Abstract
A racing pigeon (Columba livia var. domestica), a straggler from Taiwan, was sheltered in Nara Prefecture, Japan in 2020. This pigeon showed hemolysis and elevated levels of hepatobiliary and muscle enzymes. Gametocytes of Haemoproteus columbae (Apicomplexa: Haemosporida) were observed within the host erythrocytes in thin blood smears. A partial sequence of the mitochondrial cytochrome b gene amplified from blood DNA was identical to the lineage HAECOL1 previously reported from pigeons worldwide. This is the first record of H. columbae infection in a sheltered bird in Japan.
Keywords: Columbalivia var. domesticaa, Haemoproteus columbae, haemosporidian, pigeon malaria, racing pigeon
Haemoproteus Kruse, 1890 (Haemosporida: Haemoproteidae) is a genus of hematozoan parasites that infect many avian taxa [16]. There are currently seven described species that infect columbiform birds: Haemoproteus columbae Kruse, 1890; Haemoproteus multipigmentatus Valkiūnas et al., 2010; Haemoproteus multivolutinus Valkiūnas et al., 2013; Haemoproteus palumbis Barker, 1966; Haemoproteus paramultipigmentatus Valkiūnas et al., 2013; Haemoproteus sacharovi Novy and MacNeal, 1950; and Haemoproteus turtur Ortega and Berenguer, 1950 [16,17,18]. All these species are considered to be transmitted by hippoboscid flies [1, 16]. In Japan, although Haemoproteus spp. have been reported to infect rock doves (Columba livia), Japanese wood pigeon (Columba janthina janthina), red-headed wood pigeon (Columba janthina nitens), Oriental turtle dove (Streptopelia orientalis), and white-bellied green pigeon (Treron sieboldii) [7, 15], there have been few reports from the main island of Japan, and certainly no clinical cases of the disease. The present study reports a case of Haemoproteus infection in a racing pigeon, C. livia var. domestica, sheltered in Nara Prefecture, Japan, and reveals morphological and molecular features of the Haemoproteus parasite.
On July 16, 2020 (day 0), a rock dove was brought to the animal clinic in Osaka Prefecture, Japan. The bird was captured in Nara Prefecture, Japan, earlier in the year, and the leg ring information had revealed that it was a racing bird migrating from Taiwan in 2020. The bird weighed 403 g, had an appetite, and showed no abnormalities on physical examination. Blood films stained with Diff-Quick revealed hemoprotozoan infection in the erythrocytes. Investigation of the complete blood count and serum chemistry revealed elevated levels of liver and biliary duct enzymes (alkaline phosphatase, aspartate aminotransferase, gamma-glutamyl transferase, lactate dehydrogenase, and total bile acid), muscle enzymes (aspartate aminotransferase, creatine phosphokinase, and lactate dehydrogenase), and hemolysis (Table 1) when compared with standard reference values for domestic pigeons [8,9,10, 14]. Examination of feces showed a roundworm parasite and light-colored feces with yellow-green urates which was attributable to the presence of liver disorder or hemolysis (Fig. 1). Nucleic acid amplification tests conducted at a commercial laboratory were negative for Chlamydia and Mycobacterium. The bird was brought to the same hospital again (days 24, 53, 81, 112, and 140) and blood tests revealed continuously elevated liver and muscle enzymes, hemolysis, and hemoprotozoan infection (Table 1). Drugs were administered through daily drinking water for liver and bile duct disorders: clarithromycin (days 0–24, 640 mg/l), amoxicillin hydrate (days 25–140, 800 mg/l), glycyrrhizin (days 0–140, 80 mg/l), ursodeoxycholic acid (days 0–24, 160 mg/l), trepibutone (days 0–140, 48 mg/l), pravastatin in sodium (days 24–53, 8 mg/l), and diisopropylamine dichloroacetate (days 0–112, 80 mg/l).
Table 1. Hematologic parameters from a racing pigeon infected with Haemoproteus columbae.
