Abstract
Oxytocin and vasopressin systems have been studied separately in autism spectrum disorder (ASD). Here, we provide evidence from an evolutionary and neuroscience perspective about the shared mechanisms and the common roles in regulating social behaviors. We first discuss findings on the evolutionary history of oxytocin and vasopressin ligands and receptors that highlight their common origin and clarify the evolutionary background of the crosstalk between them. Second, we conducted a comprehensive review of the increasing evidence for the role of both neuropeptides in regulating social behaviors. Third, we reviewed the growing evidence on the associations between the oxytocin/vasopressin systems and ASD, which includes oxytocin and vasopressin dysfunction in animal models of autism and in human patients, and the impact of treatments targeting the oxytocin or the vasopressin systems in children and in adults. Here, we highlight the potential of targeting the oxytocin/vasopressin systems to improve social deficits observed in ASD and the need for further investigations on how to transfer these research innovations into clinical applications.
INTRODUCTION: OXYTOCIN AND VASOPRESSIN SYNTHESIS, GENE STRUCTURE, AND FUNCTION
Oxytocin (OT) and vasopressin (VP) are neuropeptides produced mainly in the supraoptic and the paraventricular nucleus (PVN) of the hypothalamus (Lucassen et al., 1997). They are released in the capillaries of the posterior pituitary and then distributed peripherally, acting as hormones, or to other brain regions, onto neurons containing their receptors, acting as neurotransmitters/neuromodulators (see Bakos et al., 2018 and references therein). Central release of OT and VP can occur through dendritic and axonal release. The dendritic release (Ludwig and Leng, 2006) is notably important to induce a positive feedback mechanism. The axonal release was discovered 40 years ago when OT and VP synapses were observed in the limbic regions of the rat brain (Buijs and Swaab, 1979).
The recent use of an optogenetic technique to induce local activation of oxytocin or vasopressin fibers (Knobloch et al., 2012; Smith et al., 2016; Hung et al., 2017) supports the idea that these peptides can be locally released in the brain. Furthermore, retro dialysis studies performed mainly in rats demonstrated that oxytocin and vasopressin can be released locally in response to social stimuli (Veenema and Neumann, 2008): for example, oxytocin is released in the PVN but not the amygdala or the lateral septum (LS) of lactating females defending their nest (Bosch et al., 2004).
Both the oxytocin and vasopressin genes are comprised by three exons that give rise to a prepropeptide (Fig. 9.1). For oxytocin, the first exon encodes the signal peptide, the oxytocin hormone, the tripeptide processing signal (GKR), and the NH2-terminal residues of neurophysin I; the second exon encodes the central part of neurophysin I; and the third exon encodes the COOH-terminal region of neurophysin I. For vasopressin, the three exons also give rise to a prepropeptide: the first exon encodes a signal peptide, the vasopressin hormone, and the NH2-terminal region of neurophysin II; the second exon encodes the central region of neurophysin II; and the third exon encodes the COOH-terminal region of neurophysin II and the glycopeptide copeptin (Melmed, 2011). The products of these processes, OT and neurophysin I, on the one hand, and VP, copeptin, and neurophysin II, on the other, are packaged in granules for axonal transport to the posterior pituitary (Brownstein et al., 1980), until their release is elicited (Renaud and Bourquet, 1991).
Fig. 9.1.
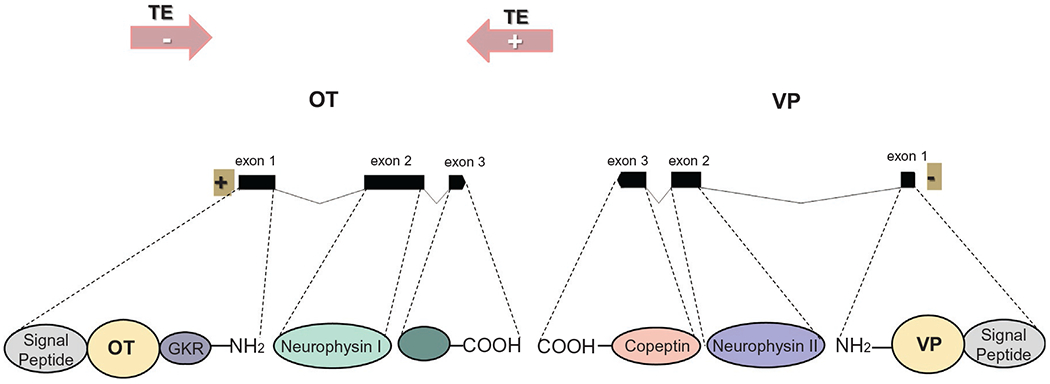
Oxytocin (OT) and vasopressin (VP) gene structure and synthesis. Local chromosomal organization of the OT-VP region. Representation of OT and VP genes (exons + introns) in human chromosome 12, DNA transposable elements (TE; pink arrows), and orientation (+, −). Each exon links with dashed lines to the gene products it encodes. GKR, glycine–lysine–arginine. Gene length scale: 100 bases.
OT and VP are involved in an array of functions that go beyond their traditional implication in uterine contractions (Magalhaes et al., 2009) and antidiuresis (Walter et al., 1967), respectively. Based on our literature review for their functions in mammals, both neuropeptides are involved in grooming (Marroni et al., 2007; Nephew and Bridges, 2008), maternal behavior (Arthur et al., 2008; Leng et al., 2008; Nephew and Bridges, 2008), blood pressure regulation (Petersson et al., 1996; Pavan de Arruda Camargo et al., 2008), social behavior (Heinrichs and Domes, 2008; Lukas et al., 2011), and memory (Weingartner et al., 1981; Larrazolo-López et al., 2008), among other functions. Other prominent functions of OT in mammals are mating (Witt and Insel, 1994; Insel and Hulihan, 1995), sperm ejaculation (Filippi et al., 2003), lactation (Leng et al., 2008), heart development (Jankowski et al., 2004), ossification (Elabd et al., 2007), digestive system regulation (Wu et al., 2003), pain perception (Yang et al., 2007), estradiol response (Jirikowski et al., 1988), drinking and eating (Verty et al., 2004), and sensory perception (Marlin et al., 2015). VP is also involved in apoptosis regulation (Chen et al., 2008), locomotion (Schank, 2009), arterial, vasoconstriction regulation (Alonso et al., 2008), and thermoregulation (Richmond, 2003).
The diverse functions of OT and VP depend on the peripheral and brain synthesis sites, release sites, and the OT and VP receptors (OTR-VPRs) distribution. The OTR-VPRs are classical seven-transmembrane G protein-coupled receptors. When peptides bind to these receptors, they cause a series of signal transduction cascades with both excitatory or inhibitory actions on Ca2+ and other messengers and transcription of specific genes.
EVOLUTIONARY HISTORY OF OXYTOCIN AND VASOPRESSIN LIGANDS AND RECEPTORS
OT and VP are adjacent paralogous genes, meaning that they are located next to each other in most vertebrate genomes (except in teleost fish) (Theofanopoulou et al., 2021), and that they likely resulted from a local duplication in the stem of vertebrates. Gwee et al. (2009) first hypothesized that since only VP is found in invertebrates and in the first vertebrates (e.g., lampreys), and OT is found for the first time in fishes (e.g., elephant sharks), it was VP that gave rise to OT, and not the other way around. Theofanopoulou et al. (2021) found evidence for this hypothesis, in that they traced DNA TEs around the OT region, but not around the VP (Fig. 9.1). TEs are known to drive gene duplications through their terminal inverted repeats, which have been found to transpose through a cut-and-paste mechanism creating an extra copy at the donor site (Wicker et al., 2007). In other words, the hypothesized ancestral VP copied and pasted itself, creating OT, and leaving TEs around it as a “remnant” of this process.
Based on the common evolutionary origin of these genes, which is supported by extensive synteny and phylogenetic analyses’ data shown in Theofanopoulou et al. (2021), we second their proposal for a universal vertebrate nomenclature. So far, the use of different gene names for each of the ligands (and receptors) in different species or lineages has brought translation between experiments on different species at a standstill. All the following terms: oxytocin (mammals), mesotocin (birds, turtles, crocodiles, frogs, and some fish), isotocin (fish), glumitocin (fish), valitocin, and aspargtocin (fish) are being used to refer to the same gene that they propose we call “oxytocin” from now on; arginine vasopressin (mammals), lysine vasopressin (mammals), phenypressin (mammals), and vasotocin (VT) (birds, crocodiles, turtles, frogs, fish, sharks, lampreys, hagfishes) are being used to refer to the same gene that they propose we call “vasotocin” from now on. Naming them “vasotocin” and “oxytocin” portrays their evolutionary history, as is standard practice for other genes that are orthologous across species (e.g., FOXP1) and paralogous within species (e.g., FOXP2, FOXP3, FOXP4). According to this practice, these two peptides would be named vasopressin1 (AVP1) and vasopressin2 (AVP2), vasotocin1 (VT1) and vasotocin2 (VT2), or oxytocin1 (OT1) and oxytocin2 (OT2). Since this would be a far-reaching shift from the existing nomenclature, Theofanopoulou et al. (2021) propose that the common origin of these genes be portrayed through the shared ending name—tocin, and paralogy conveyed through different root names oxy- and vaso-. Vasotocin is a name already used by all nonmammalian scientific communities. Although we support this new nomenclature, we have not adopted it in this chapter to avoid confusion with the nomenclature used in the rest of the chapters. We followed a similar approach for the receptors.
