Highlights
-
•
MiR-362-3p inhibited cell viability and proliferation of ovarian cancer cells.
-
•
MiR-362-3p inhibited cell migration and invasion of ovarian cancer cells.
-
•
MiR-362-3p inhibited OV growth in vivo.
-
•
LRP8 was a target of miR-362-3p.
-
•
MiR-362-3p targeting LRP8 repressed cell viability and proliferation of ovarian cancer cells.
Keywords: miR-362-3p, L, Ovarian cancer, Growth, Cancer treatment
Abstract
Background
Ovarian cancer is one of the most common female cancers, with a high incidence worldwide. Aberrant expression of low‐density lipoprotein (LDL) receptor‐related protein 8 (LRP8) and microRNA (miR)-362-3p is involved in the pathogenesis of different cancers.
Methods
We aimed to elucidate the underlying mechanism of the miR-362-3p-LRP8 axis in ovarian cancer. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to examine miR-362-3p and LRP8 expression in ovarian cancer tissues and cells. The luciferase assay was used to determine the relationship between miR-362-3p and LRP8. The function of overexpression of miR-362-3p and LRP8 was determined by assessing the cell viability using the cell counting kit 8 (CCK-8) assay, proliferation using 5′‑bromo-2′-deoxyuridine (BrdU) assay, migration using wound healing assay, invasion using transwell assay, and the protein expression levels of matrix metalloproteinase (MMP)-2, MMP9, and integrin α5 or β1 using western blotting assays in ovarian cancer cells.
Results
miR-362-3p expression levels were decreased in ovarian cancer tissues and cells and negatively correlated with LRP8 levels. Overexpression of miR-362-3p dramatically repressed cell growth. Furthermore, overexpression of LRP8 significantly facilitated the proliferation, migration, and invasion of ovarian cancer cells, which counteracted the inhibitory effect of miR-362-3p on ovarian cancer cell growth.
Conclusions
We reported that miR-362-3p hampered cell growth by repressing LRP8 expression in ovarian cancer cells. Our results provide new insights into ovarian cancer, involving both miR-362-3p and LRP8, which can be used as potential biomarkers for the treatment of ovarian cancer.
Introduction
Ovarian cancer is one of the most common female cancers, with a high incidence worldwide [1]. Unfortunately, at least 70% of patients with ovarian cancer have a poor prognosis and low survival rate [2]. With the development of clinical treatment by surgery, radiotherapy, and chemotherapy, patients with ovarian cancer at an early stage often have a better prognosis. However, patients at advanced stages show a high recurrence rate and poor prognosis [3]. Therefore, it is to necessary to find effective therapeutic targets for the diagnosis and treatment for patients with ovarian cancer by exploring the pathogenesis of this disease.
MicroRNAs (miRNAs) containing 19–24 nucleotides bind to the target gene 3 untranslated regions (UTRs) and inhibit gene expression [4,5]. Many studies have suggested that miRNAs are closely related to biological processes, such as cell growth and apoptosis [6,7]. MiR-362-3p regulated cellular progression in many cancer types, including cervical, breast, and renal cancers [8,9]. Evidence has shown that miR-362-3p represses cell growth and elevates cell apoptosis by inhibiting nemo-like kinase in renal cancer cells [8]. Additionally, miR-362-3p inhibits the human ether-a-go-go-related gene (hERG) expression and suppresses the development of breast cancer [9]. Notably, miR-362-3p is associated with the development of ovarian cancer [10]. Studies have reported that miR-362-3p reduces cell growth by reducing the expression of myeloid differentiation factor (MyD)−88 in ovarian cancer [10]. To the best of our knowledge, this study is the first to report that miR-362-3p might inhibit the development of ovarian cancer; however, the underlying mechanism of miR-362-3p-LRP8 remains unknown.
Low‐density lipoprotein (LDL) receptor-related protein 8 (LRP8) gene is located on chromosome 1p32.3, and consists of 22 exons. It encodes a member of the low-density lipoprotein receptor (LDLR) family, which plays a critical role in the migration of neurons during development by mediating reelin signaling [11], [12], [13]. Abnormal LRP8 expression is associated with cancer progression [12,14]. For instance, the expression of LRP8 is enhanced in breast cancer, which facilitates cell growth and predicts a poor prognosis in patients with breast cancer [14]. Another study also clarified that LRP8 significantly accelerated the growth of triple-negative breast cancer stem cells [12]. However, the effect of LRP8 on the development of ovarian cancer remains unclear.
In this study, we sought to elucidate the interactome of miR-362-3p-LRP8 in ovarian cancer. We hypothesized that miR-362-3p might hamper the cell growth, migration, and invasion of ovarian cancer cells by repressing LRP8 expression. Our study may provide a good potential therapeutic approach for patients with ovarian cancer.
Materials and methods
Tissue specimens, cells culture, and transfection
Tissues, including tumor and normal tissues, were collected from patients with ovarian cancer (n = 32) from our hospital with informed consent. Human ovarian cancer cells (CaOV3 and SKOV3) were provided by the American Type Culture Collection (ATCC) (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco, USA) at 37 °C and 5% carbon dioxide (CO2). Transfectants, including the LRP8 overexpression plasmid and empty vector, miR-362-3p mimic, and mimic NC, were purchased from RiboBio (Guangzhou, China). CaOV3 and SKOV3 cells were transfected with them using Lipo3000 (Invitrogen, USA) for 48 h and then subjected to other experiments.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA from ovarian tissues and cells was isolated using Trizol (Invitrogen, USA), and reverse transcribed to cDNA using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Cat#: AT311–02; Transgene, China). Finally, cDNA was performed using SYBR Premix Ex Taq (Takara, Japan) to measure the LRP8 expression. miRNA from ovarian tissues and cells was isolated using Isolation Kit (Thermo Fisher, USA), and RNA was reverse transcribed to cDNA using the TaqMan MicroRNA Reverse Transcription Kit (Thermo, USA). Finally, cDNA was performed using the TaqMan OpenArray Real-Time PCR Master Mix (Thermo Fisher, USA) to measure miR-362-3p expression. The 2-ΔΔCt method was used to analyze LRP8 and miR-362-3p expression normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6, respectively. The primers used are listed in Table 1.
