Abstract
Investigation of the role of animals that have recovered and survived from African swine fever (ASF) in carrying the ASF virus is currently intense and ongoing. However, no clear definition of the carrier stage has been established. The aim of the present study was to establish criteria to elucidate a clear status of survival in naturally ASF-infected domestic pigs in Vietnam. Seroconversion from previous infection was confirmed by serological assay, and the absence of the viral genome in various organs was also assured by molecular analysis of a partial p72 gene. We recognized that histopathological evidence could benefit from further insights into the status and role of the surviving animals; therefore, we performed a histopathological study on four pigs from farms with a history of ASF outbreak. We found fibrotic changes in the reparative process as the main finding in all four pigs. Immunohistochemical detection of viral protein revealed an interesting result. Despite the negative result from viral genome detection, the p30 protein gave a positive signal in the tonsils, lung, and stomach. This raises the possibility of stress-induced viral reactivation in long-term survivors and the risk of further outbreaks from human handling of contaminated carcasses.
Keywords: African swine fever, animal disease survival, contaminated carcasses, immunohistochemistry, reparative process
African swine fever (ASF) is currently considered an important transboundary disease in the global swine population. The etiologic pathogen of ASF is African swine fever virus (ASFV), a large double-stranded DNA virus member of the Asfaviridae family, genus Asfivirus [1]. This virus produces a lethally contagious hemorrhagic disease and is listed in the World Organization for Animal Health (OIE) Terrestrial Animal Health Code and must be reported at once when detected.
As stated in the OIE terrestrial manual 2019 [28], the polymerase chain reaction (PCR) is currently the most sensitive technique and can detect ASFV DNA at a very early stage of infection and several weeks after infection in recovered pigs. However, anti-ASFV antibodies can persist for several months or even years and no commercial vaccine against ASFV is yet available, Therefore, the detection of an immune response by enzyme-linked immunosorbent assay (ELISA) can help confirming a previous infection in animals, especially the suspected cases in endemic areas such as Vietnam where there is still a latent circulation of ASFV [24, 27]. For these reasons, the resulting synergism of PCR and ELISA is considered a crucial diagnostic technique for identifying recovered and asymptomatically surviving animals.
The appearance of survivors and convalescent cases highlights the importance of these animals becoming disease carriers after recovery from the disease [18, 19]. Many experimental and epidemiological studies have attempted to determine the longest duration and amount of persisting virus in tissue samples, as well as the transmissibility of virus from pigs that survive ASF infection [21]. For example, Nurmoja et al. [17] demonstrated that surviving animals were unable to enter a carrier state by commingling three sentinel wild boars with an animal that had recovered from infection with a highly virulent ASFV for 50–96 days post inoculation. All samples from the sentinels and the survivor were negative for the viral genome. No transmission from survivors to commingled sentinel domestic pigs has consistently been reported in a study of ASF long-term carrier status [20], despite detection of the viral genome in infected pigs for at least 90 days. These studies showed that the surviving animals may not be able to transmit the virus through direct contact. However, the presence of persisting virus in some tissues could possibly be the source of prolonged disease spreading.
Past studies of ASF surviving case were unable to include the importance of histopathological evidence into their scope of studies. Therefore, the aim of the present study was to evaluate the microscopic lesions in various organs in pigs that naturally have survived from ASF infection and to verify the possibility that viral antigen may persist in some tissues even in the absence of the viral genome. The overall goal was to enhance the understanding of the status and role of the surviving animals in ASF disease.
MATERIALS AND METHODS
Animal selection and sample collection
Pig farms with reports of ASF outbreak in August 2019 and from July to September 2020 were included in this study. According to the plans and countermeasures in Vietnam for the emergency prevention and control of ASF and the guidance on measures to prevent and combat ASF issued by the Ministry of Agriculture and Rural Development [7, 16], healthy animals in non-infected housing whose blood sample tested negative to ASFV by quantitative real-time PCR (qPCR) were isolated and closely monitored by veterinarian authorities. Thereafter, a single pig from each farm was selected as a surviving animal. The criteria for animal selection were as follows: 1) the animal showed no notable clinical signs, such as cutaneous hemorrhage or high fever (≥40.0°C) at 90 days or later after the ASF outbreak on the farm, 2) a serum sample showed seroconversion detected by a commercial blocking ELISA (INgezim PPA COMPAC, Ingenasa, Madrid, Spain) according to the manufacturer’s protocol, but a blood sample was negative according to qPCR, 3) a serum sample collected again one week after the first screening showed retained seroconversion by ELISA, 4) qPCR testing of blood, oropharyngeal fluid, and feces collected prior to euthanasia and multiple organ tissues from necropsy showed negative reactions. Ultimately, fifteen farms from three provinces were eligible for case screening. Pigs from 10 of the 15 farms passed the first and second criteria for selection; however, the owners had to consent to euthanasia, so only four pigs were finalized as our surviving animals. Information on the selected animals is shown in Table 1. Multiple tissue samples from various organs were fixed in 10% buffered formalin and routinely processed for histopathological examination.
