Abstract
Objectives
Recent studies show that cats could play an important role in the transmission of Leptospira species. There are few reports of leptospirosis on Prince Edward Island (PEI) and none in cats. The objective of this study was to determine the prevalence of serum antibodies against Leptospira serovars and of Leptospira DNA in the urine of a population of free-roaming cats.
Methods
Paired blood and urine samples were collected from 200 cats brought to a trap–neuter–return program. Antibody titers against six Leptospira serovars (Bratislava, Canicola, Gryppotyphosa, Hardjo, Pomona, Icterohaemorrhagiae) were determined by microscopic agglutination test. PCR was performed on urine samples to identify urine shedding of Leptospira DNA.
Results
Antibodies were detected in 20/200 cats (10%) for at least one serovar, with titers ranging from 1:50 to 1:6400 (all serovars tested, except Hardjo). Urine samples of 7/200 cats (3.5%) were PCR-positive.
Conclusions and relevance
Feral cats in PEI had a higher than expected exposure to leptospirosis and can shed DNA from pathogenic Leptospira species in urine. Further studies are needed to determine the prevalence of exposure to leptospirosis in other species on PEI and the potential role of feral cats in transmission of the disease.
Keywords: Leptospira species, microscopic agglutination test, PCR, PEI, feral cats
Introduction
Leptospirosis is a bacterial disease caused by spirochete bacteria belonging to the genus Leptospira. It has been reported in over 150 mammalian species, including humans. It is the most widespread zoonosis in the world and is considered a re-emerging disease in humans and dogs.1–7 In 2000, it was estimated that 500,000 severe cases of human leptospirosis were identified annually worldwide, with a mortality rate >10%. 7 In North America, the disease is not considered endemic and epidemiologic data are lacking. 5 There is increasing concern that more cases of human leptospirosis may develop in the future, as global warming, flooding, increased numbers of reservoir species and asymptomatic carriers have been identified as risk factors for increased prevalence of this disease. 8
Leptospirosis infection can occur either directly through contact with infected urine or indirectly through contact with a contaminated environment (soil or water sources). 9 Many wild and domestic animals are subclinically infected and serve as reservoir hosts, being a potential source of infection for incidental hosts such as humans. 9
In the past, cats were considered to be resistant to leptospirosis.10,11 Experimental infections and recent reports have demonstrated that cats can be infected with leptospires and active leptospirosis infection has been documented in rare cases.10–13 There is rising concern that cats might be a potential reservoir and that their role as a source of transmission has been underestimated. Indeed, several studies have shown that pathogenic leptospires can be found in the urine of cats, with a wide range of prevalence – from 0.8% in Thailand to 67.8% in Taiwan.14–18 Evidence of renal carriage has also been demonstrated, and in one study on Christmas Island, Australia, cats had a much higher renal carriage than rats and were considered a potential reservoir.19,20 In addition, a recent study reported isolation by culture of pathogenic Leptospira species from the urine and kidneys of naturally infected cats, adding to the evidence that cats can be a reservoir. 21 Interestingly, prevalence in geographically similar areas can vary widely, emphasizing the importance of obtaining data for each specific area.18–20, 22
There are a few reports of the presence of leptospirosis on Prince Edward Island (PEI), Canada, but none in cats.23,24 The objectives of our research were to determine the prevalence of exposure of feral cats on PEI to leptospirosis by measuring serum antibody titers and to determine the prevalence of urinary shedding by amplifying pathogenic leptospiral DNA by PCR. In addition, factors that could influence the seroprevalence and PCR status were evaluated, including age, sex, seasonality and localization.
Materials and methods
Study design
This prospective, cross-sectional study was approved by the Animal Care Committee of the Atlantic Veterinary College (AVC), University of Prince Edward Island. Feral cats presenting to the AVC feral cat spay and neuter program between July 2017 and September 2018 were enrolled in the study. Feral cats were defined as free-roaming, unowned, intact cats. They were humanely trapped the night before presentation to the AVC. The weight, sex and approximate age of each cat were recorded. Cats with deciduous teeth were classified as juveniles; all others were classified as adults. Cats were also assigned a colony number, based on the location where they were trapped. All cats underwent general anesthesia as part of the spay or neuter protocol. At that time, venipuncture was performed and a 2 ml blood sample was obtained and placed into a glass tube without additives. Serum was separated and stored at −20°C until being shipped for microscopic agglutination test (MAT) evaluation within 60 days. Urine was collected by manual expression of the bladder and stored at –4°C.