Concerning their receptors, there are six major OTR-VPRs in vertebrates (OTR/OXTR/VT3/MesoR/ITR, VTR1A/AVPR1A/V1AR/VT4/VASR, VTR1B/AVPR1B/AVPR3/V3, VTR2A/VT1/V2C/V2BR2/AVPR2.2, VTR2B/V2B/V2BR1/OTRL/AVPR2, and VTR2C/AVPR2/V2A2/AVPR2AA; for their nomenclature, we list the names proposed in Theofanopoulou et al. (2021) followed by other traditional names). While in invertebrates, there is only one receptor type (VTR), the invertebrate species have more than one receptor that might have duplicated in a species-specific manner from the same receptor type (Theofanopoulou et al., 2021). Several scientists have hypothesized that the six receptors evolved through the traditional two rounds of whole-genome duplications (Ocampo Daza et al., 2012; Lagman et al., 2013), meaning that two initial ancestral receptors got duplicated twice, making eight receptors, with two of them having been fully lost from the vertebrate genomes. According to more recent hypotheses (Mayasich and Clarke, 2016; Theofanopoulou et al., 2021), the receptors evolved through one round of whole-genome duplication, followed by several segmental duplications, a scenario that is better parsimoniously explained in the context of vertebrate genome evolution (Smith and Keinath, 2015; Theofanopoulou et al., 2021).
These evolutionary findings have direct repercussions on our understanding of the OT-VP system, which thus far has been mostly studied as two separate systems that show crosstalk. Specifically, the finding that the OTR evolved millions of years before the OT ligand suggests that the ancestral VP may have originally acted through the OTR before OT evolved. This suggestion is supported by findings that in some species OT and VP bind to the OTR at similar efficiencies; a greater response of OTR to OT over VP is found for the first time in teleost fish (Yamaguchi et al., 2012). Studying the OT-VP system as one and the same system, like for example, the dopamine system or the serotonin system, can frame studies showing crosstalk and interaction between OT, VP, and OTR-VPRs differently, as well as inform the design of new experiments targeting this system in social disorders, like autism spectrum disorders (ASD), which we discuss further.
OXYTOCIN AND VASOPRESSIN LIGAND AND RECEPTOR DISTRIBUTION IN THE HUMAN AND MOUSE BRAIN
We searched in the Human Protein Atlas (Thul et al., 2017) (http://www.proteinatlas.org) for brain gene expression patterns of the OT and VP ligands and the OTR-VPRs present in humans (Homo sapiens) and mice (Mus musculus) (OTR, AVPR1A, AVPR1B, AVPR2). For human data, we used the GTEx and FANTOM5 datasets. For mouse data, we used the HPA mouse brain RNA-Seq dataset. Overall, gene expression was similar in the brain tissues of both human and mice brains. An observation of their profiling combined can shed light to these genes’ expression in the mammalian brain.
High OT and VP expression was found in both the human and mouse hypothalamus. Other regions such as the basal ganglia and the amygdala show instead much less expression. This points to an almost exclusive gene expression in the hypothalamus and to the great reliance of the OT-VP system to the synaptic transmission of these products through neurons expressing their receptors, at least in the mammalian brain (Fig. 9.2).
Fig. 9.2.
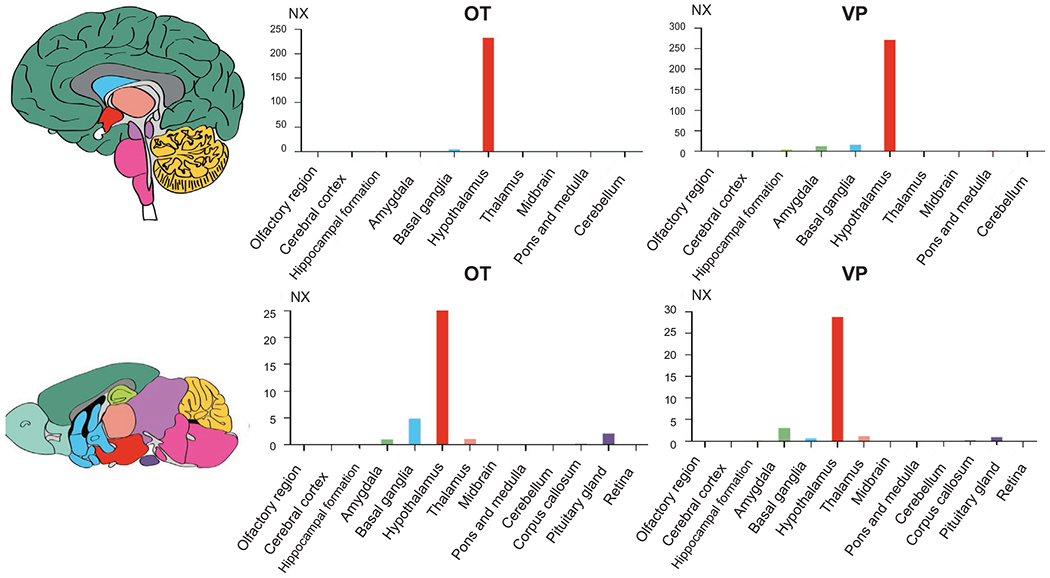
OT and VP gene expression in brain regions of the human (top) and mouse (bottom) brains. Color coding is based on brain region, and the bar shows the highest expression among the subregions included. Consensus normalized expression (NX) levels were created for the brain regions by combining the data from two transcriptomics datasets (GTEx and FANTOM5) in human and pTPM (protein-coding transcripts per million) of the individual HPA mouse dataset samples in mouse.
Although OTR has a lower region specificity, it shows high expression in several brain regions. In humans, OTR is highly expressed in the midbrain, the basal ganglia, and the hypothalamus. In mice, OTR is highly expressed in the amygdala, the basal ganglia, and the hippocampus (Figs. 9.3 and 9.4). OTR in the hippocampus has been shown to be involved in social recognition and social memory (Raam et al., 2017), something that may point to a species-specific enhancement of such functions.
Fig. 9.3.
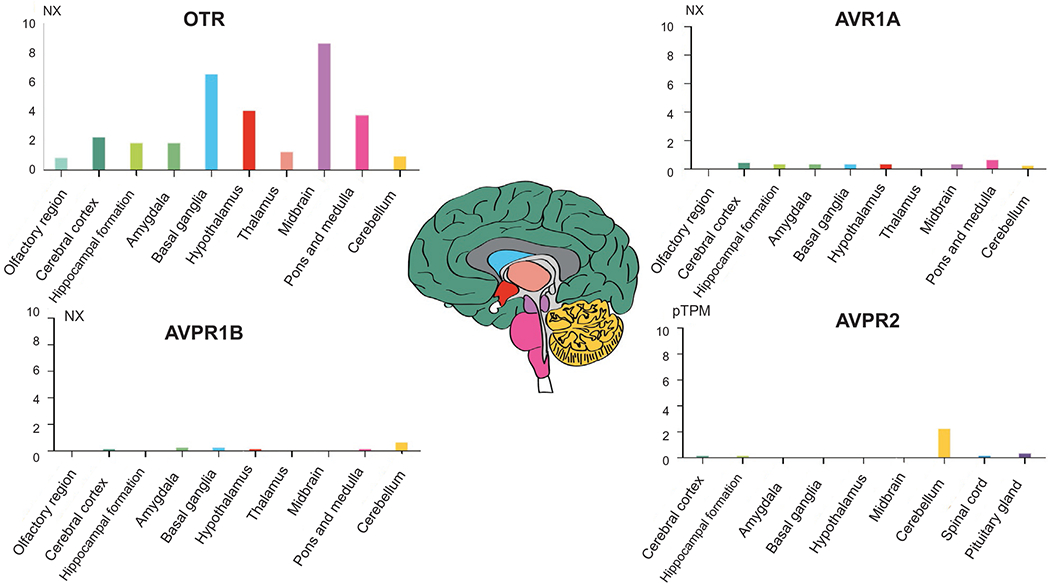
OTR, AVPR1A, AVPR1B, and AVPR2 gene expression in the brain regions of the human brain. Color coding is based on brain region and the bar shows the highest expression among the subregions included. Consensus normalized expression (NX) levels were created for the brain regions for OTR, AVPR1A, and AVPR1B expression by combining the data from two transcriptomics datasets (GTEx and FANTOM5); pTPM (protein-coding transcripts per million) levels, corresponding to mean values of the different individual samples for respective subregions generated by GTEx were created for AVPR2.
Fig. 9.4.
