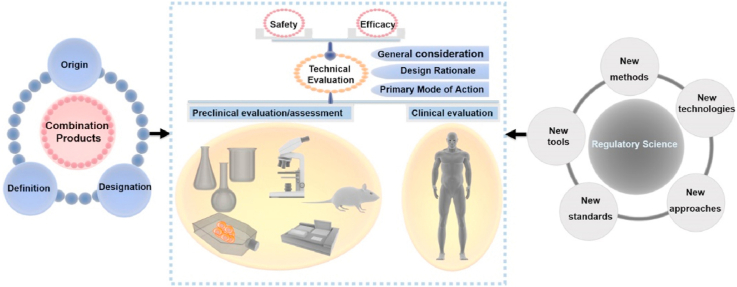
Abstract
Combination products with a wide range of clinical applications represent a unique class of medical products that are composed of more than a singular medical device or drug/biological product. The product research and development, clinical translation as well as regulatory evaluation of combination products are complex and challenging. This review firstly introduced the origin, definition and designation of combination products. Key areas of systematic regulatory review on the safety and efficacy of device-led/supervised combination products were then presented. Preclinical and clinical evaluation of combination products was discussed. Lastly, the research prospect of regulatory science for combination products was described. New tools of computational modeling and simulation, novel technologies such as artificial intelligence, needs of developing new standards, evidence-based research methods, new approaches including the designation of innovative or breakthrough medical products have been developed and could be used to assess the safety, efficacy, quality and performance of combination products. Taken together, the fast development of combination products with great potentials in healthcare provides new opportunities for the advancement of regulatory review as well as regulatory science.
Keywords: Combination products, Definition, Designation, Safety and efficacy, Regulatory science
Graphical abstract
Highlights
-
•
Full overview of the origin, definition and designation of combination products.
-
•
Regulatory review and evaluation of the safety and efficacy of combination products.
-
•
Regulatory science and its future perspectives of combination products.
1. Introduction
A biomaterial is “a material designed to take a form that can direct, through interactions with living systems, the course of any therapeutic or diagnostic procedure” [1]. The development of biomaterial science and engineering has created many medical products, such as long-term implants [[2], [3], [4], [5], [6]], high-efficient drug delivery systems [[7], [8], [9], [10]], and hydrogel-based devices with various functions [6,[10], [11], [12]]. As raw materials and components, biomaterials could directly or indirectly affect the safety and performance of medical products [1]. As a result, the ultimate goal of biomaterial research and development is to contribute human healthcare by providing safe and effective pharmaceutical drugs, medical devices, biological products, as well as combination products. Many state-of-art biomaterial research projects in fact are targeting for potential applications as combination products.
Medical devices are defined by regulations, and classified and regulated according to their complexity and the level of risks to the public [13,14]. According to the level of risks (from low to high) [[13], [14], [15]], the classifications of medical devices are divided into class I, class II and class III, and the regulatory requirements and control level also increased accordingly [[13], [14], [15]]. For example, the procedures to approve medical devices in China can be categorized into filing (for Class I devices) and registration (for the rest) [13,15]. In the US, Class I and II devices (without exemptions) should be registered by a 510k route for marketing [14]. Class III devices should be registered by a premarket approval application [14].
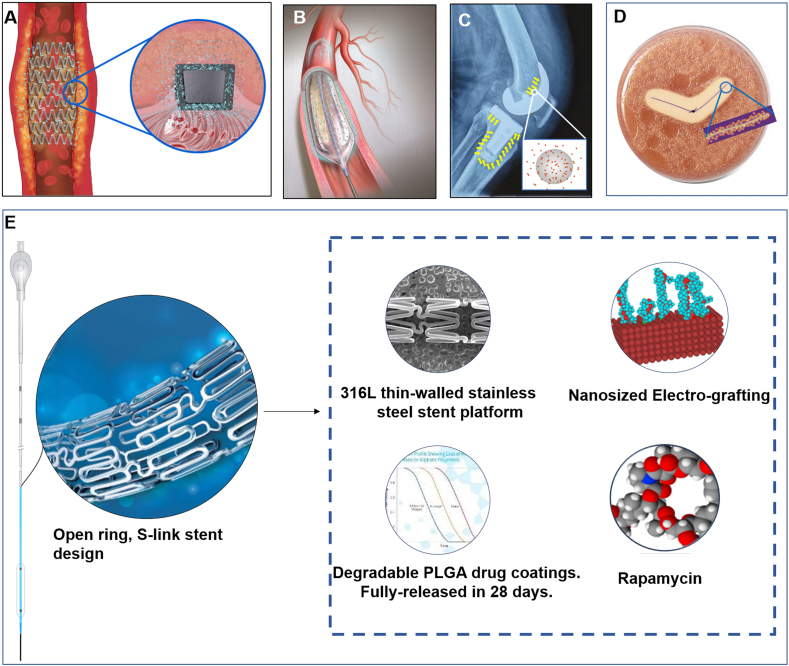
With the continuous development of technology to satisfy unmet clinical needs, combination products have developed into a unique form of medical products, such as those used in cardiovascular, orthopedic and neurological applications (see Fig. 1). At present, the marketed and known combination products include drug-eluting stents, catheters with antibacterial coatings, antibiotic-loaded bone cements and antibacterial/anti-inflammatory wound stickers (see Fig. 1). In addition, more combination products such as tissue engineering medical products [16], bioartificial liver devices [17], and chip-containing smart pills [18] are also being developed. These combination products all have their therapeutic advantages, relying on cutting-edge innovative technologies.
Fig. 1.
Representative diagrams of combination products. A. drug-eluting stents. B. catheters with antibacterial coatings. C. antibiotic-loaded bone cements for total knee replacement. D. stitches containing antibacterial ingredients and their inhibition halo. E. Intracranial drug-eluting stent system. Images were provided by the manufacturers.
The innovative and complex nature of combination products presents difficulties and challenges not only to academia and industry, but also to the regulatory agencies. This article aimed at addressing the needs of research, development, translation and regulation of combination products. Firstly, we introduced the origin, definition and designation of combination products. Then, the regulatory review was presented and discussed focusing on the safety and efficacy evaluation of device-led/supervised combination products. Finally, future research directions of regulatory science for combination products were proposed.
2. Origin, definition and designation
Combination products with any two or three products of medical devices, pharmaceuticals and biological products combined emerged in 1970s. These products have their own unique effectiveness. For example, antibiotic-loaded bone cements, made originally by Buchholz and Engelbrech in 1970, could reduce postoperative infection rate from 6% to 1.6% in artificial total hip replacement [19]. Drug-elution stents, i.e. drug-coated scaffolds, could prevent neointima hyperplasia in blood vessels [20]. Herein, combination products were growing rapidly in many product areas, including antibiotic-loaded bone cements [21,22], drug-eluting stents [20,23], prefilled syringes [24,25], nicotine patches [26], and balloon catheters filled with radioactive liquid suspensions [27].
The U.S. Food and Drug Administration (FDA) has regulated combination products since 1970 [28]. For addressing regulatory issues with these products, the U.S. Food, Drug, and Cosmetic Act was revised in 1990 [29]. These products were defined as “constitut(ing) a combination of a drug, device, or biological product” [29,30]. With the development of manufacturing technology, the universe and complexity of combination products are becoming increasingly prevalent. In 1991, combination products were firstly defined in Code of Federal Regulations Title 21 3.2(e) (21 CFR 3.2(e)) (Table 1) [31]. Combination products range from physical or chemical combinations, to products packaged together and products that are separately packaged but need to be used together [29]. Combination products were reported to account for about 30% of medical products [32]. Although the composition of combination products has been approved separately, the use of combination products may also pose additional risks. The addition of drugs or biological components may result in new intended uses and/or constitute different technical characteristics from the same variety of instruments, causing different safety and efficacy issues. Therefore, the development and complexity of combination products calls for efficient regulation to ensure their safety and efficacy. For the best regulation of combination products, the definition and designation of combination products should be firstly understood and examined.
Table 1.
The Definition of combination products in the U.S., China and Japan.
| Nation | Name | Definition |
|---|---|---|
| United States | Combination products | “A product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity; Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products; A drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect.” [31] |
| China | Drug/device combination products | “A product made up of drugs and medical devices and produced as a single entity.” [43] |
| Japan | Combination products | “The products marketed as a single drug, medical device, or cellular and tissue-based product that combine two or more types of drug, device, processed cell, etc. that are expected to fall under the category of drugs, medical devices, or cellular and tissue-based products if marketed individually.” [46] |
The Food and Drug Administration Modernization Act (FDAMA) of 1997 passed the regulation of combination products [33]. On December 24, 2002, FDA established Office of Combination Products (OCP) to ensure the prompt assignment and the timely and effective premarket review of combination products, according to modified section 503(g) in the Medical Device User Fee and Modernization Act of 2002 (MDUFMA) [34,35]. On July 22, 2013, FDA issued “Current Good Manufacturing Practice Requirements for Combination Products” (21 CFR Part 4) [36]. FDA's regulation of combination products was updated and modernized in the 21st Century Cures Act [29].
