Abstract
This study evaluated the changes in eggshell mechanical properties, ultrastructure, calcium metabolism-related serum indices, and gene expression in eggshell gland during eggshell formation between laying ducks in the peak (young duck) and late phase (aged duck) of production. A total of 84 healthy young (31 wk of age) and 84 healthy aged (65 wk of age) Longyan laying ducks were each divided into 6 replicates of 14 birds, and caged individually. All the ducks were fed in one house with the same corn-soybean meal-based diet for 5 wk. The eggshell mechanical properties (shell proportion, thickness, breaking strength, and fracture toughness) and chemical components (matrix proteins, calcium, phosphorus, and magnesium) decreased in aged laying ducks (P < 0.05). Shell structural indices: total thickness, effective thickness and its proportion decreased, whereas mammillary knob width and its proportion increased (P < 0.05). The regulation values of early fusion, cuffing, caps, and total score of mammillary knobs were higher in aged laying ducks relative to the young ducks (P < 0.05). During the initial, growth and terminal stages of eggshell formation, shell thickness and breaking strength (terminal), shell weight, and its proportion (terminal) decreased in aged laying ducks (P < 0.05). Ultrastructural changes during shell formation indicated that the mammillary-knob density and effective thickness decreased (P < 0.05). Decreases occurred in serum content of phosphorus (growth), and estradiol and calcium contents (terminal) (P < 0.05). Relative expression of Ca2+ transporter and HCO3− exchanger, and matrix proteins genes decreased in aged laying ducks (P < 0.05) at all stages of eggshell formation. Collectively, the decreased incidence of early fusion and caps, increased thickness and width of mammillary knobs, and decreased effective thickness are the crucial differences leading to the compromised mechanical properties of eggshell in the late laying period. A disturbed regulation of calcium metabolism and uterine expression of ion transporters, especially for HCO3− exchange of aged laying ducks likely contribute to age-induced ultrastructural deterioration of the eggshell.
Key words: mechanical property, shell ultrastructure, laying duck, peak and late laying phase
INTRODUCTION
The eggshell has biological and economic significance for the poultry industry and its quality has always been a major concern to the quality and safety of egg products (Samiullah et al., 2014). It is of particular importance for duck eggs because most are processed into preserved or salted eggs, and eggshell plays a vital role in the process. With aging, the number of cracked eggshells increases and leads to much economic loss in poultry industry (Travel et al., 2011). It is essential to understand the age-related changes of shell quality in commercial laying ducks in order to improve it in aged laying ducks by nutritional modulations.
The eggshell ultrastructure has been widely recognized as the vital part to its quality recently, as reported that reduced shell quality of old laying hens could be attributed to changes of shell ultrastructure with the aging of hens (Feng et al., 2020). Eggshell is a highly ordered structure comprising membranes (inner and outer), mammillary layer, palisade layer, vertical crystal layer and cuticle (Solomon, 2010), which result from the sequential precipitation of mineral carbonate and organic matrix during 3 stages of mineralization (the initial, growth, and the terminal) (Fernandez et al., 2001). The key roles of ultrastructure have been increasingly recognized in determining mechanical properties and quality of the shell (Athanasiadou et al., 2018). The thickness of the palisade layer and the organization of calcite crystals in this layer (Radwan, 2016), and the thickness and density of mammillary konbs (Dunn et al., 2012) determined the shell breaking strength. The calcified eggshell, the major component of avian eggshell, is the predominant contributor to the mechanical properties of the eggshell, as confirmed by observations that the size, shape and orientation of the calcite crystals significantly influences the structure and quality of an eggshell (Rodríguez-Navarro et al., 2002). The organic matrix, primarily proteins (70%) and polysaccharides (11%) (Heaney and Robinson, 1976) play crucial roles in modulating ultrastructure and mechanical properties of eggshell by affecting the growth rate of calcification and the orientation of crystals (Fernandez et al., 2001). During the highly precisely controlled calcification process, amounts of calcium and bicarbonate ions and precursors of organic matrix are secreted into uterine fluid, where the organic and mineral phases interact and form the eggshell (Dominguez-Vera et al., 2000). As reported, the ionic precursors are continuously supplied from the blood through transepithelial transport in the uterus, and the transepithelial transfer of calcium is mainly via numerous transporters, such as TRPV Ca2+ channels, CALB1, ATP2A2 (Bar, 2009). It also involves many transcellular transporters of other ionic species (Na+/HCO3−, SLC4A4, A5; HCO3−/Cl– exchanger, SLC26A9; H+ pump, ATP6V1B2, C2), which participate in the process of calcium secretion and in the maintenance of cellular ionic homeostasis (Nys, 2018). Furthermore, 1,25-(OH)2VD3 and estradiol are key factors modulating calcium homeostasis and affect shell formation and quality (Curl et al., 1985). 1,25-(OH)2D3 may affect the ultrastructure of the mammillary layer by regulating the expression of uterine genes (Zhang et al., 2019).
In laying hens, several studies have studied the age-related variations in shell quality and ultrastructure (Park and Sohn., 2018; Wistedt et al., 2019; Feng et al., 2020). The physical and mechanical properties of the eggshell declined (Fathi et al., 2019), and the incidence of confluence and early fusion in the mammillary layer decreased with age (Samiullah et al., 2014). Wistedt et al (2019) observed that the decreased gland density and a shift in the balance between ERα and ERβ in the shell gland, co-occurring with a dramatic drop-in duodenal carbonic anhydrase activity, were possibly the key factors accounting for the age-related changes in shell. Damage of endometrial tissue impairs the processes of ion translocation and the crystallization during eggshell formation, resulting in large and nonuniform mammillary knobs, and decreased shell quality in older hens (Park and Sohn., 2018). Feng et al. (2020) used uterine transcriptome analysis to reveal that altered gene expression of matrix proteins along with the compromised immune function in the uterus of laying hens in the late phase of production may give rise to age-related impairment of eggshell ultrastructure and its mechanical properties. Few studies thus far have studied age-related changes of shell quality in laying ducks. Though similar processes of eggshell formation are observed in laying hens and ducks, there are slight differences between the 2 species (Panheleux et al., 1999), and unpublished data from this laboratory indicate significant differences between hen and duck eggshell in the contents of phosphorus (0.19 vs. 0.29 %), copper (0.53 vs. 17.7 mg/kg), manganese (0.22 vs. 0.68 mg/kg), magnesium (0.29 vs. 0.09%), and matrix proteins (284 vs. 191 ug/g).