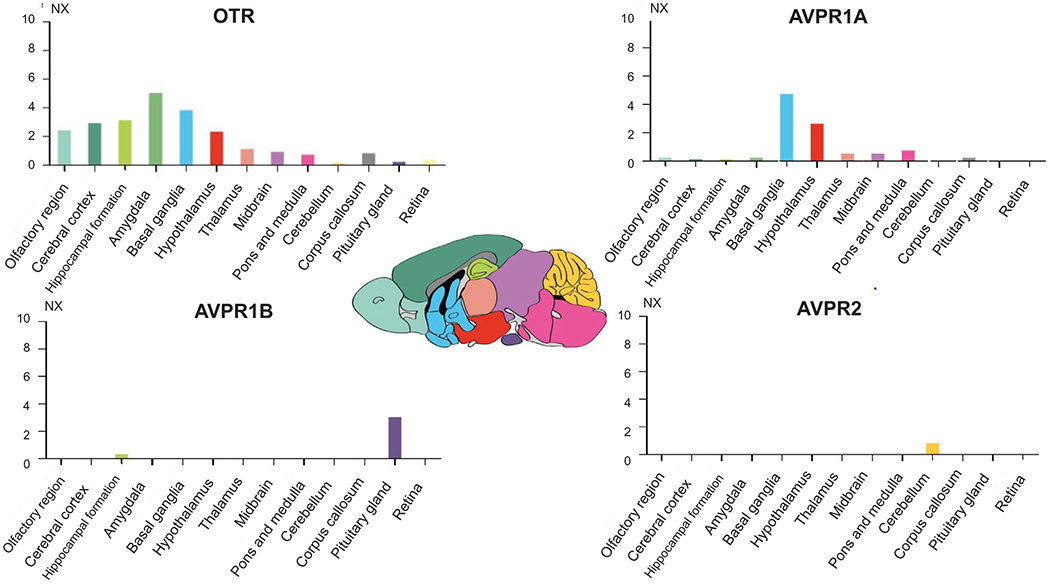
OTR, AVPR1A, AVPR1B, and AVPR2 gene expression in the brain regions of the mouse brain. Color coding is based on brain region and the bar shows the highest expression among the subregions included. Consensus normalized expression (NX) levels were created for the brain regions by pTPM (protein-coding transcripts per million) of the individual HPA Mouse dataset samples in mouse.
AVPR1A is overall less detected in the brain. It can be found in the mouse hypothalamus and basal ganglia. In humans and mice, it is expressed, although at low levels, in the amygdala, pons and medulla, and other areas (Figs. 9.3 and 9.4). AVPR1B is not enriched in any brain areas in humans. However, in humans, there are low expression levels in the cerebellum, and even lower in the amygdala, basal ganglia, and hypothalamus. In mice, AVPR1B expression is the highest in the pituitary gland of the hypothalamus with some expression in the hippocampus (Figs. 9.3 and 9.4). AVPR1B’s expression in the hypothalamus has been suggested to have specific functions in the regulation of stress, aggressive, and social behaviors, possibly through interaction with the adrenal axis (Stevenson and Caldwell, 2012).
The less studied AVPR2 is enriched in the cerebellum of both species. Its functional role is unknown. Some early study in rats (Kato et al., 1995) showed that AVPR2 is constitutively expressed in the granular layer of the cerebellum throughout brain development, concluding that since the granular layer sends output signals to the Purkinje cells, it is possible that AVPR2 could have an important role in regulating Purkinje cells (Figs. 9.2 and 9.3).
OXYTOCIN AND VASOPRESSIN REGULATE SOCIAL BEHAVIORS
Gene blockade and activation studies (knockout, pharmacological, chemogenetic, optogenetic)
To investigate the potential role of OT and VP in social behavior, scientists generated mice lacking OT, VP or lacking their receptors. More than 20 years ago, transgenic OT knockouts (KO) were shown to display social memory deficits that could be reversed by exogeneous administration of oxytocin (Ferguson et al., 2000). Interestingly, OTR-KO mice also present a wide range of social deficits with decreased ultrasonic vocalizations and mobility in pups, social discrimination deficits, and increased aggressivity in adults, as well as an alteration of maternal behavior (Takayanagi et al., 2005). The negative social impact of OTR deficiency is not limited to mice. OTR-KO prairie voles present both decreased interest for social novelty and exaggerated repeated behaviors (Horie et al., 2018), two symptoms that are reminiscent of the deficits observed in ASD. Interestingly, mice lacking only one copy of the OTR gene also display decreased sociability, indicating that even a partial decrease of OTR expression alters social behavior (Sala et al., 2011). Altogether, these results demonstrate that the activity of the OT system is necessary for social behavior.
One naturally occurring deficiency can be found in the Brattleboro strain of rats that have lost their ability to produce VP due to a genetic mutation. This strain has been extensively studied and has revealed social discrimination deficits, compared to other strains, which could be restored by intraseptal administration of VP (Engelmann and Landgraf, 1994). Similarly, mice lacking the AVPR1A present social discrimination deficits that are rescued by restoring AVPR1A expression in the LS using a viral strategy (Bielsky et al., 2005). Furthermore, mice lacking AVPR1B also show social discrimination deficits (Wersinger et al., 2002), suggesting that some aspects of social behaviors depend on the coordinated activity of different OTR-VPRs.
Other evidence for the role of OT and VP in regulating social behaviors come from pharmacological experiments. Pharmacological blockade of the AVPR1A in the LS impairs social discrimination, while the administration of exogenous VP extends social discrimination in rats (Veenema et al., 2012). The same manipulations also influence juvenile rat play behavior (Veenema et al., 2013) and pair bond formation in prairie voles (Liu et al., 2001). Studies also showed that OTR antagonist impairs social recognition in mice when administered in the medial amygdala (Ferguson et al., 2001) and impedes pair bond formation in socially monogamous species, such as zebra finches (Klatt and Goodson, 2013) and prairie voles (Liu and Wang, 2003). In the prairie vole, OTR activation is involved in mating-induced increase of correlated activity between the nodes of the social brain neural network (Johnson et al., 2016), as well as in consolation behavior (Burkett et al., 2016). Interestingly, OT has other central functions such as regulation of metabolism and feeding (reviewed in Spetter and Hallschmid, 2017). Recent studies suggest that OT impact on eating behavior may depend on its psychosocial function as social cues modulate the effect of oxytocin on feeding (Olszewski et al., 2016).
Similarly, studies taking advantage of chemogenomic techniques to manipulate the activity of endogeneous OT demonstrated that stimulating OT neurons is sufficient to promote social motivation and to reduce anxiety (Grund et al., 2019). It is also sufficient to socially transmit fear in mice (Pisansky et al., 2017). Inhibition of OT activity increased fear response in lactating mice (Menon et al., 2018). One recent study in mice used a chemogenetic strategy to specifically inhibit the activity of PVN OT neurons projecting to either the amygdala, the nucleus accumbens, or the prefrontal cortex and showed that only the inhibition of neurons projecting to the amygdala does impair emotion recognition (Ferretti et al., 2019). This shows that different OT neurons may project to different brain areas and thus regulate specific aspects of social behavior. It also suggests that the OT projections from PVN to amygdala are essential for emotional processes.
Other studies used optogenetic techniques to specifically manipulate the activity of endogenous OT or VP systems in specific brain areas that are part of the social or reward brain network. Knobloch et al. (2012) showed that optogenetic stimulation of oxytocinergic fibers in the amygdala attenuates fear response, while optogenetic stimulation of OT-PVN neurons increased social exploration and social memory in rats (Oettl et al., 2016). Optogenetic inhibition of OT fibers in the ventral tegmental area decreases sociability, while their stimulation gives rise to the opposite effect (Hung et al., 2017). Optogenetic stimulation of OT fibers in the auditory cortex of naïve virgin mice increased their pup retrieval behavior (Marlin et al., 2015), while stimulation of VP fibers in hippocampal CA2 enhanced social memory (Smith et al., 2016). Lastly, optogenetic inhibition of OT or VP fibers in the LS impairs social discrimination in mice (Borie et al., 2019). Interestingly in this study, the authors describe a population of neurons in the LS inhibited by OT only if previously exposed to VP. This reveals that these two neuropeptides can have a coordinated action and while most studies investigate one or the other, considering both OT and VP together would broaden our understanding of their function in the regulation of social behaviors (Borie et al., 2019).
Overall, these studies suggest that oxytocin and vasopressin play a key role in the regulation of social behavior. Their role is both necessary (blockade studies) and sufficient (activation studies) to regulate social behavior in general, while the localization and action of specific ligands and receptors in specific brain regions mediate different aspects of this umbrella term (social behavior) that includes behaviors like social recognition, social memory, social discrimination, among others.
Oxytocin/vasopressin concentration and administration studies
Several studies have measured OT-VP concentration, in an attempt to evaluate whether variation in the concentration correlates with variability in different social tasks. Although the methodology of concentration measurements in different sites (blood vs cerebrospinal fluid (CSF)) is still under debate (Lefevre et al., 2017), there is a general consensus that both OT and VP show a covariation with an array of social behaviors, like parenting (Apter-Levi et al., 2014) and interpersonal relationships (Gouin et al., 2012). Low oxytocin levels were also associated with introverted personalities and increased volume in the amygdala (Andari et al., 2014).
Beyond merely correlative results, studies have also investigated brain activity and behavioral changes induced by exogeneous administrations of OT and VP, mostly with an intranasal administration. Intranasal administration of these neuropeptides is the most efficient way of administration given that the spray penetrates the brain and does not affect peripheral organs the same way as peripheral injections would (Quintana et al., 2015). Several studies have shown that intranasal administration of OT does increase CSF concentration of OT in macaque (Dal Monte et al., 2014; Freeman et al., 2016), in oxytocin knockout mice (Smith et al., 2019), in rats and in mice (Neumann et al., 2013), and in humans (Born et al., 2002; Striepens et al., 2013), indicating that this route of administration is efficient in influencing central pathways. In addition, researchers have developed a sensitive and specific quantitative mass spectrometry assay that can distinguish a labeled OT from endogenous OT (Lee et al., 2018) and found that an intranasal labeled OT reaches the CSF and did not affect the endogenous release of OT.
