Abstract
Purpose
To determine the upgrade rate for biopsy-proven radial scars and radial sclerosing lesions (RS).
Materials and Methods
In this retrospective study, radiology and pathology databases from two tertiary breast centers were searched to identify patients with biopsy-confirmed RS between March 1, 2012, and December 31, 2017, during which all mammography was performed with digital breast tomosynthesis (DBT). Adjunct modalities such as MRI or US are performed at our centers to better characterize lesions identified at DBT. Patient demographics, imaging, needle and excisional biopsies, and follow-up data were collected at the patient level. Clopper-Pearson interval estimate for upgrade was calculated for 95% confidence using PropCIs package with R version 4.1.0 (R Foundation for Statistical Computing) (1).
Results
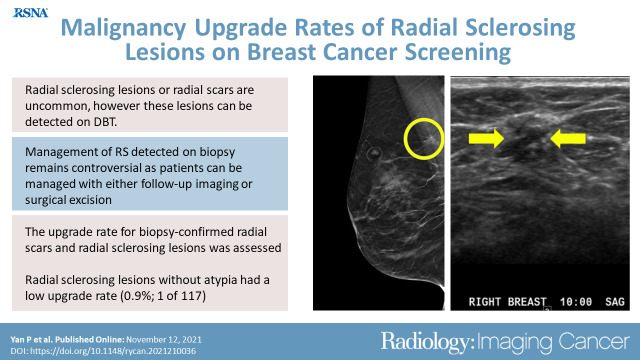
During the study period, a total of 155 885 DBT examinations were performed. From these examinations, 146 biopsy-proven RS were identified in 142 women (median age, 58 years; age range, 26–87 years). A total of 80.1% (117 of 146) of all RS did not have associated atypia or malignancy, and 19.9% (29 of 146) had associated atypia at initial biopsy. A total of 66.7% (78 of 117) of RS without atypia or malignancy were surgically excised, 25.6% (30 of 117) were followed (median, 3 years; range, 1–7 years) with benign findings on imaging, and 7.7% (nine of 117) were lost to follow-up. The rate of malignancy upgrade was 0.9% (one of 117 [95% CI: 0.02, 4.7]); one RS without concurrent atypia or malignancy demonstrated invasive carcinoma at surgical excision.
Conclusion
RS without atypia had a low upgrade rate.
Keywords: Mammography, Breast
© RSNA, 2021
Keywords: Mammography, Breast
Summary
Radial sclerosing lesions without associated atypia detected at screening had a low malignancy potential.
Key Points
■ Only one radial sclerosing lesion (RS) without atypia (0.9%; one of 117 [95% CI: 0.02, 4.7]) was upgraded to an invasive carcinoma.
■ All RS (100%; 30 of 30) without atypia followed with imaging (median, 3 years; range, 1–7 years) rather than excision were benign.
Introduction
Radial sclerosing lesions (RS) are uncommon, with a reported incidence of 0.03%–0.8% of core-needle breast biopsy results (2,3). After detection on imaging, diagnosis of RS is histologically confirmed, with histologic features frequently displaying a central fibroelastotic core with radiating spokes of ducts and lobules (4). There is unifying evidence that digital breast tomosynthesis (DBT) has improved sensitivity for lesion detection and characterization as compared with digital mammography (DM) (5–9) and has increased detection of RS (10,11).
DBT has also demonstrated higher detection rates of architectural distortions (AD) compared with DM (4,6,11–15). One of the common manifestations of RS is as AD, defined as spiculations radiating from a central point without a central mass (16). In the era of DBT, a greater proportion of DBT-detected ADs are RS compared with those detected with DM (11,13,17), with one study reporting 33% of DBT-detected ADs being RS, versus 12% of DM-detected ADs being RS (13). The positive predictive value for malignancy in DBT-detected ADs is lower than that for those detected with DM (13,18), given the greater proportion of RS.
Management of RS detected at core-needle biopsy remains controversial. RS with associated atypia have higher rates of malignant upgrade compared with RS without atypia (19–22) and are often excised. Multiple studies (23–25) suggest that RS found alone without associated atypia rarely upgrade to malignancy, with respective upgrade rates of 0% (zero of 80), 0.5% (one of 219), and 1.0% (one of 96). Most of these studies were prior to DBT or when DBT was less commonly available. As more patients are imaged with DBT and more RS are identified, breast imagers need methods to assess optimal patient management options. Concerns of patient management raise the question of whether patients with RS without atypia may be better served with follow-up imaging rather than surgical excision. This decision is particularly relevant as more RS are discovered with DBT biopsy (11), and many patients routinely undergo excision for RS without atypia (26).
We hypothesized that in the era of DBT, the rate of malignant upgrade for RS without atypia would be low enough to support imaging follow-up over surgical excision as the most optimal management practice. Given the rarity of these lesions, the estimates provided here are intended to inform future power and meta-analyses.
