Abstract
The Japanese Catheter Ablation (J‐AB) registry, started in August 2017, is a voluntary, nationwide, multicenter, prospective, observational registry, performed by the Japanese Heart Rhythm Society (JHRS) in collaboration with the National Cerebral and Cardiovascular Center using a Research Electronic Data Capture system. The purpose of this registry is to collect the details of target arrhythmias, the ablation procedures, including the type of target arrhythmias, outcomes, and acute complications in the real‐world settings. During the year of 2019, we have collected a total of 80 795 procedures (mean age of 65.2 years and 66.4% male) from 425 participant hospitals. Detailed data are shown in Figures and Tables.
Keywords: catheter ablation, complication, J‐AB, REDCap, registry
J‐AB is a prospective nationwide multicenter registry designed to collect clinical variables, successful ablation rate and sort‐term outcome, aiming to register all catheter ablation cases performed in Japan. We successfully collected 80 795 procedures during the year of 2019.

Catheter ablation has become an established therapy for the management of various cardiac arrhythmias and the procedure number has been dramatically increasing. However, little is known about the details of target arrhythmias, the ablation procedures, including the type of target arrhythmias, outcomes, and acute complications in the real‐world settings.
There are several preceding registries of catheter ablation, but the majority of which collected data from selected centers and/or selected arrhythmia and/or specified months to reveal the current status of ablations. 1 , 2 , 3 Accordingly, we conducted a nationwide, multicenter, prospective, observational registry in Japan, named Japanese Catheter Ablation (J‐AB) registry, aiming to register all catheter ablation cases in Japan. 4 This registry has been performed by the Japanese Heart Rhythm Society (JHRS) in collaboration with the National Cerebral and Cardiovascular Center using a Research Electronic Data Capture (REDCap) system. This study has been performed under the approval from the Institutional Review Board (IRB) of the National Cerebral and Cardiovascular Center (M28‐114‐7, approved at December 21, 2016), Japan, along with the IRBs of all participating hospitals. All participants were provided informed consent either by a written paper or by an opt out fashion and could withdraw their consent at any time. This study was also registered in the UMIN Clinical Trial Registry (UMIN 000028288) and ClinicalTrials.gov (NCT03729232). This J‐AB registry started in August 2017, since then the number of participating hospitals has increased to over 400 at the end of 2019. Annual data during the year of 2018 have been already reported, 5 and now we report here the annual report of the results during the year of 2019. Figure 1 shows that the cumulative number of registered hospitals and the patients during the year of 2019. Figure 2 shows that the number and rate of the target arrhythmias. AF procedure was the most common (73.8% of all ablation procedures) in 2019. Patient characteristics, acute outcomes, and acute complications of all and AF procedures are shown in Tables 1, 2, and 3, respectively. Compared to the previous reports in Japan and other countries, 1 , 2 , 3 acute complications during hospitalization were similar or low. In the Spanish Catheter Ablation Registry, the rate of all complications was 2.0%‐2.6% for all ablation procedures, and 3.4%‐5.1% for AF ablation. In the US report, the overall complication rate was 5.46% and the in‐hospital mortality rate was 0.15% for AF ablation. In the J‐CARAF during the years from 2011 to 2016, total major complications occurred in 3.0% of the AF ablation procedures.
FIGURE 1.
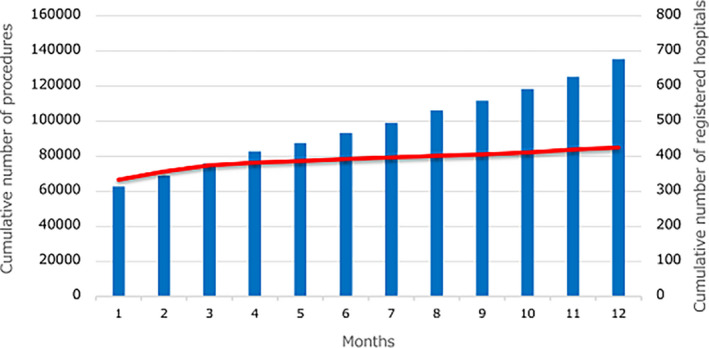
Cumulative number of registered hospitals (red line) and the patients (blue bars) during the year of 2019
FIGURE 2.