Table 1. Information of selected animals from criteria as surviving pigs.
| Case no. | Age | Province | Farm size (Standing pig population) |
Period after outbreaka | Test at day of screening |
Test at day of sacrifice |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA |
qPCRb |
ELISA |
qPCRc |
||||||||
| Result | Ct value | Result | % blockingd | Result | Ct value | Result | |||||
| 1 | 31 weeks | Hung Yen | 1,000 | 4 months | + | nde | - | 100.85 | + | nd | - |
| 2 | 25 weeks | Ha Nam | 500 | 3.5 months | + | nd | - | 102.45 | + | nd | - |
| 3 | 20 weeks | Ha Nam | 800 | 3 months | + | nd | - | 100.43 | + | nd | - |
| 4 | 21 weeks | Hung Yen | 500 | 3 months | + | nd | - | 101.36 | + | nd | - |
aApproximate period from report of outbreak until study. bQuantitative real-time PCR detection of African swine fever from blood sample. cQuantitative real-time PCR detection of African swine fever from blood sample, oropharyngeal fluid, feces, and multiple organs tissue. d% blocking was calculated according to the manufacturer’s instruction. end: not detected.
Quantitative real-time PCR (qPCR)
ASFV DNA extraction was performed from the blood, secretion/excretion samples, and 10% suspensions of homogenized tissues with a nucleic acid extraction kit (DNeasy Blood & Tissue Kit, Qiagen, Hilden, Germany). The qPCR reactions were conducted in duplicate in two independent assays, as described by King et al. [13] and Tignon et al. [23]. Taqman® probes and primers were used for detection of 250 bp [13] and 159 bp [23] of the C-terminal end of the ASFV p72 gene. The analyzed tissue samples in all pigs were tongue, brain, lung, heart, liver, gall bladder, kidney, urinary bladder, uterus, stomach, small intestine, large intestine, bone marrow, tonsil, spleen, and lymph nodes including the retropharyngeal, submandibular, gastrohepatic, mesenteric, inguinal, pulmonary, splenic (except pig no. 1 and pig no. 4), renal (except pig no. 2 and pig no. 4), and hepatic lymph nodes (except pig no. 1 and pig no. 2).
Histopathology and immunohistochemistry (IHC) examination
Formalin-fixed, paraffin-embedded tissue samples were consecutively sectioned at a thinness of 3–4 μm. Multiple areas from the lung, heart, liver, kidney, urinary bladder, stomach, small intestine, large intestine (except pig no. 1), uterus (except pig no. 4), brain, spleen, lymph nodes, and tonsils (except pig no. 1) were examined for all surviving animals. The tongue from pig no. 1 and the ureter and spinal cord tissue from pig no. 2 were also evaluated. Slides were stained using a routine hematoxylin-eosin (HE) protocol for histopathological examination. The IHC staining procedure for ASFV antigen detection followed the protocol of Izzati et al. [12]. The rabbit polyclonal ASFV phosphoprotein p30 antibody (Alpha Diagnostic International, San Antonio, TX, USA) was used as the primary antibody. Spleen and lung sections from a pig with confirmed ASFV infection by previous IHC and qPCR [12] were used as positive controls. Purified rabbit serum (negative control rabbit, ready-to-use; Dako Cytomation, Santa Clara, CA, USA) was used as a negative isotype control. Tissue sections from various tissues of a pig in ASFV-free farm collected in our previous study [12] were used as a negative antigen control. Double IHC staining for the ASFV phosphoprotein p30-Iba 1 (FUJIFILM Wako Pure Chemical Corp., Richmond, VA, USA), -vimentin (Dako, Tokyo, Japan), -AE1/AE3 pancytokeratin (Dako) were conducted on the selected stomach section of one examined pig. The procedure followed the protocol of Van Diep et al. [25]. The first reaction stained with ASFV phosphoprotein p30 was visualized using 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA) chromogen. The second reaction either stained with Iba 1, Vimentin, and AE1/AE3 was visualized by the product of 4-Chloro-1-naphthol (Tokyo Chemical Industry, Tokyo, Japan).
Ethical approval
Animals in this study were euthanized humanely by anesthetic method using pentobarbital sodium prior to electrocution method. The authors confirm the strict adherence to the ethical policies of the journal, as noted on the journal’s author guidelines and the appropriate ethical review committee approval has been received. The Animal Ethics Committees in Vietnam National University of Agriculture (VNUA-2020-01) approved this study procedure.
