Version Changes
Revised. Amendments from Version 1
Information on the historical annual average incidence of dengue in Medellin and Bello has been added to the background section. Minor clarifications have been made to the descriptions of Wolbachia deployment and monitoring methods. A case definition for the severe dengue secondary endpoint of the interrupted time series analysis has been included. Additional detail has been provided on the statistical methods for the ITS analysis. The study timeline has been removed to reflect an extension to the case-control study period in order to attain the target sample size, given a lower than expected initial event rate. An additional per-protocol analysis for the case-control study has been added. A discussion has been added of the potential for spillover effects from an interruption of dengue transmission in the case-control early release zone, into adjacent untreated areas. Two new authors have been added: Estefanía Muñoz and Suzanne M Dufault.
Abstract
Background: Dengue, chikungunya and Zika are viral infections transmitted by Aedes aegypti mosquitoes, and present major public health challenges in tropical regions. Traditional vector control methods have been ineffective at halting disease transmission. The World Mosquito Program has developed a novel approach to arbovirus control using Ae. aegypti stably transfected with the Wolbachia bacterium, which have significantly reduced ability to transmit dengue, Zika and chikungunya in laboratory experiments. Field releases in eight countries have demonstrated Wolbachia establishment in local Ae. aegypti populations.
Methods: We describe a pragmatic approach to measuring the epidemiological impact of city-wide Wolbachia deployments in Bello and Medellín, Colombia. First, an interrupted time-series analysis will compare the incidence of dengue, chikungunya and Zika case notifications before and after Wolbachia releases, across the two municipalities. Second, a prospective case-control study using a test-negative design will be conducted in one quadrant of Medellín. Three of the six contiguous release zones in the case-control area were allocated to receive the first Wolbachia deployments in the city and three to be treated last, approximating a parallel two-arm trial for the >12-month period during which Wolbachia exposure remains discordant. Allocation, although non-random, aimed to maximise balance between arms in historical dengue incidence and demographics. Arboviral disease cases and arbovirus-negative controls will be enrolled concurrently from febrile patients presenting to primary care, with case/control status classified retrospectively following laboratory diagnostic testing. Intervention effect is estimated from an aggregate odds ratio comparing Wolbachia-exposure odds among test-positive cases versus test-negative controls.
Discussion: The study findings will add to an accumulating body of evidence from global field sites on the efficacy of the Wolbachia method in reducing arboviral disease incidence, and can inform decisions on wider public health implementation of this intervention in the Americas and beyond.
Trial registration: ClinicalTrials.gov: NCT03631719. Registered on 15 August 2018.
Keywords: Wolbachia, dengue, chikungunya, Zika, vector-borne disease, disease surveillance, interrupted time series, Colombia
Abbreviations
BG trap: BioGents Sentinel trap; CI: cytoplasmic incompatibility; ELISA: enzyme-linked immunosorbent assay; IRB: Institutional Review Board; PECET: Programa de Estudio y Control de Enfermedades Tropicales; qPCR: qualitative polymerase chain reaction; SAE: serious adverse event; WEI: Wolbachia exposure index; WHO: World Health Organisation; wMel: Wolbachia pipientis; WMP: World Mosquito Program
Background
Dengue is a major public health challenge in tropical regions, with 50 – 100 million symptomatic cases estimated to occur each year 1, 2 . The World Health Organisation (WHO) cites a 30-fold increase in global incidence during the past 50 years 1 , and among endemic regions the greatest relative increase in dengue disease burden over the past two decades has been seen in Latin America 2 . The primary vector for dengue, the Aedes aegypti mosquito, also transmits the chikungunya and Zika viruses. Chikungunya emerged in epidemic fashion in several Indian Ocean islands in 2004 before spreading to southern Europe and South and South East Asia, then in 2013 re-emerged in epidemics in the Caribbean and several Latin American countries 3 . Following Zika virus outbreaks in the Western Pacific in 2013 and in Latin America in 2015 4 , it was declared a public health emergency of international concern by the WHO in 2016 5 as evidence accumulated that congenital Zika virus infections can result in severe outcomes including foetal death and severe microcephaly. No specific treatment for dengue, chikungunya or Zika currently exists. Although a vaccine against dengue (Sanofi Dengvaxia ®) was licensed in 2015, the WHO recommends vaccination only in persons with proven past dengue infection 6, 7 . While efforts to develop a safe and effective vaccine continue, the WHO has emphasised the need for innovations in vector control to achieve reductions in dengue virus transmission and disease burden 8 . The evidence base for the effectiveness of commonly used vector control interventions is limited, with few having been rigorously evaluated against a clinical disease endpoint 9 . This highlights a vital need for carefully designed studies to evaluate vector control methods for arboviral and other vector-borne diseases 10 .
The World Mosquito Program (WMP; formerly the Eliminate Dengue Program) is an international research collaboration that is delivering a paradigm shift in the control of arboviral diseases transmitted by Ae. aegypti mosquitoes. Our method utilises Wolbachia, obligate intracellular endosymbionts that are common in insect species 11– 14 but were not present in Ae. aegypti mosquitoes until they were stably transinfected in the laboratory. In insects, Wolbachia is maternally inherited and manipulates insect reproduction to favour its own population dissemination via cytoplasmic incompatibility (CI). Strikingly, the presence of Wolbachia in Ae. aegypti mosquitoes reduces their ability to transmit viruses including dengue, Zika, chikungunya, and yellow fever 15– 17 . Introgression of Wolbachia into wild Ae. aegypti populations is thus expected to severely reduce the vectorial capacity of local mosquito populations to transmit these arboviral infections. WMP’s field teams release male and female Wolbachia-infected Ae. aegypti as eggs or adults over a number of weeks. These mosquitoes then breed with the wild mosquito population and over time, through the actions of CI, the prevalence of Wolbachia in the local mosquito population increases, until such time as the majority of mosquitoes in the area carry Wolbachia. The WMP has demonstrated reduced vector competence in Wolbachia-infected mosquitoes obtained from the field, using human dengue viremic blood and a novel read-out to measure infectious mosquito saliva 18 . Wolbachia viral interference effects were found for all four DENV serotypes, resulting in estimated reductions of 66–75% in the basic reproduction number R 0 for DENV-1-4. Reductions of this magnitude are predicted to result in local elimination of DENV transmission in most epidemiological settings 18 .