The current study, therefore, has examined age-related changes in eggshell physical and mechanical properties, ultrastructure, calcium metabolism-related serum indices, and gene expression in eggshell gland during eggshell formation in commercial laying ducks.
MATERIALS AND METHODS
Experimental Design and Sample Collection
A total of 84 healthy young (31 wk of age) and 84 healthy aged (65 wk of age) Longyan laying ducks (green-colored eggs), obtained commercially from Shendan Agriculture and Animal Husbandry Co. Ltd. (Wuhan, China) and reared in one house with similar configurations. During the experiment, birds in each age group were divided into 6 replicates of 14 birds each in a randomized block design, and caged individually. All ducks were fed the same corn-soybean meal basal diet (apparent metabolizable energy 10.51 MJ/kg, crude protein 17.0%, calcium 3.7%, total phosphorus 0.59%, available phosphorus 0.37%, methionine 0.41% and lysine 0.88%) and were provided with feed and water ad libitum with exposure to 16 h of light/d and the control temperature for 5 wk. All ducks remained in good health during the feeding period with none being culled or requiring medication. The egg production in young and aged ducks was 91.7 ± 1.01% and 78.8 ± 2.15%, respectively.
At the end of the feeding period (36 wk and 70 wk), 10 eggs from each replicate that collected on 2 successive d were chosen to measure mechanical properties, chemical composition, and ultrastructure of the whole shell.
At 9.5, 15.5, and 20.5 h postoviposition (PO), corresponding stages of formation of the mammillary knobs, palisade layer, and vertical crystal layer, respectively, 1 bird from each replicate was euthanized by cervical dislocation. Blood samples were taken from the wing vein into noncoated evacuated tubes, incubated in a 37°C water bath while tilted at a 45° angle for 3 h, and then centrifuged at 3,000 × g for 10 min to harvest serum. Eggs were taken from the sampled ducks to measure the mechanical properties and ultrastructure of their eggshells. The target area of eggshell gland was taken to measure gene expression. Each treatment had 6 replicates with 1 egg or eggshell gland at each sampling time.
Mechanical Properties of the Whole Eggshell and During Formation
Eggshell thickness and breaking strength were determined using an Egg Shell Thickness Gauge and Egg Force Reader (Israel Orka Food Technology Ltd., Ramat Hasharon, Israel). The eggshell fracture toughness (N/mm3/2) was measured according to the formulas of Zhang et al. (2017). Fracture toughness is influenced by the nature and magnitude of inherent defects within a material and is formulated as [0.777 × (2.388 + (2.9934 (6/R))] (F/T3/2), where R is the radius of curvature (mm), F is the breaking strength (N), and T is the eggshell thickness (mm). Eggshell weight was measured after washing its interior membrane and drying overnight at room temperature. The eggshell ratio was calculated as eggshell weight/egg weight × 100. The eggs taken from sampled ducks in the 3 different postoviposition time at the end of the experiment to assess changes in eggshell mechanical properties during its formation. Each treatment had 6 replicates with 1 egg at each sampling time.
Ultrastructure of the Whole Eggshell and During Formation
Two pieces of each eggshell ∼ 0.5 cm2 from its equatorial section were subjected to scanning electronic microscopy (SEM; FEI Quanta 600, Thermo Fisher Scientific Ltd., Portland, OR). Before imaging, both the inside and outside of each eggshell was washed with distilled water to remove dirt, and then each portion was dried overnight. To observe the eggshell ultrastructure by SEM, samples were first mounted onto copper blocks and then coated with gold powder. The effective thickness (combined palisade, vertical crystal, and cuticle sections, μm), mammillary thickness (μm), and width (μm) of the mammillary knobs were measured using the SEM ruler according to Zhang et al. (2017). The mammillary thickness was taken as the length from the top of the membrane to the bottom of the palisade layer. The average width of the mammillary knobs was calculated as the length of the mammillary knobs/the number of the mammillary knobs. The total thickness referred to the combined effective and mammillary thickness. The effective and mammillary layers (%) are the thickness of each layer relative to the total thickness. Each treatment had 6 replicates of 10 eggs each, and 2 samples were examined for each egg, with 2 images taken for each sample.
At the end of the experiment, the changes in eggshell ultrastructure during its formation were measured. The density of the mammillary knobs was measured at 9.5 h PO. The mammillary and effective thickness, and mammillary knob width were measured at 18.5 h PO. The total thickness, effective thickness, mammillary thickness, and mammillary knob width were measured at 20.5 h PO. Mammillary knob density was counted and expressed as the number of knobs per unit. The effective and mammillary thickness, and mammillary knob width were measured using the SEM ruler. Each treatment had 6 replicates with 1 egg each, and for each egg 6 samples from the sharp, equatorial, and blunt areas of the eggs were examined, with 3 photographs taken for each sample.