Materials and Methods
This was a Health Insurance Portability and Accountability Act–compliant retrospective study that was approved by our institutional review board. The need for informed consent was waived.
Study Sample
We conducted a retrospective review of our radiology and pathology databases at two tertiary breast centers to capture all instances of patients of any age with “radial scar,” “radial sclerosing lesion,” or “complex sclerosing lesion” identified at core-needle biopsy of the breast between March 1, 2012, and December 31, 2017. We included all patients who had histologically verified RS, radial scar, or complex sclerosing lesion, excluding sclerosing adenosis, sclerosed papillomas, and incidental RS (for example, those completely excised and separate from the imaging target). Pathology reports were reviewed, and lesions were separated into two cohorts: those without associated malignancy or atypia and those with associated atypia. Lesions with other associated nonatypia pathologic features were categorized as RS without associated atypia. Pathologic slides were not rereviewed for this study.
Image Acquisition
DBT images were obtained with Selenia Dimensions units (Hologic), US with iU22 (Philips Healthcare), and MRI pre- and postcontrast enhancement with dedicated breast coils at 1.5 T (Siemens Magnetom Symphony) or 3 T (Siemens Verio).
Data Collection
The electronic medical record was accessed to identify the following: (a) patient age, (b) initial imaging findings prompting biopsy, (c) imaging modalities where findings were visible, (d) Breast Imaging Reporting and Data System (BI-RADS) assessment, (e) imaging modality used for biopsy, (f) biopsy needle gauge, (g) number of samples taken and pathologic results of needle biopsy, (h) results of excisional biopsy if performed, (i) time interval to most recent available follow-up imaging, (j) follow-up imaging modalities, and (k) follow-up imaging findings through October 2020.
Data were entered into the Health Insurance Portability and Accountability Act–compliant REDCap database (Vanderbilt University) (27). Upgrade to malignancy was defined as discovery of ductal carcinoma in situ (DCIS) or invasive carcinoma at the same site as RS upon surgical excision.
Imaging and Biopsy Interpretation
Imaging performed during the study period included screening and diagnostic DBT mammography (full-field digital mammography and DBT, 2012–2014; DBT and synthesized mammography, 2014–2017), US (diagnostic and screening), and MRI (high-risk screening and extent of disease in new cancer diagnoses). Imaging was interpreted by one of 13 fellowship-trained board-certified breast radiologists with 1–25 years of experience (including A.P.L. and L.D.). Image-guided biopsies were performed using the modality in which the lesion of interest was best visualized and was performed by the same group of breast radiologists. Histopathologic findings were interpreted by fellowship-trained board-certified pathologists, and histopathologic results for all lesions were collected from needle biopsy and surgical excisional biopsy reports. The interpreting pathologist determined whether the core-needle biopsy showed radial scar, complex sclerosing lesion, atypia, or malignancy according to standard pathologic standards applied in clinical practice. Radiologic-histopathologic concordance and discordance were determined by the radiologist performing the biopsy by assessing if histopathologic findings were representative of the imaging findings. At our institution, cases of RS are generally not presented in multidisciplinary conferences. In the case of discordance, the radiologist and pathologist may confer personally.
Statistical Analysis
Data were analyzed to determine the rate of malignancy upgrade upon surgical excision of RS. Given the rarity of these lesions, estimates are intended for subsequent meta and power analyses and, as such, are provided as counts and percentages. Clopper-Pearson interval estimate for upgrade was calculated for 95% confidence using PropCIs package with R 4.1.0 (R Foundation for Statistical Computing) (1). Comparisons between present study data and pooled results from the literature were conducted using generalized linear modeling assuming a binomial distribution with the GLIMMIX procedure with SAS software version 9.4 (SAS Institute). Demographics are provided at the patient level. Analyses were conducted by the statistical author (G.L.B.).
Results
Demographics
A total of 155 885 screening DBT were performed during the study period. A total of 142 patients with 146 biopsy-proven RS were identified from a search of the pathology database. One patient had three RS and two patients had two RS. All patients were women with median age of 58 years (range, 26–87 years). Average predicted lifetime risk of breast cancer for patients for whom Gail score was obtainable (n = 73) was 12.0% (range, 2.3%–38.6%).
