Abstract
Background
Auditory hallucinations (AH) are typically associated with schizophrenia (SZ), but they are also prevalent in bipolar disorder (BD). Despite the large body of research on the neural correlates of AH in SZ, the pathophysiology underlying AH remains unclear. Few studies have examined the neural substrates associated with propensity for AH in BD. Investigating AH across the psychosis spectrum has the potential to inform about the neural signature associated with the trait of AH, irrespective of psychiatric diagnosis.
Methods
We compared resting state functional magnetic resonance imaging data in psychosis patients with (n = 90 AH; 68 SZ, 22 BD) and without (n = 55 NAH; 16 SZ, 39 BD) lifetime AH. We performed region of interest (ROI)-to-ROI functional connectivity (FC) analysis using 91 cortical, 15 subcortical, and 26 cerebellar atlas-defined regions. The primary aim was to identify FC differences between patients with and without lifetime AH. We secondarily examined differences between AH and NAH within each diagnosis.
Results
Compared to the NAH group, patients with AH showed higher FC between cerebellum and frontal (left precentral gyrus), temporal [right middle temporal gyrus (MTG), left inferior temporal gyrus (ITG), left temporal fusiform gyrus)], parietal (bilateral superior parietal lobules), and subcortical (left accumbens, left palldium) brain areas. AH also showed lower FC between temporal lobe regions (between right ITG and right MTG and bilateral superior temporal gyri) relative to NAH.
Conclusions
Our findings suggest that dysconnectivity involving the cerebellum and temporal lobe regions may be common neurofunctional elements associated with AH propensity across the psychosis spectrum. We also found dysconnectivity patterns that were unique to lifetime AH within SZ or bipolar psychosis, suggesting both common and distinct mechanisms underlying AH pathophysiology in these disorders.
Keywords: AH, Auditory hallucinations; NAH, No lifetime history of auditory hallucinations; SZ, Schizophrenia; BD, Bipolar disorder; HC, Healthy control; rsfMRI, Resting state functional magnetic resonance imaging; FC, Functional connectivity; ROI, Region of interest
Keywords: Auditory hallucinations, Schizophrenia, Bipolar disorder, Resting state functional magnetic resonance imaging, Functional connectivity, Cerebellum
1. Introduction
Auditory hallucinations (AH) are often intrusive, distressing, and associated with increased suicide risk (Dugre et al., 2018, Falloon and Talbot, 1981, Hor and Taylor, 2010) and poor long-term recovery among patients with psychosis (Goghari et al., 2013, Goghari and Harrow, 2016). They can also persist in spite of treatment (Shergill et al., 1998, Agid et al., 2011). Given the potentially debilitating nature of AH, better understanding of the underlying pathophysiology is necessary to inform the development of more targeted and effective treatments.
Although AH are typically associated with schizophrenia (SZ), they are not disease specific and can occur in a range of neuropsychiatric disorders (Pierre, 2010, Waters et al., 2012). In bipolar disorder (BD), AH is present in approximately 34% (Shinn et al., 2012) of BD patients, though different rates have been reported (Baethge et al., 2005, Black and Nasrallah, 1989, Braunig et al., 2009, Carlson and Goodwin, 1973, Chaturvedi and Sinha, 1990, Goghari and Harrow, 2016, Hammersley et al., 2003, Keck et al., 2003, Kumari et al., 2013, Pini et al., 2004, Shinn et al., 2012, Taylor and Abrams, 1973, Taylor and Abrams, 1975, Tillman et al., 2008). There are other significant clinical, neurobiological, and genetic similarities between the two disorders Argyelan et al., 2014, International Schizophrenia Consortium et al., 2009, Lichtenstein et al., 2009, Pini et al., 2004, Skudlarski et al., 2013, Yamada et al., 2020. However, it remains unclear the degree to which the mechanisms underlying AH in SZ and BD are shared or distinct.
The neuroimaging literature on AH in SZ serves as a rational starting point for investigating the pathophysiology of AH across diagnoses. Early studies of AH in SZ examined brain abnormalities during the experience of AH (i.e., symptom capture studies) (e.g., Dierks et al., 1999, Silbersweig et al., 1995), underlying state AH. However, neurobiological trait factors associated with AH—for example, alterations in brain connectivity (Bullmore et al., 1997, Feinberg, 1982), whether due to abnormal neuronal migration, pruning, and/or activity-dependent changes, that set the individual up for impaired perceptual and other information processing, and greater propensity for AH—are also important. Identifying neurobiological trait features associated with clinically significant lifetime AH history may have greater relevance for treatments better aimed at prevention and early intervention.
The dysconnectivity hypothesis of SZ, initially proposed by Wernicke in 1894 (Pillmann, 2007, Wernicke, 1906) and Bleuler in 1911 (Bleuler, 1911), suggests that a “dysconnected brain” – or one with abnormal functional integration – may increase proneness or vulnerability to symptoms of psychosis, including AH. Contemporary models propose that AH result from: failure to inhibit an overactive auditory cortex (Hugdahl, 2009), failure to suppress unstable memory representations that become intrusive and unintentionally activated (Waters et al., 2006), misattribution of inner speech as originating externally due to impaired corollary discharge (Feinberg, 1978, Ford and Mathalon, 2005), increased synchrony between bilateral auditory cortical areas (Steinmann et al., 2014), abnormal interactions between auditory cortex and default mode network (Northoff and Qin, 2011), and/or impaired coordinating (Schmahmann, 1991, Schmahmann, 1998, Andreasen et al., 1996, Andreasen et al., 1999, Andreasen, 1999) and predictive (Pinheiro et al., 2020) functions of the cerebellum, among other mechanisms [see reviews by (Allen et al., 2008) and (Ćurčić-Blake et al., 2017)]. Many of these models can be viewed in the context of dysconnectivity, i.e., altered connections between frontal control and auditory cortical areas, between frontal control areas and the hippocampus, between brain areas that generate speech (e.g., Broca’s) and areas that perceive or comprehend it (e.g., auditory cortex and Wernicke’s area), between auditory cortices in bilateral hemispheres, between auditory cortex and nodes of the default mode network such as medial prefrontal cortex, and between the cerebellum and other brain areas, respectively.
Resting state fMRI (rsfMRI) provides an effective way to study functional connectivity (FC)– and by extension, dysconnectivity – between areas of the brain that may be associated with a disease state or symptom dimension. Consistent with some of the proposed AH models, prior rsfMRI studies investigating AH in SZ have reported altered FC involving auditory, speech, and language-related regions, anterior cingulate cortex, hippocampal formation, insula striatum, and nodes of the default mode network, among other brain areas (e.g., see review by Alderson-Day et al., 2015). However, in spite of the large literature on AH, the pathophysiology underlying AH remains unclear. Many previous studies have constrained their investigations of FC using a priori seed regions based on specific AH models. But taking a step back and analyzing the data using a more agnostic approach may provide new insights.
Moreover, though research into the neural correlates of AH in SZ has proliferated, similar studies on AH in BD are more scarce. A handful of structural neuroimaging studies of AH or related symptoms in BD have been conducted (Ekman et al., 2017, James et al., 2011, Mørch-Johnsen et al., 2018, Neves et al., 2016, Song et al., 2015, Zhuo et al., 2020). With respect to rsfMRI, two studies to date have examined functional relationships between distributed brain areas associated with hallucinations in BD (Okuneye et al., 2020, Schutte et al., 2021). Okuneye and colleagues Okuneye et al. (2020) investigated whether FC of auditory and language cortices was associated with current hallucination severity across a combined sample of patients with psychosis as well as within specific diagnoses (SZ, schizoaffective disorder, BD, HC) or biomarker-based biotypes; however, this study used a non-agnostic seed-based FC approach focusing on auditory/language cortices, and also examined hallucinations in any modality, not necessarily AH. Schutte and colleagues Schutte et al., (2021) were innovative in including individuals with lifetime hallucinations across a wide psychosis continuum (SZ with hallucinations, BD with hallucinations, BD without psychosis, and non-clinical individuals with hallucinations); however, the focus again was hallucinations in any sensory modality, not AH. And, more critically, each group was compared only to non-hallucinating healthy control participants rather than to diagnosis-matched patients without hallucinations, thus limiting inferences about FC patterns specific to hallucinations. Another recent study investigated global functional connectivity density (gFCD) associated with AH in BD (Qiu et al., 2020); however gFCD, which uses rsfMRI to measure the total number of functional connections between one voxel and all other voxels in the brain, does not inform about the functional relationships between specific brain areas.
In the current study, we investigated FC abnormalities associated with the propensity for AH in patients across the psychosis spectrum (SZ spectrum and BD with psychotic features) using an unbiased approach that is agnostic to both specific brain regions and diagnosis. The primary goal was to compare trait-level differences in whole-brain brain architecture between psychosis patients with and without AH propensity, as indicated by a lifetime history of AH. We secondarily sought to examine aberrant connectivity patterns associated with lifetime AH within each of the two diagnostic categories. We predicted that we would find FC abnormalities that are common to psychosis patients with lifetime AH, as well as those that are more specific to AH in SZ or BD.
