Abstract
We investigated the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among dogs in the Tokyo area via enzyme-linked immunosorbent assay (ELISA) using the spike protein as the target antigen. Plasma samples from 494 household dogs and blood-donor dogs were tested from July 2020 to January 2021. Of these samples, three showed optical densities that were higher than the mean plus two standard deviations of the mean of the negative-control optical densities (ODs). Of these three samples, only the sample with the highest OD by ELISA was confirmed positive by virus neutralization testing. The positive dog presented no SARS-CoV-2-related symptoms. The positivity rate of SARS-CoV-2 infections among dogs in the Tokyo area was approximately 0.2%.
Keywords: antibody, coronavirus disease 2019, dog, severe acute respiratory syndrome coronavirus 2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide since December 2019 and has caused emergencies related to public health and the economy. SARS-CoV-2 causes coronavirus disease 2019 (COVID-19), which causes mild-to-severe respiratory illness in humans and can be fatal [7]. As of May 2021, the World Health Organization reported that nearly 169 million people have been infected, and more than 3.5 million have died from COVID-19 [16]. Compared with the knowledge regarding human infections, little is known about the pathogenicity and epidemiology of SARS-CoV-2 infections in companion animals. Previous research reported that two of 15 dogs kept in households with confirmed human COVID-19 cases in Hong Kong were infected with SARS-CoV-2 [13]. Since then, SARS-CoV-2-infected dogs have been reported worldwide [1, 3]. Under experimental conditions, cats and ferrets are reported to be susceptible to the virus, while dogs are less susceptible [12]. However, almost as many dogs as cats have been naturally infected [1, 3, 11], possibly because people interact more with their dogs than with cats [11]. Although domestic dogs are often considered family members, few reports have been published on the SARS-CoV-2 seroprevalence in dogs. Clinical veterinarians should have information on the prevalence of SARS-CoV-2 infections in dogs around the area where they live to provide an adequate risk assessment for the owners, other cohabiting animals, and veterinary staff.
In humans, asymptomatic patients play important roles in natural viral transmission [4, 5, 10]. Dogs are not known to be symptomatic from SARS-CoV-2 infection; most reported cases of canine infections were asymptomatic [1, 3]; thus, asymptomatic SARS-CoV-2-infected dogs should be considered potential infection sources for humans. Asymptomatic dogs are at risk of causing domestic infections and can transmit the virus to people and animals when visiting public spaces such as animal hospitals or dog runs. As in humans, SARS-CoV-2 infection in dogs can be diagnosed by detecting the viral genome via reverse transcription (RT)-PCR. The sensitivity and specificity of RT-PCR are adequate, but the detectable period is short [12]. Therefore, serological investigations are needed to clarify the epidemiological infection status. To date, various methods have been applied to detect SARS-CoV-2 antibodies to investigate the seroprevalence in humans and animals [6, 14, 15]. Here, we developed an enzyme-linked immunosorbent assay (ELISA) using recombinant spike protein as the target antigen to investigate the seroprevalence in household dogs living in the Tokyo area.
Residue from heparinized plasma samples was collected for clinical testing from client-owned dogs at the veterinary medical center of The University of Tokyo from July 2020 to January 2021. Most of the dogs came to the center for veterinary care. Blood samples from blood-donor dogs were also used. Regarding the patients, no specific selections were made for the test except for the amount of sample available. Written consent was obtained from the owners to use the specimens for research purposes. Plasma samples were separated and stored at −20°C until analysis. A plasma sample from a SARS-CoV-2-infected dog was provided as a positive control by Dr. Genki Ishihara at the Anicom Specialty Medical Institute (Tokyo, Japan) with the owner’s consent. This dog had been kept at home and was temporarily taken to the institute because his owner was infected with SARS-CoV-2. The dog had tested positive on repeated SARS-CoV-2 PCR tests but was clinically asymptomatic. Eight plasma samples collected before the SARS-CoV-2 pandemic were used as negative controls. Commercially available recombinant spike protein produced by silkworms (Kaico, Fukuoka, Japan) was purchased as the target antigen to develop the ELISA to detect anti-SARS-CoV-2 antibodies.