RESULTS
Macroscopic findings
The typical macroscopic findings of ASF, which are hemorrhagic lesions in multiple organs were not detected in any examined pigs. There were no significant changes on spleen and the tonsils of all pigs. Mild congestion was noted on the lymph node of pig no. 1. Fibrous adhesion was seen on the hearts of pig no. 1 and no. 3. Lung of pig no. 1 showed mild irregularly distributed multiple red areas and multiple pinpoint black foci. Pig no. 2 and no. 3 had multifocal to coalescing small white foci on the lung surface. Multifocal white foci were noted only in the liver of pig no. 2.
Detection of viral gene by qPCR
The qPCR results for the blood sample, oropharyngeal fluid, feces, and multiple organ tissues of all pigs collected on the day of necropsy were all negative for ASFV.
Histopathological and immunohistochemical (IHC) examination
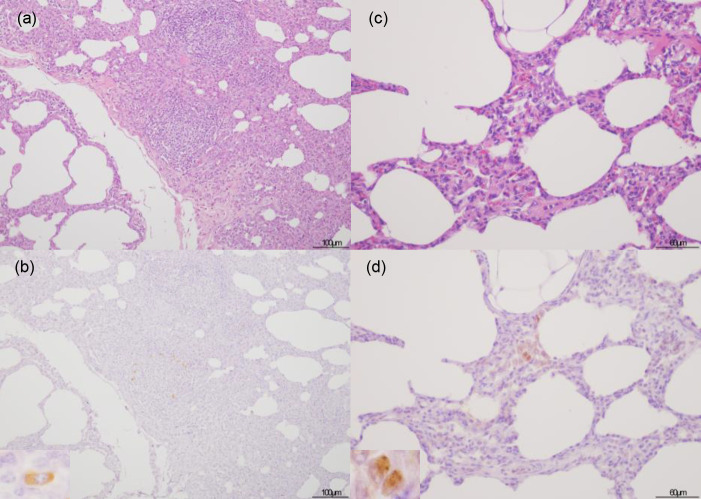
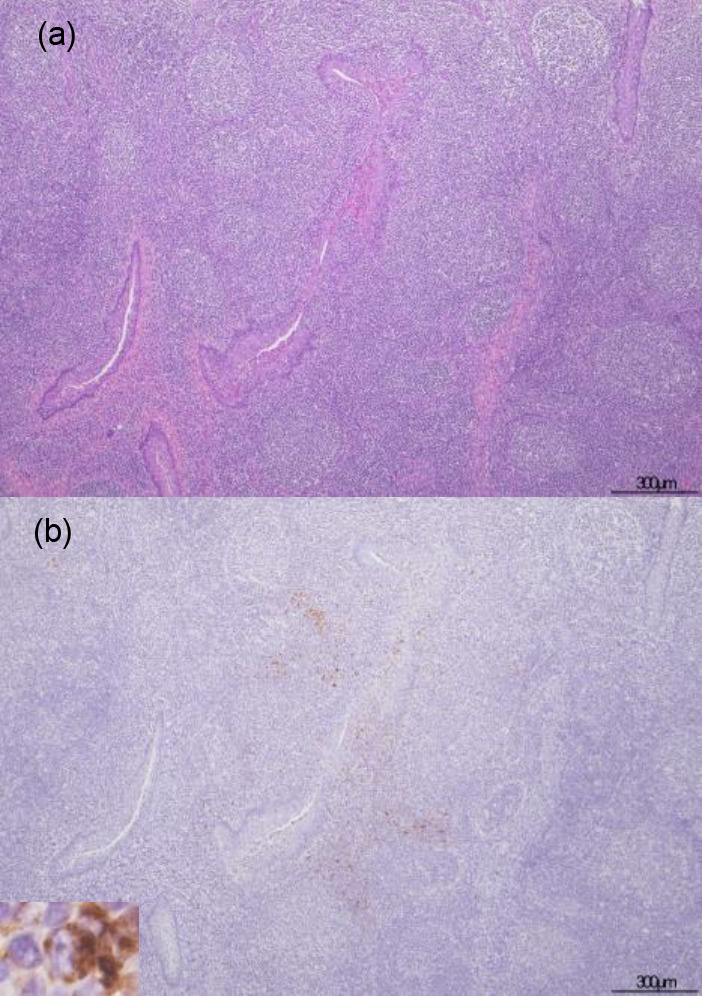
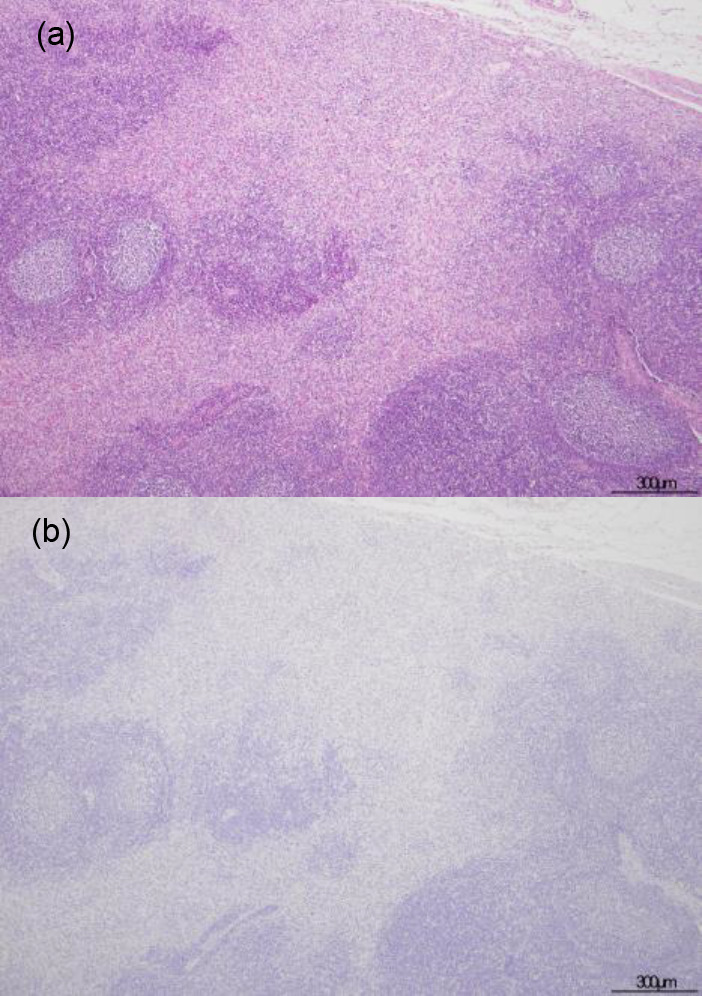
The histopathological examination revealed lesions on the lymphoid organs and predominant changes in the examined pigs (Table 2). The result of IHC examination is tabulated in Table 3. No remarkable lesions were found in the spleen and tonsils (Fig. 1a). Only one animal had a small focal apoptosis in the center of the white pulp of the spleen. Neutrophil infiltration was rarely seen in the lymphoid organ parenchyma. However, viral antigen was detected in the tonsils but not in the spleen. Most of the positive cells in the tonsils were mononuclear cells that appeared in apposition to the tonsillar crypt (Fig. 1b). Conversely, the lymph nodes of all animals showed marked fibrosis in the sinuses, especially in the subcapsular area without viral antigen presentation (Fig. 2). Mild to moderate degrees of sinus erythrocytosis were seen in pig no. 1 and no. 4. Noticeable numbers of eosinophils were generally observed in the lymph nodes of all four pigs, whereas neutrophilic infiltration was seen only in pig no. 4.