| Unit | Day 0 | Day 24 | Day 53 | Day 81 | Day 112 | Day 140 | Reference ranges | |
|---|---|---|---|---|---|---|---|---|
| ALP | U/l | 1,862 | 1,066 | 2,099 | 1,717 | 1,270 | 2,506 | 160–780a |
| AST | U/l | 661 | 263 | 150 | 162 | 119 | 114 | 45–123b |
| CK | U/l | 693 | 477 | 494 | >2,000 | 664 | 290 | 110–480b |
| GGT | U/l | 529 | 576 | 113 | 16 | 4 | 5 | 0–3b |
| LDH | U/l | 568 | 429 | 178 | 406 | 107 | 64 | 30–205b |
| BA | µmol/l | 109 | 62 | nt | nt | nt | nt | 22–60b |
| TCHO | mg/dl | 662 | 536 | 352 | 238 | 226 | 317 | na |
| TG | mg/dl | 130 | 676 | 198 | 183 | 165 | 198 | na |
| TP | g/dl | 5.4 | 6.8 | 6.2 | 4.8 | 4.4 | 4.4 | 2.1–3.5b |
| PCV | % | 52 | 52 | 65 | 63 | 61 | 60 | 40–57c |
| WBC | 103/µl | 11.0 | 11.6 | nt | nt | 12.6 | nt | 2.6–22.3c |
| Het | % | 50.9 | 44.8 | nt | nt | 61.5 | nt | 4.5–43.5c |
| Lym | % | 49.1 | 55.2 | nt | nt | 38.5 | nt | 52.5–90.5c |
| Gametocytes | Present | Present | Present | Present | Present | Present | ||
| Parasitemia | % | 3.1 | 3.3 | nt | nt | nt | nt | |
| Color of the plasma | Red | Red | Red | Red | Red | Yellow |
ALP, alkaline phosphatase; AST, aspartate aminotransferase; CK, creatine phosphokinase, GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase; BA, total bile acid; TCHO, total cholesterol; TG, tryglyceride; TP, total protein; PCV, packed cell volume; WBC, white blood cells; Het, heterophils; Lym, lymphocytes; nt, not tested; na, not available. a Lumeiji & Bruijine (1985) [8]; b Lumeiji (2008) [10]; c Scope et al. (2002) [14].
Fig. 1.

Gross findings of fecal specimens showing a roundworm and yellow-green urates.
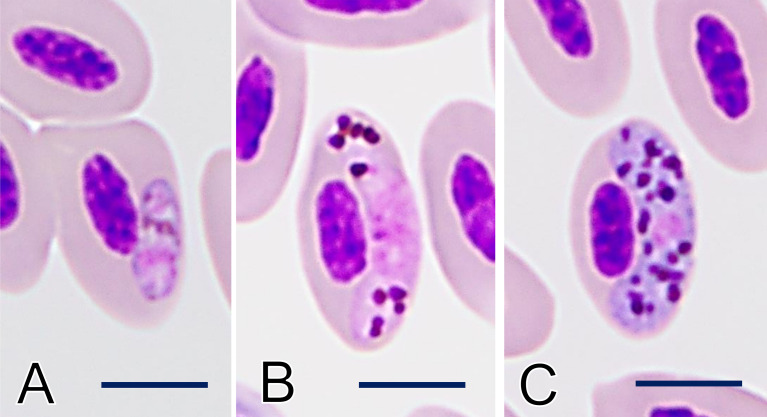
Parasitological examinations of fecal and blood specimens collected on day 0 were performed at the Nippon Veterinary and Life Science University. The parasite found on the fecal specimen was identified morphologically as pigeon roundworm, Ascaridia columbae (Nematoda: Ascaridida), commonly found in pigeons and doves. Examination of the peripheral blood film by light microscopy revealed the presence of Haemoproteus gametocytes in 3.3% of the erythrocytes, including young (0.2%; 1/415) and mature gametocytes (2.9%; 12/415) (Fig. 2A–C). Mature gametocytes were sausage-shaped, halteridial in position, and touched the nuclei and envelope of erythrocytes (Fig. 2B and 2C). Pigmented granules in the macrogametocyte cytoplasm were round, unequal in size, and sometimes aggregated into compressed masses, with approximately 30 randomly dispersed per parasite. The parasite nucleus was small and was located at the median center. The granules in the microgametocytes were round and frequently exceeded 1 µm in diameter, and aggregated in the peripheral cytoplasm. Based on the morphological keys by Valkiūnas et al. [16,17,18], the present parasite was identified as the gametocyte of H. columbae. To examine the genetic characteristics of H. columbae, a partial fragment of the mitochondrial cytochrome b gene (cytb) was amplified and sequenced. Genomic DNA was extracted from the blood using the QIAmp DNA Blood Mini Kit (Qiagen, Venlo, Netherlands). The cytb was amplified by PCR using the primer set HAEMF (5ʹ- ATGGTGCTTTCGATATATGCATG-3ʹ) and HAEMR2 (5ʹ- GCATTATCTGGATGTGATAATGGT-3ʹ) [2]. Reactions were performed using TaKaRa Ex Taq polymerase (TaKaRa Bio, Kusatsu, Japan). The amplification program consisted of initial denaturation at 95°C for 5 min, followed by 40 cycles at 95°C for 30 sec, 50°C for 30 sec, and 72°C for 1 min, with a final extension step at 72°C for 10 min. The products were electrophoresed on 1.5% agarose gel, purified with ExoSaP-IT (Applied Biosystems, Waltham, MA, USA), and sequenced on a 3730x DNA analyzer (Applied Biosystems), after labeling with BigDye terminator (Applied Biosystems), using PCR primers. The 478 bp-long sequence was deposited in the DNA Data Bank of Japan under Accession no. LC647343. Comparison with the International Nucleotide Sequence Databases using the BLAST program showed 100% identity with the haplotype HAECOL1 (Accession no. KU131583), which is reported in C. livia from a wide range of tropical and subtropical areas [3, 6, 12, 13].
