derived from XOR contributed to the oxidation of macromolecules and the activation of cell death signals that can lead to cerebrovascular events [36].
A 5-year retrospective cohort study of 6476 healthy adults from Japan showed that the development of hypercholesterolemia, as well as hypertriglyceridemia, can be predicted by the presence of an elevated baseline serum uric acid level and its increase over a 5-year period. High levels of intracellular uric acid can induce increased mitochondrial oxidative stress and promote de novo lipogenesis and triglyceride synthesis. In addition, uric acid can reduce both triglyceride accumulation and fatty acid oxidation by inhibiting AMP-kinase activity [37].
A study based on data from the Italian Society of Hypertension including 162 patients reported a U-shaped correlation between uricemia and microvascular remodeling, which was probably mediated by the activation of the mitogen-activated protein (MAP) kinase pathway and by vascular smooth muscle cell proliferation; uricemia was also associated reduced endothelial function and NO availability. These results agreed with several experimental models of asymptomatic hyperuricemia and suggest that elevated levels of serum uric acid are associated with organ damage due to oxidative stress of the vascular wall and inflammation leading to remodeling of small vessels also in humans [38].
4. Hyperuricemia, hypertension and cardiovascular diseases
A retrospective cohort study calculated that the cumulative incidences of hypertension over 5 years in a population of 3584 prehypertensive Japanese adults was 25.3%. Hyperuricemia in the normotensive population resulted an independent risk factor for developing hypertension. Among prehypertensive subjects, a level of serum uric acid higher than 7.0 mg/dl in men and 5.0 mg/dl in women further significantly increased the cumulative incidence of hypertension. This increased risk for the development of hypertension from prehypertension carried out by hyperuricemia is significantly higher in women than in men [39].
The British Regional Heart Study followed 3440 white European adult men for an average of 15 years between 1978 and 2000, evaluating the presence of cardiovascular risk factors, concluding that the most unfavorable risk profile for heart failure belongs to hypertensive subjects with hyperuricemia only when being on antihypertensive treatment. However, the results of this study suggest that older hypertensive patients should be routinely monitored for serum uric acid level [40].
A systematic review and meta-analysis based on seventeen cohort studies investigated the prognostic value of hyperuricemia in hypertensive subjects. As a result, hyperuricemia slightly increases the risk of diabetes and CVD in patients with established hypertension. In addition, a consistent relationship between hyperuricemia and an increased risk of all-cause mortality, including mortality from CVD, but not from stroke, was reported [41].
A recent study enrolling 1114 newly diagnosed hypertensive patients analyzed the interaction of hyperuricemia with insulin resistance in promoting vascular damage and demonstrates that increased arterial stiffness is associated with their synergistic action. However, a causal relationship cannot be established and other factors, such as increased ROS production and inflammation, should be considered [42].
Hyperuricemia induces arteriosclerosis and hypertension through its pro-inflammatory, pro-oxidative and vasoconstrictive actions, thus representing more than a marker of cardiometabolic diseases and contributing to their development at least in part with a causal role. Indeed, intracellular uric acid may induce oxidative stress by stimulating NADPH oxidase activity. It also induces both inflammation, by activating the production of COX2, thromboxane and chemokines, and vasoconstriction, by stimulating the renin-angiotensin system and inhibiting NO-dependent vasodilation [43].
A hypothesis has been formulated on the mechanism of uric acid-induced hypertension indicating intracellular urate as a key pathogenic factor. The increase in intracellular uric acid promotes oxidative stress by inducing MAP kinases and NADPH oxidase, as well as inflammatory responses such as the production of cytokines, endothelin and thromboxane with a result of vasoconstriction [44].
Hyperuricemia reduces the availability of endothelial NO, giving rise to endothelial dysfunction, and activates the renin-angiotensin-aldosterone system in the kidney, thus causing an increase in blood pressure. These mechanisms are fully effective in early-stage hypertension. This may explain why the results of available studies to control blood pressure with serum urate-lowering treatments appear controversial and the best results have been obtained in a young population at the onset of hypertensive disease [45].
In vitro, animal and clinical studies supported hyperuricemia as a risk factor for hypertension, kidney disease and metabolic syndrome and suggested its contribution to the development of these diseases. The greatest effect of hyperuricemia on the induction of hypertension has been seen in young people, before renal microvascular changes occurred. However, it was not established whether uric acid plays a causal role in cardiovascular and kidney disease [19]. An increasing body of data associates hyperuricemia not only with hypertension but also with metabolic syndrome, as well as with renal and CVD. Various clinically relevant evidences support the idea that hyperuricemia can be an independent risk factor for CVD. Some studies recommend lowering the serum uric acid level even below the limit previously considered to be regular [46].
A population study was conducted with a median follow-up of 11 years on 23,467 Italians to identify cut-off values of uricemia for cardiovascular outcomes. Hyperuricemia proved to be an independent risk marker for prediction of total mortality with a cut-off value > 4.7 mg/dl, after adjustment for potential confounding variables. After sex stratification, a cut-off value for fatal myocardial infarction >5.26 mg/dl, was significant for women only [47,48]. These results agreed with other large population-based prospective studies, showing a significant association between hyperuricemia and cardiovascular events, in which the suggested limit of uricemia was even lower: less than 5 mg/dl for men and 2–4 mg/dl for women [49]. However, in a meta-analysis study of 30 randomized controlled trials involving 18,585 hyperuricemic patients, although the urate lowering therapy with XOR inhibitors decreases the relative risk of major adverse cardiovascular events by 6.0%, no further improvement is observed in the subgroup with uric acid level <5 mg/dl [50].
Persistent hyperuricemia can lead to urate precipitation and crystal formation leading to gouty arthritis and urolithiasis. The presence of gout is 6-fold prevalent among patients hospitalized for cardiovascular events compared to the admitted Spanish adult population [51].