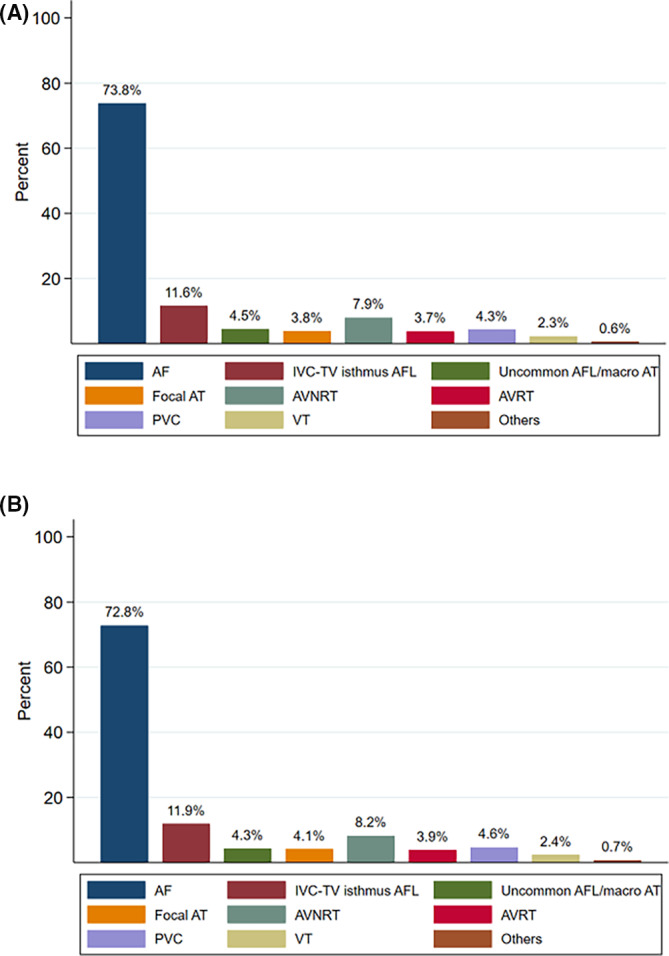
The proportion of the target arrhythmias in the J‐AB registry. (A) The J‐AB registry 2019 (80,795 procedures). (B) The J‐AB registry 2018 (55,525 procedures). Abbreviations: AF, atrial fibrillation; IVC, inferior vena cava; TV, tricuspid valve; AFL, atrial flutter; AT, atrial tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reentrant tachycardia; PVC, premature ventricular contraction; VT, ventricular tachycardia
TABLE 1.
Patient characteristics
| Atrial fibrillation (AF) | Atrial flutter (AFL)/Atrial tachycardia (AT) | |||||||
|---|---|---|---|---|---|---|---|---|
| All procedures | All AF | Paroxysmal AF (PAF) | Non‐PAF | All AFL/AT | IVC‐TV isthmus‐dependent AFL a | Uncommon AFL macro‐AT a | Focal AT a | |
| N | 80 795 | 59 624 | 35 343 | 24 138 | 13 661 | 8838 | 3132 | 2686 |
| Age, mean ± SD | 65.2 ± 13.1 | 66.9 ± 10.5 | 67.1 ± 10.9 | 66.6 ± 10.1 | 67.6 ± 12.9 | 68.1 ± 12.0 | 68.9 ± 11.9 | 64.9 ± 15.9 |
| Gender, male | 53 657 (66.4%) | 41 558 (69.7%) | 23 220 (65.7%) | 18 244 (75.6%) | 9213 (67.4%) | 6689 (75.7%) | 1819 (58.1%) | 1250 (46.5%) |
| BMI, mean ± SD | 23.9 ± 3.8 | 24.2 ± 3.7 | 23.9 ± 3.6 | 24.7 ± 3.8 | 23.5 ± 3.8 | 23.6 ± 3.7 | 23.5 ± 3.8 | 22.7 ± 3.8 |
| Heart diseases | 15 505 (19.2%) | 10 816 (18.1%) | 5649 (16.0%) | 5153 (21.4%) | 4033 (29.5%) | 2520 (28.5%) | 1385 (44.2%) | 629 (23.4%) |
| IHD | 5110 (6.3%) | 3609 (6.1%) | 2167 (6.1%) | 1437 (6.0%) | 1100 (8.1%) | 784 (8.9%) | 267 (8.5%) | 135 (5.0%) |
| Cardiomyopathy | 4592 (5.7%) | 3249 (5.4%) | 1296 (3.7%) | 1951 (8.1%) | 1002 (7.3%) | 609 (6.9%) | 334 (10.7%) | 158 (5.9%) |
| Valve disease | 2538 (3.1%) | 1501 (2.5%) | 731 (2.1%) | 763 (3.2%) | 1229 (9.0%) | 691 (7.8%) | 560 (17.9%) | 187 (7.0%) |
| CHD | 985 (1.2%) | 430 (0.7%) | 242 (0.7%) | 188 (0.8%) | 527 (3.9%) | 306 (3.5%) | 231 (7.4%) | 103 (3.8%) |
| Ventricular tachycardia (VT) | ||||||
|---|---|---|---|---|---|---|
| Atrioventricular nodal reentrant tachycardia | Atrioventricular reentrant tachycardia | Premature ventricular contraction | VT due to ischemic cardiomyopathy | VT due to nonischemic cardiomyopathy | VT due to CHD | |
| N | 6409 | 3000 | 3501 | 433 | 502 | 18 |
| Age, mean ± SD | 58.7 ± 16.7 | 48.9 ± 20.0 | 57.8 ± 16.1 | 68.6 ± 10.1 | 63.6 ± 13.1 | 41.7 ± 13.7 |
| Gender, male | 2701 (42.1%) | 1926 (64.2%) | 1894 (54.1%) | 404 (93.3%) | 396 (78.9%) | 16 (88.9%) |