A 24 International Units (IU) of OT-intranasal administration was found to increase the time of eye fixation (Guastella et al., 2008), suggesting an important involvement of OT in the social attention and emotion recognition mechanisms. Vasopressin plays a role in sociosexual cue recognition. Twenty IU of intranasal VP administration increased the speed at which men detected sexual words over other types of words (Guastella et al., 2011), while it also increased the memory for happy and angry but not neutral faces (Guastella et al., 2010b). An important series of results were obtained by studying the response to the “prisoner’s dilemma” and its neural correlates in after intranasal administration of OT (24UI) or VP (20UI). The prisoner’s dilemma is a standard example of a game where two individuals should testify either by betraying the other or remaining silent. First studies were performed in males and showed that both OT and VP administration increased the functional connectivity between the amygdala and the anterior insula, both being important components of the decision-making neural network. OT administration increases the response of the caudate nucleus in response to unreciprocated cooperation and, behaviorally, it induces an increased rate of cooperation following noncooperative moves (Rilling et al., 2012). In a replication study involving males and females, they found that while OT induces an increase of the activity of the caudate/putamen in males, it induces a decrease of such activity in females when both partners were cooperative. In the same conditions, the intranasal administration of VP increased activity in the insula in males, but decreased it in females (Feng et al., 2015). Further studies confirmed that the neural correlates of OT administration are sex dependent, with OT playing a critical role in the regulation of the activity of the social brain network in this social game, since it significantly changes its nodes’ functional connectivity (nucleus accumbens, amygdala, insula, septum, ventral tegmental area, orbitofrontal cortex) (Rilling et al., 2018).
Epigenetic studies
Studies on epigenetic modifications of our genes of interest can shed light on the specific impact these genes might have. DNA methylation patterns have been extensively studied on the OTR since it can provide a window for the outcomes of OTR transcription repression (Mamrut et al., 2013; Harony-Nicolas et al., 2014). High levels of OTR methylation have been associated with a decreased neural response in regions supporting social perception and emotion processing, such as the amygdala or the insula, but also with a decrease in the functional coupling between the amygdala and brain areas involved in emotion regulation (Puglia et al., 2015, 2018). This suggests that the oxytocin system may be involved in the attenuation of fear response and that variation in the methylation of the OTR could partially account for the natural variability in emotion processing (Krol et al., 2019). An increase in DNA methylation of the OTR was more generally associated with impairments in social, cognitive, and emotional cognition (Maud et al., 2018). Interestingly, epigenetic markers on the OTR could be modulated by environmental factors (Kumsta et al., 2013).
Single-nucleotide polymorphism (SNP) studies
Another naturally occurring source of variability in the activity of the OT-VP system are single-nucleotide polymorphisms. Different polymorphisms in the OTR have been associated with reduced plasma oxytocin levels and a low parental contact (Feldman et al., 2012), or on the contrary, with positive parenting and increased activity in the orbitofrontal and anterior cingulate cortex (Michalska et al., 2014), empathy (Wu et al., 2012), reaction to betrayal (Tabak et al., 2014), and trust (Nishina et al., 2015). Interestingly, OTR SNPs have also been associated with interindividual variability in OTR expression in specific brain areas, such as the nucleus accumbens in prairie voles (King et al., 2016).
Polymorphisms in the AVPRs are linked to variability in social behavior. Variation in the promotor region of the AVPR1A was shown to be associated with variation in the mating system of voles. Prairie voles or pine voles, which are socially monogamous, present a repetitive sequence of DNA in 5′ UTR of the AVPR1A, while this sequence is absent in the promiscuous montane vole and meadow vole (Hammock and Young, 2002). Interestingly, this variation is also associated with a different pattern of AVPR1A brain expression, specifically with an increase in the ventral pallidum, an increase that was also induced in a transgenic mouse that was carrying the 5′ UTR repetitive sequence (Young et al., 1999). Another repetitive sequence in 5′ UTR of AVPR1A, called RS3, has been associated with chimpanzee personality variation: the long form of the gene has been linked to dominance and conscientiousness (Hopkins et al., 2012), while the short form to a higher level of extraversion (Wilson et al., 2017); its influence on personality traits has been similarly reported for bonobo (Staes et al., 2016). Interestingly, a variation of a similar microsatellite region correlates with levels of AVPR1A binding in some brain areas of the prairie vole (Hammock et al., 2005). Furthermore, in human, the long RS3 repeat was associated with higher levels of AVPR1A RNA in the hippocampus and the length of this region is also associated with levels of altruistic behavior (Knafo et al., 2008).
In humans, polymorphisms in the AVPR1A have also been associated with trust and reciprocity (Nishina et al., 2019), and polymorphisms in the AVPR1B receptor gene have been associated with levels of aggressivity in children (Luppino et al., 2014) or empathy and prosociality in adults (Wu et al., 2015) (see also Theofanopoulou et al., 2018).
OXYTOCINERGIC AND VASOPRESSINERGIC SYSTEMS ARE IMPAIRED IN ASD AND RELATED ANIMAL MODELS
Nonhuman animal models
In order to understand the pathophysiology underlying deficits observed in ASDs, animal models aiming at recapitulating the main features of ASD have been developed. An ideal animal model would respond to three criteria: construct validity (causes of the pathology are the same in the model and the pathology), face validity (symptoms are similar to the disease), and predictive validity (treatments effective in the models and in the pathology would match). Since the etiology of ASD is not fully understood yet and in the absence of a solid treatment, the second criterion remains the most confident. Indeed, the three aspects of ASD-related deficits are considered to define a good model of ASD: deficits of social interaction, communication, and exaggerated repetitive behaviors.
When it comes to etiology, we can segregate the models into three categories (Bey and Jiang, 2014): genetics (modification of genes or group of genes known for their involvement in ASD), environmental, and naturally occurring strains’ variations. Interestingly, while most of these models initially do not directly target the OT or VP systems, alterations in these systems have often been observed (Peñagarikano, 2017). For example, loss of function of the FRM1 gene (causing fragile X syndrome in humans; one of the most common genetic form of autism) in mice induces a reduction of social approach, an alteration of communication, and repeated behaviors (Kazdoba et al., 2014), but also a decrease in OT and VP expression in the PVN (Francis et al., 2014). A reduction of OT immunoreactivity in the supraoptic nucleus and of the circulating OT levels have also been observed in rats subjected to a neonatal treatment with valproic acid (Dai et al., 2018), a treatment known to induce social behavior deficits relevant to ASD. Furthermore, as previously mentioned, mice lack one (Sala et al., 2013) or both alleles (Sala et al., 2011; Pobbe et al., 2012) of the OTR present social behavior deficits, patterns of repeated behavior, and neuronal hyperexcitability, all being features that make them up as a good model of ASD.
Human patients
OT and VP have been investigated as potential biomarkers for autism. More than 20 years ago, a first study revealed that plasma OT levels were lower in ASD children than in age-matched controls (Modahl et al., 1998). This result was since replicated in a small cohort (Andari et al., 2010), and in a cohort of more than 80 ASD children and 80 age-matched controls which also showed that in the ASD population, the higher the OT concentration, the less the impairment of verbal communication (Zhang et al., 2016), suggesting a role of oxytocin in vocal aspects of communication (Theofanopoulou et al., 2017). Nevertheless, other studies did not find differences in plasma OT levels between ASD and controls, suggesting that the OT system may actually play more of a regulatory role in both the physiology and the pathology (Parker et al., 2014). Lastly, OT concentration in saliva was positively correlated with secure attachment in ASD patients, which might be due to differential functional coupling between the amygdala and the hippocampus (Alaerts et al., 2019).
When it comes to VP, plasma levels are in many studies similar in children with and without ASD (Carson et al., 2015; Zhang et al., 2016), but according to Shou et al. (2017), plasma levels of VP positively correlate with brain morphological alterations in ASD patients, such as increased left amygdala and hippocampal volume, and decreased bilateral hypothalamus volume association between blood VP concentrations and behavioral phenotypes in ASD has also been demonstrated: VP levels negatively correlated with the visual and listening response score of CARS (Shou et al., 2017) and positively correlated with the theory of mind scores (Carson et al., 2015). To summarize, the association between OT and VP circulating levels and the diagnosis of autism is still unclear, but in autistic populations, the levels of circulating hormones are predictive of symptoms’ severity more often than not. Two studies found decreased CSF VP levels in autistic children, compared to controls (Oztan et al., 2018; Parker et al., 2018), something not observed in the case of OT (Oztan et al., 2018).
As it follows from our review of SNP studies with respect to social behavior, ASD patients have been shown to present specific variants, compared to controls, in the OTR, AVPR1A, and AVPR1B genes (Yang et al., 2010; Di Napoli et al., 2014; Francis et al., 2016; Uzefovsky et al., 2019). Similarly, specific methylation patterns are present in ASD population with specific behavioral phenotypes. Earlier, in a pilot study, Gregory et al. (2009) found an increased methylation of the OTR in ASD patients hand in hand with decreased OTR mRNA levels in the temporal cortex of postmortem tissues (Gregory et al., 2009). In a recent functional magnetic resonance imaging (fMRI) study, it has been shown that adults with ASD have a higher level of methylation in the first intron of OTR, which was associated with hypoconnectivity between cortical areas implicated in theory of mind and self-awareness deficits in ASD (Andari et al., 2020). Also, the study showed, for the first time, that a variation in a CpG site (not hypermethylated in ASD) in the exon area of OTR is associated with ASD symptom severity in social responsiveness, and with a hyperconnectivity between brain networks involved in reward processing (such as the ventral striatum and the ventromedial prefrontal cortex) (Andari et al., 2020). This hyperconnectivity was associated with a subtype of ASD that have restricted patterns of interests (such as computer, programming, astrophysics). DNA methylation of the OTR can become indeed a promising biomarker for ASD and for moderating the effects of OT treatment efficacy. More studies are needed to confirm this hypothesis.