Combination products are a unique regulatory category [31,37]. FDA published a proposed rule for the definition of “mode of action” (MOA) and “primary mode of action” (PMOA) [34]. Combination products include more than one type of regulated article. Each constituent part has one MOA. The most important MOA is PMOA. The rule also sets forth an algorithm to assign combination products to an agency when the agency cannot determine the most important therapeutic action [34]. If the classification of combination products is unclear or in dispute, a sponsor can submit pre-request for designation (RFD) or RFD to OCP according to 21 CFR part 3 to obtain a formal classification determination [38]. FDA's determination is mainly based on statutory definition, PMOA/MOA and the degree of innovation or the future use risks. OCP assigns audit responsibility for combination products to the Lead Center- Center for Biologics Evaluation and Research (CBER), Center for Drug Evaluation and Research (CDER) or Center for Devices and Radiological Health (CDRH) [38].
In China, a notice in 2002 firstly mentioned drug-containing medical devices and began the regulation of combination products [39]. Drug/device combination products with main effects as medical devices shall be regulated as Class III medical devices, and drugs in combination products should have drug registration certificate issued by National Medical Products Administration (NMPA) [39,40]. “Issues related to registration management of products combining drugs and medical devices (Repealed)” firstly noticed the definition of products combining drugs and medical devices [41]. NMPA announced on the registration of drug/device combination products in November 2009 (No. 16 of 2009) [42]. For the first time, drug/device combination products were defined in China. In 2021, NMPA stated “Announcement on the registration of drug/device combination products” (No. 52 of 2021) [43]. The announcement did not change the definition of drug/device combinations (see Table 1), but deleted the requirements that drugs in imported combination products should be approved previously by NMPA or countries (regions) of origin.
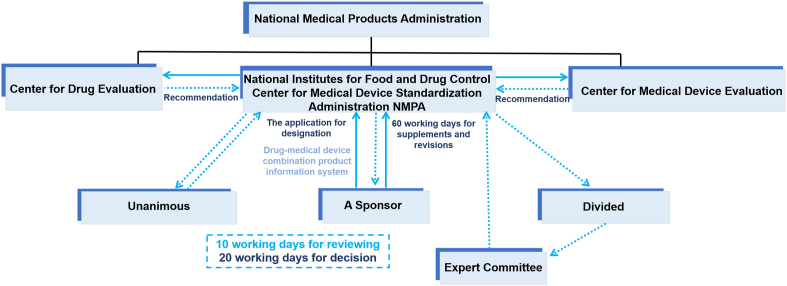
For the designation of drug/device combination products, drug-led combination products should be registered as drugs, and medical device-led combination products should be registered as medical devices [43]. In 2019, NMPA issued the “announcement on adjusting the relevant matters concerning the definition of the attributes of drug/device combination products” (No. 28 of 2019) [44]. This announcement has been consolidated into No. 52 of 2021 [43] (see Fig. 2). The Center for Medical Device Standards Administration of NMPA (CMDSA) is responsible for the attributes-definition of drug/device combination products. The CMDSA has established a coordination system for the definition of attributes with the Center for Medical Device Evaluation of NMPA (CMDE) as well as the Center for Drug Evaluation (CDE). The CMDE is the lead center for medical device-led combination products, and the CDE is the lead center for drug-led combination products [43,44]. If the designation of combination products is unclear or in dispute, the CMDSA would consult with experts in the field and/or hold expert committee meetings for proper designations.
Fig. 2.
The application process of attributes-definition of drug/device combination products in China [43].
As aforementioned, the definition of combination products is different in the United States and China. When searching for that in other countries, the definition of combination products also varies from country to country (Table 1). The European Union does not have a universal definition of combination products, nor does it classify pharmaceutical combination products into a unit category [45]. In Europe, devices intended to administer both devices and medicinal products formed single integral medicinal products, which are intended exclusively for use in the given combination on the market [44]. In Japan, the definition of combination products is similar to that in the United States. Combination products refer to the combination of two or more regulated components of drugs, medical devices, or biological products (known in Japan as cellular and tissue-based product) [46]. Because these three types of products are regulated separately in the U.S. and Japan, it is more appropriate to describe combination products in the U.S. and Japan in terms of “combination products”. Moreover, unlike emphasizing a single entity in China, the form of combination products in Japan and the United States could be a single entity, joint packaging and cross-labeling. Therefore, the definition of combination products is related to the specific national regulatory systems of different countries. The biggest difference in the definition of combination products among the three countries is the regulatory scope of combination products. However, the attributes-definition of combination products means how the combination products should be regulated. Therefore, the designation of combination products also needs further attention.
3. Regulatory review/technical evaluation
Regulation and supervision of medical products should be throughout their life cycles. The core issue of regulatory review is how to evaluate the safety and efficacy of regulated products. Combination products are typical interdisciplinary products relating to medical device and drugs. Safety is defined as “Freedom from unacceptable risk”, and efficacy is defined as “the ability of a medical device or IVD medical device to provide clinically significant results in a significant portion of the target population” [47]. The evaluation of combination products should follow the basic principles for research and development of these products. Given the complexity of definition and designation of combination products, this article focuses on medical device-led/supervised combination products. The regulatory review/technical evaluation of the safety and efficacy of device-led/supervised combination products is the focus of this section.
3.1. General consideration
The key issues for the technical review of combination products are the risk identification and the overall risk-benefit evaluation of final products. If drugs or medical devices in combination products have been individually approved by the regulatory authorities, the risks of these individual parts have been evaluated. The previous evaluation could be used as supporting information to further support the safety and efficacy of the combination products. If combination products have new MOA, new indications, new target populations and new methods of use, for individually approved components, they need to be assessed through scientific evidence. For example, adding steroids to pacing conductors can reduce the inflammatory reaction at the wire tissue interface, and reduce polarization potential, pacing threshold, and pacing energy consumption [48,49]. The methods and intended use of steroids on electrode conductors are different from what they were approved separately as drugs. Scientific evidence is needed to support their claimed efficacy. In addition, while analyzing and discussing the clinical benefits of combination products after adding pharmaceutical ingredients, it is also necessary to analyze the potential new risks that may be associated with them. For example, medical implants added or coated with antibiotics could achieve antibacterial efficacy [50,51]. However, low-level antibiotic exposure could induce resistance, which should not be ignored in the evaluation of such combination products [52]. Therefore, risks for adding drugs, especially new risks, should be the focus during evaluation.
3.2. Design rationale and primary mode of action (PMOA)
For combination products, the design rationale of adding a specific drug should be fully analyzed and demonstrated. For example, coronary stents are usually added with drugs including paclitaxel, rapamycin and its derivatives to reduce the restenosis rate caused by neointima hyperplasia [53]. However, which drug should be included in the drug-eluting stents should be demonstrated by the design rationale of the products. Similar examples also include hyaluronic acid gel with lidocaine, which could be more effective for pain relief than hyaluronic acid alone [54], and antibiotic loaded bone cement, which could reduce hip and knee prosthetic joint infections [55].
For combination products, it is necessary to study whether their PMOA is led by the drug or the device component. The PMOA is the means by which the product achieves the desired therapeutic efficacy. The PMOA of a combination product is the means by which the product is expected to make the greatest contribution to the overall intended therapeutic efficacy. Combination product based on the PMOA of medical device is a medical device-led product. For these products, drugs play a supporting role on the PMOA of medical devices. It is necessary to study the principle of roles of drugs in the products, the mechanisms to achieve the intended use and the duration of action [56].
3.3. Preclinical evaluation
3.3.1. Interactions of different components in combination products
The interactions of different components in combination products should be considered firstly. Addition of drugs to a device product may change the original product's production process, storage conditions, indications, contraindications and precautions. For example, heparin-containing oxygenators [57], triclosan-containing sutures [58], coronary stents with paclitaxel [59] could achieve anticoagulant, antibacterial, and inhibition of local cell hyperproliferation, respectively. However, their drug loading processes may affect the mechanical properties and surface properties of the final devices [60,61]. When a device is used as a drug carrier, its production additives, processes or storage conditions may have an impact on drug activity, drug release, and overall product quality. For example, the production process and critical control points of antibiotic-loaded bone cements may include the effect of mixing process, the distribution of the drugs, and the effects of terminal sterilization on the drugs' performance [62].
Considering the local use of drugs with medical devices, drug safety evaluation should be conducted on the carrier polymers (if any) and processing aids. The composition of drugs and their polymer carriers play very important roles in the final combination products. The following focuses on drug-eluting stents as an example. The drugs in the drug eluting coronary stents are designed to be released and absorbed at the target site of blood vessels [63]. Although drug concentration in the blood plasma after the implantation of drug eluting coronary stent is low, the concentration in the local tissue of the target vascular wall (especially when the stent is overlapped) may be much higher than that in the blood after the product is systemically used [63,64]. Polymer carriers should be able to coat enough drugs on the stent, and also affect the release profile of drugs from the stents [60,65,][66]] [][60], [65], [66][]. Therefore, it is necessary to carry out research on drug dose density selection, total dose concentration selection in a single stent, drug and carrier polymer formation selection, coating stability, and in vitro/in vivo release characteristics of drugs to evaluate the overall toxicology risks of final products.