Colombia, located in the northwestern region of South America, is home to one-tenth of the population of Latin America. The Ae. aegypti mosquito is highly prevalent and dengue is endemic. In 2010 Colombia recorded its largest dengue outbreak with more than 150,000 confirmed cases, 217 deaths, and simultaneous circulation of all four dengue serotypes 19 . The first autochthonous chikungunya case was detected in Colombia in September 2014 20, 21 and the first case of Zika in October 2015 22 . Since then, numerous cases of both have been reported in the country.
The protocol presented in the current paper describes a pragmatic approach to measuring the efficacy of large-scale Wolbachia deployments in reducing the burden of arboviral diseases, in the municipalities of Medellín and Bello in northwestern Colombia, which have urban populations of 2.2 million in an area of ~100km 2 and 476,000 in ~20km 2, respectively. The mean annual incidence of notified dengue cases in the seven years 2010–2016 prior to the start ofscaled Wolbachia deployments was 298 per 100,000 population in Medellin (range 38 – 771 per 100,000) and 188 per 100,000 population in Bello (range 36 – 446 per 100,000). The mean annual incidence of notified dengue cases in the seven years 2010–2016 prior to the start of scaled Wolbachia deployments was 298 per 100,000 population in Medellin (range 38 – 771 per 100,000) and 188 per 100,000 population in Bello (range 36 – 446 per 100,000). Staged deployment at the city-wide scale and within a relatively short time frame was favoured over a randomised controlled trial or other randomised design both by funders and local stakeholders. This was driven by what was, at the time of project conception, an urgent need for novel scalable strategies to combat the threat of Zika, and also a desire for the flexibility to optimise methods for scaled deployment under operational conditions, rather than the more restrictive implementation required for a formal randomised controlled trial. The proposed strategy for evaluating the impact of these staged deployments on the incidence of arboviral disease is a combination of an interrupted time-series analysis of notified arboviral disease incidence in all of Medellín and Bello, together with a more rigorous test-negative design study implemented in a sub-section of the Medellín municipal area. The primary aim of the study is to investigate whether large-scale deployments of Wolbachia-infected Ae. aegypti mosquitoes in Medellín and Bello, Colombia, lead to a measurable reduction in arboviral disease incidence.
Methods
Two complementary approaches will be used to evaluate the disease impact of Wolbachia releases in Medellín and Bello:
-
i
An interrupted time-series analysis utilising routine disease surveillance data collected by the Medellín and Bello Health Secretariats, which aims to compare incidence of dengue, chikungunya and Zika pre- and post- Wolbachia release. This analysis will be applied separately to Medellín and Bello.
-
ii
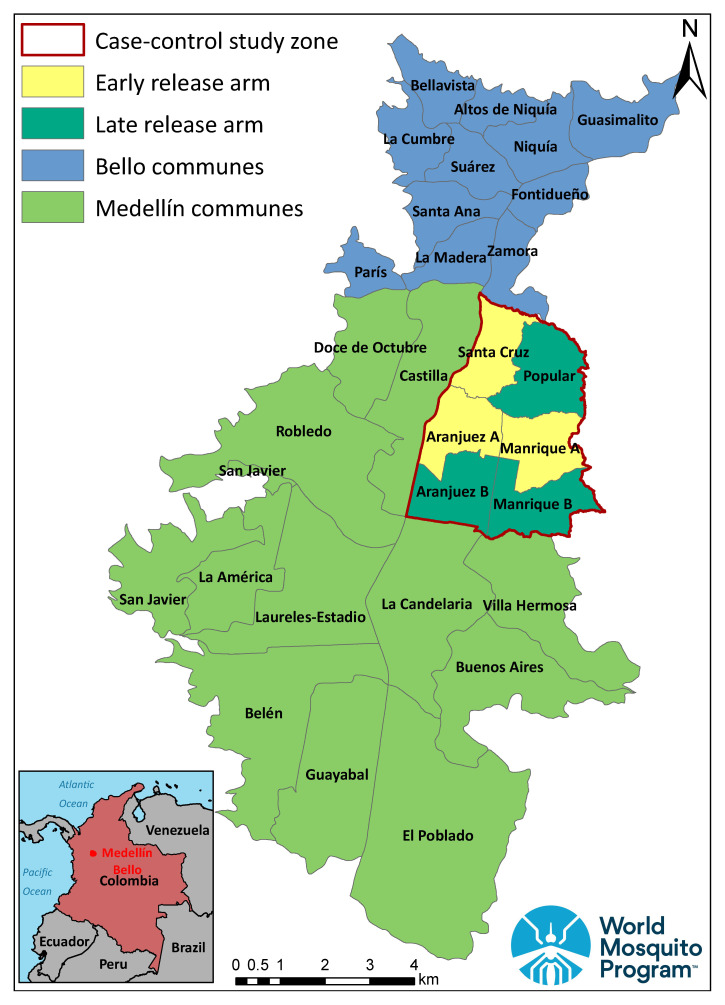
A prospective case control study using a test-negative design, which aims to quantify the reduction in disease incidence among people living within a Wolbachia-treated zone compared with an untreated zone that has a similar dengue risk profile at baseline. This study will be conducted in only one quadrant of Medellín ( Figure 1).
Figure 1. Deployment of Wolbachia across Medellín and Bello, combining pragmatic staged deployment (light green and blue) with a test-negative design study in a focused study area of ‘early’ (yellow) and ‘late’ (dark green) release zones (produced in ArcMap version 10.5, ESRI, CA).
Wolbachia deployment
Wolbachia-containing adult Ae. aegypti mosquitoes and eggs will be deployed sequentially through the study area, starting with the early-release zone of the case-control study (yellow shading in Figure 1; produced in ArcMap version 10.5, ESRI, CA), followed by releases in parts of Medellín and Bello outside the case-control area (light green and blue shading in Figure 1), then lastly in the late-release area of the case-control study (dark green shading in Figure 1). For the purpose of analysis, release zones will be considered Wolbachia-treated from the date of completion of releases.