MAT
A MAT was performed on each serum sample by a commercial laboratory (Diagnostic Center for Population and Animal Health, Michigan State University, Lansing, MI, USA). The samples were tested for Leptospira interrogans serovars Bratislava, Canicola, Icterohaemorrhagiae, Hardjo and Pomona, and for Leptospira kirschneri serovar Grippotyphosa, belonging to serogroups Australis, Canicola, Icterohaemorrhagiae, Sejroe, Pomona and Grippotyphosa. Titers ⩾1:50 were considered positive.
PCR assay
Urine samples (1–2 ml) were processed within 72 h of collection by a commercial laboratory (IDEXX Reference Laboratories, Markham, ON, Canada). The target gene used in this PCR assay is the outer membrane lipoprotein expressed by pathogenic Leptospira species, with a reported sensitivity of 92% and specificity of 99% (R Chan, IDEXX Laboratory, 2017, personal communication).
In parallel, the remaining urine (volumes ranging from 1 to 20 ml) was processed within 24 h of collection on site (Biomedical Sciences Department, AVC, University of Prince Edward Island). Each urine sample was centrifuged (20,000 g at –4°C) for 10 mins. The supernatant was discarded and the pellet was washed with 500 µl phosphate-buffered saline (PBS). After a second centrifugation, the supernatant was discarded and the pellet was resuspended in 200 µl PBS. Total DNA was extracted with the PureLink Genomic DNA MinKit (Invitrogen), following the manufacturer’s instructions (mammalian cells lysate protocol). DNA concentration and quality were determined using the NanoDrop 2000 Spectrophotometer (ThermoFisher Scientific). The samples were kept at –20°C until PCR analysis.
DNA was amplified by multiplex PCR using two sets of previously described primers. 25 G1–G2 and B64I–B64II primers, targeting specific pathogenic genes, can amplify a 285 base pair (bp) DNA fragment from L interrogans serovars Pomona, Canicola, Hardjo, Icterohaemorrhagiae, Autumnalis and Bratislava, and a 352 bp DNA fragment from L interrogans serovar Sejroe and L kirschneri serovar Gripptyphosa.
PCR components were 12.5 µl Econotaq Plus Green Master Mix (Lucigen), 2 µl primer (working concentration of 0.8 µM), 5.5 µl nuclease-free water and 5 µl of extracted DNA for a total volume of 25 µl. Each run contained standard negative (nuclease-free water, DNA from PCR-negative feline urine) and positive (pathogenic Leptospira DNA, DNA from PCR-positive feline urine) controls. The negative biological control was a cat housed and used as a blood donor at AVC, with no outdoor access and with a negative urine PCR performed at the commercial laboratory. The positive biological control was urine spiked with pathogenic Leptospira DNA. The amplification protocol was carried out in a thermocycler under the following conditions: initial polymerase activating temperature of 95°C for 12 mins, followed by 35 cycles of denaturing at 95°C for 20 s, annealing at 55°C for 30 s, elongatation at 72°C for 40 s and a final elongation step at 72°C for 7 mins. PCR amplicons were subjected to electrophoresis on a 2% agarose gel with ethidium bromide, with a 100 bp ladder run concurrently.
Statistical analyses
The sample size was calculated using the formula n = z2 × p × (1 – p)/d2, with n being the required sample size, z the standard score (1.96 for a 95% confidence interval), p the expected prevalence and d the precision in proportion of one. Based on previous published studies of seroprevalence in cats in Quebec and the small amount of canine leptospirosis confirmed at the AVC veterinary diagnostic laboratory, we calculated a required sample size of 200 cats, with an estimated prevalence of 5% and a 3.5% precision.16,24 The majority of data are presented with descriptive and summary statistics. Fisher’s exact test was used to compare seroprevalence and urinary shedding by sex and age. The association between seasonality and seroprevalence percentage was tested with a χ2 test.