Ultrastructure in Mammillary Layer
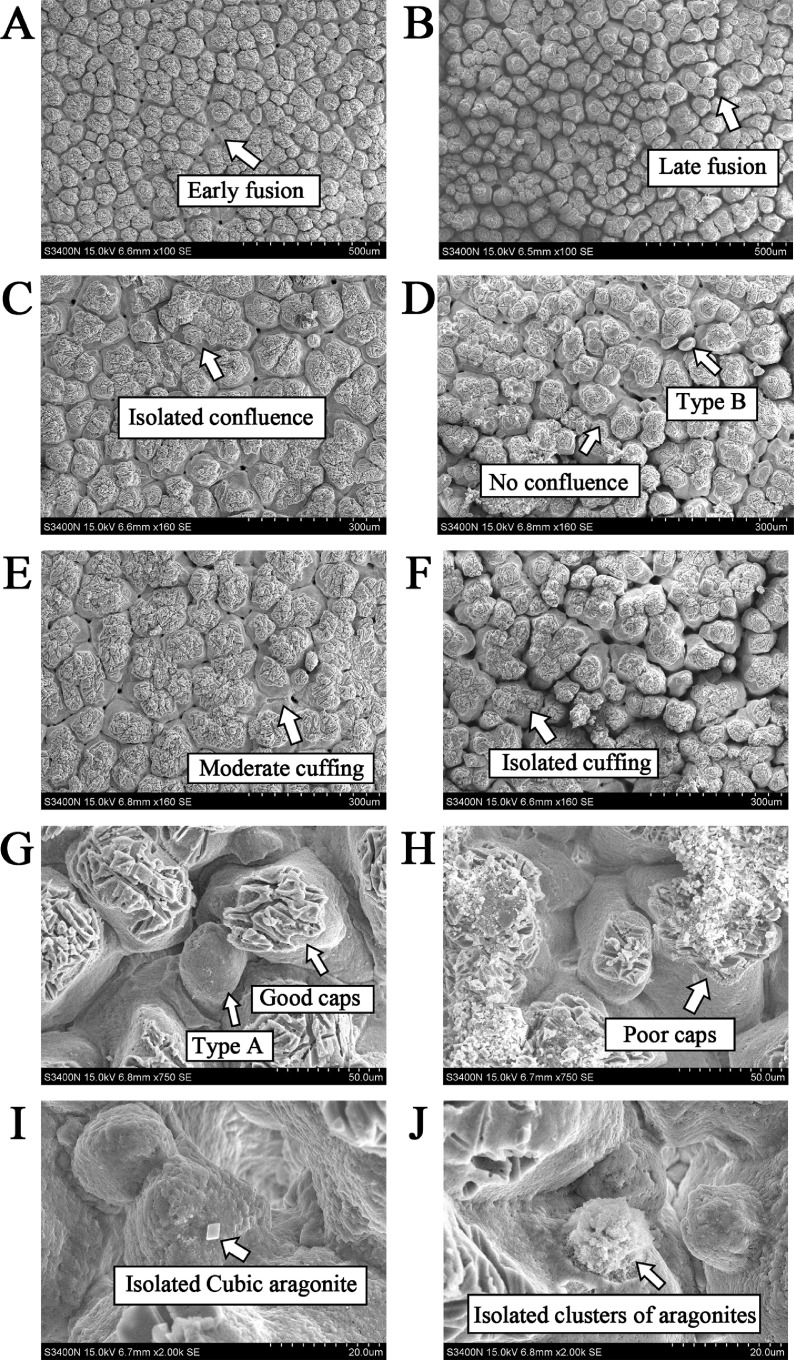
The ultrastructure of mammillary knobs was measured by the rules from Bain (1990). The scoring criteria for ultrastructure of mastoid are given in Additional file 1. The lower total score showed that an increase in cone layer quality characteristics. The organization of the mammillary layer was firstly assessed in terms of its overall visual appearance, including the presence or absence of pitting, and its severity. The depressions, erosions, and pin holes were successively higher in score and poorer in quality. The cap score was depended on the predominant degree of etching and the distance between neighboring caps. Shells with flat, poorly etched caps, or a low cap to cone ratio were given the highest score, conversely the lowest score. If the individual mammillary caps had become confluent with one another, the confluent structure was formed, which the extensive confluence had the lowest score. Early fusion of adjacent mammillae might be desirable in terms of improving shell strength, and so this was given a lower score than where the adjacent mammillary columns predominantly fused later. Type B and type A were especially spherical bodies that did not make any direct contribution to the mammillary layer. The lower the frequency of their appearance, the lower the score in the evaluation. Cuffing was believed to be formed at some point after the mammillary knobs had begun to fuse, so its appearance was considered beneficial. Aragonite was most commonly found in the intermammillary spaces that existed between adjacent cones which also didnot make any contribution to the mammillary layer.
Chemical Composition in Eggshell
Organic matrix proteins in eggshell were extracted and determined according to the method of Panheleux et al. (2000). The eggshell contents of calcium, phosphorus, and magnesium, copper, manganese, and zinc were dissolved and determined as previously described (Zhang et al., 2017). The contents of calcium, phosphorus, magnesium, copper, manganese, and zinc were analyzed using flame atomic absorption spectrophotometry (Zeenit700P, Analytik Jena, Jena, Germany), and the content of phosphorus was measured by ammonium phosphomolybdate colorimetric method using a spectrophotometer (UV-2700, Shimadzu, Kyoto, Japan).
Gene Expression of Transporters in Eggshell Gland
The RNA samples were reverse transcribed with the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction to prepare cDNA. The mRNA expression of target genes was examined by qRT-PCR using CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA) with a 20 μL PCR reaction mixture (primer concentration: 0.3 μM) according to instructions of the iTaq Universal SYBER Green Supermix (TaKaRa, Tokyo, Japan). Primers used in this study are shown in Table 1. The relative mRNA expression levels were normalized to avian β-actin by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
The genes information of iron transporter and matrix proteins in uterus.
| Genes | Primer sequence (5’-3’) | Annealing temperature (°C) |
|---|---|---|
| β-actin | Forward: AATGGCTCCGGTATGTGCAA Reverse: GGCCCATACCAACCATCACA |
60 |
| CALB1 | Forward: TAATGCTCAGGATGTTTGAT Reverse: ATGCTTTTCTTGTATGTCGC |
60 |
| ATP2A2 | Forward: CTATTGGATGTTATGTTGGT Reverse: ATGACAGGTAGTGAGATTTT |
60 |
| CA2 | Forward: AAACTTTGACCCTACTGGAC Reverse: CTCTGACTTCTCTGCTCTTC |
60 |
| SLC4A4 | Forward: CAGACCAAGAAGTCCAACCT Reverse: GTCAGAGCACCCAACATAAC |
60 |
| SLC4A5 | Forward: AGCACCAAACACCACTAACG Reverse: ATCCACACCACAAAACAGCA |
60 |
| SLC4A7 | Forward: TGGTTGGTGTTATGTTGGGT Reverse: CAGGTCAGGTTGATGTTTGG |
60 |
| SLC26A9 | Forward: GGCGTGGGATTTTCCGTGTT Reverse: GCAGCTTCGTTGGTGGTTGC |
60 |
| ATP6V1B2 | Forward: CCAACCATTGAACGCATTAT Reverse: GTGCAGCTGTCTGTCCACAT |
60 |
| ATP6V1C2 | Forward: CTCTGAGTACCTCATCACGC Reverse: GCTCTTCCTTTTCACATTTC |
60 |
| SPP1 (OPN) | Forward: CAGTTCTTTGCTTATGCCTTATCAG Reverse: GCCAGGTCATTCTGCGGGT |
60 |
| OC-116 | Forward: GAGGAACCAGACGCAGATAAAGAAG Reverse: GTTTTCAGGCTTGGGGCTGTA |
60 |
CALB1 (Calbindin D28K): Ca2+ intracellular transporter; ATP2A2 (Endoplasmic reticulum calcium ATPase 2): Ca2+ ATPase; CA2 (Carbonic anhydrase 2): catalyze HCO3− formation; SLC4A4 (Solute carrier family 4 member 4): Na + /HCO3− cotransporter; SLC4A5 (Solute carrier family 4 member 5): Na + /HCO3− cotransporter; SLC4A7 (Solute carrier family 4 member 7): Na + /HCO3− cotransporter; SLC26A9 ((Solute carrier family 26 member 9): HCO3− /Cl − exchanger; ATP6V1B2 (Vacuolar H ATPase B subunit osteoclast isozyme): H+ pump; ATP6V1C2 (Vacuolar H ATPase B subunit osteoclast isozyme): H+ pump; SPP1(OPN, Osteopontin): mineralization inhibitor; OC-116 (Ovocleidin 116): core protein of dermatan sulfate proteoglycan.