An initial period of Wolbachia deployment was undertaken in the early-release zone of the case-control area and in parts of Bello between April and December 2017, resulting initially in a high prevalence of Wolbachia. However, Wolbachia levels declined after cessation of releases. We believe this was due to fitness issues with the Wolbachia-infected mosquitoes, specifically that they were less resistant to insecticides than the wild population. Insectary processes were subsequently updated and a new colony of Wolbachia mosquitoes was produced that matched the wild-type insecticide resistance profile. Subsequent rounds of deployments were then conducted in these areas between mid-2018 and late-2019. It is this deployment period that will be considered for the purpose of the analyses described in this protocol.
Wolbachia monitoring strategy
Wolbachia prevalence is monitored through a network of BG-Sentinel adult mosquito traps (BioGents) that are evenly spaced throughout all of Bello and Medellin, including the case-control study area at a density of approximately 16 BG traps per km 2. BG traps are serviced weekly, with trapped mosquitoes screened for Wolbachia at weekly, fortnightly or monthly intervals throughout the duration of the study, depending on the stage of release and establishment. BG traps that do not catch any mosquitoes in three consecutive weeks are moved to another location. Trapped mosquitoes will be identified using microscopy. Individual Ae. aegypti mosquitoes (male and female) will be tested for Wolbachia by quantitative polymerase chain reaction (qPCR) assay. The Wolbachia prevalence in screened Ae. aegypti will be reported aggregated to the zone (i.e. early- and late-release zone in case-control area) or commune (for parts of the city outside of the case-control area) level, calculated as the total number of Ae. aegypti mosquitoes that tested positive for Wolbachia aggregated across all BG traps in the zone/commune, divided by the total number of Ae. aegypti mosquitoes that were screened in that zone/commune.
Epidemiological study 1: Interrupted time-series analysis using notifiable disease surveillance data
Notifiable disease surveillance data. In Medellín and Bello, routine public health surveillance for dengue is passive. There are more than 400 public and private health institutions that routinely report clinically-suspected and laboratory-confirmed dengue cases to the Secretary of Health as part of the Epidemiological Surveillance System. Approximately 10% of clinically-suspected dengue cases from hyperendemic or epidemic territories in Colombia are laboratory tested, however this proportion varies and was as high as >60% in 2016 in Medellín. Laboratory evidence suggestive of dengue is usually acquired via detection of anti-dengue IgM antibodies. Laboratory testing for chikungunya and Zika is not routinely performed in Colombia and is only done to demonstrate viral circulation in the area rather than for diagnostic purposes.
For the interrupted time-series analysis, disaggregate (line-listed) data will be requested for notified (clinically-suspected) dengue, chikungunya and Zika cases, and also the subset of dengue cases with IgM ELISA test results, from 2009 to 2025 for both Medellín and Bello. The dataset will include age, sex, address of primary residence, date of illness onset, date of notification, reporting health clinic, disease severity, hospitalisation, death, and, where available, geo-coordinates of the primary residence, type of diagnostic test performed, diagnostic test result, and final diagnostic classification.
Primary and secondary endpoints. The primary endpoint is the incidence of all dengue case notifications to the Epidemiological Surveillance System.
The secondary endpoints are: i) the number of cases who were IgM test positive for dengue; ii) the incidence of severe dengue cases reported to the surveillance system; and iii) the incidence of Zika and chikungunya cases reported to the surveillance system.
Severe dengue is defined as any case of dengue that has one or more of the following manifestations: i) shock or respiratory distress due to severe plasma leakage; ii) severe bleeding according to the evaluation of the treating physician; or iii) severe organ involvement, such as liver damage, impaired consciousness, myocarditis, or other organ involvement.
Statistical analysis. For the primary endpoint and secondary endpoints above, the impact of Wolbachia deployment on disease incidence will be evaluated using an interrupted time series analysis of arbovirus cases reported to the Epidemiological Surveillance System before and after Wolbachia establishment. A generalised estimating equation (GEE) approach will be used to model monthly case counts as the outcome variable, with an offset for population size and controlling for seasonality and inter-annual variation using flexible cubic splines or polynomial functions. The outcome distribution is assumed to be negative binomial to allow for overdispersion. The Wolbachia intervention effect will be modelled firstly as a binary predictor comparing dengue incidence pre- vs post-intervention to estimate the level change in incidence following the Wolbachia intervention. An additional analysis will consider Wolbachia frequency as a continuous covariate or categorised into quintiles of exposure reflecting the measured Wolbachia prevalence in the local mosquito population. Robust standard errors will be used to account for clustering of cases by commune. An autoregressive correlation structure will be specified to account for temporal autocorrelation. This analysis will be done separately for Medellín and Bello, 12 months after the completion of releases and each 12 months thereafter until five years post-intervention. The staged nature of releases across communes allow the pre-intervention period in each commune to serve as a contemporaneous untreated comparator for the treated communes, in a simple uncontrolled ITS analysis, until all communes are Wolbachia-treated. A controlled ITS analysis will also be undertaken, using other Colombian municipalities with synchronous historical dengue time series as an untreated comparator.
There is a possibility that the inference estimation of the GEE approach is affected by the modest number of clusters (18 in Medellin and 11 in Bello) each with a large number of observations. A mixed-effects negative binomial model will be used to check for small sample size issues in inference estimation. A difference in estimates between the GEE approach and mixed-effects model suggests small sample size could be a biasing factor, and depending on the direction of the discrepancy, may be related to a tendency towards inflated Type 1 error rates when using the GEE technique with small cluster numbers or inappropriate modelling assumptions in the mixed-effects model. Additional follow-up analyses may be required.
Epidemiological study 2: Prospective case control study
Study design. The prospective clinic-based case control study uses a test-negative design. The impact of Wolbachia deployments on arboviral disease incidence will be assessed by comparing the exposure distribution (probability of living in a Wolbachia-treated (early-release) vs. untreated (late-release) area) among virologically-confirmed arboviral disease cases presenting to a network of primary healthcare clinics, against the exposure distribution among patients with febrile illness of non-arboviral aetiology presenting to the same network of clinics in the same temporal window. Arboviral disease cases and arbovirus-negative controls will be sampled concurrently from within the population of patients who reside in the case-control area and present with febrile illness to the study clinic network, with case or control status classified retrospectively based on the results of laboratory diagnostic testing. The distribution of Wolbachia exposure in the sampled arbovirus negative controls is assumed to reflect the distribution of Wolbachia exposure in the underlying source population that gave rise to cases, as long as a core assumption is met that the relative propensity to seek healthcare for febrile illness at the study clinics in early-versus late-release arms is the same for arboviral disease cases as other febrile illness controls. This should be upheld if cases and controls are clinically indistinguishable until laboratory diagnosis. The concurrent sampling of cases and controls means that the odds of Wolbachia-exposure among sampled arboviral disease cases relative to febrile controls (i.e. odds ratio), is an unbiased estimate of the relative incidence of medically-attended arboviral disease in Wolbachia early-release versus late-release areas (i.e. relative risk or incidence rate ratio), from which protective efficacy can be estimated directly.