Fifty microliters of the recombinant protein (5 μg/ml) diluted in 0.1 M carbonate-bicarbonate buffer (pH 9.6) was added to 96-well ELISA plates (H Type Multi well Plate for ELISA, Sumitomo Bakelite, Tokyo, Japan) and incubated overnight at 4°C. The wells were washed three times with phosphate-buffered saline (PBS) containing 0.02% Tween-20 (PBS-T) and blocked with 200 μl of blocking buffer (Blocking One, Nakalai Tesque, Kyoto, Japan) for 2 hr at 37°C. The wells were then washed three times with PBS-T. Samples were diluted at 1:100 in Tris-buffered saline (pH 7.6) containing 0.1% Tween-20 and 0.05% Blocking One (as a dilution buffer) and incubated at 37°C for 1 hr. After washing three times, 100 μl of peroxidase-conjugated protein A/G (Thermo Fisher Scientific, Waltham, MA, USA) diluted at 1:4,000 in dilution buffer was added to each well and incubated at 37°C for 1 hr. The plates were washed five times, then 50 μl of 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Nakalai Tesque, Kyoto, Japan) was added. The plates were incubated for 15 min at room temperature. After incubation, the reaction was stopped by adding 50 μl of stop solution (Nakalai Tesque). The optical density (OD) was measured at 405 nm. When the OD values were higher than the mean OD of the negative control samples plus three standard deviations (SDs; cut off=1.440), the samples were considered antibody-positive.
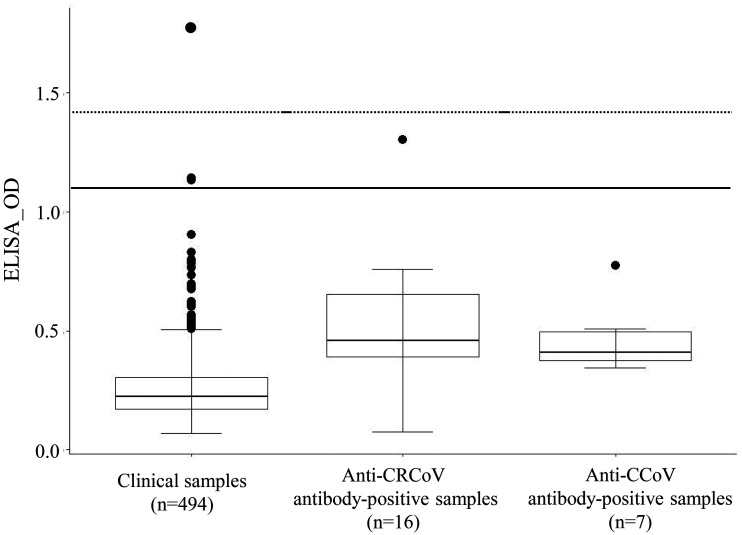
Next, 494 clinical samples were tested for anti-SARS-CoV-2 antibodies. Among these samples, three showed OD values that were higher than the mean OD plus two SDs of the negative-control ODs (Table 1, Fig. 1). For these three samples, titers of the neutralizing antibody for SARS-CoV-2 were examined using a virus neutralization test (VNT) [8]. Briefly, the SARS-CoV-2 strain, WK-521 (lineage B), was propagated in Vero E6/TMPRSS2 cells. The complete genome of the strain was deposited in Global Initiative on Sharing All Influenza Data (EPI_ISL_408667). Viral titers were determined by the 50% tissue culture infectious dose (TCID50) using Vero E6/TMPRSS2 cells. Heat-inactivated sample sera (56°C, 30 min) were serially diluted two-fold and incubated with an equal volume of 2 × 103 TCID50/ml of the virus. The mixtures were incubated for 1 hr at 37°C. VeroE6/TMPRSS2 cells were prepared in 96-well plates, and the mixtures were inoculated in duplicate into each well. After incubating for 5–6 days, the cells were fixed in 10% buffered formalin (Fujifilm Wako Pure Chemicals, Osaka, Japan) at room temperature for 1 hr and stained with 0.037% methylene blue (Fujifilm Wako Pure Chemicals). The neutralizing antibody titer was defined as the highest dilution that inhibited 100% of the cytopathic effects. Neutralizing antibody (titer=1:5) was detected in a sample from a SARS-CoV-2 PCR-positive dog. Of the three clinical samples tested, only the sample with the highest OD by ELISA (from a 3-year-old chihuahua) tested positive on the VNT (titer=1:40). The dog had been admitted to the veterinary hospital for orthopedic care and had shown no SARS-CoV-2-related clinical symptoms according to its medical records. The owner’s infection status was not investigated. Two samples showed the presence of anti-spike-protein antibody regardless of the absence of neutralizing antibodies against SARS-CoV-2. Thus, we examined the antibody cross-reactivity against that of other coronaviruses that infect dogs (i.e., canine coronavirus [CCoV] and canine respiratory coronavirus [CRCoV]; Fig. 1). CCoV is an alphacoronavirus that causes mild-to-severe diarrhea in puppies [2]. CRCoV is a betacoronavirus, as is SARS-CoV-2. CRCoV