Table 1.
The PCR primers used in this study.
| Gene | Primers sequence (5′−3′) |
|---|---|
| miR-362-3p | F: AACACACCTATTCAAGGATTCA |
| R: ACGTGACACGTTCGGAGAATT | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT | |
| LRP8 | F: TCCTGCCGAGAAGTTAAGCTG |
| R: AAGAACGCAAGTCCCATCCC | |
| GAPDH | F: CATGGCCTTCCGTGTTCCTA |
| R: TGTCATCATACTTGGCAGGTTT |
Cell counting kit 8 (CCK-8) detection
Approximately 5 × 103 transfected CaOV3 and SKOV3 cells were seeded into 96-well plates. Cell viability was examined using a CCK-8 kit (Cat#: C0037; Beyotime, China). Before 24, 48, and 72 h, 10 µL CCK-8 solution was added to the plates and incubated for 2 h in a cell culture incubator. Finally, OD450 was detected on a multimode-plate reader (Thermo Fisher, USA).
5′‑bromo-2′-deoxyuridine (BrdU) detection
Approximately 2 × 104 transfected CaOV3 and SKOV3 cells were seeded into 96-well plates. Cell proliferation was examined using a BrdU kit (Cat#: 6813; CST, USA). When the cells reached 70% density, they were cultured in BrdU (Cat#: 6813, CST, USA) labeling solution for 6 h of incubation. Afterwards, the cells were fixed and denatured using a fixation/denaturation solution. Then, the cells were incubated with the BrdU antibody for 2 h. Subsequently, the secondary horseradish peroxidase (HRP)-mouse antibody was used to incubate the cells for another 2 h. Finally, the cells were treated with the HRP substrate, tetramethylbenzidine (TMB), and OD450 was detected on a multimode-plate-reader (Thermo Fisher, USA).
Wound healing detection
Approximately 2 × 104 transfected CaOV3 and SKOV3 cells were seeded into 6-well plates. When cells reached up to 70% density, a scratch across the plate was made using a sterile pipette, at the same time, the redundant cells were washed away. At 0 and 24 h, images were obtained under a light microscope, and the distance of the cell migration was recorded.
Cell invasion detection
Matrigel (Corning, USA) was added to the upper transwell chamber (Cat#: #3244; Coring, USA) of 6-well plates, and 10% FBS-containing medium was added to the lower chambers. The transfected CaOV3 and SKOV3 cells were seeded into the upper chamber. After 48 h, the invaded cells were fixed with 4% paraformaldehyde and treated with 0.1% crystal violet for 20 min. Invasive cells were photographed under a microscope and counted.
Luciferase detection
The pmiRGLO vectors containing LRP8 3′-UTRs wild type (WT) or mutant (MUT) sequences were purchased from Genechem (Shanghai, China). Approximately 5 × 103 CaOV3 and SKOV3 cells were seeded in 24-well plates overnight. Next, the cells were treated with pmiRGLO LRP8 3′-UTR WT, MUT1, or MUT2 plasmids, and miR-362-3p mimic or NC was used to treat the cells using Lipo3000. After 48 h, the luciferase assay kit (Cat#: RG027; Beyotime, China) was used to examine the relative luciferase activity normalized to renal luciferase activity.
RNA pull-down assay
Biotin-labeled miR-362-3p probe (Bio-miR-362-3p) and negative control probe (Bio-NC), purchased from Sangon (Shanghai, China), were incubated with magnetic beads at 4 °C overnight. CaOV3 and SKOV3 cells were lysed, and lysates were collected. Then, the cell lysates were incubated with magnetic beads for 1 h at 25 °C. After purification, mRNA enrichment was detected using qRT-PCR.
Animal models
The animal experiments in this study were approved by the Animal Ethics Committee of our hospital and were carried out in accordance with the relevant guidelines and regulations of the Animal Protection and Use Committee. Five-week-old female nude mice purchased from Hunan SJA Laboratory Animal Company (Hunan, China) were housed in a room free of specific pathogens. Cell suspensions of CaOV3 cells transfected with miR-362-3p mimic or mimic-NC were stably injected into the subcutaneous area of nude mice at a dose of 2 × 105 cells/100 μL. From day 8, tumor size was measured weekly and tumor volume was calculated, with V = 0.5 × length × width2. After 29 d, the mice were sacrificed for tumor weight detection and immunohistochemical analysis.
Ki67 staining assay
Xenograft tumors were fixed with 10% formaldehyde, embedded in paraffin, sliced, dewaxed, and hydrated. Citrate buffer (0.01 M) was used to measure antigen retrieval, and hydrogen peroxide (H2O2) solution (3%) was incubated with sections at 25 °C for 15 min. Subsequently, goat serum (5%) was incubated with the sections for 30 min at 25 °C. The sections were then incubated with the primary anti-Ki67 antibody (1:100; Geneseed Biotech, Guangzhou, China) overnight at 4 °C, and with the secondary antibody for 30 min at 25 °C. After staining with diaminobenzidine (DAB) and hematoxylin, the sections were sealed with neutral resin. The Ki67 positive signal was observed under a microscope (Olympus, Tokyo, Japan).
Western blotting analysis
Proteins from transfected CaOV3 and SKOV3 cells were isolated by incubating with the radioimmunoprecipitation assay (RIPA) buffer (Cat#: #20–188; Sigma, USA) on ice for 30 min. The same quantity of proteins was separated by 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), and proteins on the gel were transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline with Tween 20 (TBST), and anti-LRP8 (1:1000, Cat#: ab235909), anti-MMP2 (1:1000, Cat#: ab181286), anti-MMP9 (1:1000, Cat#: ab228402), anti-integrin α5 or β1 polyclonal (1:500, Cat#: BS-2016R; Thermo Fisher Scientific, USA), and anti-GAPDH (1:2000, Cat#: ab8245) antibodies were used to incubate the membranes overnight at 4 °C. The secondary antibodies, anti-HRP-Rabbit for LRP8 and anti-HRP-Mouse for GAPDH, were used to incubate the membranes for 1 h. All antibodies were obtained from Abcam (Cambridge, UK). An enhanced chemiluminescence system (Pierce Biotech, USA) was used to develop the protein bands. Relative LRP8 protein expression was normalized to that of GAPDH.