Study setting. The case-control study will be conducted only within a focused study area in northeast Medellín, including six contiguous release zones within four communes ( Figure 1), with a total population of 580,000 and area 15km 2. Among these six release zones, three have been allocated non-randomly as the first zones in Medellín to receive Wolbachia deployments (early-release), and three as the last (late-release), such that a parallel two-arm trial is approximated for the period during which Wolbachia exposure remains discordant between arms. There are no buffer areas between treatment arms, but natural borders (roads, rivers, non-residential areas) were used to define study arm boundaries as much as possible, to limit the spatial spread of Wolbachia from treated areas into untreated areas, and of wild-type mosquitoes into Wolbachia treated areas. No attempt will be made to alter the routine dengue prevention and vector control activities conducted by public and private agencies throughout the case-control study area.
Allocation of the intervention. The allocation of the six zones into two arms was done in a way that maximises balance between the arms with respect to measured factors that may be associated with baseline dengue risk, including historical dengue incidence, population characteristics, and geographical area ( Table 1).
Table 1. Allocation of the six release zones into ‘early’ and ‘late’ release arms, maximizing balance between the two arms in baseline factors that may predict dengue risk.
| Ratio of baseline characteristics in late/early arms
(Ratio of 1 is perfectly balanced) |
||||||
|---|---|---|---|---|---|---|
| ‘Early’ Arm | ‘Late’ Arm | Aggregate dengue
incidence 2013–2016 |
Population | Area | % population <15
years |
Socio-economic
status |
| Aranjuez A,
Manrique A, Santa Cruz |
Aranjuez B,
Manrique B, Popular |
1.18 | 1.02 | 1.20 | 1.00 | 1.11 |
Study participants. Participants will be invited to participate, by trained research staff, from within the population of patients presenting with undifferentiated fever to a network of primary health care facilities that serve the population who reside in the study area. Participants (or their guardian if <18 years) must provide written informed consent, and meet the following inclusion criteria to be eligible for the study: fever (either self-reported or objectively measured as ≥38° C), with a date of onset between 1–4 days prior to the day of presentation to the health care facility; aged ≥3 years old; and lived (i.e. slept) in the study area for the 10 days preceding illness onset. Participants will not be eligible for inclusion if localizing features suggestive of a specific diagnosis (e.g. severe diarrhoea, otitis, pneumonia) are identified. An individual presenting to the clinic on repeat occasions for different febrile episodes will be eligible for enrolment during each different episode. However, an individual may only be enrolled once during a single illness episode, which is defined as illness occurring within 4 weeks of a previous febrile episode.
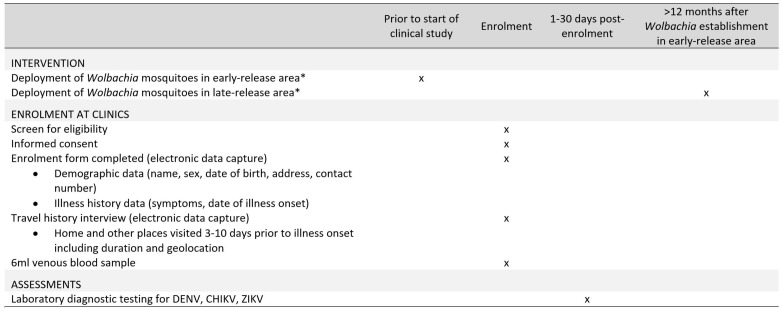
Data and sample collection. A unique identifier will be assigned to each participant at enrolment. Basic demographic details, eligibility against the inclusion criteria, illness onset date, and a retrospective travel history will be recorded in a standardised electronic data collection form. Figure 2 summarises the data and sample to be collected from each participant.
Figure 2. Schedule of enrolment, data collection and assessments (SPIRIT Figure).
*Routine dengue prevention and vector control activities will not be altered in treated or untreated areas. DENV: dengue virus; CHIKV: chikungunya virus; ZIKV: Zika virus.
A brief travel history interview will be conducted at enrolment to determine the main places visited by each participant within the 3–10 days prior to illness onset, i.e. the incubation period for dengue. The duration of time spent at home, work or school, and other visited locations during the hours of 5am to 9pm in the 8-day period will be recorded, and the geographic coordinates of those locations derived by geo-locating them on a digital map, with the assistance of the study participant. These data will be used to determine the proportion of time spent in Wolbachia treated and untreated areas, for the per-protocol analysis.
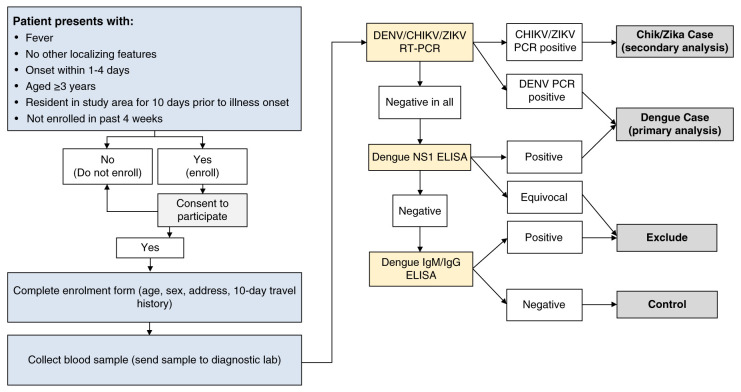
A single 6 ml venous blood sample will be collected from all consenting participants on the day of enrolment. Blood samples from all participants will be transferred to the project laboratory on the day of collection and batch-tested within one month to determine case or control status ( Figure 3).
Figure 3. Flowchart of data and sample collection procedures and diagnostic algorithm.