3.3.2. Qualitative, quantitative and in vitro release of drugs in combination products
The rationale for determining drug content/dose in combination products should be investigated. In wound dressing products with antibacterial ingredients, the dosage of antibacterial ingredients is determined by the minimum inhibitory concentration and safety thresholds for the whole body [67,68]. For antibiotic-loaded bone cements, antibiotic content, antibiotic delivery volume and rate, topical antibiotic concentration change, the minimum inhibitory concentration (MIC), resistant variants value concentration (MPC value), resistance caused by low-dose local antibiotics, and the effective release time should be evaluated [62]. If the content of a drug refers to a current combination product, the research should be conducted on the impact of design differences between the current and predicted products. Structural design and carrier material formulations could change the release rate of drugs [66,69,][70]] [][66], [69], [70][]. For combination products that achieve functions by releasing drugs to the intended site (such as sustained release, controlled release or other release methods), such as drug-eluting stents, drug-containing balloon catheters, and silver-containing dressings, drug release studies are needed [[71], [72], [73], [74], [75], [76], [77]]. In vitro release study can be used to evaluate the stability of final products and coatings [[77], [78], [79], [80]]. For combination products containing bioactive substances, such as orthopedic devices containing osteoinductive agents and growth factors and surgical instruments containing heparin coating, studies on MOA are needed. For example, qualitative and quantitative investigation could be conducted through the research of content, activity, and efficacy of bioactive substances [81,82].
3.3.3. Biological evaluation
For combination products, biological evaluation of final products could reference to the series of ISO 10993 standards. Drugs may affect the results of biological evaluation tests. Drug coatings of drug-elution stents may also have an impact on biocompatibility after stents are placed [83,84]. As a result, researchers need to combine the MOA of the drugs and clinical benefits to demonstrate whether the biological risks introduced by the drugs are acceptable. Drugs in combination products typically have a local effect. If drugs in combination products were approved previously for indications different from that of the combination products, the safety of the final combination product should be evaluated in conjunction with drug safety data. However, it is necessary to consider whether the new combination will cause any change of the established or understood safety and efficacy of drugs. If the exposed local or systemic drug concentration of combination products is greater than the approved drug dose, additional safety studies may be needed, such as the neurotoxicity of intracranial devices [85]. For some combination products, such as drug-containing balloon catheters, since body contact time and frequency of the drug may be different from that of the device, it may be not accurate to use single contact method to assess the biological risk [86].
3.3.4. Animal studies
According to technical features of combination products, animal studies need to be designed to include in vivo drug release dynamics and in vivo pharmacodynamics. For example, the animal studies of a drug eluting stent should include the performance of drug delivery, systemic toxicity, local toxicity, screening of drug doses, formulation and coating thickness of carrier polymer, and drug release as well as pharmacokinetics [[87], [88]] [[,88]. For animal studies on silver-containing wound dressing products, animal models and wound or disease models must be identified. The wound or disease models should cover the intended use of the product. The application method and frequency and the outcome measures and their rationale and standards should be evaluated by animal studies. Whether the addition of silver affects the local healing of the tissue also need to be investigated. The absorption, distribution, metabolism and excretion of silver in the animal body need to be studied to evaluate the accumulation and existence of silver in the body [89].
3.4. Clinical evaluation
Before carrying out clinical evaluation of combination products, it is necessary to clarify and understand their MOA and PMOA, intended efficacy, potential risks, and adverse events. Such examples include but not limited to the followings. Is the intended use of combination products consistent with, similar to, or significant different from the components of combination products? Do combination products expand or exceed the intended use or claim more clinical benefits than their components when used alone? For the instruction for use of combination products, have the route of drug administration, drug release, and local/systemic drug exposure range in combination products changed compared to when the drug is used alone or may the change bring new risks?
“Standards for quality control of medical device clinical trials” should be followed to conduct clinical evaluation of combined products [90]. Combining the risks and benefits of combination products, a reasonable clinical evaluation path can be selected to demonstrate the safety and efficacy, and to provide a basis for the Products' Instructions for use. The key components of clinical trial protocols for combination products include sample size, statistical methods, clinical endpoints, intended uses, efficacy claims, and the number of clinical studies if multiple trials are included. The purpose of adding pharmaceutical ingredients to devices is often to improve product functions or reduce adverse events related to the product uses. It is necessary to demonstrate the products’ clinical risks and benefits. It is also necessary to combine the performance characteristics and intended efficacy of combination products to demonstrate their science and adequacy of the clinical design. For example, due to the differences in the goals of clinical treatments, different drug-eluting stents may have different safety and efficacy evaluation outcome measures [91]. If the combination products contain new active drug ingredients, special attention should also be paid to the safety of drugs in clinical trials. Preliminary studies might be necessary to characterize the pharmacokinetics and metabolism of the drug as well as to determine the safety of the drug for human use, prior to the clinical study of the combination product [92]. These studies might not be necessary for drugs with previous approval for use in human or with available human safety information.
Taken together, the goal of regulation and supervision of combination products is to scientifically and comprehensively evaluate and ensure their safety and efficacy. Their regulatory review and technical evaluation are complex, scientifically demanding, and systematic with multi-step and multi-level assessments. Thus, regulatory science for combination products should be pursued and executed.
4. Regulatory science for combination products
As aforementioned, the safety and efficacy evaluation of combination products is difficult and complex. In the 21st century, the concept of regulatory science has gradually been accepted by FDA and later developed to an important field to ensure the safety and efficacy of medical products [93]. In August 2011, the FDA developed its Strategic Plan for Regulatory Science [94]. To promote research in regulatory science, FDA not only established a dedicated office, but also worked with the universities. In China, NMPA launched its Regulatory Science Action Plan (RSAP) in April 2019 [95]. “Combination Products” was among the first batch of nine key regulatory science projects initiated by NMPA. Based on the concept initiated by FDA [93], regulatory science of medical product is the science of developing new tools, new technologies, new methods, new standards, and new approaches to assess the safety, efficacy, quality and performance of medical products throughout their life cycles. This section looks into the current and future prospects of regulatory science for combination products from new tools and technologies, new standards, new methods, and new approaches.
4.1. New tools and technologies
4.1.1. Computational Models and Simulation
Computational Models and Simulation (CM&S) used in medical devices is to apply computational modeling science and simulation technology to evaluate medical products. In FDA, the research on CM&S associated with medical devices conducted by Office of Science and Engineering Laboratories (OSEL) is “Credibility of Computational Models Program”, which is used to minimize the risk of erroneous decisions based on computational predictions [96,97]. CM&S could also be used to simulate the clinic settings for virtual patients and to reduce clinical trials.
CM&S could reveal the interaction between products and patients, and reduce adverse reactions by adequate quantification of risks and security testing. CM&S could also reduce the cost and duration of clinical research. For example, computational simulation of clinically-relevant bioprosthetic valve performance metrics could provide scientific evidence for accurate and long-term deformation performance of transcatheter heart valves [98]. CM&S could assess the performance of bioprosthetic heart valves (BHV) because of excellent agreement between the computational and experimental results for the outcome measures commonly used to assess BHV performance [99]. A multimodal imaging-based anatomical model of the human head and neck could be used to analyze the safety and efficacy of medical products [100]. CM&S instead of clinical trials was applied in the regulatory approval of Advisa SR to demonstrate safety [101].
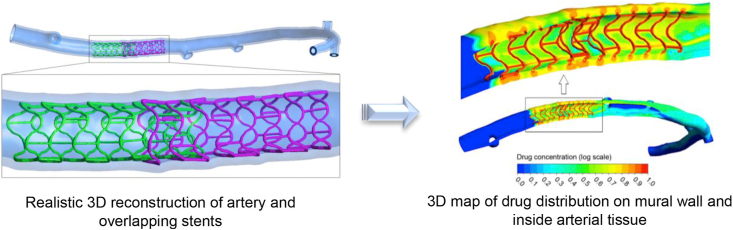
CM&S is successfully used to study drug deposition in coronary arteries with overlapping drug-eluting stents, solving the problems of complex three-dimensional geometry of deployed stents together with the drug transport (see Fig. 3) [64]. CM&S is also applied to study mechanics and drug release properties and model flow-mediated drug transport of combination products, such as drug-eluting stents [60,102,][103]] [][60], [102], [103][]. The results of CM&S could provide scientific evidence and recommendations for the clinical practice of combination products.
Fig. 3.
Combined high-resolution, multi-scale ex vivo computed tomography with a flow and mass transfer computational model for studying drug deposition in coronary arteries with overlapping drug-eluting stents. Reprinted with the permission from 2016, Elsevier [64].