Manifestation on Imaging
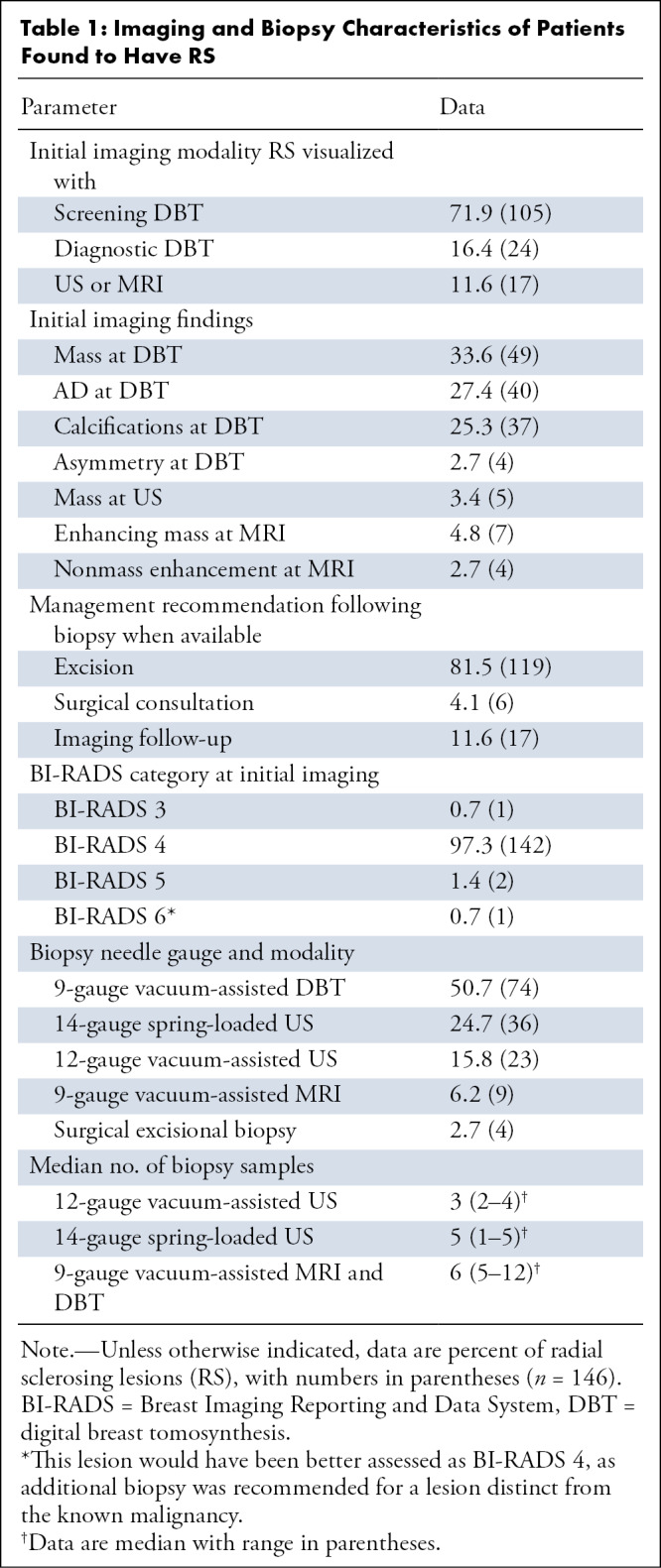
Of our cohort, the majority (71.9%, 105 of 146) of RS were found at screening DBT; 16.4% (24 of 146) were found at diagnostic DBT. Of the 146 biopsy-proven RS, 33.6% (49 of 146) manifested on DBT images as a mass, 27.4% (40 of 146) as an AD, 25.3% (37 of 146) as calcifications, and 2.7% (four of 146) as asymmetry. A total of 3.4% (five of 146) were best visualized at US as a mass; 4.8% (seven of 146) were best visualized as an enhancing mass on MR images and 2.7% (four of 146) as nonmass enhancement. All patients underwent screening or diagnostic DBT initially, though the diagnostic lesion was better visualized with other modalities in the above cases of US or MRI findings (Table 1).
Table 1:
Imaging and Biopsy Characteristics of Patients Found to Have RS
Imaging Assessment
A majority of the lesions (97.3%, 142 of 146) were categorized as BI-RADS category 4 by the radiologist, indicating a suspicious abnormality where biopsy should be considered. A total of 0.7% (one of 146) of the radiographic lesions were assessed as BI-RADS 3; 1.4% (two of 146) were assessed as BI-RADS 5, and 0.7% (one of 146) were assessed as BI-RADS 6 (patient with newly diagnosed breast cancer who underwent additional biopsy for indeterminate microcalcifications). This lesion would have been better assessed as BI-RADS 4, as additional biopsy was recommended for a lesion distinct from the known malignancy.
The radiologist who performed the biopsy recommended excision for 81.5% (119 of 146) of the detected RS. For 4.1% (six of 146) of the lesions, the radiologist recommended surgical consultation for consideration of excision versus imaging follow-up. For 11.6% (17 of 146) of the lesions, the radiologist recommended imaging follow-up. One lesion (one of 146, 0.7%) went directly to excision, as needle biopsy was not feasible. The radiologist recommendations were not available in 2.0% (three of 146), and 2.7% (four of 146) were assessed as discordant and recommended for excision, including one RS with atypia (Table 1).