Statistical Analysis
Replicate (each replicate contained 10 eggs) served as the experimental unit for analysis of the whole eggshell mechanical property, ultrastructure, and chemical composition quality data; the average of the 1 sampled duck in each replicate was the experimental unit for other variables during eggshell formation. Unpaired t-tests (2 tailed) were used to analyze the significant differences between groups using SPSS (version 16.0 for Windows; SPSS Inc., Chicago, IL). Data are presented as means with standard deviations (SD) and statistical significance was defined as a P value < 0.05.
RESULTS
Age-Related Variation in Mechanical Properties and Chemical Composition of Eggshells
As shown in Table 2, the mechanical properties including the shell proportion, breaking strength, thickness without membrane, membrane thickness, and fracture toughness, decreased in aged ducks compared to the younger ducks (P < 0.05). The contents in eggshell of matrix proteins, calcium, phosphorus, and magnesium decreased (P < 0.05), but copper, manganese, and zinc contents were unchanged (P > 0.05) in aged laying ducks.
Table 2.
Mechanical properties and chemical composition of eggshells in young (36 wk of age) and aged (70 wk of age) ducks.
| Variables1 | Young duck | Aged duck | P-value |
|---|---|---|---|
| Mechanical properties | |||
| Egg weight (g) | 66.6 ± 0.79b | 71.5 ± 2.0a | <0.001 |
| Shell weight (g) | 6.11 ± 0.125 | 6.26 ± 0.217 | 0.125 |
| Shell proportion (%) | 9.19 ± 0.152a | 8.75 ± 0.205b | <0.001 |
| Breaking strength (N) | 40.1 ± 1.98a | 37.3 ± 1.41b | 0.006 |
| Shell thickness (without membrane, mm) | 0.354 ± 0.012a | 0.322 ± 0.007b | <0.001 |
| Membrane thickness (mm) | 0.105 ± 0.003a | 0.049 ± 0.003b | <0.001 |
| Fracture toughness (N/mm3/2) | 512 ± 34.0a | 460 ± 20.9b | 0.003 |
| Chemical composition | |||
| Matrix proteins (mg/g) | 9.94 ± 2.14a | 3.85 ± 1.01b | <0.001 |
| Calcium (%) | 37.2 ± 0.62a | 36.2 ± 0.24b | 0.004 |
| Phosphorus (%) | 0.17 ± 0.012a | 0.15 ± 0.019b | 0.047 |
| Magnesium (%) | 0.26 ± 0.009a | 0.23 ± 0.015b | 0.002 |
| Copper (μg/g) | 13.8 ± 1.32 | 13.1 ± 1.77 | 0.456 |
| Manganese (μg/g) | 0.72 ± 0.08 | 0.65 ± 0.05 | 0.088 |
| Zinc (μg/g) | 1.69 ± 0.418 | 1.45 ± 0.416 | 0.340 |
Values within a row with no common superscripts differ significantly (P < 0.05)
Mean of 6 replicates (10 eggs per replicate) per treatment.
Age-Related Ultrastructural Variation in Eggshell and Mammillary Layer
The comparison of ultrastructural variations in eggshell and mammillary layer between young and aged laying ducks is shown in Table 3. The mammillary knob width increased, the total and effective thickness, and its proportion in contrast to total thickness decreased in eggs from aged ducks (P < 0.05). The mammillary thickness was not affected but its proportion increased (P < 0.05). The scanning electron micrographs of mammillary knobs in young (left) and aged (right) ducks are shown in Figure 1. The regulation values of early fusion, cuffing, caps, and total score of mammillary knobs were higher in aged ducks relative to the young ducks (P < 0.05).
Table 3.
Ultrastructural variables in eggshell and mammillary layer in young (36 wk of age) and aged (70 wk of age) ducks.
| Variables1 | Young duck | Aged duck | P-value |
|---|---|---|---|
| Eggshell ultrastructure | |||
| Mammillary knob width (μm) | 96.6 ± 5.30b | 109 ± 7.88a | 0.008 |
| Mammillary thickness (μm) | 109 ± 8.43 | 109 ± 8.21 | 0.916 |
| Effective thickness (μm) | 234 ± 5.64a | 205 ± 14.2b | 0.001 |
| Total thickness (μm) | 344 ± 6.60a | 313 ± 19.5b | 0.011 |
| Mammillary layer (%) | 31.8 ± 2.05b | 34.7 ± 1.73a | 0.024 |
| Effective layer (%) | 68.2 ± 2.05a | 65.3 ± 1.73b | 0.024 |
| Ultrastructure of mammillary layer | |||
| Mammillae density | 224.18 ± 5.98 | 221.47 ± 2.31 | 0.679 |
| Confluence | 3.68 ± 0.10 | 3.74 ± 0.07 | 0.617 |
| Type B | 1.25 ± 0.19 | 1.28 ± 0.28 | 0.782 |
| Type A | 1.25 ± 0.07 | 1.23 ± 0.08 | 0.854 |
| Aragonite | 1.07 ± 0.05 | 1.21 ± 0.09 | 0.150 |
| Early fusion | 1.44 ± 0.66b | 2.38 ± 0.14a | < 0.001 |
| Late fusion | 2.83 ± 0.16 | 2.59 ± 0.16 | 0.296 |
| Cuffing | 3.19 ± 0.17b | 3.92 ± 0.27a | 0.038 |
| Pitted | 1.28 ± 0.41 | 1.54 ± 0.45 | 0.242 |
| Caps | 1.86 ± 0.12b | 2.36 ± 0.44a | 0.022 |
| Total score | 17.86 ± 0.29b | 20.27 ± 0.28a | < 0.001 |
Values within a row with no common superscripts differ significantly (P < 0.05)
Mean of 6 replicates (10 eggs per replicate) per treatment.
Figure 1.
Scanning electron micrographs showing a mammillary view of eggshell in young (left) and aged (right) ducks. A: Early fusion (magnification, 100 ×); B: Late fusion (100 ×); C: Isolated confluence (160 ×); D: No confluence and Type B (160 ×); E: Moderate cuffing (160 ×); F: Isolated cuffing (160 ×); G: Good caps (a) and Type A (b) (750 ×); H: Poor caps (750 ×); I: Isolated Cubic aragonite (2000 ×); J: Isolated clusters of aragonites (2000 ×).