Results
Paired blood and urine samples were obtained from 200 feral cats from 70 different locations on PEI (see Figure 1 and Table 1 in the supplementary material). Eighty-three were females (41.5%) and 117 were males (58.5%). Seventy-one were classified as juveniles (35.5%) and 129 as adults (64.5%).
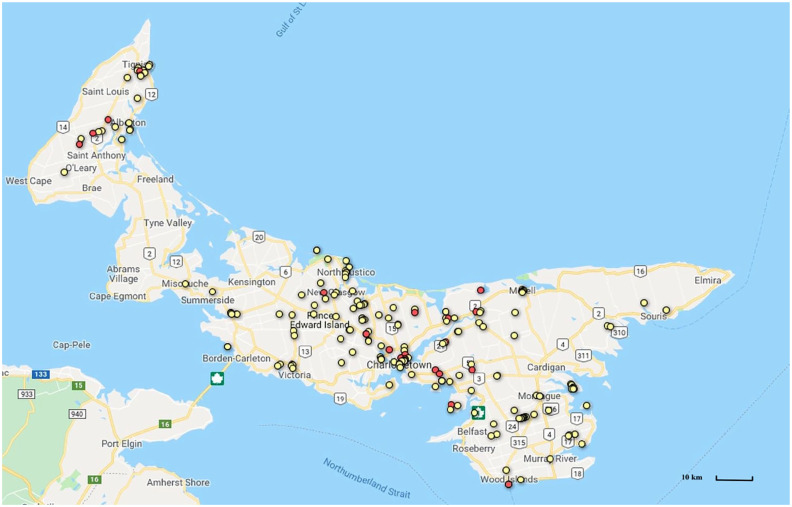
Figure 1.
Geographic distribution of the negative (yellow dots) and positive (red dots) microscopic agglutination tests for Leptospira species in 200 feral cats on Prince Edward Island. The numbers correspond to the highway route numbers
Seroprevalence (MAT results)
Twenty of 200 cats (10%; 95% CI 6.2–15) had positive antibody titers (⩾1:50). Seroprevalence for the different serovars was variable (Table 1).
Table 1.
Seroprevalence of six Leptospira serovars in 200 feral cats on Prince Edward Island
| Serovar | Number of positive samples | Seroprevalence (%) | 95% confidence interval |
|---|---|---|---|
| Bratislava | 3 | 1.5 | 0.3–4.3 |
| Canicola | 9 | 4.5 | 2.1–8.4 |
| Grippotyphosa | 9 | 4.5 | 2.1–8.4 |
| Hardjo | 0 | 0 | – |
| Icterohaemorrhagiae | 11 | 5.5 | 2.8–9.6 |
| Pomona | 3 | 1.5 | 0.3–4.3 |
The most common serovars were Icterohaemorrhagiae, followed by Grippotyphosa and Canicola. Less common serovars were Bratislava and Pomona. Antibodies against serovar Hardjo were not detected.
The majority of positive reactions had titer values of 1:50 or 1:100 (Table 2). Antibody titers ranged from 1:50 to ⩾1:6400, with the highest titers recorded for Canicola. Thirteen serum samples (6.5%) were found to react to only one serovar and seven samples (3.5%) reacted to more than one serovar. Of these seven samples, one reacted to two serovars, four reacted to three serovars and two reacted to four serovars. High titers of ⩾1:400 were found in six cats against serovar Canicola and in one cat against serovar Pomona.
Table 2.