Blue boxes indicate participant recruitment and enrolment activities undertaken at clinics. Yellow boxes indicate the laboratory diagnostic testing to be performed at the project laboratory, the results of which (white boxes) will be used to classify participants (grey boxes) as virologically confirmed dengue, Zika or chikungunya cases, arbovirus-negative controls, or excluded due to inability to rule out arbovirus infection according to the algorithm shown. DENV: dengue virus; CHIKV: chikungunya virus; ZIKV: Zika virus; PCR: polymerase chain reaction; NS1: non-structural protein 1; ELISA: enzyme-linked immunosorbent assay; IgM/IgG: immunoglobulin M/G.
Laboratory investigations. An internally controlled triplex RT-qPCR assay (Bio-Rad) will be used to detect dengue, chikungunya and Zika viruses in serum samples from all enrolled participants. Dengue NS1 ELISA (Dengue Early ELISA, Panbio) will be performed according to the manufacturer’s instructions, on serum samples which have tested negative by DENV RT-qPCR. Dengue IgM and IgG capture ELISA (IgM/IgG Capture ELISA, Panbio) will be performed on serum samples which have tested negative by DENV RT-qPCR and NS1, and would otherwise be classified as controls, to determine whether they have detectable dengue IgM or IgG antibodies indicating potentially acute secondary dengue or another cross-reactive flavivirus infection in order to prevent misclassification. The IgG capture ELISA is designed with cutoffs to detect only high IgG titers consistent with acute secondary dengue (or cross-reactive flavivirus) infections, not past dengue virus infections. All research diagnostic investigations will be performed by the Programa de Estudio y Control de Enfermedades Tropicales (PECET), at the University of Antioquia, Colombia.
Case and control classification. Dengue cases are defined as patients with virologically-confirmed DENV infection, meeting the clinical criteria for enrolment and also with a positive result in NS1 ELISA or DENV RT-qPCR. Controls are patients meeting the clinical criteria for enrolment, but with negative test results for DENV RT-qPCR, CHIKV RT-qPCR, ZIKV RT-qPCR, DENV NS1 ELISA, and DENV IgM and IgG ELISA ( Figure 3).
For the secondary endpoints, Zika or chikungunya cases are defined as patients with virologically-confirmed Zika or chikungunya infections, meeting the clinical criteria for enrolment and with a positive result in ZIKV RT-qPCR or CHIKV RT-qPCR, respectively, and controls are defined as above.
Expected study duration. Pilot clinic-based sampling of febrile patients commenced in November 2017, with enrolment into the intention-to-treat dataset after completion of Wolbachia releases in the early-release area. The study will continue to enrol participants until such time as Wolbachia deployment commences in the late-release area, which will not be before 2021, i.e. enrolment can continue even when the target sample size is reached.
Power calculations. It is estimated that 88 test-positive cases plus four times as many controls will be sufficient to detect a 50% reduction in dengue incidence with 80% power. Thus, we set the target sample size as 100 test-positive dengue cases and expect that by including in the analysis all participants enrolled after Wolbachia is established in the early zone, there will be in excess of 400 test-negative controls for 100 test-positive cases. Although we expect the true effect of Wolbachia on dengue transmission may be greater than a 50% reduction, the observable reduction in effect is expected to be lower because individuals are likely to spend a substantial proportion of their time outside their release zone of residence. These sample size estimates are based on standard formulae for calculating sample size/power in a case control study. They align with the proposed approach for estimating the intervention effect.
Statistical analysis. The analyses described here will be performed on datasets of cases and controls defined firstly using the primary endpoint of virologically-confirmed dengue cases, and then using the endpoints of virologically-confirmed chikungunya and Zika cases.
The intention-to-treat analysis will consider Wolbachia exposure as a binary classification based on residence in the early or late-release area. Residence will be defined as the primary place of residence during the 10 days prior to illness onset. The intervention effect will be estimated from an aggregate odds ratio (for data aggregated across all three Wolbachia-release zones within each study arm) comparing the exposure odds (residence in the Wolbachia early-release area) among test-positive cases versus test-negative controls. The null hypothesis is that the odds of residence in a Wolbachia early-release area is the same among test-positive cases as test-negative controls.
The per-protocol analysis will consider Wolbachia exposure as a quantitative index based on measured Wolbachia prevalence in local Ae. aegypti mosquitoes in the locations visited by the participant during the 10 days prior to illness onset, both within the case control area and in elsewhere in Medellín and Bello. The per-protocol analysis therefore allows for Wolbachia exposure to vary in a location over time, and also accounts for human mobility, in terms of the exposure-time that individuals spend outside their area of residence as reported in the travel history interview at enrolment. A weighted ‘ Wolbachia exposure index’ (WEI) will be defined for each participant, as follows. The aggregate Wolbachia prevalence for each release zone will be calculated each month from all Ae. aegypti trapped in that zone. Time spent outside a Wolbachia release area will be treated as not Wolbachia exposed. The WEI for each participant will then be calculated by multiplying the zone-level Wolbachia prevalence (in the month of participant enrolment) at each of the locations visited, by the proportion of time spent at each location, to give a value on a continuous scale from 0 to 1.
An additional per-protocol analysis will be conducted in which the WEI is calculated using only the zone-level Wolbachia prevalence in the participant’s cluster of residence (in the month of participant enrolment), ignoring the participant’s recent travel history. This recognises that dengue exposure risk may be higher at home versus other locations, rather than assuming an even distribution of exposure risk across daytime hours and locations visited.
Cases and controls will be classified by strata of their WEI (e.g. 0-0.2; 0.2-0.4; 0.4-0.6; 0.6-0.8; 0.8-1). This acknowledges that the WEI is not a highly precise measure and serves to reduce error in exposure classification. This analysis can also account for the temporal matching of arboviral disease cases and test-negative controls: risk sets of cases and controls will be defined by frequency matching enrolled confirmed arboviral disease cases to arbovirus-negative controls with illness onset in the same quarter of the year. In the unlikely event that a minimum of four controls cannot be found for a case within the same quarter, the window for matching can be extended until four controls are identified, for that case only. For a time-adjusted analysis, a Cox proportional hazard model will be fitted, which can incorporate the temporal case-control risk sets and participants’ WEI stratum as a categorical variable, using time since completion of Wolbachia releases as the time scale.