Table 2. Histopathological lesions of lymphoid organs and predominant changes in the pigs survived from the African swine fever outbreak in Vietnam.
| Organ | Histopathological findings | No. 1 | No. 2 | No. 3 | No. 4 |
|---|---|---|---|---|---|
| Spleen | Focal apoptosis | - | - | - | + |
| Inflammatory cell infiltration | + | - | - | + | |
| Focal congestion | - | - | + | - | |
| Tonsil | Lymphoid atrophy | ns | - | - | - |
| Lymph node | Subcapsular fibrosis | +++ | ++ | ++ | ++ |
| Inflammatory cell infiltration | - | + | - | ++ | |
| Lung | Edema | + | - | - | + |
| Fibrosis | ++ | ++ | + | +++ | |
| Hemorrhage | - | - | - | ++ | |
| Focal alveolar septum collapse | + | - | - | - | |
| Inflammatory cell infiltration | ++ | ++ | - | - | |
| Bronchus-associated lymphoid tissue hyperplasia | + | + | - | - | |
| Heart | Pericardial fibrosis | +++ | - | ++ | +++ |
| Inflammatory cell infiltration | + | - | - | ++ | |
-: no lesion; +: mild; ++: moderate; +++: severe; ns: no sample [12].
Table 3. Semi-quantitative examination of African swine fever virus antigen by immunohistochemistry study in surviving pigs in Vietnam.
| Organ | Surviving pigs |
|||
|---|---|---|---|---|
| Case no. 1 | Case no. 2 | Case no. 3 | Case no. 4 | |
| Spleen | 0 | 0 | 0 | 0 |
| Tonsil | ns | + | ++ | +++ |
| Lymph node | 0 | 0 | 0 | 0 |
| Liver | 0 | 0 | 0 | 0 |
| Lung | + | + | + | + |
| Brain | 0 | 0 | 0 | 0 |
| Heart | 0 | 0 | 0 | 0 |
| Stomach | ++ | +++ | 0 | +++ |
| Large intestine | ns | 0 | 0 | 0 |
| Kidney | 0 | 0 | 0 | 0 |
| Urinary bladder | 0 | 0 | 0 | 0 |
Number of positive staining in labelled cells counted in 3 fields under ×400 magnifications (light microscope, field number 18); 0: no positive cells; +: 1–10 positive cells; ++: 11–20 positive cells; +++: 21–30 positive cells; ++++: 31–40 positive cells; +++++: >41 positive cells; ns: no sample [12].