TREATMENTS FOR ASD TARGETING THE OXYTOCINERGIC AND VASOPRESSINERGIC SYSTEMS
Treatments targeting the oxytocin system in adults
In nonhuman animal models
Oxytocin treatments administered to mouse models of ASD improve their social deficits. Mice lacking the CNTNAP2 gene, whose mutation causes cortical dysplasia and focal epilepsy syndrome with 70% of ASDs comorbidity, present stereotypic movements, behavioral inflexibility, communication and social behavior abnormalities (Peñagarikano et al., 2011), but also a decreased number of OT neurons in the PVN (Peñagarikano et al., 2015). In these animals, juvenile social interaction and social preference were improved by OT intraperitoneal or intranasal administrations by a mechanism depending on the activation of the OTR itself (Peñagarikano et al., 2015). Interestingly, melanocortin four receptor agonists, which activate paraventricular oxytocinergic neurons and induce central OT release (Sabatier, 2006), can also improve the social deficits of CNTNAP2 deficient mice, suggesting that inducing an endogenous release of OT can be an efficient strategy. Acute or subchronic OT treatment was reported to be efficient in improving the deficits of social interactions in different models of ASD (Peñagarikano, 2017), including SHANK3 deficient rats (Harony-Nicolas et al., 2017), the prenatal valproic acid-induced mouse model of autism (Hara et al., 2017), or mouse lines that naturally present phenotypes relevant to core ASD symptoms, such as BALB/cByJ and C58/J lines (Teng et al., 2013).
In human patients
As early as 2003, the potential impact of OT to improve deficits observed in ASD patients was evaluated. In a cohort of 15 adults with Asperger Syndrome, 4h of continuous synthetic OT infusion decreased repetitive behaviors (Hollander et al., 2003). A few years later, the same group showed that intravenous OT administration improved the ability of patients to assign emotional significance to speech intonation (Hollander et al., 2007). Acute intranasal OT administration to adults with high functioning autism was found to increase eye fixation, cooperative interaction, and trust during social ball games (Andari et al., 2010). This result suggests that OT could improve both attention to social cues and social cognition in people with ASD. Also, Andari et al. (2016) found that intranasal administration of OT enhances the blood–oxygen level-dependent (BOLD) activity of early visual areas during the perception of facial stimuli in adults with ASD. The authors also found that the treatment modulates the BOLD activity of the amygdala during a social ball-game in a context-dependent manner (Andari et al., 2016). The treatment enhances the activity of reward areas in response to positive stimuli (good player’s face) and insula area in response to negative stimuli (bad player’s face) (Andari et al., 2016). Since these pioneering studies, more studies have followed investigating the potential of OT treatment in ASD, with several replications (Guastella et al., 2010a; Watanabe et al., 2014; Auyeung et al., 2015). Long-term administration of OT in ASD improves clinical symptoms based on the oxytocin dosage and genetic background of the OTR (Kosaka et al., 2016). However, continuous administration of OT has some contradictory results in the literature (Ooi et al., 2017), with some showing no significant effects of OT on primary measures (Anagnostou et al., 2012; Dadds et al., 2014), and others showing improvements in secondary measures (such as RMET) (Anagnostou et al., 2012) and primary measures (Watanabe et al., 2015). Few issues need to be resolved before assessing the true clinical effects of OT in ASD. There is a considerable lack of reliable and sensitive clinical measures that can detect changes over time of the core symptoms of ASD. The ability to measure the effects of drugs on objective and quantitative assessments is needed. Also, deciding on the dose and duration of treatment is crucial for enhancing OT treatment effects. Also, using a precision medicine approach and targeting specific subtypes of ASD who can benefit the most from OT treatment can be essential.
Treatments targeting the oxytocin system during development
In nonhuman animal models
OT has an important function in mammals, which is related to birth. Indeed, in mammals, birth is a stressful experience which carries high risks for the brain of the infants because it requires a change in the oxygenation mode. While in adults, GABA is known as the main inhibitory neurotransmitter, during fetal and early postnatal development, it has a depolarizing action and is the main source of excitatory inputs to immature neurons. On the day that precedes birth in rats, the number of cells excited by GABA decreases due to OT activity (Tyzio et al., 2006). Interestingly, in two animal models of ASD (rats exposed to valproate in utero and mice carrying the fragile X mutation), this transient switch of GABA is not observed indicating that such abnormality might be due to early alterations in the OT system (Tyzio et al., 2014).
Interestingly, early life experiences shape the OT system and perinatal manipulation of OT has long-term consequences. Neonatal manipulation of male prairie voles on the day of birth can induce a decrease of OT immunoreactivity in the paraventricular and supraoptic nuclei and changes in OTR binding in the bed nucleus of the stria terminalis or LS. Injection of OT on postnatal day 1 can alter anxiety, alloparental behavior, pair bond formation, and OT immunoreactivity in the PVN in adults (Carter et al., 2009). Furthermore, a single intraperitoneal administration of an OTR antagonist on the first day of life induces a decrease of AVPR1A binding sites in brain areas such as the preoptic area, the bed nucleus of the stria terminalis, and the LS (Bales et al., 2007) and an increase of immunoreactivity to VP in the PVN, confirming that there is crosstalk between the different types of ligands and receptors.
Scientists investigated the potential of a transient OT treatment in early life on the improvement of social behavior deficits of mouse models of autism or other related neurodevelopmental disorders. In CNTNAP2 deficient mice, while OT treatments had an acute impact on social deficits, a daily intranasal OT treatment performed from postnatal day 7–21 improved social preference even 9 days after the end of the treatment, an improvement that was associated with a normalization of OT immunoreactivity in the PVN (Peñagarikano et al., 2015). A study performed on MAGEL2 KO mice, a mouse model of Prader–Willi syndrome (PWS) that has an increased prevalence in autism (Schaaf et al., 2013), showed that intranasal OT administration during the seven first postnatal days was sufficient to prevent the social and learning deficits normally developed in adult MAGEL2 KO mice. In this study, the impact of the treatment was evaluated in adults, 3–4 months after the end of the treatment (Meziane et al., 2015). This treatment also normalized some aspects of the oxytocin system such as OT binding in the LS and OT immunoreactivity in the amygdala, dorso-vagal complex, and LS. Similar results were recently obtained in the valproic acid rat model, where a treatment during the seven first postnatal days restored communication at the end of the treatment and social preference and self-grooming in young adults, with these effects being associated with a restoration of OT immunoreactivity in the paraventricular and supraoptic nuclei (Dai et al., 2018).
In human patients
Early-life treatment of ASD is challenging because the diagnosis of ASD is usually not performed before 2 years. Nevertheless, some neurodevelopmental disorders that are caused by genetic alterations can be diagnosed close to birth. It is the case of PWS, a hypothalamic disorder that is characterized by intellectual disabilities, repeated and compulsive behaviors and hyperphagia, and some comorbidities with autism (Dykens et al., 2011). An early study indicated that OT may play a role in the disease as it found a 42% decrease in the density of PVN OT neurons in postmortem tissue from PWS patients (Swaab et al., 1995). Furthermore, it was recently shown that 7 days of treatment with intranasal OT in PWS infants under 6 months of age improved both feeding and social skills and increased the connectivity in the right superior orbitofrontal area (Tauber et al., 2017). The treated infants were followed for 2 years after the treatment and when compared with age-matched nontreated PWS patients, they displayed higher social skills and engagement in relationships. Interestingly, another study performed on PWS indicates that the beneficial impact of OT treatment on social and food-related behavior was dependent on the age of the patients, with the beneficial impact being limited to children less than 11 years old (Kuppens et al., 2016).
In autism patients aged 12–19 years old, a single intranasal OT administration enhanced performance in the “reading the mind in the eyes” test, suggesting an acute improvement of emotion recognition (Guastella et al., 2010a). In a study of long-tern administration (7 months) of intranasal OT, improvement of both communication and social interaction skills was detected (Tachibana et al., 2013). Furthermore, one study performed in children aged between 12 and 18 years old did not report beneficial effects on primary outcomes (Guastella et al., 2015). Studies involving fMRI provide a structural and functional support for these beneficial effects: studies showed that OT increases the activity of areas involved in social reward processing and theory of mind (i.e., striatum, nucleus accumbens, left posterior superior temporal sulcus, and left premotor cortex) when ASD children were presented with pictures of faces, but decreased it when presented with pictures of nonsocial objects (Gordon et al., 2013), suggesting a selective social effect of OT. More studies are needed to better understand the chronic effects of OT on the brain and behavior in order to maximize treatment efficacy. Also, targeting the endogenous OT system with other drugs can be a promising avenue.
