Abstract
Canine gastroesophageal intussusception (GEI) is a rare and life-threatening condition that requires prompt diagnosis and treatment. A 19-day-old Siberian Husky with a 4-day history of regurgitation was diagnosed with GEI based on the findings of computed tomography (CT) performed without anesthesia. Endoscopic reduction of intussusception was impossible; thus, surgical reduction by traction of the duodenum was performed. CT revealed improvement of megaesophagus 82 days postoperatively. Eleven months postoperatively, fluoroscopy showed recovery to nearly normal esophageal motility. Two years postoperatively, no clinical signs were reported. CT is useful to diagnose GEI in neonate puppies with poor abdominal fat and to assess the gastric edema and the anatomical association of stomach with other organs. Fluoroscopy is helpful for evaluating postoperative esophageal motility.
Keywords: canine, computed tomography, fluoroscopy, gastroesophageal intussusception, megaesophagus
Canine gastroesophageal intussusception (GEI) is a rare condition involving invagination of the stomach into the caudal esophageal lumen, with or without the spleen, duodenum, pancreas, and/or omentum [1, 15]. GEI most commonly affects male dogs younger than 3 months of age, of medium or large breeds, with an apparent overrepresentation of the German Shepherd breed [1, 8, 15]. Although the exact cause of this condition is unclear, it is considered to be related to some esophageal abnormalities including megaesophagus, abnormal esophageal motility, and laxity of the esophageal hiatus, because German Shepherds have a higher prevalence of esophageal abnormalities [1, 20].
The clinical signs associated with GEI include regurgitation or vomiting immediately after feeding, dyspnea, hematemesis, hypersalivation, abdominal discomfort, and sudden death [1, 8, 13, 15, 18]. These signs are related to the presence of the stomach and other organs in the thoracic cavity, the obstructive nature of the intussusception, vascular compromise of invaginated organs, and associated aspiration pneumonia [1, 8, 13, 18, 20].
For the diagnosis of GEI, assessment of clinical signs, thoracic radiography, positive contrast esophagography, and esophageal endoscopy are usually performed. However, they cannot assess invaginated organs other than the stomach or the severity of edema of the intussuscepted stomach [1, 4, 6,7,8,9,10,11,12,13,14,15,16,17,18,19].
The short-term prognosis of canine GEI may be poor because many dogs are immature at the time of diagnosis and their condition may have deteriorated, as shown by the severity of their clinical signs. Long-term prognosis is regarded as poor even after reduction of GEI because associated megaesophagus may not resolve in most cases and the dogs usually need lifelong nutritional management by feeding from an elevated position [6, 9, 12, 17, 19]. There are a few reports of dogs that did not require feeding management after reduction of GEI, but there has been no information on the objective evaluation of postoperative esophageal function [4, 7]. This report describes the successful management of GEI in the youngest dog reported to be treated and serves as an example of the spontaneous resolution not only of megaesophagus, but also of decreased esophageal motility.
A 19-day-old, intact male Siberian Husky dog weighing 1.28 kg presented to the Hokkaido University Veterinary Teaching Hospital with a 4-day history of frequent regurgitation immediately after nursing. An initial physical examination revealed lethargy, severely reduced skin turgor, and increased abdominal tenderness on palpation.
The blood examination findings were hypoalbuminemia (2.4 g/dl [normal range 2.6−3.5 g/dl]), hyponatremia (132 mmol/l [normal range 141−152 mmol/l]), hypochloremia (93 mmol/l [normal range 102−117 mmol/l]), hypokalemia (3.2 mmol/l [normal range 3.8−5.0 mmol/l]), and increased C-reactive protein levels (3.65 mg/dl [normal range 0.00−1.00 mg/dl]). Other findings were mild hyperglycemia (157 mg/dl [normal range 75−128 mg/dl]), leukocytosis (21,140 /µl [normal range 6,000−16,000 /µl]) characterized by neutrophilia, and a decreased hematocrit (25.9% [normal range 37.0−55.0%]) with reticulocytosis.