Statistical analysis
The data in this study were analyzed by paired t-test and one-way analysis of variance (ANOVA) for comparison of two groups and multiple groups, respectively, using GraphPad 8.0 (GraphPad, USA). The correlation between miR-362-3p and LRP8 was analyzed using Pearson's correlation analysis. Data are presented as the mean ± standard deviation (SD) from triplicate experiments. Statistical significance was set at P < 0.05.
Results
Potential regulator in ovarian cancer: miR-362-3p-LRP8 axis
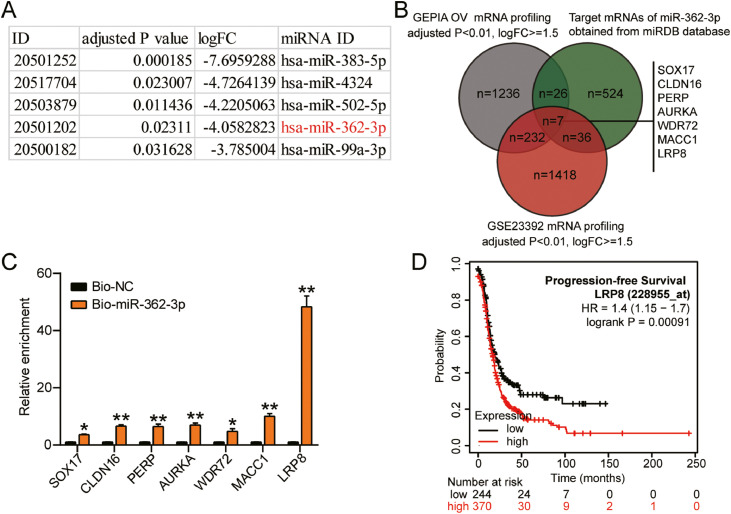
GSE119055 miRNA profiling in ovarian cancer was analyzed and 27 significantly downregulated miRNAs were identified using the criteria of adjusted P < 0.05, and logFC ≤ –1.5. The top five most significantly downregulated miRNAs are shown in Fig. 1A. We identified it as a significant tumor suppressor [9,15,16]. In 2020, the tumor suppressor role of miR-362-3p in ovarian cancer was reported for the first time [10]. Nonetheless, the role of miR-362-3p in ovarian cancer has not been thoroughly studied. Thus, we intended to conduct a study to investigate its function in ovarian cancer. To identify a downstream effector in ovarian cancer, we first predicted the targets of miR-362-3p based on the miRDB database. Then, we analyzed the mRNA profiling in ovarian cancer from the GSE23392 data series (criteria: adjusted P < 0.01, logFC ≥ 1.5), and obtained 1693 differentially expressed genes (DEGs). Another DEG list was obtained from Gene Expression Profiling Interactive Analysis (GEPIA) ovarian cancer data (criteria: adjusted P < 0.01, logFC ≥ 1.5). We intersected the three datasets, and seven genes were identified: SRY-box transcription factor 17 (SOX17), claudin 16 (CLDN16), p53 apoptosis effector related to PMP-22 (PERP), Aurora kinase A (AURKA), WDR72, metastasis-associated in colon cancer 1 (MACC1), and LRP8 (Fig. 1B). Moreover, RNA pull-down assay was used to explore the degree of enrichment of these genes in Bio-miR-362-3p. The results showed that LRP8 had the highest enrichment level in the Bio-miR-362-3p group (Fig. 1C). Furthermore, a high level of LRP8 was shown to predict poor progression-free survival outcomes among patients with ovarian cancer (Fig. 1D). In addition, LRP8 has been identified as a tumor driver in breast cancer [12,14]. Nonetheless, the role of LRP8 in ovarian cancer has not yet been reported. Thus, we aimed to study the potential role of the miR-362-3p-LRP8 axis in ovarian cancer.
Fig. 1.
miR-362-3p and LRP8 were speculated to be critical participants in OV.
(A) The significantly downregulated miRNAs (top 5) from GSE119055 data analysis, given in a downregulation descending order. (B) The intersection between the DEGs list of GEPIA OV data, the DEGs list of GSE23392 data analysis, and the predicted results of the targets of miR-362-3p from miRDB database. (C) RNA pull-down assay was used to assess the enrichment of SOX17, CLDN16, PERP, AURKA, WDR72, MACC1, and LRP8 in Bio-miR-362-3p or Bio-NC. (D) The progression-free survival prognosis result of OV patients from kmploter OV data (http://kmplot.com/, [29]).