Age-Related Variation in Mechanical Properties of Calcified Shell During Formation
During eggshell deposition stages, the shell thickness was decreased (P < 0.05) in aged ducks at 9.5, 15.5 and 19.5 h PO relative to young ducks (Table 4). The shell breaking strength and weight of the calcified shells were lower (P < 0.05), but the egg weight was higher (P < 0.05) in aged ducks at 19.5 h PO relative to those of young birds.
Table 4.
Mechanical properties in calcified shell during formation in young (36 wk of age) and aged (70 wk of age) ducks.
| Time postoviposition | Eggshell calcification phases | Calcified shell properties1 | Young duck | Aged duck | P-value |
|---|---|---|---|---|---|
| 9.5 h | Initial | Shell thickness (mm) | 0.132 ± 0.017a | 0.103 ± 0.019b | 0.030 |
| shell weight (g) | 0.775 ± 0.254 | 0.482 ± 0.185 | 0.060 | ||
| 15.5 h | Growth | Shell thickness (mm) | 0.158 ± 0.012a | 0.140 ± 0.012b | 0.030 |
| shell weight (g) | 2.768 ± 0.339 | 2.594 ± 0.392 | 0.430 | ||
| 20.5 h | Terminal | Shell breaking strength (N) | 16.3 ± 3.66a | 8.18 ± 1.63b | 0.003 |
| Shell thickness (mm) | 0.192 ± 0.022a | 0.156 ± 0.007b | 0.004 | ||
| Shell weight (g) | 4.97 ± 0.39a | 4.34 ± 0.22b | 0.040 | ||
| Egg weight (g) | 58.5 ± 2.46b | 63.4 ± 2.47a | 0.017 |
Values within a row with no common superscripts differ significantly (P < 0.05)
Mean of 6 replicates (1 egg per replicate) per treatment.
Age-Related Ultrastructural Variations in Calcified Shell During Formation
There was a significant decrease (P < 0.05) in the mammillary knob density in aged ducks at 9.5 h PO relative to young ducks (Table 5). At 15.5 h PO, the effective thickness of calcified eggshells decreased (P < 0.05), and the width of the knobs in the mammillary layer tended to increase (P = 0.076) in aged ducks compared with those of young ducks, but knob thickness did not change (P > 0.05) with duck age. At 20.5 h PO, the effective thickness of the calcified eggshells decreased (P < 0.05), and its proportion with respect to the total thickness tended to decrease (P = 0.075) in aged ducks relative to young ducks. The width and thickness of mammillary knobs did not change with duck age (P > 0.05), however, the proportion of the mammillary thickness with respect to the total thickness tended to increase (P = 0.075) in aged ducks compared with those of young birds.
Table 5.
Ultrastructural variables in calcified shell during formation in young (36 wk of age) and aged (70 wk of age) ducks.
| Time postoviposition | Eggshell calcification phases | Calcified shell ultrastructure1 | Young duck | Aged duck | P-value |
|---|---|---|---|---|---|
| 9.5 h | Initial | Mammillary knob density (/mm2) | 588 ± 25.6a | 524 ± 34.6b | 0.004 |
| 15.5 h | Growth | Mammillary knob width (μm) | 82.9 ± 7.51 | 91.3 ± 5.39 | 0.076 |
| Mammillary thickness (μm) | 99.4 ± 4.80 | 102 ± 9.24 | 0.636 | ||
| Effective thickness (μm) | 89.2 ± 7.07a | 59.3 ± 3.34b | <0.001 | ||
| 20.5 h | Terminal | Mammillary knob width (μm) | 87.3 ± 3.64 | 83.0 ± 4.90 | 0.118 |
| Mammillary thickness (μm) | 94.2 ± 11.4 | 91.8 ± 14.5 | 0.757 | ||
| Effective thickness (μm) | 215 ± 18.1b | 189 ± 16.4a | 0.026 | ||
| Total thickness (μm) | 309 ± 28.3 | 281 ± 29.4 | 0.120 | ||
| Mammillary layer (%) | 30.4 ± 1.45 | 32.6 ± 2.22 | 0.075 | ||
| Effective layer (%) | 69.6 ± 1.45 | 67.4 ± 2.22 | 0.075 |
Values within a row with no common superscripts differ significantly (P < 0.05)
Mean of 6 replicates (1 egg per replicate) per treatment.
Age-Related Variation in Serum Indices During Formation
The serum calcium content was lower in aged ducks in the initial (7.04 vs. 7.81, P = 0.085) and terminal (6.17 vs. 7.10, P < 0.05) deposition stages relative to those of young birds (Table 6). A significant decrease (5.17 vs. 7.57, P < 0.05) in the serum phosphorus content in aged ducks relative to young ducks was only observed at the linear deposition stage. The serum estradiol content decreased in aged ducks compared with young ducks in the growth (28.7 vs. 35.4, P = 0.087) and terminal (55.1 vs. 43.8, P < 0.05) deposition stages.
Table 6.
Calcium-related indices in serum during shell formation in young (36 wk of age) and aged (70 wk of age) ducks.
| Time postoviposition | Eggshell calcification phases | Serum calcium-related indices1 | Young duck | Aged duck | P-value |
|---|---|---|---|---|---|
| 9.5 h | Initial | Calcium (mmol/mL) | 7.81 ± 0.036 | 7.04 ± 0.170 | 0.085 |
| Phosphorus (mmol/mL) | 5.90 ± 0.527 | 4.85 ± 0.639 | 0.233 | ||
| Estradiol (pg/mL) | 32.4 ± 2.63 | 32.2 ± 2.09 | 0.935 | ||
| 1,25-(OH)2VD3 (ng/mL) | 1.74 ± 0.17 | 1.85 ± 0.14 | 0.636 | ||
| 15.5 h | Growth | Calcium (mmol/mL) | 6.88 ± 0.271 | 6.48 ± 0.167 | 0.237 |
| Phosphorus (mmol/mL) | 7.57 ± 0.667a | 5.17 ± 0.719b | 0.035 | ||
| Estradiol (pg/mL) | 35.4 ± 3.34 | 28.7 ± 1.26 | 0.087 | ||
| 1,25-(OH)2VD3 (ng/mL) | 1.75 ± 0.14 | 1.49 ± 0.12 | 0.192 | ||
| 20.5 h | Terminal | Calcium (mmol/mL) | 7.10 ± 0.287a | 6.17± 0.245b | 0.034 |
| Phosphorus (mmol/mL) | 7.62 ± 0.597 | 7.46 ± 0.518 | 0.840 | ||
| Estradiol (pg/mL) | 55.1 ± 2.93a | 43.8 ± 2.42b | 0.014 | ||
| 1,25-(OH)2VD3 (ng/mL) | 2.40 ± 0.22 | 2.13 ± 0.15 | 0.339 |
Values within a row with no common superscripts differ significantly (P < 0.05)
Mean of 6 replicates (1 duck per replicate) per treatment.