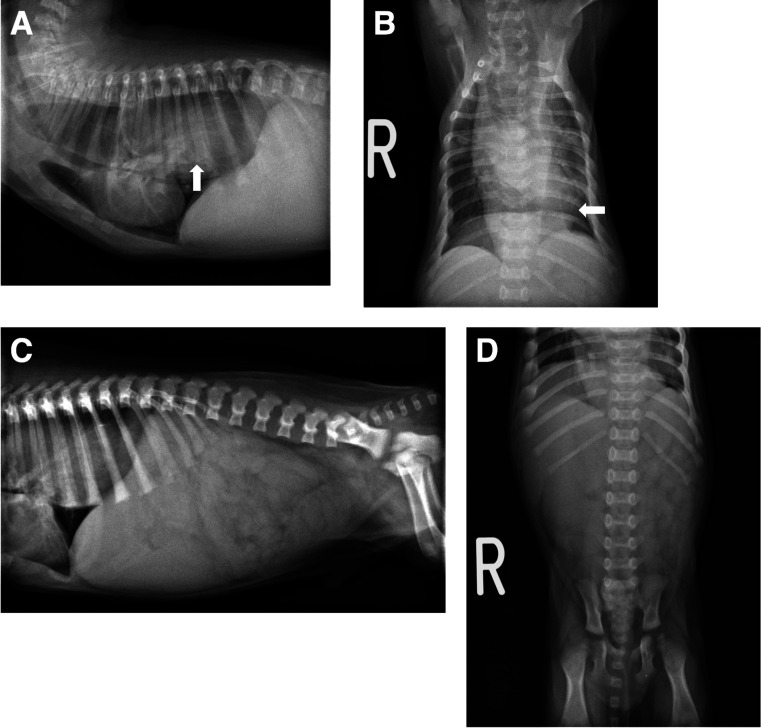
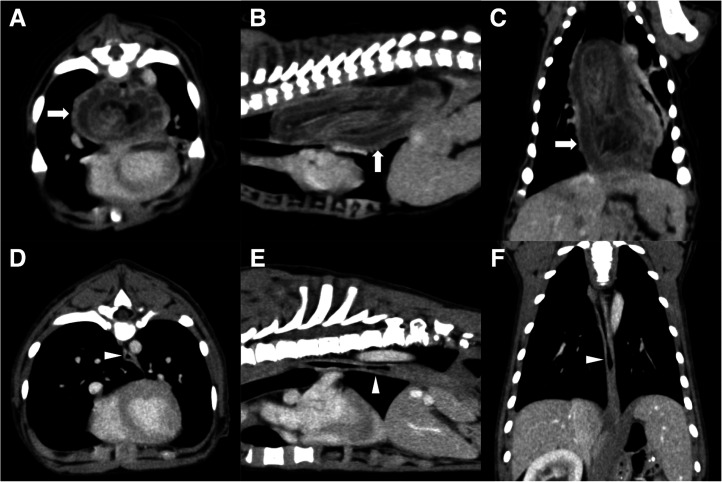
Thoracic and abdominal radiographs showed a severely distended esophagus with an intraluminal soft tissue opacity in the caudal portion and a ventrally displaced trachea (Fig. 1A and 1B). Although abdominal viscera were unclear because of poor accumulation of fat, the stomach was not visible in the cranial abdomen (Fig. 1C and 1D). From these findings, esophageal obstruction caused by GEI was suspected. Due to the regurgitation and risk of aspiration, a contrast esophagram was avoided. For a more detailed evaluation, the puppy was immobilized by tightly wrapped with blankets without disturbing breathing and a thoracic and abdominal computed tomography (CT) examination was performed without anesthesia using an 80-row multi-slice CT system (Aquilion PRIME; Canon Medical Systems Corp., Otawara, Japan) with 0.3 mm slice thickness scanning parameters and a 0.3 mm reconstruction interval. For a contrast-enhanced study, 2 ml/kg nonionic contrast agent (OMNIPAQUE 300 INJECTION; Daiichi-Sankyo Pharmaceutical Co., Ltd., Tokyo, Japan) was administered via an indwelling intravenous cannula placed in the right cephalic vein for 20 sec and the scan was carried out 40 sec after administration. The CT images showed a severely dilated thoracic esophagus and invagination of a large portion of the stomach into it with a homogeneously contrast-enhanced and diffusely thickened wall ranging from 5 mm to 11 mm, indicating diffuse edema of stuck stomach (Fig. 2A–C). No other organs were identified in the esophageal lumen. Based on these findings, a definitive diagnosis of GEI was made. Other abnormal findings were the absence of the left kidney and mild dilation of the right renal pelvis and ureter; however, no urinary obstruction was observed.