miR-362-3p hampered the proliferation, migration, and invasion of ovarian cancer cells
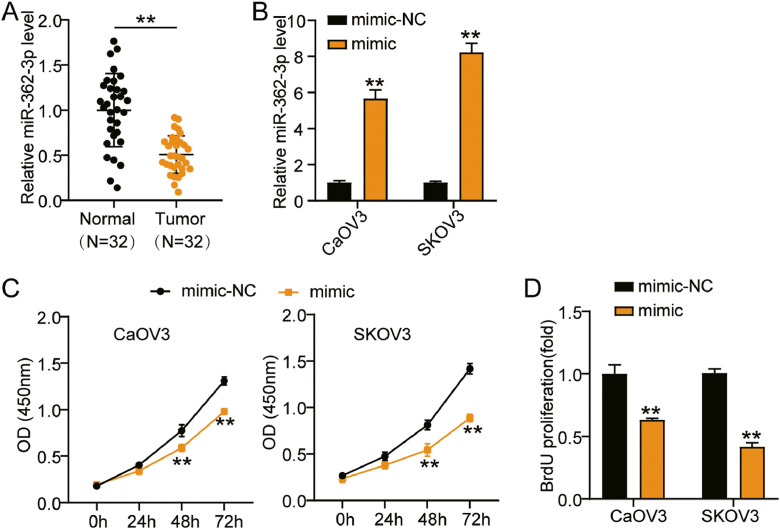
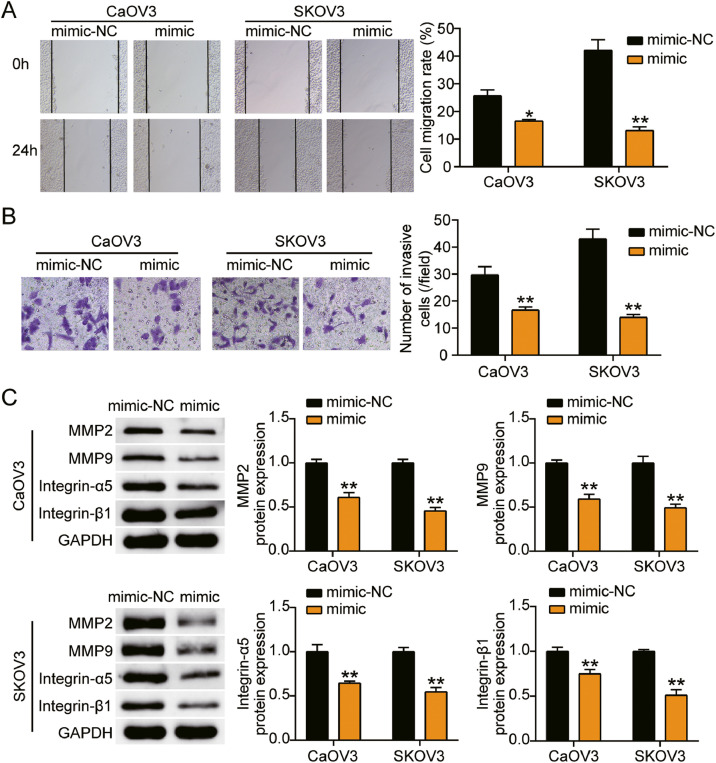
We first found that miR-362-3p levels were reduced by 50% in ovarian cancer tissues (Fig. 2A). Because CaOV3 and SKOV3 cells had the lowest levels, we transfected the miR-362-3p mimic and mimic-NC into CaOV3 and SKOV3 cells. The results illustrated that the mimic groups promoted approximately 6-fold and 8-fold miR-362-3p levels compared with NC groups in CaOV3 and SKOV3 cells, respectively (Fig. 2B). For functional studies, the mimic groups conspicuously prevented cell viability compared to the NC groups in both cells (Fig. 2C). Furthermore, the mimic groups showed approximately 50% repressed cell proliferation compared to the NC groups in both cells (Fig. 2D). On the other hand, the mimic groups showed nearly 30% and 60% decreased cell migration compared with NC groups in CaOV3 and SKOV3 cells, respectively (Fig. 3A). Moreover, the mimic groups displayed the same tendency of cell invasion as the NC groups in both cell types (Fig. 3B). Moreover, the protein expression levels of invasion markers (matrix metalloproteinase (MMP)−2, MMP-9 and α5/β1-integrin) were detected by western blotting. The protein levels of MMP-2, MMP-9, integrin-α5, and integrin-β1 were lower in the mimic group than the NC group (Fig. 3C). Taken together, miR-362-3p hampered the growth of ovarian cancer cells.
Fig. 2.
MiR-362-3p inhibited cell viability and proliferation of ovarian cancer cells.
(A) RT-qPCR detection of miR-362-3p expression in ovarian cancer tissues and normal tissues. (B) Measurement of miR-362-3p expression in CaOV3 and SKOV3 cells transfected with mimic-NC and miR-362-3p mimic by RT-qPCR. (C) Cell viability was detected in CaOV3 and SKOV3 cells transfected with mimic-NC and miR-362-3p mimic by CCK-8 assay. (D) Cell proliferation was detected in CaOV3 and SKOV3 cells transfected with mimic-NC and miR-362-3p mimic. **, P < 0.001. NC, negative control; mimic, miR-362-3p mimic.
Fig. 3.
MiR-362-3p inhibited cell migration and invasion of ovarian cancer cells.
(A) Cell migration was detected in CaOV3 and SKOV3 cells transfected with mimic-NC and miR-362-3p mimic. (B) Cell invasion was detected in CaOV3 and SKOV3 cells transfected with mimic-NC and miR-362-3p mimic. (C) MMP-2, MMP-9, integrin-α5 and integrin-β1 protein level were measured by western blot assay in CaOV3 and SKOV3 cells transfected with mimic-NC and miR-362-3p mimic. *, P < 0.05; **, P < 0.001. NC, negative control; mimic, miR-362-3p mimic.
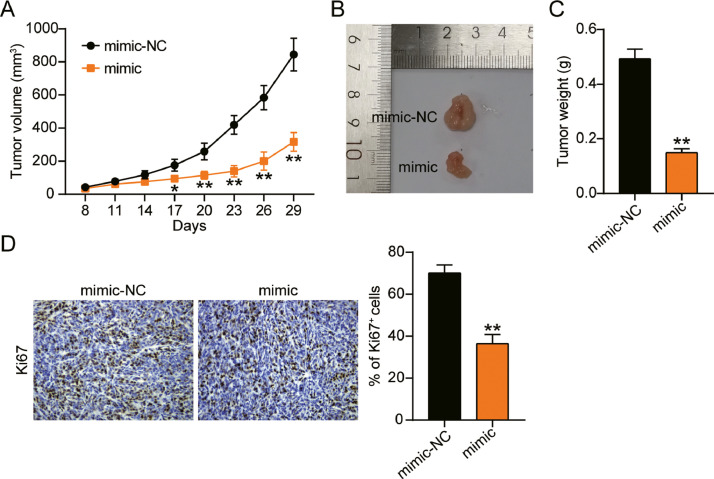
miR-362-3p inhibited ovarian cancer growth in vivo
The effect of miR-362-3p on ovarian cancer was monitored by in vivo experiments in nude mice. The tumor growth curve showed a significant reduction in tumor volume in the mimic group compared to that in the mimic-NC group (Fig. 4A). In addition, tumor dissection and weight measurement showed that miR-362-3p overexpression reduced the tumor size and weight (Fig. 4BC). The expression of Ki67 in xenograft tumor tissues was detected using IHC. Compared with the mimic-NC group, the positive signal of Ki67 was decreased in the mimic group (Fig. 4D). These data suggest that miR-362-3p inhibits the growth of ovarian cancer in vivo.
