Abstract
Introduction
Endocardial catheter ablation (ECA) for atrial fibrillation (AF) has limited efficacy. Hybrid convergent procedure (HCP) with both epicardial and endocardial ablation is a novel strategy for AF treatment. In this meta‐analysis, we aimed to evaluate the efficacy and safety of HCP in AF ablation.
Method
We performed a comprehensive literature search for studies that evaluated the efficacy and safety of HCP compared with ECA for AF. The primary outcome was freedom of atrial arrhythmia (AA). The secondary outcome was the periprocedural complication rate. Pooled relative risk (RR) and corresponding 95% confidence intervals (CIs) were calculated using the random effects model.
Results
A total of eight studies, including 797 AF patients (mean age: 60.7 ± 9.8 years, 366 patients with HCP vs. 431 patients with ECA alone), were included. HCP showed a higher rate of freedom of AA compared with ECA (RR: 1.48, 95% CI: 1.13–1.94, p = .004). However, HCP was associated with higher rates of periprocedural complications (RR: 3.64, 95% CI: 2.06–6.43; p = .00001). Moreover, the HCP had a longer procedure time and postprocedural hospital stay.
Conclusions
Although hybrid ablation was associated with a higher success rate, this should be judged for increased periprocedural adverse events and extended hospital stay. Prospective large‐scale randomized trials are needed to validate these results.
Keywords: ablation, atrial fibrillation, endocardial, epicardial, hybrid
Graphical abstract showing the outcomes of our study. Although hybrid ablation was associated with a higher success rate, this should be judged for increased periprocedural adverse events and extended hospital stay.

Abbreviations
- AA
atrial arrhythmia
- AADs
antiarrhythmic medications
- AF
atrial fibrillation
- CI
confidence interval
- ECA
endocardial catheter ablation
- HCP
hybrid convergent procedure
- LA
left atrium
- LAPW
left atrial posterior wall
- Per‐LSPer AF
persistent or long‐standing persistent AF
- PVs
pulmonary veins
- RR
relative risk
1. INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia and continues to be a worldwide health burden. 1 The prevalence of AF in the United States is rising, with 12.1 million people are expected to have AF by 2030. 1 Conventional endocardial catheter ablation (ECA) is the mainstay interventional treatment modality of AF and targets mostly pulmonary veins (PVs) isolation. 2 However, the success rate of ECA is still unsatisfactory, with only half of the patients attaining freedom from atrial tachyarrhythmias at 1‐year follow‐up. 2 Furthermore, the creation of endocardial transmural lines during the procedure possesses a possible risk of esophageal, lung, and phrenic nerve injury. 3
Since its advent in the late 1980s, epicardial ablation for AF with various surgical approaches has continued to evolve. 4 The relative safety varies with the different techniques used, with comparable success rates to the ECA approach. 4
Hybrid convergent procedure (HCP), which combines both epicardial and endocardial ablation approaches, has emerged to utilize the advantages of both approaches. During the last decade, HCP has gained increasing acceptance in clinical practice, with several reports of durable antiarrhythmic outcomes. 5 However, studies analyzing the clinical outcomes of HCP are currently limited by small sample sizes. Therefore, we conducted this meta‐analysis to evaluate all the available evidence to better assess the efficacy and safety of the hybrid convergent ablation HCP for atrial fibrillation.
2. METHODS
2.1. Data sources and search strategy
We performed a comprehensive search for published studies indexed in PubMed/MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from inception to March 30, 2021. We also performed a manual search for additional relevant studies using references of the included articles. The following search terms were used: (“atrial fibrillation” or “AF”), (“hybrid” or “convergent” or “surgical‐transcatheter” or “thoracoscopic‐transcatheter” or “epicardial”), and (“endocardial ablation” or “catheter ablation” or “radiofrequency ablation”). The search was not limited by language, study design, or country of origin. Table S1 describes the full search term used in each database searched.
2.2. Study selection
We followed the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) and the meta‐analysis of observational studies in epidemiology (MOOSE) guidelines to screen the studies. 6 , 7 We included full texts and abstracts of randomized controlled trials, cohort studies, and case–control studies. We excluded single‐arm studies, animal studies, case reports, case series, reviews, editorials, and letters to editors. Two investigators (MM and AB) independently screened and selected the studies for the final review. Discrepancies were resolved by a third investigator (AA).