2. Methods
2.1. Participants
We compared rsfMRI data in psychosis patients with lifetime auditory hallucinations (AH) and psychosis patients with no lifetime history of AH (NAH). Data were collected from men and women ages 18–65 years with diagnoses of schizophrenia spectrum disorders (SZ; including schizophrenia and schizoaffective disorder) or bipolar disorder with psychotic features (BD). Patients were recruited from in- and outpatient clinical services at McLean Hospital, a private psychiatric hospital in Belmont, MA, USA, affiliated with Harvard Medical School. Data were collected as part of an ongoing study approved by the Mass General Brigham Institutional Review Board, which oversees human subjects research at McLean. Exclusion criteria included significant medical or neurological illness, pregnancy, electroconvulsive treatment in the previous 3 months, history of head trauma with significant loss of consciousness, and contraindications to MRI. Subsets of this dataset have been previously described (Baker et al., 2014, Shinn et al., 2017).
Patients underwent comprehensive clinical assessments within 24 h of scanning. The Structured Clinical Interview for the DSM-IV-TR (SCID) (First et al., 1995) was administered to confirm diagnoses. In addition, we used SCID item B16 (“Did you ever hear things that other people couldn’t, such as noises or the voices of people whispering or talking?”) to categorize patients into AH and NAH groups. The initial sample, prior to quality control, consisted of 114 psychosis patients (86 SZ, 28 BD) with AH and 71 psychosis patients (19 SZ, 52 BD) categorized as NAH.
We also administered the Young Mania Rating Scale (YMRS) (Young et al., 1978), the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Åsberg, 1979), and the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) to assess the severity of mood and psychotic symptoms in the past month. The scores for the PANSS total, PANSS positive, and YMRS were calculated after excluding the score for the hallucination-related items (PANSS item P3 and YMRS item 8 for content). We calculated total medication load (TML) (Phillips et al., 2008)—a composite score of all psychotropic medications a patient was on at the time of scanning– in addition to chlorpromazine (CPZ) equivalent doses, since many patients were on multiple medications, including antidepressants and mood stabilizers in addition to antipsychotics.
To serve as a point of reference for interpretation, we analyzed rsfMRI data from 75 healthy control (HC) participants. HC data were selected from an existing database of 2292 adults, aged 18–83 years, who were scanned using identical pulse sequences on an identical scanner, and selected to match the psychosis patients on age, sex, handedness, and image signal-to-noise ratio (SNR) (Holmes et al., 2015). Exclusion criteria for HC participants included any past or current psychiatric illnesses, any current medical or neurological illness, pregnancy, history of head trauma with significant loss of consciousness, and MRI contraindications.
2.2. Image acquisition
Imaging was performed on a Siemens 3-Tesla Tim-Trio scanner with a 12-channel phased-array head coil. Functional data were acquired using gradient-echo echoplanar imaging sensitive to blood oxygenation level–dependent (BOLD) contrast. Participants were instructed to remain still, stay awake, and keep their eyes open. No fixation image was used, but patients were monitored via eye tracking video to ensure that eyes remained open during functional scans.
The echoplanar imaging parameters were repetition time, 3000 ms; echo time, 30 ms; flip angle, 85°; 3 mm voxels; field of view, 216; and 47 axial sections interleaved with no gap. Functional runs lasted 6.2 min (124 time points). Whole-brain coverage was achieved with sections aligned to the anterior commissure–posterior commissure plane using an automated alignment procedure, ensuring consistency among participants. Structural data included high-resolution, multiecho, magnetization-prepared, gradient-echo (ME-MPRAGE) T1-weighted images allowing increased contrast through weighted averaging of four derived images.
2.3. Image analysis
We used the Conn functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) version 17e (www.nitrc.org/projects/conn) with SPM12 and Matlab 2016a for analysis of our rsfMRI data. The first four time points of the resting BOLD image were discarded to account for magnet stabilization. Other preprocessing steps included realignment, slice-time correction, segmentation, normalization into Montreal Neurological Institute (MNI) space, and smoothing with a 9 mm kernel.
Rigorous methods for handling artifact are required for valid interpretation of FC analyses, as rsfMRI data are frequently contaminated by head motion and other non-neuronal sources of noise (Power et al., 2011, Van Dijk et al., 2012). For data quality assurance, we calculated signal-to-noise ratio (SNR; mean ± SD of the mean slice intensity time series). In addition, we identified outlier time points in the rsfMRI scans using the ARtifact Detection Tool (ART), with z set to > 3 SD from mean global activation, subject-motion threshold > 1 mm, and rotation threshold 0.05. Participants with either SNR < 100 or > 20 outlier time points (i.e., without at least 100 time points, or 300 s of rsfMRI data) were excluded from analysis (n = 22 AH, n = 16 NAH). Two AH participants were missing symptom severity information, so were removed from further analysis as well. Among the 90 AH and 55 NAH patients who met our inclusion cut-off for data quality, there was no difference between the AH (mean 3.5, range 0–19) and NAH (2, 0–15) groups in ART outliers (p = 0.859). HC were also screened for motion outliers using ART (mean 4, range 0–15).
For the 145 cases meeting our data quality criteria, we took several steps to further denoise the rsfMRI data. For each case, we regressed out ART outliers (“scrubbing”), the six rigid body realignment parameters and their first derivatives, and the five principal components of white matter signal and five principal components of cerebrospinal fluid (CSF) signal using the CompCor noise reduction method (Behzadi et al., 2007) in Conn. White matter and CSF signals were extracted using white matter and CSF masks generated from segmentation of each participant’s anatomical images. After the noise regression steps, band-pass temporal filtering (0.008, 0.09) was applied.
We performed ROI-to-ROI FC analysis using the atlas ROI’s in Conn. These consist of 132 ROI’s consisting of 91 cortical and 15 subcortical regions as defined by the Harvard-Oxford maximum likelihood atlas, and 26 cerebellar regions defined by the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). This set of ROI’s, which comprise the default parcellation distributed with Conn, provides good whole-brain coverage which is critical for our agnostic analysis approach, has sufficient anatomical specificity, has anatomically-based boundaries which are easy to interpret, and enables results to be compared with other studies using these atlases.
As the primary aim was to identify FC features associated with lifetime AH, we compared the two patient groups (AH vs. NAH). We secondarily examined differences between AH and NAH within each diagnosis (SZ, BD). Third, we explored the contrast between SZAH vs. SZNAH and BDAH vs. BDNAH. As clinical and demographic differences between the AH and NAH groups could bias findings (e.g., results could reflect differences related to diagnosis more than AH), we adjusted for between-group differences in clinical and demographic variables (Table 1) by including them as covariates in the general linear model of all FC analyses. Given the detrimental effect of head motion in rsfMRI studies, we also covaried for ART outliers in all FC analyses. For all analyses, the significance threshold was set at p < 0.05, two-sided, false discovery rate (FDR)-corrected for multiple comparisons.
Table 1.
Participant Characteristics.
| Lifetime AH | NAH | Statistic | Significance | |
|---|---|---|---|---|
| Sample Size (N = 145) | 90 | 55 | ||
| DSM-IV-TR Primary Diagnosis | χ2 = 30.242 | p = 3.8 × 10-8 | ||
| Schizophrenia (SZ), No. (%) | 68 (76%) | 16 (29%) | – | – |
| Bipolar Disorder (BD), No. (%) | 22 (24%) | 39 (71%) | – | – |
| Age, median (range), y | 29 (18–57) | 26 (18–62) | z = -0.373 | p = 0.709 |
| Female, No. (%) | 31 (38%) | 16 (29%) | χ2 = 0.447 | p = 0.504 |
| Not Right-Handed, No. (%) | 11 (12%) | 5 (11%) | χ2 = 0.341 | p = 0.559 |
| Hallucinations in Other Modalities (SCID) | 48 (53%) | 11 (20%) | χ2 = 15.717 | p = 7.4 × 10-5 |
| Visual Hallucinations, No. (%) | 37 (41%) | 5 (9%) | χ2 = 17.011 | p = 3.7 × 10-5 |
| Tactile Hallucinations, No. (%) | 29 (32%) | 7 (13%) | χ2 = 6.952 | p = 0.008 |
| Olfactory & Gustatory Hallucinations1, No. (%) | 10 (11%) | 2 (4%) | p = 0.133 | |
| PANSS P3 Hallucination Item2, median (range) | 3.5 (1–7) | 1 (1–6) | z = 20.421 | p = 0.0001 |
| PANSS Total Score3, median (range) | 58 (32–102) | 54 (29–84) | z = -1.537 | p = 0.124 |
| Positive3, median (range) | 14 (6–29) | 14 (6–32) | z = -0.541 | p = 0.589 |
| Negative, median (range) | 12 (7–40) | 9 (7–28) | z = -3.521 | p = 4.3 × 10-4 |
| General Psychopathology, median (range) | 29.5 (16–56) | 28 (16–46) | z = -1.140 | p = 0.254 |
| YMRS3, median (range) | 6 (0–30) | 14 (0–37) | z = -3.149 | p = 0.002 |
| MADRS, median (range) | 15.5 (0–44) | 12 (0–40) | z = -1.859 | p = 0.063 |
| Chlorpromazine Equivalent, median (range) | 300 (0–1959) | 200 (0–1175) | z = -1.774 | p = 0.076 |
| Total Medication Load, median (range) | 3 (0–10) | 4 (0–11) | z = -0.668 | p = 0.504 |
| Current Substance Abuse or Dependence | ||||
| Alcohol, No. (%) | 5 (6%) | 2 (4%) | χ2 = 0.274 | p = 0.601 |
| Cannabis, No. (%) | 13 (14%) | 9 (16%) | χ2 = 0.098 | p = 0.755 |
| Stimulant and/or Cocaine1, No. (%) | 2 (2%) | 1 (2%) | p = 1.000 | |
| RsfMRI ART Outlier Time Points | 3.5 (0–19) | 2 (0–15) | z = -0.178 | p = 0.859 |
Abbreviations: AH = Lifetime auditory hallucinations group; NAH = No lifetime auditory hallucinations group; SCID = Structured Clinical Interview for the DSM-IV-TR; PANSS = Positive and Negative Syndrome Scale; YMRS = Young Mania Rating Scale; MADRS = Montgomery-Asberg Depression Rating Scale; ART = ARtifact Detection Tool in Conn functional connectivity toolbox.