Hyperuricemia contributed to the onset and progression of atherosclerosis and hypertension and showed a significantly positive correlation with related CVD, such as atrial fibrillation, coronary artery disease and heart failure, as suggested by numerous clinical studies and meta-analyzes. A recent review analyzed both the molecular mechanisms through which high intracellular levels of uric acid induce oxidative stress, inflammation and endoplasmic reticulum stress and various clinical studies about the correlation between hyperuricemia and CVD [52]. Uric acid increased ROS production in cardiomyocytes and reduced their viability by inhibiting the signaling pathway of both the phosphatidylinositol 3-kinase (PI3K)/Akt and the nuclear factor erythroid 2-related factor 2 (NfE2), the latter inducing the expression of antioxidant proteins, thus promoting oxidative stress [53]. Also, uric acid induced inflammatory response of macrophages, vascular smooth muscle cells and endothelial cells by increasing the expression of high mobility group box chromosomal protein 1 (HMGB1) and of the receptor for advanced glycation end products (RAGE), as well as activating the NLRP3 inflammasome via inducing nuclear factor κB (NF-κB) and HIF-1α expression, which upregulate IL-1β production (Fig. 3A). Uric acid induces the secretion of IL-1β via the mammalian target of rapamycin (mTOR) signaling pathway by suppressing the phosphorylation of the AMP-activated protein kinase (AMPK) which in turn inhibits mTOR [30]. Uric acid can induce endothelial cell and cardiomyocyte apoptosis through the activation of calpain-1 and the consequent endoplasmic reticulum stress [54]. Lastly, uric acid inhibited ubiquitination and proteasomal degradation of voltage-gated potassium channel subfamily 1 number 5 (Kv1.5) proteins in HL-1 mouse atrial myocytes through overexpression of the heat shock protein 70 (Hsp70), thus increasing the channel currents leading to the induction of arrhythmias such as atrial fibrillation [55].
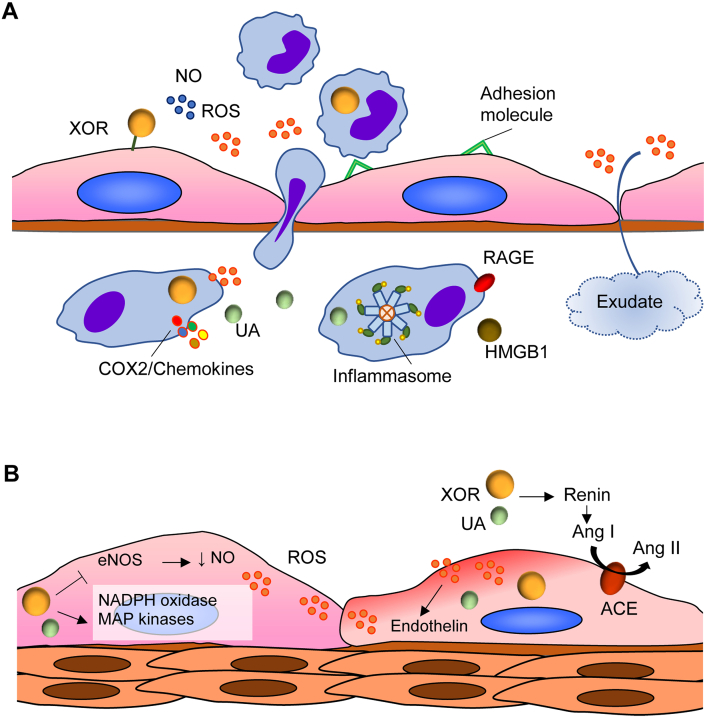
Fig. 3.
A) Xanthine oxidoreductase (XOR)-elicited inflammation. XOR-generated reactive oxygen species (ROS) and nitric oxide (NO) activate endothelial cells, which increase their permeability and express adhesion molecules for leukocytes. The cytokines released by the activated leukocytes upregulate macrophage XOR expression. This XOR contributes to the antibacterial defense during phagocytosis through its products and the consequent formation of RNS [9]. Uric acid induces inflammation by activating the production of cyclooxygenase 2 (COX2), thromboxane and chemokines [43]. Also, uric acid activates the NACHT, LRR and PYD domain-containing protein 3 (NLRP3) inflammasome and induces the expression of high mobility group box chromosomal protein 1 (HMGB1) and of the receptor for advanced glycation end products (RAGE) [52]. B) XOR-induced vasoconstriction. Pro-atherosclerotic stimuli augment ROS production by increasing the expression and activity of endothelial XOR and activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. In turn, ROS oxidize and inactivate tetrahydrobiopterin causing decoupling of endothelial nitric oxide synthase (eNOS), which increases ROS formation [23]. In addition, an increased level of uric acid inside endothelial cells activates NADPH oxidase, which generates ROS and decreases the NO bioavailability, thus causing endothelial dysfunction since NO induces vasodilation and inhibits leukocyte adhesion, platelet aggregation and the proliferation of vascular smooth muscle cells [30]. Hyperuricemia upregulates the renin-angiotensin system, thus augmenting the formation of angiotensin I (Ang I), which is converted in angiotensin II (Ang II) by endothelial angiotensin-converting enzyme (ACE). Moreover, hyperuricemia favors oxidative stress of the vascular wall and inflammation leading to remodeling of small vessels also in humans probably mediated by the activation of the mitogen-activated protein (MAP) kinase pathway and vascular smooth muscle cell proliferation [38].
5. Xanthine oxidoreductase activity and cardiovascular diseases
The activity of XOR and its products impact on the redox balance and are associated with various effects on vascular endothelium and vessel walls, which can have a significant relevance on the cardiovascular system. XOR-derived ROS and oxidative stress may cause endothelial dysfunction that promotes the development and progression of atherosclerosis. In addition, the intracellular pro-oxidant effect of uric acid in the vascular system may stimulate proliferation of the smooth muscle cells, activate the renin-angiotensin system and inhibit the synthesis of nitric oxide, thus promoting hypertension and CVD (Fig. 3B) [16].
Alterations in the post-translational modification of cardiomyocyte filaments may lead to contractile dysfunction. In spontaneously hypertensive-heart failure rats, an overproduction of XOR-derived ROS caused the hyponitrosylation of the myocyte ryanodine receptor 2 that is involved in excitation-contraction coupling. This deficient S-nitrosylation was associated to impaired contractility and Ca2+ leak from sarcoplasmic reticulum, a hallmark of cardiac dysfunction. XOR inhibition reversed these abnormality [3].
Blood pressure was significantly higher in eNOS-deficient mice than in wild-type mice and it was further increased by XOR inhibition with febuxostat in eNOS-deficient mice, but not in wild-type mice. In eNOS-deficient mice, dietary nitrate had a blood pressure lowering effect that was abolished by the administration of febuxostat, while the same dietary supplement increased the blood pressure of wild animals and XOR inhibition reversed this effect. These results indicate that both XOR nitrite reductase activity ensures NO availability in the absence of eNOS and XOR products help support blood pressure [56].