2.3. Data extraction
We extracted the following data from the final studies: the last name of the first author, publication year, study design, country of origin, follow‐up duration, sample size, efficacy endpoints (the freedom of atrial arrhythmia by the time of the last follow‐up), and safety endpoints (including periprocedural complications such as pericardial effusion, atrio‐esophageal fistula, cerebrovascular accident, and death). Also, we extracted data for the number of patients who underwent HCP or ECA, their age, and baseline comorbidities (including diabetes mellitus, hypertension, body mass index) and preprocedural characteristics (including left ventricular ejection fraction [LVEF], left atrial [LA] diameter, percentage of persistent or long‐standing persistent [Per‐LSPer] AF, pervious treatment with amiodarone, previous ablations, and CHA2DS2‐VASc). Finally, we extracted procedural details, endocardial, and fluoroscopic times as well as the postprocedural hospital stay.
2.4. Outcomes
The primary outcome of our meta‐analysis was freedom of atrial arrhythmia (AA) by the time of the last follow‐up. Total AA is defined as a composite of AF, sustained atrial tachycardia (AT), and atypical atrial flutter (AFL) after the index procedure.
Our secondary outcome was the rate of periprocedural complications through 30 days of the index procedure. Complications include the development of stroke, bleeding events that required intervention, pericardial effusion, cardiac tamponade, atrio‐esophageal fistula, infections, phrenic nerve paralysis, and death.
2.5. Statistical analysis
The meta‐analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre). The random effects model was used to calculate the weighted pooled risk ratio (RR) and corresponding 95% confidence intervals (CI). We performed a subgroup analysis for the primary outcome based on the timing of HCP and the use of antiarrhythmic medications. We also performed a subgroup analysis for the safety outcome based on the surgical access used in the HCP. A p value of <.05 was considered statistically significant. Heterogeneity was assessed using the Higgins I 2 index, where I 2 values >50% implied the presence of substantial heterogeneity. 8
2.6. Quality assessment
We assessed the quality of the included studies using the Newcastle‐Ottawa Scale for observational studies and the Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2) for RCTs. 9 , 10 Two authors (MM and OS) independently assessed each study for bias. Discrepancies were resolved by consensus. We did not evaluate for publication bias in our study because of the limited number of included studies. 11
3. RESULTS
3.1. Study selection
A total of 667 studies were retrieved by our search strategy. Among these, 72 were eligible for the systematic review. Subsequently, we excluded 64 studies that were not relevant, had insufficient data, single‐arm studies, or being a prognostic study. Finally, eight studies met our inclusion criteria and were included in the meta‐analysis. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Figure 1 shows the PRISMA flow chart that illustrates how the final studies were selected.