Variables with p-values in bold are included as covariates, along with ARtifact detection Tool (ART) outlier time points, in AH vs. NAH functional connectivity analyses.
Fisher’s exact test due to cell sizes < 5.
PANSS P3 Hallucination Item assessed current symptom severity, i.e., severity of hallucinations in the past month; therefore, patients with lifetime but no current AH could have a P3 score of 1 (absent) or 2 (minimal). P3 asks about hallucinations in any modality, and is not specific to AH; therefore, patients in the NAH group could have P3 scores > 2.
Symptom scale total calculated by excluding hallucination-related item.
For all FC abnormalities, we extracted the Fisher-transformed correlation coefficients in BOLD signal between each ROI-pair. We did not include HC participants in FC analyses, as we were interested in identifying FC abnormalities associated specifically with AH, rather than those associated with psychotic disorders more broadly; hence, the contrasts of interest were between AH and NAH psychosis patients. Nevertheless, for each FC abnormality identified by comparing the AH and NAH groups, we extracted the Fisher-transformed coefficients from HC’s and present them alongside the values for AH and NAH as a point of reference.
3. Results
3.1. Participant characteristics
As shown in Table 1, the AH and NAH groups were comparable with respect to age, sex, and handedness. As expected, the severity of hallucinations in the past month, as measured by the PANSS P3 hallucination item, was significantly higher in the lifetime AH group than in the NAH group (median 3.5 vs. 1; p = 0.0001). The two groups also differed significantly on diagnosis and severity of visual and tactile hallucinations, negative symptoms, and manic symptoms. The rates of DSM-IV-TR alcohol or substance abuse and dependence were not statistically significant between the AH and NAH groups. TML was not significantly different, but there was a trend for the AH group to take higher doses of antipsychotic medications than the NAH group, as measured by chlorpromazine equivalent dose. To minimize confounding by these demographic and clinical differences between the AH and NAH groups (i.e., diagnosis, lifetime history of hallucinations in non-auditory modalities, PANSS negative subscale, YMRS score, MADRS score, CPZ equivalents), we included these variables, as well as a measure of head motion (i.e., ART outliers), as covariates in all FC analyses.
3.2. Functional connectivity of AH vs. NAH across the psychosis spectrum
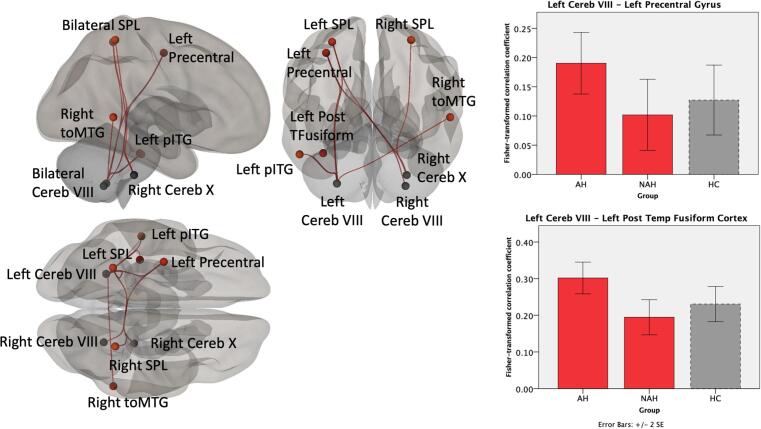
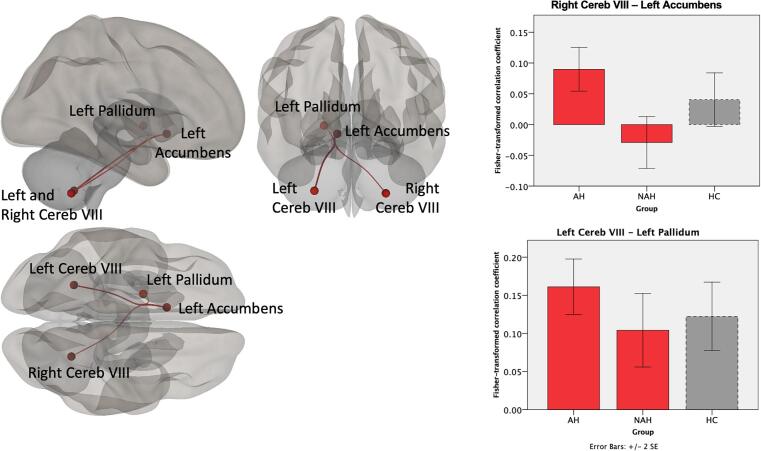
We found that psychosis patients with AH have connectivity abnormalities involving multiple, distributed brain regions (Fig. 1). Relative to NAH, psychosis patients with AH demonstrated higher FC between the cerebellum and several cerebral cortical (Fig. 2) and subcortical (Fig. 3) brain areas. The cerebellar regions involved were left and right cerebellar lobule VIII and right cerebellar lobule X. Examination of the Fisher-transformed correlation coefficients showed that AH patients had higher cerebellar-cortical and cerebellar-subcortical FC compared to both NAH and HC. Notably, the NAH group had lower FC even relative to HC.
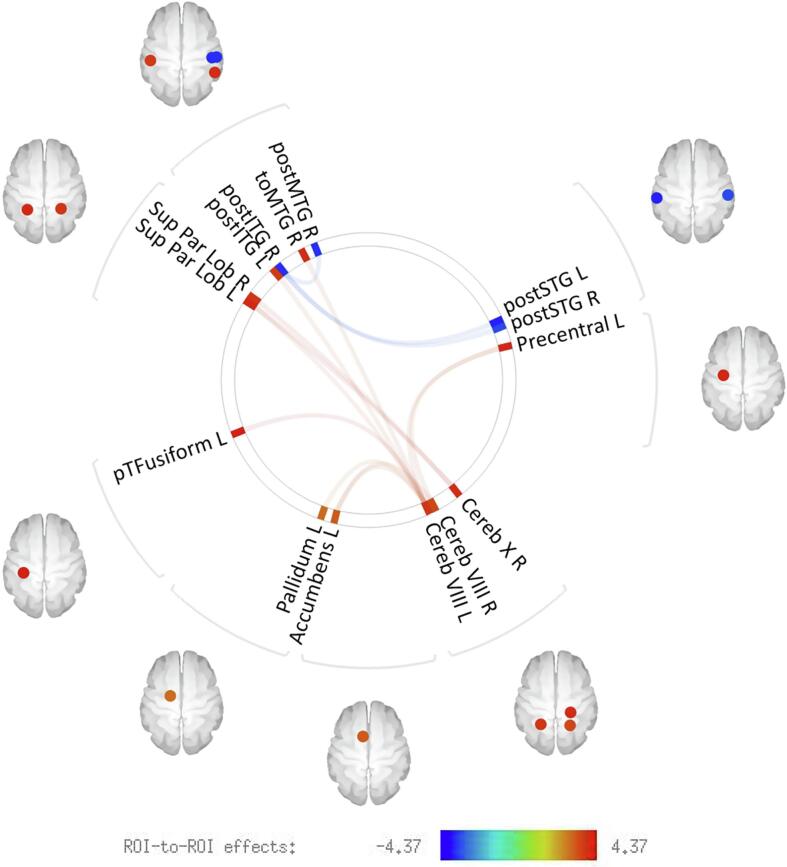
Fig. 1.