Fig. 1.
Hematoxylin & eosin and immunohistochemical staining for African swine fever phosphoprotein p30 in tonsils. The inset show African swine fever virus-positive cell. No remarkable changes were noticed in the tonsils of case no. 4 (a). Positive staining of mononuclear cells was detected in apposition to the tonsillar crypt in case no. 4 (b).
Fig. 2.
Hematoxylin & eosin and immunohistochemical staining for African swine fever phosphoprotein p30 in lymph nodes. Marked fibrosis predominantly presented in the sinuses, especially in the subcapsular area of case no. 2 (a). Antigen-labeled cell was not present in the lesion of case no. 2 (b).
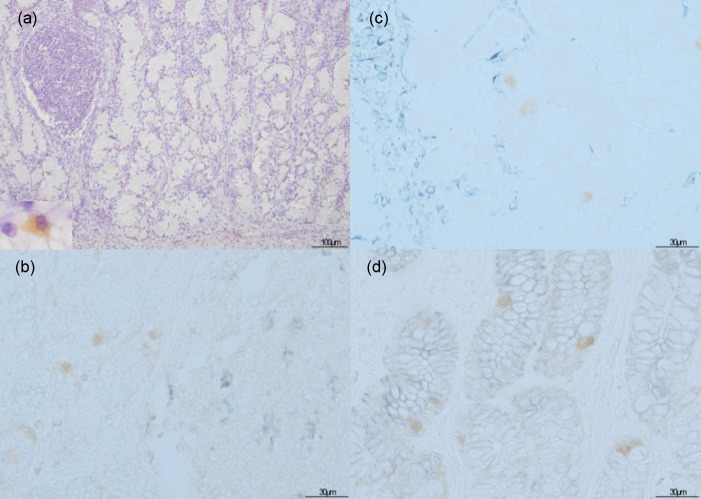
The lung showed lesions with scarce viral antigen. The common lesion seen in all four pigs was a thickening of the alveolar septum by fibrous tissue. Some mononuclear inflammatory cells were simultaneously observed in lung parenchyma in two pigs (no. 1 and no. 2), indicating an interstitial pneumonia condition. Bronchus-associated lymphoid tissue hyperplasia was also encountered in these two pigs (Fig. 3). Focal alveolar septal collapse and edematous interlobular septa were noted in pig no. 1. IHC revealed only limited numbers of macrophages were labeled in all four pigs (Fig. 3b and 3d).
Fig. 3.
Hematoxylin & eosin and immunohistochemical staining for African swine fever phosphoprotein p30 in lungs. The inset show African swine fever virus-positive cell. Bronchus-associated lymphoid tissue hyperplasia appeared in case no. 2 (a) with positive cells (b). Thickened alveolar septum due to fibrous tissue was apparent in case no. 3 (c) with presentation of viral antigen-labeled cells in the alveoli (d).
In the heart where ASFV protein were not detected, the epicardium (visceral pericardium) was thickened by distinct fibrous tissue in three pigs. However, two pigs (no. 1 and no. 4) had mixing of macrophages, lymphocytes and neutrophils infiltrated into the fibrous tissue, indicating a fibrinous visceral pericarditis condition. No inflammatory cells were detected in the neovascularized fibrous tissue in pig no. 3.
In other tissues and organs, none of the four pigs developed any pronounced lesions. However, three pigs showed incidental pathological changes, namely a small amount of lymphocyte infiltration into the gastric mucosa of pig no. 1 and into the renal parenchyma of pig no. 4, and severe portal bridging fibrosis with overwhelming eosinophil infiltration of pig no. 2. Those other organ tissues did not show positive reactions in any cell type, except for the amorphous cells of the gastric gland in the stomachs of three pigs (Fig. 4a). Nevertheless, the double IHC staining of stomach from pig no. 4 revealed that the ASFV antigen-labeled cells were neither Iba 1- (Fig. 4b), vimentin- (Fig. 4c), nor AE1/AE3-expressing cells (Fig. 4d). Tissue sections of unaffected pig from ASFV-free farm showed no significant pathological changes and no signal of viral antigen nor non-specific staining.
Fig. 4.
Immunohistochemical (IHC) staining for African swine fever (ASF) phosphoprotein p30 (a) and double IHC staining for ASF phosphoprotein p30 as the brown color with Iba-1 (b), vimentin (c), and AE1/AE3 (d) as the purplish grey color in stomach of case no. 4. The inset show African swine fever virus-positive cell. A positive reaction occurred in amorphous cells in the gastric glands (a). No double stained cells were observed (b–d).