FIGURE 1.
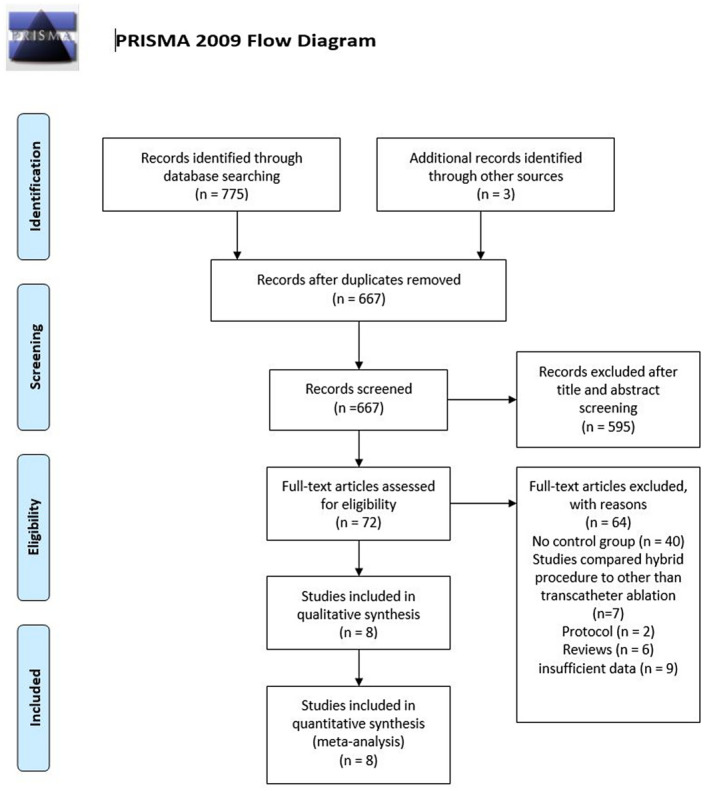
PRISMA flow diagram for the selection of studies
3.2. Study characteristics
Table 1 shows the characteristics of the eight studies that were included in our meta‐analysis. The studies included a total of 797 AF patients, of whom 366 underwent HCP and 431 underwent ECA. The studies were published between 2011 and 2020. Based on the country of origin, four studies originated from the United States, one from South Korea, one from Slovenia, one from the United Kingdom, and one multicentric from the United States and the United Kingdom. Based on study design, three studies were randomized controlled trials, four were retrospective cohorts, and one was a prospective cohort. All the included studies were full‐text publications. The mean age was 60.7 ± 9.8 years, and males represented 77.4% of total patients. Around 93% of the entire study population had Per‐LSPer AF, 27% treated previously with amiodarone, and 18.7% underwent prior ablation for AF. Table 2 summarizes the baseline comorbidities and preprocedural characteristics, including LVEF, LA diameter, and CHA2DS2‐VASc score.
TABLE 1.
Characteristics of studies included in the meta‐analysis
| Study | Design | Origin | Follow‐up duration, mean ± SD or mean | Inclusion criteria | AAD use | AA detection |
|---|---|---|---|---|---|---|
| DeLurgio, 2020 | RCT | Multicentric, United States, United Kingdom | 18 |
‐ Age 18–80 ‐ Symptomatic persistent AF ‐ Refractory or intolerant to AAD ‐ LA size ≤6.0 cm |
Allowed |
‐ 24‐h Holter at 6 and 12 months ‐ 7‐day Holter at 18 months |
| Edgerton, 2016 | RCT | United States | 24 |
‐ LSPAF ‐ LAD >4.5 cm ‐ Failed AAD |
Allowed | EKG, 7‐day Holter |
| Genev, 2017 | Retrospective Cohort | United States | 24 | ‐ Not received prior invasive AF therapy | Allowed | NR |
| Hwang, 2018 | Retrospective Cohort | South Korea | 25 |
‐ Symptomatic drug‐refractory nonvalvular AF ‐ No prior history of ablation for AF |
Allowed | 24‐h Holter |
| Jan, 2018 | RCT | Slovenia | 30.5 ± 6.9 | ‐ Paroxysmal AF | Allowed | Implantable loop recorder, monitoring |
| Kress, 2016 | Retrospective Cohort | United States | 16 | ‐ Persistent or LSP AF | Allowed | EKG, loop recorder, Holter |
| Maclean, 2020 | Retrospective Cohort | United Kingdom | 30.5 ± 13.3 |
‐ Persistent AF ‐ No previous cardiac surgery, abdominal surgery, or a contraindication to anticoagulation |
Allowed | EKG, 72‐h Holter, Deviinterrogation if pacemaker in place. |
| Mahapatra, 2011 | Prospective cohort | United States | 20.7 ± 4.5 |
‐ Persistent or LSP AF ‐ Failed at least one AAD and one catheter ablation |
Allowed | EKG, 7‐day Holter, 24‐h Holter, telephone. |
Abbreviations: AA, atrial arrythmia (AF, Atrial flutter, or atrial tachycardia); AAD, antiarrhythmic medications; AF, atrial fibrillation; LA, left atrium; LAD, left atrium diameter; LSP, long standing persistent.
TABLE 2.