ROI-to-ROI functional connectivity of patients with (AH) and without lifetime auditory hallucinations (NAH) across the psychosis spectrum. Compared to NAH patients, AH patients showed multiple regions (cerebral cortical and subcortical) that were functional hyperconnected to cerebellar regions, and hypoconnectivity between temporal lobe areas. Results were adjusted for motion, diagnosis, lifetime hallucinations in other modalities, negative symptom severity, mania severity, depression severity, and antipsychotic exposure as measured by chlorpromazine equivalents. The significance threshold was set at p < 0.05, two-sided, false discovery rate (FDR)-corrected for multiple comparisons.
Fig. 2.
Cerebello-cerebral cortical functional connectivity associated with lifetime AH across the psychosis spectrum. Compared to NAH patients, AH patients showed higher functional connectivity between cerebellum and regions in frontal, temporal, and parietal areas. The bar graphs show the Fisher-transformed Pearson’s correlation between example ROI-pairs, with healthy control data presented in dashed lines as a point of reference. The data show patients with AH to have increased connectivity relative to both NAH and also healthy controls.
Fig. 3.
Cerebello-subcortical functional connectivity associated with lifetime AH across the psychosis spectrum. Compared to NAH patients, AH patients showed higher functional connectivity between cerebellum and left nucleus accumbens and left pallidum. The bar graphs show the Fisher-transformed Pearson’s correlation between the two significant ROI-to-ROI findings, with healthy control data presented in dashed lines as a point of reference.
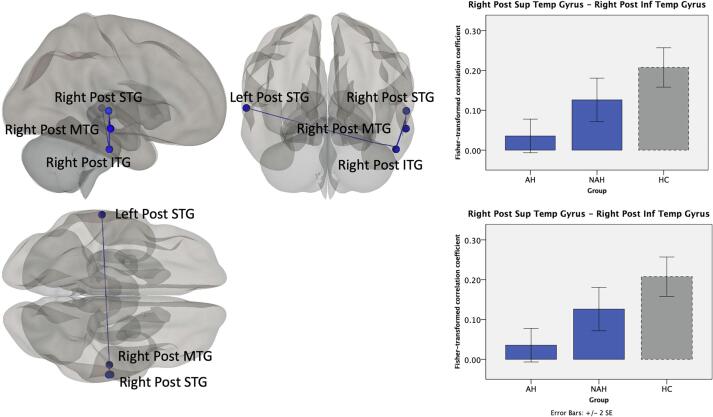
In addition, lifetime AH was associated with lower FC between temporal lobe areas (Fig. 4). This pattern was seen mostly with temporal lobe gyri in the right hemisphere, i.e., between right posterior inferior temporal gyrus (pITG) and right posterior superior temporal gyrus (pSTG) and between right pITG and right posterior middle temporal gyrus (pMTG). However, lower FC between temporal lobe areas was also observed between hemispheres, between right pITG and left pSTG. Fisher-transformed correlation coefficients between the temporal lobe region pairs shows that AH patients have lower FC relative to both NAH patients and HC, with the NAH group showing FC intermediate between AH and HC.
Fig. 4.
Functional connectivity between temporal lobe regions in association with lifetime AH across the psychosis spectrum. Compared to NAH patients, AH patients showed lower functional connectivity between temporal lobe areas within the right hemisphere as well as between hemispheres. The bar graphs show the Fisher-transformed Pearson’s correlation between example ROI-pairs, with healthy control data presented in dashed lines as a point of reference.
3.3. Functional connectivity of AH vs. NAH within the schizophrenia spectrum
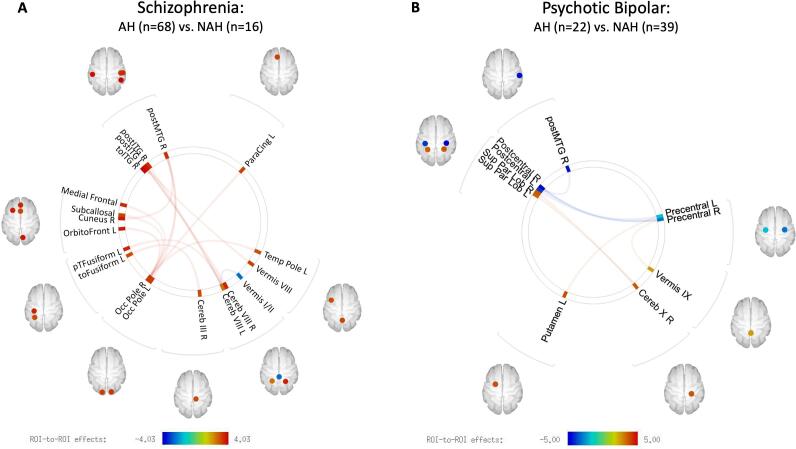
Compared to SZNAH patients (n = 16), SZAH patients (n = 68) showed higher FC between cerebellar and temporal lobe areas, between the occipital pole bilaterally and temporal and frontal regions, and between right cuneus and right pMTG (Fig. 5A, Supplementary Table 1). The SZAH vs. SZNAH analysis yielded only one lower FC finding, between cerebellar vermis I/II and left cerebellar lobule VIII.
Fig. 5.
Functional connectivity of AH vs. NAH within each diagnostic category. (A) Schizophrenia AH vs. schizophrenia NAH. (B) Bipolar psychosis AH vs. bipolar psychosis NAH. (See supplementary materials for Fisher-transformed correlation coefficients.)
3.4. Functional connectivity of AH vs. NAH within psychotic bipolar disorder
BDAH patients (n = 22) also showed cerebellar FC abnormalities relative to BDNAH patients (n = 39), though fewer than the number observed within SZ (Fig. 5B, Supplementary Table 2). Compared to BDNAH patients, BDAH patients showed higher FC between right cerebellar lobule X and bilateral superior parietal lobules, and higher FC between cerebellar vermix IX and right precentral gyrus. BDAH patients were also characterized by lower FC within the sensorimotor network, specifically between pre- and postcentral gyri, lower FC between right pMTG and right postcentral gyrus, and higher FC between left putamen and left precentral gyrus compared to BDNAH patients.
3.5. Functional connectivity of schizophrenia AH vs. bipolar disorder AH
FC between the right cerebellum lobule VI and bilateral middle temporal gyri distinguished the SZ AH group from the BD AH group (Supplementary Material for details).
4. Discussion
In this study, we took an unbiased approach to investigating whole-brain resting state functional connectivity (FC) associated with lifetime history of AH across the psychosis spectrum, and report connectivity abnormalities involving multiple, distributed brain regions. Across the psychosis spectrum, lifetime AH was associated with increased connectivity of the cerebellum with frontal, temporal, parietal, and subcortical regions, and decreased connectivity between temporal lobe regions. Further exploration of AH within each diagnostic category revealed increased cerebello-temporal connectivity in SZAH vs. SZNAH, and increased cerebello-parietal connectivity in BDAH vs. BDNAH, among other FC abnormalities. As we compared psychosis patients with AH vs. those without AH, rather than comparing psychosis patients with AH to HC, our findings allow us to draw inferences about FC patterns that are specific to AH and not a general characteristic of psychotic disorders.
Across the psychosis spectrum as well as within SZ and BP, a common underlying neurofunctional element associated with AH in our sample was cerebellar dysconnectivity. Our finding of AH-related cerebellar dysconnectivity is not surprising when considered in the context of the associative functions of the cerebellum. Although the cerebellum was historically thought to mediate only motor functions such as balance and coordination, we now know that the cerebellum also has reciprocal connections with myriad cerebral cortical regions that coordinate non-motor functions (Buckner et al., 2011, Middleton and Strick, 1994). Indeed, mapping of the cerebellum using FC has revealed that only a small portion of the cerebellum is involved in motor processing; the majority is dedicated to higher-order associative functions mediated by large-scale networks including the fronto-parietal network, dorsal attention network, salience network, and default mode network (Buckner et al., 2011).