Fig. 2.
Intraerythrocyte gametocyte of Haemoproteus columbae in the thin blood film. (A) Young gametocyte. (B) Microgametocyte. (C) Macrogametocyte. Giemsa stain. Bar=5 µm.
Haemoproteus columbae has a complex life cycle involving two hosts: merogony in internal organs and gametogony in red blood cells of pigeons, and sporogony in the midguts of the pigeon fly Pseudolynchia canariensis [16]. Cepeda et al. (2019) showed the full lifecycle of the lineage H. columbae HAECOL1 [4]. Merogony is found mainly in the lungs and liver and causes pneumonia and hepatitis, respectively. Gametocytes appear in the peripheral blood 19 to 20 days post infection (d.p.i.), followed by a rapid increase 22 to 25 d.p.i. and a rapid decrease 29 to 30 d.p.i., and then return to the chronic phase a week later. In the chronic phase, parasitemia is low (<10%) and lasts for over several months. In the present case, the infection phase is considered to be chronic because the parasitemia is maintained at a low level. Histological examination of a biopsy is necessary to determine whether the prolonged elevation of liver and muscle enzymes is due to merogony-induced disorder or some other disease such bacterial and viral infections and poisoning [10]. The treatment of Haemoproteus infections has not been sufficiently studied. Treatment with buparvaquone, a second-generation hydroxynaphthoquinone antiprotozoal drug related to atvaquone and parvaquone, reduces the number of gametocytes [5]; however, complete cure is difficult and is not approved for use in animals in Japan.
Infection with H. columbae is widely reported in tropical and subtropical areas, which coincides with the distribution of pigeon flies [16]. On the other hand, because pigeon flies are not seen in main island of Japan [19], there are no reports on the detection of H. columbae there (Table 2) [7, 11, 15, 20]. Matsumura louse fly, Ornithomya avicularia aobatonis (Diptera: Hippoboscidae), often parasitizes pigeons in main island of Japan, but the species is considered an unsuitable host for H. columbae [1]. Therefore, it is presumed that the present case was sucked by pigeon flies carrying H. columbae in Taiwan and then migrate to main island of Japan.
Table 2. Survey and documentation of Haemoproteus infection in rock dove, Columba livia, in Japan.
| Localities | Origin | Diagnosic method | Infection status (species) | References |
|---|---|---|---|---|
| Hyogo | Wild | Microscopy | 0/278 | [11] |
| Hokkaido | Wild | PCR | 0/27 | [20] |
| Okinawa | na | PCR | 1/2 (H. columbae) | [15] |
| Kanto region | na | PCR | 0/15 | [15] |
| Kanto region | Wild | PCR | 0/34 | [7] |
na: not available.
In summary, this is the first molecularly confirmed case of H. columbae in the main island of Japan. The present case demonstrated the potential risk of transboundary introduction of H. columbae by racing pigeons.
CONFLICT OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Baker J. R.1967. A review of the role played by the Hippoboscidae (Diptera) as vectors of endoparasites. J. Parasitol. 53: 412–418. doi: 10.2307/3276603 [DOI] [PubMed] [Google Scholar]
- 2.Bensch S., Stjernman M., Hasselquist D., Östman O., Hansson B., Westerdahl H., Pinheiro R. T.2000. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. Biol. Sci. 267: 1583–1589. doi: 10.1098/rspb.2000.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chagas C. R. F., Guimarães L. O., Monteiro E. F., Valkiūnas G., Katayama M. V., Santos S. V., Guida F. J. V., Simões R. F., Kirchgatter K.2016. Hemosporidian parasites of free-living birds in the São Paulo Zoo, Brazil. Parasitol. Res. 115: 1443–1452. doi: 10.1007/s00436-015-4878-0 [DOI] [PubMed] [Google Scholar]
- 4.Cepeda A. S., Lotta-Arévalo I. A., Pinto-Osorio D. F., Macías-Zacipa J., Valkiūnas G., Barato P., Matta N. E.2019. Experimental characterization of the complete life cycle of Haemoproteus columbae, with a description of a natural host-parasite system used to study this infection. Int. J. Parasitol. 49: 975–984. doi: 10.1016/j.ijpara.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 5.el-Metenawy T. M.1999. Therapeutic effects of some antihaematozoal drugs against Haemoproteus columbae in domestic pigeons. Dtsch. Tierarztl. Wochenschr. 106: 72. [PubMed] [Google Scholar]