Number of positive microscopic agglutination tests (⩾1:50) for six different Leptospira serovars in 200 feral cats on Prince Edward Island
| Serogroup | Serovar | 1:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:3200 | ⩾1:6400 |
|---|---|---|---|---|---|---|---|---|
| Australis | Bratislava | 3 | ||||||
| Canicola | Canicola | 2 | 1 | 1 | 1 | 4 | ||
| Grippotyphosa | Grippotyphosa | 6 | 1 | 2 | ||||
| Sejroe | Hardjo | |||||||
| Icterohaemorrhagiae | Icterohaemorrhagiae | 8 | 3 | |||||
| Pomona | Pomona | 2 | 1 |
There were more female than male cats with positive antibody titers, but the difference was not statistically significant (14.4% vs 6.8%; P = 0.09). There were more adult than juvenile cats with positive antibody titers and the difference was considered significant (13.2% vs 4.2%; P <0.05).
Positive titers were found in various locations throughout PEI (Figure 1). No obvious clusters of positive cats were found. Results were also compared based on the time of the year, with three time periods considered, based on seasonal patterns of leptospirosis cases in Canada: June–August (5%), September–November (9.3%) and December–May (17%). 26 Although there were more positive samples in December–May, the seroprevalence did not vary statistically with seasonality (χ2 test, P = 0.11).
Individual demographic, geographic and laboratory characteristics for the 20 positive cats can be found in Table 2 in the supplementary material.
DNA PCR results
The urine samples of five cats (2.5%; 95% CI 0.8–5.7) were PCR-positive when the samples were processed at the commercial laboratory. Seven urine samples (3.5%; 95% CI 1.4–7.1) were PCR-positive when processed on site and included the five positive samples processed at the commercial laboratory. DNA quality was considered adequate for all samples. PCR-negative controls tested negative and PCR-positive controls tested positive. All seven samples produced a PCR product of 285 bp. All PCR positive cats had antibody titers ⩾1:200, apart from one cat that was negative for serology. The demographics and laboratory characteristics of PCR-positive cats are presented in Table 3.
Table 3.
Demographic and laboratory characteristics of seven PCR-positive cats for the presence of Leptospira species in urine, among 200 feral cats tested on Prince Edward Island
| Sex | Age | Month of collection | MAT titers | Location |
|---|---|---|---|---|
| Male | Juvenile | October | Bratislava 1:50 Grippotyphosa 1:50 Icterohaemorrhagiae 1:100 Canicola >1:6400 |
Charlottetown |
| Female | Adult | November | Grippotyphosa 1:200 | Mount Stewart |
| Female | Adult | January | Negative | Ebenezer |
| Male | Adult | April | Bratislava 1:50 Grippotyphosa 1:50 Icterohaemorrhagiae 1:50 Canicola >1:6400 |
Bell Heights |
| Female | Adult | June | Canicola 1:3200 | Springvale |
| Female | Juvenile | February | Grippotyphosa 1:100 Icterohaemorrhagiae 1:50 Canicola >1:6400 |
Elmsdale |
| Female | Adult | October | Canicola 1:800 | Bloomfield |
MAT = microscopic agglutination test
Discussion
The seroprevalence for feline exposure to Leptospira species obtained in this study was 10%, which is similar to prevalences reported in most other studies in North America, ranging from around 5% to 17%, apart from one study from Quebec where the prevalence was 25%.16,27–31 However, the cut-off value used in our study for antibody titers was 1:50, which is lower than in most other studies and could have overestimated the prevalence in comparison to other studies. Indeed, the seroprevalence would be 6% instead of 10% if the cut-off value had been 1:100.