Patients with essential hypertension had the serum level of XOR activity significantly higher than dialyzed patients with chronic renal disease or control subjects [57]. Serum XOR activity was significantly increased in obese adolescents as compared to healthy weight peers and had a significantly positive association with body mass index z-score, waist circumference and the serum level of oxidized LDL, as well as a negative association with monocyte chemoattractant protein-1, adiponectin and HDL [58]. These correlations with cardiovascular risk factors suggest that XOR activity may play a role in increasing cardiovascular risk.
In a prospective study of patients with chronic kidney disease or chronic hemodialysis followed for three years, the serum level of XOR activity, but not uric acid, resulted an independent predictor of cardiovascular events. These results suggest that XOR itself plays a role in CVD related to chronic kidney disease by inducing oxidative stress [59].
Both XOR and uric acid were independently associated and independent predictors for the onset and progression of albuminuria in patients with type 2 diabetes mellitus, possibly because ROS production and induction of inflammatory cytokines damage the glomerular filtration barrier [60]. Weight loss in patients with severe obesity was associated with a decrease in circulating XOR and uric acid, but the lowering in serum uric acid level was not mediated by the reduction in XOR activity [61].
In a population-based study of cardiac patients, plasma XOR activity level had a significantly positive association with body mass index, serum level of liver enzymes and glycated hemoglobin (HbA1c) and a negative association with renal function and left ventricular hypertrophy, as well as a U-shaped association with ventricular ejection fraction and elevated serum level of type B natriuretic peptide, regardless of various confounding factors, including uricemia [62]. Similarly, the level of XOR activity in serum correlated with circulating hepatic transaminases and insulin resistance indices but not with the serum uric acid level, which also had no correlation with insulin resistance index in Japanese patients with type 2 diabetes mellitus and metabolic syndrome [63]. In fact, uricemia is more determined by renal function and the consequent level of excretion than by XOR activity, which most influences the intracellular concentration of uric acid. A U-shaped association was also observed between abnormally high or low plasma XOR activity and chronic heart failure severity and clinical outcome after adjustment for confounding risk factors in a study with 484 subjects followed for 3 years. Although the pathological mechanism is not elucidated, abnormal plasma XOR activity worsens cardiac prognosis by identifying high-risk chronic heart failure patients [64].
A cross-sectional study of 193 Japanese with lifestyle-related diseases, such as alcohol consumption, smoking, diabetes mellitus, hypertension and dyslipidemia, showed that plasma XOR activity was associated with insulin resistance as determined based on the homeostasis model assessment index, regardless of visceral adiposity and adiponectin level [65]. Among the 301 outpatients with CVD in a prospective study, those with diabetes mellitus belonged to the group with a high plasma XOR level. Diabetes mellitus was independently associated with high plasma XOR activity. In diabetic patients, body mass index independently correlated with high plasma XOR activity. Moreover, in these patients, plasma H2O2 was significantly higher than in patients without high plasma XOR activity and obesity, suggesting that the increased XOR activity is responsible for the high plasma H2O2 level [66].
In heart failure, XOR activity is upregulated as a consequence of its increased expression induced by inflammatory cytokines, as well as of the augmented substrate supply, due to hypoxia, catabolic dominance, insulin resistance, cell death and cachexia, leading to an increment in the production of both uric acid and ROS. Although uric acid is an unquestioned marker of a number of pathological heart conditions, urate-lowering therapies other than XOR inhibition fail to exert the beneficial effects that would be expected if hyperuricemia had a main pathogenic role in heart failure [67].
In a prospective study that included 257 patients with heart failure with preserved ejection fraction and a median follow-up period of 809 days, high XOR activity was suggested to be an independent risk factor for major adverse cardiovascular events, being significantly associated with them, after adjustment for confounding factors, regardless of the state of hyperuricemia. The results obtained from this study suggest that XOR inhibition can improve the clinical outcomes of these patients only in the presence of high plasma XOR activity [68].
Recently, the focus has been moved from uric acid to XOR as a true risk factor or at least as a more reliable biomarker for metabolic, renal and CVD because of the association between the serum XOR activity level and smoking, obesity, dyslipidemia, insulin resistance and liver dysfunction [69,70].
A general population-based cohort study including 1631 Japanese demonstrated that serum XOR activity level was independently associated with body mass index, diabetes mellitus, dyslipidemia and uricemia. Furthermore, subjects in the top quartile of XOR activity were associated with a high risk of CVD after adjustment for baseline characteristics. The results of this cross-sectional study suggest that high XOR activity is a marker of cardiovascular risk at least in Japanese population [71].
Myocardial fibrosis and adverse ventricular remodeling can lead to heart failure. These cardiac transformations may in part result from the endothelial-mesenchymal transition, a shift of the phenotype from the endothelial to the mesenchymal cell that is stimulated by TGF-β via SMAD-2/3/4 and the Slug signaling pathway. During the evolution of inflammation to fibrosis, XOR can contribute to this process by producing both uric acid and ROS, which in addition trigger cardiomyocyte autophagy and apoptosis. An upregulation of XOR activity is found in many of the prevalent comorbidity of heart failure such as ageing, diabetes mellitus, metabolic syndrome, hypertension and obesity, which act through the endothelium of the coronary microcirculation and induce a pro-inflammatory state. The increased XOR activity in heart failure, caused by hyperinsulinemia, hypoxia and increased substrate supply, due to cell death and the prevalence of catabolism, generates an imbalance with NOS activity, thus reducing the myocardial mechanical efficiency [72].
In a prospective study in acute heart failure, 187 patients were subdivided based on plasma XOR activity determined at admission with a cut-off value of 100 pmol/h/ml. The low XOR group had a significant worse prognosis than the high XOR group within 365 days. A decrease in XOR activity level within 14 days after admission due to heart failure treatment has a positive prognostic significance, while an insufficient reduction in XOR activity is associated with an increase in further heart failure events. These results suggest a relationship between plasma XOR level and liver/gut edema from right heart failure, which can induce hypoxia and metabolic/respiratory acidosis. In other words, the plasma XOR level reflects insufficiently compensated acute heart failure [73].