Prior research has drawn on this newer understanding of the cerebellum’s associative functions and implicated dysfunction in cerebello-thalamo-cortical circuits in psychosis. Schmahmann’s dysmetria of thought model suggests that abnormal modulation of cognitive and affective processes by the cerebellum may underlie psychotic symptoms (Schmahmann, 1991, Schmahmann, 1998). Similarly, Andreasen’s cognitive dysmetria model suggests that misconnections between the cerebral cortex, thalamus, and cerebellum lead to dysmetria or incoordination of mental activity which, in turn, contribute to psychotic symptoms such as AH (Andreasen et al., 1996, Andreasen et al., 1999, Andreasen, 1999). Given the contributions of the cerebellum to auditory and other perceptual processing (Baumann et al., 2015), cerebellar involvement in AH and other perceptual abnormalities makes sense. In particular, the cerebellum is thought to play a key role in feedforward models (Ramnani, 2006), and it has been proposed that the receipt of an imprecise or delayed corollary discharge, imprecise comparison of predicted vs. actual sensory feedback, and/or insufficient updating of the forward model by the cerebellum could lead to AH (Pinheiro et al., 2020). Consistent with these frameworks implicating aberrant cerebellar function in psychosis and AH, we (Brady et al., 2019, Shinn et al., 2015, Shinn et al., 2017) and others (Bernard et al., 2017, Cao et al., 2018, Chen et al., 2013, Collin et al., 2011, Ferri et al., 2018, Guo et al., 2015, Guo et al., 2018, Liu et al., 2011, Repovs et al., 2011, Wang et al., 2016, Zhuo et al., 2018, Lee et al., 2019) have previously reported disruptions in cerebro-cerebellar FC in psychotic disorders. Cerebellar dysconnectivity has also been reported in whole-brain rsfMRI studies investigating AH (Clos et al., 2014, Chang et al., 2015, Mallikarjun et al., 2018, Zhao et al., 2018, Itahashi et al., 2018, Chen et al., 2019), more specifically, and been shown to normalize with theta-burst transcranial magnetic stimulation to the left temporoparietal junction (Chen et al., 2019). Similarly, a recent lesion mapping study found that brain lesions causing hallucinations were located within a single functionally connected network defined by positive FC with regions in the cerebellum— consistent with our finding of cerebellar hyperconnectivity—and negative FC with right superior temporal sulcus (Kim et al., 2021). Convergent with the FC literature on cerebellar abnormalities in AH, a voxel based morphometry study investigating abnormalities of cerebellar structure in AH found lower gray matter volume in right lobule VIIIa in patients with versus those without persistent AH (Cierpka et al., 2017). Moreover, a meta-analysis of AH symptom capture studies (Zmigrod et al., 2016), as well as a fMRI study of healthy hallucinators (Powers et al., 2017), found that the cerebellum is functionally activated during AH.
Notably, dysconnectivity involving the cerebellum was not among the results of two recent studies examining FC associated with hallucinations across the continuum of psychosis (Okuneye et al., 2020, Schutte et al., 2021). However, one of these studies employed parcellations that excluded the cerebellum (Schutte et al., 2021), and the second used a seed-based approach that focused on auditory and language regions (Okuneye et al., 2020). While seed-based FC analysis is important for testing specific hypotheses, such a strategy is, by definition, constrained to evaluate only those FC patterns associated with the a priori defined seed region(s) of interest while ignoring other potentially important functional connections. Neuroimaging research tends to have a corticocentric bias (Parvizi, 2009). Perhaps as a result of such bias, as well as the historical understanding of the cerebellum as a brain area involved primarily in motor functions, the cerebellum has been relatively neglected in the AH literature (Pinheiro et al., 2020). The identification in the current study of cerebellar dysconnectivities associated with AH across the psychosis spectrum illustrates a valuable role for unbiased investigational approaches, especially when the pathophysiological mechanism(s) underlying a condition or dimension are unclear, and also suggests that the cerebellum ought to be considered for analysis in future studies of AH.
Many of the frequently cited AH models feature altered connections involving frontal control areas (Hugdahl, 2009, Waters et al., 2006); auditory, speech, and language areas (Feinberg, 1978, Hugdahl, 2009, Northoff and Qin, 2011, Steinmann et al., 2014)); the hippocampus and related limbic regions involved in memory (Waters et al., 2006); and nodes of the default mode network (Northoff and Qin, 2011), among other brain areas. Thus, in addition to the results we found, we had expected to find abnormal FC involving the inferior frontal gyrus, anterior cingulate cortex, dorsolateral prefrontal cortex, medial prefrontal cortex, and other higher order cerebral cortical association areas. We did not observe dysconnectivity directly affecting these brain areas. However, there is regional specificity to cerebellar topography (Makris et al., 2005), and many of our findings of cerebellar dysconnectivity involved posterior lobules in the lateral hemisphere of the cerebellum (VIII, X), which are associated with cognitive and other higher order associative functions. Neuroimaging studies have demonstrated that the posterior cerebellum, specifically lobules VI – X, is generally associated with language, cognitive, and higher-order executive processes, while the anterior cerebellar lobe is more responsible for motor functions (Stoodley and Schmahmann, 2009, Kansal et al., 2017).
In addition to the cerebellum, our results also implicated dysconnectivity of temporal regions in AH across the psychosis spectrum. We found altered connectivity between temporal lobe regions within a single hemisphere, between hemispheres, and between temporal regions and the cerebellum. There was hypoconnectivity between temporal lobe regions, and cerebello-temporal hyperconnectivity, in AH vs. NAH. Multiple studies Clos et al., 2014, Gavrilescu et al., 2010, Hoffman et al., 2011, Liemburg et al., 2012, Oertel-Knochel et al., 2013, Shinn et al., 2013, Sommer et al., 2012, Vercammen et al., 2010, Wolf et al., 2011—including studies in patients across the continuum of SZ and BD56, (Schutte et al., 2021), as well as in individuals with non-clinical voice hearing (Diederen et al., 2012, Lutterveld et al., 2013, Schutte et al., 2021)—have described abnormal connectivity involving temporal regions in association with AH. Meta-analyses of functional activation studies have reported abnormal activations in the auditory cortex (Kompus et al., 2011) and language-related regions (Jardri et al., 2010) associated with AH. In addition, a quantitative meta-analysis of voxel-based morphometry studies showed that AH severity was significantly associated with gray matter volume reductions in the left superior temporal gyrus, including the primary auditory cortex, and also marginally with right superior temporal gyrus (Modinos et al., 2012). Moreover, as mentioned, a lesion mapping study that found that lesions causing hallucinations were defined by connectivity to both cerebellum and right superior temporal sulcus (Kim et al., 2021). Our finding of abnormal cerebello-temporal FC more specifically suggests that, consistent with dysmetria models, AH may result from altered ability of the cerebellum to modulate or coordinate activity in auditory, speech, and language areas in the temporal lobe.
While our findings show evidence of dysconnectivity associated with AH in patients across the psychosis spectrum, we also observed FC features that were unique to AH in BD. The cerebello-temporal FC abnormalities observed in SZAH were generally consistent with the findings we observed in lifetime AH across the psychosis spectrum. By contrast, AH in BD was characterized by higher cerebello-parietal FC, vermis-precentral FC, and putamen-precentral FC, as well as lower precentral-postcentral gyri FC and pMTG-postcentral gyrus FC. In addition to cerebellar FC abnormalities, our results point to significant FC abnormalities involving somatosensory and motor cortices in BDAH. Though Schutte and colleagues Schutte et al. (2021) compared BD with hallucinations to HC, rather than to BD without hallucinations, they similarly reported decreased FC in the sensorimotor network to be among the many FC abnormalities (i.e., fronto-temporal, fronto-central, and between bilateral frontal lobes) that characterized BD patients with hallucinations. It is more difficult to compare our findings with those of Okuneye and colleagues Okuneye et al. (2020), who constrained their analysis to auditory/language cortex FC. Nevertheless, Okuneye and colleagues included pMTG as part of the unimodal auditory association cortex (AAC) seed and found that left AAC showed greater FC with a right MTG/angular gyrus cluster that included the right homologue of Wernicke’s area in BD. They did not observe pMTG-postcentral FC abnormalities in BDAH as we did. The discrepant results could be related to our studying AH while Okuneye and colleagues, like Schutte and colleagues, examined FC hallucinations in any sensory modality. Regardless of differences in the specific results, an important point that is highlighted by all three studies, including ours, is that there do appear to exist distinctive FC features that are unique to AH/hallucinations in BD. This suggests that while some elements of AH pathophysiology may be shared across SZ and BD, they are not identical. In the current study, we went one step further to also directly examine the contrast between AH in SZ and BD; however, the results of this exploratory analysis, which involved contrasting four subgroups with small and unequal samples [(68 SZAH vs. 16 SZNAH) vs. (22 BDAH vs. 39 BDNAH)] require replication and should be interpreted with caution. That said, it is possible that the FC differences between SZAH and BDAH could reflect differences in the phenomenology of AH in SZ and BD. Distinct phenomenological subtypes may arise from different pathophysiological mechanisms (McCarthy-Jones et al., 2014), and a recent study investigating AH phenomenology found differences in affective and non-affective psychosis (Toh et al., 2020). Unfortunately, we did not collect data about AH phenomenology in this sample to examine associations between FC and specific characteristics of AH.
This study investigates FC between specific brain areas associated with AH in BD, an area with limited research to date compared to the sizeable literature relating to FC of AH in SZ. Our unbiased approach to FC analysis, agnostic to specific brain areas or diagnosis, is a strength of this study. Furthermore, we directly compared psychosis patients with and without AH, unlike many studies that purport to identify brain abnormalities associated with AH yet compare patients with AH to healthy control participants. However, this study also has several limitations. First, the prevalence of lifetime AH is higher among individuals with SZ than among people with BD, and this was reflected in a smaller number of BD patients with AH in our sample. However, our analyses controlled for diagnosis so that results were more likely to reflect differences related to AH than diagnosis. Second, BD is characterized by fluctuations in mood, and different BD mood states are associated with different patterns of FC (Brady Jr. et al., 2017). In order to recruit as large a BD sample as possible, we recruited BD patients in heterogenous mood states (i.e., manic, depressed, or euthymic). However, we controlled for the severity of manic and depressive symptoms, along with differences in hallucinations in other modalities and negative symptom severity in our analyses. Third, we studied patients who were medicated, and there was a trend-level difference in antipsychotic exposure between AH and NAH patients. However, restricting recruitment to unmedicated individuals is difficult, given the practical and ethical challenges of withholding medications from psychosis patients. We decided, instead, to include chlorpromazine equivalent antipsychotic dose as a covarate in our analyses.