4.1.2. Artificial intelligence
Artificial intelligence (AI) is a powerful tool to gain insights from a large amount of data from the real-world use and daily healthcare experience with computational models, expert systems and machine learning (ML) [104]. Consequently, healthcare could be importantly transformed by AI. According to FDA, AI is defined as “the science and engineering of making intelligent machines, especially intelligent computer programs” [104,105]. As an AI technique, ML can be used to create new software algorithms based on real world data [104]. AI and ML technologies could create medical products to better improve patient care and assist healthcare providers [104]. AI/ML could be involved in the research and development of pharmaceuticals throughout the lifespan, such as design, drug screening, prediction of the physicochemical properties, bioactivity and toxicity, quality regulation, and clinical evaluation (see Fig. 4) [[106], [107], [108], [109], [110]]. In medical devices, AI/ML-based medical devices are developed to contribute in image acquisition and processing, earlier disease detection and diagnosis, prognosis, risk assessment, new patterns identification on human physiology, and personalized diagnostics and therapeutics [105]. GI Genious is the first device that help detect potential signs of colon cancer using AI [111]. Abilify MyCite (aripiprazole tablets with sensor) is an FDA-approved pill using AI and has the ability to digitally track patient medications [112].
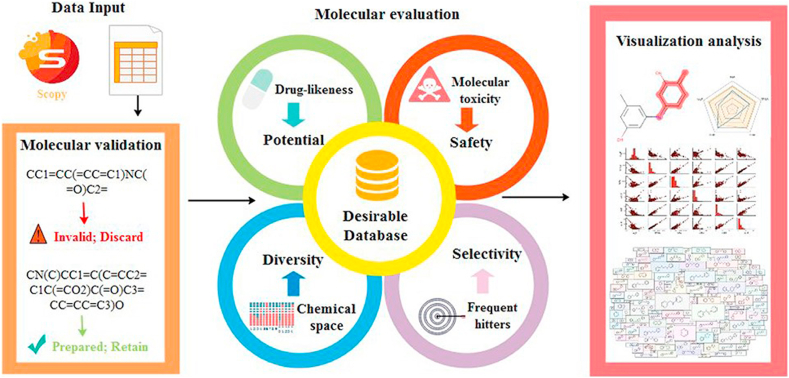
Fig. 4.
AI-based drug, toxic and proprietary prediction software platform. Reprinted with the permission from 2020, Oxford University Press [110].
4.1.3. Organs-on-chips
Human organs-on-chips are microfluidic devices created with microchip manufacturing methods, which linked with living human cells [[113], [114], [115]]. Human organs-on-chips could accurately mimic the physiology and mechanical environment in human body by reproduce blood and air flow in their tiny fluidic channels [113,115]. Organ-on-chips could recapitulate the multicellular architectures, vascular perfusion, tissue-tissue interfaces and physicochemical microenvironments of human organs (see Fig. 5) [113,116,117]. The human airway chip could recreate the lung environment, such as airways and blood vessels, which could be used to study the effect of different drug and pathogens on lung function [118]. Recently, organ-on-chips could model organ-level physiology and disease and develop cancer-on-a-chip models [113]. Organ-on-chips could make progress in tissue development, disease etiology and organ physiology, which is valuable for the research in toxicity testing, molecular MOA, and drug/medical device shielding [5]. Organ-on-chips could be used in adsorption, distribution, metabolism, elimination and toxicity (ADMET) testing, PK/PD modeling, efficacy testing and drug discovery [113,117,][119]] [][113], [117], [119][] (see Fig. 5). These functions could be of value for clinical trials and progressively replacement of animal-based tests [113]. Besides organs-on-chips, other 3D biomimetic cultures [120], such as 3D printing organ-specific tissues [121,122] and cell sheets [123,124], could also serve the platforms for the testing and evaluation of medical products including combination products.
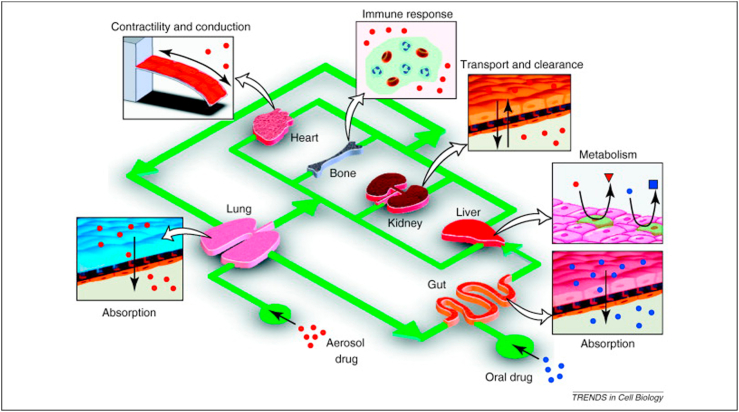
Fig. 5.
Organ-on-chips could be used in ADMET testing, PK/PD modeling, efficacy testing and drug discovery. Reprinted with the permission from 2011, Elsevier [117].
As aforementioned, CM&S, AI/ML and Organ-on-chips are new tools and technologies for research and development as well as efficacy and safety evaluation of combination products. New tools and technologies such as organs-on-chips have their unique advantages, such as replacing part of conventional animal studies, reducing animal studies, and improving study efficiency. New tools and technologies such as computation modeling may also be used to reduce the sample size of clinical studies, thereby promoting safer and faster market-entry of new products. With advances in computer science, engineering and biological research, these new tools and technologies may gradually develop to a primary platform for preclinical testing and evaluation of medical products. In the coming future, these tools and technologies will be developed alongside conventional tools to evaluate and regulate advanced medical products, such as combination products.
4.2. New standards
According to the document produced by International Medical Device Regulators Forum (IMDRF), standards are defined as “part of regulatory compliance, their development can benefit from these Essential Principles of Safety and Performance” [47]. International standards reflect the best experience of industry, researchers, consumers and regulators around the world and serve as an important basis for regulatory compliance assessments, providing an effective way to simplify and coordinate regulatory processes worldwide [47]. The fast development of combination products calls for new standards and their own standard system to provide guides, test methods and specifications for common and repeated use [125]. New standards and their standard system also provide consistent and accurate results, which is extremely helpful to the safe and effective supervision and technical review of combination products. ISO 12417-1 sets a good example of standard of a combination product by specifying minimum requirements for vascular device-drug combination products [92].
Existing standards face challenge for combination products in product testing, developing performance characteristics, testing methods, scientific protocols, product standards and specifications. A standard can be “a physical object/material (e.g., reference materials/reference standard), a document (e.g., documentary standard that defines terms and describes the use of rules, conditions, guidelines or characteristics for products or related processes and production methods, as well as related quality management systems, a test method or a specification) or a set of reference data” [126]. Therefore, the development of standards could be divided to several levels. Standards development could further accelerate product development by providing a platform for the convergence of diverse scientific approaches to promote evaluation and regulation of combination products.
4.3. New methods
Evidence-based research is the research using approaches such as systematic review and meta-analysis that were developed by evidence-based medicine [127]. Evidence-based research is an important method for preclinical and clinical research of medical products. This method provides scientific evidence for the evaluation of the safety, efficacy, quality and performance of medical devices [[128], [129], [130], [131], [132]]. Evidence-based research can be used to prove safety and effectiveness to the greatest extent and eliminate the unnecessary burden of repeated research [128,129]. Evidence-based research can provide fully cumulative evidence about the safety and efficacy of medical products (see Fig. 6) [128,129]. The evidence can assess feasibility, benefits and risks of clinical translation of novel medical products [129], and can help to identify the main risks of medical products for the regulatory review and technical evaluation [128].
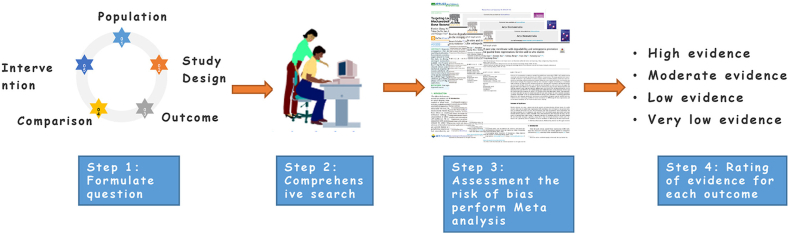
Fig. 6.
The standard process of conducting evidence-based research. Reprinted with the permission from 2021, Elsevier [129].
Evidence-based research can evaluate the quality of evidence for combination products and provide scientific basis for determining the drug selection and dose in the final products, which is one of the critical points whether drugs can play the expected roles in the clinical use. In addition, novel polymeric materials as drug carriers are one of the evaluation focuses for combination products. Because the drugs may also be used in the novel polymeric materials, how to evaluate their safety has become the focus of research of combination products. Evidence-based research methods such as systematic reviews and meta-analyses could provide useful methods to perform such safety evaluations [[133], [134], [135]].