6. Urate-lowering therapies and xanthine oxidoreductase inhibition
A cohort study including 14,000 hyperuricemic Danish patients showed that allopurinol-treated subjects had significantly less cardiovascular outcome, i.e., acute myocardial infarction, stroke, cardiovascular death and all-cause mortality, than those without allopurinol treatment [74].
Over the past five years, the number of publications regarding urate-lowering therapies, including uricosuric drugs, XOR inhibitors and recombinant uricase, has been steadily growing, but this issue cannot be addressed here and should be redirected to dedicated reviews [75]. There are meta-analyzes on clinical trials including a large number of subjects and there are excellent reviews that attempt to answer the question of whether hyperuricemia is only an indicator or rather one of the causes in CVD and whether a urate-lowering therapy is recommended in asymptomatic hyperuricemia and to which categories of patients. Another problem to solve is whether XOR represents a better indicator, or even the contributing cause in CVD, rather than uric acid, because of XOR induction of oxidative stress, inflammation and endothelial dysfunction. As a consequence, the question is if the most promising therapeutic strategy to choose for CVD is to inhibit XOR, either by allopurinol or febuxostat, or not. No definitive answers are available at the moment. While antioxidant therapies give disappointing results, competitive XOR inhibitor treatments improve heart failure symptoms only in hyperuricemic patients [76]. Accordingly, uric acid lowering-drugs are efficient in reducing blood pressure only in populations with both hypertension and hyperuricemia. However, population-based genetic association analyses indicate that hypertension is associated with XOR genetic polymorphisms, while no association with major urate transporters is present. This finding suggests that a high intracellular concentration of uric acid produced by XOR activity is crucial in the development and progression of hypertension regardless of hyperuricemia [45].
Patients with gout treated with XOR inhibitors showed significantly improved flow-mediated dilation of the brachial artery after reaching target serum uric acid. The improvement in the endothelial-dependent arterial response was present only in patients without established cardiovascular risk factors and comorbidities, such as hypertension and hyperlipidemia. These results suggest that the reason why urate-lowering therapies give discordant results could be the low level of focus on the choice of the target population [77].
The level of uricemia shows a strong direct correlation with hypertension, atherosclerotic CVD, heart failure and atrial fibrillation. Furthermore, various pathogenetic mechanisms, such as endothelial and vascular dysfunction mediated by oxidative stress and the reduced availability of NO, can be triggered through the inflammatory response caused by soluble or crystallized uric acid. Recent European guidelines on arterial hypertension include uric acid as a factor influencing cardiovascular risk, suggesting a possible causal link between hyperuricemia and CVD. However, careful examination of various meta-analysis studies on urate-lowering therapy with a large number of subjects does not provide definitive results [78]. Accordingly, although hyperuricemic patients have a higher risk and a worse prognosis of heart failure and XOR activity is increased in heart failure, the available evidence does not allow to distinguish whether uric acid and/or XOR are causal risk factors or just CVD markers [79].
In randomized controlled trials, whilst XOR inhibition moderately improved the condition of chronic heart failure patients, it increased the risk of cardiovascular death, particularly when the inhibition was obtained with the more selective and potent inhibitor febuxostat [80]. This finding could be explained by considering that both competitive and non-competitive XOR inhibitors block purine oxidation along with XOR nitrite-reduction activity, thus aggravating heart conditions by reducing NO availability and increasing oxidative stress. Furthermore, a more intense lowering of urates may be associated with higher mortality due to the loss of the antioxidant capacity of the plasma that is mostly dependent on uric acid [81].
7. Conclusions
The role of XOR activity and products in the onset and evolution of CVD has been intriguing basic researchers as well as metabolic, renal and cardiac clinicians for a long time and still awaits definitive answers. The reason for this incessant attention lies in the biological effects of the main products of XOR activity: uric acid, ROS and NO. Uric acid has an antioxidant function in serum, while intracellularly it has a pro-oxidant effect. It increases the expression of inflammatory cytokines and the renin-angiotensin pathway, so hyperuricemia is associated with established cardiovascular risk factors such as hypertension, dyslipidemia, type 2 diabetes, chronic kidney disease and metabolic syndrome. ROS can play a signaling role in various pathways including immune and inflammatory responses or they can be cytotoxic and lead to oxidative stress, depending on their concentration. NO is a neurotransmitter and inhibits the contraction of vascular smooth muscle thus having a vasodilating effect. It also participates in inflammation by generating cytotoxic peroxynitrite together with ROS and contributes to the regulation of cardiac contractility.
Much research has focused on hyperuricemia, but the results have not been satisfactory. An explanation may be that this parameter is influenced by several factors: genetic basis, diet, drug intake, urate transporters in the intestine and mainly by the renal function that modulates its excretion. The correlation of hyperuricemia with most CVD risk factors makes it difficult to find an independent and meaningful relationship with CVD after eliminating all possible confounding factors, such as age, gender, kidney function, as well as diabetes and dyslipidemia. There is a suspicion that significance for hyperuricemia as a causal risk factor for CVD is not achieved due to over-adjustment [82,83].
Recently the focus has shifted to the intracellular concentration of uric acid and its production by XOR, which also generates ROS. Therefore, rather than focusing on urate-lowering therapies, growing interest points to XOR inhibition, which not only helps control patients' uricemia, but also reduces the level of ROS production and the consequent risk of oxidative stress and chronic inflammation, the role of which in the onset and aggravation of CVD are well known. For this reason, plasma XOR activity has been suggested as a new biomarker of metabolic disorders. Furthermore, XOR may deserve the role of a risk factor, while uric acid may simply be a risk marker for CVD [69].
On the basis of the data collected so far, the most advisable urate-lowering therapy seems to be that with XOR competitive inhibitors, but in the first place, it remains to be clarified what is the optimal level of uricemia. Second, there are few cases in which XOR inhibitor therapy has been shown to significantly improve the prognosis of patients at risk of CVD, although higher uric acid levels are often associated with worse outcomes. This suggests continuing to search for the precise clinical conditions in which limiting the production of uric acid and ROS can be helpful. Unfortunately, the inhibition of XOR also involves the reduced production of NO, which in certain circumstances alters the delicate balance that allows the correct regulation of the vascular tone and of the heart pump. In conclusion, we are still far from being able to suggest pharmacological control of asymptomatic hyperuricemia, while surveillance of the uricemia parameter is highly recommended in all patients with CVD.