Baseline patient characteristics included in the meta‐analysis
| No of studies | All Patients (N = 797) | HCP (N = 366) | ECA (N = 431) | p‐value | |
|---|---|---|---|---|---|
| Age, year | 8 | 60.7 ± 9.8 | 61.5 ± 10.1 | 60 ± 9.5 | .03 |
| Male | 8 | 77.4% (617/797) | 83.1% (304/366) | 72.6% (313/431) | <.01 |
| BMI | 5 | 34.9 ± 12.3 | 35.2 ± 12.4 | 34.7 ± 12.3 | NS (0.63) |
| Hypertension | 7 | 62.3% (443/711) | 64.1% (207/323) | 60.8% (236/388) | NS (0.37) |
| Diabetes mellitus | 6 | 17% (95/558) | 12.7& (28/221) | 19.9% (67/337) | .03 |
| CHADS2 score | 5 | 1.8 ± 1.9 | 1.7 ± 1.5 | 1.9 ± 2.2 | NS (0.22) |
| LA diameter, cm | 6 | 4.5 ± 0.85 | 4.5 ± 0.8 | 4.5 ± 0.9 | NS (1.00) |
| LVEF, % | 7 | 55.5 ± 10.2 | 55.3 ± 10.2 | 55.7 ± 10.3 | NS (0.6) |
| Per‐LSPer AF, % | 7 | 92.9% (653/703) | 93% (320/344) | 92.7% (333/359) | NS (0.89) |
| Previous amiodarone | 3 | 27.2% (70/257) | 25.5% (36/141) | 29.3% (34/116) | NS (0.5) |
| Previous ablation | 6 | 18.7% (129/688) | 21.1% (67/318) | 16.7% (62/370) | NS (0.15) |
| Procedure time, min | 5 | 266.1 ± 84.98 | 304.9 ± 78.8 | 224.1 ± 70.3 | <.0001 |
| Endocardial time, min | 5 | 139.8 ± 83.3 | 112.6 ± 56.4 | 169.3 ± 96.8 | <.0001 |
| Fluoroscopy time, min | 5 | 34.1 ± 26.9 | 29.8 ± 25 | 38.8 ± 28.2 | <.0001 |
Abbreviations: BMI, Body mass index; ECA, Endocardial catheter ablation; HCP, hybrid convergent procedure; LA, Left atrium; LVEF, Left ventricular ejection fraction; Per‐LSPer, Persistent‐ longstanding persistent.
All studies defined AF recurrence as any atrial arrhythmia lasting more than 30 s after the 3‐month blanking period. Follow‐up duration ranged from 16 to 30.5 months.
Genev et al. additionally compared the HCP with another invasive procedure (Complete Cox‐maze), 14 whereas the rest of the studies compared HCP with ECA. Five studies compared the freedom of AA with or without antiarrhythmic medications (AADs). 12 , 13 , 15 , 16 , 18 Five studies reported procedural, endocardial, and fluoroscopy times. 12 , 13 , 16 , 17 , 19 Three studies reported the average postoperative hospital stay. 15 , 17 , 19
3.3. Procedural characteristics
Five studies performed HCP via transdiaphragmatic subxiphoid incision, two via thoracoscopy, and one via mini‐thoracotomy approach. Three studies performed staged HCP, whereas the rest conducted concomitant endocardial and epicardial HCP. The detailed ablation lesion sets, and sequence of each study were summarized in Table S2.
The assessment of success rates of HCP and ECA was made mainly through Holter monitoring ranging from 24 h to 7 days. Only two studies utilized implantable loop recorder monitoring, 16 , 17 and one study interrogated the pacemaker devices when available. 18
3.4. Primary outcomes
All the included studies reported the rate of freedom of atrial arrhythmia by the time of the last follow‐up (average of 24 months). HCP showed a higher rate of freedom of AA compared with ECA (RR: 1.48, 95% CI: 1.13–1.94, p = .004). However, significant heterogeneity was found (I 2 = 77%, p < .0001) (Figure 2). A sensitivity analysis was conducted by removing one study at a time to reduce heterogeneity and found no significant change in our results (Figure S1). Furthermore, subgroup analysis based on the HCP surgical access failed to improve the observed heterogeneity.
FIGURE 2.

Forest plot comparing hybrid convergent procedure and endocardial catheter ablation regarding the freedom of atrial arrhythmia by the time of the last follow‐up
3.5. Secondary outcomes
The rate of periprocedural complications was higher in the HCP group compared with the ECA group (RR: 3.64, 95% CI: 2.06–6.43, p = .00001). No significant heterogeneity was found in the measurement of the safety outcome (I 2 = 0%, p = .74) (Figure 3). Forty‐five adverse events were observed in the HCP group compared with only 17 events in the ECA group. Most of the reported complications were procedure‐related bleeding, pericardial effusion, and cardiac tamponade. Furthermore, five deaths were reported in the HCP group. The causes of death included esophageal fistula, large thromboembolic stroke, gastrointestinal bleeding about 2 weeks after the procedure, sudden death at home, and procedure‐related death. No mortality was observed in the ECA group.