| BMI, mean ± SD | 22.9 ± 3.9 | 23.0 ± 3.9 | 23.7 ± 3.9 | 24.2 ± 3.8 | 23.5 ± 4.0 | 25.6 ± 5.1 |
| Heart diseases | 479 (7.5%) | 195 (6.5%) | 633 (18.1%) | 396 (91.5%) | 452 (90.0%) | 17 (94.4%) |
| IHD | 155 (2.4%) | 55 (1.8%) | 221 (6.3%) | 16 (3.2%) | 0 (0%) | |
| Cardiomyopathy | 71 (1.1%) | 33 (1.1%) | 252 (7.2%) | 14 (3.2%) | 0( 0%) | |
| Valve disease | 63 (1.0%) | 24 (0.8%) | 60 (1.7%) | 18 (4.2%) | 22 (4.4%) | 0( 0%) |
| CHD | 45 (0.7%) | 29 (1.0%) | 23 (0.7%) | 1 (0.2%) | 6 (1.2%) | |
Abbreviations: BMI, Body Mass Index; CHD, congenital heart disease; IHD, ischemic heart disease; SD, Standard Deviation
Multiple choices allowed.
TABLE 2.
Acute outcomes
| Pulmonary vein isolation for atrial fibrillation (n = 58 429) | |
|---|---|
| Ablation system n (%) | |
| RF alone | 43 047 (73.67%) |
| Balloon alone (Cryo, hot, laser) | 10 464 (17.91%) |
| RF + Balloon combination | 4586 (7.85%) |
| Others | 168 (0.29%) |
| Missing | 164 (0.28%) |
| Patient with a first session (n = 47 726) | |
| Success | 47 462 (99.45%) |
| Unsuccess | 186 (0.39%) |
| Already isolated | 60 (0.13%) |
| Unknown | 18 (0.04%) |
| Patient with second session (n = 8863) | |
| Success | 7448 (84.03%) |
| Unsuccess | 19 (0.21%) |
| Already isolated | 1388 (15.66%) |
| Unknown | 8 (0.09%) |
| Additional ablation only | 577 (6.09%) |
| Patient with third session (n = 2090) | |
| Success | 1138 (64.40%) |
| Unsuccess | 4 (0.23%) |
| Already isolated | 625 (35.37%) |
| Additional ablation only | 319 (15.26%) |
| IV‐TV isthmus‐dependent atrial flutter (n = 8838) | |
| Success | 8776 (99.30%) |
| Unsuccess | 59 (0.67%) |
| Unknown | 3 (0.03%) |
| Uncommon atrial flutter/atrial tachycardia (n = 3132) | |
| Complete success | 2650 (84.61%) |
| Partial success | 319 (10.19%) |
| Unsuccess | 103 (3.29%) |
| Unknown | 60 (1.92%) |
| Focal atrial tachycardia (n = 2686) | |
| Complete success | 2238 (83.32%) |
| Partial success | 313 (11.65%) |
| Unsuccess | 101 (3.76%) |
| Unknown | 34 (1.27%) |
| Atrioventricular nodal reentrant tachycardia by slow‐fast (n = 5574) | |
| Complete success | 5457 (97.90%) |
| Partial success | 70 (1.26%) |
| Unsuccess | 29 (0.52%) |
| Unknown | 18 (0.32%) |
| Atrioventricular nodal reentrant tachycardia by fast‐slow (n = 581) | |
| Complete success | 558 (96.04%) |
| Partial success | 18 (3.10%) |
| Unsuccess | 3 (0.52%) |
| Unknown | 2 (0.34%) |