A total of 79.5% (93 of 117) of the lesions without atypia at initial biopsy were recommended for excision and 0.9% (one of 117) of these were upgraded at excision. This was assessed as concordant following needle biopsy and was recommended for excision.
Biopsy Modalities
Details of image-guided biopsies, including needle type and number of samples obtained, are shown in Table 1.
Histopathologic Assessment
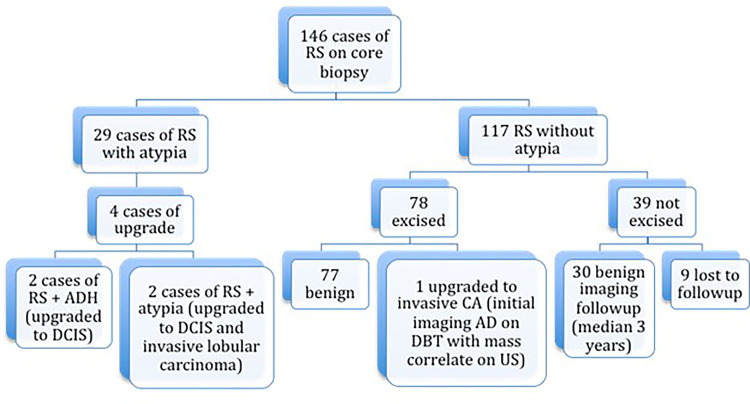
Of the 146 biopsy-proven RS lesions, 80.1% (117 of 146) demonstrated radial scar without associated atypia or cancer at the biopsy site and were classified as RS without atypia. The remaining 19.9% (29 of 146) had concurrent atypia at the biopsy site (Fig 1).
Figure 1:
Flow diagram of cases of radial sclerosing lesions (RS) and radial scars with and without atypia at core biopsy and results of excision or imaging follow-up. AD = architectural distortions, ADH = atypical ductal hyperplasia, CA = carcinoma, DBT = digital breast tomosynthesis, DCIS = ductal carcinoma in situ.
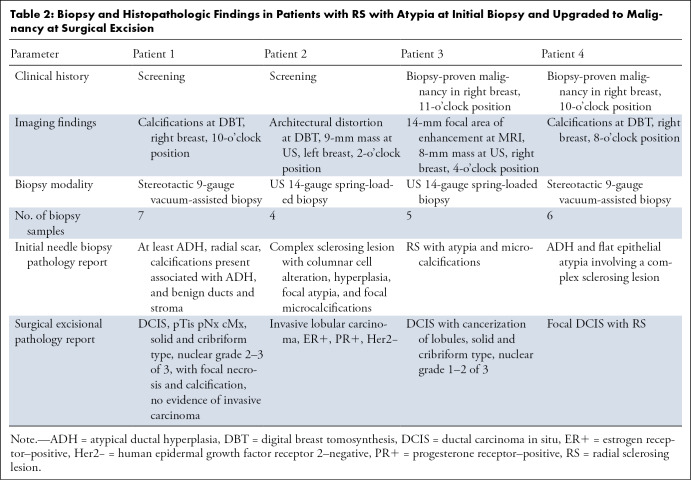
Of the 29 RS with atypia, 14% (four of 29) were upgraded to DCIS or carcinoma at the time of surgical excision. The remaining 86% (25 of 29) had no upgrade at surgical excision. Of the RS with atypia at initial biopsy, 41% (12 of 29) had atypia at excision: 21% (six of 29) had lobular neoplasia, and 48% (14 of 29) had atypical ductal hyperplasia, with some having more than one type of atypia at the biopsy site. Specific pathologic features at needle biopsy and surgical excision of the four RS lesions with atypia that were upgraded at surgical excision are presented in Table 2.
Table 2:
Biopsy and Histopathologic Findings in Patients with RS with Atypia at Initial Biopsy and Upgraded to Malignancy at Surgical Excisio
Of the 117 RS lesions without atypia, 66.7% (78 of 117) were surgically excised. The remaining 33.3% (39 of 117) were either lost to follow-up (7.7%, nine of 117) or showed benign findings at follow-up imaging (25.6%, 30 of 117), with median follow-up of 3 years (range, 1–7 years). One hundred percent (30 of 30) of RS lesions without atypia that were followed with imaging alone were benign.