Treatments targeting the vasopressin system
In nonhuman animal models
In animal models of autism, VP treatments have been less investigated than OT treatments. In the CNTNAP2 deficient mice, VP treatment was found to improve the social deficits, but this effect was mainly mediated by the OTR, as its blockade abolished the improvement induced by VP treatment (Peñagarikano et al., 2015). This is probably due to the important promiscuity of the OT and VP systems (Song and Albers, 2017). Similarly, in the OTR deficient mice, both OT and VP treatment were found to have a beneficial impact on the social deficits. This is due, in both cases, to the activation of AVPR1A (Sala et al., 2011), indicating that when the oxytocin system is impaired, it is possible that the VP system takes over to make up for the impairment. It is thus possible that the impact of OT treatment partly relies on its binding to VPRs. In a recent study performed in MAGEL2 KO mice, it was found that stimulation of VP but not OT fibers in the LS could restore social behavior in a social habituation–dishabituation task, suggesting that VP also plays a role by itself (Borie et al., 2019).
In human subjects
In recent studies, the vasopressinergic system was targeted in order to improve the social symptoms of ASDs. A 4-week intranasal VP administration in children with ASD (6–13 years old) improved social abilities in the social responsiveness scale and decreased anxiety and some repetitive behaviors (Parker et al., 2019). Balovaptan, a AVPR1A antagonist that can be administered orally, improved socialization and communication scores in high functioning men with ASD (Bolognani et al., 2019) after a 12-week treatment. Interestingly, both agonists and antagonists of VPRs showed the beneficial impact. It is nevertheless important to note that the age of the patients differs in the two cohorts and that more studies are necessary to fully understand how and when to target the vasopressinergic system to improve the phenotype of ASD.
Oxytocin and behavioral therapies
Studies indicate that cognitive behavioral therapy can improve behavioral phenotypes in children with ASD (Remington et al., 2007; Kurz et al., 2018). In a mouse model of autism with blunted reward processing, a behavioral therapy based on the association of positive reinforcement to social interaction rescued mice’ social preference behaviors. Interestingly, this behavioral therapy normalized OT and VP systems in the reward and social circuitry, suggesting that such a behavioral therapy could indirectly modulate the activity of OT and VP (Pujol et al., 2018). In humans, it has been shown that endogenous OT release can occur in response to social interaction. For example, according to a pilot study, a daily 20 min massage performed by the mother of children with ASD was sufficient to increase salivary OT concentration in both the children and their mothers (Tsuji et al., 2015). The possibility of shaping the OT and VP release systems through behavioral therapies alone, or in combination with exogenous treatments, opens a new field of research that is bound to give informative results in the close future.
CONCLUSION
In this chapter, we have reviewed findings on the evolutionary history of oxytocin and vasopressin ligands and receptors that highlight their common origin and clarify the evolutionary background of the crosstalk between them. Understanding both neuroendocrine systems, although named differently, but evolutionary derived from the same “vasotocinergic” system, helps decipher the neurobiology of social functioning and the establishment of promising treatments. Additionally, we have reviewed the evidence on the brain distribution of the ligands and the receptors in humans and mice, which has shown that they are expressed in regions that have been associated with social behaviors. We have then reviewed studies exhaustively in humans and nonhuman animal models showing how oxytocin and vasopressin regulate social behaviors, including gene blockade and activation studies, studies on their concentration centrally and peripherally, as well administration, epigenetic, and variation studies. We lastly reviewed the literature targeting the involvement/impairment of OT, VP, and OTR-VPRs in ASD, as well as ASD treatments involving these molecules. We conclude that the OT/VP system is one of the most promising systems to uncover treatments for social disorders given its pivotal role in social cognition and emotional processing.
References
- Alaerts K, Bernaerts S, Vanaudenaerde B et al. (2019). Amygdala–hippocampal connectivity is associated with endogenous levels of oxytocin and can be altered by exogenously administered oxytocin in adults with autism. Biol Psychiatry 4: 655–663. 10.1016/j.bpsc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Alonso G, Gallibert E, Lafont C et al. (2008). Intrahypothalamic angiogenesis induced by osmotic stimuli correlates with local hypoxia: a potential role of confined vasoconstriction induced by dendritic secretion of vasopressin. Endocrinology 149: 4279–4288. 10.1210/en.2008-0387. [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Chaplin W et al. (2012). Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism 3:16. 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel J-R, Zalla T et al. (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A 107: 4389–4394. 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Schneider FC, Mottolese R et al. (2014). Oxytocin’s fingerprint in personality traits and regional brain volume. Cereb Cortex 24: 479–486. 10.1093/cercor/bhs328. [DOI] [PubMed] [Google Scholar]
- Andari E, Richard N, Leboyer M et al. (2016). Adaptive coding of the value of social cues with oxytocin, an fMRI study in autism spectrum disorder. Cortex 76: 79–88. 10.1016/j.cortex.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Andari E, Nishitani S, Kaundinya G et al. (2020). Epigenetic modification of the oxytocin receptor gene: implications for autism symptom severity and brain functional connectivity. Neuropsychopharmacology 45: 1150–1158. 10.1038/s41386-020-0610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter-Levi Y, Zagoory-Sharon O, Feldman R (2014). Oxytocin and vasopressin support distinct configurations of social synchrony. Brain Res 1580: 124–132. 10.1016/j.brainres.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Arthur P, Taggart MJ, Zielnik B et al. (2008). Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. J Physiol 586: 6063–6076. 10.1113/jphysiol.2008.164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Heinrichs M et al. (2015). Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl Psychiatry 5: e507. 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos J, Srancikova A, Havranek T et al. (2018). Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast 2018: 1–9. 10.1155/2018/4864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ et al. (2007). Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience 144: 38–45. 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey AL, Jiang Y (2014). Overview of mouse models of autism spectrum disorders. Curr Protoc Pharmacol 66: 5.66.1–5.66.26. 10.1002/0471141755.ph0566s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Ren X et al. (2005). The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47: 503–513. 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bolognani F, del Valle Rubido M, Squassante L et al. (2019). A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci Transl Med 11: eaat7838. 10.1126/scitranslmed.aat7838. [DOI] [PubMed] [Google Scholar]
- Borie AM, Dromard Y, Dufner D et al. (2019). Control of social withdrawal of mice deficient for the autism gene Magel2 by restoration of vasopressin-oxytocin dialogue in septum. bioRxiv 800425. 10.1101/800425. [DOI] [Google Scholar]
- Born J, Lange T, Kern W et al. (2002). Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5: 514–516. 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Krömer SA, Brunton PJ et al. (2004). Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 124: 439–448. 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H (1980). Synthesis, transport, and release of posterior pituitary hormones. Science 207: 373–378. 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF (1979). Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res 204: 355–365. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV et al. (2016). Oxytocin-dependent consolation behavior in rodents. Science 351: 375–378. 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Garner JP, Hyde SA et al. (2015). Arginine vasopressin is a blood-based biomarker of social functioning in children with autism. PLoS One 10: e0132224. 10.1371/journal.pone.0132224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H et al. (2009). Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci 31: 332–341. 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Volpi S, Aguilera G (2008). Anti-apoptotic actions of vasopressin in H32 neurons involve map kinase transactivation and bad phosphorylation. Exp Neurol 211: 529–538. 10.1016/j.expneurol.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi A et al. (2014). Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord 44: 521–531. 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- Dai Y-C, Zhang H-F, Schön M et al. (2018). Neonatal oxytocin treatment ameliorates autistic-like behaviors and oxytocin deficiency in valproic acid-induced rat model of autism. Front Cell Neurosci 12: 355. 10.3389/fncel.2018.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J et al. (2014). CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One 9: e103677. 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Napoli A, Warrier V, Baron-Cohen S et al. (2014). Genetic variation in the oxytocin receptor (OXTR) gene is associated with Asperger syndrome. Mol Autism 5: 48. 10.1186/2040-2392-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM, Lee E, Roof E (2011). Prader–Willi syndrome and autism spectrum disorders: an evolving story. J Neurodev Disord 3: 225–237. 10.1007/s11689-011-9092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd SK, Sabry I, Hassan WB et al. (2007). Possible neuroendocrine role for oxytocin in bone remodeling. Endocr Regul 41: 131–141. [PubMed] [Google Scholar]
- Engelmann M, Landgraf R (1994). Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav 55: 145–149. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O et al. (2012). Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry 72: 175–181. 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC et al. (2015). Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav 9: 754–764. 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF et al. (2000). Social amnesia in mice lacking the oxytocin gene. Nat Genet 25: 284–288. 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR et al. (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V, Maltese F, Contarini G et al. (2019). Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr Biol 29: 1938–1953. 10.1016/j.cub.2019.04.070. [DOI] [PubMed] [Google Scholar]
- Filippi S, Vignozzi L, Vannelli GB et al. (2003). Role of oxytocin in the ejaculatory process. J Endocrinol Invest 26: 82–86. [PubMed] [Google Scholar]