Fig. 1.
Right lateral and ventrodorsal radiographic images of the thorax and abdomen. (A, B) The thoracic esophagus is severely distended with an intraluminal soft tissue opacity in the caudal portion (arrow) and a ventrally displaced trachea. (C, D) Abdominal viscera were unclear and the gastric silhouette was not visible in the cranial abdomen.
Fig. 2.
Computed tomography findings on transverse, dorsal, and sagittal plane images at the soft tissue window with contrast media on the day of presentation and at 82 days postoperatively. (A−C) The dilated thoracic esophagus involves the stomach with a homogeneously contrast-enhanced and diffusely thickened wall (arrow) on the day of presentation. (D−F) The stomach is seen at the normal position and esophageal dilation has improved (arrowhead) at 82 days postoperatively.
Initial treatment for dehydration and electrolyte imbalances consisted of intravenous (IV) saline (Isotonic Sodium Chloride Solution; Terumo Corp., Tokyo, Japan) supplemented with potassium chloride (5 ml/kg/hr) (KCL Corrective Injection 1 mEq/ml; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) and 2% glucose (Otsuka Glucose Injection 50%; Otsuka Pharmaceutical Co., Ltd.). Additional medications included famotidine (0.5 mg/kg subcutaneously [SC]) (Gaster Injection, 10 mg; Astellas Pharma Inc., Tokyo, Japan) as a gastric mucosa protectant and ampicillin (20 mg/kg IV) (Viccillin 0.25 g for injection; Meiji Seika Pharma Co., Ltd., Tokyo, Japan) as a prophylactic antibiotic.
Esophageal endoscopy was performed under general anesthesia the same day. The puppy was premedicated with fentanyl (3 µg/kg IV) (Fentanyl injection; Daiichi-Sankyo Pharmaceutical Co., Ltd.) and anesthesia was induced with propofol (8 mg/kg IV) (Propofol 1% Injection 20 ml; Pfizer Co., Ltd., Tokyo, Japan). The puppy was intubated and maintained with inhaled isoflurane (ISOFLU; DS Pharma Animal Health Co., Ltd., Osaka, Japan). The endoscopy revealed a dilated, fluid-filled proximal esophagus and a large intraluminal mass consistent with the mucosal surface of the stomach in the midthoracic esophagus. Because the stomach was stuck deeply in the esophagus, reduction of the intussusception using the endoscope failed. Therefore, surgical reduction was performed immediately. Exploration of the abdomen found that the esophageal hiatus was enlarged and the entire stomach had telescoped into the thoracic esophagus. Gentle traction of the duodenum resulted in the reduction of the stomach into the abdominal cavity. Esophagoscopy after the reduction revealed a dilated, inflamed, and congested esophagus. No other invaginated organ was observed during abdominal exploration. A gastrostomy tube (PEG Feeding Tubes; Smiths Medical ASD Inc., Weston, MA, USA) was placed surgically in the left abdominal wall, and gastropexy with a 3–0 polydioxanone suture (PDS II; Ethicon Inc., Somerville, NJ, USA) was performed.
After an uneventful recovery from anesthesia, the patient’s condition improved immediately. Feeding breast milk via the gastrostomy tube was started 1 hr postoperatively. Famotidine (0.5 mg/kg SC, q 12 hr) and ampicillin (20 mg/kg IV, q 12 hr) were continued for 3 days.