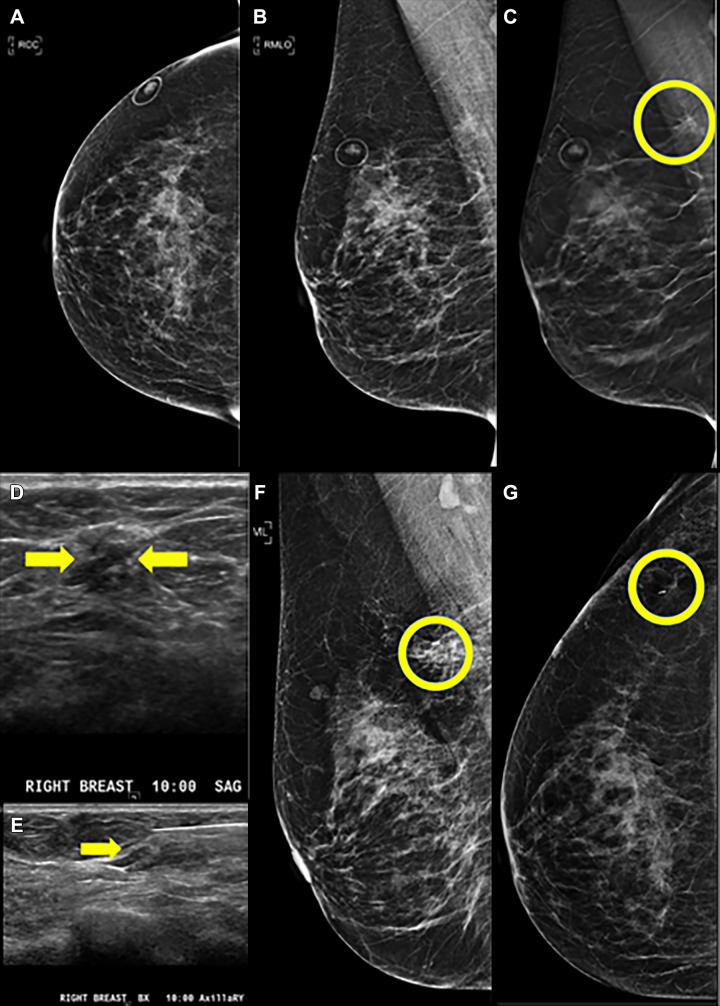
One RS without atypia at biopsy was upgraded to invasive carcinoma (0.9%, one of 117 [95% CI: 0.02, 4.7]). This lesion presented as AD at DBT (Fig 2A–2C) with a corresponding mass at US (Fig 2D, 2E). Mammogram obtained after biopsy clip placement shows the clip in the abnormality (Fig 2F, 2G), but the US biopsy image (Fig 2E) shows the needle slightly superficial to the mass. This patient had a history of contralateral breast cancer treated with segmental excision and radiation therapy 7 years prior. At excisional biopsy, a 7-mm estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, invasive tubular carcinoma Nottingham grade I of III with no lymphovascular invasion was identified adjacent to, but distinct from, the biopsy needle track. Sampling error likely contributed to this upgrade.
Figure 2:
Images in a 66-year-old woman with previous history of left breast cancer treated with segmental excision and radiation therapy 7 years prior. Digital breast tomosynthesis (DBT) helped detect radial sclerosing lesion (RS) without atypia in the contralateral (right) breast, which was upgraded to invasive carcinoma after surgical excision. (A) Craniocaudal (CC) digital mammogram and (B) mediolateral oblique (MLO) digital mammogram as well as (C) MLO DBT views. Architectural distortion with possible associated mass in the upper right breast posteriorly was optimally observed on DBT MLO view (circle). (D, E) Sagittal and biopsy US images show a subtle 7-mm hypoechoic mass (arrows) corresponding to the DBT finding. (F, G) Postbiopsy mediolateral and laterally exaggerated CC digital mammograms demonstrate clip placement within the area of architectural distortion. Core biopsy returned radial scar, which was assessed as concordant. Surgical excision was recommended. Final surgical excisional pathologic analysis demonstrated a 7-mm estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, invasive tubular carcinoma Nottingham grade I of III with no lymphovascular invasion, which was identified adjacent to, but distinct from, the biopsy needle track.
Discussion
Conventional two-dimensional mammography has been a stalwart of breast radiology practice. With the advent of DBT, this new technology is now the predominant form of screening in 41.8% of hospital referral regions in the United States and has improved the sensitivity of screening mammography and our ability to characterize lesions (5–9,28). As widespread clinical use of DBT has identified more RS (4,6,11–13), appropriate management of newly detected RS remains unclear. The purpose of this study was to determine the rate of upgrade to malignancy in RS without atypia and with atypia during a study period when all mammography was performed with DBT. We found that the majority (80.1%, 117 of 146) of RS were without concurrent atypia or malignancy and that the rate of malignancy upgrade among those lesions was minimal (0.9%, one of 117). In contrast, the rate of upgrade was 14% (four of 29) for RS with associated atypia. The proportion of RS without atypia or malignancy that we found (80.1%) is comparable to other studies (reported at 75% [91 of 157], 81% [77 of 95], and 87% [76 of 88]) (or 80.1% [95% CI: 62.3, 90.8] vs pooled: 71.8% [95% CI: 60.2, 81.0]; P = .19) (29–31, respectively). The proportion of RS with associated atypia we found (19.9%, 29 of 146) was also comparable to other studies reporting 25% (66 of 157), 19% (18 of 95), and 13% (12 of 88) (or 19.9% [95% CI: 9.2, 37.7] vs pooled: 28.2% [95% CI: 19.0, 39.8]; P = .19) (29–31, respectively).