Contributor Information
Letizia Polito, Email: letizia.polito@unibo.it.
Massimo Bortolotti, Email: massimo.bortolotti2@unibo.it.
Maria Giulia Battelli, Email: mariagiulia.battelli@unibo.it.
Andrea Bolognesi, Email: andrea.bolognesi@unibo.it.
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., Bonny A., Brauer M., Brodmann M., Cahill T.J., Carapetis J., Catapano A.L., Chugh S.S., Cooper L.T., Coresh J., Criqui M., DeCleene N., Eagle K.A., Emmons-Bell S., Feigin V.L., Fernández-Solà J., Fowkes G., Gakidou E., Grundy S.M., He F.J., Howard G., Hu F., Inker L., Karthikeyan G., Kassebaum N., Koroshetz W., Lavie C., Lloyd-Jones D., Lu H.S., Mirijello A., Temesgen A.M., Mokdad A., Moran A.E., Muntner P., Narula J., Neal B., Ntsekhe M., Moraes de Oliveira G., Otto C., Owolabi M., Pratt M., Rajagopalan S., Reitsma M., Ribeiro A.L.P., Rigotti N., Rodgers A., Sable C., Shakil S., Sliwa-Hahnle K., Stark B., Sundström J., Timpel P., Tleyjeh I.M., Valgimigli M., Vos T., Whelton P.K., Yacoub M., Zuhlke L., Murray C., Fuster V., GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. Erratum in: J. Am. Coll. Cardiol. 2021, 77, 1958-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouch S.H., Botha-Le Roux S., Delles C., Graham L.A., Schutte A.E. Inflammation and hypertension development: a longitudinal analysis of the African-PREDICT study. Int. J. Cardiol. Hypertens. 2020;7:100067. doi: 10.1016/j.ijchy.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy J., Galano J.M., Durand T., Le Guennec J.Y., Lee J.C. Physiological role of reactive oxygen species as promoters of natural defenses. Faseb. J. 2017;31:3729–3745. doi: 10.1096/fj.201700170R. [DOI] [PubMed] [Google Scholar]
- 4.Daiber A., Andreadou I., Oelze M., Davidson S.M., Hausenloy D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021;163:325–343. doi: 10.1016/j.freeradbiomed.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Terao M., Garattini E., Romão M.J., Leimkühler S. Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes. J. Biol. Chem. 2020;295:5377–5389. doi: 10.1074/jbc.REV119.007741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelli M.G., Bolognesi A., Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta. 2014;1842:1502–1517. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Lin J., Xu P., LaVallee P., Hoidal J.R. Identification of proteins binding to E-Box/Ku86 sites and function of the tumor suppressor SAFB1 in transcriptional regulation of the human xanthine oxidoreductase gene. J. Biol. Chem. 2008;283:29681–29689. doi: 10.1074/jbc.M802076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5:546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortolotti M., Polito L., Battelli M.G., Bolognesi A. Xanthine oxidoreductase: one enzyme for multiple physiological tasks. Redox Biol. 2021;41:101882. doi: 10.1016/j.redox.2021.101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino T., Okamoto K., Kawaguchi Y., Matsumura T., Eger B.T., Pai E.F., Nishino T. The C-terminal peptide plays a role in the formation of an intermediate form during the transition between xanthine dehydrogenase and xanthine oxidase. FEBS J. 2015;282:3075–3090. doi: 10.1111/febs.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in drug metabolism: beyond a role as a detoxifying enzyme. Curr. Med. Chem. 2016;23:4027–4036. doi: 10.2174/0929867323666160725091915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol. 2013;1:353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battelli M.G., Bortolotti M., Bolognesi A., Polito L. Pro-aging effects of xanthine oxidoreductase products. Antioxidants (Basel) 2020;9:839. doi: 10.3390/antiox9090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwabara M. Hyperuricemia, Cardiovascular disease, and hypertension. Pulse (Basel) 2016;3:242–252. doi: 10.1159/000443769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler R.G., Camandola S., Feldman N.H., Yoon J.S., Haran J.B., Arguelles S., Mattson M.P. Uric acid enhances longevity and endurance and protects the brain against ischemia. Neurobiol. Aging. 2019;75:159–168. doi: 10.1016/j.neurobiolaging.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battelli M.G., Bortolotti M., Polito L., Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2018;1864:2557–2565. doi: 10.1016/j.bbadis.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Battelli M.G., Bortolotti M., Polito L., Bolognesi A. Metabolic syndrome and cancer risk: the role of xanthine oxidoreductase. Redox Biol. 2019;21:101070. doi: 10.1016/j.redox.2018.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R.J., Sánchez-Lozada L.G., Andrews P., Lanaspa M.A. Perspective: a historical and scientific perspective of sugar and its relation with obesity and diabetes. Adv. Nutr. 2017;8:412–422. doi: 10.3945/an.116.014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson R.J., Bakris G.L., Borghi C., Chonchol M.B., Feldman D., Lanaspa M.A., Merriman T.R., Moe O.W., Mount D.B., Sanchez Lozada L.G., Stahl E., Weiner D.E., Chertow G.M. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National kidney Foundation. Am. J. Kidney Dis. 2018;71:851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmon D.B., Mandler W.K., Sipula I.J., Dedousis N., Lewis S.E., Eckels J.T., Du J., Wang Y., Huckestein B.R., Pagano P.J., Cifuentes-Pagano E., Homanics G.E., Van't Erve T.J., Stefanovic-Racic M., Jurczak M.J., O'Doherty R.M., Kelley E.E. Hepatocyte-specific ablation or whole-body inhibition of xanthine oxidoreductase in mice corrects obesity-induced systemic hyperuricemia without improving metabolic abnormalities. Diabetes. 2019;68:1221–1229. doi: 10.2337/db18-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Shehri S.S., Duley J.A., Bansal N. Xanthine oxidase-lactoperoxidase system and innate immunity: biochemical actions and physiological roles. Redox Biol. 2020;34:101524. doi: 10.1016/j.redox.2020.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid. Med. Cell Longev. 