Data management. Clinical study data will be stored in a custom designed relational database hosted on a secure web-based server. Role-based, tiered access permissions will be used to control access to the clinical database and associated data capture applications. User logs will document the activities of all users. An audit trail will be preserved within the database to capture the history of any changes made to data records after their initial capture.
Data collected from participants in the case-control study will be captured through standardised electronic data capture forms and digital mapping interfaces, deployed as web-based applications on mobile tablets. Laboratory diagnostic results will be captured directly from laboratory assay output and uploaded to a web-based application for storage in the same relational database. Validity controls will be applied at the point of data capture into electronic forms, by predefining value ranges, specifying categorical option lists, and minimizing the use of free text fields. The use of carefully designed electronic forms will facilitate the coding of participant responses at the point of data collection. Quality control in the form of logic and consistency checks will be applied at the point of data capture into an electronic form and at the point of upload into the web-based database. All data relating to the case-control study, including field entomology and epidemiological data, will be retained indefinitely, and for a minimum of five years after study completion, in accordance with International Council for Harmonization on Good Clinical Practice requirements.
Monitoring of adverse events. Any severe adverse events (SAE) associated with collection of blood samples from study participants will be reported to the relevant institutional ethics committees within three days of notification. Standard SAE reporting forms will be used.
Study governance. A steering committee will be assembled to provide operational and strategic advice on planning and operations of the study. A WHO-convened independent evaluation group will review the study within a year of active enrolment as part of a program-wide evaluation of WMP Colombia activities in Medellín and Bello. The independent evaluation group will report its findings to all stakeholders at completion of the review.
Ethical considerations. The study protocol (version 3.0) and the informed consent document have been reviewed and approved by the Institutional Review Boards (IRBs) of the IPS Universitaria of Universidad de Antioquia, Colombia (No. 115, 25 Oct 2017 and No. 127, 12 Oct 2018), and Monash University, Melbourne (ID 11534). Any future protocol amendments will be submitted for review and approval by the same IRBs, prior to implementing protocol changes. The trial protocol was registered on ClinicalTrials.gov (NCT03631719) on 15 th August 2018.
Confidentiality of participant information will be strictly maintained at all times by the participating investigators, research staff and the sponsoring institution (Universidad de Antioquia) by means of a coded ID number. This confidentiality is extended to cover testing of biological samples in addition to all laboratory specimens, reports, data collection forms and log books, and geolocated records relating to participating subjects. All records that contain names or other personal identifiers, such as informed consent forms, will be stored separately from study records while identified by ID numbers. All local databases will be secured with password-protected access systems. No information concerning the study or the data will be released to any unauthorised third party, without prior written approval of the sponsoring institution. Clinical or personal information will not be released without written permission of the subject, except as necessary for monitoring by an ethical review board or regulatory agencies. Reporting of study results will not be done in any way that permits identification of individual participants, or the location of their homes or other visited locations.
Current study status
Participant recruitment into the prospective case-control study commenced in early 2019 and is ongoing. Wolbachia deployments across the two municipalities are ongoing through to 2020, and collation and analysis of disease surveillance data will continue until 2025.
Dissemination of study results
Analysis and reporting of the results of the prospective case-control study will occur only at completion of participant enrolment, and subject to the prior approval of the steering committee; there will be no interim analysis or dissemination of results. The interrupted time series analysis will be conducted 12 months after the completion of releases and annually thereafter. Findings from both studies will be submitted for peer review and publication in an appropriate open access journal, together with aggregate supporting data.
Discussion
Mosquito suppression remains the primary method used to control dengue virus transmission. A recent evaluation found little reliable evidence for the effectiveness of any dengue vector control method, and concluded that standardised studies of higher quality must be prioritised 9 . Aedes aegypti mosquitoes are the primary vectors of dengue, chikungunya and Zika viruses, therefore evidence-based interventions targeting this species have the potential to reduce multiple arboviral diseases where they co-circulate. The study described here will evaluate the impact of Wolbachia-infected mosquitoes on dengue and other arboviral diseases in Bello and Medellín municipalities, Colombia, using a combination of routinely collected disease surveillance data throughout the municipalities, and a prospective clinic-based test-negative study focused in one area of Medellín.
The test-negative design, a variant on the case-control design, which has been widely applied in non-randomised influenza vaccine effectiveness studies, uses outcome-based concurrent sampling of dengue cases and non-dengue controls to measure the efficacy endpoint 23– 26 . Effect estimates (odds ratios) from a test-negative design are equivalent to direct estimates of relative risk in the source population, under the assumption that the distribution of test-negative illness is not associated with the intervention, and that test-negative controls are allowed to include participants who may be classified as dengue cases at other times during the study period.
The interrupted time-series design is commonly used to evaluate the impact of public health interventions introduced at a population level and targeting population-level health outcomes 27– 29 . A series of repeat observations over time is analysed to establish a baseline trend which is assumed to be ‘interrupted’ by the introduction of an intervention. The subsequent post-intervention trend is compared to the counterfactual that would be expected in the absence of the intervention based on the baseline trend. A quantitative estimate of the intervention effect is derived from a segmented linear regression model of the outcome of interest (e.g. dengue case count or incidence) as a function of time, in which the intervention status is captured by a binary variable coded 0 for pre-intervention time points and 1 for post-intervention time points 30, 31 . Seasonality and other secular trends (e.g. ENSO) and time-varying confounders can also be controlled for by the inclusion of additional model parameters. A limitation of using notifiable disease surveillance data is the imperfect specificity of the clinical case definition used for notifications, meaning an unknown and time-varying proportion of notified cases are not true dengue infections. Inconsistent reporting practices, outbreaks of non-dengue febrile diseases, or other factors may induce secular trends in the surveillance data that are independent of, but contemporaneous with, the Wolbachia intervention and thus may influence our ability to estimate the true intervention effect from time series data. A subset of the notified dengue cases in Colombia have supportive laboratory diagnostic results, but these have several limitations: i) laboratory testing can be infrequent, particularly during outbreaks, ii) the cross-reactivity of IgM serology between dengue and Zika limits the utility of serological data where Zika co-circulates, and iii) no virological confirmation (PCR or NS1 antigen detection) of cases is performed. We therefore base our primary analysis on all notified dengue cases (suspected and dengue IgM test positive). The benefits of using these routinely collected surveillance data include the availability of a long time series, reduced costs for data collection and timely acquisition of data.