The upgrade rate for RS associated with atypia in this study was 14%, which is within the range of upgrade rates reported previously (upgrade rate of 35% [38 of 108] for RS with atypical ductal hyperplasia [32] and upgrade rates of 17% [one of six] and 10% for RS with atypia [five of 49] [33,34]). These upgrade rates support surgical excision as an optimal management decision for patients with RS with atypia at biopsy.
Our very low upgrade rate of 0.9% (one of 117) for RS without atypia at biopsy strongly supports imaging follow-up over surgical excision as the best treatment for these patients. Similar upgrade rates have been reported in other studies (0% [zero of 92], 0.9% [one of 107], 1.9% [two of 105] 1% [one of 97]) for RS without atypia or malignancy at biopsy (26,32,33,35, respectively). In one study of DBT-biopsied RS by Martaindale et al, a similar upgrade rate of 2.8% (one of 36) was reported for cases without atypia at biopsy (33). Interestingly, Rakha et al demonstrated that the upgrade rate for RS and concurrent atypical ductal hyperplasia was similar to the upgrade rate of atypical ductal hyperplasia alone (32), supporting the hypothesis that atypical ductal hyperplasia is likely the main contributor to malignant potential.
An earlier study from our center (23) identified 100 RS without atypia before the widespread use of DBT; 0% (zero of 100) of these were upgraded to malignancy, though only 41 underwent surgical excisional biopsy. In contrast, in this current study, the majority of RS were detected with DBT, and the majority of biopsies were obtained with DBT guidance. This is not surprising, as DBT has been shown to help detect more AD and RS (4,6,11–13).
As Cohen et al postulated, biopsy sampling error may contribute to radiologic-histopathologic discordance and to false-negative findings (31,32,36). Our single upgraded RS without atypia was 7 mm in size and had three samples taken with a 12-gauge needle. Excisional histopathologic analysis demonstrated the biopsy needle tracked adjacent to, but not involving, the invasive carcinoma. In addition, US images from the biopsy show the needle was likely superficial to the targeted mass, thus sampling error likely contributed to this upgrade. While some studies have shown that taking more than 12 samples at biopsy (19,37) reduces the likelihood of missed associated carcinoma, AD poses a challenge with regard to sampling error, as the spiculations and distortion can extend over a fairly substantial distance. Another study demonstrated that a majority of RS upgrades had a needle track that missed the nearby malignancy by 6 mm or less (37). The single upgraded lesion in this study likely falls into this category. Precise needle targeting is critical to decreasing false-negative biopsy results.
In our study, 30 RS lesions without atypia did not undergo surgical excision; all had stable, benign imaging findings for a median duration of 3 years following biopsy, strongly supporting benignity. Another study demonstrating similar results is Kraft et al, where 50 of 50 patients undergoing active surveillance instead of excision did not progress on follow-up imaging (35). This is a larger cohort than in previous studies followed with imaging alone rather than surgical excision (23,33, 34, 38). For example, in Martaindale et al, 13 lesions were followed successfully for a median of 18 months with benign findings (33). The majority (66.7%, 78 of 117) of RS without atypia in our study were surgically excised. Replacing surgical excisions with follow-up imaging could have positive mental, emotional, and cosmetic outcomes for patients, with minimal risk of a missed cancer. Notably, we had nine patients who were lost to follow-up. This emphasizes the need to recommend follow-up with imaging carefully; establishing benignity in vulnerable populations who may have sporadic health care access necessitates a shared discussion of the feasibility of imaging follow-up. Increasing data regarding the minimal upgrade rates of RS without atypia in the DBT era may help inform management recommendations, potentially allowing more lesions to safely be followed with imaging rather than undergoing surgical excision.
This study was limited in its ability to recommend management practice given the small sample size. These are inherent limitations when studying an uncommon pathologic state at a small number of centers, though it does examine a larger cohort than some previous studies (33,35,39). In addition, nine patients were lost to follow-up; the upgrade rate could thus potentially be higher than the one reported. The radiologists interpreting imaging and performing needle biopsies were all fellowship-trained breast radiologists; thus, the results may not generalize to a general radiology practice. Additionally, we did not consider how other factors, such as the presence of multiple radial scars, distantly located concurrent cancer, or the unique breast cancer risk of the individual, may affect outcomes. With the small numbers of cases, it would be difficult to discern significance for any of these metrics. The one case of upgrade did have previously diagnosed contralateral breast cancer 7 years prior.