There are a number of reasons why 1:50 was used as a cut-off value in this study. There is no consensus on the most appropriate cut-off value to choose in cats, and cats are thought to respond to infection with low antibody titers, ranging from 1:30 to 1:400, as has been demonstrated in experimental and naturally occurring infections.16,30–35 Indeed, in a study from Taiwan, 67.8% of cats were shedding leptospires in their urine, but only 9.3% had antibodies detected by MAT. 14 Low antibody titers could also represent cross-reactions from serovars not tested or not yet described. 17 In addition, a study in bovines showed good specificity and sensitivity for MAT with a ⩾1:50 cut-off, when compared to microbiological culture. 36 For these reasons, a cut-off value of 1:50 was chosen in this study, as proposed by other investigators.18,31,32,35
In this study, antibody titers varied widely among seropositive cats. High titers, defined as titers ⩾1:400, with some reaching the maximal dilution performed (>1:6400), were found in eight cats. This is in contrast to most other studies, where titers were <1:400, but similar to what was reported in a study from Quebec.16,30–35 Such high titers could represent a recent or active infection. 29 However, the majority of cats had low antibody titers. As discussed above, low antibody titers have been documented in cats not only exposed to, but also infected with Leptospira species, and there is concern that in some cases the low magnitude may be due to cross-reactions from serovars not tested. 18 It is also unknown how long titers can persist in cats and low titers could represent old exposure. 18
In the tested population of feral cats from PEI the highest prevalence was found for the serovar Icterohemorrhagiae, which would suggest rats as the source of infection for some of the cats.1,4,37 The serovar Grippotyphosa was also predominant, which could reflect the presence of a reservoir in wild small mammals such as raccoons, skunks or voles.16,38 Interestingly, nearly half of the cats had positive antibody titers against the serovar Canicola, with most of them being extremely high titers (⩾1:3200). Although this serovar has been reported in cats, it is not commonly found in this species. 39 Although dogs are known to be the maintenance host, it has been described in swine and some human cases.40–42 Canicola has also been detected in cattle in Brazil. 43 This serovar has not been reported in wildlife studies, including studies of red foxes and coyotes.38,44 This raises the question as to whether dogs on PEI could serve as a reservoir of leptospirosis, despite very few reported clinical cases.24,39,45
These results should be interpreted with caution. It has been shown that the predominant serogroup with a titre ⩾1:100 predicted correctly only 46.4% of all serovars isolated in humans. 17 Cross-reactivity to non-vaccinal serogroups was demonstrated in dogs, as well as in cats.28,46 The study in cats showed not only that antibodies against serovars (and serogroups) not contained in the vaccine were present, but also that antibodies against serovars contained within the vaccine were not detected. As such, it is possible that the highest titers found in our population of cats are not representative of the specific serovar they were exposed to.
Our results did not show any statistical difference in the distribution of males and females among seropositive cats, but more adults than juveniles had positive antibody titers. This was not unexpected, as it has been shown that older age is associated with higher antibody titers, probably due to longer possible exposure times.10,47,48
One of the goals of our study was to determine if there were geographic clusters of urinary shedding or seropositivity on PEI. The cats in our study came from various locations throughout PEI and no single location was over-represented. Future studies should not be restrained to a narrow specific area.
Surprisingly, the seroprevalence was not statistically different during the three periods of the year used for analysis. It has been shown that most canine cases of leptospirosis in Canada, are diagnosed between August and November, and there is a correlation with rainfall 3 months prior. 26 However, this seasonality was reported for clinical cases and not a survey of seroprevalence. In addition, winter was relatively mild during our study period, which might have explained the outbreak of canine cases in Nova Scotia during that winter. 49
Urinary excretion of leptospires was confirmed by positive urine PCR in seven cats when performed on site but only five positive results when performed by a commercial laboratory. This difference is most likely explained by immediate extraction of DNA on site in comparison to a delay of 24–72 h off site. Studies have shown that degradation of DNA occurs when fresh urine is stored at 4°C or lower temperatures, making recovery of DNA more difficult. 50 False positives would also be possible but seem unlikely, given that the PCR used on site has been shown to be 100% specific and negative controls were consistently negative. 25
The prevalence of urinary shedding was low and similar to those reported in other studies.16,17 It was also much lower than the seroprevalence, which is not unexpected, as intermittent shedding is common. 4 Only one cat that was PCR positive was MAT negative, which could be explained by a chronic carrier state. 14 The presence of urinary shedding by feral cats raises public health concerns and further studies are warranted to accurately determine risks.