DISCUSSION
The role of surviving or subclinically infected animals in maintaining and transmitting ASFV in domestic pigs has been a controversial issue since the revelation of a reservoir status in ASF-resistant wild African suids and the presence of a small portion of seropositive pigs (0.9–1.3%) in abattoirs [4, 21]. Studies on pig-to-pig transmission have focused on blood, nasal secretions, and rectal excretions since these samples are a great resource of circulating ASFV [6, 8]. In our study, the examined pigs that survived the outbreak for up to 90 to 120 days showed negative results for qPCR of blood samples, oropharyngeal fluids, and feces. This implied the absence of viremia and no capacity for viral transmission by these surviving pigs.
Izzati et al. [12] reported that hemorrhages in multiple organs are the main lesions of acute and subacute ASF outbreaks in Vietnam. As expected, a histopathological study of the surviving pigs in the same/nearby provinces investigated in this study revealed a healing process of those organs. The predominant lesions detected by microscopy indicated mild to moderate degrees of fibrosis in several organs, including subcapsular fibrosis in the lymph nodes, lung parenchyma, and epicardium. A fibrotic lesion is considered to represent a reparative process following severe hemorrhage and edema in those organs [14]. Although clinical signs or gross lesions related to ASF infection was not presented, the duplication of independent seropositive result provided the evidence of these pigs previously being exposed to ASFV. This could imply that the infectious stage was over and these animals had survived from the disease.
Fibrinous visceral pericarditis and pneumonia have occasionally been reported in the chronic form of ASF that is related to secondary infection in immunocompromised animals [22]. In the present study, highly pathogenic-porcine reproductive and respiratory syndrome virus, classical swine fever virus, salmonellosis, and swine erysipelas were ruled out by PCR and reverse transcription-PCR (data not shown); however, other opportunistic pathogens might have disrupted the reparative processes in the heart and the lung.
In the lung, the numbers of positive cells in affected lungs were generally lower in surviving pigs than in the diseased pigs [12]. In fact, these cells were extremely rare and resembled alveolar or interstitial macrophages, not the pulmonary intravascular macrophages (PIMs) common in ASFV-infected lungs [12]. This might suggest that ASFV particles were not circulating in the bodies of these pigs, a possibility that is supported by the absence of viremia evident by qPCR of the blood samples. In addition, the study of cytokine expression in ASF revealed that the pulmonary lesions coincided with the high numbers of non-infected and infected PIMs releasing interleukin-1 alpha and tumor necrosis factor-alpha. On the contrary, the role of alveolar macrophages in pathogenesis in the lung is thought to be insignificant [5, 22]. Together with the possibility of secondary infection, this makes the relation between the remaining positive cells and pulmonary lesions debatable. However, since neutrophils were not detected in any lung samples, bacterial infection may not have much attribution to the lesions.
In the stomach, the viral antigen found in some cells in the gastric gland of the surviving pigs was higher than in the diseased pigs. Non monocyte-macrophage (m-MØ) cells, such as fibroblasts, reticular cells, and smooth muscle cells, were discovered to harbor immature and mature viral particles during the middle and final phases of acute ASF [3, 10, 11]. The double IHC results in this study convinced that these viral antigen-labeled cells in stomach are neither macrophage, mesenchymal, nor epithelial cell. Therefore, the cell type of the positive cells in the stomach of the surviving pigs remains obscure. However, the numbers of such cells were unrelated to the lesion in stomach both in the diseased pigs and surviving pigs. This supports the hypothesis of the role of ASFV-labeled cell in the stomach may insignificantly contribute to the pathogenesis of ASF [11].
The fact that IHC detected a positive signal for the p30 protein while qPCR could not detect the existence of the partial p72 viral genome raises questions about the latency status of ASFV. We confirmed the result by performing qPCR in duplicate in two independent assays validated by the OIE [28]. As discussed by Tignon et al. [23], the assay has a detection limit between 5.7 and 57 ASFV genomes per reaction. Hence, a reasonable postulation is that the scarcity of viral content in these surviving animals led to the negative test results.
The qPCR results indicated that the p72-encoding gene (B646L), as an indicator of viral replication at late time points post-infection, had not yet been expressed in these animals [15]. Meanwhile, p30, an early protein involved in internalization of the virus into the cell, was labeled by IHC [2, 9]. Taken together, our data suggest that surviving pigs might possess an ability to repress the typical ASFV replication pathway. However, since conventional PCR did not detect the p30-encoding gene (CP204L), which was probably due to very low viral copies (data not shown), further investigation is required using other assays that can confirm gene expression at the level of individual cells to determine the stage of infection of intracellular ASFV. Ballester et al. [3] successfully detected intranuclear ASFV DNA in m-MØ cells and infection in several cell types by in situ hybridization. Nevertheless, the use of the digoxigenin-labeled oligonucleotide probe that is complementary to the gene sequence encoding p30 was impractical in their study. Therefore, more efficient probes specific for CP204L are needed to ascertain the possibility of a latent period of ASFV persistence in long-term surviving animals.