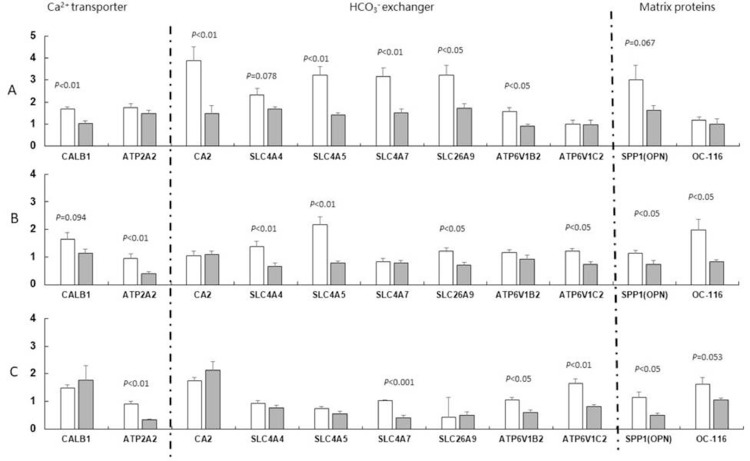
Age-Related Variation of Gene Expression in Eggshell Gland During Formation
Among the genes of Ca2+ transport in the shell gland, transcripts of CALB1 decreased in aged ducks in the initial (P < 0.01) and growth (P = 0.094) deposition stages, and ATP2A2 transcripts decreased in growth (P < 0.01) and terminal (P < 0.01) deposition stages in comparison to young ducks (Figure 2). For those of HCO3− exchange, expression of CA2 (initial, P < 0.01), SLC4A4 (initial, P = 0.078; growth, P < 0.01), SLC4A5 (initial and growth, P < 0.01), SLC4A7 (initial, P < 0.01; terminal, P < 0.001), SLC26A9 (initial and growth, P < 0.05), ATP6V1B2 (initial and terminal, P < 0.05), and ATP6V1C2 (linear, P < 0.05; terminal, P < 0.01) decreased in aged, relative to young, laying ducks during eggshell formation. The SPP1 matrix proteins decreased in initial (P = 0.067), growth (P < 0.05) and terminal (P < 0.05) deposition stages, and OC-116 transcripts decreased in the growth (P < 0.05) and terminal (P = 0.053) deposition stages.
Figure 2.
Relative gene expression of ion transporters and matrix proteins in the uterus in the initial (A), growth (B), and terminal (C) deposition stages. CALB1 (Calbindin D28K): Ca2+ intracellular transporter; ATP2A2 (Endoplasmic reticulum calcium ATPase 2): Ca2+ ATPase; CA2 (Carbonic anhydrase 2): catalyze HCO3− formation; SLC4A4 (Solute carrier family 4 member 4): Na + /HCO3−cotransporter; SLC4A5 (Solute carrier family 4 member 5): Na + /HCO3−cotransporter; SLC4A7 (Solute carrier family 4 member 7): Na + /HCO3−cotransporter; SLC26A9 (Solute carrier family 26 member 9): HCO3− /Cl − exchanger; ATP6V1B2 (Vacuolar H ATPase B subunit osteoclast isozyme): H+ pump; ATP6V1C2 (Vacuolar H ATPase B subunit osteoclast isozyme): H+ pump; SPP1(OPN, Osteopontin): mineralization inhibitor; OC-116 (Ovocleidin 116): core protein of dermatan sulfate proteoglycan.
Mean of 6 replicates (1 duck per replicate) per treatment.
DISCUSSION
Consistent with the findings in hens (Sirri et al., 2018; Fathi et al., 2019; Wistedt et al., 2019), the aged ducks studied here had increased egg weight, but decreased shell proportion, thickness, breaking strength, and fracture toughness. As reported, there was a highly significant positive correlation between the breaking force and either eggshell toughness or shell thickness, and regression analyses suggested that eggshell toughness was the best predictor for breaking force, followed by shell thickness (Fathi et al., 2019). These changes indicted that the physical and mechanical properties of eggshell were decreased in aged ducks, which mainly results from the deterioration of ultrastructure, as found previously (Park and Sohn, 2018; Feng et al., 2020) and here; the effective thickness decreased, but the thickness and width of mammillary knobs increased with aging. The current study showed that the scores of early fusions, cuffing, caps, and total score of mammillary knobs increased in aged laying ducks, which implied decreased frequency of early fusion and cups, and more structural deterioration of mammillary knobs in aged rather than young ducks. Bain (1990) reported that the increased frequency of early fusion and decreased incidence of cuffing and caps structure of the mammillary knobs indicated their structure was much tougher and could better resist external stress. Similarly, the mammillary thickness and knob width were significantly higher in weak-shelled eggs than hard-shelled eggs (Zhang et al., 2019). In addition, there was increased frequency of the type B mammillary knobs (Park and Sohn, 2018) and a reduction in the incidence of early fusion in the mammillary layer in aged compared to young hens (Feng et al., 2020). Induced molt could improve the porosity and the mammillae density, increase the frequency of confluent and cuffing mammillae, thus improve shell ultrastructure and quality in late-phase laying hens (Gongruttananun, 2018). Above all, the changes of shell ultrastructure, especially in the mammillary knobs, accounted for most of the decreased mechanical and physical properties of eggshell in aged laying ducks. In this respect, intervening the shell structure in its formation particularly the mammillary layer could be the effective way to improve shell quality in aged laying ducks.