- 6.Hala M. N. T., Mona M. I. A., Heba M. A.2020. Phylogenetical analysis of partially sequenced cytb gene of Haemoproteus columbae in pigeons and its pathological lesions in Egypt. Iran J. Vet. Res. 21: 203–210. [PMC free article] [PubMed] [Google Scholar]
- 7.Inumaru M., Murata K., Sato Y.2017. Prevalence of avian haemosporidia among injured wild birds in Tokyo and environs, Japan. Int. J. Parasitol. Parasites Wildl. 6: 299–309. doi: 10.1016/j.ijppaw.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeij J. T., de Bruijne J. J.1985. Blood chemistry reference values in racing pigeons (Columba livia domestica). Avian Pathol. 14: 401–408. doi: 10.1080/03079458508436241 [DOI] [PubMed] [Google Scholar]
- 9.Lumeij J. T., Meidam M., Wolfswinkel J., Van der Hage M. H., Dorrestein G. M.1988. Changes in plasma chemistry after drug-induced liver disease or muscle necrosis in racing pigeons (Columba livia domestica). Avian Pathol. 17: 865–874. doi: 10.1080/03079458808436508 [DOI] [PubMed] [Google Scholar]
- 10.Lumeij J. T.2008. Avian clinical biology. pp. 839–872. In: Clinical Biochemistry of Domestic Animals, 6th ed. (Kaneko, J. J., Harvey, J. W. and Bruss, M. L. eds.), Academic Press, Cambridge. [Google Scholar]
- 11.Murata K.2002. Prevalence of blood parasites in Japanese wild birds. J. Vet. Med. Sci. 64: 785–790. doi: 10.1292/jvms.64.785 [DOI] [PubMed] [Google Scholar]
- 12.Nebel C., Harl J., Pajot A., Weissenböck H., Amar A., Sumasgutner P.2020. High prevalence and genetic diversity of Haemoproteus columbae (Haemosporida: Haemoproteidae) in feral pigeons Columba livia in Cape Town, South Africa. Parasitol. Res. 119: 447–463. doi: 10.1007/s00436-019-06558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosyadi I., Salasia S. I. O., Argamjav B., Sato H.2021. Impact of subclinical Haemoproteus columbae infection on farmed domestic pigeons from central Java (Yogyakarta), Indonesia, with special reference to changes in the hemogram. Pathogens 10: 440. doi: 10.3390/pathogens10040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scope A., Filip T., Gabler C., Resch F.2002. The influence of stress from transport and handling on hematologic and clinical chemistry blood parameters of racing pigeons (Columba livia domestica). Avian Dis. 46: 224–229. doi: 10.1637/0005-2086(2002)046[0224:TIOSFT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K., Sumiyama D., Kanazawa T., Sato Y., Murata K.2019. Prevalence and molecular phylogeny of avian malaria parasites in Columbiformes in Japan. Jpn. J. Zoo Wildl. Med. 24: 65–71 (in Japanese). doi: 10.5686/jjzwm.24.65 [DOI] [Google Scholar]
- 16.Valkiūnas G.2004. Avian Malaria Parasites and Other Haemosporidia. CRC Press, Boca Raton. [Google Scholar]
- 17.Valkiūnas G., Santiago-Alarcon D., Levin I. I., Iezhova T. A., Parker P. G.2010. A new Haemoproteus species (Haemosporida: Haemoproteidae) from the endemic Galapagos dove Zenaida galapagoensis, with remarks on the parasite distribution, vectors, and molecular diagnostics. J. Parasitol. 96: 783–792. doi: 10.1645/GE-2442.1 [DOI] [PubMed] [Google Scholar]
- 18.Valkiūnas G., Iezhova T. A., Evans E., Carlson J. S., Martínez-Gómez J. E., Sehgal R. N. M.2013. Two new Haemoproteus species (Haemosporida: Haemoproteidae) from columbiform birds. J. Parasitol. 99: 513–521. doi: 10.1645/12-98.1 [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T., Tsuda Y., Sato Y., Murata K.2011. Pigeon louse fly, Pseudolynchia canariensis (Diptera: Hippoboscidae), collected by dry-ice trap. J. Am. Mosq. Control Assoc. 27: 441–443. doi: 10.2987/11-6183.1 [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura A., Koketsu M., Bando H., Saiki E., Suzuki M., Watanabe Y., Kanuka H., Fukumoto S.2014. Phylogenetic comparison of avian haemosporidian parasites from resident and migratory birds in northern Japan. J. Wildl. Dis. 50: 235–242. doi: 10.7589/2013-03-071 [DOI] [PubMed] [Google Scholar]