Evidence-based research may also provide references for the clinical research design of combination products. In pre-market clinical research, controls are often one of the followings: pure medical devices without any drug content, predicate similar drug-containing medical devices or clinically recognized gold-standard treatments. In addition, the primary endpoint measures are determined by the intended uses and claimed efficacy of the product. However, these clinical studies are limited by the number of patients and observation time. It may not be possible to fully assess whether the combination product can obtain expected benefits of the added drugs, and whether the product adverse events are fully identified. Therefore, it is necessary to accumulate long-term follow-up clinical data to conduct evidence-based research. For example, a systematic and meta-analysis in the American Journal of Cardiology confirmed that the efficacy of the drug-coated balloons (DCBs) on coronary in-stent restenosis (ISRs) are not better than second-generation drug-eluting stents [136]. However, DCBs also have irreplaceable advantages of “intervention without implantation”, such as the treatment of small vascular lesions with small diameters, and ISRs that have previously been treated with stents.
Real world evidence (RWE) is defined as “the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD)” [137]. Real-world research could provide supplementary evidence besides evidence from traditional clinical trials [138]. Compared with traditional clinical trials, real-world research has the advantages of large amount of data, rich data dimension, wide coverage of patient population, long follow-up time, good external authenticity of treatment process and result evaluation, and good extrapolation of research results. As the recognized value continues to increase, RWE will certainly facilitate the regulatory approval processes for combination products. For example, it could be used to evaluate the long-term safety and effectiveness of high-risk combination products, and to support the potential modification of indications of the combination products.
4.4. New approaches
Countries around the world all pay close attentions to innovative medical products, and have issued a number of policies and regulations to encourage innovative products research and development. Breakthrough Therapy/Device Designation is an FDA program intended to accelerate the development and review of new drugs and medical devices for serious or life-threatening diseases [139]. Such designation was provided by Food and Drug Administration Safety and Innovation Act (FDASIA), signed on July 9, 2012 [140]. This new designation is a novel review path of FDA, following fast track, accelerate approval and priority review [139,141]. In Japan, the amended Pharmaceutical Affairs Law established regulations and controls of regenerative medicine products [142]. Pharmaceuticals and Medical Devices Agency (PMDA) in Japan has established a Regulatory Science Center in 2018 to further strengthen the review and safety of regenerative medicine products [142].
China also encourages the development of innovative medical products. From 2014 to 2018, the State Council of the People's Republic of China and NMPA issued several documents about innovative medical devices [[143], [144], [145], [146], [147]]. Announcement of NMPA on Special Review Procedures for Innovative Medical Devices set up a special approval path for innovative medical devices [143]. NMPA also issued the medical device priority approval procedure for special medical devices [144]. These regulations and policies have promoted the development and commercialization of combination products. Until July 2021, NMPA has approved 115 innovative medical devices from 96 companies, nine of which are combination products. In addition, four combination products were also approved through priority review.
5. Conclusion
The advancement of innovative biomaterials and technologies has promoted the research and development of combination products, which was originated in 1970s. The definition and designation of combination products depend on the regulations specified by regulatory authorities. The goal of the regulatory review of combination products is to evaluate and ensure their safety and efficacy. A systematic approach of regulatory review of combination products should focus on their design rationale, PMOA, preclinical evaluation and clinical evaluation. However, there are difficulties in the designation of combination products with controversial MOA. Different definition and regulation in different countries bring about new regulatory difficulties. The future combination products may be more complex and innovative with novel compositions, technologies and intended uses. Novel combination products may bring new benefits as well as risks. Advanced regulatory evaluation system and post-market surveillance system are required for the regulation of innovative combination products. The difficulty and complexity of regulatory evaluation of combination products present both challenges and opportunities to the regulatory authorities, calling for the development of regulatory science. Promising research areas in regulatory science of combination products include new tools and technologies (CM&S, AI and organ-on-chips), new standards, new methods such as evidence-based research and new approaches including the designation of innovative or breakthrough products programs. Regulatory inputs especially contributions of regulatory science will greatly benefit the future development of combination products.
CRediT authorship contribution statement
Jiaxin Tian: Conceptualization, Investigation, Writing – original draft, review & editing, Visualization. Xu Song: Conceptualization, Investigation, Writing – original draft, review & editing, Visualization. Yongqing Wang: Investigation. Maobo Cheng: Investigation. Shuang Lu: Investigation. Wei Xu: Investigation. Guobiao Gao: Investigation. Lei Sun: Investigation. Zhonglan Tang: Investigation. Minghui Wang: Writing – review & editing. Xingdong Zhang: Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgement
The authors would like to thank the National Medical Products Administration of China, as well as the group members of “technical evaluation of drug-device combination products”. This study was supported by China's Action Plan on Scientific Drug Administration (technical evaluation of drug-device combination products), National Natural Science Foundation of China (NSFC, No. 32001002), National Key Research and Development Program of China (No. 2017YFE0102600), Sichuan Major Science and Technology Project on Biotechnology and Medicine (No. 2018SZDZX0018), Sichuan University Postdoctoral Interdisciplinary Innovation Startup Found.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jiaxin Tian, Email: tianjx@cmde.org.cn.
Xu Song, Email: xusong2016@scu.edu.cn.
References
- 1.Williams D., Zhang X. Elsevier; 2019. II - Biomaterials and Biomedical Materials, Definitions of Biomaterials for the Twenty-First Century; pp. 15–23. [DOI] [Google Scholar]
- 2.Zhang X., Zhou P., Zhang J., et al. In: Bioceramics and the Human Body. Ravaglioli A., Krajewski A., editors. Springer Netherlands; Dordrecht: 1992. A study of hydroxyapatite ceramics and its osteogenesis; pp. 408–416. [DOI] [Google Scholar]
- 3.Yang Z., Yuan H., Tong W., et al. Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: variability among different kinds of animals. Biomaterials. 1996;17(22):2131–2137. doi: 10.1016/0142-9612(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 4.Manivasagam G., Dhinasekaran D., Rajamanickam A. Biomedical implants: corrosion and its prevention -A review. Recent Pat Nanotechnol. 2010;2(1):40–54. doi: 10.2174/1877610801002010040. [DOI] [Google Scholar]
- 5.Khademhosseini A., Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11(10):1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 6.Coury A. Claudio Migliaresi and Antonella Motta. CRC Press (Taylor & Francis); 2014. Tissue engineering: scope, products, and commercialization strategies, chapter 17, scaffolds for tissue engineering: biological design, materials, and fabrication; pp. 614–625. [Google Scholar]
- 7.Stewart M.P., Sharei A., Ding X., et al. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y., Aimetti A.A., Langer R., et al. Bioresponsive materials. Nat Rev Mater. 2016;1:16075. doi: 10.1038/natrevmats.2016.75. [DOI] [Google Scholar]
- 9.Song X., Wan Z., Chen T., et al. Development of a multi-target peptide for potentiating chemotherapy by modulating tumor microenvironment. Biomaterials. 2016;108:44–56. doi: 10.1016/j.biomaterials.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Tsou Y.H., Khoneisser J., Huang P.C., et al. Hydrogel as a bioactive material to regulate stem cell fate. Bioact Mater. 2016;1(1):39–55. doi: 10.1016/j.bioactmat.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastrup C.J., Nahrendorf M., Figueiredo J.L., et al. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(52):21444–21449. doi: 10.1073/pnas.1217972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu A.C., Chen H., Chan D., et al. Scalable manufacturing of biomimetic moldable hydrogels for industrial applications. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113(50):14255–14260. doi: 10.1073/pnas.1618156113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The State Council of the People's Republic of China Regulation on the supervision and administration of medical devices, Decree of the State Council of the People's Republic of China. 2021. http://www.gov.cn/gongbao/content/2021/content_5595920.htm (No 793)