Fig. 4.
MiR-362-3p inhibited OV growth in vivo.
(A) The xenograft tumor volume in nude mice injected with CaOV3 treated with mimic-NC or mimic were monitored. (B) The xenograft tumors in each mice were photographed. (C) xenograft tumor weight in nude mice were detected. (D) Ki67 expression in xenograft tumors was measured by IHC assay. *, P < 0.05; **, P < 0.001. NC, negative control; mimic, miR-362-3p mimic.
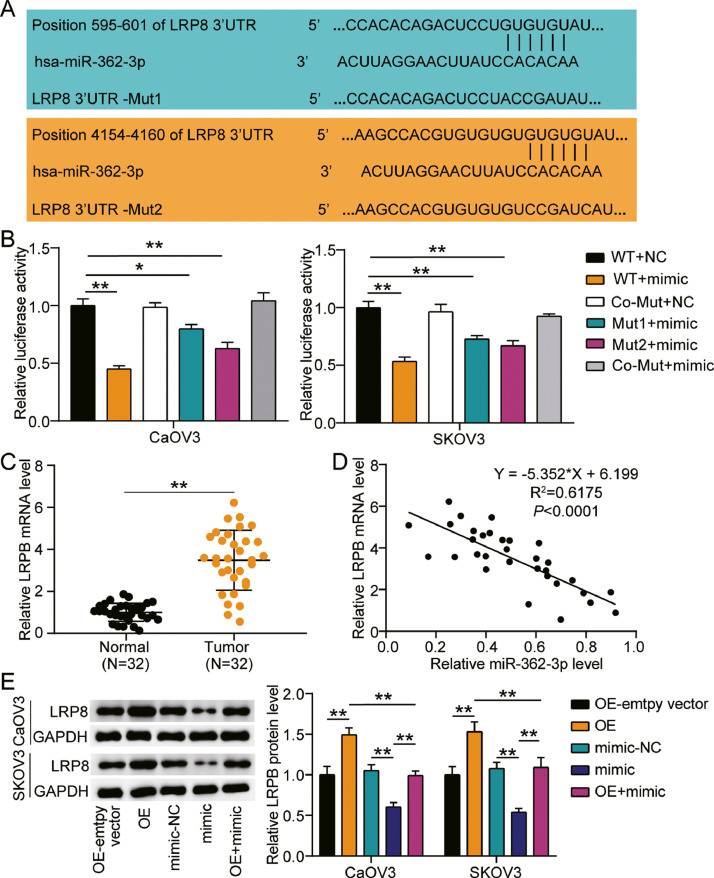
LRP8 was a target of miR-362-3p
Two binding sequences between LRP8 and miR-362-3p are shown in Fig. 5A using TargetScan Human 7.2. Thus, we transfected WT LRP8 3′-UTR and the two mutant LRP8 3′-UTR plasmids, and miR-362-3p-mimic or NC into CaOV3 and SKOV3 cells. The results showed that the luciferase activities of LRP8 3′-UTR WT and mimic groups decreased by 50%, while one of the mutant LRP8 3′-UTR plasmids with mimic treatment showed nearly 20% downregulated luciferase activity, and the mutant LRP8 3′-UTR plasmid groups showed no difference in both cells (Fig. 5B). In addition, LRP8 expression in the ovarian cancer tumor tissues showed approximately 4-fold upregulation compared to that in the normal tissues (Fig. 5C). At the same time, a negative correlation between miR-362-3p and LRP8 expression in ovarian cancer tumor tissues was observed (Fig. 5D). To corroborate the role of the miR-362-3p-LRP8 axis in ovarian cancer, we further transfected LRP8 overexpression (OE), OE-empty vector, miR-362-3p mimic, mimic-NC, and OE+ mimic into CaOV3 and SKOV3 cells. The results showed a nearly 1.5-fold increase in LRP8 protein expression in the OE groups, while 40% decreased LRP8 protein expression in the mimic groups in both cell lines. However, there was no difference in LRP8 protein expression between the OE+ mimic group and the OE-empty vector or mimic-NC groups (Fig. 5E).
Fig. 5.
LRP8 was a target of miR-362-3p.
(A) Bioinformatics analysis showed the predicted binding sequences of LRP8 3′-UTR. (B) Dual luciferase assay was performed in cells co-transfected with plasmids LRP8-WT or LRP8-MUT1 or LRP8-MUT2 and miR-NC or miR-362-3p mimic in CaOV3 and SKOV3 cells. (C) RT-qPCR analysis of LRP8 expression in ovarian cancer tissues and normal tissues. (D) Correlation analysis between the miR-362-3p expression and LRP8 expression in the ovarian cancer tumor tissues. (E) Measurement of LRP8 protein expression in CaOV3 and SKOV3 cells transfected with OE-empty vector, mimic-NC, OE, mimic and mimic+ OE by western blot. *, P < 0.05; **, P < 0.001. WT, wild-type; MUT, mutant; NC, negative control; OE, LRP8 overexpression; mimic, miR-362-3p mimic; mimic+ OE, miR-362-3p mimic+ LRP8 overexpression.