The pragmatic approach described here to evaluate disease impact arose from the imperative from funders and local stakeholders to achieve rapid scale-up of Wolbachia deployments and to retain flexibility in the release sequence and methods, in the context of the declaration of the Zika public health emergency at the time of project conception and funding 8 . These imperatives precluded implementation of the proposed test-negative design study across all of Medellín/Bello, given the time required to obtain approvals, establish clinical enrolment processes, train staff, and then maintain an untreated comparison area for the duration of clinical enrolment. Randomised allocation of the early and late Wolbachia release areas in the focused case-control study was also not feasible, given the small number of zones within the case-control area. In general, there is greater potential for selection bias in non-randomised studies than in randomised studies, which, in the context of the current study, may present as a differential distribution of non-arboviral febrile illness (i.e. test-negative controls) by study arm due to chance imbalance in the care-seeking populations between study arms or differential propensity for care-seeking among those in areas where Wolbachia is released compared with untreated areas. As long as a core assumption of the test-negative design is met that the relative propensity to seek healthcare for undifferentiated febrile illness at a study clinic, in early vs late zones, is the same for dengue cases as for other febrile illness controls, then an imbalance in participant enrolment between early and late zones should not in itself bias the results.
A staged approach to city-wide deployments following a ‘stepped-wedge’ design was also considered in planning the disease impact assessment for Medellín, however this carried a requirement to define a deployment sequence that maintained balance between ‘already treated’ and ‘not yet treated’ areas in factors associated with baseline dengue risk, to avoid selection bias. This had resource implications for the field entomology and community engagement teams’ activities that were inconsistent with the necessarily pragmatic approach to deployment in Medellín and Bello needed to achieve large-scale coverage within a short time frame.
The non-blinded deployment of the intervention means community members may alter their care seeking or vector control behaviour due to the belief that they are protected from dengue by Wolbachia. Blinding was determined to be cost prohibitive in this study as it would have doubled the resources and time required to conduct field releases of mosquitoes. Under the test-negative design, test-positive cases and test-negative controls are drawn from the same population of patients presenting to health clinics with febrile illness, who are clinically indistinguishable at the time of enrolment. Thus, the threat of bias due to non-blinding in the test-negative study is considered minimal as long as any modified behaviour applies equally to test-positive cases and test-negative controls. Routine vector control by health authorities were not altered as part of the study, and it is possible that some public health activities or change in community behaviour occurred which impacts arboviral disease incidence independently of Wolbachia, leading to an under- or over-estimation of the Wolbachia intervention effect in our study.
Contamination between adjacent Wolbachia-treated and untreated areas is a potential challenge in measuring the effectiveness of the Wolbachia intervention, and could theoretically arise from three sources: human mobility, mosquito movement, or spillover of the intervention effect due to broad suppression of dengue transmission. Given the highly focal nature of dengue transmission in urban settings 32– 34 it is unlikely, though possible, that interruption of dengue transmission in the Wolbachia-treated early-release area could suppress transmission also in the untreated late-release area. Human mobility may also confound the estimation of Wolbachia’s impact on arboviral disease, whereby individuals in whom the efficacy endpoint is measured may spend a proportion of their time outside their allocated study area (i.e. their area of primary residence) resulting in at least some exposure misclassification. There are no buffer zones separating the early-release and late-release areas of the case control study, nor the other areas of Medellín and Bello where staged deployments will occur, and Wolbachia spread from one area to another is possible but also measurable. The combined result of human mobility, Wolbachia contamination and any spillover effect is that the exposure status of the populations in the nominally ‘treated’ and ‘untreated’ areas become more similar to each other, thereby diluting any estimated intervention effect towards the null. By powering the case-control study to detect a relatively conservative effect size of 50%, we have allowed for some of this effect dilution while targeting a reduction in dengue incidence of public health significance. The case-control study per-protocol analysis, where recent travel history is documented and a quantitative Wolbachia exposure status is calculated for each participant, will account for both for the time spent in areas away from home and the local measure of Wolbachia prevalence in visited areas.
Inter-annual fluctuations in dengue transmission mean that the case-control study might fall in a period of lower incidence just by chance. Nevertheless, the target sample size of 100 dengue test-positive cases is seen as feasible even in the event of a low transmission period. The interrupted time-series analysis, which spans a longer period than the case-control study, is expected to have at least three to five years of post- Wolbachia release data, and thus should be more tolerant to periods of low dengue transmission.
The generalisability of the current study’s findings to other dengue endemic settings will likely be influenced by setting-specific factors such as the local entomological and climate context, which influences Wolbachia introgression. Generalisability may also depend on the local distribution of circulating DENV serotypes and the intensity of virus transmission, which might influence the observed impact of Wolbachia on arboviral disease incidence. A cost-effectiveness analysis is underway in Indonesia, and the findings are expected to help inform cost optimisation for different deployment scenarios, target settings, and scale-up methods that can be applied to other countries. If the results of the current study do demonstrate a reduction in arboviral disease incidence associated with Wolbachia deployment in Medellín and Bello, a key next step would be to scale this intervention into wider public health implementation in Colombia and elsewhere in the Latin American region, using epidemiological, ecological and cost-effectiveness data to inform an optimal strategy for scaled deployment.
Ethics approval and consent to participate
This study has been approved by the Institutional Review Boards of the IPS Universitaria of Universidad de Antioquia, Colombia, and Monash University, Melbourne. Written informed consent will be obtained from all participants, or their guardian where the participant is a minor (<18 years), prior to enrolment in the clinic-based case-control study. The interrupted time-series analysis uses pre-existing non-identifiable disease surveillance data, which does not require individuals’ consent.
Data availability
No data is associated with this article.
Acknowledgements
The authors acknowledge the contributions of Peter Ryan (World Mosquito Program) for the planning and implementation of Wolbachia deployments in Medellín and Bello, Tomas Santamaria (World Mosquito Program) for administrative support, and Rita Almanza and Carlos Montes (Medellín Health Secretariat) for support and provision of Medellin surveillance data.