FIGURE 3.
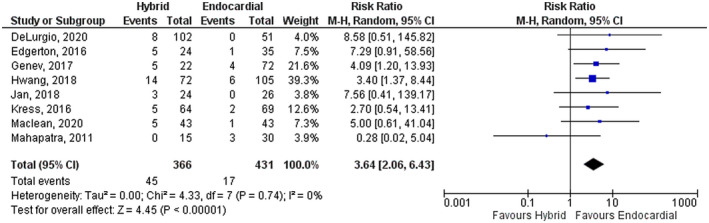
Forest plot comparing hybrid convergent procedure and endocardial catheter ablation regarding the periprocedural adverse events
3.6. Subgroup analyses
The first subgroup analysis was conducted for the periprocedural complications based on the access of the HCP (transdiaphragmatic vs. thoracoscopy vs. mini‐thoracotomy). Thoracoscopy group of HCP showed better safety profile (RR: 1.42, 95% CI: 0.13–15.41, p = .77) (Figure 4). However, the test for subgroup differences was not significant (I 2 = 0%, chi‐square = 0.87, degrees of freedom = 2, p‐value = .65).
FIGURE 4.

Subgroup analysis comparing hybrid convergent procedure and endocardial catheter ablation regarding the periprocedural complications based on the access of the hybrid convergent procedure
The second subgroup analysis was conducted for the freedom of AA based on the timing of the HCP (concomitant vs. staged approaches). HCP and ECA had similar AA freedom rates based on the concomitant (RR: 1.25, 95% CI: 0.83–1.88, p = .29) and staged (RR: 1.53, 95% CI: 0.98–2.38, p = .06) approaches (Figure S2). The test for subgroup differences was also not significant (I 2 = 0%, chi‐square = 0.44, degrees of freedom = 1, p value = .51).
The third subgroup analysis was conducted for the freedom of AA based on the use of AADs and showed high heterogeneity among both studies with AADs use (I 2 = 61%, p‐value = .04) and studies without AADs use (I 2 = 53%, p‐value = .07). HCP and ECA had similar AA freedom rates based on the AADs use (RR 1.05, 95% CI 0.61–1.80, p = .87) and without AADs use (RR 1.47, 95% CI 0.99–2.18, p = .06) (Figure S3).
3.7. Additional analyses
HCP was associated with significantly prolonged hospital stay compared with the ECA group (mean difference [MD], 3.91 [days], 95% CI: 1.68–6.14; p = .0006) (Figure S4).
HCP had longer procedure time than ECA (MD: 89.93 [min], 95% CI: 47.71–131.94, p = .0001); however, the endocardial time was shorter in the HCP group (MD: −73.74 [min.], 95% CI: −119.51, −27.96, p = .002). No difference was found regarding the fluoroscopy time (MD: −6.07 [min], 95% CI: −23.18, 11.05, p = .49). Significant heterogeneity was observed in all reported outcomes (Figure S5).
3.8. Quality assessment
We assessed the quality of the included studies by using the Newcastle‐Ottawa Scale for cohort studies and the Revised Cochrane risk‐of‐bias tool for randomized controlled trials, as shown in Tables S3 and S4. All studies scored low to moderate in quality assessment.
4. DISCUSSION
This study was a systematic review and meta‐analysis of all studies investigating the efficacy and safety of the hybrid convergent procedure compared to the conventional endocardial catheter ablation for atrial fibrillation. Our meta‐analysis demonstrated that the HCP significantly improved the freedom of atrial tachyarrhythmia. However, it was associated with higher perioperative complications, but HCP through thoracoscopy access showed a better safety profile. Furthermore, HCP was associated with longer procedure time and hospital stay.