| Atrioventricular nodal reentrant tachycardia by other (n = 581) | |
| Complete success | 339 (90.40%) |
| Partial success | 20 (5.33%) |
| Unsuccess | 7 (1.87%) |
| Unknown | 9 (2.40%) |
| Atrioventricular reentrant tachycardia by kent (n = 2951) | |
| Complete success | 2840 (96.24%) |
| Unsuccess | 85 (2.88%) |
| Unknown | 26 (0.88%) |
| Premature ventricular contraction (n = 3501) | |
| Complete success | 2642 (75.46%) |
| Partial success | 602 (17.20%) |
| Unsuccess | 228 (6.51%) |
| Unknown | 29 (0.83%) |
| Idiopathic ventricular tachycardia (n = 781) | |
| Complete success | 595 (76.18%) |
| Partial success | 122 (15.62%) |
| Unsuccess | 42 (5.38%) |
| Unknown | 22 (2.82%) |
| Ventricular tachycardia due to ischemic cardiomyopathy (n = 433) | |
| Complete success | 272 (62.82%) |
| Partial success | 117 (27.02%) |
| Unsuccess | 20 (4.62%) |
| Unknown | 24 (5.54%) |
| Ventricular tachycardia due to nonischemic cardiomyopathy (n = 502) | |
| Complete success | 289 (57.57%) |
| Partial success | 156 (31.08%) |
| Unsuccess | 40 (7.97%) |
| Unknown | 17 (3.39%) |
| Ventricular tachycardia due to CHD (n = 18) | |
| Complete success | 10 (55.56%) |
| Partial success | 7 (38.89%) |
| Unsuccess | 1 (5.56%) |
Abbreviations: CHD, congenital heart disease; IVC, inferior vena cava; RF, radiofrequency ablation; TV, tricuspid valve.
TABLE 3.
Acute complications: All procedures and AF procedures
| All procedures | AF procedures | |
|---|---|---|
| N | 80 795 | 59 624 |
| Complications during hospitalization | 2023 (2.50%) | 1633 (2.74%) |
| Major bleeding (BARC>=2) | 902 (1.12%) | 700 (1.17%) |
| Cardiac tamponade | 532 (0.66%) | 380 (0.64%) |
| Embolism | 149 (0.18%) | 128 (0.21%) |
| Phrenic nerve paralysis | 212 (0.26%) | 205 (0.34%) |
| Esophagus | 147 (0.18%) | 146 (0.24%) |
| Esophagus ulcer | 20 (0.02%) | 19 (0.03%) |
| Gastric hypomotility | 127 (0.16%) | 127 (0.21%) |
| Atrioesophageal fistula | 0 (0) | 0 (0) |
| Pericarditis | 99 (0.12%) | 84 (0.14%) |
| Sick sinus syndrome | 134 (0.17%) | 110 (0.18%) |
| Atrioventricular block | 65 (0.08%) | 17 (0.03%) |
| Death during hospitalization | 89 (0.11%) | 34 (0.06%) |
| Cardiac death | 58 (0.07%) | 18(0.03%) |
| Related to ablation therapy | 2 (0.002%) | 1 (0.002%) |
| Non‐cardiac death | 31 (0.04%) | 16(0.03%) |
| Related to ablation therapy | 1 (0.001%) | 0 (0) |
Abbreviations: AF, atrial fibrillation; BARC, Bleeding Academic Research Consortium.