Funding Statement
This work was supported by the Wellcome Trust working in partnership with the UK Department for International Development (Grant 102591/Z/13/A), United States Agency for International Development (Grant AID-OAA-AA-16-00081), and Bill & Melinda Gates Foundation (Grant OPP1159497).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved
References
- 1. World Health Organisation: Global strategy for dengue prevention and control 2012–2020.Geneva: WHO;2012. Reference Source [Google Scholar]
- 2. Stanaway JD, Shepard DS, Undurraga EA, et al. : The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–23. 10.1016/S1473-3099(16)00026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rolph MS, Foo SS, Mahalingam S: Emergent chikungunya virus and arthritis in the Americas. Lancet Infect Dis. 2015;15(9):1007–8. 10.1016/S1473-3099(15)00231-5 [DOI] [PubMed] [Google Scholar]
- 4. Weaver SC, Costa F, Garcia-Blanco MA, et al. : Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. 10.1016/j.antiviral.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organisation: Outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barre syndrome.2016; Accessed 2 May 2018. Reference Source [Google Scholar]
- 6. World Health Organisation: WHO Global Advisory Committee on Vaccine Safety Statement on Dengvaxia (CYD-TDV).2017; Accessed 2 May 2018. Reference Source [Google Scholar]
- 7. World Health Organisation: Revised SAGE recommendations on use of dengue vaccine.2018; Accessed 2 May 2018. Reference Source [Google Scholar]
- 8. World Health Organization: Mosquito (vector) control emergency response and preparedness for Zika virus.2016; Accessed 2 May 2018. Reference Source [Google Scholar]
- 9. Bowman LR, Donegan S, McCall PJ: Is Dengue Vector Control Deficient in Effectiveness or Evidence?: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10(3):e0004551. 10.1371/journal.pntd.0004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson AL, Boelaert M, Kleinschmidt I, et al. : Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015;31(8):380–90. 10.1016/j.pt.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 11. Hilgenboecker K, Hammerstein P, Schlattmann P, et al. : How many species are infected with Wolbachia?--A statistical analysis of current data. FEMS Microbiol Lett. 2008;281(2):215–20. 10.1111/j.1574-6968.2008.01110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Neill SL, Pettigrew MM, Sinkins SP, et al. : In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol. 1997;6(1):33–9. 10.1046/j.1365-2583.1997.00157.x [DOI] [PubMed] [Google Scholar]
- 13. Rousset F, Vautrin D, Solignac M: Molecular identification of Wolbachia, the agent of cytoplasmic incompatibility in Drosophila simulans, and variability in relation with host mitochondrial types. Proc Biol Sci. 1992;247(1320):163–8. 10.1098/rspb.1992.0023 [DOI] [PubMed] [Google Scholar]
- 14. Stouthamer R, Breeuwer JA, Hurst GD: Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. 10.1146/annurev.micro.53.1.71 [DOI] [PubMed] [Google Scholar]
- 15. Dutra HL, Rocha MN, Dias FB, et al. : Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19(6):771–4. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson KN: The Impact of Wolbachia on Virus Infection in Mosquitoes. Viruses. 2015;7(11):5705–17. 10.3390/v7112903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rainey SM, Shah P, Kohl A, et al. : Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol. 2014;95(Pt 3):517–30. 10.1099/vir.0.057422-0 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson NM, Kien DT, Clapham H, et al. : Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7(279): 279ra37. 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villar LA, Rojas DP, Besada-Lombana S, et al. : Epidemiological trends of dengue disease in Colombia (2000–2011): a systematic review. PLoS Negl Trop Dis. 2015;9(3):e0003499. 10.1371/journal.pntd.0003499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez M, Gomez S: Chikungunya en Colombia, el inicio de la transmisión autóctona, 2014. IQUEN. 2014;19(18):260–79. Reference Source [Google Scholar]
- 21. National Health Institute: Chikungunya Public Health Surveillance Protocol.2017; Accessed 5 Nov 2018. Reference Source [Google Scholar]
- 22. National Health Institute: Zika Public Health Surveillance Protocol.2017; Accessed 5 Nov 2018. Reference Source [Google Scholar]
- 23. De Serres G, Skowronski DM, Wu XW, et al. : The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18(37): pii: 20585. 10.2807/1560-7917.es2013.18.37.20585 [DOI] [PubMed] [Google Scholar]
- 24. Foppa IM, Haber M, Ferdinands JM, et al. : The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31(30):3104–9. 10.1016/j.vaccine.2013.04.026 [DOI] [PubMed] [Google Scholar]
- 25. Jackson ML, Nelson JC: The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–8. 10.1016/j.vaccine.2013.02.053 [DOI] [PubMed] [Google Scholar]
- 26. Orenstein EW, De Serres G, Haber MJ, et al. : Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36(3):623–31. 10.1093/ije/dym021 [DOI] [PubMed] [Google Scholar]
- 27. Bernal JL, Cummins S, Gasparrini A: Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55. 10.1093/ije/dyw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gasparrini A, Gorini G, Barchielli A: On the relationship between smoking bans and incidence of acute myocardial infarction. Eur J Epidemiol. 2009;24(10):597–602. 10.1007/s10654-009-9377-0 [DOI] [PubMed] [Google Scholar]
- 29. Steinbach R, Perkins C, Tompson L, et al. : The effect of reduced street lighting on road casualties and crime in England and Wales: controlled interrupted time series analysis. J Epidemiol Community Health. 2015;69(11):1118–24. 10.1136/jech-2015-206012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Linden A: Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15(2):480–500. 10.1177/1536867X1501500208 [DOI] [Google Scholar]
- 31. Lagarde M: How to do (or not to do) ... Assessing the impact of a policy change with routine longitudinal data. Health Policy Plan. 2012;27(1):76–83. 10.1093/heapol/czr004 [DOI] [PubMed] [Google Scholar]
- 32. Morrison AC, Minnick SL, Rocha C, et al. : Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4(5):e670. 10.1371/journal.pntd.0000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoang Quoc C, Henrik S, Isabel RB, et al. : Synchrony of Dengue Incidence in Ho Chi Minh City and Bangkok. PLoS Negl Trop Dis. 2016;10(12):e0005188. 10.1371/journal.pntd.0005188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salje H, Lessler J, Endy TP, et al. : Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci U S A. 2012;109(24):9535–8. 10.1073/pnas.1120621109 [DOI] [PMC free article] [PubMed] [Google Scholar]