RS without atypia diagnosed at core-needle biopsy after DBT screening had a very low (0.9%) rate of upgrade to malignancy. Excision of RS without atypia may not be warranted; imaging follow-up of these lesions may be a reasonable management plan instead.
Authors declared no funding for this work.
Disclosures of conflicts of interest: P.Y. No relevant relationships. L.D. RSNA News editorial board member. G.L.B. No relevant relationships. A.P.L. No relevant relationships.
Abbreviations:
- AD
- architectural distortions
- BI-RADS
- Breast Imaging Reporting and Data System
- DBT
- digital breast tomosynthesis
- DCIS
- ductal carcinoma in situ
- DM
- digital mammography
- RS
- radial sclerosing lesion
References
- 1. Scherer R, Scherer MR. Package ‘PropCIs’. 2018.
- 2. Kim EMH, Hankins A, Cassity J, et al. Isolated radial scar diagnosis by core-needle biopsy: Is surgical excision necessary? Springerplus 2016;5(1):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nassar A, Conners AL, Celik B, Jenkins SM, Smith CY, Hieken TJ. Radial scar/complex sclerosing lesions: a clinicopathologic correlation study from a single institution. Ann Diagn Pathol 2015;19(1):24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ray KM, Turner E, Sickles EA, Joe BN. Suspicious Findings at Digital Breast Tomosynthesis Occult to Conventional Digital Mammography: Imaging Features and Pathology Findings. Breast J 2015;21(5):538–542. [DOI] [PubMed] [Google Scholar]
- 5. Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013;14(7):583–589. [DOI] [PubMed] [Google Scholar]
- 6. Dibble EH, Lourenco AP, Baird GL, Ward RC, Maynard AS, Mainiero MB. Comparison of digital mammography and digital breast tomosynthesis in the detection of architectural distortion. Eur Radiol 2018;28(1):3–10. [DOI] [PubMed] [Google Scholar]
- 7. Bernardi D, Caumo F, Macaskill P, et al. Effect of integrating 3D-mammography (digital breast tomosynthesis) with 2D-mammography on radiologists’ true-positive and false-positive detection in a population breast screening trial. Eur J Cancer 2014;50(7):1232–1238. [DOI] [PubMed] [Google Scholar]
- 8. Gennaro G, Hendrick RE, Toledano A, et al. Combination of one-view digital breast tomosynthesis with one-view digital mammography versus standard two-view digital mammography: per lesion analysis. Eur Radiol 2013;23(8):2087–2094. [DOI] [PubMed] [Google Scholar]
- 9. Lei J, Yang P, Zhang L, Wang Y, Yang K. Diagnostic accuracy of digital breast tomosynthesis versus digital mammography for benign and malignant lesions in breasts: a meta-analysis. Eur Radiol 2014;24(3):595–602. [DOI] [PubMed] [Google Scholar]
- 10. Lång K, Nergården M, Andersson I, Rosso A, Zackrisson S. False positives in breast cancer screening with one-view breast tomosynthesis: An analysis of findings leading to recall, work-up and biopsy rates in the Malmö Breast Tomosynthesis Screening Trial. Eur Radiol 2016;26(11):3899–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rochat CJ, Baird GL, Lourenco AP. Digital Mammography Stereotactic Biopsy versus Digital Breast Tomosynthesis-guided Biopsy: Differences in Biopsy Targets, Pathologic Results, and Discordance Rates. Radiology 2020;294(3):518–527. [DOI] [PubMed] [Google Scholar]
- 12. Zuley ML, Bandos AI, Ganott MA, et al. Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology 2013;266(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography. AJR Am J Roentgenol 2017;209(5):1162–1167. [DOI] [PubMed] [Google Scholar]
- 14. Durand MA, Wang S, Hooley RJ, Raghu M, Philpotts LE. Tomosynthesis-detected architectural distortion: management algorithm with radiologic-pathologic correlation. RadioGraphics 2016;36(2):311–321. [DOI] [PubMed] [Google Scholar]
- 15. Partyka L, Lourenco AP, Mainiero MB. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: initial clinical experience. AJR Am J Roentgenol 2014;203(1):216–222. [DOI] [PubMed] [Google Scholar]
- 16. American College of Radiology . ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2014. [Google Scholar]
- 17. Freer PE, Wang JL, Rafferty EA. Digital breast tomosynthesis in the analysis of fat-containing lesions. RadioGraphics 2014;34(2):343–358. [DOI] [PubMed] [Google Scholar]
- 18. Alshafeiy TI, Nguyen JV, Rochman CM, Nicholson BT, Patrie JT, Harvey JA. Outcome of Architectural Distortion Detected Only at Breast Tomosynthesis versus 2D Mammography. Radiology 2018;288(1):38–46. [DOI] [PubMed] [Google Scholar]