The present study has raised awareness of the residual ASFV in the tonsils, lungs and stomach of qPCR-negative seroconverted pigs. However, further analyzes of the infectivity of ASFV in surviving animal must be pursued. In addition, the risk of retransmission from these pigs must be taken into serious consideration, since corticosteroid-induced reactivation of ASFV was observed in surviving pigs up to six months after infection [26]. Finally, contamination from the ASFV-presented organs through human activities also possess the possibility of further outbreaks especially in offal meat consumption countries.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Acknowledgments
We would like to thank farms’ owners and all veterinarians involved in this study. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP17H04639 and the Ministry of Science and Technology (project DAKH-02/19-DT.02), Socialist Republic of Vietnam.
REFERENCES
- 1.Alonso C., Borca M., Dixon L., Revilla Y., Rodriguez F., Escribano J.M. and ICTV Report Consortium. 2018. ICTV virus taxonomy profile. Asfarviridae. J. Gen Virol. 99: 613–614. [DOI] [PubMed] [Google Scholar]
- 2.Andrés G.2017. African swine fever virus gets undressed: New insights on the entry pathway. J. Virol. 91: e01906–e01916. doi: 10.1128/JVI.01906-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballester M., Galindo-Cardiel I., Gallardo C., Argilaguet J. M., Segalés J., Rodríguez J. M., Rodríguez F.2010. Intranuclear detection of African swine fever virus DNA in several cell types from formalin-fixed and paraffin-embedded tissues using a new in situ hybridisation protocol. J. Virol. Methods 168: 38–43. doi: 10.1016/j.jviromet.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 4.Beltrán-Alcrudo D., Arias M., Gallardo C., Kramer S., Penrith M. L.2017. African swine fever: detection and diagnosis–A manual for veterinarians, no.19, Food and Agriculture Organization of the United Nations, Rome. [Google Scholar]
- 5.Carrasco L., Núñez A., Salguero F. J., Díaz San Segundo F., Sánchez-Cordón P., Gómez-Villamandos J. C., Sierra M. A.2002. African swine fever: Expression of interleukin-1 alpha and tumour necrosis factor-alpha by pulmonary intravascular macrophages. J. Comp. Pathol. 126: 194–201. doi: 10.1053/jcpa.2001.0543 [DOI] [PubMed] [Google Scholar]
- 6.Davies K., Goatley L. C., Guinat C., Netherton C. L., Gubbins S., Dixon L. K., Reis A. L.2017. Survival of African swine fever virus in excretions from pigs experimentally infected with the Georgia 2007/1 isolate. Transbound. Emerg. Dis. 64: 425–431. doi: 10.1111/tbed.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Animal Health of Vietnam (DAH). 2019. African swine fever in Vietnam. https://rr-asia.oie.int/wp-content/uploads/2019/12/3-asf-situation-in-vietnam.pdf [accessed on May 31, 2021].
- 8.Guinat C., Gogin A., Blome S., Keil G., Pollin R., Pfeiffer D. U., Dixon L.2016. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet. Rec. 178: 262–267. doi: 10.1136/vr.103593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez-Puertas P., Rodríguez F., Oviedo J. M., Brun A., Alonso C., Escribano J. M.1998. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 243: 461–471. doi: 10.1006/viro.1998.9068 [DOI] [PubMed] [Google Scholar]
- 10.Gómez-Villamandos J. C., Hervás J., Moreno C., Carrasco L., Bautista M. J., Caballero J. M., Wilkinson P. J., Sierra M. A.1997. Subcellular changes in the tonsils of pigs infected with acute African swine fever virus. Vet. Res. 28: 179–189. [PubMed] [Google Scholar]
- 11.Gómez-Villamandos J. C., Bautista M. J., Sánchez-Cordón P. J., Carrasco L.2013. Pathology of African swine fever: the role of monocyte-macrophage. Virus Res. 173: 140–149. doi: 10.1016/j.virusres.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 12.Izzati U. Z., Inanaga M., Hoa N. T., Nueangphuet P., Myint O., Truong Q. L., Lan N. T., Norimine J., Hirai T., Yamaguchi R.2020. Pathological investigation and viral antigen distribution of emerging African swine fever in Vietnam. Transbound. Emerg. Dis. 00: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King D. P., Reid S. M., Hutchings G. H., Grierson S. S., Wilkinson P. J., Dixon L. K., Bastos A. D. S., Drew T. W.2003. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 107: 53–61. doi: 10.1016/S0166-0934(02)00189-1 [DOI] [PubMed] [Google Scholar]
- 14.Kumar R., Abbas A., Delancey A., Malone E.2010. Tissue renewal, repair, and regeneration. pp. 79–110. In: Robbins and Cotran Pathologic Basis of Disease, 8th ed. (Schmitt. W. and Gruliow, R. eds.), Saunders Elsevier, Philadelphia. [Google Scholar]
- 15.Lithgow P., Takamatsu H., Werling D., Dixon L., Chapman D.2014. Correlation of cell surface marker expression with African swine fever virus infection. Vet. Microbiol. 168: 413–419. doi: 10.1016/j.vetmic.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Agriculture and Rural Development (MARD). 2019. Guidance on supplementing a number of measures to prevent and combat African swine fever. https://thuvienphapluat.vn/cong-van/Tai-nguyen-Moi-truong/Cong-van-5169-BNN-TY-2019-huong-dan-bo-sung-bien-phap-phong-chong-benh-Dich-ta-lon-Chau-Phi-428874.aspx [assessed on May 31, 2021].