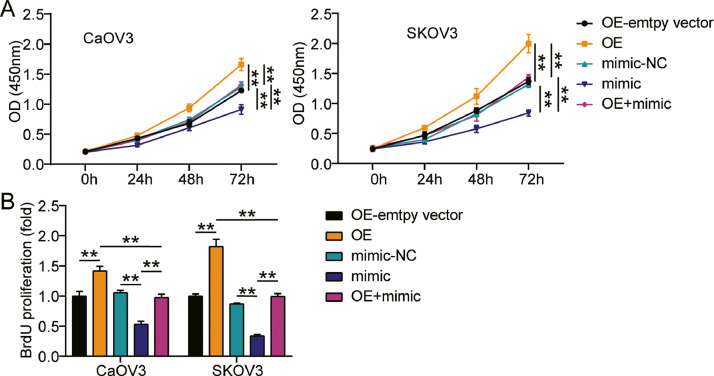
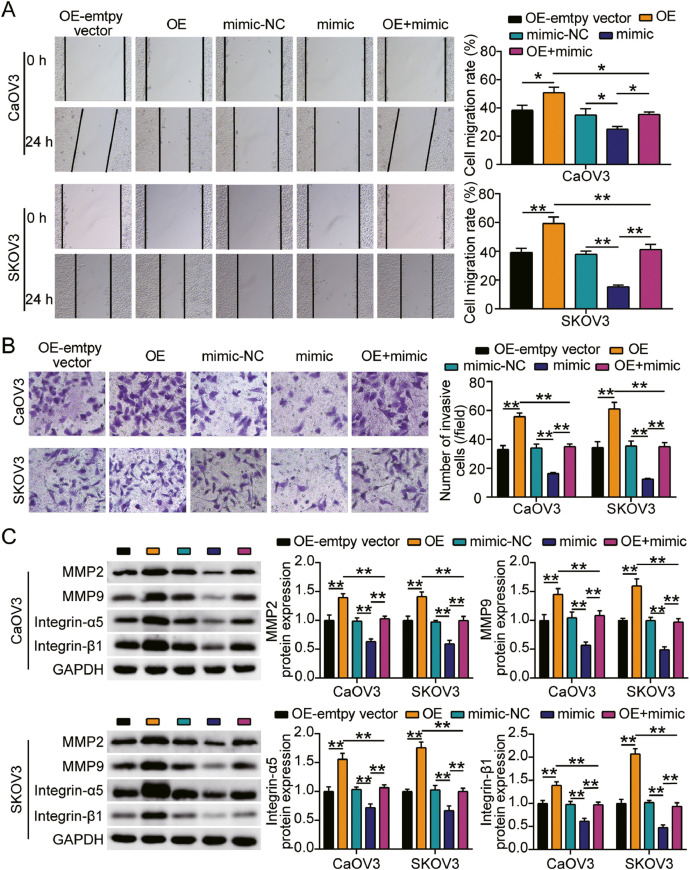
miR-362-3p targeting LRP8 suppressed the proliferation, migration, and invasion of ovarian cancer cells
We further examined the function of the miR-362-3p-LRP8 axis in ovarian cancer and found that the OE groups displayed accelerated cell viability compared with OE-empty vector groups, while cells transfected with mimic+ OE counteracted the oncogenic effect of LRP8 overexpression (Fig. 6A). In addition, the OE groups showed approximately 1.5-fold enhanced cell proliferation compared with the OE-empty vector groups, while cells transfected with mimic+ OE reversed the effect of LRP8 overexpression (Fig. 6B). Furthermore, the OE groups displayed a 1.3-fold increase in cell migration compared with the OE-empty vector groups, while the mimic+ OE groups showed no effect (Fig. 7A). Additionally, the OE groups showed a 2-fold increase in cell migration compared with the OE-empty vector groups, while the effect in the mimic+ OE groups was diminished (Fig. 7B). Finally, western blot analysis showed that compared with OE-empty vector groups, the protein levels of MMP-2, MMP-9, integrin-α5, and integrin-β1 in the OE groups were increased, while no significant changes were observed in the mimic+ OE groups (Fig. 7C). Collectively, these results suggest that miR-362-3p targeting LRP8 suppresses the proliferation, migration, and invasion of ovarian cancer cells.
Fig. 6.
MiR-362-3p targeting LRP8 repressed cell viability and proliferation of ovarian cancer cells.
(A) Cell viability was detected in CaOV3 and SKOV3 cells transfected with OE-empty vector, mimic-NC, OE, mimic and mimic+ OE by CCK-8 assay. (B) Cell proliferation was detected in CaOV3 and SKOV3 cells transfected with OE-empty vector, mimic-NC, OE, mimic and mimic+ OE by BrdU assay. *, P < 0.05; **, P < 0.001. NC, negative control; mimic, miR-362-3p mimic; OE, LRP8 overexpression; mimic+ OE, miR-362-3p mimic+ LRP8 overexpression.
Fig. 7.
MiR-362-3p targeting LRP8 suppressed cell migration and invasion of ovarian cancer cells.
(A) Cell migration was detected in CaOV3 and SKOV3 cells transfected with OE-empty vector, mimic-NC, OE, mimic and mimic+ OE by BrdU assay. (B) Cell invasion was determined in CaOV3 and SKOV3 cells transfected with OE-empty vector, mimic-NC, OE, mimic and mimic+ OE. (C) MMP-2, MMP-9, integrin-α5 and integrin-β1 protein level were measured by western blot assay in CaOV3 and SKOV3 cells transfected with OE-empty vector, mimic-NC, OE, mimic and mimic+ OE. *, P < 0.05; **, P < 0.001. NC, negative control; mimic, miR-362-3p mimic; OE, LRP8 overexpression; mimic+ OE, miR-362-3p mimic+ LRP8 overexpression.
Discussion
We found that miR-362-3p levels were reduced, while LRP8 levels were significantly upregulated in ovarian cancer tissues and cells. Overexpression of miR-362-3p inhibited ovarian cancer cell proliferation, migration, and invasion, but upregulation of LRP8 had the opposite effect in ovarian cancer cells. Mechanistically, miR-362-3p reduced cell growth by suppressing the expression of LRP8 in ovarian cancer cells.