2016;2016:3527579. doi: 10.1155/2016/3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 24.Battelli M.G., Polito L., Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis. 2014;237:562–567. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vasc. Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., Daiber A. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushiyama A., Nakatsu Y., Matsunaga Y., Yamamotoya T., Mori K., Ueda K., Inoue Y., Sakoda H., Fujishiro M., Ono H., Asano T. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic syndrome components such as atherosclerosis and nonalcoholic steatohepatitis. Mediat. Inflamm. 2016;2016:8603164. doi: 10.1155/2016/8603164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomura J., Busso N., Ives A., Matsui C., Tsujimoto S., Shirakura T., Tamura M., Kobayashi T., So A., Yamanaka Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014;4:4554. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruhashi T., Hisatome I., Kihara Y., Higashi Y. Hyperuricemia and endothelial function: from molecular background to clinical perspectives. Atherosclerosis. 2018;278:226–231. doi: 10.1016/j.atherosclerosis.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y., Yanagida T., Onda A., Tsukui D., Hosoyamada M., Kono H. Soluble uric acid promotes atherosclerosis via AMPK (AMP-Activated protein kinase)-mediated inflammation. Arterioscler. Thromb. Vasc. Biol. 2020;40:570–582. doi: 10.1161/ATVBAHA.119.313224. [DOI] [PubMed] [Google Scholar]
- 31.Rattazzi M., Donato M., Bertacco E., Millioni R., Franchin C., Mortarino C., Faggin E., Nardin C., Scarpa R., Cinetto F., Agostini C., Ferri N., Pauletto P., Arrigoni G. l-Arginine prevents inflammatory and pro-calcific differentiation of interstitial aortic valve cells. Atherosclerosis. 2020;298:27–35. doi: 10.1016/j.atherosclerosis.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Duni A., Liakopoulos V., Rapsomanikis K.P., Dounousi E. Chronic kidney disease and disproportionally increased cardiovascular damage: does oxidative stress explain the burden? Oxid. Med. Cell Longev. 2017;2017:9036450. doi: 10.1155/2017/9036450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeMarco V.G., Aroor A.R., Sowers J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng T.C., Wang C.C., Kao T.W., Chan J.Y., Yang Y.H., Chang Y.W., Chen W.L. Relationship between hyperuricemia and lipid profiles in US adults. BioMed Res. Int. 2015;2015:127596. doi: 10.1155/2015/127596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayachandran M., Qu S. Harnessing hyperuricemia to atherosclerosis and understanding its mechanistic dependence. Med. Res. Rev. 2021;41:616–629. doi: 10.1002/med.21742. [DOI] [PubMed] [Google Scholar]
- 36.Ganji M., Nardi V., Prasad M., Jordan K.L., Bois M.C., Franchi F., Zhu X.Y., Tang H., Young M.D., Lerman L.O., Lerman A. Carotid plaques from symptomatic patients are characterized by local increase in xanthine oxidase expression. Stroke. 2021;52:2792–2801. doi: 10.1161/STROKEAHA.120.032964. [DOI] [PubMed] [Google Scholar]
- 37.Kuwabara M., Borghi C., Cicero A.F.G., Hisatome I., Niwa K., Ohno M., Johnson R.J., Lanaspa M.A. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int. J. Cardiol. 2018;261:183–188. doi: 10.1016/j.ijcard.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Masi S., Georgiopoulos G., Alexopoulos G., Pateras K., Rosada J., Seravalle G., De Ciuceis C., Taddei S., Borghi C., Grassi G., Rizzoni D., Virdis A. The study groups on the uric acid right for heArt health urrah, micro-and macro-circulation of the Italian society of hypertension siia. The complex relationship between serum uric acid, endothelial function and small vessel remodeling in humans. J. Clin. Med. 2020;9:2027. doi: 10.3390/jcm9072027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuwabara M., Hisatome I., Niwa K., Hara S., Roncal-Jimenez C.A., Bjornstad P., Nakagawa T., Andres-Hernando A., Sato Y., Jensen T., Garcia G., Rodriguez-Iturbe B., Ohno M., Lanaspa M.A., Johnson R.J. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. 2018;71:78–86. doi: 10.1161/HYPERTENSIONAHA.117.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wannamethee S.G., Papacosta O., Lennon L., Whincup P.H. Serum uric acid as a potential marker for heart failure risk in men on antihypertensive treatment: the British Regional Heart Study. Int. J. Cardiol. 2018;252:187–192. doi: 10.1016/j.ijcard.2017.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin T., Zhou X., Wang J., Wu X., Li Y., Wang L., Huang H., Li J. Hyperuricemia and the prognosis of hypertensive patients: a systematic review and meta-analysis. J. Clin. Hypertens. 2016;18:1268–1278. doi: 10.1111/jch.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassano V., Crescibene D., Hribal M.L., Pelaia C., Armentaro G., Magurno M., Toscani A., Miceli S., Andreozzi F., Maio R., Perticone M., Sesti G., Perticone F., Sciacqua A. Uric acid and vascular damage in essential hypertension: role of insulin resistance. Nutrients. 2020;12:2509. doi: 10.3390/nu12092509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borghi C., Agabiti-Rosei E., Johnson R.J., Kielstein J.T., Lurbe E., Mancia G., Redon J., Stack A.G., Tsioufis K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020;80:1–11. doi: 10.1016/j.ejim.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Lozada L.G., Rodriguez-Iturbe B., Kelley E.E., Nakagawa T., Madero M., Feig D.I., Borghi C., Piani F., Cara-Fuentes G., Bjornstad P., Lanaspa M.A., Johnson R.J. Uric acid and hypertension: an update with recommendations. Am. J. Hypertens. 2020;33:583–594. doi: 10.1093/ajh/hpaa044. Erratum in: Am. J. Hypertens. 