Fourth, as mentioned, AH phenomenology has the potential to provide important clues about differences in underlying pathophysiological mechanisms, and we did not collect details about the characteristics of patients’ AH. Fifth, we also did not collect data on AH experiences during scanning and thus could not control for potential state-related AH effects that may confound FC associated with lifetime, or trait, AH. Sixth, AH can occur in non-psychiatric samples, and we did not confirm the absence of lifetime AH in HC participants, as their data were drawn from a study unrelated to psychosis. The AH status of the HC group may be less relevant in the current study, however, as we did not directly compare AH or NAH with HC; rather, we included the Fisher-transformed coefficients from the HC group merely as a point of reference. Finally, it has been shown that functional and structural connectivity results can vary according to the choice of parcellation scheme (Craddock et al., 2012, Zalesky et al., 2010), and we used anatomical atlas-based brain parcellations with ROI’s that may lack sufficient spatial specificity and functional homogeneity. However, the set of Harvard-Oxford and AAL parcellations in Conn provides good whole-brain coverage, which was important for our agnostic approach, has high interpretability owing to its anatomically based parcellations, and enables integration of findings with other studies that have used these atlases. A related issue is that the findings of this study rely on group averaged data and, as such, may not capture unique variations across patients that are related to AH. The use of finer grained parcellations, and ideally those that are individually-based, should be employed in future studies to not only improve spatial specificity, but also help lay the groundwork for the development of precision medicine approaches to treating AH.
While recognizing the limitations of this study, our findings advance our understanding of functional connectivity abnormalities associated with AH as a trait. Specifically, our findings point to the significance of hyperconnectivity involving the cerebellum and hypoconnectivity between temporal lobe regions in individuals with a history of lifetime AH across the psychosis spectrum. We also found dysconnectivity patterns that were unique to lifetime AH within SZ or bipolar psychosis, suggesting both common and distinct mechanisms underlying AH pathophysiology in these disorders.
CRediT authorship contribution statement
Melissa Hwang: Formal analysis, Investigation, Visualization, Writing – original draft. Youkyung S. Roh: Investigation, Data curation. Jessica Talero: Investigation. Bruce M. Cohen: Writing – review & editing. Justin T. Baker: Data curation. Roscoe O. Brady: Investigation, Writing – review & editing. Dost Öngür: Conceptualization, Resources, Investigation, Supervision, Writing – review & editing. Ann K. Shinn: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are grateful to the patients who participated in this study, the staff at the McLean Imaging Center for their technical support with scanning, and to Alfonso Nieto-Castañon, PhD at Boston University and the Massachusetts Institute of Technology (MIT) for his support with Conn. Preliminary results from this study were presented at the 2019 Congress of the Schizophrenia International Research Society in Orlando, FL (abstract published in Schizophrenia Bulletin 2019; 45 supplement 2: S157).
Funding
This work was supported by the National Institutes of Health (NIH) K23MH100611 and R21MH121831, the Brain and Behavior Research Foundation, and the Eleanor and Miles Shore Harvard Medical School Fellowship to AKS; NIH K23MH104515 to JTB; and NIH R01MH116170 to ROB.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102893.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Dierks T., Linden D.E., Jandl M., et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22(3):615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Dugre J.R., Guay J.P., Dumais A. Risk factors of compliance with self-harm command hallucinations in individuals with affective and non-affective psychosis. Schizophr Res. 2018;195:115–121. doi: 10.1016/j.schres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Falloon I.R., Talbot R.E. Persistent auditory hallucinations: coping mechanisms and implications for management. Psychol Med. 1981;11(2):329–339. doi: 10.1017/s0033291700052144. [DOI] [PubMed] [Google Scholar]
- Hor K., Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 Suppl):81–90. doi: 10.1177/1359786810385490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari V.M., Harrow M., Grossman L.S., Rosen C. A 20-year multi-follow-up of hallucinations in schizophrenia, other psychotic, and mood disorders. Psychol Med. 2013;43(6):1151–1160. doi: 10.1017/S0033291712002206. [DOI] [PubMed] [Google Scholar]
- Goghari V.M., Harrow M. Twenty year multi-follow-up of different types of hallucinations in schizophrenia, schizoaffective disorder, bipolar disorder, and depression. Schizophr Res. 2016;176(2–3):371–377. doi: 10.1016/j.schres.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., Murray R.M., McGuire P.K. Auditory hallucinations: a review of psychological treatments. Schizophr Res. 1998;32(3):137–150. doi: 10.1016/s0920-9964(98)00052-8. [DOI] [PubMed] [Google Scholar]
- Agid O., Arenovich T., Sajeev G., Zipursky R.B., Kapur S., Foussias G., Remington G. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439–1444. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- Pierre J.M. Hallucinations in nonpsychotic disorders: toward a differential diagnosis of “hearing voices”. Harv Rev Psychiatry. 2010;18(1):22–35. doi: 10.3109/10673220903523706. [DOI] [PubMed] [Google Scholar]
- Waters F., Allen P., Aleman A., Fernyhough C., Woodward T.S., Badcock J.C., Barkus E., Johns L., Varese F., Menon M., Vercammen A., Laroi F. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38(4):683–693. doi: 10.1093/schbul/sbs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn A.K., Pfaff D., Young S., Lewandowski K.E., Cohen B.M., Öngür D. Auditory hallucinations in a cross-diagnostic sample of psychotic disorder patients: a descriptive, cross-sectional study. Compr Psychiatry. 2012;53(6):718–726. doi: 10.1016/j.comppsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini S., de Queiroz V., Dell'Osso L., Abelli M., Mastrocinque C., Saettoni M., Catena M., Cassano G.B. Cross-sectional similarities and differences between schizophrenia, schizoaffective disorder and mania or mixed mania with mood-incongruent psychotic features. Eur Psychiatry. 2004;19(1):8–14. doi: 10.1016/j.eurpsy.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Black D.W., Nasrallah A. Hallucinations and delusions in 1,715 patients with unipolar and bipolar affective disorders. Psychopathology. 1989;22(1):28–34. doi: 10.1159/000284576. [DOI] [PubMed] [Google Scholar]
- Cao H., Chén O.Y., Chung Y., Forsyth J.K., McEwen S.C., Gee D.G., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Carrión R.E., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Thermenos H., Tsuang M.T., van Erp T.G.M., Walker E.F., Hamann S., Anticevic A., Woods S.W., Cannon T.D. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat. Commun. 2018;9(1):3836. doi: 10.1038/s41467-018-06350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G.A., Goodwin F.K. The stages of mania. A longitudinal analysis of the manic episode. Arch. Gen. Psychiatry. 1973;28(2):221–228. doi: 10.1001/archpsyc.1973.01750320053009. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S.K., Sinha V.K. Recurrence of hallucinations in consecutive episodes of schizophrenia and affective disorder. Schizophr Res. 1990;3(2):103–106. doi: 10.1016/0920-9964(90)90042-6. [DOI] [PubMed] [Google Scholar]
- Hammersley P., Dias A., Todd G., Bowen-Jones K., Reilly B., Bentall R.P. Childhood trauma and hallucinations in bipolar affective disorder: preliminary investigation. Br J Psychiatry. 2003;182(6):543–547. doi: 10.1192/bjp.182.6.543. [DOI] [PubMed] [Google Scholar]
- Keck P.E., McElroy S.L., Havens J.R., Altshuler L.L., Nolen W.A., Frye M.A., Suppes T., Denicoff K.D., Kupka R., Leverich G.S., Rush A.J., Post R.M. Psychosis in bipolar disorder: phenomenology and impact on morbidity and course of illness. Compr Psychiatry. 2003;44(4):263–269. doi: 10.1016/S0010-440X(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Argyelan M., Ikuta T., DeRosse P., Braga R.J., Burdick K.E., John M., Kingsley P.B., Malhotra A.K., Szeszko P.R. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull. 2014;40(1):100–110. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baethge C., Baldessarini R.J., Freudenthal K., Streeruwitz A., Bauer M., Bschor T. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136–145. doi: 10.1111/bdi.2005.7.issue-210.1111/j.1399-5618.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- Taylor M.A., Abrams R. The phenomenology of mania. A new look at some old patients. Arch. Gen. Psychiatry. 1973;29(4):520–522. doi: 10.1001/archpsyc.1973.04200040066011. [DOI] [PubMed] [Google Scholar]
- Taylor M.A., Abrams R. Acute mania. Clinical and genetic study of responders and nonresponders to treatments. Arch. Gen. Psychiatry. 1975;32(7):863–865. doi: 10.1001/archpsyc.1975.01760250055005. [DOI] [PubMed] [Google Scholar]
- Tillman R., Geller B., Klages T., Corrigan M., Bolhofner K., Zimerman B. Psychotic phenomena in 257 young children and adolescents with bipolar I disorder: delusions and hallucinations (benign and pathological) Bipolar Disord. 2008;10(1):45–55. doi: 10.1111/j.1399-5618.2008.00480.x. [DOI] [PubMed] [Google Scholar]