Following conventional endocardial ablation, the recurrence rate of AF varies significantly among different studies; early recurrences (within the first 3 months) occur in almost half of the patients after ECA. 20 Late recurrence (after 3 months) was observed in more than 40% of patients as detected by continuous rhythm monitoring in the CIRCA‐DOSE trial. 2 Thus, repeated procedures and the use of maintenance antiarrhythmic medications are usually necessary to achieve acceptable success rates. 21 The rate of repeated ablation procedures may reach up to 80%. 22 Despite added strategies beyond PV isolation, the success rate did not remarkably improve. 23
A combined epicardial and endocardial ablation strategy (the hybrid convergent procedure) has been developed to fill the gap of the conventional endocardial catheter ablation. Surgical access (usually through transdiaphragmatic subxiphoid incision, a mini‐thoracotomy, or thoracoscopic approaches) is used to access into the pericardial space to facilitate epicardial ablation, mainly to isolate the posterior wall of the left atrium, 5 after which endocardial ablation is done by an electrophysiologist to complete the isolation of the pulmonary veins. Furthermore, verifications of the epicardial ablation efficacy can be done during the endocardial portion, and further ablation to isolate the mitral isthmus, cavotricuspid isthmus, or coronary sinus can be done if necessary. 5 This combined approach facilitates the isolation of both the posterior wall of LA and the PVs; both share the same embryological origin and the arrhythmogenic electrophysiologic potential. 24
In our study, the rate of late AA recurrence for the ECA group was significantly higher than HCP (51.86% vs. 29.25%, respectively). The enhanced success risk of HCP could be explained by additional ablation of arrhythmogenic targets beyond the isolation of pulmonary veins and robust lesion formation through direct epicardial‐catheter contact. 25 However, the ECA strategy showed a better safety profile than the HCP. Our results showed that the procedural‐related complications rate was almost three times higher in HCP (12.3% in HCP vs. 3.9 in ECA). Our results are consistent with Khan et al., who reported an overall HCP complication rate of 10%. 26 Furthermore, the meta‐analysis by Pearman et al. showed no difference between the hybrid procedure and epicardial ablation in the prevention of AA recurrence; moreover, the HCP was associated with higher complication rates (7.3% vs. 2.8%, RR = 2.6). 27 The safety and efficacy of HCP could be potentially affected by the various approaches to the procedure. The review article by Khoynezhad et al. summarized the outcomes of 15 studies that utilized the HCP in AF ablation 28 which showed significant variability in the AF freedom outcome (ranging between 19% and 94%) and the safety profile (reported complications rate up to 24%). This significant heterogeneity could be explained by different surgical accesses used, lesion sets applied, the timing of the HCP, type of energy used, and the exclusion of LAA. In our subgroup analysis, thoracoscopic access of HCP showed a better safety profile; however, it was not statistically significant compared with the other two approaches. Furthermore, our results showed that AA freedom rate was similar regardless of whether the HCP was a concomitant or a staged procedure.
Our study showed significant differences in other key outcomes such as the procedure time and the hospital stay. These results are consistent with the meta‐analysis by Zhang et al., which showed a statistically significant difference in these outcomes favoring the ECA strategy. 29 However, our meta‐analysis included four more studies including two more RCTs, with a larger number of included patients (n = 797 patients vs. 331), thus leading to a more robust conclusion on the utility of HCP. Furthermore, we investigated the impact of the procedure access on the rate of periprocedural complications which showed that HCP through thoracoscopy access might have a better safety profile.
For HCP to be successful, it needs a multidisciplinary convergent “team” that integrates skills from cardiothoracic surgery and electrophysiology as well as perioperative coordination because it may require medications adjustment and deal with the potential postoperative complications. In addition, effective patient selection is an important aspect of success. During the HCP, epicardial ablation should be the first component. Under endoscopic observation, a closed‐irrigation, unipolar RF catheter device is typically utilized for epicardial ablation. 30 To access the left atrial posterior wall (LAPW), the device is placed by a pericardioscopic cannula and moved in the pericardial space with the use of the cannula and endoscope. As the temperature approaches 60°C, the RF energy delivery should achieve 124 coagulation, but not to the point that tissue vaporization occurs. Lesions are typically overlapped across the whole LAPW to improve contiguity and transmurality, minimizing gaps and resulting in a uniform zone of electrical silence. Then, the endocardial component complements the epicardial one by touching up the LAPW lesion set if needed based on an electro‐anatomic map and by performing additional ablation as required based on the individual patient procedure and clinical characteristics. 30
There are certain limitations to our meta‐analysis. First, the ablation procedures were not standardized among the included studies; however, in most of the study cohort, the posterior wall of the left atrium and the pulmonary veins were ablated. Second, the success rate of ablative procedures was assessed with different approaches. In most of the studies, only symptomatic recurrences proven by EKG or Holter monitor were counted as failures; only two studies employed an implantable loop recorder to confirm the AA recurrence. Third, the included trials were of a single‐blinded design. Therefore, investigator bias cannot be undermined. Last, we could not perform publication bias due to the small number of included studies.