2020, 33, 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piani F., Cicero A.F.G., Borghi C. Uric acid and hypertension: prognostic role and guide for treatment. J. Clin. Med. 2021;10:448. doi: 10.3390/jcm10030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borghi C., Rosei E.A., Bardin T., Dawson J., Dominiczak A., Kielstein J.T., Manolis A.J., Perez-Ruiz F., Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015;33:1729–1741. doi: 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 47.Casiglia E., Tikhonoff V., Virdis A., Masi S., Barbagallo C.M., Bombelli M., Bruno B., Cicero A.F.G., Cirillo M., Cirillo P., Desideri G., D'Elia L., Ferri C., Galletti F., Gesualdo L., Giannattasio C., Iaccarino G., Lippa L., Mallamaci F., Maloberti A., Mazza A., Muiesan M.L., Nazzaro P., Palatini P., Parati G., Pontremoli R., Quarti-Trevano F., Rattazzi M., Rivasi G., Salvetti M., Tocci G., Ungar A., Verdecchia P., Viazzi F., Volpe M., Grassi G., Borghi C. Working group on uric acid and cardiovascular risk of the Italian society of hypertension (SIIA). Serum uric acid and fatal myocardial infarction: detection of prognostic cut-off values: the URRAH (Uric acid right for heart health) study. J. Hypertens. 2020;38:412–419. doi: 10.1097/HJH.0000000000002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maloberti A., Giannattasio C., Bombelli M., Desideri G., Cicero A.F.G., Muiesan M.L., Rosei E.A., Salvetti M., Ungar A., Rivasi G., Pontremoli R., Viazzi F., Facchetti R., Ferri C., Bernardino B., Galletti F., D'Elia L., Palatini P., Casiglia E., Tikhonoff V., Barbagallo C.M., Verdecchia P., Masi S., Mallamaci F., Cirillo M., Rattazzi M., Pauletto P., Cirillo P., Gesualdo L., Mazza A., Volpe M., Tocci G., Iaccarino G., Nazzaro P., Lippa L., Parati G., Dell'Oro R., Quarti-Trevano F., Grassi G., Virdis A., Borghi C. Working group on uric acid and cardiovascular risk of the Italian society of hypertension (SIIA). Hyperuricemia and risk of cardiovascular outcomes: the experience of the URRAH (uric acid right for heart health) project. High Blood Pres. Cardiovasc. Prev. 2020;27:121–128. doi: 10.1007/s40292-020-00368-z. [DOI] [PubMed] [Google Scholar]
- 49.Kuwabara M., Hisatome I., Niwa K., Bjornstad P., Roncal-Jimenez C.A., Andres-Hernando A., Kanbay M., Johnson R.J., Lanaspa M.A. The optimal range of serum uric acid for cardiometabolic diseases: a 5-year Japanese cohort study. J. Clin. Med. 2020;9:942. doi: 10.3390/jcm9040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ying H., Yuan H., Tang X., Guo W., Jiang R., Jiang C. Impact of serum uric acid lowering and contemporary uric acid-lowering therapies on cardiovascular outcomes: a systematic review and meta-analysis. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.641062. Erratum in: Front. Cardiovasc. Med. 2021, 8, 723626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calabuig I., Gómez-Garberí M., Andrés M. Gout is prevalent but under-registered among patients with cardiovascular events: a field study. Front. Med. 2020;7:560. doi: 10.3389/fmed.2020.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu W., Cheng J.D. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front. Pharmacol. 2020;11:582680. doi: 10.3389/fphar.2020.582680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun X., Jiao H., Zhao J., Wang X., Lin H. Unexpected effect of urate on hydrogen peroxide-induced oxidative damage in embryonic chicken cardiac cells. Free Radic. Res. 2017;51:693–707. doi: 10.1080/10715762.2017.1362106. [DOI] [PubMed] [Google Scholar]
- 54.Yan M., Chen K., He L., Li S., Huang D., Li J. Uric acid induces cardiomyocyte apoptosis via activation of calpain-1 and endoplasmic reticulum stress. Cell. Physiol. Biochem. 2018;45:2122–2135. doi: 10.1159/000488048. [DOI] [PubMed] [Google Scholar]
- 55.Taufiq F., Maharani N., Li P., Kurata Y., Ikeda N., Kuwabara M., Otani N., Miake J., Hasegawa A., Tsuneto M., Shirayoshi Y., Ninomiya H., Saitoh T., Nakai A., Yamamoto K., Hisatome I. Uric acid-induced enhancements of Kv1.5 protein expression and channel activity via the Akt-HSF1-Hsp70 pathway in HL-1 atrial myocytes. Circ. J. 2019;83:718–726. doi: 10.1253/circj.CJ-18-1088. [DOI] [PubMed] [Google Scholar]
- 56.Peleli M., Zollbrecht C., Montenegro M.F., Hezel M., Zhong J., Persson E.G., Holmdahl R., Weitzberg E., Lundberg J.O., Carlström M. Enhanced XOR activity in eNOS-deficient mice: effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic. Biol. Med. 2016;99:472–484. doi: 10.1016/j.freeradbiomed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Boban M., Kocic G., Radenkovic S., Pavlovic R., Cvetkovic T., Deljanin-Ilic M., Ilic S., Bobana M.D., Djindjic B., Stojanovic D., Sokolovic D., Jevtovic-Stoimenov T. Circulating purine compounds, uric acid, and xanthine oxidase/dehydrogenase relationship in essential hypertension and end stage renal disease. Ren. Fail. 2014;36:613–618. doi: 10.3109/0886022X.2014.882240. [DOI] [PubMed] [Google Scholar]
- 58.Tam H.K., Kelly A.S., Metzig A.M., Steinberger J., Johnson L.A. Xanthine oxidase and cardiovascular risk in obese children. Child. Obes. 2014;10:175–180. doi: 10.1089/chi.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gondouin B., Jourde-Chiche N., Sallee M., Dou L., Cerini C., Loundou A., Morange S., Berland Y., Burtey S., Brunet P., Guieu R., Dussol B. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron. 2015;131:167–174. doi: 10.1159/000441091. [DOI] [PubMed] [Google Scholar]
- 60.Klisic A., Kocic G., Kavaric N., Jovanovic M., Stanisic V., Ninic A. Xanthine oxidase and uric acid as independent predictors of albuminuria in patients with diabetes mellitus type 2. Clin. Exp. Med. 2018;18:283–290. doi: 10.1007/s10238-017-0483-0. [DOI] [PubMed] [Google Scholar]
- 61.Richette P., Poitou C., Manivet P., Denis J., Bouillot J.L., Clément K., Oppert J.M., Bardin T. Weight loss, xanthine oxidase, and serum urate levels: a prospective longitudinal study of obese patients. Arthritis Care Res. 2016;68:1036–1042. doi: 10.1002/acr.22798. [DOI] [PubMed] [Google Scholar]