- 19. Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol 2002;179(5):1179–1184. [DOI] [PubMed] [Google Scholar]
- 20. Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med 1999;340(6):430–436. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Ranade A, Zhao C. Pathologic findings of follow-up surgical excision for radial scar on breast core needle biopsy. Hum Pathol 2016;48(76):80. [DOI] [PubMed] [Google Scholar]
- 22. Sohn VY, Causey MW, Steele SR, Keylock JB, Brown TA. The treatment of radial scars in the modern era--surgical excision is not required. Am Surg 2010;76(5):522–525. [PubMed] [Google Scholar]
- 23. Kalife ET, Lourenco AP, Baird GL, Wang Y. Clinical and radiologic follow-up study for biopsy diagnosis of radial scar/radial sclerosing lesion without other atypia. Breast J 2016;22(6):637–644. [DOI] [PubMed] [Google Scholar]
- 24. Leong RY, Kohli MK, Zeizafoun N, Liang A, Tartter PI. Radial scar at percutaneous breast biopsy that does not require surgery. J Am Coll Surg 2016;223(5):712–716. [DOI] [PubMed] [Google Scholar]
- 25. Resetkova E, Edelweiss M, Albarracin CT, Yang WT. Management of radial sclerosing lesions of the breast diagnosed using percutaneous vacuum-assisted core needle biopsy: recommendations for excision based on seven years’ of experience at a single institution. Breast Cancer Res Treat 2011;127(2):335–343. [DOI] [PubMed] [Google Scholar]
- 26. Farshid G, Buckley E. Meta-analysis of upgrade rates in 3163 radial scars excised after needle core biopsy diagnosis. Breast Cancer Res Treat 2019;174(1):165–177. [DOI] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richman IB, Hoag JR, Xu X, et al. Adoption of digital breast tomosynthesis in clinical practice. JAMA Intern Med 2019;179(9):1292–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chou WYY, Veis DJ, Aft R. Radial scar on image-guided breast biopsy: is surgical excision necessary? Breast Cancer Res Treat 2018;170(2):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quinn EM, Dunne E, Flanagan F, et al. Radial scars and complex sclerosing lesions on core needle biopsy of the breast: upgrade rates and long-term outcomes. Breast Cancer Res Treat 2020;183(3):677–682. [DOI] [PubMed] [Google Scholar]
- 31. Batohi B, Fang C, Michell MJ, et al. An audit of mammographic screen detected lesions of uncertain malignant potential (B3) diagnosed on initial image guided needle biopsy: how has our practice changed over 10 years? Clin Radiol 2019;74(8):653.e19–653.e25, 653.e25. [DOI] [PubMed] [Google Scholar]
- 32. Rakha E, Beca F, D’Andrea M, et al. Outcome of radial scar/complex sclerosing lesion associated with epithelial proliferations with atypia diagnosed on breast core biopsy: results from a multicentric UK-based study. J Clin Pathol 2019;72(12):800–804. [DOI] [PubMed] [Google Scholar]
- 33. Martaindale S, Omofoye TS, Teichgraeber DC, Hess KR, Whitman GJ. Imaging follow-up versus surgical excision for radial scars identified on tomosynthesis-guided core needle biopsy. Acad Radiol 2020;27(3):389–394. [DOI] [PubMed] [Google Scholar]
- 34. Gašljević G, Hertl K, Gazić B, Lamovec J, Žgajnar J. Reducing indications for radial scar surgical excision in Slovenian breast cancer screening program. Ann Diagn Pathol 2020;45(151438):151438. [DOI] [PubMed] [Google Scholar]
- 35. Kraft E, Limberg JN, Dodelzon K, et al. Radial scars and complex sclerosing lesions of the breast: prevalence of malignancy and natural history under active surveillance. Ann Surg Oncol 2021;28(9):5149–5155. [DOI] [PubMed] [Google Scholar]
- 36. Cohen MA, Newell MS. Radial scars of the breast encountered at core biopsy: review of histologic, imaging, and management considerations. AJR Am J Roentgenol 2017;209(5):1168–1177. [DOI] [PubMed] [Google Scholar]
- 37. Douglas-Jones AG, Denson JL, Cox AC, Harries IB, Stevens G. Radial scar lesions of the breast diagnosed by needle core biopsy: analysis of cases containing occult malignancy. J Clin Pathol 2007;60(3):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bacci J, MacGrogan G, Alran L, Labrot-Hurtevent G. Management of radial scars/complex sclerosing lesions of the breast diagnosed on vacuum-assisted large-core biopsy: is surgery always necessary? Histopathology 2019;75(6):900–915. [DOI] [PubMed] [Google Scholar]
- 39. Freer PE, Niell B, Rafferty EA. Preoperative tomosynthesis-guided needle localization of mammographically and sonographically occult breast lesions. Radiology 2015;275(2):377–383. [DOI] [PubMed] [Google Scholar]