- Francis SM, Sagar A, Levin-Decanini T et al. (2014). Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res 1580: 199–218. 10.1016/j.brainres.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Kim S-J, Kistner-Griffin E et al. (2016). ASD and genetic associations with receptors for oxytocin and vasopressin—AVPR1A, AVPR1B, and OXTR. Front Neurosci 10: 516. 10.3389/fnins.2016.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC et al. (2016). Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology 66: 185–194. 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Gordon I, Wyk BCV, Bennett RH et al. (2013). Oxytocin enhances brain function in children with autism. PNAS 110: 20953–20958. 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Carter CS, Pournajafi-Nazarloo H et al. (2012). Plasma vasopressin and interpersonal functioning. Biol Psychol 91: 270–274. 10.1016/j.biopsycho.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ et al. (2009). Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 7: 62. 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand T, Tang Y, Benusiglio D et al. (2019). Chemogenetic activation of oxytocin neurons: temporal dynamics, hormonal release, and behavioral consequences. Psychoneuroendocrinology 106: 77–84. 10.1016/j.psyneuen.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry 63: 3–5. 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM et al. (2010a). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 67: 692–694. 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA et al. (2010b). Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry 67: 1220–1222. 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Unkelbach C et al. (2011). Arginine vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology 36: 294–297. 10.1016/j.psyneuen.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Gray KM, Rinehart NJ et al. (2015). The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry 56: 444–452. 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- Gwee PC, Tay BH, Brenner S et al. (2009). Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol Biol 9: 1–15. 10.1186/1471-2148-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EAD, Young LJ (2002). Variation in the vasopressin V1a receptor promoter and expression: implications for inter- and intraspecific variation in social behaviour. Eur J Neurosci 16: 399–402. 10.1046/j.1460-9568.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, Lim MM, Nair HP et al. (2005). Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav 4:289–301. 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Hara Y, Ago Y, Higuchi M et al. (2017). Oxytocin attenuates deficits in social interaction but not recognition memory in a prenatal valproic acid-induced mouse model of autism. Horm Behav 96: 130–136. 10.1016/j.yhbeh.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Harony-Nicolas H, Mamrut S, Brodsky L et al. (2014). Brain region-specific methylation in the promoter of the murine oxytocin receptor gene is involved in its expression regulation. Psychoneuroendocrinology 39: 121–131. 10.1016/j.psyneuen.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Harony-Nicolas H, Kay M, du Hoffmann J et al. (2017). Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. eLife 6: e18904. 10.7554/eLife.18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G (2008). Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res 170: 337–350. 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M et al. (2003). Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology 28: 193–198. 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W et al. (2007). Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61: 498–503. 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Donaldson ZR, Young LJ (2012). A polymorphic indel containing the RS3 microsatellite in the 5’ flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes Brain Behav 11: 552–558. 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue K, Suzuki S et al. (2018). Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm Behav 111: 60–69. 10.1016/j.yhbeh.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS et al. (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science 357: 1406–1411. 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ (1995). A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci 109: 782–789. 10.1037/0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Danalache B, Wang D et al. (2004). Oxytocin in cardiac ontogeny. Proc Natl Acad Sci U S A 101: 13074–13079. 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowski GF, Caldwell JD, Pedersen CA et al. (1988). Estradiol influences oxytocin-immunoreactive brain systems. Neuroscience 25: 237–248. 10.1016/0306-4522(88)90022-X. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA et al. (2016). Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav 79: 8–17. 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Igarashi N, Hirasawa A et al. (1995). Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation 59: 163–169. 10.1046/j.1432-0436.1995.5930163.x. [DOI] [PubMed] [Google Scholar]
- Kazdoba TM, Leach PT, Silverman JL et al. (2014). Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis Res 3: 118–133. 10.5582/irdr.2014.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K et al. (2016). Variation in the oxytocin receptor gene predicts brain region specific expression and social attachment. Biol Psychiatry 80: 160–169. 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt JD, Goodson JL (2013). Oxytocin-like receptors mediate pair bonding in a socially monogamous songbird. Proc Biol Sci 280: 20122396. 10.1098/rspb.2012.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Israel S, Darvasi A et al. (2008). Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav 7: 266–275. 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC et al. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566. 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Okamoto Y, Munesue T et al. (2016). Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: a 24-week randomized clinical trial. Transl Psychiatry 6: e872. 10.1038/tp.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol KM, Puglia MH, Morris JP et al. (2019). Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev Cogn Neurosci 37: 100648. 10.1016/j.dcn.2019.100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Hummel E, Chen FS et al. (2013). Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front Neurosci 7: 83. 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens RJ, Donze SH, Hokken-Koelega ACS (2016). Promising effects of oxytocin on social and food-related behavior in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin Endocrinol (Oxf) 85: 979–987. 10.1111/cen.13169. [DOI] [PubMed] [Google Scholar]
- Kurz R, Huemer J, Muchitsch E et al. (2018). Cognitive behavioral therapy for children with autism spectrum disorder: a prospective observational study. Eur J Paediatr Neurol 22: 803–806. 10.1016/j.ejpn.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Lagman D, Ocampo Daza D, Widmark J et al. (2013). The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications. BMC Evol Biol 13: 238. 10.1186/1471-2148-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrazolo-López A, Kendrick KM, Aburto-Arciniega M et al. (2008). Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience 152: 585–593. 10.1016/j.neuroscience.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX et al. (2018). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry 23: 115–122. 10.1038/mp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Mottolese R, Dirheimer M et al. (2017). A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci Rep 7: 17222. 10.1038/s41598-017-17674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ (2008). Oxytocin and the maternal brain. Curr Opin Pharmacol 8: 731–734. 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121: 537–544. 10.1016/S0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z (2001). Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav Neurosci 115: 910–919. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Van Heerikhuize JJ, Guldenaar SE et al. (1997). Unchanged amounts of vasopressin mRNA in the supraoptic and paraventricular nucleus during aging and in Alzheimer’s disease. J Neuroendocrinol 9: 297–305. 10.1046/j.1365-2826.1997.t01-1-00583.x. PMID: 9147293. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G (2006). Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7: 126–136. 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO et al. (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36: 2159–2168. 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino D, Moul C, Hawes DJ et al. (2014). Association between a polymorphism of the vasopressin 1B receptor gene and aggression in children. Psychiatr Genet 24: 185–190. 10.1097/YPG.0000000000000036. [DOI] [PubMed] [Google Scholar]
- Magalhaes JKRS, Carvalho JCA, Parkes RK et al. (2009). Oxytocin pretreatment decreases oxytocin-induced myometrial contractions in pregnant rats in a concentration-dependent but not time-dependent manner. Reprod Sci 16: 501–508. 10.1177/1933719108329954. [DOI] [PubMed] [Google Scholar]
- Mamrut S, Harony H, Sood R et al. (2013). DNA methylation of specific CpG sites in the promoter region regulates the transcription of the mouse oxytocin receptor. PLoS One 8: e56869. 10.1371/journal.pone.0056869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA et al. (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520: 499–504. 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroni SS, Nakano FN, Gati CDC et al. (2007). Neuroanatomical and cellular substrates of hypergrooming induced by microinjection of oxytocin in central nucleus of amygdala, an experimental model of compulsive behavior. Mol Psychiatry 12: 1103–1117. 10.1038/sj.mp.4002015. [DOI] [PubMed] [Google Scholar]
- Maud C, Ryan J, McIntosh JE et al. (2018). The role of oxytocin receptor gene (OXTR) DNA methylation (DNAm) in human social and emotional functioning: a systematic narrative review. BMC Psychiatry 18: 154. 10.1186/s12888-018-1740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayasich SA, Clarke BL (2016). The emergence of the vasopressin and oxytocin hormone receptor gene family lineage: clues from the characterization of vasotocin receptors in the sea lamprey (Petromyzon marinus). Gen Comp Endocrinol 226: 88–101. 10.1016/j.ygcen.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Melmed S (2011). The pituitary, Elsevier Inc; 10.1016/C2009-0-61488-4. [DOI] [Google Scholar]
- Menon R, Grund T, Zoicas I et al. (2018). Oxytocin signaling in the lateral septum prevents social fear during lactation. Curr Biol 28: 1066–1078.e6. 10.1016/j.cub.2018.02.044. [DOI] [PubMed] [Google Scholar]
- Meziane H, Schaller F, Bauer S et al. (2015). An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol Psychiatry 78: 85–94. 10.1016/j.biopsych.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Decety J, Liu C et al. (2014). Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Front Behav Neurosci 8: 21. 10.3389/fnbeh.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D et al. (1998). Plasma oxytocin levels in autistic children. Biol Psychiatry 43: 270–277. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS (2008). Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav 91: 77–83. 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI et al. (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38: 1985–1993. 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Nishina K, Takagishi H, Inoue-Murayama M et al. (2015). Polymorphism of the oxytocin receptor gene modulates behavioral and attitudinal trust among men but not women. PLoS One 10: e0137089. 10.1371/journal.pone.0137089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina K, Takagishi H, Takahashi H et al. (2019). Association of polymorphism of arginine-vasopressin receptor 1A (AVPR1a) gene with trust and reciprocity. Front Hum Neurosci 13: 230. 10.3389/fnhum.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo Daza D, Lewicka M, Larhammar D (2012). The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, inclucing two distinct V2 subtypes. Gen Comp Endocrinol 175: 135–143. 10.1016/j.ygcen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Oettl L-L, Ravi N, Schneider M et al. (2016). Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron 90: 609–621. 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Klockars A, Levine AS (2016). Oxytocin: a conditional anorexigen whose effects on appetite depend on the physiological, behavioural and social contexts. J Neuroendocrinol 28: 10.1111/jne.12376. [DOI] [PubMed] [Google Scholar]