In addition, the mechanical properties and ultrastructure of calcified shells were dynamically changed during eggshell formation, and these variations were in consistent with the whole eggshells in the present study. For example, the thickness of calcified shells in the initial, growth, and terminal deposition stages decreased in aged laying ducks, mammillary knob density decreased in the initial stage, and effective thickness increased in the growth and terminal stages. These changes indicated that age-related changes in shell mechanical properties and ultrastructure in laying ducks were apparent throughout the stages of shell formation, and possibly stemmed from changes in capacity for calcium transport, and organic matrix synthesis and secretion in the eggshell gland in aged laying ducks. This speculation was supported by the current findings of decreased contents of matrix proteins, calcium and phosphorus in the whole eggshell, and decreased serum phosphorous content, and decreased serum contents of calcium and estradiol in the growth and terminal stages, respectively. Eggshell biomineralization is regulated by calcium homeostasis in hens, in turn affected by vitamin D metabolites and sex steroid hormones (Bar, 2008; Nys, 2018). Furthermore, eggshell calcium content significantly decreased with increasing hen age (Park and Sohn, 2018). Age-related impairment of the mammillary layer in the initial stage of shell formation in hens in the late phase of production resulted mainly from the altered gene expression of matrix proteins along with the compromised immune function in the uterus (Feng et al., 2020). In addition, the current study found decreased gene expression in the uterus of transporters related with Ca2+ and especially HCO3− transfer and matrix proteins in aged relative to young ducks. Duan et al (2015) had reported that the polymorphisms in ion transport genes were associated with mechanical characteristics of eggshell, and the sodium channel gene family (SCNN) played an essential role in cation transportation and was significantly associated with eggshell traits and influenced eggshell quality (Fan et al., 2013). Gene expression of SCNN transporters did not change with aging, but CALB1 and SPP1 were decreased here. Similarly, dietary supplementation with sodium bicarbonate increased duodenal expression of CALB1 and improved calcium absorption and eggshell quality of laying hens during peak production (Jiang et al., 2015). The expression of CALB1 and SPP1 decreased, and the thickness of the palisade layer decreased and the eggshell surface became rougher (Zhu et al., 2020). The OC-116 and SPP1 were also expressed lower values in low strength shells than normal strength shells (Zhang et al., 2015). Furthermore, there were significant associations between gene polymorphism and eggshell quality and indicated the influence of organic matrix proteins on eggshell fabric: OC-116 was associated with shell thickness and elastic modulus, SPP1 with fracture toughness (Dunn et al., 2009). Thus, the decreased expression of OC-116 and SPP1 in uterus partly accounted for the decrease in shell thickness and fracture toughness in aged ducks. During the 3 stages of deposition, expression of different genes was greatest in the initial period of calcification, corresponding to the activation of shell calcification. It was suggested that the initiation period of calcification rather than the growth or termination periods determined eggshell strength (Zhang et al., 2019). Overall, in aged ducks, impaired shell ultrastructure, especially the mammillary layer is due to changes in gene expression of ion transporters and matrix proteins in the uterus. On the other hand, the large and nonuniform mammillary knobs in possibly due to damaged endometrial tissue, as noted in aged laying hens (Park and Sohn, 2018). Thus, it is achievable to ameliorate reduced shell quality with aging by modulating calcium metabolism and matrix proteins in laying ducks.
As inferred from previous studies (Nys, 2018), serum 1,25-(OH)2D3 and estradiol influences calcium metabolism in hens and 1,25-(OH)2D3 affects the ultrastructure of the mammillary layer by regulating the expression of uterine genes; it was significantly higher in the initiation period of hard-shelled eggs than weak-shelled eggs (Zhang et al., 2019). For this reason, serum estradiol and 1,25-(OH)2D3 was determined during eggshell formation stages in the ducks examined here. Serum estradiol content decreased in aged ducks in the terminal deposition stages, as was the serum content of calcium. In addition, the contents of matrix proteins, calcium, phosphorus and magnesium in eggshells all decreased with aging of laying ducks, which implied roles for these factors underlying the reduced shell quality in the late phase of production. In contrast, calcium, phosphorus and matrix protein in eggshell were not changed between young and aged laying hens (Feng et al., 2020), indicating a greater influence of these factors in duck eggshells than in chicken eggshells.
The present study with young and aged laying ducks suggested that decreased incidence of early fusion and caps, increased thickness and width of mammillary knobs, and decreased effective thickness are the crucial variations leading to the compromised physical and mechanical properties of eggshells in the late laying period. A disturbed regulation of calcium metabolism and expression of ion transporters, especially for those for HCO3− exchange in uterus of aged ducks likely contribute to age-related ultrastructural deterioration of the eggshell.
ACKNOWLEDGMENTS
We sincerely thank W. Bruce Currie from Cornell University for his help in the presentation of this manuscript. This study was supported by the National Natural Science Foundation of China (3180131540), Natural Science Foundation of Guangdong Province (2019A1515012231), China Agricultural Research System of MOF and MARA (CARS-42-K13), National Key Research and Development program (2018YFD0501504, 2018YFE0128200), Key Project of the Science and Technology program of Guangzhou City (201904020001), Science and Technology program of Guangdong Province (2019A050505007), Modern Agricultural Industry Technology System Innovation Team of Guangdong Province (2019KJ137), Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (201601TD, 202106TD, R2017YJ-YB3005, R2018QD-073).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101573.