The puppy was discharged 3 days postoperatively. Reassessment 10 days postoperatively showed no regurgitation and a slight body weight gain to 1.38 kg. The blood examination revealed persisting hypoalbuminemia (1.9 g/dl), hyponatremia (140 mmol/l), leukocytosis (22,300 /µl) characterized by a neutrophilia, and low hematocrit (31.6%). Potassium and chloride concentrations were within their normal ranges at 4.8 mmol/l and 107 mmol/l, respectively. Thoracic radiographs showed evidence of megaesophagus. Oral feeding of breast milk and food paste from an elevated position was started in combination with feeding via the gastrostomy tube the same day.
A follow-up examination was performed 28 days postoperatively. The owner reported no regurgitation after oral feeding, and the dog had a gradual body weight gain to 2.64 kg. A fluoroscopic study of the esophagus was performed in right lateral recumbency using a standard fluoroscopy unit (POPULUS So; Hitachi Medical Corp., Tokyo, Japan) at a frame rate of 4 frames per second. Barium-mixed food paste was administered in 5–10 ml doses, and 3 to 5 swallows were observed with at least 1 bolus entering the stomach. Some of paste remained within the esophagus, consistent with reduced esophageal motility. The owner was instructed to stop the feeding via the gastrostomy tube and continue oral feeding from the elevated position.
The next follow-up examination 82 days postoperatively showed no regurgitation and a body weight gain to 6.36 kg. The blood examination revealed mildly decreased hematocrit (36.2%), however, all other parameters, including the concentration of albumin (3.1 g/dl), were within their normal ranges. Reassessment of megaesophagus under general anesthesia using CT showed an improvement of the dilation of the esophagus to nearly normal, and the gastrostomy tube was subsequently removed (Fig. 2D–F). A fluoroscopic study of the esophagus performed the same day showed the unimpeded passing of the barium-mixed food paste and stagnation of the barium-soaked solid food in the thoracic esophagus, indicating partial functional restoration of the esophagus.
After 11 months postoperatively, the dog showed good nutrition with a body weight of 20.9 kg. A fluoroscopic study showed the unimpeded passing of both soft and solid food through the esophagus. The esophageal transit speeds of solid food in the cranial and caudal thoracic esophagus were 5.0 cm/sec and 4.6 cm/sec, respectively. During the fluoroscopic study, the lumen of the thoracic esophagus expanded to a maximum diameter of 18 mm during the transit of food and shrunk to less than 7 mm after the passage of food. The owner was instructed to feed the dog commercially available dry food and water from a normal position.
Two years after surgery, the dog could eat in a normal position and the owner noticed no regurgitation. The dog had good nutrition with a body weight of 21.2 kg. A repeated thoracic radiograph showed no evidence of megaesophagus.
In this case, a multi-slice CT scan examination without anesthesia was effective for the definitive diagnosis of GEI. Because the patient was a 19-day-old puppy at the initial visit and had little abdominal fat to provide contrast between the abdominal organs, it was difficult to detect the precise location of the stomach by plain abdominal radiography. CT images showed the invagination of a large portion of the stomach in the caudal esophagus without the involvement of any other organs. Contrast-enhanced CT images were also helpful for understanding the precise pathology of GEI which revealed a homogeneously contrast-enhanced and diffusely thickened stomach wall, indicating the diffuse edema of the invaginated stomach wall. The thickness of the stomach wall in healthy puppies has been reported to be less than 3 mm [2]. In this case, the thickness of the stomach wall was calculated from 5 mm to 10 mm in the CT images, indicating diffuse and severe edema of the intussuscepted stomach. To the authors’ knowledge, this is the first case report describing CT images of canine GEI. A CT scan examination without anesthesia is a safe and useful modality for the diagnosis and understanding of GEI, especially for neonatal puppies that have deteriorated.
Esophageal endoscopy showed an invaginated stomach in the midthoracic esophagus; however, the pressure of the endoscopic tip was not able to reduce the intussusception in this case. One of the reasons for this was that a large portion of the stomach was profoundly stuck in the esophagus and diffuse edema of the stomach wall developed, making endoscopic reduction unsuccessful. Esophageal endoscopy is often performed not only for diagnosis, but also for the reduction of GEI [6, 7, 11, 12, 14, 17]. However, endoscopic reduction of GEI may be difficult, depending on the amount of edema present in the invaginated stomach. If there is severe edema of the invaginated stomach, it is unlikely that endoscopic reduction of the stomach will succeed, so it may be possible to choose surgical reduction over endoscopic reduction based on CT examination findings.