However, there are several strengths to our meta‐analysis. First, to our knowledge, this is the first meta‐analysis to include eight studies with three RCTs to compare the clinical outcomes of adjunctive epicardial strategy with conventional ECA strategy in terms of efficacy and safety. Second, we performed a subgroup analysis for the late atrial arrhythmia recurrence rate based on the timing of the HCP and the use of AADs. In addition, no heterogeneity was found in the measurement of our safety outcome.
In conclusion, our meta‐analysis demonstrated that the hybrid convergent procedure for AF ablation was associated with a higher success rate and reduced the risk of late atrial arrhythmia recurrence. However, this should be judged for increased periprocedural adverse events and extended hospital stay. Prospective large‐scale randomized trials are needed to validate these results.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
MM and AB conceived and designed the study and critically revised the manuscript. MM, PC, A. Abumoawad, and A. Al‐abdouh designed the study, collected, analyzed, and interpreted the data and drafted the manuscript. HA, MA, CB, OH, O. Sajdeya, and O. Srour collected the data and reviewed the literature. All authors read and approved the final manuscript.
IRB APPROVAL
This study was deemed exempt by the Institutional Review Board of the University of Toledo, as it was a meta‐analysis of published studies that included de‐identified patient information.
Supporting information
Supplementary Material
Mhanna M, Beran A, Al‐Abdouh A, Ayesh H, Sajdeya O, Srour O, et al. Hybrid convergent ablation versus endocardial catheter ablation for atrial fibrillation: A systematic review and meta‐analysis. J Arrhythmia. 2021;37:1459–1467. 10.1002/joa3.12653
Funding information
None.
REFERENCES
- 1. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–7. [DOI] [PubMed] [Google Scholar]
- 2. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 3. Gilligan DM, Joyner CA, Bundy GM. Multidisciplinary collaboration for the treatment of atrial fibrillation: convergent procedure outcomes from a single center. J Innov CRM. 2013;4:1396–403. [Google Scholar]
- 4. Sanchez JM, Al‐Dosari G, Chu S, Beygui R, Deuse T, Badhwar N, et al. Hybrid and surgical procedures for the treatment of persistent and longstanding persistent atrial fibrillation. Expert Rev Cardiovasc Ther. 2018;16:91–7. [DOI] [PubMed] [Google Scholar]
- 5. Wats K, Kiser A, Makati K, Sood N, DeLurgio D, Greenberg Y, et al. The convergent atrial fibrillation ablation procedure: evolution of a multidisciplinary approach to atrial fibrillation management. Arrhythm Electrophysiol Rev. 2020;9:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deeks J, Dinnes J, D'Amico R, Sowden A, Sakarovitch C, Song F, et al. Evaluating non‐randomised intervention studies. Health Technol Assess. 2003;7(27):1–173. [DOI] [PubMed] [Google Scholar]
- 11. Dalton JE, Bolen SD, Mascha EJ. Publication bias: the elephant in the review. Anesth Analg. 2016;123:812–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeLurgio DB, Crossen KJ, Gill J, Blauth C, Oza SR, Magnano AR, et al. Hybrid convergent procedure for the treatment of persistent and long‐standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol. 2020;13:e009288. [DOI] [PubMed] [Google Scholar]
- 13. Edgerton Z, Perini AP, Horton R, Trivedi C, Santangeli P, Bai R, et al. Hybrid procedure (Endo/Epicardial) versus standard manual ablation in patients undergoing ablation of longstanding persistent atrial fibrillation: results from a single center. J Cardiovasc Electrophysiol. 2016;27:524–30. [DOI] [PubMed] [Google Scholar]