- 14.U.S. Food and Drug Administration Classify your medical device. 2020. https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device
- 15.Liu W., Shi X., Lu Z., et al. Review and approval of medical devices in China: changes and reform. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(6):2093–2100. doi: 10.1002/jbm.b.34031. [DOI] [PubMed] [Google Scholar]
- 16.Lee M.H., Arcidiacono J.A., Bilek A.M., et al. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States, Tissue. Eng. Times Part B Rev. 2010;16(1):41. doi: 10.1089/ten.teb.2009.0449. [DOI] [PubMed] [Google Scholar]
- 17.Nicolas C.T., Joo D.J., Nelson E.D., et al. In: Encyclopedia of Tissue Engineering and Regenerative Medicine. Reis R.L., editor. Academic Press; Oxford: 2019. Stem cell therapies for liver diseases; pp. 137–145. [DOI] [Google Scholar]
- 18.Abdul Minaam D.S., Abd-Elfattah M. Smart drugs:improving healthcare using smart pill box for medicine reminder and monitoring system. Future Computing and Informatics Journal. 2018;3(2):443–456. doi: 10.1016/j.fcij.2018.11.008. [DOI] [Google Scholar]
- 19.Buchholz H.W., Elson R.A., Heinert K. Antibiotic-loaded acrylic cement. Journal of Bone & Joint Surgery-british. 1977;59(2):200–205. doi: 10.1302/0301-620X.59B2.873980. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki Y., Violaris A.G., Serruys P.W. New stent technologies. Prog. Cardiovasc. Dis. 1996;39(2):129–140. doi: 10.1016/s0033-0620(96)80022-3. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz H.W., Elson R.A., Heinert K. Antibiotic-loaded acrylic cement: current concepts. Clin. Orthop. 1984;190(190):96. doi: 10.1097/00003086-198411000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Elson R.A. Current Concepts of Infections in Orthopedic Surgery. Springer; 1985. Antibiotic-loaded acrylic cement (ALAC) pp. 247–250. [DOI] [Google Scholar]
- 23.Aggarwal R.K., Ireland D.C., Azrin M.A., et al. Antithrombotic potential of polymer-coated stents eluting platelet glycoprotein IIb/IIIa receptor antibody. Circulation. 1996;94(12):3311–3317. doi: 10.1161/01.cir.94.12.3311. [DOI] [PubMed] [Google Scholar]
- 24.Riffkin C. Panel discussion: prefilled disposable syringes. I Development aspects of prefilled disposable syringes. Bull. Parenter. Drug Assoc. 1969;23(1):6–8. [PubMed] [Google Scholar]
- 25.Jr B.G. Cost limits the use OF prefilled syringes, strip-packaged tablets. Mod. Hosp. 1965;104:138. [PubMed] [Google Scholar]
- 26.Aringa S.D. Teens anld nicotine patch therapy. AAP News. 1997;10:2. https://www.aappublications.org/content/13/10/2.5 [Google Scholar]
- 27.Thornton R.T., Bradshaw A.J., Snyder W.W. 1994. Intravascular Radiotherapy Employing a Liquid-Suspended Source; p. US5618266. [Google Scholar]
- 28.Schillinger D.C. The office of combination products: its roots, its creation, and its role. 2004. https://dash.harvard.edu/handle/1/8852096
- 29.Hunter N.L., Sherman R.E. Combination products: modernizing the regulatory paradigm. Nature reviews. Nat Rev Drug Discov. 2017;16(8):513–514. doi: 10.1038/nrd.2017.66. [DOI] [PubMed] [Google Scholar]
- 30.H.R.3095 101st congress - safe medical devices Act of 1990. 1990. https://www.congress.gov/bill/101st-congress/house-bill/3095/text
- 31.Code of Federal Regulations Title 21 3.2(e) 1991. https://ecfr.federalregister.gov/current/title-21/chapter-I/subchapter-A/part-3
- 32.Drues M. Combination products 101: a primer for medical device makers. Med Device Online. 2014 https://www.meddeviceonline.com/doc/combination-products-a-primer-for-medical-device-makers-0001 [Google Scholar]
- 33.Shen Y.T., Li C.H., Chang K.C., et al. Synthesis, optical, and mesomorphic properties of self-assembled organogels featuring phenylethynyl framework with elaborated long-chain pyridine-2,6-dicarboxamides. Langmuir. 2009;25(15):8714–8722. doi: 10.1021/la900003m. [DOI] [PubMed] [Google Scholar]
- 34.Definition of primary mode of action of a combination product. Final rule. Fed. Regist. 2005;70(164):49848–49862. https://www.govinfo.gov/content/pkg/FR-2005-08-25/pdf/05-16527.pdf [PubMed] [Google Scholar]
- 35.H.R.3580 107th congress - medical device user fee and modernization Act of 2002. 2002. https://www.congress.gov/bill/107th-congress/house-bill/3580?__cf_chl_jschl_tk__=pmd_4d974f37edfe6739ba724b69e2430b308abac69a-1627436975-0-gqNtZGzNAfijcnBszQoO
- 36.Mallia V.A., George M., Blair D.L., et al. Robust organogels from nitrogen-containing derivatives of (R)-12-hydroxystearic acid as gelators: comparisons with gels from stearic acid derivatives. Langmuir. 2009;25(15):8615–8625. doi: 10.1021/la8042439. [DOI] [PubMed] [Google Scholar]
- 37.21 U.S.C. § 353(g). Federal Food, drug, and cosmetic Act. Section 503(g). Regulation of combination products. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title21-section353&num=0&edition=prelim.
- 38.U.S. Food and Drug Administration Classification of products as drugs and devices and additional product classification issues. 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/classification-products-drugs-and-devices-and-additional-product-classification-issues#_ftn10 September 2017.
- 39.National Medical Products Administration The regulation of magnetic therapy and drug-containing medical device products. 2002. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjylqx/20020816010101116.html
- 40.National Medical Products Administration Rules for classification of medical devices. 2019. http://subsites.chinadaily.com.cn/nmpa/2019-10/11/c_415411.htm
- 41.National Medical Products Administration Issues related to registration management of products combining drugs and medical devices. 2004. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjylqx/20040408010101667.html
- 42.National Medical Products Administration Announcement on the Registration of drug/device combination products. 2009. https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/qtggtg/20091112141901388.html 2009.
- 43.National Medical Products Administration Announcement on the Registration of drug/device combination products. 2021. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210727154135199.html?type=pc&m=
- 44.National Medical Products Administration Announcement on adjusting the relevant matters concerning the definition of the attributes of drug/device combination products. 2019. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20190531180601758.html
- 45.European Medicines Agency Guideline on quality documentation for medicinal products when used with a medical device. 2021. https://www.ema.europa.eu/en/quality-documentation-medicinal-products-when-used-medical-device (Legal effective date: 1 January 2022)
- 46.Pharmaceuticals and Medical Devices Agency Handling of marketing application for combination products. 2014. https://www.pmda.go.jp/files/000153158.pdf
- 47.IMDRF website Essential principles of safety and performance of medical devices and IVD medical devices. 2018. http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-181031-grrp-essential-principles-n47.pdf
- 48.Verma A., Wilkoff B.L. Intravascular pacemaker and defibrillator lead extraction: a state-of-the-art review. Heart Rhythm. 2004;1(6):739–745. doi: 10.1016/j.hrthm.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Assad R.S., Zielinsky P., Kalil R., et al. New lead for in utero pacing for fetal congenital heart block. J. Thorac. Cardiovasc. Surg. 2003;126(1):300–302. doi: 10.1016/s0022-5223(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 50.Veerachamy S., Yarlagadda T., Manivasagam G., et al. Bacterial adherence and biofilm formation on medical implants: a review. Proc. Inst. Mech. Eng. H. 2014;228(10):1083–1099. doi: 10.1177/0954411914556137. [DOI] [PubMed] [Google Scholar]
- 51.Chouirfa H., Bouloussa H., Migonney V., et al. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37–54. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 52.Wistrand-Yuen E., Knopp M., Hjort K., et al. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018;9(1):1599. doi: 10.1038/s41467-018-04059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siontis G.C., Stefanini G.G., Mavridis D., et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386(9994):655–664. doi: 10.1016/S0140-6736(15)60657-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang C., Luan S., Panayi A.C., et al. Effectiveness and safety of hyaluronic acid gel with lidocaine for the treatment of nasolabial folds: a systematic review and meta-analysis. Aesthetic Plast. Surg. 2018;42(4):1104–1110. doi: 10.1007/s00266-018-1149-3. [DOI] [PubMed] [Google Scholar]