The present study has several limitations. The population studied could not be evaluated for clinical illness. Although all cats appeared healthy, no history, long-term observation, additional blood work or paired serum titers were available, and it was not possible to distinguish clinical illness from exposure. Follow-up urine PCR would have helped determine if the cats were chronic, sporadic or one-time shedders but could not be performed owing to limited access to the cats and limited finances. The serovars chosen in our study were based on reports in cats in Canada and in other species on PEI.16,23 However, the range of serovars tested should not be limited to local strains according to the Guidelines of International Leptospirosis Society. 18 As we limited the study to six serovars, the true seroprevalence might be higher, as false-negative results are possible.
Conclusions
We have shown that feral cats on PEI are exposed to leptospirosis, and that the seroprevalence and urinary shedding are not negligible and higher than expected. Further studies are needed to determine the prevalence of exposure to leptospirosis in other species on PEI and the potential role of feral cats as sentinels for other species or in transmission of the disease.
Supplemental Material
Distribution of the negative and positive microscopic agglutination tests for Leptospira species per location in 200 feral cats on Prince Edward Island
Demographic, geographic and laboratory characteristics of 20 cats positive for Leptospira species on Prince Edward Island, based on microscopic agglutination test
Footnotes
Accepted: 9 February 2021
Author note: Some of these data were presented as a poster at the annual forum of the American College of Veterinary Internal Medicine, Phoenix, AZ, USA, June 2019.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the AVC Internal Research fund.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
Supplementary material: The following files are available online:
Table 1: Distribution of the negative and positive microscopic agglutination tests for Leptospira species per location in 200 feral cats on Prince Edward Island.
Table 2: Demographic, geographic and laboratory characteristics of 20 cats positive for Leptospira species on Prince Edward Island, based on microscopic agglutination test.
ORCID iD: Emilia Bourassi  https://orcid.org/0000-0002-0213-928X
https://orcid.org/0000-0002-0213-928X
References
- 1. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 2003; 3: 757–771. [DOI] [PubMed] [Google Scholar]
- 2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect 2011; 17: 494–501 [DOI] [PubMed] [Google Scholar]
- 3. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 2009; 7: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levett PN. Leptospirosis. Clin Microbiol Rev 2001; 14: 296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pappas G, Papadimitriou P, Siozopoulou V, et al. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis 2008; 12: 351–357. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Leptospirosis worldwide. Wkly Epidemiol Rec 1999; 74: 237–242. [PubMed] [Google Scholar]
- 7. World Health Organization. Leptospirosis: an emerging public health problem. Wkly Epidemiol Rec 2009; 6: 45–52. [PubMed] [Google Scholar]
- 8. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis 2015; 9: e0003898. DOI: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartmann K, Egberink H, Pennisi MG, et al. Leptospira species infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arbour J, Blais MC, Carioto L, et al. Clinical leptospirosis in three cats (2001–2009). J Am Anim Hosp Assoc 2012; 48: 256–260. [DOI] [PubMed] [Google Scholar]
- 11. Fessler JF, Morter RL. Experimental feline leptospirosis. Cornell Vet 1964; 54: 176–190. [PubMed] [Google Scholar]
- 12. Mason RW, King SJ, McLachlan NM. Suspected leptospirosis in two cats. Aust Vet J 1972; 48: 622–623. [DOI] [PubMed] [Google Scholar]
- 13. Bryson DG, Ellis WA. Leptospirosis in a British domestic cat. J Small Anim Pract 1976; 17: 459–465. [DOI] [PubMed] [Google Scholar]