causes respiratory disease in dogs. Seven anti-CCoV antibody-positive samples with titers ranging from 1:320 to 1:1,280 via CCoV immunoperoxidase plaque-staining tests and 16 anti-CRCoV antibody-positive samples with titers ranging from 1:50 to ≥1:6,400 via CRCoV ELISA tests were examined via spike-protein-based SARS-CoV-2 ELISA testing. These samples were selected from stocked clinical samples at Marupi Lifetech Clinical Laboratory (Ikeda, Osaka, Japan). None of the seven anti-CCoV antibody-positive samples showed ODs higher than the mean plus 2 SDs of the negative-control ODs for the test. One of the 16 anti-CRCoV antibody-positive samples showed an OD higher than the mean plus 2 SDs for the test (Fig. 1); however, this sample tested negative for SARS-CoV-2 on the VNT. The ELISA results yielded mean ODs of 0.468 and 0.511 for anti-CCoV antibody-positive and anti-CRCoV antibody-positive serum samples, respectively. The OD values of the seven anti-CCoV and 16 anti-CRCoV antibody-positive sera were significantly higher than those of the 494 clinical samples (P<0.01; analyzed by the Wilcoxon rank-sum test). We examined the antibody titers against CRCoV in two samples that showed high OD values on the ELISA for SARS-CoV-2 but were negative on the VNT. The resulting titers were 1:1,600 and <1:50, suggesting that anti-CCoV and anti-CRCoV antibodies might cause some cross-reactivity to the SARS-CoV-2 ELISA. Although Stevanovic et al. reported finding no serological cross-reactivity between SARS-CoV-2 and CCoV or CRCoV on a spike-protein-based SARS-CoV-2 ELISA test [14], neutralization tests should still be considered to confirm SARS-CoV-2 infections in dogs. However, the number of samples should be narrowed down by ELISA because VNTs require facilities with a high biosafety level.
Table 1. Summary of ELISA to detect antibody against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in dogs.
| Number of samples |
Mean OD value | Standard deviation |
Number of samples exceeded mean + 2 OD (samples VNT positive) |
Remarks | |
|---|---|---|---|---|---|
| Clinical samples | 494 | 0.262 | 0.159 | 3 (1) | House hold dogs living in Tokyo area |
| Positive control | 1 | - | - | 1 (1) | SARS -CoV-2 PCR test positive dog |
| Negative control | 8 | 0.377 | 0.354 | 0 | Sera collected before SARS-CoV-2 pandemic |
| Anti-CRCoV Ab positive samples | 16 | 0.511 | 0.284 | 1 (0) | Anti-CRCoV antibody positive sera |
| Anti-CCoV Ab positive samples | 7 | 0.468 | 0.148 | 0 | Anti-CRCoV antibody positive sera |
OD, optical density; VNT, virus neutralization test.
Fig. 1.
Box plot with outliers on the enzyme-linked immunosorbent assay (ELISA) to detect antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein. Clinical samples of canine plasma obtained from July 2020 to January 2021 (n=494), stocked anti-canine respiratory coronavirus (CRCoV)-positive samples (n=16), and stocked canine coronavirus (CCoV) samples (n=7) were examined. The solid and dashed lines represent the mean + two standard deviations (SDs) and 3 SDs of the negative control samples, respectively.
One study reported a correlation between antibody-positive rates in animals and the COVID-19 infection density in humans [11]. At the time of our sample collection in December 2020, the SARS-CoV-2 seroprevalence of people in the Tokyo area was estimated at ~1.35% [9]. In the current study, three of 494 clinical samples showed OD values higher than the mean plus two SDs of the negative-control ODs. Only one of these three samples was confirmed positive by VNT, indicating a low SARS-CoV-2 infection rate of approximately 0.2% in household dogs in Japan.