Recently, there has been increasing evidence that non-coding RNAs, including miRNAs, long non-coding RNAs (lncRNAs), and circRNAs, play important roles in a variety of biological processes, including cell proliferation and apoptosis [17]. miRNA triggering is associated with translation inhibition or mRNA degradation of single or multiple transcripts [18]. lncRNAs/circRNAs may competitively bind to miRNAs through their miRNA response components, thus modulating miRNA target mRNA expression levels [19]. Therefore, lncRNA/circRNA-miRNA-mRNA interactions may be an important mechanism for cancer occurrence and development. This hypothesis was verified in a study on miR-362-3p. For example, circMYLK increased the expression of Rab23 by sponging miR-362-3p, thereby promoting the proliferation, invasion, and migration of hepatocellular carcinoma cells [20]. MALAT1 deletion inhibits drug resistance in renal cell carcinoma by regulating miR-362-3p-mediated GTPase-activating protein SH3 domain–binding protein 1 (G3BP1) [21]. High miR-362-3p expression was found in colorectal cancer, which induced cell cycle arrest by repressing the expression of E2F1, upstream stimulatory factor 2 (USF2), and protein tyrosine phosphatase non-receptor type 1 (PTPN1) in colorectal cancer cells [22]. Evidence has shown that miR-362-3p levels are reduced in cervical adenocarcinoma, and upregulation of miR-362-3p hampers cell growth by repressing the expression of minichromosome maintenance complex component 5 (MCM5) [23]. Furthermore, evidence has shown that miR-362-3p is inhibited by lncRNA SBF2-AS1, and upregulation of miR-362-3p could counteract the role of SBF2-AS1 by inhibiting GRB2 expression [24]. Notably, miR-362-3p was identified as an oncogene in gastric cancer, and downregulation of miR-362-3p significantly attenuated cell migration and invasion by decreasing CD82 expression in gastric cancer cells [25]. Regarding ovarian cancer, only one study revealed that miR-362-3p levels were downregulated in the tissues and cells, which could suppress cell growth and migration by reducing MyD88 expression [10]. Additionally, recent studies have also shown that miR-362-3p inhibits ovarian cancer and inhibits the occurrence and development of ovarian cancer by directly binding its target gene, SERPINE1 mRNA-binding protein 1 (SERBP1) [26]. Similar to a previous study, miR-362-3p levels were also significantly downregulated in ovarian cancer tissues and cells in our study. Furthermore, the miR-362-3p mimic prevented the growth of ovarian cancer cells. Moreover, overexpression of LRP8 effectively abrogated the ovarian cancer tumorigenesis role under miR-362-3p mimic treatment.
Recently, LRP8 has been associated with cancer pathogenesis, especially in breast cancer [11,12,14]. Lin et al. found that LRP8 was upregulated in breast cancer tissues, and downregulation of LRP8 dramatically reduced breast cancer stem cell growth [12]. Maire et al. found that depletion of LRP8 significantly inhibited cell proliferation, which is a potential target for breast cancer treatment [11]. Furthermore, LRP8 was repressed by miR-1262, and overexpression of LRP8 obviously elevated cell growth and tumor progression in breast cancer [14]. In contrast, LRP8 is downstream of apolipoprotein E2, which promotes cell growth and reduces cell apoptosis by activating the phosphorylation of extracellular signal‐regulated kinase (ERK)−1/2 signaling in pancreatic cancer cells [27]. Unfortunately, the biological role of LRP8 in ovarian cancer development remains unclear. Our study showed that LRP8 expression was significantly elevated in ovarian cancer tissues and cells. Overexpression of LRP8 dramatically facilitated the growth, migration, and invasion of ovarian cancer cells. Notably, we found that miR-362-3p downregulated LRP8 expression and attenuated the proliferation, migration, and invasion of ovarian cancer cells.
LRP8 affects cancer development through a complex regulatory network. It is downstream of apolipoprotein E2, which induces ERK1/2 phosphorylation to activate c-Myc and promotes the expression of cycle-related proteins involved in the malignant behavior of pancreatic cancer [27]. Inhibition of LRP8 inhibits the enrichment and proliferation of breast cancer stem cells in triple-negative breast cancer by weakening the Wnt/β-catenin signaling pathway [28]. In future studies, we will focus on the downstream signaling pathway of LRP8 to explore the effects of the miR-362-3p-LRP8 axis on the proliferation, invasion, and migration of ovarian cancer cells via the ERK1/2 or Wnt/β-catenin pathways. In addition, the upstream regulatory mechanism of miR-362-3p warrants further exploration.
Conclusions
In this study, we demonstrated that miR-362-3p repressed LRP8 expression to attenuate the proliferation, migration, and invasion of ovarian cancer cells. Therefore, our study provides a comprehensive examination of the roles of miR-362-3p and LRP8 in the pathological progression of ovarian cancer, which may aid in the development of effective therapeutic targets for the treatment of patients with ovarian cancer.
Declarations
Ethics approval and informed consent
The present study was approved by the Ethics Committee of Wuhan Third Hospital. The processing of clinical tissue samples is in strict compliance with the ethical standards of the Declaration of Helsinki. All patients signed written informed consent.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Chun Li: Methodology, Writing – review & editing. Yi Yang: Methodology, Writing – review & editing. Huimin Wang: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Yu Song: Formal analysis, Writing – review & editing. Huan Huang: Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Not applicable.