- 14. Fenimore A, Carter K, Lunn KF. Detection of leptospiruria in shelter cats in Colorado [abstract]. J Vet Intern Med 2012; 26: 783. [Google Scholar]
- 15. Chan KW, Hsu YH, Hu WL, et al. Serological and PCR detection of feline Leptospira in Southern Taiwan. Vector Borne Zoonotic Dis 2014; 14: 118–123. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez J, Blais MC, Lapointe C, et al. Serologic and urinary PCR survey of leptospirosis in healthy cats and in cats with kidney disease. J Vet Intern Med 2014; 28: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weis S, Rettinger A, Bergmann M, et al. Detection of Leptospira DNA in urine and presence of specific antibodies in outdoor cats in Germany. J Feline Med Surg 2017; 19: 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sprißler F, Jongwattanapisan P, Luengyosluechakul S, et al. Leptospira infection and shedding in cats in Thailand. Transbound Emerg Dis 2019; 66: 948–956. [DOI] [PubMed] [Google Scholar]
- 19. Desvars A, Naze F, Benneveau A, et al. Endemicity of leptospirosis in domestic and wild animal species from Reunion Island (Indian Ocean). Epidemiol Infect 2013; 141: 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dybing N, Jacobson C, Irwin P, et al. Leptospira species in feral cats and black rats from Western Australia and Christmas Island. Vector Borne Zoonotic Dis 2017; 17: 319–324. [DOI] [PubMed] [Google Scholar]
- 21. Alashraf AR, Lau SF, Khairani-Bejo S, et al. First report of pathogenic Leptospira spp isolated from urine and kidneys of naturally infected cats. PLoS One 2020; 15: e0230048. DOI: 10.1371/journal.pone.0230048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomard Y, Lagadec E, Humeau L, et al. Feral cats do not play a major role in leptospirosis epidemiology on Reunion Island. Epidemiol Infect 2019; 147: e97. DOI: 10.1017/S0950268819000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Til L, Dohoo I. A serological survey of leptospirosis in Prince Edward Island swine herds and its association with infertility. Can J Vet Res 1991; 55: 352–355. [PMC free article] [PubMed] [Google Scholar]
- 24. Gilroy C, MacKenzie A, MacMahon E. Update on canine leptospirosis in Atlantic Canada. Diagnostic update from Diagnostic Services Laboratory AVC. 2010; 4: 6–7. Availabe at: https://files.upei.ca/avc/diagnosticservices/newsletters/avcds_aug2010_vol4-02.pdf [Google Scholar]
- 25. Cai HY, Hornby G, Key DW, et al. Preliminary study on differentiation of Leptospira grippotyphosa and Leptospira sejroe from other common pathogenic leptospiral serovars in canine urine by polymerase chain reaction assay. J Vet Diagn Invest 2002; 14: 164–168. [DOI] [PubMed] [Google Scholar]
- 26. Ward MP. Seasonality of canine leptospirosis in the United States and Canada and its association with rainfall. Prev Vet Med 2002; 56: 203–213. [DOI] [PubMed] [Google Scholar]
- 27. Markovich JE, Ross L, McCobb E. The prevalence of leptospiral antibodies in free roaming cats in Worcester County, Massachusetts. J Vet Intern Med 2012; 26: 688–689. [DOI] [PubMed] [Google Scholar]
- 28. Shropshire SB, Veir JK, Morris AK, et al. Evaluation of the Leptospira species microscopic agglutination test in experimentally vaccinated cats and Leptospira species seropositivity in aged azotemic client-owned cats. J Feline Med Surg 2016; 18: 768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lapointe C, Plamondon I, Dunn M. Feline leptospirosis serosurvey from a Quebec referral hospital. Can Vet J 2013; 54: 497–499. [PMC free article] [PubMed] [Google Scholar]
- 30. Palerme JS, Lamperelli E, Gagne J, et al. Seroprevalence of Leptospira spp, Toxoplasma gondii, and Dirofilaria immitis in free-roaming cats in Iowa. Vector Borne Zoonotic Dis 2019; 19: 193–198. [DOI] [PubMed] [Google Scholar]
- 31. Mylonakis ME, Bourtzi-Hatzopoulou E, Koutinas AF, et al. Leptospiral seroepidemiology in a feline hospital population in Greece. Vet Rec 2005; 156: 615–616. [DOI] [PubMed] [Google Scholar]