- 62.Fujimura Y., Yamauchi Y., Murase T., Nakamura T., Fujita S.I., Fujisaka T., Ito T., Sohmiya K., Hoshiga M., Ishizaka N. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sunagawa S., Shirakura T., Hokama N., Kozuka C., Yonamine M., Namba T., Morishima S., Nakachi S., Nishi Y., Ikema T., Okamoto S., Matsui C., Hase N., Tamura M., Shimabukuro M., Masuzaki H. Activity of xanthine oxidase in plasma correlates with indices of insulin resistance and liver dysfunction in patients with type 2 diabetes mellitus and metabolic syndrome: a pilot exploratory study. J. Diabet. Investig. 2019;10:94–103. doi: 10.1111/jdi.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otaki Y., Watanabe T., Kinoshita D., Yokoyama M., Takahashi T., Toshima T., Sugai T., Murase T., Nakamura T., Nishiyama S., Takahashi H., Arimoto T., Shishido T., Miyamoto T., Kubota I. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol. 2017;228:151–157. doi: 10.1016/j.ijcard.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 65.Kurajoh M., Fukumoto S., Murase T., Nakamura T., Ishihara T., Go H., Yamamoto K., Nakatani S., Tsuda A., Morioka T., Mori K., Imanishi Y., Inaba M., Emoto M. Insulin resistance associated with plasma xanthine oxidoreductase activity independent of visceral adiposity and adiponectin level: MedCity21 health examination registry. Internet J. Endocrinol. 2019;2019:1762161. doi: 10.1155/2019/1762161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsushita M., Shirakabe A., Okazaki H., Shibata Y., Goda H., Shigihara S., Asano K., Tani K., Kiuchi K., Murase T., Nakamura T., Takayasu T., Asano M., Okajima F., Kobayashi N., Hata N., Asai K., Shimizu W. Plasma xanthine oxidoreductase (XOR) activity in cardiovascular disease outpatients. Circ. Rep. 2020;2:104–112. doi: 10.1253/circrep.CR-19-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doehner W., Jankowska E.A., Springer J., Lainscak M., Anker S.D. Uric acid and xanthine oxidase in heart failure - emerging data and therapeutic implications. Int. J. Cardiol. 2016;213:15–19. doi: 10.1016/j.ijcard.2015.08.089. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe K., Watanabe T., Otaki Y., Shishido T., Murase T., Nakamura T., Kato S., Tamura H., Nishiyama S., Takahashi H., Arimoto T., Watanabe M. Impact of plasma xanthine oxidoreductase activity in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2020;7:1735–1743. doi: 10.1002/ehf2.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furuhashi M., Koyama M., Higashiura Y., Murase T., Nakamura T., Matsumoto M., Sakai A., Ohnishi H., Tanaka M., Saitoh S., Moniwa N., Shimamoto K., Miura T. Differential regulation of hypoxanthine and xanthine by obesity in a general population. J. Diabet. Investig. 2020;11:878–887. doi: 10.1111/jdi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okuyama T., Shirakawa J., Nakamura T., Murase T., Miyashita D., Inoue R., Kyohara M., Togashi Y., Terauchi Y. Association of the plasma xanthine oxidoreductase activity with the metabolic parameters and vascular complications in patients with type 2 diabetes. Sci. Rep. 2021;11:3768. doi: 10.1038/s41598-021-83234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kotozaki Y., Satoh M., Tanno K., Ohmomo H., Otomo R., Tanaka F., Nasu T., Taguchi S., Kikuchi H., Kobayashi T., Shimizu A., Sakata K., Hitomi J., Sobue K., Sasaki M. Plasma xanthine oxidoreductase activity is associated with a high risk of cardiovascular disease in a general Japanese population. Int. J. Environ. Res. Publ. Health. 2021;18:1894. doi: 10.3390/ijerph18041894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumrić M., Borovac J.A., Kurir T.T., Božić J. Clinical implications of uric acid in heart failure: a comprehensive review. Life (Basel) 2021;11:53. doi: 10.3390/life11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okazaki H., Shirakabe A., Matsushita M., Shibata Y., Sawatani T., Uchiyama S., Tani K., Murase T., Nakamura T., Takayasu T., Asano M., Kobayashi N., Hata N., Asai K., Shimizu W. Plasma xanthine oxidoreductase activity in patients with decompensated acute heart failure requiring intensive care. ESC Heart Fail. 2019;6:336–343. doi: 10.1002/ehf2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Søltoft Larsen K., Pottegård A., Lindegaard H.M., Hallas J. Impact of urate level on cardiovascular risk in allopurinol treated patients. A nested case-control study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cicero A.F.G., Fogacci F., Kuwabara M., Borghi C. Therapeutic strategies for the treatment of chronic hyperuricemia: an evidence-based update. Medicina (Kaunas) 2021;57:58. doi: 10.3390/medicina57010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur. J. Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toprover M., Shah B., Oh C., Igel T.F., Romero A.G., Pike V.C., Curovic F., Bang D., Lazaro D., Krasnokutsky S., Katz S.D., Pillinger M.H. Initiating guideline-concordant gout treatment improves arterial endothelial function and reduces intercritical inflammation: a prospective observational study. Arthritis Res. Ther. 2020;22:169. doi: 10.1186/s13075-020-02260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cortese F., Giordano P., Scicchitano P., Faienza M.F., De Pergola G., Calculli G., Meliota G., Ciccone M.M. Uric acid: from a biological advantage to a potential danger. A focus on cardiovascular effects. Vasc. Pharmacol. 2019;120:106565. doi: 10.1016/j.vph.2019.106565. [DOI] [PubMed] [Google Scholar]
- 79.Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J. Card. Fail. 2020;26:977–984. doi: 10.1016/j.cardfail.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 80.White W.B., Saag K.G., Becker M.A., Borer J.S., Gorelick P.B., Whelton A., Hunt B., Castillo M., Gunawardhana L., Investigators C.A.R.E.S. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 2018;378:1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Gomez M.V., Bartsch L.A., Castillo-Rodriguez E., Fernandez-Prado R., Kanbay M., Ortiz A. Potential dangers of serum urate-lowering therapy. Am. J. Med. 2019;132:457–467. doi: 10.1016/j.amjmed.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Fenech G., Rajzbaum G., Mazighi M., Blacher J. Serum uric acid and cardiovascular risk: state of the art and perspectives. Joint Bone Spine. 2014;81:392–397. doi: 10.1016/j.jbspin.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Ndrepepa G. Uric acid and cardiovascular disease. Clin. Chim. Acta. 2018;484:150–163. doi: 10.1016/j.cca.2018.05.046. [DOI] [PubMed] [Google Scholar]