References
- 1.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M., Xia B., Xu Y., Zhang Y., Xu J., Lou G. Circular RNA (hsa_circ_0051240) promotes cell proliferation, migration and invasion in ovarian cancer through miR-637/KLK4 axis. Artif. Cells Nanomed. Biotechnol. 2019;47(1):1224–1233. doi: 10.1080/21691401.2019.1593999. [DOI] [PubMed] [Google Scholar]
- 3.Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527(7579):S217. doi: 10.1038/527S217a. [DOI] [PubMed] [Google Scholar]
- 4.Kiselev F.L. [MicroRNA and cancer] Mol. Biol. (Mosk.) 2014;48(2):232–242. [PubMed] [Google Scholar]
- 5.Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11(3):228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 6.Li X.T., Wang H.Z., Wu Z.W., Yang T.Q., Zhao Z.H., Chen G.L., et al. miR-494-3p regulates cellular proliferation, invasion, migration, and apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell. Mol. Neurobiol. 2015;35(5):679–687. doi: 10.1007/s10571-015-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae D.K., Park J., Cho M., Ban E., Jang M., Yoo Y.S., et al. MiR-195 and miR-497 suppress tumorigenesis in lung cancer by inhibiting SMURF2-induced TGF-β receptor I ubiquitination. Mol. Oncol. 2019;13(12):2663–2678. doi: 10.1002/1878-0261.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X., Zhong J., Li J., Su Z., Chen Y., Deng W., et al. miR-362-3p targets nemo-like kinase and functions as a tumor suppressor in renal cancer cells. Mol. Med. Rep. 2016;13(1):994–1002. doi: 10.3892/mmr.2015.4632. [DOI] [PubMed] [Google Scholar]
- 9.Assiri A.A., Mourad N., Shao M., Kiel P., Liu W., Skaar T.C., et al. MicroRNA 362-3p Reduces hERG-related current and inhibits breast cancer cells proliferation. Cancer Genom. Proteom. 2019;16(6):433–442. doi: 10.21873/cgp.20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J., Li T., Yi K., Hou M. The suppressive role of miR-362-3p in epithelial ovarian cancer. Heliyon. 2020;6(7):e04258. doi: 10.1016/j.heliyon.2020.e04258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maire V., Mahmood F., Rigaill G., Ye M., Brisson A., Némati F., et al. LRP8 is overexpressed in estrogen-negative breast cancers and a potential target for these tumors. Cancer Med. 2019;8(1):325–336. doi: 10.1002/cam4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C.C., Lo M.C., Moody R., Jiang H., Harouaka R., Stevers N., et al. Targeting LRP8 inhibits breast cancer stem cells in triple-negative breast cancer. Cancer Lett. 2018;438:165–173. doi: 10.1016/j.canlet.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asif M., Bhat S., Nizamuddin S., Mustak M.S. TG haplotype in the LRP8 is associated with myocardial infarction in south Indian population. Gene. 2018;642:225–229. doi: 10.1016/j.gene.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Li L., Qu W.H., Ma H.P., Wang L.L., Zhang Y.B., Ma Y. LRP8, modulated by miR-1262, promotes tumour progression and forecasts the prognosis of patients in breast cancer. Arch. Physiol. Biochem. 2020:1–9. doi: 10.1080/13813455.2020.1716019. [DOI] [PubMed] [Google Scholar]
- 15.Song L., Liu S., Yao H., Zhang L., Li Y., Xu D., et al. MiR-362-3p is downregulated by promoter methylation and independently predicts shorter OS of cervical squamous cell carcinoma. Biomed. Pharmacother. 2019;115 doi: 10.1016/j.biopha.2019.108944. [DOI] [PubMed] [Google Scholar]
- 16.Yang S., Zhang X., Sun Y., Shi J., Jiang D., Wang J., et al. MicroRNA-362-3p inhibits migration and invasion via targeting BCAP31 in cervical cancer. Front. Mol. Biosci. 2020;7:107. doi: 10.3389/fmolb.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santosh B., Varshney A., Yadava P.K. Non-coding RNAs: biological functions and applications. Cell Biochem. Funct. 2015;33(1):14–22. doi: 10.1002/cbf.3079. [DOI] [PubMed] [Google Scholar]
- 18.Hausser J., Zavolan M. Identification and consequences of miRNA-target interactions-beyond repression of gene expression. Nat. Rev. Genet. 2014;15(9):599–612. doi: 10.1038/nrg3765. [DOI] [PubMed] [Google Scholar]
- 19.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z., Hu Y., Zeng Q., Wang H., Yan J., Li H., et al. Circular RNA MYLK promotes hepatocellular carcinoma progression by increasing Rab23 expression by sponging miR-362-3p. Cancer Cell Int. 2019;19:211. doi: 10.1186/s12935-019-0926-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Wang Z., Chang X., Zhu G., Gao X., Chang L. Depletion of lncRNA MALAT1 inhibited sunitinib resistance through regulating miR-362-3p-mediated G3BP1 in renal cell carcinoma. Cell Cycle. 2020;19(16):2054–2062. doi: 10.1080/15384101.2020.1792667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen L.L., Tobiasen H., Holm A., Schepeler T., Ostenfeld M.S., Thorsen K., et al. MiRNA-362-3p induces cell cycle arrest through targeting of E2F1, USF2 and PTPN1 and is associated with recurrence of colorectal cancer. Int. J. Cancer. 2013;133(1):67–78. doi: 10.1002/ijc.28010. [DOI] [PubMed] [Google Scholar]
- 23.Wang D., Wang H., Li Y., Li Q. MiR-362-3p functions as a tumor suppressor through targeting MCM5 in cervical adenocarcinoma. Biosci. Rep. 2018;38(3) doi: 10.1042/BSR20180668. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Wang A., Wang J. E2F1-induced overexpression of long noncoding RNA SBF2-AS1 promotes non-small-cell lung cancer metastasis through regulating miR-362-3p/GRB2 axis. DNA Cell Biol. 2020;39(7):1290–1298. doi: 10.1089/dna.2020.5426. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q.H., Yao Y.L., Wu X.Y., Wu J.H., Gu T., Chen L., et al. Anti-miR-362-3p inhibits migration and invasion of human gastric cancer cells by its target CD82. Dig. Dis. Sci. 2015;60(7):1967–1976. doi: 10.1007/s10620-015-3563-6. [DOI] [PubMed] [Google Scholar]
- 26.Cao S., Li N., Liao X. miR-362-3p acts as a tumor suppressor by targeting SERBP1 in ovarian cancer. J. Ovarian Res. 2021;14(1):23. doi: 10.1186/s13048-020-00760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du S., Wang H., Cai J., Ren R., Zhang W., Wei W., et al. Apolipoprotein E2 modulates cell cycle function to promote proliferation in pancreatic cancer cells via regulation of the c-Myc-p21(Waf1) signalling pathway. Biochem. Cell Biol. 2020;98(2):191–202. doi: 10.1139/bcb-2018-0230. [DOI] [PubMed] [Google Scholar]
- 28.Lin C.C., Lo M.C., Moody R., Jiang H., Harouaka R., Stevers N., et al. Corrigendum to "Targeting LRP8 inhibits breast cancer stem cells in triple-negative breast cancer" [Canc. Lett. 438 (2018, Dec 1) 165-173] Cancer Lett. 2020;480:51. doi: 10.1016/j.canlet.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Gyorffy B., Lánczky A., Szállási Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer. 2012;19(2):197–208. doi: 10.1530/ERC-11-0329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.