- Braunig P., Sarkar R., Effenberger S., Schoofs N., Kruger S. Gender differences in psychotic bipolar mania. Gend Med. 2009;6(2):356–361. doi: 10.1016/j.genm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Kumari R., Chaudhury S., Kumar S. Dimensions of hallucinations and delusions in affective and nonaffective illnesses. ISRN Psychiatry. 2013;2013:1–10. doi: 10.1155/2013/616304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P., Schretlen D.J., Thaker G.K., Stevens M.C., Keshavan M.S., Sweeney J.A., Tamminga C.A., Clementz B.A., O’Neil K., Pearlson G.D. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170(8):886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P., Yip B.H., Björk C., Pawitan Y., Cannon T.D., Sullivan P.F., Hultman C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N.D., Sambataro F., Vasic N., et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J. Psychiatry Neurosci. 2011;36(6):366–374. doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Matsumoto M., Iijima K., Sumiyoshi T. Specificity and Continuity of Schizophrenia and Bipolar Disorder: Relation to Biomarkers. Curr Pharm Des. 2020;26(2):191–200. doi: 10.2174/1381612825666191216153508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D.A., Stern E., Frith C., Cahill C., Holmes A., Grootoonk S., Seaward J., McKenna P., Chua S.E., Schnorr L., Jones T., Frackowiak R.S.J. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res (Suppl). 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Frangou S., Murray R.M. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28(2-3):143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Thieme; 1906. Grundrisse der Psychiatrie. [Google Scholar]
- Pillmann F. Carl Wernicke and the neurobiological paradigm in psychiatry. Acta Neuropsychologica. 2007;5(4):246–260. [Google Scholar]
- Bleuler E. International Universities Press; New York, New York: 1911. Dementia Praecox or the Group of Schizophrenias. [Google Scholar]
- Hugdahl K. “Hearing voices”: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand J Psychol. 2009;50(6):553–560. doi: 10.1111/j.1467-9450.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- Waters F.A., Badcock J.C., Michie P.T., Maybery M.T. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11(1):65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4(4):636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- Ford J.M., Mathalon D.H. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int J Psychophysiol. 2005;58(2–3):179–189. doi: 10.1016/j.ijpsycho.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Song J., Han D.H., Kim S.M., Hong J.S., Min K.J., Cheong J.H., Kim B.N. Differences in gray matter volume corresponding to delusion and hallucination in patients with schizophrenia compared with patients who have bipolar disorder. Neuropsychiatr Dis Treat. 2015;11:1211–1219. doi: 10.2147/NDT.S80438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann S., Leicht G., Mulert C. Interhemispheric auditory connectivity: Structure and function related to auditory verbal hallucinations. Frontiers in Human Neuroscience. 2014;8:55. doi: 10.3389/fnhum.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Qin P. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res. 2011;127(1–3):202–214. doi: 10.1016/j.schres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. An emerging concept. The cerebellar contribution to higher function. Arch. Neurol. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998;2(9):362–371. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., O'Leary D.S., Cizadlo T., et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Nopoulos P., O’Leary D.S., Miller D.D., Wassink T., Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46(7):908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C. A unitary model of schizophrenia. Bleuler's “Fragmented Phrene” as schizencephaly. Archives of General Psychiatry. 1999;56(9):781. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Pinheiro A.P., Schwartze M., Kotz S.A. Cerebellar circuitry and auditory verbal hallucinations: An integrative synthesis and perspective. Neurosci Biobehav Rev. 2020;118:485–503. doi: 10.1016/j.neubiorev.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Allen P., Laroi F., McGuire P.K., Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32(1):175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Ćurčić-Blake B., Ford J.M., Hubl D., Orlov N.D., Sommer I.E., Waters F., Allen P., Jardri R., Woodruff P.W., David O., Mulert C., Woodward T.S., Aleman A. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol. 2017;148:1–20. doi: 10.1016/j.pneurobio.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B., McCarthy-Jones S., Fernyhough C. Hearing voices in the resting brain: A review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev. 2015;55:78–87. doi: 10.1016/j.neubiorev.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A., Hough M., James S., Burge L., Winmill L., Nijhawan S., Matthews P.M., Zarei M. Structural brain and neuropsychometric changes associated with pediatric bipolar disorder with psychosis. Bipolar Disord. 2011;13(1):16–27. doi: 10.1111/j.1399-5618.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- Neves M.d.C., Duarte D.G., Albuquerque M.R., Nicolato R., Neves F.S., Souza-Duran F.L.d., Busatto G., Corrêa H. Neural correlates of hallucinations in bipolar disorder. Braz J Psychiatry. 2016;38(1):1–5. doi: 10.1590/1516-4446-2014-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman C.J., Petrovic P., Johansson A.G., Sellgren C., Ingvar M., Landen M. A History of Psychosis in Bipolar Disorder is Associated With Gray Matter Volume Reduction. Schizophr Bull. 2017;43(1):99–107. doi: 10.1093/schbul/sbw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørch-Johnsen L., Nerland S., Jørgensen K.N., Osnes K., Hartberg C.B., Andreassen O.A., Melle I., Nesvåg R., Agartz I. Cortical thickness abnormalities in bipolar disorder patients with a lifetime history of auditory hallucinations. Bipolar Disord. 2018;20(7):647–657. doi: 10.1111/bdi.12627. [DOI] [PubMed] [Google Scholar]
- Zhuo C., Lin X., Wang C., Song X., Xu X., Li G., Xu Y., Tian H., Zhang Y., Wang W., Zhou C. Unified and disease specific alterations to brain structure in patients across six categories of mental disorders who experience own-thought auditory verbal hallucinations: A pilot study. Brain Res Bull. 2020;160:33–39. doi: 10.1016/j.brainresbull.2020.04.001. [DOI] [PubMed] [Google Scholar]
- Okuneye V.T., Meda S., Pearlson G.D., Clementz B.A., Keshavan M.S., Tamminga C.A., Ivleva E., Sweeney J.A., Gershon E.S., Keedy S.K. Resting state auditory-language cortex connectivity is associated with hallucinations in clinical and biological subtypes of psychotic disorders. Neuroimage Clin. 2020;27:102358. doi: 10.1016/j.nicl.2020.102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Ye J., Ji F., Li G., Li G., Ma X., Li R., Tian H., Wang L., Chen G., Xu Y., Wang W., Jiang D., Pan J., Zhuo C. Common and distinct global functional connectivity density alterations in patients with bipolar disorder with and without auditory verbal hallucination during major depressive episodes. Brain Imaging Behav. 2020;14(6):2724–2730. doi: 10.1007/s11682-019-00222-4. [DOI] [PubMed] [Google Scholar]
- Baker J.T., Holmes A.J., Masters G.A., Yeo B.T.T., Krienen F., Buckner R.L., Öngür D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn A.K., Roh Y.S., Ravichandran C.T., Baker J.T., Ongur D., Cohen B.M. Aberrant cerebellar connectivity in bipolar disorder with psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(5):438–448. doi: 10.1016/j.bpsc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. New York State Psychiatric Institute; Biometrics Research: 1995. Structured clinical interview for DSM-IV Axis I Disorders. [Google Scholar]
- Montgomery S.A., Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Parvizi J. Corticocentric myopia: old bias in new cognitive sciences. Trends Cogn Sci. 2009;13(8):354–359. doi: 10.1016/j.tics.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Travis M.J., Fagiolini A., Kupfer D.J. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.J., Hollinshead M.O., O’Keefe T.M., Petrov V.I., Fariello G.R., Wald L.L., Fischl B., Rosen B.R., Mair R.W., Roffman J.L., Smoller J.W., Buckner R.L. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2(1) doi: 10.1038/sdata.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2011;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S., Trauer T., Mackinnon A., Sims E., Thomas N., Copolov D.L. A new phenomenological survey of auditory hallucinations: evidence for subtypes and implications for theory and practice. Schizophr Bull. 2014;40(1):231–235. doi: 10.1093/schbul/sbs156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O., Borra R.J., Bower J.M., Cullen K.E., Habas C., Ivry R.B., Leggio M., Mattingley J.B., Molinari M., Moulton E.A., Paulin M.G., Pavlova M.A., Schmahmann J.D., Sokolov A.A. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14(2):197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn A.K., Baker J.T., Lewandowski K.E., Ongur D., Cohen B.M. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Frontiers in human neuroscience. 2015;9:134. doi: 10.3389/fnhum.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R.O., Gonsalvez I., Lee I., Öngür D., Seidman L.J., Schmahmann J.D., Eack S.M., Keshavan M.S., Pascual-Leone A., Halko M.A. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am J Psychiatry. 