- 17.Nurmoja I., Petrov A., Breidenstein C., Zani L., Forth J. H., Beer M., Kristian M., Viltrop A., Blome S.2017. Biological characterization of African swine fever virus genotype II strains from north-eastern Estonia in European wild boar. Transbound. Emerg. Dis. 64: 2034–2041. doi: 10.1111/tbed.12614 [DOI] [PubMed] [Google Scholar]
- 18.Okoth E., Gallardo C., Macharia J. M., Omore A., Pelayo V., Bulimo D. W., Arias M., Kitala P., Baboon K., Lekolol I., Mijele D., Bishop R. P.2013. Comparison of African swine fever virus prevalence and risk in two contrasting pig-farming systems in South-west and Central Kenya. Prev. Vet. Med. 110: 198–205. doi: 10.1016/j.prevetmed.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 19.Penrith M. L., Thomson G. R., Bastos A. D. S., Phiri O. C., Lubisi B. A., Du Plessis E. C., Macome F., Pinto F., Botha B., Esterhuysen J.2004. An investigation into natural resistance to African swine fever in domestic pigs from an endemic area in southern Africa. Rev. Sci. Tech. 23: 965–977. doi: 10.20506/rst.23.3.1533 [DOI] [PubMed] [Google Scholar]
- 20.Petrov A., Forth J. H., Zani L., Beer M., Blome S.2018. No evidence for long-term carrier status of pigs after African swine fever virus infection. Transbound. Emerg. Dis. 65: 1318–1328. doi: 10.1111/tbed.12881 [DOI] [PubMed] [Google Scholar]
- 21.Ståhl K., Sternberg-Lewerin S., Blome S., Viltrop A., Penrith M. L., Chenais E.2019. Lack of evidence for long term carriers of African swine fever virus - a systematic review. Virus Res. 272: 197725. doi: 10.1016/j.virusres.2019.197725 [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Cordón P. J., Vidaña B., Neimanis A., Núñez A., Wikström E., Gavier-Widén D.2021. Pathology of African swine fever. pp. 87–140. In: Understanding and Combating African Swine Fever (Lacolina, L., Penrith, M.-L., Bellini, S., Chenais, E., Jori, F., Montoya, M., StÅhl, K. and Gavier- Widén, D. eds.), Wageningen Academic Publishers, Wageningen. [Google Scholar]
- 23.Tignon M., Gallardo C., Iscaro C., Hutet E., Van der Stede Y., Kolbasov D., De Mia G. M., Le Potier M. F., Bishop R. P., Arias M., Koenen F.2011. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J. Virol. Methods 178: 161–170. doi: 10.1016/j.jviromet.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Tran H. T. T., Truong A. D., Ly D. V., Vu T. H., Hoang V. T., Nguyen T. C., Chu T. N., Nguyen T. H., Pham N. T., Nguyen T., Yersin A. G., Dang H. V.2020. Genetic characterization of African swine fever virus in outbreaks in Ha Nam province, Red River Delta region of Vietnam, and activity of antimicrobial products against virus infection in contaminated feed. J. Vet. Res. (Pulawy) 64: 207–213. doi: 10.2478/jvetres-2020-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Diep N., Choijookhuu N., Fuke N., Myint O., Izzati U. Z., Suwanruengsri M., Hishikawa Y., Yamaguchi R.2020. New tropisms of porcine epidemic diarrhoea virus (PEDV) in pigs naturally coinfected by variants bearing large deletions in the spike (S) protein and PEDVs possessing an intact S protein. Transbound. Emerg. Dis. 67: 2589–2601. doi: 10.1111/tbed.13607 [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson P. J.1984. The persistence of African swine fever in Africa and the Mediterranean. Prev. Vet. Med. 2: 71–82. doi: 10.1016/0167-5877(84)90050-3 [DOI] [Google Scholar]
- 27.Woonwong Y., Do Tien D., Thanawongnuwech R.2020. The future of the pig industry after the introduction of African swine fever into Asia. Anim. Front. 10: 30–37. doi: 10.1093/af/vfaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Organization for Animal Health. 2019. African swine fever. Manual of diagnostic tests and vaccines for terrestrial animals 2019. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.01_ASF.pdf [accessed on June 24, 2021].