- 55.Sebastian S., Liu Y., Christensen R., et al. Antibiotic containing bone cement in prevention of hip and knee prosthetic joint infections: a systematic review and meta-analysis. J Orthop Translat. 2020;23:53–60. doi: 10.1016/j.jot.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.U.S. Food and Drug Administration Guidance on premarket notification [510(K)] submission for short-term and long-term intravascular catheters. 1997. https://www.fda.gov/media/72722/download
- 57.Zeng W., Li Y., Wang Y., et al. In: Encyclopedia of Tissue Engineering and Regenerative Medicine. Reis R.L., editor. Academic Press; Oxford: 2019. Tissue engineering of blood vessels; pp. 413–424. [DOI] [Google Scholar]
- 58.Renko M., Paalanne N., Tapiainen T., et al. Triclosan-containing sutures versus ordinary sutures for reducing surgical site infections in children: a double-blind, randomised controlled trial. Lancet Infect. Dis. 2017;17(1):50–57. doi: 10.1016/S1473-3099(16)30373-5. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen V.P.T., Kim C., Hong S.J., et al. Comparison of clinical outcomes of two different types of paclitaxel-coated balloons for treatment of patients with coronary in-stent restenosis. Heart Ves. 2019;34(9):1420–1428. doi: 10.1007/s00380-019-01388-z. [DOI] [PubMed] [Google Scholar]
- 60.Gagliardi M. Numerical analysis of paclitaxel-eluting coronary stents: mechanics and drug release properties. Med. Eng. Phys. 2020;82:78–85. doi: 10.1016/j.medengphy.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Hou R., Wu L., Wang J., et al. Surface-degradable drug-eluting stent with anticoagulation, antiproliferation, and endothelialization functions. Biomolecules. 2019;9(2):69. doi: 10.3390/biom9020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Medical Products Administration Guidelines for technical review of the registration of acrylic bone cement for artificial joint replacement. 2020. https://www.nmpa.gov.cn/ylqx/ylqxggtg/ylqxzhdyz/20200509155301198.html
- 63.Luscher T.F., Steffel J., Eberli F.R., et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 64.Rikhtegar F., Edelman E.R., Olgac U., et al. Drug deposition in coronary arteries with overlapping drug-eluting stents. J. Contr. Release. 2016;238:1–9. doi: 10.1016/j.jconrel.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H., Li X., Deng W., et al. Drug release kinetics from a drug-eluting stent with asymmetrical coat. Front. Biosci. 2017;22:407–415. doi: 10.2741/4491. [DOI] [PubMed] [Google Scholar]
- 66.Katz G., Harchandani B., Shah B. Drug-eluting stents: the past, present, and future. Curr. Atherosclerosis Rep. 2015;17(3):485. doi: 10.1007/s11883-014-0485-2. [DOI] [PubMed] [Google Scholar]
- 67.Ragab T.I.M., Nada A.A., Ali E.A., et al. Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nano-forms: multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol. 2019;135:407–421. doi: 10.1016/j.ijbiomac.2019.05.156. [DOI] [PubMed] [Google Scholar]
- 68.Ferdous Z., Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020;21(7) doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rivadeneira J., Audisio M.C., Gorustovich A. Films based on soy protein-agar blends for wound dressing: effect of different biopolymer proportions on the drug release rate and the physical and antibacterial properties of the films. J. Biomater. Appl. 2018;32(9):1231–1238. doi: 10.1177/0885328218756653. [DOI] [PubMed] [Google Scholar]
- 70.Dukhin S.S., Labib M.E. Theory of effective drug release from medical implants based on the Higuchi model and physico-chemical hydrodynamics. Colloids Surf A Physicochem Eng Asp. 2012;409:10–20. doi: 10.1016/j.colsurfa.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tijssen R.Y.G., Kraak R.P., Lu H., et al. Evaluation of the MiStent sustained sirolimus eluting biodegradable polymer coated stent for the treatment of coronary artery disease: does uniform sustained abluminal drug release result in earlier strut coverage and better safety profile? Expet Rev. Med. Dev. 2017;14(5):325–334. doi: 10.1080/17434440.2017.1318057. [DOI] [PubMed] [Google Scholar]
- 72.Dugas T.R., Brewer G., Longwell M., et al. Nanoentrapped polyphenol coating for sustained drug release from a balloon catheter. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(3):646–651. doi: 10.1002/jbm.b.34157. [DOI] [PubMed] [Google Scholar]
- 73.Song C., Zhou C., Zhang J., et al. Ultrasound controlled paclitaxel releasing system-A novel method for improving the availability of coronary artery drug coated balloon. Cathet. Cardiovasc. Interv. 2020;96(2):E119–E128. doi: 10.1002/ccd.28564. [DOI] [PubMed] [Google Scholar]
- 74.Anderson J.A., Lamichhane S., Remund T., et al. Preparation, characterization, in vitro drug release, and cellular interactions of tailored paclitaxel releasing polyethylene oxide films for drug-coated balloons. Acta Biomater. 2016;29:333–351. doi: 10.1016/j.actbio.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 75.Rykowska I., Nowak I., Nowak R. Drug-eluting stents and balloons-materials, structure designs, and coating techniques: a review. Molecules. 2020;25(20):4624. doi: 10.3390/molecules25204624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dongargaonkar A.A., Bowlin G.L., Yang H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound-dressing and drug-delivery platform. Biomacromolecules. 2013;14(11):4038–4045. doi: 10.1021/bm401143p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu B., Han X., Zhao A., et al. Intelligent H2S release coating for regulating vascular remodeling. Bioactive materials. 2020;6(4):1040–1050. doi: 10.1016/j.bioactmat.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tao Y., Hu T., Wu Z., et al. Heparin nanomodification improves biocompatibility and biomechanical stability of decellularized vascular scaffolds. Int. J. Nanomed. 2012;7:5847–5858. doi: 10.2147/IJN.S37113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao H., Zhang F., Liang G., et al. Preparation and experimental research into retrievable rapamycin- and heparin-coated vena cava filters: a pilot study. J. Thromb. Thrombolysis. 2016;41(3):422–432. doi: 10.1007/s11239-015-1278-3. [DOI] [PubMed] [Google Scholar]
- 80.Yang L., Li L., Wu H., et al. Catechol-mediated and copper-incorporated multilayer coating: an endothelium-mimetic approach for blood-contacting devices. J. Contr. Release. 2020;321:59–70. doi: 10.1016/j.jconrel.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura S., Ito T., Okamoto K., et al. Acceleration of bone regeneration of horizontal bone defect in rats using collagen-binding basic fibroblast growth factor combined with collagen scaffolds. J. Periodontol. 2019;90(9):1043–1052. doi: 10.1002/jper.18-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Starke R.M., Thompson J., Pagani A., et al. Preclinical safety and efficacy evaluation of the pipeline vantage embolization device with shield technology. J. Neurointerventional Surg. 2020;12(10):981–986. doi: 10.1136/neurintsurg-2020-016043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin R.X., Yang D.Z., Wu J.Z. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014;4(2):175–200. doi: 10.7150/thno.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlyle W.C., McClain J.B., Tzafriri A.R., et al. Enhanced drug delivery capabilities from stents coated with absorbable polymer and crystalline drug. J. Contr. Release. 2012;162(3):561–567. doi: 10.1016/j.jconrel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levy E.I., Hanel R.A., Howington J.U., et al. Sirolimus-eluting stents in the canine cerebral vasculature: a prospective, randomized, blinded assessment of safety and vessel response. J. Neurosurg. 2004;100(4):688–694. doi: 10.3171/jns.2004.100.4.0688. [DOI] [PubMed] [Google Scholar]
- 86.Cremers B., Speck U., Kaufels N., et al. Drug-eluting balloon: very short-term exposure and overlapping. Thromb. Haemostasis. 2009;101(1):201–206. doi: 10.1160/TH08-06-0387. [DOI] [PubMed] [Google Scholar]
- 87.National Medical Products Administration Guidelines for pre-clinical research of coronary drug eluting stent. 2018. https://www.nmpa.gov.cn/ylqx/ylqxggtg/ylqxqtgg/20180511163101550.html
- 88.Chen S., Yao Z., Guan Y., et al. High nitrogen stainless steel drug-eluting stent - assessment of pharmacokinetics and preclinical safety in vivo. Bioact Mater. 2020;5(4):779–786. doi: 10.1016/j.bioactmat.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Medical Products Administration Center fro medical device evaluation, Guidelines for technical review of registration of silver dressings. 2016. https://www.cmde.org.cn/CL0063/5169.html
- 90.ISO 14155 Clinical investigation of medical devices for human subjects — good clinical practice. 2020. https://www.iso.org/standard/71690.html
- 91.National Medical Products Administration Guidelines for clinical trials of coronary drug eluting stent. 2018. https://www.cmde.org.cn/CL0063/5169.html
- 92.ISO 12417-1 2015 Cardiovascular implants and extracorporeal systems — vascular device-drug combination products — Part 1: general requirements. 2015. https://www.iso.org/standard/57697.html asssessed.
- 93.FDA SCIENCE AND MISSION AT RISK USA today magazine. 2007. http://www.psychrights.org/Articles/0711ReportonFDAScience.pdf 3.
- 94.Stevens R. Advancing regulatory science at FDA: a strategic plan. 2011. https://www.fda.gov/media/81109/download 2011.