- 32. Agunloye CA, Nash AS. Investigation of possible leptospiral infection in cats in Scotland. J Small Anim Pract 1996; 37: 126–129. [DOI] [PubMed] [Google Scholar]
- 33. Dickeson D, Love DN. A serological survey of dogs, cats and horses in south-eastern Australia for leptospiral antibodies. Aust Vet J 1993; 70: 389–390. [DOI] [PubMed] [Google Scholar]
- 34. Lucke VM, Crowther ST. The incidence of leptospiral agglutination titres in the domestic cat. Vet Rec 1965; 77: 647–648. [PubMed] [Google Scholar]
- 35. Shophet R. A serological survey of leptospirosis in cats. N Z Vet J 1979; 27: 245–236. [DOI] [PubMed] [Google Scholar]
- 36. Hernández-Rodríguez P, Díaz CA, Dalmau EA, et al. A comparison between polymerase chain reaction (PCR) and traditional techniques for the diagnosis of leptospirosis in bovines. J Microbiol Methods 2011; 84: 1–7. [DOI] [PubMed] [Google Scholar]
- 37. McKiel JA, Cousineau JG, Hall RR. Leptospirosis in wild animals in Eastern Canada with particular attention to the disease in rats. Can J Comp Med Vet Sci 1961; 25: 15–18. [PMC free article] [PubMed] [Google Scholar]
- 38. Shearer KE, Harte MJ, Ojkic D, et al. Detection of Leptospira spp in wildlife reservoir hosts in Ontario through comparison of immunohistochemical and polymerase chain reaction genotyping methods. Can Vet J 2014; 55: 240–248. [PMC free article] [PubMed] [Google Scholar]
- 39. Andre-Fontaine G. Canine leptospirosis — do we have a problem? Vet Microbiol 2006; 17: 19–24. [DOI] [PubMed] [Google Scholar]
- 40. Schuller S, Francey T, Hartmann K, et al. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract 2015; 56: 159–179. [DOI] [PubMed] [Google Scholar]
- 41. Trevejo RT, Rigau-Pérez JG, Ashford DA, et al. Epidemic leptospirosis associated with pulmonary hemorrhage –Nicaragua. J Infect Dis 1998; 178: 1457–1463. [DOI] [PubMed] [Google Scholar]
- 42. Truong QL, Seo TW, Yoon BI, et al. Prevalence of swine viral and bacterial pathogens in rodents and stray cats captured around pig farms in Korea. J Vet Med Sci 2013; 75: 1647–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miraglia F, Morais ZM, Dellagostin OA, et al. Molecular and serological characterization of Leptospira interrogans serovar Canicola isolated from dogs, swine, and bovine in Brazil. Trop Anim Health Prod 2013; 45: 117–121. [DOI] [PubMed] [Google Scholar]
- 44. Gese EM, Schultz RD, Johnson MR, et al. Serological survey for diseases in free-ranging coyotes (Canis latrans) in Yellowstone National Park, Wyoming. J Wildl Dis 1997; 33: 47–56. [DOI] [PubMed] [Google Scholar]
- 45. Miotto BA, Guilloux AGA, Tozzi BF, et al. Prospective study of canine leptospirosis in shelter and stray dog populations: identification of chronic carriers and different Leptospira species infecting dogs. PLoS One 2018; 13: e0200384. DOI: 10.1371/journal.pone.0200384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin LE, Wiggans KT, Wennogle SA, et al. Vaccine-associated Leptospira antibodies in client-owned dogs. J Vet Intern Med 2014; 28: 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Betance L, Peda A, Conan A, et al. Seroprevalence of leptospirosis in the feral cat population of St. Kitts. J Anim Res Technol 2017; 38–42. [Google Scholar]
- 48. Larsson CE, Santa Rosa CA, Larsson MH, et al. Laboratory and clinical features of experimental feline leptospirosis. Int J Zoonoses 1985; 12: 111–119. [PubMed] [Google Scholar]
- 49. Zoetis Canada. Leptospirosis: closer than you think? https://www.zoetis.ca/lepto/index.aspx (2019, accessed June 20, 2019).
- 50. Hilhorst M, Theunissen R, Van Rie H, et al. DNA extraction from long-term stored urine. BMC Nephrol 2013; 14: 238. DOI: 10.1186/1471-2369-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of the negative and positive microscopic agglutination tests for Leptospira species per location in 200 feral cats on Prince Edward Island
Demographic, geographic and laboratory characteristics of 20 cats positive for Leptospira species on Prince Edward Island, based on microscopic agglutination test