2019;176(7):512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.u., Fan G., Xu K.e., Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 2011;34(6):1430–1438. doi: 10.1002/jmri.v34.610.1002/jmri.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69(10):967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G., Hulshoff Pol H.E., Haijma S.V., Cahn W., Kahn R.S., van den Heuvel M.P. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73. doi: 10.3389/fpsyt.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.L., Tu P.C., Lee Y.C., Chen Y.S., Li C.T., Su T.P. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res. 2013;149(1–3):26–34. doi: 10.1016/j.schres.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Chen J., et al. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci. Rep. 2015;5:17275. doi: 10.1038/srep17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Guo W., Liu F., et al. Patients with first-episode, drug-naive schizophrenia and subjects at ultra-high risk of psychosis shared increased cerebellar-default mode network connectivity at rest. Sci. Rep. 2016;6:26124. doi: 10.1038/srep26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Orr J.M., Mittal V.A. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. doi: 10.1016/j.nicl.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Zhang F., Liu F., Chen J., Wu R., Chen D.Q., Zhang Z., Zhai J., Zhao J. Cerebellar abnormalities in first-episode, drug-naive schizophrenia at rest. Psychiatry Res Neuroimaging. 2018;276:73–79. doi: 10.1016/j.pscychresns.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Zhuo C., Wang C., Wang L., Guo X., Xu Q., Liu Y., Zhu J. Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging Behav. 2018;12(2):383–389. doi: 10.1007/s11682-017-9704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J., Ford J.M., Roach B.J., Turner J.A., van Erp T.G., Voyvodic J., Preda A., Belger A., Bustillo J., O'Leary D., Mueller B.A., Lim K.O., McEwen S.C., Calhoun V.D., Diaz M., Glover G., Greve D., Wible C.G., Vaidya J.G., Potkin S.G., Mathalon D.H. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med. 2018;48(15):2492–2499. doi: 10.1017/S003329171800003X. [DOI] [PubMed] [Google Scholar]
- Lee KH, Oh H, Suh JS, et al. Functional and Structural Connectivity of the Cerebellar Nuclei With the Striatum and Cerebral Cortex in First-Episode Psychosis. J Neuropsychiatry Clin Neurosci. Dec 18 2018:appineuropsych17110276. doi:10.1176/appi.neuropsych.17110276. [DOI] [PubMed]
- Clos M., Diederen K.M.J., Meijering A.L., Sommer I.E., Eickhoff S.B. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct. Funct. 2014;219(2):581–594. doi: 10.1007/s00429-013-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Xi Y.-B., Cui L.-B., Wang H.-N., Sun J.-B., Zhu Y.-Q., Huang P., Collin G., Liu K., Xi M., Qi S., Tan Q.-R., Miao D.-M., Yin H. Distinct inter-hemispheric dysconnectivity in schizophrenia patients with and without auditory verbal hallucinations. Sci. Rep. 2015;5:11218. doi: 10.1038/srep11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallikarjun P.K., Lalousis P.A., Dunne T.F., Heinze K., Reniers R.L., Broome M.R., Farmah B., Oyebode F., Wood S.J., Upthegrove R. Aberrant salience network functional connectivity in auditory verbal hallucinations: a first episode psychosis sample. Transl Psychiatry. 2018;8(1):69. doi: 10.1038/s41398-018-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Li X., Feng G., et al. Altered Effective Connectivity in the Default Network of the Brains of First-Episode, Drug-Naive Schizophrenia Patients With Auditory Verbal Hallucinations. Frontiers in human neuroscience. 2018;12:456. doi: 10.3389/fnhum.2018.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium, Purcell S.M., Wray N.R., et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi T., Mimura M., Hasegawa S., Tani M., Kato N., Hashimoto R.I. Aberrant cerebellar-default-mode functional connectivity underlying auditory verbal hallucinations in schizophrenia revealed by multi-voxel pattern analysis of resting-state functional connectivity MRI data. Schizophr Res. 2018;197:607–608. doi: 10.1016/j.schres.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Chen X., Ji G.-J., Zhu C., Bai X., Wang L.u., He K., Gao Y., Tao L., Yu F., Tian Y., Wang K. Neural Correlates of Auditory Verbal Hallucinations in Schizophrenia and the Therapeutic Response to Theta-Burst Transcranial Magnetic Stimulation. Schizophr Bull. 2019;45(2):474–483. doi: 10.1093/schbul/sby054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.Y., Hsu J., Talmasov D., Joutsa J., Soussand L., Wu O., Rost N.S., Morenas-Rodríguez E., Martí-Fàbregas J., Pascual-Leone A., Corlett P.R., Fox M.D. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry. 2021;26(4):1299–1309. doi: 10.1038/s41380-019-0565-3. [DOI] [PubMed] [Google Scholar]
- Cierpka M., Wolf N.D., Kubera K.M., Schmitgen M.M., Vasic N., Frasch K., Wolf R.C. Cerebellar Contributions to Persistent Auditory Verbal Hallucinations in Patients with Schizophrenia. Cerebellum. 2017;16(5-6):964–972. doi: 10.1007/s12311-017-0874-5. [DOI] [PubMed] [Google Scholar]
- Zmigrod L., Garrison J.R., Carr J., Simons J.S. The neural mechanisms of hallucinations: A quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2016;69:113–123. doi: 10.1016/j.neubiorev.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Powers A.R., Mathys C., Corlett P.R. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357(6351):596–600. doi: 10.1126/science.aan3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterveld R.V., Diederen K.M., Otte W.M., Sommer I.E. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum Brain Mapp. 2013;35(4):1436–1445. doi: 10.1002/hbm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Schlerf J.E., Hodge S.M., Haselgrove C., Albaugh M.D., Seidman L.J., Rauch S.L., Harris G., Biederman J., Caviness V.S., Kennedy D.N., Schmahmann J.D. MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage. 2005;25(4):1146–1160. doi: 10.1016/j.neuroimage.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2010;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Kansal K., Yang Z., Fishman A.M., Sair H.I., Ying S.H., Jedynak B.M., Prince J.L., Onyike C.U. Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain. 2017;140(3):707–720. doi: 10.1093/brain/aww327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilescu M., Rossell S., Stuart G.W., Shea T.L., Innes-Brown H., Henshall K., McKay C., Sergejew A.A., Copolov D., Egan G.F. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med. 2010;40(7):1149–1158. doi: 10.1017/S0033291709991632. [DOI] [PubMed] [Google Scholar]
- Vercammen A., Knegtering H., den Boer J.A., Liemburg E.J., Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010;67(10):912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Fernandez T., Pittman B., Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69(5):407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I.E., Clos M., Meijering A.L., Diederen K.M.J., Eickhoff S.B., Draganski B. Resting state functional connectivity in patients with chronic hallucinations. PLoS One. 2012;7(9):e43516. doi: 10.1371/journal.pone.004351610.1371/journal.pone.0043516.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg E.J., Vercammen A., Ter Horst G.J., Curcic-Blake B., Knegtering H., Aleman A. Abnormal connectivity between attentional, language and auditory networks in schizophrenia. Schizophr Res. 2012;135(1–3):15–22. doi: 10.1016/j.schres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Schutte M.J L., Bohlken M.M., Collin G., Abramovic L., Boks M.P.M., Cahn W., Dauwan M., van Dellen E., van Haren N.E.M., Hugdahl K., Koops S., Mandl R.C.W., Sommer I.E.C. Sci Rep. 2021;11(1):1108. doi: 10.1038/s41598-020-80657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn A.K., Baker J.T., Cohen B.M., Ongur D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr Res. 2013;143(2–3):260–268. doi: 10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V., Knochel C., Matura S., Prvulovic D., Linden D.E., van de Ven V. Reduced functional connectivity and asymmetry of the planum temporale in patients with schizophrenia and first-degree relatives. Schizophr Res. 2013;147(2–3):331–338. doi: 10.1016/j.schres.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Diederen K.M., Neggers S.F., de Weijer A.D., et al. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol. Med. 2012;43(8):1685–1696. doi: 10.1017/S0033291712002541. [DOI] [PubMed] [Google Scholar]
- Kompus K., Westerhausen R., Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Modinos G., Costafreda S.G., van Tol M.J., McGuire P.K., Aleman A., Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex. 2012;49(4):1046–1055. doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Toh W.L., Thomas N., Hollander Y., Rossell S.L. On the phenomenology of auditory verbal hallucinations in affective and non-affective psychosis. Psychiatry Res. 2020;290:113147. doi: 10.1016/j.psychres.2020.113147. [DOI] [PubMed] [Google Scholar]
- Brady Jr. R.O., Tandon N., Masters G.A., Margolis A., Cohen B.M., Keshavan M., Öngür D. Differential brain network activity across mood states in bipolar disorder. J Affect Disord. 2017;207:367–376. doi: 10.1016/j.jad.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Harding I.H., Cocchi L., Yücel M., Pantelis C., Bullmore E.T. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50(3):970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Craddock R.C., James G.A., Holtzheimer P.E., Hu X.P., Mayberg H.S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33(8):1914–1928. doi: 10.1002/hbm.v33.810.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.