CONFLICT OF INTEREST
Kengo Kusano: Speaker honoraria from DAIICHI SANKYO COMPANY, Ltd., Japan, Bristol‐Myers Squibb, Biotronik Japan, and Medtronic Japan, and research grants from Medtronic Japan and EP‐CRSU Co., Ltd. Teiichi Yamane: Speaker honoraria from DAIICHI SANKYO COMPANY, Ltd., Japan, Boerringer Ingelheim, Abbott Japan, Medtronic Japan, and Kaneka Corporation and research grants from Boehringer Ingelheim. Koichi Inoue: Speaker honoraria from DAIICHI SANKYO COMPANY, Ltd., Japan, Bristol‐Myers Squibb, Bayer Yakuhin, Nihon Boehringer Ingelheim, Johnson and Johnson KK, and Medtronic Japan. Koji Miyamoto: Research grant from Japan LifeLine, Abbott Japan, Speaker honoraria from Daiichi Sankyo, Boerringer Ingelheim, Bayer, Bristol‐Myers Squibb, Pfizer, Abbott Japan, and Medtronic Japan. Yu‐ki Iwasaki: Research grant from Daiichi Sankyo, Seiji Takatsuki: Research grant from Japan Lifeline, honoraria from Medtronic Japan, Daiichi Sankyo. Morio Shoda: Speaker honorarium from Medtronic Japan, and financial endowments to our clinical research division from Biotronik Japan, Medtronic Japan, Boston Scientific Japan, and Abbott Japan. Akihiko Nogami: Speaker honoraria from Abbott, Biosense Webster, and Daiichi‐Sankyo; an endowment from Medtronic and DVX. Wataru Shimizu: Research grant from Daiichi Sankyo Co, Ltd., and Nihon Boehringer Ingelheim, and Speaker honoraria from Daiichi Sankyo Co, Ltd., Bristol‐Myers Squibb Co, Ltd, Bayer Yakuhin Co, Ltd, Nihon Boehringer Ingelheim, Ono Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Novartis Pharma KK, and Medtronic Japan. None: MT, YMN, M.K, M.N, K.K, K.N, and YM
ACKNOWLEDGMENTS
The authors are grateful for the contributions of all the investigators and Yoko Sumida for the data management in the J‐AB registry.
Kusano K, Yamane T, Inoue K, Takegami M, Nakao YM, Nakai M, et al; J‐AB registry investigators . The Japanese Catheter Ablation Registry (J‐AB): Annual report in 2019. J Arrhythmia. 2021;37:1443–1447. 10.1002/joa3.12640
Funding information
Japanese Heart Rhythm Society.
REFERENCES
- 1. Ibanez Criado JL, Quesada A, Cozar R; Spanish Catheter Ablation Registry Collaborators and Appendix REGISTRY COLLABORATORS . Spanish Catheter Ablation Registry. 18th Official Report of the Spanish Society of Cardiology Working Group on Electrophysiology and Arrhythmias (2018). Rev Esp Cardiol (Engl Ed). 2019;72:1031‐42. [DOI] [PubMed] [Google Scholar]
- 2. Inoue K, Murakawa Y, Nogami A, Shoda M, Naito S, Kumagai K, et al; Japanese Heart Rhythm Society Members . Current status of catheter ablation of atrial fibrillation in Japan: summary of the 4th survey of the Japanese Catheter Ablation Registry of Atrial Fibrillation (J‐CARAF). J Cardiol. 2016;68:83–8. [DOI] [PubMed] [Google Scholar]
- 3. Tripathi B, Arora S, Kumar V, Abdelrahman M, Lahewala S, Dave M, et al. Temporal trends of in‐hospital complications associated with catheter ablation of atrial fibrillation in the United States: An update from Nationwide Inpatient Sample database (2011–2014). J Cardiovasc Electrophysiol. 2018;29:715–24. [DOI] [PubMed] [Google Scholar]
- 4. Yamane T, Inoue K, Kusano K, Takegami M, Nakao YM, Miyamoto Y, et al; J‐AB Registry Investigators . Study design of nationwide Japanese Catheter Ablation Registry: protocol for a prospective, multicenter, open registry. J Arrhythm. 2019;35:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kusano K, Yamane T, Inoue K, Takegami M, Nakao YM, Miyamoto Y, et al; J‐AB Registry Investigators . The Japanese Catheter Ablation Registry (J‐AB): a prospective nationwide multicenter registry in Japan. Annual report in 2018. J Arrhythm. 2020;36:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