Repeated fluoroscopic studies during postoperative follow-up using barium revealed a gradual recovery of esophageal function, especially based on the textures of different foods. Although food paste had been retained or passed slowly through the thoracic esophagus immediately after surgery, solid food finally passed through the esophagus smoothly 11 months after surgery. According to a previous report on esophageal transit time in healthy dogs, cranial and caudal thoracic esophageal transit speeds of kibble food in lateral recumbency were 5.30 ± 1.88 cm/sec and 4.81 ± 1.15 cm/sec, respectively [3]. In this case, the cranial and thoracic esophageal transit speeds of kibble food were 5.0 cm/sec and 4.6 cm/sec, respectively, 11 months after surgery. This result indicates the recovery of esophageal function to nearly normal. The fluoroscopic study was believed to be useful for the evaluation of esophageal function and for instructing the owner how to manage feeding after surgery.
In this case, megaesophagus and esophageal motility improved to nearly normal after resolution of GEI. Many other reported cases have shown the persistence not only of megaesophagus, but also regurgitation, indicating low esophageal motility [6, 9, 12, 17, 19]. Although the exact etiology is unclear, canine GEI has been considered to develop secondary to the active, retrograde motility initiated during vomiting as well as from underlying congenital conditions, such as megaesophagus or low esophageal motility [1, 8, 20]. For the same reasons, it has been assumed that most cases of megaesophagus will not recover even after the reduction of GEI. However, there are some reports of the clinical improvement of spontaneous megaesophagus that were probably due to the postnatal maturation of esophageal innervation and/or musculature [4, 5]. There has been one previous report of a 5-week-old puppy demonstrating spontaneous resolution of megaesophagus and esophageal function after reduction of GEI [4]. One of the possible reasons for the morphological and functional recovery of the esophagus in this case was that the patient was a 19-day-old puppy, the youngest dog reported to date with a diagnosis of GEI who was treated, thus resulting in the postoperative maturation of esophageal innervation and musculature [4, 6, 7, 9,10,11,12,13,14, 16,17,18,19]. This result indicates the possibility that megaesophagus and low motility of the esophagus may recover depending on certain factors such as age at onset and timing of treatment. If GEI is diagnosed in neonate puppies immediately after onset and treated appropriately, it may result in a good functional prognosis.
Prompt surgical treatment for acute GEI provided excellent morphologic and functional improvements of the esophagus and long-term outcome for a 19-day-old puppy. A multi-slice CT scan is useful for the diagnosis of GEI and repeated fluoroscopic assessment of esophageal function after surgery is beneficial for the management of postoperative feeding.
CONFLICT OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Applewhite A. A., Cornell K. K., Selcer B. A.2002. Diagnosis and treatment of intussusceptions in dogs. Comp. Cont. Educ. Pract. 24: 110–127. [Google Scholar]
- 2.Banzato T., Milani C., Zambello E., Zotti A.2017. Normal ultrasonographic reference values for the gastrointestinal tract in developing puppies. Res. Vet. Sci. 115: 371–373. doi: 10.1016/j.rvsc.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Bonadio C. M., Pollard R. E., Dayton P. A., Leonard C. D., Marks S. L.2009. Effects of body positioning on swallowing and esophageal transit in healthy dogs. J. Vet. Intern. Med. 23: 801–805. doi: 10.1111/j.1939-1676.2009.0325.x [DOI] [PubMed] [Google Scholar]