- Ooi YP, Weng S-J, Kossowsky J et al. (2017). Oxytocin and autism spectrum disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacopsychiatry 50: 5–13. 10.1055/s-0042-109400. [DOI] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Partap S et al. (2018). Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann Neurol 84: 611–615. 10.1002/ana.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA et al. (2014). Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci U S A 111: 12258–12263. 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Oztan O et al. (2018). Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci Transl Med 10: eaam9100. 10.1126/scitranslmed.aam9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA et al. (2019). A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med 11: eaau7356. 10.1126/scitranslmed.aau7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan de Arruda Camargo GM, Saad WA, de Arruda Camargo LA (2008). Vasopressin and angiotensin receptors of the medial septal area in the control of mean arterial pressure induced by vasopressin. J Renin Angiotensin Aldosterone Syst 9: 133–138. 10.1177/1470320308095260. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O (2017). Oxytocin in animal models of autism spectrum disorder. Dev Neurobiol 77: 202–213. 10.1002/dneu.22449. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI et al. (2011). Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities and core autism-related deficits. Cell 147: 235–246. 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu X-H et al. (2015). Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med 7: 271ra8. 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Alster P, Lundeberg T et al. (1996). Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol Behav 60: 1311–1315. 10.1016/S0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- Pisansky MT, Hanson LR, Gottesman II et al. (2017). Oxytocin enhances observational fear in mice. Nat Commun 8: 2102. 10.1038/s41467-017-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RLH, Pearson BL, Defensor EB et al. (2012). Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav 61: 436–444. 10.1016/j.yhbeh.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP et al. (2015). Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci U S A 112: 3308–3313. 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Connelly JJ, Morris JP (2018). Epigenetic regulation of the oxytocin receptor is associated with neural response during selective social attention. Transl Psychiatry 8: 116. 10.1038/s41398-018-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol CN, Pellissier LP, Clément C et al. (2018). Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities. Transl Psychiatry 8: 1–15. 10.1038/s41398-018-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Alvares GA, Hickie IB et al. (2015). Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neurosci Biobehav Rev 49: 182–192. 10.1016/j.neubiorev.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A et al. (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun 8: 2001. 10.1038/s41467-017-02173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington B, Hastings RP, Kovshoff H et al. (2007). Early intensive behavioral intervention: outcomes for children with autism and their parents after two years. Am J Ment Retard 112: 418–438. https://doi.org/10.1352/0895-8017(2007)112[418:EIBIOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Renaud LP, Bourquet CW (1991). Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol 36: 131–169. 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Richmond CA (2003). The role of arginine vasopressin in thermoregulation during fever. J Neurosci Nurs 35: 281–286. 10.1097/01376517-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD et al. (2012). Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37: 447–461. 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Chen X, Chen X et al. (2018). Intranasal oxytocin modulates neural functional connectivity during human social interaction. Am J Primatol 80: e22740. 10.1002/ajp.22740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N (2006). Alpha-melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol 18: 703–710. 10.1111/j.1365-2826.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D et al. (2011). Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a nurobehavioral model of autism. Biol Psychiatry 69: 875–882. 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Donzelli A et al. (2013). Mice heterozygous for the oxytocin receptor gene (Oxtr(+/−)) show impaired social behaviour but not increased aggression or cognitive inflexibility: evidence of a selective haploinsufficiency gene effect. J Neuroendocrinol 25: 107–118. 10.1111/j.1365-2826.2012.02385.x. [DOI] [PubMed] [Google Scholar]
- Schaaf CP, Gonzalez-Garay ML, Xia F et al. (2013). Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat Genet 45: 1405–1408. 10.1038/ng.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JC (2009). Early locomotor and social effects in vasopressin deficient neonatal rats. Behav Brain Res 197: 166–177. 10.1016/j.bbr.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Shou X-J, Xu X-J, Zeng X-Z et al. (2017). A volumetric and functional connectivity MRI study of brain arginine-vasopressin pathways in autistic children. Neurosci Bull 33: 130–142. 10.1007/s12264-017-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Keinath MC (2015). The sea lamprey meiotic map improves resolution of ancient vertebrate genome duplications. Genome Res 25: 1081–1090. 10.1101/gr.184135.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Williams Avram SK, Cymerblit-Sabba A et al. (2016). Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry 21: 1137–1144. 10.1038/mp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Korgan AC, Young WS (2019). Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol Res 146: 104324. 10.1016/j.phrs.2019.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Albers HE (2017). Cross-talk among oxytocin and arginine-vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol 51: 14–24. 10.1016/j.yfrne.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetter MS, Hallschmid M (2017). Current findings on the role of oxytocin in the regulation of food intake. Physiol Behav 176: 31–39. 10.1016/j.physbeh.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Staes N, Weiss A, Helsen P et al. (2016). Bonobo personality traits are heritable and associated with vasopressin receptor gene 1a variation. Sci Rep 6: 38193. 10.1038/srep38193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK (2012). The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav 61: 277–282. 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V et al. (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 3: 3440. 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA (1995). Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab 80: 573–579. 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Carver CS et al. (2014). Variation in oxytocin receptor gene (OXTR) polymorphisms is associated with emotional and behavioral reactions to betrayal. Soc Cogn Affect Neurosci 9: 810–816. 10.1093/scan/nst042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kagitani-Shimono K, Mohri I et al. (2013). Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol 23: 123–127. 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF et al. (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A 102: 16096–16101. 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M, Boulanouar K, Diene G et al. (2017). The use of oxytocin to improve feeding and social skills in infants with Prader-Willi syndrome. Pediatrics 139: e20162976. 10.1542/peds.2016-2976. [DOI] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL et al. (2013). Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology 72: 187–196. 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofanopoulou C, Boeckx C, Jarvis ED (2017). A hypothesis on a role of oxytocin in the social mechanisms of speech and vocal learning. Proc Biol Sci 284. 10.1098/rspb.2017.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofanopoulou C, Andirko A, Boeckx C (2018). Oxytocin and vasopressin receptor variants as a window onto the evolution of human prosociality. bioRxiv 460584. [Google Scholar]
- Theofanopoulou C, Gedman G, Cahill JA et al. (2021). Universal nomenclature for oxytocin-vasotocin ligand and receptor families. Nature. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul PJ, Åkesson L, Wiking M et al. (2017). A subcellular map of the human proteome. Science 356: eaal3321. 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Yuhi T, Furuhara K et al. (2015). Salivary oxytocin concentrations in seven boys with autism spectrum disorder received massage from their mothers: a pilot study. Front Psych 6: 58. 10.3389/fpsyt.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I et al. (2006). Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314: 1788–1792. 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC et al. (2014). Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343: 675–679. 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- Uzefovsky F, Bethlehem RAI, Shamay-Tsoory S et al. (2019). The oxytocin receptor gene predicts brain activity during an emotion recognition task in autism. Mol Autism 10: 12. 10.1186/s13229-019-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID (2008). Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res 170: 261–276. 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ (2012). Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav 61: 50–56. 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ (2013). Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology 38: 2554–2561. 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty ANA, McFarlane JR, McGregor IS et al. (2004). Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology 47: 593–603. 10.1016/j.neuropharm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Walter R, Rudinger J, Schwartz IL (1967). Chemistry and structure-activity relations of the antidiuretic hormones. Am J Med 42: 653–677. 10.1016/0002-9343(67)90087-3. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Abe O, Kuwabara H et al. (2014). Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiat 71: 166–175. 10.1001/jamapsychiatry.2013.3181. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kuroda M, Kuwabara H et al. (2015). Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 138: 3400–3412. 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- Weingartner H, Gold P, Ballenger JC et al. (1981). Effects of vasopressin on human memory functions. Science 211: 601–603. 10.1126/science.7455701. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll A-M et al. (2002). Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 7: 975–984. 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A et al. (2007). A unified classification system for eukaryotic transposable elements. Nat Rev Genet 8: 973–982. 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Wilson VAD, Weiss A, Humle T et al. (2017). Chimpanzee personality and the arginine vasopressin receptor 1A genotype. Behav Genet 47: 215–226. 10.1007/s10519-016-9822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Insel TR (1994). Increased Fos expression in oxytocin neurons following masculine sexual behavior. J Neuroendocrinol 6: 13–18. 10.1111/j.1365-2826.1994.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Wu CL, Hung CR, Chang FY et al. (2003). Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn Schmiedebergs Arch Pharmacol 367: 406–413. 10.1007/s00210-003-0690-y. [DOI] [PubMed] [Google Scholar]
- Wu N, Li Z, Su Y (2012). The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J Affect Disord 138: 468–472. 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Wu N, Shang S, Su Y (2015). The arginine vasopressin V1b receptor gene and prosociality: mediation role of emotional empathy. Psychiatry J 4: 160–165. 10.1002/pchj.102. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Kaiya H, Konno N et al. (2012). The fifth neurohypophysial hormone receptor is structurally related to the V2-type receptor but functionally similar to V1-type receptors. Gen Comp Endocrinol 178: 519–528. 10.1016/j.ygcen.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Chen JM et al. (2007). Effect of oxytocin on acupuncture analgesia in the rat. Neuropeptides 41: 285–292. 10.1016/j.npep.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Yang SY, Cho S-C, Yoo HJ et al. (2010). Family-based association study of microsatellites in the 5′ flanking region of AVPR1A with autism spectrum disorder in the Korean population. Psychiatry Res 178: 199–201. 10.1016/j.psychres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG et al. (1999). Increased affiliative response to vasopressin in mice expressing the V 1a receptor from a monogamous vole. Nature 400: 766–768. 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Zhang H-F, Dai Y-C, Wu J et al. (2016). Plasma oxytocin and arginine-vasopressin levels in children with autism spectrum disorder in China: associations with symptoms. Neurosci Bull 32: 423–432. 10.1007/s12264-016-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