Appendix. Supplementary materials
REFERENCES
- Athanasiadou D., Jiang W., Goldbaum D., Saleem A., Basu K., Pacella M.S., Böhm C.F., Chromik R.R., Hincke M.T., Rodríguez-Navarro A.B., Vali H., Wolf S.E., Gray J.J., Bui K.H., McKee M.D. Nanostructure, osteopontin, and mechanical properties of calcitic avian eggshell. Sci. Adv. 2018;4:eaar3219. doi: 10.1126/sciadv.aar3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain M.M. Univ. Scotland; Glasgow: 1990. Eggshell Strength: A Mechanical/Ultrastructural Evaluation. PhD Diss. [Google Scholar]
- Bar A. Calcium homeostasis and vitamin D metabolism and expression in strongly calcifying laying birds. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008;151:477–490. doi: 10.1016/j.cbpa.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2009;152:447–469. doi: 10.1016/j.cbpa.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Curl J.S., Thayer R., Wettemann R.P., Morrison R. Preovulatory concentrations of progesterone and estradiol in plasma and their relationships with eggshell quality in the laying hen. Poult. Sci. 1985;64:2383–2387. doi: 10.3382/ps.0642383. [DOI] [PubMed] [Google Scholar]
- Dominguez-Vera J.M., Gautron J., Garcia-Ruiz J.M., Nys Y. The effect of avian uterine fluid on the growth behavior of calcite crystals. Poult. Sci. 2000;79:901–907. doi: 10.1093/ps/79.6.901. [DOI] [PubMed] [Google Scholar]
- Duan Z., Chen S., Sun C., Shi F., Wu G., Liu A., Xu G., Yang N. Polymorphisms in ion transport genes are associated with eggshell mechanical property. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn I.C., Rodríguez-Navarro A.B., Mcdade K., Schmutz M., Preisinger R., Waddington D., Wilson P.W., Bain M. Genetic variation in eggshell crystal size and orientation is large and these traits are correlated with shell thickness and are associated with eggshell matrix protein markers. Anim. Genet. 2012;43:410–418. doi: 10.1111/j.1365-2052.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- Dunn I.C., Joseph N.T., Bain M., Edmond A., Wilson P.W., Milona P., Nys Y., Gautron J., Schmutz M., Preisinger R., Waddington D. Polymorphisms in eggshell organic matrix genes are associated with eggshell quality measurements in pedigree Rhode Island Red hens. Anim. Genet. 2009;40:110–114. doi: 10.1111/j.1365-2052.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- Fan Y.F., Hou Z.C., Yi G.Q., Xu G.Y., Yang N. The sodium channel gene family is specifically expressed in hen uterus and associated with eggshell quality traits. BMC Genet. 2013;14:90. doi: 10.1186/1471-2156-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi M.M., Galal A., Ali U.M., Abou-Emera O.K. Physical and mechanical properties of eggshell as affected by chicken breed and flock age. Br. Poult. Sci. 2019;60:506–512. doi: 10.1080/00071668.2019.1621992. [DOI] [PubMed] [Google Scholar]
- Feng J., Zhang H.J., Wu S.G., Qi G.H., Wang J. Uterine transcriptome analysis reveals mRNA expression changes associated with the ultrastructure differences of eggshell in young and aged laying hens. BMC Genomics. 2020;21:770. doi: 10.1186/s12864-020-07177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M.S., Moya A., Lopez L., Arias J.L. Secretion pattern, ultrastructural localization and function of extracellular matrix molecules involved in eggshell formation. Matrix Biol. 2001;19:793–803. doi: 10.1016/s0945-053x(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Gongruttananun N. Induced molt using cassava meal. 2. Effects on eggshell quality, ultrastructure, and pore density in late-phase laying hens. Poult. Sci. 2018;97:1050–1058. doi: 10.3382/ps/pex365. [DOI] [PubMed] [Google Scholar]
- Heaney R.K., Robinson D.S. The isolation and characterization of hyaluronic acid in egg shell. Biochim. Biophys. Acta. 1976;451:133–142. doi: 10.1016/0304-4165(76)90265-8. [DOI] [PubMed] [Google Scholar]
- Jiang M.J., Zhao J.P., Jiao H.C., Wang X.J., Zhang Q., Lin H. Dietary supplementation with sodium bicarbonate improves calcium absorption and eggshell quality of laying hens during peak production. Br. Poult. Sci. 2015;56:740–747. doi: 10.1080/00071668.2015.1113499. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nys Y. Calcium Homeostasis and Eggshell Biomineralization in Female Chicken. Elsevier. Inc.; Amsterdam: 2018. pp. 361–382. [Google Scholar]
- Panheleux M., Bain M., Fernandez M.S., Morales I., Gautron J., Arias J.L., Solomon S.E., Hincke M., Nys Y. Organic matrix composition and ultrastructure of eggshell: a comparative study. Br. Poult. Sci. 1999;40:240–252. doi: 10.1080/00071669987665. [DOI] [PubMed] [Google Scholar]
- Panheleux M., Nys Y., Willians J., Gautron J., Boldicke T., Hincke M.T. Extraction and quantification by ELISA of eggshell organic matrix proteins (ovocleidin-17, ovalbumin, ovotransferrin) in shell from young and old hens. Poult. Sci. 2000;79:580–588. doi: 10.1093/ps/79.4.580. [DOI] [PubMed] [Google Scholar]
- Park J.A., Sohn S.H. The influence of hen aging on eggshell ultrastructure and shell mineral components. Korean J. Food. Sci. Anim. Resour. 2018;38:1080–1091. doi: 10.5851/kosfa.2018.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan L.M. Eggshell quality: a comparison between Fayoumi, Gimieizah and Brown Hy-Line strains for mechanical properties and ultrastructure of their eggshells. Anim. Prod. Sci. 2016;56:908–912. [Google Scholar]
- Rodríguez-Navarro A., Kalin O., Nys Y., García-Ruiz J.M. Influence of the microstructure on the shell strength of eggs laid by hens of different ages. Br. Poult. Sci. 2002;43:395–403. doi: 10.1080/00071660120103675. [DOI] [PubMed] [Google Scholar]
- Samiullah ., Roberts J.R., Chousalkar K.K. Effect of production system and flock age on egg quality and total bacterial load in commercial laying hens. PSA. 2014;23:59–70. [Google Scholar]
- Sirri F., Zampiga M., Berardinelli A., Meluzzi A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018;97:1818–1823. doi: 10.3382/ps/pex456. [DOI] [PubMed] [Google Scholar]
- Solomon S.E. The eggshell: strength, structure and function. Br. Poult. Sci. 2010;51(Suppl 1):52–59. doi: 10.1080/00071668.2010.497296. [DOI] [PubMed] [Google Scholar]
- Travel A., Nys Y., Bain M.M. In: Pages 300-329 in Improving the Safety and Quality of Eggs and Egg Products. Nys Y., Bain M., Van I.F., editors. Woodhead Publishing Limited; Cambridge, Cambridgeshire: 2011. Effect of hen age, moult, laying environment and egg storage on egg quality. [Google Scholar]
- Wistedt A., Ridderstråle Y., Wall H., Holm L. Age-related changes in the shell gland and duodenum in relation to shell quality and bone strength in commercial laying hen hybrids. Acta. Vet. Scand. 2019;61:14. doi: 10.1186/s13028-019-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang Y., Zhang C., Xiong M., Rajput S.A., Liu Y., Qi D. The differences of gonadal hormones and uterine transcriptome during shell calcification of hens laying hard or weak-shelled eggs. BMC Genomics. 2019;20:707. doi: 10.1186/s12864-019-6017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhu F., Liu L., Zheng C.W., Wang D.H., Hou Z.C., Ning Z.H. Integrating transcriptome and genome resequencing data to identify key genes and mutations affecting chicken eggshell qualities. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.N., Wang L., Zhang H.J., Wu S.G., Qi G.H. Effect of dietary supplementation of organic or inorganic manganese on eggshell quality, ultrastructure, and components in laying hens. Poult. Sci. 2017;96:2184–2193. doi: 10.3382/ps/pew495. [DOI] [PubMed] [Google Scholar]
- Zhu M., Li H., Miao L., Li L., Dong X., Zou X. Dietary cadmium chloride impairs shell biomineralization by disrupting the metabolism of the eggshell gland in laying hens. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa025. skaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.