- 14. Genev IK, Tompkins LA, Khare MM, Farokhi F. Comparison of the efficancy and complication rates of the hybrid maze, complete cox‐maze and catheter ablation in the treatment of atrial fibrillation. J Atr Fibrillation. 2017;9:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hwang JK, Jeong DS, Gwag HB, Park K‐M, Ahn J, Carriere K, et al. Staged hybrid procedure versus radiofrequency catheter ablation in the treatment of atrial fibrillation. PLoS One. 2018;13:e0205431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jan M, Žižek D, Geršak ŽM, Geršak B. Comparison of treatment outcomes between convergent procedure and catheter ablation for paroxysmal atrial fibrillation evaluated with implantable loop recorder monitoring. J Cardiovasc Electrophysiol. 2018;29:1073–80. [DOI] [PubMed] [Google Scholar]
- 17. Kress DC, Erickson L, Choudhuri I, Zilinski J, Mengesha T, Krum D, et al. Comparative effectiveness of hybrid ablation versus endocardial catheter ablation alone in patients with persistent atrial fibrillation. JACC Clin Electrophysiol. 2017;3:341–9. [DOI] [PubMed] [Google Scholar]
- 18. Maclean E, Yap J, Saberwal B, Kolvekar S, Lim W, Wijesuriya N, et al. The convergent procedure versus catheter ablation alone in longstanding persistent atrial fibrillation: a single centre, propensity‐matched cohort study. Int J Cardiol. 2020;303:49–53. [DOI] [PubMed] [Google Scholar]
- 19. Mahapatra S, LaPar DJ, Kamath S, Payne J, Bilchick KC, Mangrum JM, et al. Initial experience of sequential surgical epicardial‐catheter endocardial ablation for persistent and long‐standing persistent atrial fibrillation with long‐term follow‐up. Ann Thorac Surg. 2011;91:1890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter sustained treatment of paroxysmal atrial fibrillation (STOP AF) trial. Circ Arrhythm Electrophysiol. 2014;7:69–75. [DOI] [PubMed] [Google Scholar]
- 21. Rostock T, Salukhe TV, Steven D, Drewitz I, Hoffmann BA, Bock K, et al. Long‐term single‐ and multiple‐procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011;8:1391–7. [DOI] [PubMed] [Google Scholar]
- 22. Verma A, Jiang C‐Y, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 23. Fink T, Schlüter M, Heeger C‐H, Lemes C, Maurer T, Reissmann B, et al. Stand‐alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long‐standing persistent atrial fibrillation: the randomized Alster‐Lost‐AF Trial (Ablation at St. Georg Hospital for Long‐Standing Persistent Atrial Fibrillation). Circulation: Arrhythmia and Electrophysiology. 2017;10:e005114. [DOI] [PubMed] [Google Scholar]
- 24. Ho SY, Cabrera JA, Sanchez‐Quintana D. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol. 2012;5:220–8. [DOI] [PubMed] [Google Scholar]
- 25. Kumar P, Kiser AC, Gehi AK. Hybrid treatment of atrial fibrillation. Prog Cardiovasc Dis. 2015;58:213–20. [DOI] [PubMed] [Google Scholar]
- 26. Khan Z, Hamandi M, Khan H, DiMaio JM, Evans M. Convergent epicardial‐endocardial ablation for treatment of long‐standing persistent atrial fibrillation: a review of literature. J Card Surg. 2020;35:1306–13. [DOI] [PubMed] [Google Scholar]
- 27. Pearman CM, Poon SS, Bonnett LJ, Haldar S, Wong T, Mediratta N, et al. Minimally invasive epicardial surgical ablation alone versus hybrid ablation for atrial fibrillation: a systematic review and meta‐analysis. Arrhythm Electrophysiol Rev. 2017;6:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khoynezhad A, Ellenbogen KA, Al‐Atassi T, Wang PJ, Kasirajan V, Wang X, et al. Hybrid atrial fibrillation ablation: current status and a look ahead. Circ Arrhythm Electrophysiol. 2017;10. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Sun H, He K, Gu J, Zheng R, Shao Y. Hybrid ablation versus transcatheter ablation for atrial fibrillation: a meta‐analysis. Medicine (Baltimore). 2019;98:e14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makati KJ, Sood N, Lee LS, Yang F, Shults CC, DeLurgio DB, et al. Combined epicardial and endocardial ablation for atrial fibrillation: Best practices and guide to hybrid convergent procedures. Heart Rhythm. 2021;18:303–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