The population of owners who visited the university’s referral veterinary hospital may have been biased; however, some speculations can be made as to why dogs have a lower seroprevalence. First, dogs are less sensitive to the infection than are cats [12]. Low angiotensin-converting enzyme 2 expression in the canine respiratory tract explains the relatively low sensitivity in dogs [17]. Second, the virus may have been transmitted to the household dogs mainly via the dogs’ owners [1, 3, 11]. Dogs are not highly susceptible to the virus, so transmission from humans to dogs may be limited. At a facility that provides pet boarding services for SARS-CoV-2-infected pet owners, only a relatively small number of dogs have been found to be infected (personal communication, Dr. Genki Ishihara, Anicom Specialty Medical Institute Inc., Tokyo, Japan). Although contact with dogs is presumed to yield only a small risk of infection, the spread of mutant strains can change the infectivity and pathogenicity in dogs. Viral detection and analysis should be continued in companion animals as long as the COVID-19 epidemic exists among humans.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Dr. Genki Ishihara (Anicom Specialty Medical Institute Inc., Tokyo, Japan) for providing the canine samples. This research was supported by grants by the University of Tokyo, Promoting practical use of measures against coronavirus disease 2019 (COVID-19).
REFERENCES
- 1.American Veterinary Medical Associations. In-depth summary of reports of naturally acquired SARS-CoV-2 infections in domestic animals and farmed or captive wildlife. https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/depth-summary-reports-naturally-acquired-sars-cov-2 [accessed on August 1, 2021].
- 2.Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M.2006. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 12: 492–494. doi: 10.3201/eid1203.050839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dróżdż M., Krzyżek P., Dudek B., Makuch S., Janczura A., Paluch E.2021. Current state of knowledge about role of pets in zoonotic transmission of SARS-CoV-2. Viruses 13: 1149. doi: 10.3390/v13061149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa N. W., Brooks J. T., Sobel J.2020. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg. Infect. Dis. 26: e201595. doi: 10.3201/eid2607.201595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buitrago-Garcia D., Egli-Gany D., Counotte M. J., Hossmann S., Imeri H., Ipekci A. M., Salanti G., Low N.2020. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 17: e1003346. doi: 10.1371/journal.pmed.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudreault N. N., Trujillo J. D., Carossino M., Meekins D. A., Morozov I., Madden D. W., Indran S. V., Bold D., Balaraman V., Kwon T., Artiaga B. L., Cool K., García-Sastre A., Ma W., Wilson W. C., Henningson J., Balasuriya U. B. R., Richt J. A.2020. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 9: 2322–2332. doi: 10.1080/22221751.2020.1833687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B.2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M.2020. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 117: 7001–7003. doi: 10.1073/pnas.2002589117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health, Labor and Welfare. 2021.https://www.mhlw.go.jp/content/000761671.pdf (in Japanease) [accessed on May 31, 2021].
- 10.Oran D. P., Topol E. J.2020. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann. Intern. Med. 173: 362–367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson E. I., Elia G., Grassi A., Giordano A., Desario C., Medardo M., Smith S. L., Anderson E. R., Prince T., Patterson G. T., Lorusso E., Lucente M. S., Lanave G., Lauzi S., Bonfanti U., Stranieri A., Martella V., Solari Basano F., Barrs V. R., Radford A. D., Agrimi U., Hughes G. L., Paltrinieri S., Decaro N.2020. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 11: 6231. doi: 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z.2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368: 1016–1020. doi: 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sit T. H. C., Brackman C. J., Ip S. M., Tam K. W. S., Law P. Y. T., To E. M. W., Yu V. Y. T., Sims L. D., Tsang D. N. C., Chu D. K. W., Perera R. A. P. M., Poon L. L. M., Peiris M.2020. Infection of dogs with SARS-CoV-2. Nature 586: 776–778. doi: 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevanovic V., Vilibic-Cavlek T., Tabain I., Benvin I., Kovac S., Hruskar Z., Mauric M., Milasincic L., Antolasic L., Skrinjaric A., Staresina V., Barbic L.2020. Seroprevalence of SARS-CoV-2 infection among pet animals in Croatia and potential public health impact. Transbound. Emerg. Dis.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernike K., Aebischer A., Michelitsch A., Hoffmann D., Freuling C., Buschmann A. B., Graaf A., Müller T., Osterrieder N., Rissmann M., Rubbenstroth D., Schön J., Schulz C., Trimpert J., Ulrich L., Volz A., Mettenleiter T., Beer M.2020. Multi-species ELISA for the detection of antibodies against SARS-CoV-2 in animals. Transbound. Emerg. Dis.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. 2021. COVID-19 Weekly Epidemiological Update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [accessed on May 31, 2021].
- 17.Zhai X., Sun J., Yan Z., Zhang J., Zhao J., Zhao Z., Gao Q., He W. T., Veit M., Shuo S.2020. Comparison of SARS-CoV-2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. J. Virol. 94: e00831–e20. doi: 10.1128/JVI.00831-20 [DOI] [PMC free article] [PubMed] [Google Scholar]