- 4.Clark G. N., Spodnick G. J., Rush J. E., Keyes M. L.1992. Belt loop gastropexy in the management of gastroesophageal intussusception in a pup. J. Am. Vet. Med. Assoc. 201: 739–742. [PubMed] [Google Scholar]
- 5.Diamant N., Szczepanski M., Mui H.1974. Idiopathic megaesophagus in the dog: reasons for spontaneous improvement and a possible method of medical therapy. Can. Vet. J. 15: 66–71. [PMC free article] [PubMed] [Google Scholar]
- 6.Graham K. L., Buss M. S., Dhein C. R., Barbee D. D., Seitz S. E.1998. Gastroesophageal intussusception in a Labrador retriever. Can. Vet. J. 39: 709–711. [PMC free article] [PubMed] [Google Scholar]
- 7.Greenfield C. L., Quinn M. K., Coolman B. R.1997. Bilateral incisional gastropexies for treatment of intermittent gastroesophageal intussusception in a puppy. J. Am. Vet. Med. Assoc. 211: 728–730. [PubMed] [Google Scholar]
- 8.Lewis D. D., Ellison G. W.1987. Intussusception in dogs and cats. Compend. Contin. Educ. Pract. Vet. 9: 523–534. [Google Scholar]
- 9.Lockwood A., Radlinksy M., Crochik S.2010. Gastroesophageal intussusception in a German shepherd. Compend. Contin. Educ. Vet. 32: E1–E4. [PubMed] [Google Scholar]
- 10.Mathis K. R., Nykamp S. G., Ringwood B. P., Martin D. M.2013. What is your diagnosis? Gastroesophageal intussusception. J. Am. Vet. Med. Assoc. 242: 465–467. doi: 10.2460/javma.242.4.465 [DOI] [PubMed] [Google Scholar]
- 11.McGill S. E., Lenard Z. M., See A. M., Irwin P. J.2009. Nonsurgical treatment of gastroesophageal intussusception in a puppy. J. Am. Anim. Hosp. Assoc. 45: 185–190. doi: 10.5326/0450185 [DOI] [PubMed] [Google Scholar]
- 12.Murphy L. A., Nakamura R. K., Miller J. M.2015. Surgical correction of gastro-oesophageal intussusception with bilateral incisional gastropexy in three dogs. J. Small Anim. Pract. 56: 630–632. doi: 10.1111/jsap.12359 [DOI] [PubMed] [Google Scholar]
- 13.Nagel C. M., Montgomery J. E., O’Connor B. P.2014. What is your diagnosis? Gastroesophageal intussusception. J. Am. Vet. Med. Assoc. 244: 279–280. doi: 10.2460/javma.244.3.279 [DOI] [PubMed] [Google Scholar]
- 14.Pietra M., Gentilini F., Pinna S., Fracassi F., Venturini A., Cipone M.2003. Intermittent gastroesophageal intussusception in a dog: clinical features, radiographic and endoscopic findings, and surgical management. Vet. Res. Commun. 27 Suppl 1: 783–786. doi: 10.1023/B:VERC.0000014271.98916.ff [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen L.2003. Stomach. pp. 631−632. In: Textbook of Small Animal Surgery, 3rd ed. (Slatter, D. H. ed.), Saunders, Philadelphia. [Google Scholar]
- 16.Roach W., Hecht S.2007. What is your diagnosis? Gastroesophageal intussusception. J. Am. Vet. Med. Assoc. 231: 381–382. doi: 10.2460/javma.231.3.381 [DOI] [PubMed] [Google Scholar]
- 17.Shibly S., Karl S., Hittmair K. M., Hirt R. A.2014. Acute gastroesophageal intussusception in a juvenile Australian Shepherd dog: endoscopic treatment and long-term follow-up. BMC Vet. Res. 10: 109. doi: 10.1186/1746-6148-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torad F. A., Hassan E. A.2015. Gastroesophageal intussusception in a 50-day-old German shepherd dog. Top. Companion Anim. Med. 30: 22–24. doi: 10.1053/j.tcam.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 19.von Werthern C. J., Montavon P. M., Flückiger M. A.1996. Gastro-oesophageal intussusception in a young German shepherd dog. J. Small Anim. Pract. 37: 491–494. doi: 10.1111/j.1748-5827.1996.tb01750.x [DOI] [PubMed] [Google Scholar]
- 20.Washabau R. J.2003. Gastrointestinal motility disorders and gastrointestinal prokinetic therapy. Vet. Clin. North Am. Small Anim. Pract. 33: 1007–1028, vi. doi: 10.1016/S0195-5616(03)00076-7 [DOI] [PubMed] [Google Scholar]