- 95.The Central People's Government of the People's Republic of China NMPA launched China's action plan on scientific drug administration. 2019. http://www.gov.cn/xinwen/2019-05/02/content_5388253.htm
- 96.U.S. Food and Drug Administration Credibility of computational models program: research on computational models and simulation associated with medical devices. 2021. https://www.fda.gov/medical-devices/medical-device-regulatory-science-research-programs-conducted-osel/credibility-computational-models-program-research-computational-models-and-simulation-associated
- 97.U.S. Food and Drug Administration Promoting innovation in medical product assessment: a risk-based framework for evaluating computational models for regulatory decision-making. 2020. https://www.fda.gov/drugs/news-events-human-drugs/promoting-innovation-medical-product-assessment-risk-based-framework-evaluating-computational-models#4
- 98.Jafar R., Labrosse M.R., Weaver J.D., et al. A computational study on deformed bioprosthetic valve geometries: clinically relevant valve performance metrics. J. Biomech. Eng. 2020;142(1) doi: 10.1115/1.4044235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J.H., Rygg A.D., Kolahdouz E.M., et al. Fluid-Structure interaction models of bioprosthetic heart valve dynamics in an experimental pulse duplicator. Ann. Biomed. Eng. 2020;48(5):1475–1490. doi: 10.1007/s10439-020-02466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ida I.M., Esra N., Esther A., et al. MIDA: a multimodal imaging-based detailed anatomical model of the human head and neck. PloS One. 2015;10 doi: 10.1371/journal.pone.0124126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faris O., Shuren J. An FDA viewpoint on unique considerations for medical-device clinical trials. N. Engl. J. Med. 2017;376(14):1350–1357. doi: 10.1056/NEJMra1512592. [DOI] [PubMed] [Google Scholar]
- 102.Saleh Y.E., Gepreel M.A., Allam N.K. Functional nanoarchitectures for enhanced drug eluting stents. Sci. Rep. 2017;7:40291. doi: 10.1038/srep40291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vijayaratnam P.R.S., Reizes J.A., Barber T.J. Flow-mediated drug transport from drug-eluting stents is negligible: numerical and in-vitro investigations. Ann. Biomed. Eng. 2019;47(3):878–890. doi: 10.1007/s10439-018-02176-y. [DOI] [PubMed] [Google Scholar]
- 104.McCarthy J. Stanford University; Stanford, CA: 2007. What Is Artificial Intelligence?http://jmc.stanford.edu/articles/whatisai/whatisai.pdf [Google Scholar]
- 105.U.S. Food and Drug Administration Artificial intelligence and machine learning in software as a medical device. 2021. https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device
- 106.Baek M., DiMaio F., Anishchenko I., et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021 doi: 10.1126/science.abj8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paul D., Sanap G., Shenoy S., et al. Artificial intelligence in drug discovery and development. Drug Discov. Today. 2021;26(1):80–93. doi: 10.1016/j.drudis.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei G.W. Protein structure prediction beyond AlphaFold. Nat Mach Intell. 2019;1(8):336–337. doi: 10.1038/s42256-019-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walker S.W.C., Anwar A., Psutka J.M., et al. Determining molecular properties with differential mobility spectrometry and machine learning. Nat. Commun. 2018;9(1):5096. doi: 10.1038/s41467-018-07616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Z.Y., Yang Z.J., Lu A.P., et al. Scopy: an integrated negative design python library for desirable HTS/VS database design. Briefings Bioinf. 2021;22(3) doi: 10.1093/bib/bbaa194. bbaa194. [DOI] [PubMed] [Google Scholar]
- 111.U.S. Food and Drug Administration FDA authorizes marketing of first device that uses artificial intelligence to help detect potential signs of colon cancer. 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-device-uses-artificial-intelligence-help-detect-potential-signs-colon
- 112.U.S. Food and Drug Administration FDA approves pill with sensor that digitally tracks if patients have ingested their medication. 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-pill-sensor-digitally-tracks-if-patients-have-ingested-their-medication
- 113.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32(8):760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 114.D. Ingber, Human Organs-on-Chips-Microfluidic devices lined with living human cells for drug development, disease modeling, and personalized medicine. https://wyss.harvard.edu/technology/human-organs-on-chips/.
- 115.U.S. Food and Drug Administration, ORGANS-ON-CHIPS technology. https://www.fda.gov/media/104288/download.
- 116.U.S. Food and Drug Administration Organs-on-chips for radiation countermeasures. 2019. https://www.fda.gov/emergency-preparedness-and-response/mcm-regulatory-science/organs-chips-radiation-countermeasures
- 117.Huh D., Hamilton G.A., Ingber D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21(12):745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Si L., Bai H., Rodas M., et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng. 2021:1–15. doi: 10.1038/s41551-021-00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Polini A., Prodanov L., Bhise N.S., et al. Organs-on-a-chip: a new tool for drug discovery. Expet Opin. Drug Discov. 2014;9(4):335–352. doi: 10.1517/17460441.2014.886562. [DOI] [PubMed] [Google Scholar]
- 120.Chen C.S. 3D biomimetic cultures: the next platform for cell biology. Trends Cell Biol. 2016;26(11):798–800. doi: 10.1016/j.tcb.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skylar-Scott M.A., Uzel S.G.M., Nam L.L., et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv. 2019;5(9) doi: 10.1126/sciadv.aaw2459. eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 123.Tang Z., Akiyama Y., Itoga K., et al. Shear stress-dependent cell detachment from temperature-responsive cell culture surfaces in a microfluidic device. Biomaterials. 2012;33(30):7405–7411. doi: 10.1016/j.biomaterials.2012.06.077. [DOI] [PubMed] [Google Scholar]
- 124.Tang Z., Akiyama Y., Yamato M., et al. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials. 2010;31(29):7435–7443. doi: 10.1016/j.biomaterials.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 125.ISO/IEC Directives . Part 2 Principles and Rules for the Structure and Drafting of ISO and IEC Documents. eighth ed. IX-ISO; 2018. https://www.iec.ch/members_experts/refdocs/iec/isoiecdir2%7Bed8.0.RLV%7Den.pdf 2018. [Google Scholar]
- 126.Arcidiacono J.A., Bauer S.R., Kaplan D.S., et al. FDA and NIST collaboration on standards development activities supporting innovation and translation of regenerative medicine products. Cytotherapy. 2018;20(6):779–784. doi: 10.1016/j.jcyt.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 127.Zhang K., Sun X., Yu J., et al. Regulatory science for medical devices and evidence-based science. Chin. J. Evidence-Based Med. 2019:527–531. doi: 10.7507/1672-2531.201903142. 019.005. [DOI] [Google Scholar]
- 128.Liu W., Xie Y., Zheng Y., et al. Regulatory science for hernia mesh: current status and future perspectives. Bioact Mater. 2021;6(2):420–432. doi: 10.1016/j.bioactmat.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.A J.Z., B Y.J., B Z.S., et al. Biodegradable metals for bone defect repair: a systematic review and meta-analysis based on animal studies. Bioact Mater. 2021;6(11):4027–4052. doi: 10.1016/j.bioactmat.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Razavi M.K., Jaff M.R., Miller L.E. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10) doi: 10.1161/CIRCINTERVENTIONS.115.002772. [DOI] [PubMed] [Google Scholar]
- 131.Yin S.H., Xu P., Wang B., et al. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: systematic review and network meta-analysis. BMJ. 2019;365:l2222. doi: 10.1136/bmj.l2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiong Y.Q., Tan J., Liu Y.M., et al. Cervical pessary for preventing preterm birth in singletons and twin pregnancies: an update systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2020:1–10. doi: 10.1080/14767058.2020.1712705. [DOI] [PubMed] [Google Scholar]
- 133.Liu J., Li L., Deng K., et al. Incretin based treatments and mortality in patients with type 2 diabetes: systematic review and meta-analysis. BMJ. 2017;357:j2499. doi: 10.1136/bmj.j2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y., Li L., Deng K., et al. Comparison of the combination of recombinant follicle-stimulating hormone and recombinant luteinizing hormone protocol versus human menopausal gonadotropin protocol in controlled ovarian stimulation: a systematic review and meta-analysis. J. Evid. Base Med. 2020;13(3):215–226. doi: 10.1111/jebm.12390. [DOI] [PubMed] [Google Scholar]
- 135.Wang W., Chen W., Liu Y., et al. Antibiotics for uncomplicated skin abscesses: systematic review and network meta-analysis. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elgendy I.Y., Mahmoud A.N., Elgendy A.Y., et al. Meta-analysis comparing the frequency of target lesion revascularization with drug-coated balloons or second-generation drug-eluting stents for coronary in-stent restenosis. Am. J. Cardiol. 2019;123(7):1186–1187. doi: 10.1016/j.amjcard.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 137.Yang B., Qian F., Li W., et al. Effects of general anesthesia with or without epidural block on tumor metastasis and mechanisms. Oncol Lett. 2018;15(4):4662–4668. doi: 10.3892/ol.2018.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klonoff D.C., Gutierrez A., Fleming A., et al. Real-world evidence should Be used in regulatory decisions about new pharmaceutical and medical device products for diabetes. J Diabetes Sci Technol. 2019;13(6):995–1000. doi: 10.1177/1932296819839996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.U.S. Food and Drug Administration Breakthrough therapy. 2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy
- 140.21 U.S.C . 2012. § 356(c). FDASIA, Pub. L. No. 112-144, 126 Stat. 993 § 901. [Google Scholar]
- 141.Kepplinger E.E. FDA's expedited approval mechanisms for new drug products. Biotechnol. Law Rep. 2015;34(1):15–37. doi: 10.1089/blr.2015.9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pharmaceutical Affairs Law Chapter 2. Pharmaceutical laws and regulations. 2018. http://www.jpma.or.jp/english/parj/pdf/2018_ch02.pdf
- 143.National Medical Products Administration Announcement of national medical products administration on special review procedures for innovative medical devices. 2018. https://www.nmpa.gov.cn/ylqx/ylqxggtg/ylqxqtgg/20180511163101550.html
- 144.National Medical Products Administration Letter seeking advice on the priority approval process for medical devices. 2016. https://www.nmpa.gov.cn/directory/web/nmpa/ylqx/ylqxjgdt/20160621151501523.html
- 145.Special Procedure of Review and Approval for Innovative Medical Devices. CFDA; 2014. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjylqx/20140207154501788.html [SYJXG] No. 13. 2014. [Google Scholar]
- 146.The State Council of the People's Republic of China, the Opinions on Reforming Review and Approval Process for Drugs and Medical Devices. 2015. http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm GUOFA No. 44) [Google Scholar]
- 147.The State Council of the People's Republic of China, Official Opinions on Deepening the Review and Approval Policies Reform and Encouraging Drug and Medical Device Innovations. 2017. http://www.gov.cn/zhengce/2017-10/08/content_5230105.htm [Google Scholar]